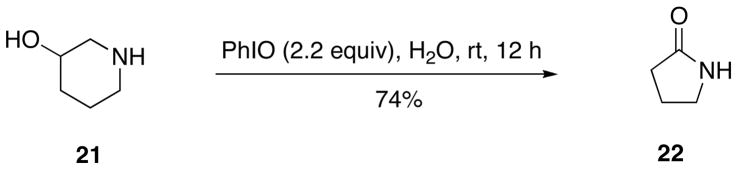1. Introduction
Starting from the early 1990’s, the chemistry of polyvalent iodine organic compounds has experienced an explosive development. This surging interest in iodine compounds is mainly due to the very useful oxidizing properties of polyvalent organic iodine reagents, combined with their benign environmental character and commercial availability. Iodine(III) and iodine(V) derivatives are now routinely used in organic synthesis as reagents for various selective oxidative transformations of complex organic molecules. Several areas of hypervalent organoiodine chemistry have recently attracted especially active interest and research activity. These areas, in particular, include the synthetic applications of 2-iodoxybenzoic acid (IBX) and similar oxidizing reagents based on the iodine(V) derivatives, the development and synthetic use of polymer-supported and recyclable polyvalent iodine reagents, the catalytic applications of organoiodine compounds, and structural studies of complexes and supramolecular assemblies of polyvalent iodine compounds.
The chemistry of polyvalent iodine has previously been covered in four books1–4 and several comprehensive review papers.5–17 Numerous reviews on specific classes of polyvalent iodine compounds and their synthetic applications have recently been published.18–61 Most notable are the specialized reviews on [hydroxy(tosyloxy)iodo]benzene,41 the chemistry and synthetic applications of iodonium salts,29,36,38,42,43,46,47,54,55 the chemistry of iodonium ylides,56–58 the chemistry of iminoiodanes,28 hypervalent iodine fluorides,27 electrophilic perfluoroalkylations,44 perfluoroorgano hypervalent iodine compounds,61 the chemistry of benziodoxoles,24,45 polymer-supported hypervalent iodine reagents,30 hypervalent iodine-mediated ring contraction reactions,21 application of hypervalent iodine in the synthesis of heterocycles,25,40 application of hypervalent iodine in the oxidation of phenolic compounds,32,34,50–53,60 oxidation of carbonyl compounds with organohypervalent iodine reagents,37 application of hypervalent iodine in (hetero)biaryl coupling reactions,31 phosphorolytic reactivity of o-iodosylcarboxylates,33 coordination of hypervalent iodine,19 transition metal catalyzed reactions of hypervalent iodine compounds,18 radical reactions of hypervalent iodine,35,39 stereoselective reactions of hypervalent iodine electrophiles,48 catalytic applications of organoiodine compounds,20,49 and synthetic applications of pentavalent iodine reagents.22,23,26,59
The main purpose of the present review is to summarize the data that appeared in the literature following publication of our previous reviews in 1996 and 2002. In addition, a brief introductory discussion of the most important earlier works is provided in each section. The review is organized according to the classes of organic polyvalent iodine compounds with emphasis on their synthetic application. Literature coverage is through July 2008.
2. Structure and Bonding
2.1. General Features
Structural aspects of polyvalent iodine compounds have previously been discussed in our original 1996 review5 and in the 1992 monograph by Varvoglis.2 More recently, general aspects of structure and bonding in hypervalent organic compounds have been summarized by Akiba in the book on Chemistry of Hypervalent Compounds62 and by Ochiai in a chapter in the volume on Hypervalent Iodine Chemistry in Topics in Current Chemistry.1 A brief summary of the key structural features of iodine(III) and iodine(V) compounds is provided below.
All known organic polyvalent iodine derivatives belong to two general structural types: (1) iodine(III) compounds 1 and 2, also named λ3-iodanes according to IUPAC recommendations, and (2) iodine(V) compounds 3, or λ5-iodanes. The iodine atom in λ3-iodanes 1 has a total 10 electrons and the overall geometry of a distorted trigonal bipyramid with two heteroatom ligands X occupying the apical positions, and the least electronegative carbon ligand R and both electron pairs residing in equatorial positions. Iodonium salts 2, which have two carbon ligands and a closely associated anionic part of the molecule, have a similar pseudo trigonal bipyramidal geometry and also belong to λ3-iodanes. In agreement with this model, the experimentally determined bond angle R–I–R in iodonium salts and ylides is close to 90°. In the hypervalent model, bonding in RIX2 uses the non-hybridized 5p orbital of iodine in the linear X–I–X bond. Such a linear three-center, four-electron (3c–4e) bond is highly polarized and is longer and weaker compared to a regular covalent bond. This bond is termed “hypervalent” and the presence of this bond in λ3-iodanes is responsible for their high electrophilic reactivity.
Organic λ5-iodanes 3 have a distorted octahedral structure with the organic group R and the electron pair in the apical positions and four heteroatom ligands X in basal positions. Two orthogonal hypervalent 3c–4e bonds accommodate all ligands X, while the apical group R is connected to iodine by a normal covalent bond using 5sp-hybridized orbital.2 In general, only λ3- and λ5-iodanes with an aromatic group R (R = aryl or hetaryl) have sufficient stability and can be isolated. A few examples of alkyl substituted λ3-iodanes stabilized by strong electron-withdrawing groups (perfluoroalkyl or arylsulfonylmethyl λ3-iodanes) have also been isolated. The stable aryl substituted λ3- and λ5-iodanes possess high chemical reactivity and are widely used in organic synthesis as oxidants and electrophilic agents, which are commonly referred to as “hypervalent iodine reagents”.

2.2. Computational Studies
A relatively small number of theoretical computational studies concerning the structure and reactivity of hypervalent iodine compounds have appeared in the last 10 years.63–76 Hoffmann and co-workers analyzed the nature of hypervalent bonding in trihalide anions by applying ideas from qualitative MO theory to computational results from density-functional calculations.63 This systematic, unified investigation showed that the bonding in all of these systems can be explained in terms of the Rundle-Pimentel scheme for electron-rich three-center bonding. The same authors reported an analysis of intermolecular interaction between hypervalent molecules, including diaryliodonium halides Ar2IX, using a combination of density functional calculations and qualitative arguments.64 Based on fragment molecular orbital interaction diagrams, the authors concluded that the secondary bonding in these species can be understood using the language of donor-acceptor interactions: mixing between occupied states on one fragment and unoccupied states on the other. There is also a strong electrostatic contribution to the secondary bonding. The calculated strengths of these halogen-halogen secondary interactions are all less than 10 kcal mol−164
The self-assembly of hypervalent iodine compounds to macrocyclic trimers was studied using MO calculations. The principal driving force for the self-assembly of iodonium units is the formation of secondary bonding interactions between iodonium units as well as a rearrangement of primary and secondary bonding around iodine to place the least electronegative substituent in the equatorial position for every iodine in the trimer.65
Kiprof has analyzed the iodine oxygen bonds of hypervalent 10-I-3 iodine(III) compounds with T-shaped geometry using the Cambridge Crystallographic Database and ab initio MO calculations. The statistical analysis of the I–O bond lengths in PhI(OR)2 revealed an average of 2.14 Å and a strong correlation between the two bond lengths.66 Further theoretical investigation of the mutual ligand interaction in the hypervalent L–I–L′ system has demonstrated that ligands’ trans influences play an important role in the stability of hypervalent molecules.67 In particular, combinations of ligands with large and small trans influences, as in PhI(OH)OTs, or of two moderately trans influencing ligands, as in PhI(OAc)2, are favored and lead to higher stability of the molecule. trans Influences also seem to explain why iodosylbenzene, (PhIO)n, adopts an oxo-bridged zigzag polymer structure in contrast to PhI(OH)2, which is monomeric.67
The structure and reactivity of several specific classes of hypervalent iodine compounds were theoretically investigated. In particular, Okuyama and Yamataka investigated the reactivity of vinyliodonium ions with nucleophiles by ab initio MO (MP2) calculations at the double-zeta (DZ) + d level.68 It was proposed that interaction of methyl(vinyl)iodonium ion with chlorine anion leads to chloro-λ3-iodane CH2=CHI(Me)Cl. Transition states for the SN2, ligand-coupling substitution, and β-elimination were found for reactions at the vinyl group. The barrier to ligand-coupling substitution is usually the lowest in the gas phase, but relative barriers to SN2 and to β-elimination change with the substituents. Effects of solvent on this reaction were evaluated by a dielectric continuum model and found to be large on SN2 but small on ligand-coupling.68
Widdowson, Rzepa and co-workers reported ab initio and MNDO-d SCF-MO computational studies of the extrusion reactions of diaryliodonium fluorides.69,71 The results of these studies, in particular, predicted that the intermediates and transition states in these reactions might involve dimeric, trimeric, and tetrameric structures. The regioselectivity of nucleophilic substitution in these reactions was investigated theoretically and supported by some experimental observations.69–71
Goddard and Su have theoretically investigated the mechanism of alcohol oxidation with 2-iodoxybenzoic acid (IBX) on the basis of density functional quantum mechanics calculations.72 It has been found that the rearrangement of hypervalent bonds, so called hypervalent twisting, is the rate-determining step in this reaction. Based on this mechanism, the authors explain why IBX oxidizes large alcohols faster than small ones and propose a modification to the reagent predicted to make it more active.72
Bakalbassis, Spyroudis, and Tsiotra reported a DFT study on the intramolecular thermal phenyl migration in iodonium ylides. The results of this study support a single-step mechanism involving a five-membered ring transition-state. The frontier-orbital-controlled migration also confirms the different thermal behavior experimentally observed for two different ylides.77
Molecular orbital computational studies of (arylsulfonylimino)iodoarenes (ArINSO2Ar′),73 benziodazol-3-ones,74 and a series of ortho-substituted chiral organoiodine(III) compounds75 have been reported in the literature. Results of these calculations were found to be in good agreement with X-ray structural data for these compounds.
In a very recent communication, Quideau and co-workers presented DFT calculations of spiroheterocylic iodine(III) intermediates to validate their participation in the PhI(OAc)2-mediated spiroketalization of phenolic alcohols.76
2.3. Experimental Structural Studies
Numerous X-ray crystal structures have been reported for all main classes of organic polyvalent iodine compounds, and the results of these studies will be briefly discussed in the appropriate sections of this review. Several general areas of structural research on hypervalent organoiodine compounds have recently attracted especially active interest. These areas, in particular, include the preparation and structural study of complexes of hypervalent iodine compounds with crown ethers78–82 or nitrogen ligands,83–85 self-assembly of hypervalent iodine compounds into various supramolecular structures,86–88 and the intramolecular secondary bonding in ortho-substituted aryliodine(V) and iodine(III) derivatives.73,89–99
Typical coordination patterns in various organic derivatives of iodine(III) in the solid state with consideration of primary and secondary bonding have been summarized by Sawyer and coworkers100 in 1986 and updated in recent publications.101–104 Structural features of organic iodine(V) compounds have been discussed in older papers of Martin and co-authors,105,106 and in numerous more recent publications on IBX and related λ5-iodanes.89,93–98,107
Several important spectroscopic structural studies of polyvalent iodine compounds in the solution have been published.108–112 Hiller and co-workers reported NMR and LC-MS study on the structure and stability of 1-iodosyl-4-methoxybenzene and 1-iodosyl-4-nitrobenzene in methanol solution.108 Interestingly, LC-MS analyses provided evidence that unlike the parent iodosylbenzene, which has a polymeric structure, the 4-substituted iodosylarenes exist in the monomeric form. Both iodosylarenes are soluble in methanol and provide acceptable 1H and 13C NMR spectra; however, gradual oxidation of the solvent was observed after several hours. Unlike iodosylbenzene, the two compounds did not react with methanol to give the dimethoxy derivative ArI(OMe)2.108
Cerioni, Mocci and co-workers investigated the structure of bis(acyloxy)iodoarenes and benzoiodoxolones in chloroform solution by 17O NMR spectroscopy and also by DFT calculations.109,110 This investigation provided substantial evidence that the T-shaped structure of iodine(III) compounds observed in the solid state is also adopted in solution. Furthermore, the “free” carboxylic groups of bis(acyloxy)iodoarenes show a dynamic behavior, observable only in the 17O NMR. This behavior is ascribed to a [1,3] sigmatropic shift of the iodine atom between the two oxygen atoms of the carboxylic groups, and the energy involved in this process varies significantly between bis(acyloxy)iodoarenes and benzoiodoxolones.110
Richter, Koser and co-workers investigated the nature of species present in aqueous solutions of phenyliodine(III) organosulfonates.111 It was shown by spectroscopic measurements and potentiometric titrations that PhI(OH)OTs and PhI(OH)OMs upon solution in water undergo complete ionization to give the hydroxy(phenyl)iodonium ion (PhI+OH in hydrated form) and the corresponding sulfonate ions. The hydroxy(phenyl)iodonium ion can combine with [oxo(aquo)iodo]benzene PhI+(OH2)O−, a hydrated form of iodosylbenzene that is also observed in the solution, producing the dimeric μ-oxodiiodine cation Ph(HO)I–O–I+(OH2)Ph and dication Ph(H2O)I+–O–I+(OH2)Ph.111
Silva and Lopes analyzed solutions of iodobenzene dicarboxylates in acetonitrile, acetic acid, aqueous methanol and anhydrous methanol by electrospray ionization mass spectrometry (ESI-MS) and tandem mass spectrometry (ESI-MS/MS).112 The major species found in the solutions of PhI(OAc)2 in acetonitrile, acetic acid, and aqueous methanol are [PhI(OAc)2Na]+, [PhI(OAc)2K]+, [PhI]+, [PhIOAc]+, [PhIOH]+, [PhIO2Ac]+, [PhIO2H]+ and the dimer [Ph2I2O2Ac]+. On the other hand, the anhydrous methanol solutions showed [PhIOMe]+ as the most abundant species. In contrast to the data obtained for PhI(OAc)2, the ESI-MS spectral data of PhI(O2CCF3)2 in acetonitrile suggests that the main species in solutions is iodosylbenzene.112
3. Iodine(III) Compounds
Iodine(III) compounds (structures 1 and 2), or λ3-iodanes according to the IUPAC nomenclature, are commonly classified by the type of ligands attached to the iodine atom.2,3,5,6 This section of the review is organized according to the traditional classification and will cover the preparation, structure, and reactivity of iodosylarenes, aryliodine(III) halides, carboxylates, sulfonates, cyclic λ3-iodanes, iodonium salts, ylides, and imides with emphasis on their synthetic application.
3.1. Iodosylarenes
3.1.1. Preparation
The most important representative of iodosylarenes, iodosylbenzene, is best prepared by alkaline hydrolysis of (diacetoxy)iodobenzene.113 The same procedure can be used for the preparation of a variety of ortho-, meta-, and para-substituted iodosylbenzenes from the respective (diacetoxy)iodoarenes (Scheme 1).90–92,108,114 This procedure, for example, was recently used for the preparation of 4-methoxyiodosylbenzene,108 4-nitroiodosylbenzene108 and pseudocyclic iodosylarenes bearing tert-butylsulfonyl91 or diphenylphosphoryl92 groups in the ortho-position.
Scheme 1.
An alternative general procedure for the preparation of iodosylarenes 7 employs the alkaline hydrolysis of (dichloroiodo)arenes under conditions similar to the hydrolysis of (diacetoxyiodo)arenes.115 A modified procedure employs aqueous tetrahydrofuran as the solvent for the hydrolysis of (dichloroiodo)arenes 6 (Scheme 2).116
Scheme 2.
Iodosylbenzene is a yellowish amorphous powder, which cannot be recrystallized due to its polymeric nature; it dissolves in methanol with depolymerization affording PhI(OMe)2.117 Heating or extended storage at room temperature results in disproportionation of iodosylbenzene to PhI and a colorless, explosive iodylbenzene, PhIO2. Drying iodosylbenzene at elevated temperatures should be avoided; a violent explosion of 3.0 g PhIO upon drying at 110 °C in vacuum has recently been reported.118
3.1.2. Structural Studies
Based on spectroscopic studies, it was suggested that in the solid state iodosylbenzene exists as a zigzag polymeric, asymmetrically bridged structure, in which monomeric units of PhIO are linked by intermolecular I•••O secondary bonds.6 The I–O bond distances of 2.04 and 2.37 Å and the C–I–O bond angle near 90° have been deduced from EXAFS analysis of polymeric iodosylbenzene.119 The polymeric structure of iodosylbenzene was also theoretically analyzed by density functional theory computations at the B3LYP level and, in particular, the importance of the presence of a terminal hydration water in its zigzag polymeric structure HO–(PhIO)n–H was established.120 The zigzag asymmetrically bridged structure of (PhIO)n has recently been confirmed by single crystal X-ray diffraction studies of the oligomeric sulfate 8 and perchlorate 9 derivatives.87,121 In particular, iodine atoms in the (PhIO)3 fragment of the oligomeric sulfate 8 exhibit a typical of trivalent iodine T-shaped intramolecular geometry with O-I-O and O-I-C bond angles close to 180° (166.54–177.99) and 90° (79.18–92.43), respectively. The I-O bond distances in the (PhIO)3 fragment of sulfate 8 vary in a broad range of 1.95 to 2.42 Å.121 The single crystal X-ray crystal study of the oligomeric perchlorate 9 revealed a complex structure consisting of pentaiodanyl dicationic units joined by secondary I•••O bonds into an infinite linear structure of 12-atom hexagonal rings.87 The oligomer 8 was prepared by the treatment of PhI(OAc)2 with aqueous NaHSO4, while product 9 precipitated from dilute aqueous solutions of PhI(OH)OTs and Mg(ClO4)2. The formation of both products can be explained by self-assembly of the hydroxy(phenyl)iodonium ions (PhI+OH in hydrated form) and [oxo(aquo)iodo]benzene PhI+(OH2)O− in aqueous solution under reaction conditions.

Ochiai and co-workers have reported the preparation, X-ray crystal structures, and useful oxidizing reactions of activated iodosylbenzene monomer complexes with 18C6 crown ether.19,78 Reaction of iodosylbenzene with HBF4–Me2O in the presence of equimolar 18C6 in dichloromethane afforded quantitatively the stable, crystalline crown ether complex 10, which is soluble in MeCN, MeOH, water, and dichloromethane. X-ray analysis revealed a protonated iodosylbenzene monomer structure 10 stabilized by intramolecular coordination with the crown ether oxygen atoms.78 The aqua complexes of iodosylarenes 11 and 12 with a water molecule coordinated to iodine(III) were prepared by the reaction of (diacetoxyiodo)benzene with trimethylsilyl triflate in the presence of 18C6 crown ether in dichloromethane. X-ray analysis of complex 11 revealed a T-shaped structure, ligated with one water molecule at the apical site of the iodine(III) atom of hydroxy(phenyl)iodonium ion, with a near-linear O–I–O triad (173.96 ). Including a close contact with one of the crown ether oxygens, the complex adopts a distorted square planar geometry around the iodine.122

The ortho-substituted iodosylarenes 13–16 bearing tert-butylsulfonyl,91 diphenylphosphoryl,92 or nitro99 groups have a monomeric, pseudocyclic structure due to the replacement of intermolecular I•••O interactions with intramolecular secondary bonding. The structure of product 13 was established by single crystal X-ray analysis.89

3.1.3. Oxidations with Iodosylarenes
Iodosylbenzene is an effective oxidizing reagent but its insolubility, due to the polymeric structure, significantly restricts its practical usefulness. The overwhelming majority of the known reactions of iodosylbenzene require the presence of a hydroxylic solvent (water or alcohols) or a catalyst (Lewis acid, bromide or iodide anions, transition metal complex, etc.) that can effectively depolymerize (PhIO)n generating the reactive monomeric species. Numerous examples of such oxidations have been reported in our previous reviews5,6 and include, for example, selective oxidation of alcohols123,124 or sulfides125 with (PhIO)n/KBr/H2O, the oxidation of silyl enol ethers to α-hydroxy- and α-alkoxy substituted of carbonyl compounds using (PhIO)n/BF3•Et2O in water or an alcohol,126,127 the generation and sequential fragmentation of radicals from alcohols or amides (e.g., 17 and 18) with the PhIO–I2 system (Scheme 3),128–130 and the oxidation of tetrahydroisoquinolines 19 by (PhIO)n/Bu4NI/H2O to the respective lactams 20 (Scheme 4).131
Scheme 3.
Scheme 4.
Several new oxidations with (PhIO)n have been recently reported. The oxidation of 3-hydroxypiperidine 21 with iodosylbenzene in water affords 2-pyrrolidinone 22 directly in good yield (Scheme 5).132 The mechanism of this reaction probably involves oxidative Grob fragmentation yielding imino aldehyde, which upon hydrolysis affords 2-pyrrolidinone by a cyclization-oxidation sequence.
Scheme 5.
Togo and co-workers have reported the preparation of α-tosyloxy ketones and aldehydes 24 in good yields from alcohols 23 by treatment with iodosylbenzene and p-toluenesulfonic acid monohydrate. This method can also be used for the direct preparation of thiazoles (25, X = S), imidazoles (25, X = NH), and imidazo[1,2-a]pyridines 26 from alcohols in good to moderate yields by the successive treatment with iodosylbenzene and p-toluenesulfonic acid monohydrate, followed by thioamides, benzamidine, and 2-aminopyridine, respectively (Scheme 6).133
Scheme 6.
The reactions of 4-acyloxybut-1-enylsilanes 27 with iodosylbenzene in the presence of BF3•OEt2 afford 4-acyloxy-2-oxobutylsilanes 28, 31 and 3-acyloxytetrahydrofuran-2-ylsilanes 29, 32 via a 1,3-dioxan-2-yl cation intermediate, which is generated by participation of the acyloxy group during the electrophilic addition of iodine(III) species to the substrate (Scheme 7).134
Scheme 7.
Ochiai and co-workers have reported several useful oxidations employing the activated iodosylbenzene species.19,78,122,135,136 The monomeric iodosylbenzene complex 10 in the presence of water can cleave the carbon-carbon double bond of indene 33 with the formation of dialdehyde 34 (Scheme 8).135 Similar oxidative cleavage of various alkenes can be performed by using iodosylbenzene in water in the presence of HBF4. This convenient procedure provides a safe alternative to the ozonolysis of alkenes.135
Scheme 8.
Reaction of 3-phenylpropanol 35 with activated iodosylbenzene complex 10 in dichloromethane in the presence of BF3•OEt2 afforded directly the 6-chromanyl(phenyl)iodonium salt 36 (isolated as a complex with 18C6 crown ether) through tandem oxidative intramolecular cyclization yielding chroman and its subsequent regioselective reaction with complex 10 leading to the final product 36 (Scheme 9).136
Scheme 9.
The oligomeric iodosylbenzene sulfate (PhIO)3•SO3 (structure 8) is a readily available, stable, and water-soluble reagent with reactivity pattern similar to activated iodosylbenzene. It reacts with alkenes, alcohols, and aryl alkyl sulfides in aqueous acetonitrile at room temperature to afford the respective products of oxidation 37–40 in good yields (Scheme 10).88
Scheme 10.
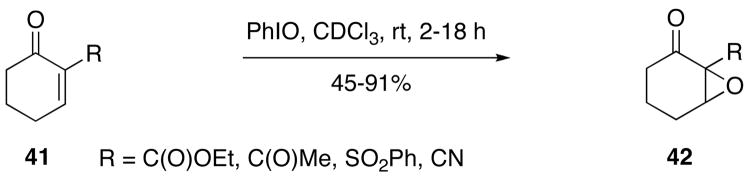
Iodosylbenzene is a useful reagent for nucleophilic epoxidation of electron-deficient alkenes, such as tetrasubstituted perfluoroalkenes137 and α,β-unsaturated carbonyl compounds.118,138 In a specific example, iodosylbenzene reacts with enones 41 to furnish the corresponding epoxides 42 in generally high yields (Scheme 11).118
Scheme 11.
Only very few ArIO other than iodosylbenzene have been used as reagents. The only exception is represented by ortho- and meta-iodosylbenzoic acids. The o-iodosylbenzoic acid (IBA) has a cyclic structure of benziodoxolone and is discussed in Section 3.7 of this review. The m-iodosylbenzoic acid has recently found some synthetic application as an efficient, safe, and recyclable oxidant.103,139,140 In particular, m-iodosylbenzoic acid in the presence of iodine is a convenient reagent for oxidative iodination of arenes at room temperature in acetonitrile solution. Separation of pure products is conveniently achieved by scavenging any aryl iodide by ion exchange with ion exchange resin IRA-900 (hydroxide form). The reduced form of the reagent, m-iodobenzoic acid, can be easily recovered from the ion exchange resin or from the basic aqueous solution by simple acidification with HCl.140
3.1.3. Transition Metal Catalyzed Oxidations
The oxidation reactions of iodosylarenes can be effectively catalyzed by metal salts and complexes.6 Iodosylbenzene is widely used as the most efficient terminal oxidant – source of oxygen in biomimetic oxidations catalyzed by metalloporphyrins and other transition metal derivatives.141–145 Recent examples of transition metal catalyzed oxidations employing iodosylbenzene include the hydroxylation of hydrocarbons,146–151 the transition metal-mediated epoxidation of alkenes,138,152–169 oxidation of alcohols170,171 or silyl ethers172 to carbonyl compounds, δ-sultone formation through Rh-catalyzed C-H insertion,173 and oxidation of organic sulfides163,174,175 to sulfoxides.
Iodosylarenes other than iodosylbenzene have also been used in the transition metal catalyzed oxidation reactions. The soluble, monomeric ortho-substituted iodosylarene 13 (see Section 3.1.2) can serve as an alternative to iodosylbenzene in the (porphyrin)manganese(III)-catalyzed alkene epoxidation reactions.157 A convenient recyclable reagent, m-iodosylbenzoic acid, selectively oxidizes primary and secondary alcohols to the respective carbonyl compounds in the presence of RuCl3 (0.5 mol%) at room temperature in aqueous acetonitrile.139 Separation of pure products in this case is achieved by simple extraction of the basic aqueous solution, and the reduced form of the reagent, m-iodobenzoic acid, can be easily recovered from the aqueous solution by simple acidification.
3.2. Fluorides
3.2.1. Preparation
A clean and selective, although relatively expensive procedure for the preparation of (difluoroiodo)arenes 43 consists of the treatment of iodoarenes with xenon difluoride in dichloromethane (Scheme 12) in the presence of anhydrous hydrogen fluoride.176,177 This method works well for the fluorination of iodoarenes with electron-donating or electron-withdrawing substituents; the latter, however, require longer reaction times. (Difluoroiodo)arenes 43 are hygroscopic and highly hydrolizable compounds, which make their separation and crystallization extremely difficult. Since xenon is the only byproduct in this reaction (Scheme 12), the resulting dichloromethane solutions contain essentially pure fluorides 43 which can be used in the subsequent reactions without additional purification. A similar procedure, but in the absence of anhydrous hydrogen fluoride, has been employed in the synthesis of some heteroaromatic iododifluorides. 2,3,5,6-Tetrafluoropyridin-4-yliodine difluoride, 4-(C5F4N)IF2 was prepared in 84% yield from by the reaction of 4-(C5F4N)I with XeF2 in dichloromethane at room temperature.178 Likewise, the fluorination of 3-iodo-4-methylfurazan with xenon difluoride in acetonitrile at room temperature was recently used for the preparation 3-(difluoroiodo)-4-methylfurazan.179
Scheme 12.
A variety of other powerful fluorinating reagents, such as F2, ClF, CF3OCl, BrF5, C6F5BrF2, C6F5BrF4, XeF2/BF3, can be used for the preparation of (difluoroiodo)arenes derived from polyfluorosubstituted iodoarenes.180–182 A convenient procedure for the preparation of (difluoroiodo)benzene and 4-(difluoroiodo)toluene consists of direct fluorination of the respective iodoarenes with the commercially available fluorinating reagent Selectfluor in acetonitrile solution.183 Various mixed (fluoroiodo)arene triflates, ArIF(OTf), can be generated in situ by fluorination of the respective iodoarenes with xenon fluorotriflate, FXeOTf.184,185
The para-substituted (difluoroiodo)arenes can be effectively prepared by the electrochemical fluorination of the respective iodoarenes.186,187 In this procedure, the electrosynthesis of ArIF2 is accomplished by the anodic oxidation of iodoarenes with Et3N•3HF or Et3N•5HF in anhydrous acetonitrile using a divided cell. This procedure works especially well for the preparation of 4-NO2C6H4IF2, which precipitates from the electrolytic solution in pure form during the electrolysis. The other para-substituted (difluoroiodo)arenes, such as TolIF2 and 4-MeOC6H4IF2, can be generated similarly and used without isolation as in-cell mediators for the following reactions.186,187
An older common procedure for the preparation of (difluoroiodo)arenes involves a one-step reaction of mercuric oxide and aqueous hydrofluoric acid with the (dichloroiodo)arenes in dichloromethane.188 The resulting solution of (difluoroiodo)arenes in dichloromethane can be used in the subsequent reactions without additional purification. A drawback of this method is the use of a large quantity of harmful HgO in order to remove the chloride ion from the reaction mixture. A convenient modified procedure without the use of HgO consists of the treatment of iodosylarenes 44 with 40–46% aqueous hydrofluoric acid (Scheme 13) followed by crystallization of products 45 from hexane.116,189 It is important that the freshly prepared iodosylarenes 44 are used in this procedure.
Scheme 13.
3.2.2. Structural Studies
Only a few examples of structural studies of organoiododifluorides, RIF2, have been reported in the literature. Single crystal X-ray diffraction studies of trifluoromethyliododifluoride, CF3IF2, revealed a distorted T-shaped structure with the I-F bond lengths 1.982(2) Å, and the F–I–F angle 165.4(2)°.190 Theoretical studies of CF3IF2 by ab initio and DFT calculations have also been reported.191 The structure of pentafluorophenyliododifluoride, C6F5IF2, has been investigated by single crystal X-ray crystallography and by multinuclear NMR, IR and Raman spectroscopy.180 The X-ray crystal and molecular structures of p-(difluoroiodo)toluene and m-(difluoroiodo)nitrobenzene have been reported in a Ph.D. dissertation in 1996.192
3.2.3. Reactions
(Difluoro)iodoarenes are powerful and selective fluorinating reagents towards various organic substrates. Various β-dicarbonyl compounds can be selectively fluorinated at the α-position by 4-(difluoroiodo)toluene and HF-amine complex.193 This fluorination can also be performed electrochemically using 4-(difluoroiodo)toluene generated in situ from iodotoluene in Et3N-5HF in an undivided cell under constant potential.187 More recently, Hara and co-workers have reported a modified procedure that allows to prepare monofluorinated products 47 from β-ketoesters, β-ketoamides and β-diketones 46 in good yields under mild conditions without the addition of the HF-amine complexes (Scheme 14).194 Ketones cannot be directly fluorinated by (difluoro)iodoarenes; however, α-fluoroketones can be prepared by the reaction of silyl enol ethers with 4-(difluoroiodo)toluene in the presence of BF3•OEt2 and the Et3N-HF complex.195
Scheme 14.
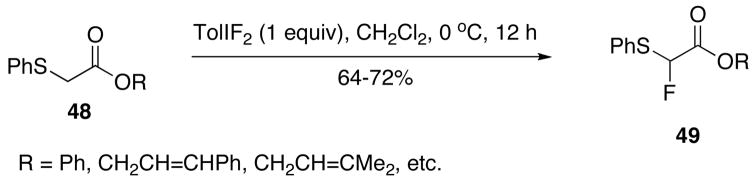
Treatment of α-phenylthio esters 48 with one equivalent of 4-(difluoroiodo)toluene affords the α-fluoro sulfides 49 in good overall yield through a fluoro-Pummerer reaction (Scheme 15).196 Addition of a second equivalent of 4-(difluoroiodo)toluene in this reaction produced α,α-difluoro sulfides and a third led to α,α-difluoro sulfoxides. This sequential fluorination-oxidation behavior was exploited in the one-pot synthesis of 3-fluoro-2(5H)-furanone starting from (3R)-3-fluorodihydro-2(3H)-furanone.196 The α-monofluorination of sulfanyl amides can be achieved by treatment of α-phenylsulfanylacetamides with one equivalent of 4-(difluoroiodo)toluene under similar conditions.197
Scheme 15.
Arrica and Wirth have reported the monofluorination of a series of α-acceptor-substituted selenides 50 using (difluoroiodo)toluene (Scheme 16).189 Although the yields of products 51 are only moderate, the reactions are usually very clean and, under the reaction conditions used, no further oxidized products are observed.
Scheme 16.
Fluorinated five- to seven-membered cyclic ethers 55–57 were stereoselectively synthesized from iodoalkyl substituted four- to six-membered cyclic ethers 52–54 by fluorinative ring-expansion reaction using (difluoroiodo)toluene (Scheme 17).198
Scheme 17.
Furrow and Myers have developed a convenient general procedure for the esterification of carboxylic acids with diazoalkanes 59 generated in situ by the oxidation of N-tert-butyldimethylsilylhydrazones 58 with (difluoroiodo)benzene (Scheme 18).199 This protocol affords various esters 60 from a broad range of carboxylic acids and, compared to the traditional esterification using diazoalkanes, offers significant advantages with regard to safety, because the diazo intermediates 59 are neither isolated nor achieve appreciable concentrations during the reaction.
Scheme 18.
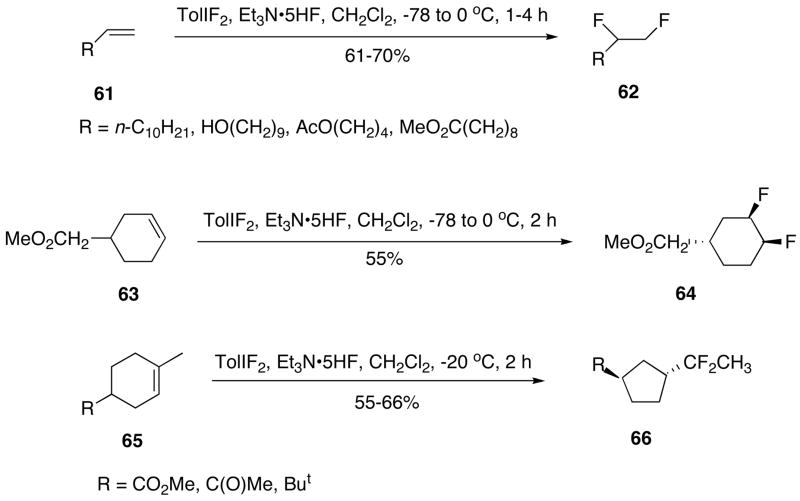
4-(Difluoroiodo)toluene reacts with terminal alkenes 61 to give vic-difluoroalkanes 62 in moderate yields (Scheme 19).200 The cyclohexene derivative 63 reacts with this reagent under similar conditions with the stereoselective formation of cis-difluoride 64.200 The observed syn-stereoselectivity of this difluorination is explained by a two-step mechanism involving the anti-addition of the reagent to the double bond through a cyclic iodonium intermediate at the first step, and then nucleophilic substitution of iodotoluene with fluoride anion in the second step. The reaction of substituted cyclic alkenes 65 with 4-(difluoroiodo)toluene and Et3N-5HF results in a fluorinating ring-contraction with the selective formation of difluoroalkyl substituted cycloalkanes 66 (Scheme 19).201
Scheme 19.
The fluorination of alkenes 67, 69 and alkynes 71 with 4-(difluoroiodo)toluene in the presence of iodine affords vic-fluoroiodoalkanes 68, 70 and fluoroiodoalkenes 72 in moderate to good yields (Scheme 20).202 This reaction proceeds in a Markovnikov fashion and with prevalent anti-stereoselectivity via the initial addition of the electrophilic iodine species followed by nucleophilic attack of fluorine anion. The analogous reaction of alkenes and alkynes with 4-(difluoroiodo)toluene in the presence of diphenyl diselenides affords the respective products of phenylselenofluorination in good yields.203
Scheme 20.
The reaction of 4-(difluoroiodo)toluene with 5-halopentynes with a four-, five-, or six-membered carbocycle 73 afforded the ring-expanded (E)-δ-fluoro-β-halovinyl iodonium tetrafluoroborates 74 stereoselectively in high yields (Scheme 21).204 This reaction proceeds via a sequence of λ3-iodanation-1,4-halogen shift-ring enlargement-fluorination steps.
Scheme 21.
4-(Difluoroiodo)toluene and other (difluoroiodo)arenes are commonly employed as reagents for the preparation of iodonium salts (see also Section 3.9).205–208 Especially useful is the reaction of potassium organotrifluoroborates with 4-(difluoroiodo)toluene affording various iodonium tetrafluoroborate salts under mild conditions.205
3.3. Chlorides
3.3.1. Preparation
The most general approach to (dichloroiodo)arenes involves the direct chlorination of iodoarenes with chlorine in a suitable solvent, such as chloroform or dichloromethane.209 This method can be applied to the large scale (20–25 kg) preparation of PhICl2 by the reaction of iodobenzene with chlorine at −3 to +4 °C in dichloromethane.210 The direct chlorination of iodoarenes 75 and 77 has recently been used for the preparation of 4,4′-bis(dichloroiodo)biphenyl 76 and 3-(dichloroiodo)benzoic acid 78 (Scheme 22), which are convenient recyclable hypervalent iodine reagents.211
Scheme 22.
In order to avoid the use of elemental chlorine, the chlorination of iodoarenes can be effected in situ in aqueous hydrochloric acid in the presence of an appropriate oxidant, such as KMnO4, activated MnO2, KClO3, NaIO3, concentrated HNO3, NaBO3, Na2CO3•H2O2, Na2S2O8, CrO3, and the urea-H2O2 complex.212–214 For example, the chlorination of iodoarenes in a biphasic mixture of carbon tetrachloride and concentrated hydrochloric acid in the presence of Na2S2O8 affords the corresponding (dichloroiodo)arenes in 60–100% crude yields.213 A recently reported convenient and mild approach to (dichloroiodo)arenes 80 consists of the chlorination of iodoarenes 79 using concentrated hydrochloric acid and aqueous sodium hypochlorite (Scheme 23).215 Sodium chlorite, NaClO2, can also be used in this procedure; however, in this case the chlorination takes longer time (3 hours at room temperature) and the yields of products 80 are generally lower.215
Scheme 23.
The other synthetic approaches to (dichloroiodo)arenes are represented by the one-pot oxidative iodination/chlorination of arenes with iodine and the appropriate oxidant in hydrochloric acid216 and by the treatment of iodosylbenzene with trimethylsilyl chloride.217,218
(Dichloroiodo)arenes are generally isolated as light and heat sensitive yellow crystalline solids, which are insufficiently stable for extended storage even at low temperatures.
3.3.2. Structural Studies
Several X-ray crystallographic studies of organoiododichlorides, RICl2, have been reported in the literature. The first X-ray crystal structures of PhICl2219 and 4-ClC6H4ICl2220 published in 1953 and 1956 were imprecise by modern standards. More recently, a good quality structure of PhICl2 obtained at low temperature has been reported.221 The molecule of PhICl2 has the characteristic T-shape with primary I–Cl bond distances of 2.47 Å and 2.49 Å, and Cl–I–C bond angles of 87.8 and 89.2°. In the solid state the molecules form an infinite zig-zagged chain, in which one of the chlorine atoms interacts with the iodine of the next unit with an intermolecular I•••Cl secondary bond distance of 3.42 Å. The coordination of iodine is distorted square planar with the lone pairs occupying the trans-positions of a pseudooctahedron.221
X-ray structures of two sterically encumbered (dichloroiodo)arenes, 2,4,6-Pri3C6H2ICl2222 and ArICl2 [Ar = 2,6-bis(3,5-dichloro-2,4,6-trimethylphenyl)benzene]223 have been reported. Both molecules have the expected T-shaped geometry; the latter molecule has Cl–I–C angles of 89.4(3) and 92.1(3) ° and I–Cl distances of 2.469(4) and 2.491(4) Å. The secondary I•••Cl bond distance in this compound is 3.816 Å, which indicates a significant reduction of intermolecular association as compared to PhICl2.223 The recently reported X-ray crystal structure of o-nitrobenzeneiododichloride, 2-NO2C6H4ICl2, does not show any significant intramolecular interaction between the iodine(III) center and the oxygen atom of the nitro group in the ortho position (I•••O bond distance 3.0 Å).99
X-ray structure of the PhICl2 adduct with tetraphenylphosphonium chloride, [Ph4P]+[PhICl3]−, has been reported.224 The [PhICl3]− anions in this structure have a planar coordination environment at the iodine atom. The I–Cl bond length of the chlorine atom trans to the Ph group is much longer (3.019 Å) than the bond distance to the cis Cl atoms (2.504 Å).224
X-ray crystal structures of two perfluoroalkyliododichlorides, CF3CH2ICl2 and CHF2(CF2)5CH2ICl2, have been reported.225 In comparison to PhICl2, which has a simple chain structure, perfluoroalkyliododichlorides have more complicated structures in which weak interactions between chains, coupled with aggregation of perfluoro groups, result in the formation of layers.
3.3.3. Reactions
(Dichloroiodo)arenes have found practical application as reagents for chlorination or other oxidative transformations of various organic substrates. Chlorinations of alkanes with (dichloroiodo)arenes proceed via a radical mechanism and generally require photochemical conditions or the presence of radical initiators in solvents of low polarity, such as chloroform or carbon tetrachloride.5 The chlorination of alkenes may follow a radical or ionic mechanism depending on the conditions.211,226–228 For example, norbornene reacts with (dichloroiodo)benzene under radical conditions in nonpolar solvents with the formation of 1,2-dichlorides as the only detectable products.226 In contrast, reactions of (dichloroiodo)benzene with various monoterpenes in methanol have an ionic mechanism and afford the respective products of chloromethoxylation of the double bond with high regio- and stereoselectivity.228 Likewise, the reaction of 4,4′-bis(dichloroiodo)biphenyl 76 with styrene derivatives 81 in methanol affords exclusively the products of electrophilic chloromethoxylation 82 (Scheme 24).211
Scheme 24.
(Dichloroiodo)arenes can also be used for the chlorination of electron-rich aromatic compounds. Aminoacetophenone 83 is selectively chlorinated with (dichloroiodo)benzene to give product 84 in good yield (Scheme 25). This process can be scaled up to afford 24.8 kg of product 84 with 94% purity.210
Scheme 25.
(Dichloroiodo)toluene was found to be a suitable chlorinating agent in the catalytic asymmetric chlorination of β-keto esters 85, catalyzed by the titanium complex 86, leading to the respective α-chlorinated products 87 in moderate to good yields and enantioselectivities (Scheme 26). The enantioselectivity of this reaction showed a remarkable temperature dependence, and the maximum selectivity was obtained at 50 °C.229
Scheme 26.
The reaction of N-protected pyrrolidine 88 with 4-nitrobenzeneiododichloride affords α-hydroxy-β,β-dichloropyrrolidine 89 as the main product (Scheme 27) via a complex ionic mechanism involving a triple C–H bond activation. This oxidative pathway has been demonstrated to be general for several saturated, urethane protected nitrogen heterocyclic systems.218
Scheme 27.
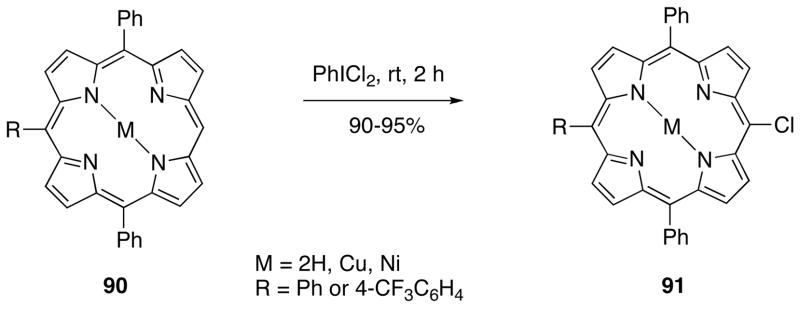
Treatment of 5,10,15-trisubstituted porphyrins 90 with (dichloroiodo)benzene affords the corresponding meso-chlorinated porphyrins 91 (Scheme 28).230 The reactions of trisubstituted Zn-porphyrins lead to the products of coupling, meso, meso-linked bisporphyrins, along with the meso-chlorinated products. The chlorination of 5,10,15,20-tetraarylporphyrins, in which all meso-positions are substituted, under similar conditions affords β-monochlorinated products in high yields.230
Scheme 28.
(Dichloroiodo)arenes have been applied in various oxidative transformations of organic substrates. An efficient and mild procedure has been described for the oxidation of different types of alcohols to carbonyl compounds using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) as the catalyst and (dichloroiodo)benzene as a stoichiometric oxidant at 50 °C in chloroform solution in the presence of pyridine.215 Under these conditions 1,2-diols are oxidized to α-hydroxy ketones or α-diketones depending upon the amount of PhICl2 used. A competitive study has shown that this system preferentially oxidizes aliphatic secondary alcohols over aliphatic primary alcohols.215
A simple and mild system using bis(dichloroiodo)biphenyl 76 in combination with tetraethylammonium bromide at room temperature has been developed for selective debenzylation of sugars. Acetates, benzoate, and sensitive glycosidic linkages are unaffected under the reaction conditions. A specific example of the debenzylation of benzyl 4-O-benzoyl 2,3-O-isopropylidene-α-L-arabinopyranoside 92 is shown in Scheme 29.231
Scheme 29.
An efficient route to the 3-iodo-4-aryloxypyridinones 95, which are highly potent non-nucleoside inhibitors of HIV-1 reverse transcriptase, has been developed starting from 4-hydroxy substituted pyridinone 93 and (dichloroiodo)arenes 94 (Scheme 30).232,233
Scheme 30.
Various organic substrates, such as enol silyl ethers, ketene silyl acetals, β-dicarbonyl compounds,234 alkynes,235 and para-unsubstituted phenols and naphthols,236 can be effectively thiocyanated with the combination reagent PhICl2/Pb(SCN)2. More recently, Prakash and co-workers have reported an improved method for the thiocyanation of 2-arylindan-1,3-diones, phenols, and anilines using a reagent combination of (dichloroiodo)benzene and potassium thiocyanate in dry dichloromethane.237 For example, the para-unsubstituted phenols and anilines 96 are efficiently converted under these reaction conditions to the respective p-thiocyanato derivatives 97 in high yields (Scheme 31).
Scheme 31.
Very recently, Zhang and co-workers have reported the application of (dichloroiodo)benzene in combination with sodium azide for the effective synthesis of carbamoyl azides from aldehydes.238
(Dichloroiodo)benzene is commonly used as a reagent for the oxidation or chlorination of various transition metal complexes. Recent examples include the oxidation of d8•••d10 heterobimetallic Pt(II)-Au(I) complex to give the d7-d9 Pt(III)-Au(II) complex containing a Pt(III)-Au(II) bond,239 and oxidations or chlorinations of palladium,240,241 cobalt,242 vanadium,243 and molybdenum244 complexes. Several examples of Pd-catalyzed chlorinations of organic substrates using (dichloroiodo)benzene have also been reported.245,246
3.4. [Bis(acyloxy)iodo]arenes
[Bis(acyloxy)iodo]arenes, ArI(O2CR)2, are the most important, well investigated, and practically useful organic derivatives of iodine(III). Two of them, (diacetoxyiodo)benzene, commonly abbreviated as DIB, PID, PIDA (phenyliodine diacetate), IBD, or IBDA (iodosobenzene diacetate) and [bis(trifluoroacetoxy)iodo]benzene, abbreviated as BTI or PIFA [(phenyliodine bis(trifluoroacetate)], are commercially available and widely used oxidizing reagents. In this review, the abbreviations DIB and BTI, originally suggested by Varvoglis,2 will be used. Over a thousand research papers dealing mainly with various synthetic applications of DIB and BTI have been published since the year of 2000. The use of [bis(acyloxy)iodo]arenes as precursors to other iodine(III) compounds and as the reagents for oxidation of alkynes, allenes, alkenes, enolizable ketones, electron-rich aromatic compounds, alcohols, organic derivatives of nitrogen, phosphorus, sulfur, selenium, tellurium, and other organic substrates has been discussed in previous reviews.2,5,6 In this section, the preparation, structural studies, and typical recent examples of synthetic applications of [bis(acyloxy)iodo]arenes are overviewed.
3.4.1. Preparation
Two general approaches are used for the preparation of [bis(acyloxy)iodo]arenes: (1) the oxidation of iodoarenes in the presence of a carboxylic acid, and (2) a ligand exchange reaction of the readily available DIB with an appropriate carboxylic acid. The most common and practically important representative of [bis(acyloxy)iodo]arenes, DIB, is usually prepared by the oxidation of iodobenzene with peracetic acid in acetic acid.247 A similar peracid oxidation of substituted iodobenzenes can be used for the preparation of other [bis(acyloxy)iodo]arenes. In particular, the polymer-supported analogs of DIB have been prepared by treatment of poly(iodostyrene) or aminomethylated poly(iodostyrene) with peracetic acid,30,248–250 and the ion-supported [bis(acyloxy)iodo]arenes, imidazolium derivatives 98 and 99, have been prepared by the peracetic oxidation of the appropriate aryliodides.251,252 Likewise, various [bis(trifluoroacetoxy)iodo]arenes can be synthesized in high yield by the oxidation of the respective iodoarenes with peroxytrifluoroacetic acid in trifluoroacetic acid.253–255

A modification of this method consists of the oxidative diacetoxylation of iodoarenes in acetic or trifluoroacetic acid using appropriate oxidants, such as periodates,256–258 sodium percarbonate,259 m-chloroperoxybenzoic acid,260–264 potassium peroxodisulfate,265,266 H2O2-urea,267 Selectfluor,183 and sodium perborate.264,268–274 The oxidation of iodoarenes with sodium perborate in acetic acid at 40 °C is the most simple and general procedure that has been used for a small scale preparation of numerous (diacetoxyiodo)-substituted arenes and hetarenes.264,268–274 This method can be improved by performing the perborate oxidation in the presence of trifluoromethanesulfonic acid.275 A further convenient modification of this approach employs the interaction of arenes 100 with iodine and potassium peroxodisulfate in acetic acid (Scheme 32).276 The mechanism of this reaction probably includes the oxidative iodination of arenes, followed by diacetoxylation of ArI in situ leading to (diacetoxyiodo)arenes 101.
Scheme 32.
The second general approach to [bis(acyloxy)iodo]arenes is based on the ligand exchange reaction of a (diacetoxyiodo)arene (usually DIB) with the appropriate carboxylic acid. A typical procedure consists of heating DIB with a non-volatile carboxylic acid RCO2H in the presence of a high boiling solvent, such as chlorobenzene (Scheme 33).277–282 The equilibrium in this reversible reaction can be shifted towards the synthesis of the product 102 by distillation under reduced pressure of the relatively volatile acetic acid formed during the reaction. This procedure, in particular, has recently been used for the preparation of the glutamate-derived diacyloxyiodobenzenes 103,278 protected amino acid derivatives 104,280 the cinnamate derivative 105,282 and 3-methylfurazan-4-carboxylic acid derivative 106.283
Scheme 33.
The reactions of DIB with stronger carboxylic acids usually proceed under milder conditions at room temperature. A convenient procedure for the preparation of BTI consists of simply dissolving DIB in trifluoroacetic acid and evaporating to a small volume.284 In a related method, used for the preparation of a series of PhI(OCOCO2R)2, DIB is treated with oxalyl chloride in the respective alcohol, ROH.285
[Bis(acyloxy)iodo]arenes are generally colorless, stable microcrystalline solids, which can be easily recrystallized and stored for extended periods of time without significant decomposition.
3.4.2. Structural Studies
Numerous structural reports on [bis(acyloxy)iodo]arenes were summarized in earlier reviews.2,5,6 In general, single crystal X-ray structural data for [bis(acyloxy)iodo]benzenes indicate a pentagonal planar coordination of iodine within the molecule, combining the primary T-shaped iodine(III) geometry with two secondary intramolecular I•••O interactions with the carboxylate oxygens.286 X-ray crystal structures of four new compounds, 1,3,5,7-tetrakis[4-(diacetoxyiodo)phenyl]adamantane 107,260 tetrakis[4-(diacetoxyiodo)phenyl]methane 108,261 3-[bis(trifluoroacetoxy)iodo]benzoic acid 109,103 and 1-(diacetoxyiodo)-2-nitrobenzene 110,99 have been reported in the recent literature.

In the molecule of trifluoroacetate 109, the C–I bond length is 2.083 Å, the primary I–O bond lengths are 2.149 and 2.186 Å, and the intramolecular secondary I•••O interactions with the carboxylate oxygens have distances of I(1)•••O(5) 3.146 Å and I(1)•••O(4) 3.030 Å; these five intramolecular interactions result in the pentagonal planar coordination of iodine within the molecule.103 In addition to the five intramolecular interactions, an intermolecular coordination of iodine atom to one the carboxylic oxygens of the neighboring molecule is also present with a distance of 3.023 Å. It is interesting to note that the presence of the meta-carboxylic group does not have any noticeable effect on the molecular geometry of compound 109, which is very similar to the X-ray crystal structure of [bis(trifluoroacetoxy)iodo]benzene.286 The X-ray crystal structure of 1-(diacetoxyiodo)-2-nitrobenzene 110 does not show any significant intramolecular interaction between the iodine(III) center and the oxygen atom of the nitro group in the ortho position (I•••ONO bond distance 3.11 Å).99
The 17O NMR study of bis(acyloxy)iodoarenes in chloroform has confirmed that the T-shaped structure of iodine(III) compounds observed in the solid state is also adopted in solution.109,110 The carboxylic groups of bis(acyloxy)iodoarenes show a dynamic behavior, which is explained by a [1,3] sigmatropic shift of the iodine atom between the two oxygen atoms of the carboxylic groups.110
3.4.3. Oxidation of Alcohols
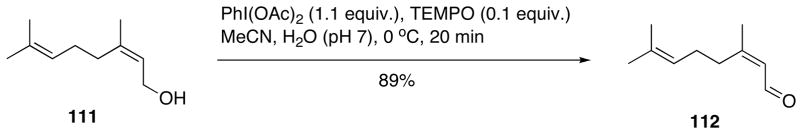
An efficient procedure for the oxidation alcohols with DIB in the presence of catalytic amounts of TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl), originally developed by Piancatelli, Margarita and co-workers,287 has been frequently used in recent years.264,288–293 An optimized protocol, published in Organic Synthesis for the oxidation of nerol 111 to nepal 112 (Scheme 34), consists of the treatment of the alcohol 111 solution in buffered (pH 7) aqueous acetonitrile with DIB and TEMPO (0.1 equivalent) at 0 °C for 20 minutes.288
Scheme 34.
This procedure exhibits a very high degree of selectivity for the oxidation of primary alcohols to aldehydes, without any noticeable overoxidation to carboxylic acids, and a high chemoselectivity in the presence of either secondary alcohols or of other oxidizable moieties.287 A similar oxidation procedure has been used for the oxidation of (fluoroalkyl)alkanols, RF(CH2)nCH2OH, to the respective aldehydes,289 in the one-pot selective oxidation/olefination of primary alcohols using DIB-TEMPO system and stabilized phosphorus ylides,290 and in the chemo-enzymatic oxidation-hydrocyanation of γ,δ-unsaturated alcohols.291 Other [bis(acyloxy)iodo]arenes can be used instead of DIB in the TEMPO catalyzed oxidations, such as the recyclable monomeric 1,3,5,7-tetrakis[4-(diacetoxyiodo)phenyl]adamantane 107260 and biphenyl- and terphenyl-based (diacetoxyiodo)arenes,264 and the polymer-supported DIB.292,293 Further modifications of this method include the use of polymer-supported TEMPO,294 fluorous-tagged TEMPO,295,296 ion-supported TEMPO,297 and TEMPO immobilized on silica.291
Based on the ability of the DIB-TEMPO system to selectively oxidize primary alcohols to the corresponding aldehydes in the presence of secondary alcohols, Forsyth and co-workers have developed selective oxidative conversion of a variety of highly functionalized 1°,2°-1,5-diols into the corresponding δ-lactones.298 A representative example of converting substrate 113 to the δ-lactone 114 is shown in Scheme 35. Monitoring of this reaction showed the initial formation of the intermediate lactol species, which then undergoes further oxidation to the lactone.298 A similar DIB-TEMPO promoted γ-lactonization has recently been utilized in the asymmetric total synthesis of the antitumor (+)-eremantholide A.299
Scheme 35.
[Bis(acyloxy)iodo]arenes in the presence of KBr in water can oxidize primary and secondary alcohols analogously to the PhIO/KBr system.124 The oxidation of primary alcohols affords carboxylic acids or esters,123,300 while the oxidation of secondary alcohols under similar conditions results in the formation of the respective ketones in excellent yields.261 In a specific example, primary alcohols 115 are readily oxidized to methyl esters 116 upon treatment with polystyrene-supported DIB in the presence of KBr in the acidic aqueous methanol solution (Scheme 36).300 Aldehydes can be converted to methyl esters by a similar procedure using DIB and NaBr.301
Scheme 36.
The oxidation of various primary and secondary alcohols with the ion-supported [bis(acyloxy)iodo]arene 99 (1.4 equivalents) in the ionic liquid [emim]+[BF4]− (1-ethyl-3-methylimidazolium tetrafluoroborate) in the presence of bromide anion selectively affords the respective carbonyl compounds without overoxidation to carboxylic acids.251
Molecular iodine can serve as an efficient catalyst in the oxidation of secondary alcohols to ketones and primary alcohols to carboxylic acids using DIB as an oxidant in acetonitrile solution.302 The oxidation of primary alcohols or aldehydes with the DIB/I2 system in methanol solution affords the respective methyl esters in excellent yields.303
Only a few examples of uncatalyzed oxidation of alcohols with [bis(acyloxy)iodo]arenes have been reported.249,304,305 Substituted benzyl alcohols can be oxidized by BTI in aqueous acetic acid to the corresponding benzaldehydes.304 Vicinal fullerene diol is oxidized to fullerene dione in 80% yield by DIB in benzene at 35 °C.305 Various vicinal diols 117 (13 examples) can be oxidized to aldehydes 118 using polymer-supported DIB (Scheme 37).249 Protecting groups such as OAc, OR, OBn, OBz, and isopropylidene in the substrates are stable under these reaction conditions. cis-1,2-Cyclohexandiol is converted to 1,6-hexandial in this reaction.249
Scheme 37.
3.4.4. Oxidative Functionalization of Carbonyl Derivatives and Unsaturated Compounds
In the 1980s Moriarty and co-workers have developed a particularly useful methodology for the oxidative α-functionalization of enolizable carbonyl compounds or their enol ethers using DIB or other hypervalent iodine oxidants.306–309 The applications of this methodology in organic synthesis, especially in the chemistry of heterocyclic compounds, have been summarized in several reviews.9,37,40,310 Ochiai and co-workers have recently reported a catalytic variant of α-acetoxylation of ketones based on the in situ generation of DIB from iodobenzene using m-chloroperbenzoic acid (mCPBA) as a terminal oxidant.311 In a typical example, the oxidation of a ketone with mCPBA (2 equiv.) in acetic acid in the presence of a catalytic amount of PhI (0.1 equiv.), BF3•OEt2 (3 equiv.) and water (5 equiv.) at room temperature under argon affords the respective α-acetoxy ketone in 63–84% isolated yield. p-Methyl- and p-chloroiodobenzene can also serve as efficient catalysts in the α-acetoxylation of ketones using mCPBA as a terminal oxidant.311
The oxidative functionalization of silyl enol ethers 119 with DIB as oxidant and N-aminophthalimide 120 as external nucleophile has recently been employed in the stereoselective synthesis of trans-α-ketohydrazones 121 in good yields under mild conditions (Scheme 38).312 The mechanism of this reaction involves the initial formation of α-ketohydrazines, which are further oxidized by DIB to give the final ketohydrazones 121.
Scheme 38.
Numerous recent examples of oxidative transformations of alkenes using [bis(acyloxy)iodo]arenes have been reported.138,282,313–318 [Bis(trifluoroacetoxy)iodo]benzene reacts with alkenes in the absence of any additive or catalyst affording bis(trifluoroacetates), which can be converted into the corresponding diols or carbonyl compounds by hydrolysis.313,319 For example, cyclohexene reacts with BTI in dichloromethane under reflux conditions to give cis-1,2-bis(trifluoroacetate) 122 in almost quantitative yield (Scheme 39). In the case of bicyclic alkenes, such as norbornene or benzonorbornadiene 123, the rearranged products (e.g. 124) are predominantly formed.313 Similar rearranged products are formed in the reactions of alkenes with DIB in the presence of strong acids.314
Scheme 39.
[Bis(acyloxy)iodo]arenes can be used as the oxidants in organocatalytic, asymmetric epoxidation of α,β-unsaturated aldehydes using imidazolidinone catalyst 126.138 In a specific example, the reaction of aldehyde 125 with DIB affords epoxide 127 with good enantioselectivity (Scheme 40).
Scheme 40.
A procedure for the preparation of aromatic aldehydes 129 from isopropenylbenzenes 128 and zeolite-supported DIB under microwave irradiation (Scheme 41) has been reported. This method was used for a clean and reproducible preparation of piperonal, vanillin and p-anisaldehyde in generally high yields and selectivities.315
Scheme 41.
In the 1990s, Tingoli and co-workers have found a general approach to various arylselenated products by the reaction of unsaturated compounds with diaryl diselenides and DIB.320–323 Several further modifications of this reaction have recently been reported.282,316–318 The reaction of gem-aryl-disubstituted methylenecyclopropanes with diphenyl diselenide and DIB produced the corresponding bis-phenylselenated rearranged products in moderate yields under mild conditions.318 A multicomponent reaction of allenes 130, diaryl diselenides, DIB, and alcohols or acids affords 3-functionalized-2-arylselenyl substituted allyl derivatives 131 in moderate yields (Scheme 42).316
Scheme 42.
Nifantiev and co-workers reported an improved preparative method for homogeneous azidophenylselenylation of glycols by the reaction with DIB, diphenyldiselenide, and trimethylsilyl azide. In a representative example, the reaction of tri-O-benzyl-galactal 132 with DIB/Ph2Se2/TMSN3 in dichloromethane under mild conditions affords the corresponding selenoglycoside 133 in moderate yield (Scheme 43).317 The noncarbohydrate alkenes, such as styrene and substituted cyclopentenes, can also be azidophenylselenated under these conditions.
Scheme 43.
The selenodecarboxylation of cinnamic acid derivatives 134 with diaryldiselenides promoted by DIB in acetonitrile affords vinyl selenides 135 in moderate yields (Scheme 44). A similar reaction of arylpropiolic acids gives respective alkynyl selenides in 60–90% yields.282
Scheme 44.
Kirschning and co-workers have developed several experimental procedures for the stereoselective bromoacetoxylation or iodoacetoxylation of alkenes based on the interaction of DIB with iodide or bromide anions.324,325 The actual reacting electrophilic species in these reactions are the diacetylhalogen(I) anions, (AcO)2I− and (AcO)2Br−, which can also be prepared as the polymer-supported variant.326–328 A similar iodocarboxylation of alkenes using amino acid-derived iodobenzene dicarboxylates 104 selectively affords the respective amino acid esters 136 in moderate yields (Scheme 45).280
Scheme 45.
Iodine in combination with [bis(acyloxy)iodo]arenes can be used for the oxidative iodination of aromatic and heteroaromatic compounds.6,329 A mixture of iodine and BTI in acetonitrile or methanol iodinates the aromatic ring of methoxy substituted alkyl aryl ketones to afford the products of electrophilic monoiodination in 68–86% yield.330 1-Iodoalkynes can be prepared in good to excellent yields by the oxidative iodination of terminal alkynes with DIB, potassium iodide, and copper(I) iodide.331 A solvent-free, solid state oxidative halogenation of arenes using DIB as the oxidant has recently been reported.332 A recyclable reagent, [bis(trifluoroacetoxy)iodo]benzoic acid 109, can also be used as the oxidant in the oxidative iodination reactions.103,333 Substituted pyrazoles 137 can be iodinated to the corresponding 4-iodopyrazole derivatives 138 by treatment with iodine and DIB or polymer-supported DIB at room temperature (Scheme 46).334
Scheme 46.
Oxidative thiocyanation of the electron-rich aromatic compounds, including phenol ethers, dimethyl aniline, thiophene and N-methylindole, can be performed using ammonium thiocyanate and DIB as the oxidant at room temperature in acetonitrile solution.335 Likewise, the direct cyanation of a wide range of electron-rich heteroaromatic compound, such as pyrroles, thiophenes, and indoles, can be achieved under mild conditions using [bis(acyloxy)iodo]arenes and trimethylsilyl cyanide as the cyanide source.262,263 In a specific example, the N-tosylpyrroles 139 are selectively cyanated at the 2-position using [bis(trifluoroacetoxy)iodo]benzene and trimethylsilyl cyanide to afford products 140 in good yields (Scheme 47).263
Scheme 47.
BTI in the presence of tert-butyl hydroperoxide can oxidize various aromatic hydrocarbons to afford the corresponding quinones.336 For example, naphthalene is oxidized to1,4-naphthaquinone in a moderate yield upon treatment with BTI (1.5 equiv.) and tert-butyl hydroperoxide (5 equiv.) for 3 hours at −30 °C.336 The introduction of hydroxy, alkoxy and acetoxy groups to the activated aromatic ring using [bis(acyloxy)iodo]arenes as oxidants has also been reported. N-Arylamides can be hydroxylated in the para position by BTI in trifluoroacetic acid at room temperature.337 The oxidation of 2,5-dihydroxyacetophenone with DIB in different alcohols leads to a regioselective alkoxylation, providing a convenient route for the synthesis of 6-alkoxy-2,5-dihydroxyacetophenones.338 Likewise, the DIB-promoted oxidation of 6-hydroxyflavone and 6-hydroxyflavanones in acetic acid leads to regioselective acetoxylation affording the respective 5-acetoxylated products in 53–63% yield.339
Applications of [bis(acyloxy)iodo]arenes in the oxidative transformations of phenolic compounds and in the biaryl coupling reaction will be discussed in Sections 3.4.6 and 3.4.7.
3.4.5. Oxidative Cationic Cyclizations, Rearrangements, and Fragmentations
DIB and BTI are commonly used as the reagents in various cationic cyclizations, rearrangements, and fragmentations.6 The cyclizations, induced by hypervalent iodine reagents, are particularly useful in the synthesis of heterocycles. Tellitu and Domínguez have developed a series of BTI-promoted intramolecular amidation reactions, generalized in Scheme 48, leading to various five, six and seven-membered heterocycles 143.340–353 Experimental evidence supports the ionic mechanism of this reaction, involving N-acylnitrenium intermediates 142 generated in the initial reaction of the amide 141 with the hypervalent iodine reagent.340
Scheme 48.
This methodology with some variations (Scheme 48) has been utilized by Tellitu, Domínguez and co-workers in the synthesis of the following heterocyclic systems: heterocycle-fused quinolinone derivatives,341 1,4-benzodiazepin-2-ones,342 benzo-, naphtho-, and heterocycle-fused pyrrolo[2,1-c][1,4]diazepines,343 2,3-diarylbenzo[b]furans,344 quinolinone or pyrrolidinone derivatives,345 dibenzo[a,c]phenanthridines,346 thiazolo-fused quinolinones,347 isoindolinone and isoquinolin-2-one derivatives,348 indoline derivatives,349 5-aroyl-pyrrolidinones,350,351 and indazolone derivatives.352,353 Recent representative examples include the preparation of indoline derivatives 145 from anilides 144,349 pyrrolidinones 147 from alkynylamides 146,350,351 and indazol-3-ones 149 from anthranilamides 148 (Scheme 49).352,353
Scheme 49.
Similar DIB or BTI induced cyclizations of the appropriate amide or amine precursors have been used in numerous useful synthetic transformations, such as: the synthesis of highly substituted pyrrolin-4-ones via BTI-mediated cyclization of enaminones,354 the synthesis of 2-substituted-4-bromopyrrolidines via DIB-induced intramolecular oxidative bromocyclization of homoallylic sulfonamides in the presence of KBr,355 the preparation of 2-(N-acylaminal) substituted tetrahydropyrans by DIB-induced oxidative cyclization of hydroxy-substituted N-acyl enamines,356 the preparation of 1,2,4-thiadiazoles by the reaction of DIB or BTI with 1-monosubstituted thioureas,357,358 the synthesis of azaspirocyclic synthetic intermediates via the BTI-induced nitrenium ion cyclizations,359–365 the preparation of lactams and spiro-fused lactams from the reaction of N-acylaminophthalimides and BTI,366 the stereocontrolled preparation of highly substituted lactams and N-hydroxy lactams from appropriate hydroxamates and BTI,365 the synthesis of 1,2,4-triazolo[4,3-a][1,8]naphthyridines using DIB-oxidation of 1,8-naphthyridin-2-ylhydrazones in the solid state,367 the synthesis of various substituted 1,2,4-triazolo[4,3-a]pyrimidines by the DIB-oxidation of the appropriate 2,4-pyrimidinylhydrazones,368–370 the preparation of thiazolo[2,3-c]-s-triazoles by the reaction of arenecarbaldehyde-4-arylthiazol-2-ylhydrazones with poly[(4-diacetoxyiodo)styrene],371 the synthesis of pyrrolidino[60]fullerene from the DIB-promoted reaction between C60 and amino acid esters,372 1,3,4-oxadiazoles from acylhydrazones by BTI oxidation,373–375 the synthesis of 1-aryl-4-methyl-1,2,4-triazolo[4,3-a]quinoxalines from arenecarboxaldehyde-3-methyl-2-quinoxalinylhydrazones,376,377 the synthesis of 1-benzoyltetrahydroisoquinoline derivatives using polymer-supported BTI.378 Likewise, the preparation of benzopyrano- and furopyrano-2-isoxazoline derivatives from 2-allyloxybenzaldoximes by DIB oxidation,379 the synthesis of various N-substituted indole derivatives via BTI-mediated intramolecular cyclization of enamines,380 the synthesis of 2-substituted benzothiazoles via the oxidative cyclization of thiobenzamides,381 the preparation of 2,3-diphenylquinoxaline-1-oxide from benzil-α-arylimino oximes using DIB,382 the synthesis of 1-(5-aryl-[1,3,4]oxadiazol-2-ylmethyl)-3-(4-methoxyphenyl)-1H-[1,8]naphthyridin-2-ones by oxidative cyclization of [2-oxo-3-(4-methoxyphenyl)-2H-[1,8]naphthyridin-1-yl]acetic acid arylidenehydrazides with alumina-supported DIB under microwave irradiation,383 the synthesis of 2,5-disubstituted-1,3,4-oxadiazoles by via BTI-mediated oxidative cyclization of aldazines,384 the preparation of 2-substituted oxazolines from aldehydes and 2-amino alcohols using DIB as an oxidant,385 the synthesis of 3,4-bis(1-phenyl-3-arylpyrazolyl)-1,2,5-oxadiazole-N-oxides by the DIB oxidation of pyrazole-4-carboxaldehyde oximes,386 the synthesis of 2-arylbenzimidazoles from phenylenediamines and aldehydes via a one-step process using DIB as an oxidant,387 the DIB-mediated efficient synthesis of imidazoles from α-hydroxy ketones, aldehydes and ammonium acetate,388 the preparation of dihydrooxazole derivatives by DIB-promoted 1,3-dipolar cycloaddition reactions of phthalhydrazide,389 and the synthesis of seco-psymberin/irciniastatin A via a DIB-mediated cascade cyclization reaction.390 Very recently, Togo and Moroda have reported a DIB-mediated cyclization reaction of 2-aryl-N-methoxyethanesulfonamides using iodobenzene as a catalyst (5–10 mol%) and m-chloroperoxybenzoic acid as the stoichiometric oxidant.391
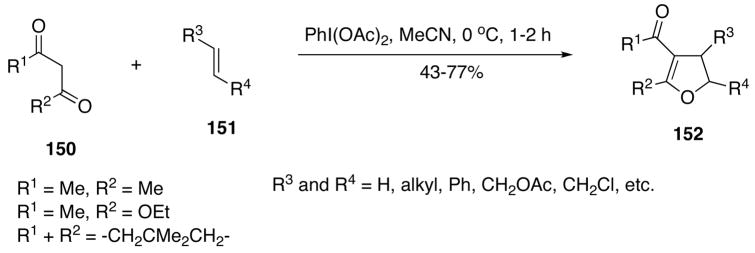
Several examples of the DIB or BTI-induced cyclizations of non-amine substrates have also been reported. The DIB-mediated oxidative addition of 1,3-dicarbonyl compounds 150 to various alkenes 151 allows an efficient one-pot synthesis of 2,3-dihydrofuran derivatives 152 (Scheme 50).392 A variety of alkenes and cycloalkenes bearing electron-withdrawing or electron-donating substituents can be used in this cyclization.
Scheme 50.
Wirth and co-workers reported the lactonization of 4-phenyl-4-pentenoic acid 153 upon treatment with DIB (Scheme 51).393 The mechanism of this reaction includes electrophilic lactonization induced by the addition of the iodine(III) electrophile to the double bond of substrate 153 followed by 1,2-phenyl migration leading to the final rearranged lactone 154. The same group reported a one-pot procedure for the conversion of alkenes into 1,1-dicyanocyclopropane derivatives by treatment with DIB and 1,1-dicyanopropane.394
Scheme 51.
Kita and co-workers developed a facile and efficient synthesis of lactols 156 via an oxidative rearrangement reaction of 2,3-epoxy alcohols 155 with BTI (Scheme 52).395–397 This BTI-induced oxidative transformation has been utilized in the synthesis of several lactones and in the asymmetric synthesis of the marine γ-lactone metabolite (+)-tanikolide.395,396
Scheme 52.
A DIB-induced domino reaction of the vicinal unsaturated diol 157 afforded cyclic ene-acetal 158 (Scheme 53), which was further utilized in the synthesis of a norsesquiterpene spirolactone/testosterone hybrid.398
Scheme 53.
Iglesias-Arteaga and co-workers reported several DIB-promoted oxidative transformations of steroidal substrates.399–401 In particular, the treatment of (25R)-3α-acetoxy-5β-spirostan-23-one 159 with DIB in basic methanol leads to F-ring contraction via Favorskii rearrangement to afford product 160 (Scheme 54).399
Scheme 54.
The treatment of steroidal substrate 161 with DIB and boron trifluoride etherate in acetic acid led to the introduction of an axial acetoxy group at position C-23 of the side chain,400 while a similar reaction of the same substrate 161 with DIB and BF3•OEt2 in formic acid unexpectedly produced the equatorial formate 162 mixed with products of rearrangement 163 and 164 (Scheme 55).401
Scheme 55.
The DIB-promoted oxidative iodolactonization of pentenoic acids 165 in the presence of tetrabutylammonium iodide proceeds smoothly at room temperature to afford lactones 166 in high yields.402 Based on this reaction, a convenient approach has been developed for the iodolactonization using iodobenzene as a catalyst (Scheme 56). In this procedure, DIB is generated in situ using a catalytic amount of iodobenzene with sodium perborate monohydrate as the stoichiometric oxidant. A variety of unsaturated acids including δ-pentenoic acids 167, δ-pentynoic acids and δ-hexynoic acid gave high yields of the respective lactones (e.g. 168) using this organocatalytic methodology (Scheme 56).402
Scheme 56.
Kita and co-workers reported a mild and efficient fragmentation reaction of β-amino alcohols 169 and α-amino acids 170 upon treatment with [bis(trifluoroacetoxy)iodo]pentafluorobenzene leading to N,O-acetals 171 (Scheme 57). This method has been utilized in an improved synthesis of the key intermediate of discorhabdins.403,404
Scheme 57.
Kozlowski and co-workers reported an unusual DIB-promoted oxidative rearrangement of cis- and trans-1,5-diazadecalins. In a specific example, upon treatment with DIB in aqueous NaOH, 1,5-diaza-trans-decalin 172 undergoes oxidation along with fragmentation to yield the ring-expanded bislactam 173 (Scheme 58).405
Scheme 58.
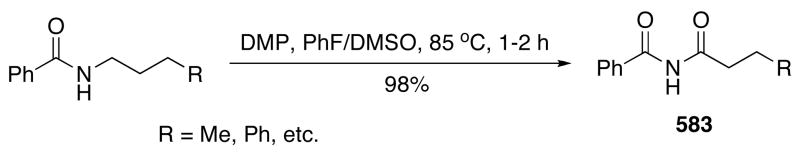
A stereoselective synthesis of 5–7 membered cyclic ethers can be achieved by deiodonative ring-enlargement of cyclic ethers having an iodoalkyl substituent. For example, the reaction of tetrahydrofuran derivative 174 with (diacetoxyiodo)toluene proceeds under mild conditions to afford ring-expanded product 175 (Scheme 59). The use of hexafluoroisopropanol (HFIP) as solvent in this reaction is critically important.406
Scheme 59.
[Bis(acyloxy)iodo]arenes can serve as excellent oxidants in Hofmann-type degradation of aliphatic or aromatic carboxamides to the respective amines. DIB is a superior reagent for the Hofmann rearrangement of protected asparagines.407 This procedure was used for the preparation of optically pure Nα-n-Boc-L-α,β-diaminopropionic acid 177 from asparagine 176 in hundred kilogram quantities (Scheme 60).408 Other examples include the oxidative rearrangement of anthranilamides or salicylamides 178 to the respective heterocycles 179,409 and the preparation of alkyl carbamates of 1-protected indole-3-methylamines 181 from the corresponding acetamides 180 (Scheme 60).410
Scheme 60.
BTI has also been used as a reagent for the Hofmann rearrangement, as illustrated by the conversion of amide 182 to the respective amine 183 (Scheme 61).411 A similar BTI-induced Hofmann rearrangement has been used for the preparation of both enantiomers of trans-2-aminocyclohexanecarboxylic acid from trans-cyclohexane-1,2-dicarboxylic acid.412
Scheme 61.
3.4.6. Oxidative Dearomatization of Phenolic Substrates
[Bis(acyloxy)iodo]arenes are commonly used as the reagents for various synthetically useful oxidative transformations of phenolic compounds.32,34,50,51,53,60 DIB is the reagent of choice for the oxidation of various substituted o- and p-hydroquinones to the corresponding benzoquinones. The oxidation generally proceeds in methanol solution at room temperature, and the yield of benzoquinones is almost quantitative.413 Gladysz and Rocaboy have reported the application of fluorous (diacetoxyiodo)arenes in oxidations of hydroquinones to quinones; in this procedure the fluorous reagents can be conveniently recovered by simple liquid/liquid biphase workups.273 Particularly useful is the oxidative dearomatization of 4- or 2-substituted phenols (e.g. 184 and 188) with DIB or BTI in the presence of an appropriate external or internal nucleophile (Nu) leading to the respective cyclohexadienones 187 or 189 according to Scheme 62. The mechanism of this reaction most likely involves the initial formation of the phenoxyiodine(III) species 185 followed by elimination of PhI and the generation of cationic phenoxenium intermediates 186, which finally combine with the nucleophile.5,414
Scheme 62.
Various nucleophiles, such as water,415 alcohols,76,413,416–418 fluoride ion,419 carboxylic acids,418,420,421 amides,422 oximes,423 and electron-rich aromatic rings,424,425 have been used successfully in this reaction (Scheme 62) in either an inter- or intra-molecular mode. Recent examples of this reaction in the inter-molecular mode include the oxidative ipso-fluorination of p-substituted phenols 190 (or a similar ipso-fluorination of p-substituted anilines426) using pyridinium polyhydrogen fluoride, Py•(HF)x, in combination with DIB or BTI,427 and the methoxylation of various phenolic substrates, such as 191, using DIB in methanol (Scheme 63).428–430 This reaction can be further improved by using phenol trimethylsilyl ethers instead of phenols as the substrates. It was shown that the oxidation of trimethylsilyl ethers 192 affords p-quinols 193 in greatly improved yields due to the minimization of oligomer side products formation compared to the oxidation of free phenol.431
Scheme 63.
Very recently, Quideau and co-workers have reported the preparation of versatile chiral substrates for asymmetric synthesis through the DIB induced spiroketalization of phenols with a chiral substituted ethanol unit O-tethered to the ortho position.76 This reaction has been successfully utilized in the asymmetric total synthesis of the natural product (+)-biscarvacrol.
Quideau and co-workers have developed a BTI-mediated regioselective protocol for the oxidative dearomatization of 2-alkoxyarenols in the presence of external carbon-based nucleophiles.432–435 This is a synthetically valuable process, as illustrated by the BTI-mediated oxidative nucleophilic substitution of the 2-alkoxynaphthol 194 with the silyl enol ether 195 leading to the highly functionalized naphthoid cyclohexa-2,4-dienone 196 (Scheme 64), which is an important intermediate product in the synthesis of aquayamycin-type angucyclinones.434,435
Scheme 64.
The DIB or BTI-induced phenolic oxidation in the intra-molecular mode provides an efficient approach to synthetically valuable polycyclic products. Representative examples of oxidative phenolic cyclizations promoted by [bis(acyloxy)iodo]arenes are shown in Scheme 65. In particular, the oxidative cyclization of phenolic oxazolines 197 affords synthetically useful spirolactams 198,51,436 the oxidation of enamide 199 leads to the spiroenamide 200, which is a key intermediate product in the total synthesis of annosqualine,437 and the spirocyclic product 202 has been prepared by a BTI-induced oxidation of catechol 201 in a key step of the total synthesis of the marine sesquiterpene quinone (+)-puupehenone.438
Scheme 65.
Additional examples of the DIB or BTI-induced oxidative phenolic cyclizations include the following studies: the asymmetric total syntheses of the pentacyclic Stemona alkaloids tuberostemonine and didehydrotuberostemonine,439 the fully stereocontrolled total syntheses of (−)-cylindricine C and (−)-2-epicylindricine C,440,441 the asymmetric total syntheses of platensimycin,442 the total synthesis of a potent antitumor alkaloid, discorhabdin A,443 the total synthesis of the amaryllidaceae alkaloid (+)-plicamine using solid-supported reagents,444 the construction of oxygenated indole, quinoline, and phenanthridine alkaloid motifs,445 DIB-mediated regioselective aza benzannulation of nitrogen-tethered 2-methoxyphenols,446 the investigation of oxidative dearomatization of resorcinol derivatives leading to valuable cyclohexa-2,5-dienones,447 the development of enantioselective organocatalytic oxidative dearomatization methodology,448 the development of a flow process for the multi-step synthesis of the alkaloid natural product oxomaritidine,449 the synthesis of carpanone using solid-supported reagents and scavengers,450 and the studies on ring expansions of a spirocyclohexadienone system.451
Kita and co-workers have reported a catalytic variant of the oxidative spirocyclization reaction based on the in situ regeneration of a [bis(trifluoroacetoxy)iodo]arene from iodoarene using m-chloroperbenzoic acid (mCPBA) as a terminal oxidant.452 In a typical example, the oxidation of the phenolic substrate 203 with mCPBA in dichloromethane in the presence of a catalytic amount of p-[bis(trifluoroacetoxy)iodo]toluene (0.01 equiv.) and trifluoroacetic acid at room temperature affords the respective spirolactone 204 in good yield (Scheme 66). A variety of other [bis(trifluoroacetoxy)iodo]arenes can be used as catalysts in this reaction [e.g. BTI, 4-MeOC6H4I(OCOCF3)2 and 2,4-F2C6H3I(OCOCF3)2] and different acidic additives (acetic acid, BF3•OEt2, TMSOTf, molecular sieves), but the TolI(OCOCF3)2/CF3CO2H system generally provides the best catalytic efficiency. Under these optimized conditions, a variety of phenolic substrates 205 was oxidized to spirolactones 206 in the presence of catalytic amounts of p-iodotoluene (Scheme 66).452 Likewise, the amide derivatives of phenolic substrates 205 can be catalytically oxidized to the respective N-fused spirolactams using catalytic amounts of p-iodotoluene and mCPBA as a terminal oxidant.453 A similar catalytic procedure has been reported for the oxidation of 4-alkoxyphenols to the corresponding 1,4-quinones using a catalytic amount of 4-iodophenoxyacetate in the presence of oxone as a co-oxidant in an aqueous acetonitrile solution.454
Scheme 66.
Very recently, Kita and co-workers reported the first enantioselective spirocyclization reaction of the ortho-substituted phenolic substrates using chiral aryliodine(III) diacetate having a rigid spirobiindane backbone.455
The oxidative dearomatization of substituted phenols 188 bearing electron-releasing substituents R, such as methoxy group, at their ortho-position(s) leads to 6,6-disubstituted cyclohexa-2,4-dienones 189 (see Scheme 62), which can be conveniently utilized in situ as dienes in Diels-Alder reactions.418,421,456 When the oxidation of phenols is performed in the absence of an external dienophile, a dimerization via [4+2] cycloaddition often occurs spontaneously at ambient temperature to afford the corresponding dimers with an extraordinary level of regio-, site-, and stereoselectivity. A detailed experimental and theoretical investigation of such hypervalent iodine induced Diels–Alder cyclodimerizations has recently been published by Quideau and co-workers.456 A representative example of an oxidative Diels–Alder cyclodimerization of a phenolic substrate 207 to the dimer 208 is shown in Scheme 67.
Scheme 67.
When the oxidation is performed in the presence of an external dienophile, the respective products of [4+2] cycloaddition are formed.457–461 Typical examples are illustrated by a one-pot synthesis of several silyl bicyclic alkenes 211 by intermolecular Diels-Alder reactions of 4-trimethylsilyl substituted masked o-benzoquinones 210 derived from the corresponding 2-methoxyphenols 209,457 and by the hypervalent iodine-mediated oxidative dearomatization/Diels-Alder cascade reaction of phenols 212 with allyl alcohol affording polycyclic acetals 213 (Scheme 68).458 The BTI-promoted tandem phenolic oxidation/Diels-Alder reaction has been utilized in the stereoselective synthesis of the bacchopetiolone carbocyclic core.459
Scheme 68.
A mechanistic investigation of the oxidation of 2,6-dimethylphenol using different oxidizing systems has shown that DIB is the most efficient reagents for the oxidative coupling leading to 3,5,3′,5′-tetramethyl-biphenyl-4,4′-diol. A reaction mechanism was proposed which involved an initial formation of a [bis(phenoxy)iodo]benzene intermediate followed by its radical fragmentation and then radical coupling and comproportionation/redox reaction steps.462
3.4.7. Oxidative Coupling of Electron-Rich Aromatic Substrates
The interaction of phenol ethers 214 or other electron-rich aromatic substrates with BTI leads to the generation of cation radical intermediates 215, which combine with external or internal nucleophiles affording the products of dearomatization 216 or coupling 217 according to Scheme 69. Kita and co-workers have recently published a detailed mechanistic study of this process (Scheme 69) for a specific reaction of oxidative cyclization of electron-rich aromatics with the intramolecular hydroxyl group.463 In this study, the formation of the cation radical intermediates 215 (R-Nu = -CH2CH2CH2OH) was experimentally confirmed by ESR spectroscopy, and the factors determining the ratio of products 216 and 217 and their consequent transformations were clarified.
Scheme 69.
The direct nucleophilic substitution of electron-rich phenol ethers using BTI and Lewis acid and involving aromatic cation radical intermediates was originally developed by Kita and coworkers in 1994.464 Since then this procedure with some variations has been extensively applied by Kita and other researchers for various oxidative transformations, such as the synthesis of biaryls,465–472 spirodienones,467,473–475 quinone imines,476 sulfur-containing heterocycles,477 and chromans.478 Specific recent examples of the oxidative coupling of phenolic ethers include the oxidative biaryl coupling of various N-substituted 1-benzyltetrahydroisoquinolines 218 to the corresponding aporphines 219,468 the oxidative cyclization of 3,4-dimethoxyphenyl 3,4-dimethoxyphenylacetate 220 leading the seven-membered lactone 221,469 and the conversion of phenol ether derivatives 222 to the products of intramolecular coupling 223 using a combination of BTI and heteropoly acid (Scheme 70).466 A similar oxidative coupling reaction of benzyltetrahydroisoquinolines (laudanosine derivatives) using BTI and heteropoly acid has been used in an efficient synthesis of morphinandienone alkaloids.479 A catalytic version of the intermolecular oxidative coupling of phenolic ethers using BTI (0.125 equivalents) as a catalyst and mCPBA as the stoichiometric oxidant has also been reported.452 Very recently, Kita and co-workers have reported a new H2O2/acid anhydride system for the iodoarene-catalyzed intramolecular C-C cyclization of phenolic derivatives.480
Scheme 70.
The non-phenolic electron-rich aromatic substrates can also be oxidatively coupled using [bis(acyloxy)iodo]arenes. Kita and co-workers reported facile and efficient oxidative coupling reaction of alkylarenes 224 leading to alkylbiaryls 225 using a combination of BTI and BF3•OEt2 (Scheme 71).481 Similarly, multiply iodinated biaryls can be prepared in good yields by the BTI-induced direct oxidative coupling reaction of the iodinated arenes.482
Scheme 71.
Oxidation of N-aromatic methanesulfonamides 226 with DIB in the presence of thiophene in trifluoroethanol or hexafluoroisopropanol affords the respective coupling products 227 in good yield.483 Likewise, the head-to-tail dimers 229 can be selectively prepared by the hypervalent iodine oxidation of 3-substituted thiophenes 228,484,485 and bipyrroles 231 can be regioselectively synthesized by oxidative dimerization of pyrroles 230 with BTI in the presence of bromotrimethylsilane (Scheme 72).486
Scheme 72.
3.4.8. Radical Cyclizations, Rearrangements and Fragmentations
Useful synthetic methodologies are based on the cyclization, rearrangement or fragmentation of the alkoxyl radicals generated in the reaction of alcohols with [bis(acyloxy)iodo]arenes in the presence of iodine under photochemical conditions or in the absence of irradiation.5,6 Suàrez and co-workers have applied this methodology in various useful transformations of carbohydrate derivatives, such as the synthesis of polyhydroxy piperidines and pyrrolidines related to carbohydrates,129 the synthesis of alduronic acid lactones,487 the syntheses of chiral dispiroacetals from carbohydrates,488 and the synthesis of α-iodoalkyl esters from carbohydrates.489 Recent examples include the synthesis of 1,1-difluoro-1-iodo alditols 233,490 2-azido-1,2-dideoxy-1-iodo-alditols 235,491,492 and chiral vinyl sulfones 237493 by fragmentation of carbohydrate anomeric alkoxyl radicals generated from the respective carbohydrates 232, 234 and 236 (Scheme 73).
Scheme 73.
The intramolecular hydrogen abstraction reactions promoted by alkoxy radicals in carbohydrates are particularly useful for the stereoselective synthesis of various polycyclic oxygen-containing ring systems.128,494–497 This reaction can be illustrated by the intramolecular 1,8-hydrogen abstraction between glucopyranose units in disaccharide 238 promoted by alkoxyl radicals and leading to the 1,3,5-trioxocane derivative 239 (Scheme 74).494
Scheme 74.
Boto and Hernandez have reported a short and efficient synthesis of chiral furyl carbinols from carbohydrates, such as 240, based on the alkoxyl radicals fragmentation reaction leading to the intermediate product 241 (Scheme 75).498 The same authors have developed an efficient procedure for the selective removal from carbohydrate substrates of methoxy protecting groups next to hydroxy groups by treatment with the DIB-I2 system.499
Scheme 75.
The treatment of 1-alkynylcycloalkanols 242 with poly[styrene(iodosodiacetate)] and iodine affords (Z)-2-(1-iodo-1-organyl)methylenecycloalkanones 243 resulting, probably, from the alkoxyl radical promoted ring expansion reaction (Scheme 76).500 The mechanism of the β-scission reactions of the 1-alkylcycloalkoxyl radicals generated from alkylcycloalkanols by treatment with the DIB-I2 under photochemical conditions has been investigated by Bietti and co-workers.501
Scheme 76.
A mild and highly efficient one-pot synthesis of aryl glycines 245 from easily available serine derivatives 244 has been reported (Scheme 77).502 The method is based on the β-fragmentation of a primary alkoxyl radical, generated on treatment of the serine derivative with DIB and iodine, immediately followed by the addition of the nucleophile. This methodology is also applicable to the synthesis of other uncommon amino acids.502
Scheme 77.
The one-pot radical fragmentation-phosphorylation reaction of α-amino acids or β-amino alcohols (e.g. 246) affords α-amino phosphonates 247 in good yields (Scheme 78). This reaction was applied to the synthesis of potentially bioactive phosphonates.503
Scheme 78.
The radical decarboxylation of carboxylic acids on treatment with DIB-I2 allows to introduce iodine or other functional group into nitrogen heterocycles under mild conditions.504,505 For example, the decarboxylation of β- and γ-amino acids 248 under these conditions affords iodinated heterocycles 249 (Scheme 79). This reaction was applied to the synthesis of bioactive products, such as opioid analogs, imino sugars and new antifungal agents.504
Scheme 79.
Kita and co-workers developed a simple and reliable method for the direct construction of biologically important aryl lactones 251 from carboxylic acids 250 using a combination of DIB with KBr (Scheme 80). The mechanism of this reaction includes the initial generation of the carbonyloxy radical followed by the intramolecular benzylic hydrogen abstraction and cyclization.506
Scheme 80.
Conjugate addition of radicals generated by decarboxylative fragmentation of(diacyloxyiodo)benzene 103 to dehydroamino acid derivatives (e.g. 252) has been used by Sutherland and Vederas in the synthesis of diaminopimelic acid analogues 253 (Scheme 81).278
Scheme 81.
Barluenga and co-workers reported a direct iodination of alkanes 254 by the reaction with DIB-I2 in the presence of t-butanol under photochemical or thermal conditions (Scheme 82).507 This reaction can be used for the preparation of alkyliodides 255 in excellent yields by direct C–H bond activations in cyclic or non-cyclic alkanes and at the benzylic position. The presence of an alcohol (e.g., t-butanol) is essential for an efficient alkane activation.
Scheme 82.
The alkoxy radical fragmentation with DIB in the presence of iodine was also used in a facile synthesis of (n+3) and (n+4) ring-enlarged lactones as well as of spiroketolactones from n-membered cycloalkanones.508
Useful synthetic methodologies are based on the cyclization or rearrangement of the nitrogen-centered radicals generated in the reaction of the appropriate amides with DIB in the presence of iodine.130,509–511 Specific examples are illustrated by the synthesis of bicyclic spirolactams 257 from amides 256,509 and the preparation of the oxa-azabicyclic systems (e.g. 259) by the intramolecular hydrogen atom transfer reaction promoted by carbamoyl and phosphoramidyl radicals generated from the appropriately substituted carbohydrates 258 (Scheme 83).510
Scheme 83.
3.4.9. Oxidations of Nitrogen, Phosphorus, and Sulfur Compounds
DIB and BTI have found wide application for the oxidation of organic derivatives of such elements as nitrogen, sulfur, selenium, tellurium, and others.5,6 The use of [bis(acyloxy)iodo]arenes for the oxidation of organonitrogen compounds leading to the generation of the N-centered cationic or radical intermediates and their subsequent cyclizations and rearrangements (e.g. Hofmann rearrangement) is discussed in previous sections of this review (see Sections 3.4.5 and 3.4.8). Additional recent examples include the DIB induced oxidation of aromatic amines to imines applied for deprotection of protected amino diols,512 the N-acylation of 1,3-disubstituted thioureas using DIB,513 the DIB oxidation of 1,2-dicarbethoxyhydrazine to diethyl azodicarboxylate as a key step of an organocatalytic Mitsunobu reaction,514 the BTI oxidations of phenylhydrazones leading to regeneration of the carbonyl function,515 the low temperature generation of diazocompounds by the reaction of BTI with hydrazones,516 the preparation of N-aroyl-N′-arylsulfonylhydrazines by oxidation of aromatic aldehyde N-arylsulfonylhydrazones with BTI,517 and conversion of oximes into nitroso compounds using p-bromo(diacetoxyiodo)benzene.518
[Bis(acyloxy)iodo]arenes have been used for the oxidation of various organosulfur compounds. Organic sulfides are selectively oxidized to the respective sulfoxides by DIB or the polymer-supported DIB in water in the presence of KBr.519 The recyclable reagent, 3-[bis(trifluoroacetoxy)iodo]benzoic acid 109, can oxidize organic sulfides to the respective sulfoxides at room temperature in aqueous acetonitrile.103 Thioacetals and thioketals are efficiently cleaved to carbonyl compounds with BTI or DIB under mild conditions. This reaction is especially useful for the selective deprotection of either thioacetals or thioketals and is compatible with a variety of other functional groups.520–524
Makowiec and Rachon investigated the reactivity of DIB toward trivalent phosphorus nucleophiles. It was found that both H-phosphonates and secondary phosphine oxides react with DIB in alcohols in the presence of sodium alkoxides yielding trialkyl phosphates and alkyl phosphinates, respectively. A mechanism of these reactions involving an initial addition of a phosphorus(III) nucleophile to the iodine(III) center has been proposed.525
3.4.10. Transition Metal Catalyzed Reactions
The oxidations with [bis(acyloxy)iodo]arenes can be effectively catalyzed by transition metal salts and complexes. DIB is occasionally used instead of iodosylbenzene as the terminal oxidant in biomimetic oxygenations catalyzed by metalloporphyrins and other transition metal complexes.526–528 Primary and secondary alcohols can be selectively oxidized to the corresponding carbonyl compounds by DIB in the presence of transition metal catalysts, such as RuCl3,139,529 Ru(Pybox)(Pydic) complex,530 polymer-micelle incarcerated ruthenium catalysts,531 chiral-Mn(salen)-complexes,532,533 Mn(TPP)CN/Im catalytic system,534 and (salen)Cr(III) complexes.535 Kirschning and co-workers have recently reported the use of the recyclable reagent, phenylsulfonate-tagged DIB, in the RuCl3-catalyzed oxidation of alcohols.536 The epoxidation of alkenes, such as stilbenes, indene and 1-methylcyclohexene, using DIB in the presence of chiral binaphthyl ruthenium(III) catalysts (5 mol%) has also been reported. The chemoselectivity and enantioselectivity of this reaction was found to be low (4% ee).537
The mechanisms and applications of palladium-catalyzed reactions of DIB and other hypervalent iodine reagents in synthetically useful organic transformations were recently reviewed by Deprez and Sanford.18 Particularly useful are the Pd-catalyzed oxidation reactions, including the oxidative functionalization of C-H bonds and the 1,2-aminooxygenation of olefinic substrates.538–552 Representative examples of these catalytic oxidations are illustrated by the selective acetoxylation of C-H bonds adjacent to coordinating functional groups (e.g., pyridine in substrate 260),538 and by the Pd(OAc)2-catalyzed intramolecular aminoacetoxylation in the reaction of γ-aminoolefins (e.g., cinnamyl alcohol derived tosyl carbamate 261) with DIB (Scheme 84).539 The key mechanistic step in these catalytic transformations includes the DIB promoted oxidation of Pd(II) to the Pd(IV) species, as proved by the isolation and X-ray structural identification of stable Pd(IV) complexes prepared by the reaction of PhI(O2CPh)2 with Pd(II) complexes containing chelating 2-phenylpyridine ligands.553
Scheme 84.
Yan and co-workers have developed an efficient procedure for synthesis of symmetrical conjugated diynes 263 from terminal alkynes 262 using DIB as oxidant under palladium-catalyzed conditions (Scheme 85).554,555
Scheme 85.
3.5. Organosulfonates
A detailed discussion of the literature on the preparation, structural studies and synthetic applications of aryliodine(III) compounds derived from strong inorganic acids can be found in our previous reviews.5,6 The aryliodine(III) compounds ArI(OX)2 that are derived from strong acids HOX, such as H2SO4, HNO3, HClO4, CF3SO3H, HSbF6 and HPF6, usually lack stability and can only be generated at low temperature, under absolutely dry conditions. Traces of moisture immediately convert these compounds into μ-oxo-bridged derivatives or more complex polymeric structures (see structures 8 and 9 in Section 3.1.2). For example, the unstable and extremely hygroscopic phenyliodine(III) sulfates PhIO•SO3 and (PhIO)2•SO3 can be generated from PhIO and SO3 or Me3SiOSO2Cl under absolutely dry conditions,556–558 while the partially hydrolyzed, stable oligomeric sulfate (PhIO)3•SO3 (structure 8) is conveniently prepared by the treatment of PhI(OAc)2 with aqueous NaHSO4.88
[Hydroxy(organosulfonyloxy)iodo]arenes, ArI(OH)OSO2R, are the most common, well investigated, and practically useful aryliodine(III) derivatives of strong acids. The most important of them, [hydroxy(tosyloxy)iodo]benzene (HTIB or Koser’s reagent), is commercially available and is commonly used as an oxidizing reagent in organic synthesis.41 In this section, the preparation, structural studies, and recent examples of synthetic applications of [hydroxy(organosulfonyloxy)iodo]arenes are overviewed.
3.5.1. Preparation
Various [hydroxy(tosyloxy)iodo]arenes are readily prepared by a ligand exchange reaction of (diacetoxyiodo)arenes with p-toluenesulfonic acid monohydrate in acetonitrile (Scheme 86).75,103,257,260,261,559,560 This method has recently been applied to the synthesis of [hydroxy(tosyloxy)iodo]heteroaromatic derivatives (e.g., 264 and 265),560 the derivatives with various substituted aromatic groups (e.g. 266 and 267),103,257,560 and the recyclable hypervalent iodine reagents 268 and 269.260,261 A convenient modified procedure for the preparation of various [hydroxy(sulfonyloxy)iodo]arenes consists of the one-pot reaction of iodoarenes and mCPBA in the presence of sulfonic acids in a small amount of chloroform at room temperature.561 This modified procedure was recently used for the preparation of new biphenyl- and terphenyl-based recyclable organic trivalent iodine reagents 270 and 271.264
Scheme 86.
A similar procedure using 4-nitrobenzenesulfonic acid, methanesulfonic acid, or 10-camphorsulfonic acid leads to the corresponding organosulfonyloxy analogs.559,562 A solvent-free, solid-state version of this reaction is carried out by simple grinding of ArI(OAc)2 with the appropriate sulfonic acid in an agate mortar followed by washing the solid residue with diethyl ether.563 This solid-state procedure has been used for the preparation of HTIB and several other [hydroxy(organosulfonyloxy)iodo]arenes in 77–98% yields. A polymer-supported [hydroxy(tosyloxy)iodo]benzene can be prepared similarly by treatment of poly[(diacetoxy)iodo]styrene with p-toluenesulfonic acid monohydrate in chloroform at room temperature.564,565
The highly electrophilic phenyliodine(III) trifluoromethanesulfonate (PhIO)2•Tf2O, which is also known as Zefirov’s reagent, may be prepared either by the exchange reaction of (diacetoxy)iodobenzene with trifluoromethanesulfonic acid,566 or by the combination of two equivalents of iodosobenzene with one equivalent of triflic anhydride.567 This triflate has an oxo-bridged structure and is isolated as a relatively stable yellow microcrystalline solid that can be handled for brief periods in air and stored under a nitrogen atmosphere. It can be conveniently generated in situ from PhIO and triflic anhydride or trimethylsilyl triflate and immediately used in the subsequent reactions;568 the extended storage of this reagent in the presence of trifluoromethanesulfonic acid results in self-condensation with the formation of oligomeric products.569
3.5.2. Structural Studies
Single-crystal X-ray structural data for HTIB show the T-shaped geometry around the iodine center with almost collinear O-ligands and two different I-O bonds of 2.47 Å (I-OTs) and 1.94 Å (I-OH).570 The presence of a substituent in the phenyl ring does not have any noticeable effect on the molecular geometry of [hydroxy(tosyloxy)iodo]arenes. The recently reported X-ray structure of 3-[hydroxy(tosyloxy)iodo]benzoic acid 267 is very similar to the structure of HTIB. The I-OTs bond distance in tosylate 267, (2.437 Å), is significantly longer than the I-OH bond distance of 1.954 Å, which is indicative of some ionic character of this compound. In addition to the three intramolecular bonds, a weaker intermolecular coordination of iodine atom to one of the sulfonyl oxygens of the neighboring molecule is found with a distance of 2.931 Å. No intermolecular interaction involving a meta carboxylic group is present in molecule 267.103
The solution studies of HTIB in water by spectroscopic measurements and potentiometric titrations indicate complete ionization to a hydroxy(phenyl)iodonium cation (PhI+OH in hydrated form) and tosylate anion.111
3.5.3. Reactions
The functionalization of carbonyl compounds at an α-carbon represents the most typical reaction of [hydroxy(organosulfonyloxy)iodo]arenes (Scheme 87).41 Recent examples of synthetic application of this procedure include the following: the preparation of α-mesyloxyketones for the photochemical synthesis of highly functionalized cyclopropyl ketones,571 the one-step conversion of ketones into α-azidoketones using HTIB and sodium azide,572,573 the one-pot conversion of ketones into β-keto sulfones using HTIB and sodium arene sulfinate under solvent-free conditions,574 the solvent-free synthesis of α-tosyloxy β-keto sulfones using HTIB,575 direct α-hydroxylation of ketones using HTIB or polymer-supported HTIB in dimethyl sulfoxide-water,576,577 the use of HTIB in the synthesis of 1,4-diaryl-2-(arylamino)-but-2-ene-1,4-diones,578 the high yield preparation of dicarboxylic acid dimethyl esters from cycloalkanones using [hydroxy(2,4-dinitrobenzenesulfonyloxy)iodo]benzene,579 the ionic liquid-accelerated one-pot synthesis of 2-arylimidazo[1,2-a]pyrimidines,580 the HTIB mediated stereoselective synthesis of bicyclic ketones,581 the HTIB promoted synthesis of 6-arylimidazo[2,1-b]thiazoles,582 the synthesis of thiazole-2(3H)-thiones through [hydroxy(tosyloxy)iodo]benzene,583 the HTIB promoted synthesis of 2-substituted 4,5-diphenyloxazoles under solvent-free microwave irradiation conditions,584 the preparation of oxazoles from ketones and amides using [hydroxy(2,4-dinitrobenzenesulfonyloxy)iodo]benzene,585 the one-pot preparation of 2,4,5-trisubstituted oxazoles from ketones, nitriles, and aryliodine(III) triflates generated in situ from iodoarene, mCPBA and triflic acid,586 the preparation of flavones from flavanones using HTIB,587 the synthesis of isoflavones from 2′-benzoyloxychalcones using polymer-supported HTIB,588 the preparation of 3-tosyloxychromanones by the reaction of HTIB with chromanone and 2-methylchromanone,589 the HTIB promoted one-pot synthesis of 3-carbomethoxy-4-arylfuran-2-(5H)-ones from ketones,590 the HTIB mediated synthesis of 2-aryl-7-cyano(ethoxycarbonyl)-6-methylthio-1H-imidazo[1,2-b]pyrazoles from 5-amino-4-cyano(ethoxycarbonyl)-3-methylthio-1H-pyrazole and acetophenones,591,592 the synthesis of imidazo[2,1-a]isoquinolines using [hydroxy(2,4-dinitrobenzenesulfonyloxy)iodo]benzene,593 and the microwave-promoted solvent-free oxidation of α-methylene ketones to α-diketones.594
Scheme 87.
Recent modifications of this procedure (Scheme 87) include the use of solvent-free reaction conditions,563,575 application of ionic liquids as solvents,595–597 the use of recyclable reagents 267–271,103,260,261,264 the use of heterocycle-based reagents 264 and 265,560 and the catalytic α-oxytosylation of ketones using mCPBA as stoichiometric oxidant and iodoarenes as catalysts in the presence of p-toluenesulfonic acid.598–601
HTIB has been used in various oxidative rearrangements and fragmentations. Justik and Koser have reported a study of an oxidative rearrangement that occurs upon the treatment of arylalkenes 272 with HTIB in 95% methanol affording the corresponding α-aryl ketones 273 in generally high yields (Scheme 88). This oxidative rearrangement is general for acyclic and cyclic arylalkenes and permits the regioselective syntheses of isomeric α-phenyl ketone pairs.602
Scheme 88.
A similar HTIB induced oxidative rearrangement has recently been utilized in the regioselective synthesis of 6-prenylpolyhydroxyisoflavone (wighteone)603 and in a diastereoselective total synthesis of (±)-indatraline.604 In particular, the key intermediate product 275 in the synthesis of wighteone was prepared by the oxidative rearrangement of 3′-iodotetraalkoxychalcone 274,603 and the key step in the synthesis of (±)-indatraline involved the HTIB promoted diastereoselective ring contraction of a 1,2-dihydronaphthalene 276 to construct the indane ring system 277 (Scheme 89).604 A similar oxidative rearrangement of 3-cinnamoyl-4-hydroxy-6-methyl-2H-pyran-2-ones with HTIB in dichloromethane followed by cyclization was used by Prakash and co-workers for the direct conversion of o-hydroxychalcones into isoflavone derivatives.605
Scheme 89.
The HTIB induced oxidative rearrangement of alkenes can be effectively used in ring expansion reactions. Justik and Koser have investigated the oxidative ring expansions of alkylidenebenzocycloalkenes 278 to β-benzocycloalkenones 279 using HTIB in 95% methanol (Scheme 90).606 This reaction allows the efficient conversion of alkenes 278, which can be conveniently prepared from the respective α-benzocycloalkenones by Wittig olefination, to the homologous β-benzocycloalkenones 279 containing six, seven and eight-membered rings.
Scheme 90.
Silva and co-workers reported a similar HTIB-promoted ring expansion of 1-vinylcycloalkanol derivatives leading to seven- or eight-membered rings. In a specific example, the reaction of the unsaturated TMS ether 280 with excess HTIB affords benzocycloheptanone derivative 281 in high yield (Scheme 91).607
Scheme 91.
HTIB is commonly used for the oxidative functionalization of arenes, alkenes and alkynes. Koser, Telu and Laali investigated the oxidative substitution reactions of polycyclic aromatic hydrocarbons with iodine(III) sulfonate reagents.608 Various polycyclic arenes, such as pyrene, anthracene, phenanthrene, perylene and others, undergo regioselective oxidative substitution reactions with iodine(III) sulfonate reagents in dichloromethane at room temperature to give the corresponding aryl sulfonate esters in moderate to good yields. The reaction of polycyclic aromatic hydrocarbons with HTIB in the presence of trimethylsilyl isothiocyanate leads to the regioselective thiocyanation of the PAH nucleus, as illustrated by the reaction of anthracene shown in Scheme 92.608
Scheme 92.
Dihydropyridone derivatives 282 can be efficiently iodinated to afford products 283 by the treatment with N-iodosuccinimide (NIS) in the presence of HTIB (Scheme 93).609
Scheme 93.
Poly[4-(hydroxy)(tosyloxy)iodo]styrene can be used in the halotosyloxylation reaction of alkynes with iodine or N-bromosuccinimide (NBS) or N-chlorosuccinimide (NCS) (Scheme 94).610 The polymer reagent can be regenerated and reused.
Scheme 94.
HTIB can also be used in the oxidative rearrangements and fragmentations of various nitrogen-containing compounds. Similar to [bis(trifluoroacetoxy)iodo]benzene, HTIB can be applied in the intramolecular cyclization reactions involving N-acylnitrenium intermediates 142 (see Scheme 48 in Section 3.4.5).366,611 For example, spirodienones 285 bearing the 1-azaspiro[4.5]decane ring system were synthesized from N-methoxy-3-(4-halophenyl)propanamides 284 via the intramolecular ipso-cyclization of a nitrenium ion generated with HTIB in trifluoroethanol (Scheme 95).611 The HTIB-promoted cyclizations of the appropriate amides were also utilized in the preparation of 2,1-benzothiazine derivatives from sulfonamides612 and in the synthesis of (−)-lapatin B via oxidative cyclization of N,N-diacetylglyantrypine.613
Scheme 95.
Similar to [bis(acyloxy)iodo]arenes (see Section 3.4.5), HTIB can serve as excellent oxidant in Hofmann-type degradation of carboxamides to the respective amines.614–616 In a recent example, primary alkyl- and benzylcarboxamides were converted to the corresponding alkylammonium tosylates with poly[4-hydroxy(tosyloxy)iodo]styrene in acetonitrile at reflux in yields ranging from 60% to 90%.617 Likewise, the recyclable reagents 267103 and 268260 (see Section 3.5.1) have been used to convert p-nitrobenzamide 286 and phenylacetamide 288 to the respective aniline 287 and benzylammonium tosylate 289 in good yields under mild reaction conditions (Scheme 96).103,260
Scheme 96.
Benzylic alcohols can be oxidized with HTIB under solvent-free microwave irradiation conditions to afford the corresponding aldehydes or ketones in excellent yields.618 The glucal derivative 290 was oxidized to the enone 291 by treatment with HTIB in acetonitrile (Scheme 97).619
Scheme 97.
Aryl ketones 292 can be converted to the corresponding substituted benzoic acids 293 by sequential treatment with [hydroxy(2,4-dinitrobenzenesulfonyloxy)iodo]benzene and urea-hydrogen peroxide in [bmim]BF4 ionic liquid (Scheme 98).620
Scheme 98.
Yan and co-workers reported a catalyst- and base-free Suzuki-type coupling reaction of sodium tetraphenylborate with HTIB or other λ3-iodanes. This non-catalytic coupling affords the respective biaryls in good yields in water solution or solvent-free under microwave irradiation.621–623
HTIB and other sulfonate derivatives of iodosylbenzene have also found wide application for the preparation of various iodonium salts.
3.6. Nitrogen Substituted λ3-Iodanes
The noncyclic aryliodine(III) derivatives with an iodine nitrogen bond usually lack stability and, with a few exceptions, cannot be isolated as individual compounds. The chemistry of these compounds was discussed in our previous reviews.5,6 In particular, several examples of aryliodine(III) amides, ArI(NHCOR)2, derived from phthalimide, succinimide, glutarimide, and saccharine have been reported by Varvoglis and co-workers.624–626 Aryliodine(III) amides ArI(NHCOR)OAc and ArI(NHCOR)OTs bearing one N-ligand at iodine are plausible intermediates in the Hofmann-type degradation of amides with [bis(acyloxy)iodo]arenes or [hydroxy(tosyloxy)iodo]benzene.614 In most cases, these intermediates are highly unstable and instantaneously rearrange at room temperature with loss of iodobenzene to give isocyanates.
The noncyclic azidoiodanes, PhI(N3)X (X = OAc, Cl, OTMS, etc.) or PhI(N3)2, were proposed as reactive intermediates in the widely used azidation reactions involving the combination of iodosylbenzene or (diacetoxy)iodobenzene with trimethylsilyl azide or sodium azide.5 Attempts to isolate these intermediates always resulted in fast decomposition at −25 to 0 °C with the formation of iodobenzene and dinitrogen; however, low-temperature spectroscopy and the subsequent chemical reactions in situ provided some experimental evidence toward the existence of these species. The final proof for the existence of azidoiodanes was provided by the preparation and the single-crystal X-ray structure determination of stable azidobenziodoxoles.627
(Diazidoiodo)benzene, PhI(N3)2, generated in situ from PhIO/TMSN3 has found some practical application as an efficient reagent for the introduction of the azido function into organic molecules.6 Magnus and co-workers reported the synthetically useful azidation of triisopropylsilyl enol ethers 294 affording β-azido adducts 295 and the azidation of N,N-dimethylarylamines 296 to give N-azidomethyl derivatives 297 in excellent yields (Scheme 99).628–630
Scheme 99.
More recently, Bols and co-workers have found that the PhI(OAc)2/TMSN3 system is similar in reactivity to IN3 and can promote high-yield azidations of ethers, aldehydes and benzal acetals at 0 °C to room temperature in acetonitrile.631 For example, the azidation of ethers 298 under these conditions leads to benzylic azides 299, while the aldehydes 300 initially afford the unstable acyl azides 301, which are converted to carbamoyl azides 302 via the Curtius rearrangement upon heating with an excess of TMSN3 (Scheme 100). These azidations proceed through a radical mechanism and involve the initial generation of PhI(N3)2. It is essential for the reaction that TMSN3 is added subsequent to the mixture of PhI(OAc)2 and the substrate; mixing of TMSN3 and PhI(OAc)2 before adding the substrate completely fails to produce any azidation products, presumably because the generated intermediate azidoiodane species decompose before the reaction.631
Scheme 100.
Austin and co-workers utilized the PhI(N3)2 mediated vicinal diazidation of a double bond in the key step of the total synthesis of (±)-dibromophakellstatin. The key syn-diazide 304 was prepared by the treatment of pyrazinone 303 with the PhI(OAc)2/TMSN3 system followed by the addition of tetraethylammonium iodide (Scheme 101).632 Under these conditions, the initially generated PhI(N3)2, further reacts with the iodide anion leading to the in situ formation of the diazido iodate anion, (N3)2I−,633 which serves as the actual azidating species in this reaction.
Scheme 101.
The interaction of the PhI(OAc)2/NaN3 system with organic ditellurides can be used for the generation of the organotellurenyl radicals. This reaction has been utilized in the synthesis of organyltellurophosphates 307 by the treatment of diorganyl phosphites 306 and diorganyl ditellurides 305 with (diacetoxyiodo)benzene and sodium azide in dichloromethane at room temperature (Scheme 102).634
Scheme 102.
3.7. Stabilized Alkyl Substituted λ3-Iodanes
Alkyl substituted λ3-iodanes, RIX2, in general lack stability and can exist only as short-lived reactive intermediates in the oxidations of alkyliodides.5,6 The thermal stability of alkyliodosyl derivatives can be substantially increased by steric or electronic modification of the alkyl moiety preventing decomposition of the molecule by either elimination or nucleophilic substitution pathways. Most commonly such a stabilization is achieved by the introduction of electron-withdrawing substituents, such as fluorine atoms or a sulfonyl group, into the alkyl moiety. Especially well-investigated and important representatives of stabilized alkyl substituted λ3-iodanes are [bis(trifluoroacetoxy)iodo]perfluoroalkanes 308,44,417,635–639 [hydroxy(sulfonyloxy)iodo]perfluoroalkanes 309,640,641 1-[bis(trifluoroacetoxy)iodo]-1H,1H-perfluoroalkanes 310,642 1-[hydroxy(sulfonyloxy)iodo]-1H,1H-perfluoroalkanes 311,643,644 [bis(trifluoroacetoxy)iodo](arylsulfonyl)methane derivatives 312,645 and fluoroalkyliododichlorides 313.225

The trifluoroacetate derivatives 308, 310, and 312 are usually prepared by the oxidation of appropriate iodides with 80% hydrogen peroxide and trifluoroacetic anhydride followed by removal of the volatile products in vacuum (yield 97–98%).637,638,640 A convenient procedure for the preparation of [bis(trifluoroacetoxy)iodo]perfluoroalkanes 308 by the oxidation of commercial perfluoroalkyl iodides using urea-hydrogen peroxide complex in a mixture of trifluoroacetic anhydride and trifluoroacetic acid at −5 to 0 °C was recently reported.417 Trifluoroacetates 308 and 310 can be converted to sulfonates 309 and 311 by treatment with the appropriate sulfonic acid.640,644 In contrast to the starting trifluoroacetates 308 and 310, sulfonates 309 and 311 have a substantially higher thermal stability and are not water sensitive; they can be purified by crystallization from acetonitrile, and can be stored for several months in a refrigerator.
Single crystal X-ray diffraction studies of several representatives of stabilized alkyl substituted λ3-iodanes have previously been reported, namely: trifluoromethyliodine(III) difluoride, CF3IF2 (see Section 3.2.2),190 trifluoromethyliodine(III) dichloride, CF3ICl2,646 trifluoromethyliodine(III) chloride fluoride, CF3I(Cl)F,647 [bis(trifluoroacetoxy)iodo]trifluoromethane, CF3I(OCOCF3)2,648 trifluoromethyliodine(III) chloride trifluoroacetate, CF3I(Cl)OCOCF3,649 [bis(methoxy)iodo]trifluoromethane, CF3I(OMe)2,650 methoxy(trifluoromethyl)iodine(III) chloride, CF3I(Cl)OMe,650 fluoroalkyliododichlorides 313 (see Section 3.3.2),225 and the bis(trifluoroacetate) CF3CH2I(OCOCF3)2.651 In particular, the bis(trifluoroacetate) CF3CH2I(OCOCF3)2 has a distorted T-shaped coordination similar to other known dicarboxylates, but forms a previously unknown tetrameric array of molecules due to strong intermolecular I•••O contacts.651
[Bis(trifluoroacetoxy)iodo]perfluoroalkanes 308 are the most practically useful representatives of stabilized alkyl substituted λ3-iodanes. Trifluoroacetates 308 have found practical application as starting compounds for the preparation of (perfluoroalkyl)aryliodonium salts, which are useful electrophilic perfluoroalkylating reagents.44 Recently, Tesevic and Gladysz have demostrated the utility of [bis(trifluoroacetoxy)iodo]perfluoroalkanes 308 with a long fluorous alkyl chain (n = 7–12) as convenient recyclable oxidants.637,638 Similarly to [bis(trifluoroacetoxy)iodo]benzene and (diacetoxyiodo)benzene (see Section 3.4.6) [bis(trifluoroacetoxy)iodo]perfluoroalkanes can serve as excellent reagents for the oxidation of phenolic substrates. The reduced form of the reagent, the respective iodoperfluoroalkane, can be efficiently separated from the reaction mixture using fluorous techniques and reused. In a specific example, reagents 308 (n = 8, 10, 12) can rapidly oxidize 1,4-hydroquinones 314 to the respective quinones 315 in methanol at room temperature (Scheme 103). Subsequent addition of a fluorous solvent, such as perfluoro(methylcyclohexane), results in a liquid/liquid biphase system. The product quinones 315 are generally isolated in about 95% yields from the methanol phase, and iodoperfluoroalkanes 316 are isolated in 98–99% yields from the fluorous phase. The recovered iodoperfluoroalkanes 316 may be reoxidized to the initial reagents 308 in 97% yield and reused.637
Scheme 103.
Westwell and co-workers investigated the oxidation of hydroxylated stilbenes 317 using [bis(trifluoroacetoxy)iodo]perfluorohexane (Scheme 104).417 Instead of the expected products of the phenolic oxidation, diaryl-1,2-dimethoxyethanes 318 as mixtures of diastereoisomers were isolated in moderate yields from this reaction. The perfluorohexyl iodide by-product (bp 140 °C) could be removed simply by evaporation of the reaction mixture under reduced pressure.417
Scheme 104.
[Bis(trifluoroacetoxy)iodo]perfluoroalkanes 308 (n = 7, 8, 10, 12) are effective and easily recyclable reagents for the oxidation of aliphatic and benzylic secondary alcohols 319 to ketones 320 in the presence of aqueous KBr and the absence of organic or fluorous solvents (Scheme 105).638 The reduced form of the reagent, the respective iodoperfluoroalkane 316, can be efficiently isolated from the reaction mixture in 96–98% yield by adding 3–5 volumes of methanol and separating the resulting fluorous/methanolic liquid/liquid biphase system. The recovered iodoperfluoroalkane 316 can be reoxidized to reagent 308 and reused.638
Scheme 105.
It is noteworthy that the fluorous reagents 308 oxidize secondary alcohols in the presence of bromide ions much more rapidly than other iodine(III) compounds (e.g., iodosylbenzene or DIB) under similar conditions. The higher reactivity may in part be ascribed to the directly bound electron-withdrawing perfluoroalkyl substituent in compounds 308, which enhance its oxidizing strength.638
3.8. Iodine(III) Heterocycles
The most important iodine(III) heterocycles are represented by various derivatives of benziodoxole 321 and benziodazole 322.24 The collective name “benziodoxoles” is commonly used for heterocycles 321 with iodine and oxygen incorporated in the five-membered ring and various substituents X attached to iodine. The first derivatives of benziodoxole, 1-hydroxy-1,2-benziodoxol-3-(1H)-one (321, X = OH, 2R = O)652 and 1-chloro-1,2-benziodoxol-3-(1H)-one (321, X = Cl, 2R = O),653 were prepared over 100 years ago by oxidation or chlorination of 2-iodobenzoic acid. In the mid-1980’s, 1-hydroxybenziodoxoles have attracted considerable interest and research activity mainly due to their excellent catalytic activity in the cleavage of reactive phosphate esters.33 More recently, various new benziodoxole derivatives were synthesized and their usefulness as reagents for organic synthesis was demonstrated.24 In contrast to benziodoxoles, the analogous five-membered iodine-nitrogen heterocycles, benziodazoles 322, have received much less attention and, moreover, their structural assignment in some cases was not reliable. The most important and readily available derivative of benziodazole, 1-acetoxybenziodazole (322, X = OAc, R = H), was first prepared in 1965 by the peracetic oxidation of 2-iodobenzamide,654 and the correct structure of this compound was reported in 1997.655

X-ray molecular structures were reported for numerous benziodoxole derivatives 321.100,101,627,656–668 In general, the five-membered ring in benziodoxole is highly distorted with almost linear alignment of the two electronegative ligands. The I-O bond length in benziodoxolones (321, 2R = O) varies in a wide range from 2.11 Å in carboxylates (321, X = m-ClC6H4CO2)661 to 2.48 Å in the phenyl derivative (321, X = Ph),100 which indicates considerable changes in the ionic character of this bond. The endocyclic C-I-O bond angle is typically around 80°, which is a significant deviation from the expected angle of 90° for the normal T-shaped geometry of hypervalent iodine. The examples of recently reported X-ray structures of benziodoxoles include phosphoranyl-derived benziodoxoles 323,101 1-bromobenziodoxoles 324,666 and 1-trifluoromethylbenziodoxoles 325.667,668 Benziodoxoles 323 and 325 were prepared by a standard ligand exchange procedure starting from the appropriate 1-acetoxybenziodoxole and a phosphonium ylide or CF3SiMe3, respectively,101,667,668 while 1-bromobenziodoxoles 324 were synthesized in 56–60% yield by oxidative bromination of the appropriate iodoarenes with N-bromosuccinimide.666

The structural parameters of benziodazoles (322, X = OAc or Ph) in general are similar to those of benziodoxoles.74,102,655 The synthesis and structural studies of N-functionalized benziodazoles were recently reported.102 1-Acetoxybenziodazoles 327 were prepared by the peracetic oxidation of 2-iodobenzamides 326 derived from alanine or valine (Scheme 106).102
Scheme 106.
The alanine derivative 328 was further converted to phenyliodonium salt 329, which, according to X-ray data, has a pseudo-cyclic structure with an I•••O distance of 2.56 Å in the benziodoxole ring.102 The treatment of pseudo-benziodoxole 329 with sodium bicarbonate affords 1-phenylbenziodazole 330 (Scheme 107), whose structural parameters are very similar to the structure of the previously reported 1-phenylbenziodoxole (321, X = Ph). In particular, the benziodazole ring system in compound 330 is essentially planar and has a relatively long I-N bond of 2.445 Å. This structural study of benziodazole-based phenyliodonium derivatives 329 and 330 provides insight into facile interchange between benziodazole and benziodoxole ring systems under acidic or basic conditions.102
Scheme 107.
The distinctive feature of heterocyclic λ3-iodanes is the considerably higher stability than that of their acyclic analogs. This stabilization is usually explained by the bridging of the apical and the equatorial positions by a five-membered ring, and also by the better overlap of the lone pair electrons on the iodine atom with the p-orbitals of the benzene ring.656,669 The greater stability of benziodoxoles enabled the preparation and isolation of otherwise unstable iodine(III) derivatives with I–Br,656,666 I–OOR,670–674 I–N3,627,675,676 I–CN,664,665,677 and I–CF3 bonds.667,668 These various benziodoxole derivatives have found practical application as the reagents for oxidative functionalization of organic substrates. For example, the stable 1-azidobenziodoxoles (321, X = N3) can be used as efficient reagents for direct azidation of an unactivated C–H bond in alkanes,627,675,676 while 1-tert-butylperoxy-1,2-benziodoxol-3(1H)-one (321, X = OOBut) is a useful oxidant with numerous synthetic applications.670–674 Ochiai and co-workers have recently demonstrated that 1-tert-butylperoxy-1,2-benziodoxol-3(1H)-one is a particularly useful radical reagent for the generation of α-oxy carbon-centered radicals from cyclic ethers and acetals.674,678
Togni and co-workers have found that 1-trifluoromethylbenziodoxole 331 is a useful reagent for electrophilic trifluoromethylation of nucleophilic substrates. This reagent, in particular, reacts with β-ketoesters 332 under mild conditions in the presence of potassium carbonate affording α-trifluoromethylated product 333 in good yield (Scheme 108).667,668 Likewise, this mild electrophilic trifluoromethylation reagent can be used to transfer a CF3 group to other C-centered nucleophiles, such as α-nitro esters, to S-centered nucleophiles,668 and secondary or primary aryl- and alkylphosphines.679
Scheme 108.
Very recently, Hu and co-workers have reported the preparation of the reagent’s 331 analog bearing PhSO2CF2- substituent on the iodine atom. This new benziodoxole derivative was found to act as the electrophilic (phenylsulfonyl)difluoromethylating reagent for a variety of S-nucleophiles under mild reaction conditions.680
3.9. Iodonium Salts
Iodonium salts, R2I+ X−, are defined as positively charged 8-I-2 species with two carbon ligands and a negatively charged counter ion. X-ray structural data for the overwhelming majority of iodonium salts show a significant secondary bonding between the iodine atom and the anion with average bond distances within a range of 2.3 to 2.7 Å, which results in a pseudo trigonal bipyramidal geometry similar to λ3-iodanes with one carbon ligand. In agreement with this model, the experimentally determined bond angle R–I–R in iodonium salts is close to 90°.6 The most common and well investigated class of these compounds are diaryliodonium salts, known for over one hundred years and extensively covered in previous reviews. In the 1980s and 1990s significant research activity was focused on aryliodonium derivatives, Ar(R)I+ X−, bearing alkynyl-, alkenyl-, or fluoroalkyl groups as ligand R. These aryl substituted iodonium salts are particularly useful reagents for the electrophilic transfer of ligand R to electron-rich organic substrates. The high reactivity of phenyliodonium salts, Ph(R)I+ X−, in these reactions is explained by the “hyperleaving group ability” of the PhI group, which, has a leaving group ability about 106 times greater than triflate.681
Stable iodonium salts have found numerous practical applications, such as cationic photoinitiators in polymer chemistry,682–685 and as biologically active compounds. A summary of the biological properties of iodonium salts is provided in our 1996 review.5 In a specific example, a recent study of the in vitro activities of several iodonium salts against oral and dental anaerobes has demonstrated that their activities are comparable to that of chlorhexidine and these compounds may be suitable for incorporation into an oral mouthwash.686
In this section, the preparation and chemistry of iodonium salts will be discussed with emphasis on recent synthetic applications.
3.9.1. Alkyl- and Fluoroalkyliodonium Salts
Similar to the alkyl substituted λ3-iodanes (see Section 3.7), iodonium salts with one or two aliphatic groups generally lack stability.6 The presence of electron-withdrawing groups in the alkyl group of iodonium salts has a pronounced stabilizing effect. The most stable derivatives of this type are fluoroalkyl(aryl)iodonium salts 334, 335 and (arylsulfonylmethyl)iodonium triflates 336. The preparation of fluoroalkyl(aryl)iodonium salts and their application as electrophilic fluoroalkylating reagents was reviewed by Umemoto.44 Iodonium salts 334–336 are usually prepared by the reaction of the appropriate bis(trifluoroacetates) 308, 310 and 312 (Section 3.7) with benzene in the presence of trifluoromethanesulfonic or other strong acid.6 The structure of iodonium triflate 336 (Ar = Tol) was unambiguously established by a single-crystal X-ray analysis.645

The preparation of fluoroalkyliodonium salts 337 by the reaction of bis(trifluoroacetates) 310 with benzene and triflimide acid was recently reported (Scheme 109).225,651,687 The structure of trifluoroethyl(phenyl) iodonium salt 337 (n = 1) was established by a single-crystal X-ray analysis.225 In contrast to fluoroalkyliodonium triflates 335, compounds 337 are stable to water and can be used for fluoroalkylations in aqueous media.
Scheme 109.
Compounds 337 are especially useful as reagents for fluoroalkylation of amino acids and peptides.651,687–691 For example, the reaction of iodonium salt 337 (n = 7) with the tert-butyl carboxyl ester of tyrosine 338 in the presence of collidine results in quantitative formation of the monoalkylation product 339 (Scheme 110).687,690 Due to this reactivity, iodonium salts 337 can be used as fluorous capping reagents for facile purification of peptides synthesized on the solid phase.687,691
Scheme 110.
3.9.2. Aryl- and Heteroaryliodonium Salts
Diaryliodonium salts belong to the most common and well investigated class of iodine(III) compounds, and the chemistry of these compounds has been extensively covered in previous reviews.5,6 In this section, the preparative methods and recent examples of synthetic applications of diaryliodonium and heteroaryliodonium salts, Ar2I+X−, are overviewed. Numerous X-ray structures of aryliodonium salts have been reported in the older literature. The more recent structural studies include the X-ray structure reports on (2-methoxy-5-methylphenyl)(4-methoxy-2-methylphenyl)iodonium trifluoroacetate,692 diaryl zwitterionic iodonium compound PhI+C6H4-4-SO2N−Tf,693 1-naphthylphenyliodonium tetrafluoroborate and 1-naphthylphenyliodonium tetrakis(pentafluorophenyl)gallate,694 and the study of structural and electronic characteristics of thienyl(aryl)iodonium triflates.695
3.9.2.1 Preparation of aryliodonium salts
Diaryliodonium tetrafluoroborates 341 and 343 can be conveniently prepared by the boron-iodine(III) exchange reaction of (diacetoxyiodo)arenes with tetraarylborates 340696 or arylboronic acids 342697,698 followed by the treatment with a saturated sodium tetrafluoroborate solution (Scheme 111). Recent modification of this procedure consists of the treatment of arylrifluoroborates, ArBF3−K+, with (difluoroiodo)arenes under mild conditions.205 Likewise, fluoroorganoiodonium tetrafluoroborates (C6F5)2I+BF4−, (4-C5F4N)2I+BF4− and [C6F5(4-C5F4N)I+BF4− can be prepared by interaction of the appropriate (difluoroiodo)arenes with fluorinated organodifluoroboranes, ArfBF2, in dichloromethane at 0 to 20 °C.178
Scheme 111.
An alternative procedure consists of a similar tin-iodine(III) and silicon-iodine(III) exchange reaction of (diacetoxyiodo)arenes or iodosylbenzene with tetraphenylstannane699 or trimethylsilylbenzene699 in the presence of boron trifluoride etherate.
Frohn and co-workers reported the preparation of a perfluoroaryliodonium salt, (C6F5)2I+ AsF6−, by the electrophilic arylation of C6F5I with a stable pentafluorophenylxenonium hexafluoroarsenate, C6F5Xe+AsF6−.700
Numerous experimental procedures for the preparation of symmetrical and unsymmetrical diaryl- and hetaryliodonium sulfates and organosulfonates have been reported.3,5,6 The most common synthetic approach to unsymmetric diaryl- and hetaryl(aryl)iodonium tosylates is based on the reactions of [hydroxy(tosyloxy)iodo]arenes with arenes,701 aryl- or hetaryltrimethylsilanes,702,703 aryltributylstannanes,257,704,705 or arylboronic acids.706 The reaction of HTIB with arylstannanes proceeds under milder conditions compared to arylsilanes and is applicable to a wide range of arenes with electron-withdrawing substituents. Arylboronic acids in general have some advantage over arylstannanes in the case of the electron-rich heterocyclic precursors.706
Various unsymmetrically functionalized diaryliodonium triflates 346 can be synthesized by the reaction of iodosylbenzene707 or (diacetoxyiodo)arenes 344708 with arenes 345 in trifluoromethanesulfonic acid (Scheme 112).708 This simple procedure affords diaryliodonium triflates in relatively high yields, but it is limited to aromatic substrates that are not sensitive to strong acids. Moreover, the formation of the p-phenylene type oligomeric iodonium salts as side products may occur upon the reaction of (diacetoxyiodo)benzene with trifluoromethanesulfonic acid.569 In a milder and a more selective variation of this procedure (diacetoxyiodo)benzene is reacted with arylboronic acids in the presence of triflic acid at −30 °C to afford aryl(phenyl)iodonium triflates in 74–97% yields.706
Scheme 112.
Several modified procedures for the preparation of diaryliodonium triflates have recently been reported. Kitamura and Hossain have developed a direct preparation of diaryliodonium triflates in good yields from iodoarenes and aromatic substrates using K2S2O8 as an oxidant in a one-pot reaction.709 Further modification of this procedure involves the reaction of arenes with elemental iodine and K2S2O8 in trifluoroacetic acid, followed by treatment with sodium triflate (Scheme 113).710,711
Scheme 113.
Olofsson and co-workers have developed a general and efficient one-pot synthesis of symmetrical and unsymmetrical diaryliodonium triflates 349 from both electron-deficient and electron-rich arenes 348 and aryl iodides 347 using mCPBA as the oxidant and triflic acid (Scheme 114).712–714 The electron-rich diaryliodonium tosylates are prepared similarly using toluenesulfonic acid instead of triflic acid as the additive.714 Symmetrical diaryliodonium triflates can be synthesized by a modified one-pot procedure from iodine, arenes, mCPBA and triflic acid under similar conditions.712,713 A similar procedure based on a one-pot reaction of arylboronic acids, aryl iodides, mCPBA and BF3•Et2O has recently been used for regioselective synthesis of unsymmetrical diaryliodonium tetrafluoroborates.715
Scheme 114.
Skulski and Kraszkiewicz have recently reported a new method for the preparation of various symmetrical diaryliodonium bromides (in 15–88% crude yields) directly from arenes by the reaction of ArH with NaIO4 in sulfuric acid followed by the addition of KBr.716
A very mild and general method for the preparation of diaryl- and heteroaryliodonium triflates is based on iodonium transfer reactions of iodine(III) cyanides with the respective aryl- or heteroarylstannanes.253,255,717,718 Specifically, (dicyano)iodonium triflate 350, generated in situ from iodosyl triflate and TMSCN, reacts with tributyltin derivatives of aromatic and heteroaromatic compounds affording the corresponding symmetrical iodonium salts under very mild conditions (Scheme 115).717,718
Scheme 115.
Aryl(cyano)iodonium triflates (e.g. 351) can be used in a similar iodonium exchange with stannylated aromatic precursors affording various mixed diaryl or aryl(heteroaryl) iodonium salts.253,255,695 In a recent study, Tykwinski, Hinkle and co-workers have utilized this iodonium transfer reaction in the preparation of a series of mono- and bithienyl(aryl)iodonium triflates 352 with increasingly electron-withdrawing substituents on the aryl moiety (Scheme 116).695
Scheme 116.
The preparation of several macrocyclic iodonium triflates, such as rhomboids 355, a square 358, and a pentagon 359 was recently reported (Scheme 117).719 The rhomboid shaped molecules 355 were prepared by the treatment of compounds 353 and 354 with trimethylsilyl triflate. The reaction of dication 356 with compound 357 in the presence of Me3SiOTf gave an iodonium containing molecular square 358 in 70% yield.254,719 In addition, a pentagon-shaped macrocycle 359 was prepared in 60% yield from precursors 356 and 353. The structures of these iodonium-containing charged macrocycles were established using elemental analysis, multinuclear NMR and mass spectrometry. These iodonium-containing macromolecules may find potential application in nanotechnology.719
Scheme 117.
A very mild and selective approach to aryl- and hetaryliodonium chlorides 362 is based on the reaction of the appropriate aryllithium 360 (generated in situ from bromoarenes and butyllithium) with trans-(chlorovinyl)iodonium dichloride 361 (Scheme 118).720–724 The iodonium transfer reagent 361 is prepared by the reaction of iodine trichloride with acetylene in concentrated hydrochloric acid;722 this compound is extremely unstable and should be handled and stored with proper safety precautions.721 The iodonium transfer procedure with reagent 361 is particularly useful for the preparation of bis(hetaryl)iodonium chlorides 364 from the appropriate nitrogen heterocycles 363 (Scheme 118).721
Scheme 118.
3.9.2.2 Reactions of aryliodonium salts
The most important and synthetically useful reactions of aryliodonium salts include the direct electrophilic arylations of various nucleophiles, the transition metal mediated cross-coupling reactions, and the reactions involving the generation and trapping of the benzyne intermediates.
Numerous examples of the rections of aryliodonium salts with such nucleophiles as thiosulfonate anions, fluoride anion, malonates, and silyl enol ethers under polar, non-catalytic conditions are provided in our previous reviews.5,6 In more recent papers, the electrophilic arylations of sodium arenesulfinates,725 potassium carbonotrithioates,726 and benzazoles727 using diaryliodonium salts in ionic liquids, and the arylations of anilines,728 sodium tetraphenylborate729 and vinylindiums730 have been reported.
The mechanism of solvolysis of methoxy-substituted diaryliodonium tetrafluoroborates, ArI+Ph −BF4, in methanol and 2,2,2-trifluoroethanol has recently been investigated.731 The solvolysis products include alkoxide substitution products (ArOR and PhOR) as well as iodoarenes (PhI and ArI). The ratios of products, ArOR/PhOR, range from 8/2 to 4/6. The results of this study provide experimental evidence against the formation of aryl cation under these conditions and support the pathways via ligand coupling or SNAr2 mechanisms involving a solvent molecule as a nucleophile in the transition state.731
The reactions of aryliodonium salts with fluoride anion have recently been used for the preparation of fluorine-18 labelled aromatic compounds.258,705,732 In a specific example, the 18F labelled compound 366 was prepared by the reaction of diaryliodonium salt 365 with the radioactive 18F anion (Scheme 119). Compound 366 is used as a positron emission tomography (PET) ligand for imaging peripheral-type benzodiazepine receptor.705
Scheme 119.
Reactions of arylation of carbon nucleophiles using aryliodonium salts are particularly important. Compounds containing an active methylene group, such as malonates, or the respective carbanions formed in situ, react smoothly with diaryliodonium salts to yield α-arylated products.733,734 Aggarwal and Olofsson have developed a direct asymmetric α-arylation of prochiral ketones using chiral lithium amide bases and diaryliodonium salts.721 In a specific example, the deprotonation of cyclohexanone derivative 367 using chiral Simpkins’ (R,R)-base followed by the reaction with pyridyl iodonium salt 364 gave the arylated product 368 in 94% enantiomeric excess (Scheme 120). This reaction (Scheme 120) has been employed in a short total synthesis of the alkaloid (−)-epibatidine.721
Scheme 120.
Ozanne-Beaudenon and Quideau reported a regioselective dearomatizing phenylation of phenols and naphthols using diaryliodonium salts.735,736 For example, the treatment of naphthols 369 substituted at the ortho position by a small electron-donating group with diphenyliodonium chloride leads to their regioselective ortho-phenylation to give products 370 (Scheme 121). The mechanism of this reaction involves a nonradical direct coupling of the ligands on the hypervalent iodine center.735 The formation of phenol ethers due to the O-phenylation can also occur when the reaction of phenolate anion with diphenyliodonium chloride is carried out in a polar aprotic solvent such as dimethylformamide.735
Scheme 121.
The O-arylation of the appropriate phenols using symmetrical iodonium salts has been utilized in the synthesis of hydroxylated and methoxylated polybrominated diphenyl ethers, some of which are related to natural products.737,738 In particular, several polybrominated diphenyl ethers 373 were prepared by the reaction of iodonium salt 371 with phenols 372 in N,N-dimethylacetamide solution under basic conditions (Scheme 122).737
Scheme 122.
Arylations with aryliodonium salts can be effectively catalyzed by transition metals. Aryliodonium salts can serve as efficient reagents in the copper-catalyzed arylation of lithium enolates,739 thiophenes,740 5-aryl-2H-tetrazole,741 and uracil nucleosides.742
Palladium salts and complexes are efficient catalysts in the cross-coupling reactions of diaryliodonium salts with organoboron compounds,743,744 organostannanes,745 silanes,746 organolead triacetates,747 organobismuth(V) derivatives,748 carbon monoxide,749 allylic alcohols,750 functionalized allenes,751,752 Grignard reagents,753 alkenes,754,755 terminal alkynes,756 and with arenecarboxylic acids via decarboxylative cross-coupling reaction.757 Particularly interesting is the palladium-catalyzed directed C-H activation/phenylation of substituted 2-phenylpyridines and indoles with aryliodonium salts recently reported by Sanford and co-workers.698,758 In a representative example, 2-pyridyl substituted substrates 374 are selectively phenylated to the ortho-position affording products 375 in good yields (Scheme 123). Preliminary mechanistic experiments have provided evidence in support of a rare Pd(II)/(IV) catalytic cycle for this transformation.698 The preparation of stable triorganyl Pd(IV) complexes by the electrophilic arylation of palladium(II) bipyridine complexes using Ph2I+ TfO− was reported by Canty and co-workers.759
Scheme 123.
Kitamura and co-workers reported the preparation and uses of several efficient benzyne precursors based on aryliodonium salts.760–764 In particular, phenyl[2-(trimethylsilyl)phenyl]iodonium triflate (376) is readily prepared by the reaction of 1,2-bis(trimethylsilyl)benzene with the PhI(OAc)2/TfOH reagent system.760 The treatment of reagent 376 with tetrabutylammonium fluoride in dichloromethane at room temperature generates benzyne, which can be trapped with a diene to afford the respective benzyne adducts in high yields.760 Recent examples of synthetic application of reagent 376 as benzyne precursor include O-arylation of carboxylic acids leading to aryl esters 377,765 preparation of 2-aryl-substituted nitriles 379 by arylation of nitriles 378 via a benzyne reaction,766 and cycloaddition/elimination reaction of thiophene S-oxide 380 with benzyne leading to product 381 (Scheme 124).767 Reagent 376 was also used in the synthesis of spiro(imidazolidine-2,3′-benzo[b]thiophene) by a one-pot reaction of benzyne, aryl isothiocyanates and N-heterocyclic carbenes,768 and for the preparation of benzo[b]seleno[2,3-b]pyridines by the reaction of acetic acid 2-selenoxo-2H-pyridin-1-yl esters with benzyne.769
Scheme 124.
The efficient acylbenzyne precursors, [5-acyl-2-(trimethylsilyl)phenyl]iodonium triflates 382 have recently been prepared by the reaction of the appropriate 1,2-bis(trimethylsilyl)benzenes with the PhI(OAc)2 in the presence of trifluoromethanesulfonic acid in dichloromethane at room temperature. Treatment of these reagents with Bu4NF in dichloromethane generates acylbenzynes 383, which can be trapped by furan to give adducts 384 in high yield (Scheme 125).763
Scheme 125.
Lee and co-workers reported the preparation of oxadisilole-substituted benzyne precursors, such as iodonium triflate 386, from benzobisoxadisilole 385 and the PhI(OAc)2/TfOH reagent system.770 The treatment of reagent 386 with Bu4NF in THF and diisopropylamine at room temperature generates oxadisilole-substituted benzyne 387, which can be trapped with furan to afford adduct 388 in good yield (Scheme 126).
Scheme 126.
Ko, Kang and co-workers have reported the generation and trapping of 1,2-dehydrocarborane, the carborane analog of benzyne.771 The 1,2-dehydrocarborane precursor, phenyl[o-(trimethylsilyl)carboranyl]iodonium acetate, was readily prepared by the reaction of [o-(trimethylsilyl)carboranyl]lithium and PhI(OAc)2. 1,2-Dehydrocarborane was efficiently generated from phenyl[o-(trimethylsilyl)carboranyl]iodonium acetate by treatment with CsF in ether and trapped with dienes such as anthracene, naphthalene, norbornadiene and 2,5-dimethylfuran to give the respective 1,2-dehydrocarborane adducts in high yield.771
3.9.3. Alkenyliodonium Salts
The chemistry of alkenyliodonium salts was extensively covered in several recent reviews by Ochiai,36,38 Okuyama,47,54,55 and Zefirov and co-authors.46 This section of our review will summarize the important recent developments in the preparation and synthetic application of alkenyliodonium salts.
3.9.3.1 Preparation of alkenyliodonium salts
Boron trifluoride-catalyzed silicon-iodine(III) exchange reaction of organosilanes 389 with iodosylarenes followed by treatment with aqueous NaBF4 constitutes the most general method for synthesis of alkenyl(aryl)iodonium tetrafluoroborates 390 (Scheme 127).697,772,773 This reaction proceeds under mild conditions and in a stereospecific manner with retention of configuration of organosilanes.
Scheme 127.
A similar borane-iodine(III) exchange of organoboronic acids 391 with iodosylbenzene or (diacetoxyiodo)benzene in the presence of boron trifluoride etherate is an efficient alternative method for a selective preparation of alkenyl(phenyl)iodonium tetrafluoroborates 392 in excellent yields (Scheme 128).774,775
Scheme 128.
(E)-β-Fluoroalkenyl(tolyl)iodonium tetrafluoroborates 393 are conveniently synthesized by the treatment of terminal alkynes with 4-iodotoluene difluoride in the presence of boron trifluoride etherate (Scheme 129).206 This reaction occurred instantaneously at −78 °C to give fluoroalkenyliodonium salts 393 in good yields with high stereoselectivity. Likewise, various alkenyliodonium organosulfonates can be synthesized via electrophilic addition of the appropriate hypervalent iodine reagents to alkynes.184,776,777
Scheme 129.
(E)-β-Fluoroalkenyl(phenyl)iodonium tetrafluoroborates 395 can be stereoselectively prepared by the reaction of alkynyl(phenyl)iodonium salts 394 with aqueous HF in good yields (Scheme 130).778,779 The method is applicable to the synthesis of fluoroalkenyliodonium salts having functional groups such as ketone, ester, and chloride.
Scheme 130.
A very general and mild procedure for the stereospecific synthesis of alkenyliodonium organosulfonates 398 involves the reaction of aryl(cyano)iodonium triflates and tosylates 397 with stannylated alkenes 396 (Scheme 131).780,781
Scheme 131.
The polymer-supported alkenyliodonium tosylates 401 can be prepared by the treatment of polystyrene-based resin 399 with 3-aminocrotonate esters 400 (Scheme 132).782 The similar monomeric α-acyl-β-aminoalkenyl(phenyl)iodonium tosylates have been synthesized by the reaction of amino substituted α, β-unsaturated ketones with [hydroxy(tosyloxy)iodo]benzene.783
Scheme 132.
3.9.3.2. Reactions of alkenyliodonium salts
Alkenyl(phenyl)iodonium salts are very reactive compounds because of the excellent leaving group ability of the phenyliodonium moiety (1012 times greater than for iodine itself) combined with its high electron-withdrawing properties (the Hammett substituent constant σm for the PhI+ group is 1.35).784 Several research groups have recently been involved in the mechanistic studies of nucleophilic substitution in alkenyliodonium salts.785–790 Various mechanisms, including SN1, SN2, ligand coupling, and Michael addition-elimination have been observed in these reactions. The mechanistic aspects of the reactions of vinylic iodonium salts with nucleophiles have been reviewed by Okuyama47,791 and by Ochiai.36,38
Particularly interesting is the recently reported observation of cyclohexyne intermediates 403 as products of β-elimination in the reactions of 1-cyclohexenyl(phenyl)iodonium salts 402 with mild bases such as tetrabutylammonium acetate, fluoride ion, alkoxides, and amines in aprotic solvents.784,785,792 Cyclohexynes 403 could be effectively trapped with tetraphenylcyclopentadienone to give products of [4+2] cycloaddition 404 in high yields (Scheme 133). Cycloheptyne intermediates can be generated under similar conditions from the appropriate iodonium precursors.784,789,793
Scheme 133.
Alkenyl(phenyl)iodonium salts have found synthetic application as alkenylating reagents in the reactions with various nucleophilic substrates. In most cases these reactions proceed with predominant retention of configuration via the addition-elimination mechanism or ligand coupling on the iodine. Recent examples of alkenylations of nucleophiles under non-catalytic conditions include the stereoselective reactions of alkenyliodonium salts with sodium selenide, sodium sulfide, sodium azide, potassium thiocyanate,794 and benzotriazole.795 In a specific example, functionalized β-enamines 405 have been prepared by the reaction of polymer-supported alkenyliodonium tosylates 401 with various nucleophiles at room temperature (Scheme 134).782
Scheme 134.
(E)- and (Z)-(fluoroalkenyl)boronates 407, 409 were prepared stereospecifically by the reaction of (E)- or (Z)-(2-fluoroalkenyl)iodonium salts 406, 408 with di(p-fluorophenoxy)alkylboranes, followed by transesterification to pinacol esters (Scheme 135). The mechanism of this reaction involves the initial generation of 2-fluoroalkylideneiodonium ylide by the α-deprotonation of iodonium salts with LDA followed by its reaction with with di(p-fluorophenoxy)alkylboranes.796,797
Scheme 135.
Only a few examples of non-catalytic alkenylation of carbon nucleophiles are known. In particular, enolate anions derived from various 1,3-dicarbonyl compounds can be vinylated with cyclohexenyl (410) and cyclopentenyl iodonium salts to afford products 411 (Scheme 136).798
Scheme 136.
The selectivity of the alkenylation reactions and the yields of products can be dramatically improved by carrying out the reaction of alkenyliodonium salts with carbon nucleophiles in the presence of transition metal compounds in stoichiometric or catalytic amounts. In the presence of a copper(I) catalyst iodonium salts selectively react with iodide anion,778,779 organoborates,799 Grignard reagents,800 and terminal alkynes801 to afford the respective cross-coupling products in high yields with complete retention of configuration. A recent example of such a reaction is represented by the copper-mediated cross-coupling of H-phosphonates 413 with vinyliodonium salts 412 leading to 2-arylvinylphosphonates 414 under mild conditions (Scheme 137).802
Scheme 137.
Alkenyliodonium salts can be used as highly reactive reagents for Heck-type olefination,803,804 Sonogashira-type coupling with alkynes,778,805 and similar palladium-catalyzed cross-coupling reactions.206,779,806 In a specific example, (Z)-β-fluoro-α, β-unsaturated esters 416 were stereoselectively synthesized from (Z)-2-fluoro-1-alkenyliodonium salts 415 by the Pd-catalyzed methoxycarbonylation reaction (Scheme 138).806 The reaction proceeded at room temperature and various functional groups on the substrate can tolerate the reaction conditions.
Scheme 138.
Reactions of alkenyliodonium salts with strong bases may lead to the generation of an alkylidenecarbene via a base-induced α-elimination. Alkylidenecarbenes generated by this method can undergo a 1,5-carbon-hydrogen insertion, providing a useful route for the construction of substituted cyclopentenes.807–809 In a recent example, an efficient synthesis of fluorocyclopentenes 418 by the reaction of (Z)-(2-fluoroalkenyl)iodonium salts 417 with potassium tert-butoxide has been developed (Scheme 139). The mechanism of this reaction involves the initial generation of (α-fluoroalkylidene)carbenes which give fluorocyclopentenes via 1,5-C–H insertion.807
Scheme 139.
3.9.4. Alkynyliodonium Salts
The chemistry of alkynyliodonium salts was exhaustively covered in several previous reviews.29,42,810 Therefore, this section will only summarize the important recent developments in the preparation and synthetic application of alkynyliodonium salts.
3.9.4.1 Preparation of alkynyliodonium salts
The most common approach to alkynyl(phenyl)iodonium tetrafluoroborates employs the reaction of iodosylbenzene with alkynylsilanes in the presence of boron trifluoride etherate followed by treatment with aqueous NaBF4.811,812 Varvoglis, Koumbis and co-workers have recently used this procedure for the preparation of several ortho-substituted arylethynyl(phenyl)iodonium terafluoroborates 420 from alkynylsilanes 419 (Scheme 140).813
Scheme 140.
A modified procedure for the synthesis of alkynyl(phenyl)iodonium tetrafluoroborates 422 reported by Hara and co-workers consists of the direct reaction of terminal alkynes 421 with iodosylbenzene, 42% aqueous solution of tetrafluoroboric acid, and a catalytic amount of mercury oxide (Scheme 141).814
Scheme 141.
Yoshida and coauthors have reported a facile preparation of iodonium salts 424 by the reaction of potassium organotrifluoroborates 423 with (difluoroiodo)arenes under mild conditions (Scheme 142).205
Scheme 142.
Alkynyl(phenyl)iodonium tosylates are commonly prepared by gentle heating of [hydroxy(tosyloxy)iodo]benzene with terminal alkynes in chloroform or dichloromethane.812,815,816 This method is also applicable to the synthesis of alkynyliodonium mesylates and 4-nitrobenzenesulfonates by the reaction of the appropriate [hydroxy(organosulfonyloxy)iodo]benzenes with terminal alkynes under similar conditions.815
The most versatile method of preparation of alkynyl(phenyl)iodonium triflates 427 employs the iodonium transfer reaction between cyano(phenyl)iodonium triflate 426 and alkynylstannanes 425 under very mild conditions (Scheme 143).817 This procedure is particularly useful for the preparation of various complex, functionalized alkynyliodonium derivatives, such as compounds 428, 429,818 430,819 431,820 and 432.821 Compounds 428–432 are formed under these very mild conditions in high yields (80–90%) and can be used in subsequent transformations without additional purification.
Scheme 143.
An alternative general procedure for the selective preparation of alkynyl(phenyl)iodonium triflates in moderate yields employs the reaction of alkynylsilanes or alkynylstannanes with Zefirov’s reagent (see Section 3.5.1).813,822 This method is also applicable to the synthesis of the parent ethynyl(phenyl)iodonium triflate.823
3.9.3.2. Reactions of alkynyliodonium salts
Reactions of alkynyliodonium salts with nucleophiles proceed via an addition-elimination mechanism involving alkylidene carbenes as key intermediates. Depending on the structure of the alkynyliodonium salt, specific reaction conditions, and the nucleophile employed, this process can lead to a substituted alkyne due to the carbene rearrangement, or to a cyclic product via intramolecular 1,5-carbene insertion.42 Both of these reaction pathways have been widely utilized in organic synthesis.
Alkynyl(phenyl)iodonium salts have found synthetic application for the preparation of various substituted alkynes by the reaction with the appropriate nucleophiles, such as: enolate anions,822,824 selenide and telluride anions,825–827 dialkylphosphonate anions,828 benzotriazolate anion,829 imidazolate anion,830 N-functionalized amide anions,831–833 and transition metal complexes.834–838 Specific recent examples are represented by the preparation of N-alkynyl carbamates 435 by alkynylation of carbamates 433 using alkynyliodonium triflates 434 (Scheme 144),832 synthesis of ynamides 437 by the alkynylation/desilylation of tosylanilides 436 using trimethylsilylethynyl(phenyl)iodonium triflate (Scheme 145),833 and the preparation of Ir(III) σ-acetylide complex 439 by the alkynylation of Vaska’s complex 438 (Scheme 146).834
Scheme 144.
Scheme 145.
Scheme 146.
Alkynyl(phenyl)iodonium salts can be efficiently coupled with organocopper reagents,839 or with organoboronic acids or organostannanes in the presence of Cu(I) catalysts.840,841 Specifically, the copper iodide-catalyzed cross- and carbonylative coupling reactions of alkynyliodonium salts 441 with arylboronic acids 440 or organostannanes 443 under mild conditions afford arylacetylenes 442 and aryl alkynyl ketones 444 in high yields (Scheme 147).841 Interestingly, alkynyliodonium tetrafluoroborates 441 are more efficient in these coupling reactions than the corresponding iodonium triflates and tosylates.
Scheme 147.
A variety of five-membered heterocycles can be prepared efficiently by inter- or intramolecular addition/cyclizations of appropriate nucleophiles with alkynyliodonium salts via alkylidene carbene intermediates.29,42,810 The intermolecular variant of this cyclization has recently been utilized in the synthesis of 3-substituted-5,6-dihydroimidazo[2,1-b]thiazoles,842 2-substituted imidazo[1,2-a]pyrimidines,843 and 2-substituted-imidazo[1,2-a]pyridines.844 In a specific example, 2-substituted-imidazo[1,2-a]pyridines 447 were synthesized in good yield by cyclocondensation of alkynyl(phenyl)iodonium tosylates 445 with 2-aminopyridine 447 under mild conditions (Scheme 148). The mechanism of this cyclization involves initial nucleophilic addition of the amino group of 2-aminopyridine to the triple bond of the alkynyliodonium salt followed by generation and subsequent cyclization of the intermediate alkylidene carbene. 844
Scheme 148.
Ochiai and co-workers have investigated the mechanism for the one-pot synthesis of 2,4-disubstituted thiazoles 450 by cyclocondensation of alkynyliodonium salts 448 with thioureas or thioamides 449 (Scheme 149).845 This reaction was originally reported by Wipf and Venkatraman in 1996.846 Ochiai and co-workers have isolated and identified by X-ray analysis intermediate products 453 (as mesylate or tetrafluoroborate salts), which suggests the mechanism involving Michael addition of sulfur nucleophile 449 to alkynyliodonium salt 448 giving intermediate ylide 451 followed by the 1,2-rearrangement of sulfenyl groups in the resulting alkylidene carbene 452 (Scheme 149).845
Scheme 149.
The intramolecular variant of the alkylidene carbene cyclization is achieved by the treatment of functionalized alkynyliodonium salts with the appropriate nucleophile. Recent examples are represented by the preparation of various functionalized 2,5-dihydrofurans by treatment of 3-alkoxy-1-alkynyl(phenyl)iodonium triflates with sodium benzenesulfinate,821 by the utilization of the alkylidene carbene cyclization in the total syntheses of natural products agelastatin A and agelastatin B,819 and by the preparation of the tricyclic core of (±)-halichlorine through the use of an alkynyliodonium salt/alkylidenecarbene/1,5 C-H insertion sequence.820 In particular, Wardrop and Fritz have utilized the sodium benzenesulfinate induced cyclization of the generated in situ alkynyliodonium triflate 454 leading to dihydrofuran 455 (Scheme 15), which is a key intermediate product in the total synthesis of (±)-magnofargesin.821
Feldman and co-workers have applied the sodium p-toluenesulfinate induced cyclizations of alkynyliodonium salts 456 and 431 for the preparation of compounds 457 and 458 (Scheme 151), the key intermediates in the total syntheses of agelastatins819 and (±)-halichlorine, respectively.820
Scheme 151.
3.10. Iodonium Ylides
The first preparation of an iodonium ylide by the reaction of dimedone and (difluoroiodo)benzene was reported by Neiland and co-workers in 1957.847 Since then a large number of stable iodonium ylides have been prepared, and many synthetic applications have emerged. The chemistry of iodonium ylides was overviewed in several reviews devoted to the reactions of carbenes.56–58 This section will summarize the preparation and structural studies of iodonium ylides and important recent developments in their synthetic applications.
3.10.1. Preparation and Structure
The most common and relatively stable structural types of iodonium ylides, namely phenyliodonium bis(organosulfonyl)methides, PhIC(SO2R)2 and the dicarbonyl derivatives PhIC(COR)2, are generally prepared by a reaction of (diacetoxyiodo)benzene with the appropriate disulfone or dicarbonyl compound under basic conditions.848–850 The vast majority of iodonium ylides have low thermal stability and can be handled only at low temperature or generated and used in situ. Several structural types of ylides, however, are sufficiently stable for X-ray structural analysis. Single crystal X-ray structural parameters have been reported for 3-phenyliodonio-1,2,4-trioxo-1,2,3,4-tetrahydronaphthalenide 459,851 3-phenyliodonio-2,4-dioxo-1,2,3,4-tetrahydro-1-oxanaphthalenide 460,851 mixed phosphonium iodonium ylides 461852 and 462,853 mixed arsonium iodonium ylides 463,854 cyclic iodonium ylide 464,855 and phenyliodonium bis(trifluoromethanesulfonyl)methide 465.856 In particular, the X-ray structural analysis for phenyliodonium bis(trifluoromethanesulfonyl)methide 465 shows a geometry typical for an iodonium ylide with the I–C ylide bond length of about 1.9 Å and an C-I-C bond angle of 98°.856

Ochiai and coworkers have recently reported the intermolecular transylidation reactions between halonium ylides under thermal or catalytic conditions, which allow to synthesize a variety of iodonium ylides 467 (Scheme 152). The transylidations of bromonium 466 to iodonium 467 ylides proceed under thermal conditions and probably involve generation of a reactive carbene intermediate.857 The heating of phenyliodonium bis(trifluoromethylsulfonyl)methylide 465 in a large amount of an iodoarene in the presence of 5 mol% of rhodium(II) acetate as a catalyst results in the transfer of the bis(trifluoromethylsulfonyl)methylidene group to the iodine(I) atom to afford a substituted aryliodonium ylide 467 in a good yield. Reversible nature of the catalytic intermolecular transylidation makes it possible to evaluate the thermodinamic stability of aryliodonium ylides.858
Scheme 152.
A mechanistic study of 1,4 alkyl group migration in hypervalent halonium ylides was recently reported by Moriarty and co-authors. In particular, it was found that the rhodium(II)-acetate-catalyzed decomposion of either 1,3-cyclohexanedione phenyliodonium ylide or 5,5-dimethyl-1,3-cyclohexanedione phenyliodonium ylide in the presence of alkyl halides yields the corresponding 3-alkoxy-2-halocyclohex-2-enones via a 1,4 alkyl group migration shown to be concerted and intramolecular.859
The monocarbonyl iodonium ylides 469 can be quantitatively generated in situ from the (Z)-(2-acetoxyvinyl)iodonium salts 468 via an ester exchange reaction with ethoxylithium in THF at −78 °C (Scheme 153). 1H NMR measurements indicate that ylides 469 are stable up to −30 °C, and they can be conveniently used in the subsequent transformations without isolation.860–862
Scheme 153.
The unstable ylides PhIC(H)NO2863,864 and PhIC(CO2Me)NO2865,866 can be generated in situ from nitromethane and methyl nitroacetate, respectively, and used in the rhodium(II) carbenoid reactions without isolation.
3.10.2. Reactions
Iodonium ylides can serve as convenient precursors to the respective carbene intermediates under thermal, photochemical, or catalytic conditions. A detailed discussion of the reaction mechanisms and synthetic applications of iodonium ylides as carbene precursors can be found in the 2004 review of Muller.58
Several new uncatalyzed reactions of iodonium ylides have recently been reported.867–873 Koser and co-workers have found that the treatment of electron-rich aromatic substrates, such as anthracene, pyrene, 2-alkylthiophenes, and 1,4-dimethoxybenzene with phenyliodonium bis(carbonyl)methylides in the presence of BF3•Et2O leads to bis(carbonyl)alkylation of the aromatic nucleus.867 For example, the reactions of 2-alkylthiophenes 470 with ylides 471 afford products 472 in 15–39% isolated yield (Scheme 154).
Scheme 154.
The reaction of disulfonyl iodonium ylide 473 with alkyl iodides 474 affords functionalized iodides 475 in moderate yield (Scheme 155). The mechanism of this reaction most likely involves the initial transylidation with the formation of unstable alkyliodonium ylides, RCH2I=C(SO2Ph)2, which then undergo the intramolecular Stevens rearrangement forming iodides 475.868
Scheme 155.
Spyroudis and co-workers have reported the reaction of the phenyliodonium ylide of 2-hydroxy-1,4-naphthoquinone 459 with amines 476 in refluxing dichloromethane to afford good yields of the indanedione 2-carboxamides 477 (Scheme 156). This reaction proceeds through initial carbene formation, followed by a ring-contraction leading to an intermediate α,α′-dioxoketene,874 which reacts with amines 476 to afford the final amides 477.869 The analogous products are formed when ylide 459 is reacted with amino esters, ureas, amino alcohols, aminophenols, and indole derivatives under thermal conditions.870,871
Scheme 156.
Li and co-workers have developed a mild and general synthesis of substituted benzofurans by the cycloaddition of iodonium ylides with arynes generated from 2-(trimethylsilyl)aryl triflates and CsF. In a specific example, 2-(trimethylsilyl)aryl triflates 478 smoothly react with iodonium ylides 479 in the presence of CsF at room temperature giving benzofurans 480 in moderate to good yields (Scheme 157).872
Scheme 157.
Ochiai and co-workers have found that the interaction of monocarbonyl iodonium ylides 482, generated by the ester exchange of (Z)-(2-acetoxyvinyl)iodonium salts 481 with EtOLi, with organoboranes results in the formation of ketones 484, probably via the intermediate formation of the hitherto unknown α-boryl ketones 483 (Scheme 158).861
Scheme 158.
The mixed phosphonium-iodonium ylides, such as the tosylate 485, represent a potentially useful class of reagents that combine in one molecule synthetic advantages of a phosphonium ylide and an iodonium salt.854,875–878 Specifically, phosphorane-derived phenyliodonium tosylate 485 can react with soft nucleophiles, such as iodide, bromide, benzenesulfinate, and thiophenolate anions, with a selective formation of the respective α-functionalized phosphonium ylides 486 (Scheme 159), which can be further converted to alkenes by the Wittig reaction with aldehydes.875,876 The analogous arsonium-iodonium ylides (e.g. 463) have a similar reactivity toward nucleophiles.854,877,879
Scheme 159.
The carbenoid reactions of iodonium ylides can be effectively catalyzed by rhodium(II) or copper complexes.56–58 The product composition in the rhodium(II) catalyzed reactions of iodonium ylides was found to be identical to that of the corresponding diazo compounds, which indicates that the mechanism of both processes is similar and involves metallocarbenes as key intermediates as it has been unequivocally established for the diazo decomposition.849 Recent examples of the transition metal catalyzed carbenoid reactions of iodonium ylides are represented by the following publications: Rh(II)- or Cu(I)-catalyzed cyclopropanation reactions using the unstable ylides PhIC(H)NO2863 and PhIC(CO2Me)NO2865,866 generated in situ from nitromethane and methyl nitroacetate; Rh(II)-catalyzed three-component coupling of an ether with a nitromethane-derived carbenoid generated from PhIC(H)NO2;864 Rh(II)- or Cu(II)-catalyzed insertion of carbene into alkenyl C-H bond in pyrroles,880 flavones,881 and highly phenylated ethylenes;882 Rh(II)-catalyzed reaction of iodonium ylides with conjugated compounds leading to efficient synthesis of dihydrofurans, oxazoles, and dihydrooxepines;883 synthesis of various heterocycles by Rh(II)-catalyzed reactions of iodonium ylides with vinyl ethers, carbon disulfide, alkynes, and nitriles;884 Rh(II)-catalyzed reaction of iodonium ylides with electron-deficient and conjugated alkynes leading to substituted furans;885 efficient synthesis of β-substituted α-haloenones by Rh(II)-catalyzed reactions of iodonium ylides with benzyl halides and acid halides;886 Rh(II)- or Cu(II)-catalyzed generation/rearrangement of onium ylides of allyl and benzyl ethers via iodonium ylides;887 and Rh(II)- or Cu(II)-catalyzed stereoselective cycloaddition of disulfonyl iodonium ylides with alkenes leading to 1,2,3-trisubstituted benzocyclopentenes888 or functionalized indanes.889–891
The metal-catalyzed carbenoid decomposition of iodonium ylides can be applied in asymmetric reactions. 865,892–894 For example, the copper(II)-catalyzed intramolecular C–H insertion of phenyliodonium ylide 487 in the presence of chiral ligands followed by hydrolysis and decarboxylation affords product 488 in moderate yield with up to 72% ee (Scheme 160).894
Scheme 160.
A palladium-catalyzed coupling reaction of iodonium ylides 489 with aryl boronic acids 490 was reported. The mild reaction conditions and convenient synthetic accessibility of iodonium ylides 489 make this method a valuable tool for the preparation of diversified 3-aryl-4-hydroxycoumarins 491 (Scheme 161).895
Scheme 161.
3.11. Iodonium Imides
The chemistry of iodonium imides (also known as iminoiodanes) has been reviewed by Dauban and Dodd in 2003.28 Aryliodonium imides 494 are best prepared by the reaction of (diacetoxyiodo)arenes 492 with the respective amides 493 under basic conditions (Scheme 162).28,73,222,896–900 Most iodonium imides are stable at room temperature but their storage under an inert atmosphere at low temperature is recommended. They are thermally sensitive and some of them are even claimed to be explosive. Violent decomposition frequently occurs at the melting point.28
Scheme 162.
Single-crystal X-ray structural data have been reported for several N-tosyliminoiodanes, namely, PhI=NTs,222,901 2,4,6-Me3C6H2I=NTs,222 and 2-MeC6H4I=NTs.898 Similar to iodosylarenes (see Section 3.1.2), iminoiodanes have a linear polymeric, asymmetrically bridged structure with the T-shaped geometry around the iodine centers. In the case of PhI=NTs, the monomeric units are bridged by I-N interactions, while in the more sterically hindered 2,4,6-Me3C6H2I=NTs the bridging atom is the oxygen of the tosyl group.222 Protasiewicz and coworkers have reported the preparation and X-ray structure of highly soluble, ortho-sulfonyl substituted aryliodonium imide 2-ButSO2C6H4I=NTs, in which the intramolecular secondary I•••O bond replaces the intermolecular interactions that are typical of the other iminoiodanes.90
Aryliodonium imides have found synthetic applications as useful nitrene precursors under thermal or catalytic conditions in amidation and imidation reactions of various organic substrates and in the aziridination of alkenes.28 Only a few examples of the reactions of aryliodonium imides in the absence of transition metal catalysts have been published in the recent literature. Che and coworkers have reported the aziridination of alkenes with phenyliodonium imides generated in situ from N-substituted hydrazines 495 and (diacetoxyiodo)benzene under mild conditions (Scheme 163).902 This reaction affords aziridines 496 in good to excellent yields (up to 99%), and conversions. The practicality and simplicity of this C-N bond formation protocol was exemplified by its application to the aziridination of cholesteryl acetate 497 in a stereoselective manner (Scheme 164).902 A similar reaction of the PhI(OAc)2/N-substituted hydrazine 495 system has been used in the nitrene mediated metal-free ring expansions of alkylidenecyclopropanes and alkylidenecyclobutanes.903
Scheme 163.
Scheme 164.
Wirth, Desaize and Richardson have published a detailed study of the aziridination of alkenes with the PhI(OAc)2/N-substituted hydrazine 495 system and, in particular, reported tentative evidence that this reaction (Scheme 163) proceeds through the formation of an aminoiodane that reacts directly with the alkene.904 Furthermore, the authors of this publication904 have analyzed the requirements to make this reaction catalytic in iodoarene. This reaction requires an oxidant that will oxidize iodoarenes but that does not oxidize alkenes, and it is possible that no such oxidant actually exists. However, a method in which the hypervalent iodine reagent can be recycled without the need for reisolation is possible.904
The transition metal catalyzed amidation of C–H bonds in saturated or unsaturated substrates represents one of the most common reactions of aryliodonium imides.6,28 Recent examples of this reaction using PhI=NTs as the nitrene precursor are represented by the following publications: the highly efficient Ru(II) porphyrin catalyzed C-H bond amidation of aldehydes,905 the aromatic C-H amidation mediated by a diiron complex,906 the AuCl3-catalyzed nitrene insertion into aromatic and benzylic C-H bonds,907 the silver-catalyzed intermolecular and intramolecular amidation of C-H bond in saturated hydrocarbons,908,909 the α-amidation of cyclic ethers catalyzed by Cu(OTf)2,910 the mechanistic study of catalytic intermolecular amination of C-H bonds,911 the nitrene insertion into the sp3 C-H bonds of alkylarenes and cyclic ethers or the sp2 C-H bonds of benzene using a copper-homoscorpionate complex,912 the Co(II)-catalyzed allylic amidation reactions,913 the Ru(II) porphyrin-catalyzed amidation of aromatic heterocycles,914 and the non-heme iron-catalyzed amidation of aromatic substrates.915 The enantioselective amidation of a C–H bond can also be achieved in the presence of the chiral (salen)manganese(III) complexes. For example, the amidation of substrate 498 occurs at the benzylic C-H bond to afford product 499 with good enantioselectivity (Scheme 165).916
Scheme 165.
Aryliodonium imides are efficient nitrene precursors in the transition metal-catalyzed aziridination of alkenes.6,28 Particularly important is the application of PhINTs in the asymmetric aziridination of alkenes using copper catalysts with chiral dinitrogen ligands.917–924 In a specific example, the PhINTs promoted asymmetric aziridination of alkene 500 affords chiral aziridine 501 in over 99% ee (Scheme 166).921
Scheme 166.
The aziridination and amidation reactions of aryliodonium imides can be efficiently catalyzed by the Rh(II) complexes.925–930 Dirhodium(II) tetrakis[N-tetrafluorophthaloyl-(S)-tert-leucinate], Rh2(S-TFPTTL)4, is an exceptionally efficient catalyst for enantioselective aminations of silyl enol ethers 502 with iodonium imide 503 providing α-amido ketones 504 in high yields and with enantioselectivities of up to 95% ee (Scheme 167). The effectiveness of this catalytic protocol has been demonstrated by an asymmetric formal synthesis of (−)-metazocine.925 The same catalyst has also been used for the asymmetric synthesis of phenylglycine derivatives by enantioselective amidation of silylketene acetals with aryliodonium imides.926
Scheme 167.
Sanford and co-workers have recently reported the carbon-nitrogen bond-forming reactions of palladacycles with aryliodonium imides.931 In particular, palladium(II) complexes (e.g. 505) containing bidentate cyclometalated chelating ligands react with PhINTs at room temperature to insert the tosylimino group into the Pd-C bond (Scheme 168). This tosylimino insertion reaction has been applied to palladacyclic complexes of azobenzene, benzo[h]quinoline, and 8-ethylquinoline. The newly aminated organic ligands can be liberated from the metal center by protonolysis with a strong acid.931
Scheme 168.
The imido group can be efficiently transferred to the sulfur atom in organic sulfides or sulfoxides,932–935 or the nitrogen atom in aromatic nitrogen heterocycles using aryliodonium imides in the presence of copper, ruthenium, or iron complexes.936,937 Specific examples are represented by the selective N-imidation of aromatic nitrogen heterocycles (e.g. 506) catalyzed by carbonyl[meso-tetrakis(p-tolyl)porphyrinato]ruthenium(II) [Ru(II)(TPP)(CO)] (Scheme 169),936 and the iron-catalyzed imination of sulfoxides (e.g. 507) and sulfides (Scheme 170).932
Scheme 169.
Scheme 170.
4. Iodine(V) Compounds
The chemistry of organic iodine(V) compounds, or λ5-iodanes according to the IUPAC nomenclature, in general has been less developed in comparison with the λ3-iodanes.6 The first comprehensive review on the synthetic applications of hypervalent iodine(V) reagents has appeared in 2006,22 and a specialized review on iodoxybenzoic acid (IBX) was published by Wirth in 2001.938 There has been a very significant recent interest in the cyclic λ5-iodanes, mainly IBX and Dess-Martin periodinane (DMP), which have found broad practical application as mild and selective reagents for the oxidation of alcohols and some other useful oxidative transformations.938 Despite their importance, IBX and DMP are not perfect reagents and have some disadvantages. IBX is potentially explosive and is insoluble in common organic solvents due to the strong intermolecular secondary bonding creating a three-dimensional polymeric structure, while DMP is highly sensitive to moisture. Several IBX derivatives and analogs with improved properties have been developed in the last 5–6 years and utilized in organic synthesis. In particular, the highly soluble and non-explosive pseudo-cyclic derivatives of IBX, as well as their polymer-supported analogs, have been introduced. This section of our review will summarize the preparation and structure of λ5-iodanes and overview important recent developments in their synthetic applications.
4.1. Non-Cyclic and Pseudocyclic Iodylarenes
Iodylarenes, ArIO2, which are also known as iodoxy compounds, are commonly prepared by direct oxidation of iodoarenes with strong oxidants or by disproportionation of iodosylarenes. It is assumed that the initial oxidation of ArI usually leads to iodosylarenes, ArIO, which then slowly disproportionate to ArI and ArIO2 upon gentle heating, or even at room temperature.92,256,939 The most common oxidizing reagents that are used for the preparation of iodylarenes from iodoarenes include sodium hypochlorite, sodium periodate, dimethyldioxirane, and oxone. In particular, Skulski and Kraszkiewicz reported an improved method for the preparation of various iodylarenes 509 from the corresponding iodoarenes 508 using sodium periodate as the oxidant dissolved in boiling 30% aqueous acetic acid (Scheme 171).939 Iodylarenes 509 usually precipitate from the reaction mixture and can be additionally purified by recrystallization from hot water or other solvents. Dry iodylarenes are potentially hazardous compounds, which may explode upon impact, scratching with a spatula, or heating, and therefore should be handled with appropriate precautions.
Scheme 171.
A new facile methodolology for the preparation of noncyclic iodylarenes using peracetic acid as an oxidant in the presence of catalytic amounts of ruthenium trichloride has recently been reported.529,940 This new procedure allows the preparation of several previously unknown iodylarenes 509 bearing strongly electron-withdrawing CF3 groups in the aromatic ring.940
Iodylbenzene, PhIO2, has a polymeric structure, which makes it insoluble in the majority of organic solvents, with the exception of DMSO. X-ray crystal structural investigations of PhIO2 revealed infinite polymeric chains with strong I•••O secondary intermolecular interactions.941 Iodylbenzene and other noncyclic iodylarenes in general have found only very limited practical application due to their low stability and explosive properties.22
Aryliodyl derivatives bearing an appropriate substituent in the ortho-position to the iodine are characterized by the presence of a pseudocyclic structural moiety due to a strong intramolecular secondary bonding between the hypervalent iodine center and the oxygen atom in the ortho-substituent. Compared to the non-cyclic aryliodyl derivatives, pseudocyclic iodine(V) compounds have much better solubility, which is explained by a partial disruption of their polymeric nature due to the redirection of secondary bonding.89,91
Protasiewicz and co-workers have recently reported the preparation of a soluble ortho-phosphoryl stabilized aryliodyl derivative 511, which was obtained by the hypochlorite oxidation of the appropriate aryliodide 510 (Scheme 173).92 Single crystal X-ray analysis of compound 511 has shown a close contact of the phosphoryl oxygen atom and the iodine(V) atom with a distance of 2.612 Å, which is significantly shorter than the I•••O distance of 3.291 Å determined for the unoxidized aryliodide 510.92
Scheme 173.
The previously unknown esters of 2-iodoxybenzoic acid (IBX-esters, 513) were prepared by the hypochlorite oxidation of the readily available esters of 2-iodobenzoic acid 512 (Scheme 174) and isolated in the form of stable microcrystalline solids.95,96 This procedure allows for the preparation of products 513 derived from various types of alcohols, such as primary, secondary, and tertiary alcohols, adamantanols, optically active menthols and borneol. X–Ray data on products 513 revealed a pseudo-benziodoxole structure in which the intramolecular I•••O secondary bonds partially replace the intermolecular I•••O secondary bonds disrupting the polymeric structure characteristic of PhIO2941 and other previously reported iodylarenes.96 This structural feature substantially increases the solubility of these compounds in comparison to other iodine(V) reagents and affects their oxidizing reactivity. IBX-esters can oxidize alcohols to the respective aldehydes or ketones in the presence of trifluoroacetic acid or boron trifluoride etherate.96 Isopropyl 2-iodoxybenzoate 513 (R = Pri) is a particularly useful reagent for the clean and selective oxidation of organic sulfides to sulfoxides.942 This reaction proceeds without over-oxidation to sulfones and is compatible with the presence of the hydroxy group, double bond, phenol ether, benzylic carbon, and various substituted phenyl rings in the molecule of organic sulfide.
Scheme 174.
Methyl 2-iodoxybenzoate 513 (R = Me) can be further converted to the diacetate 514 or a similar bis(trifluoroacetate) derivative by treatment with acetic anhydride or trifluoroacetic anhydride, respectively. Single crystal X-ray diffraction analysis of methyl 2-[(diacetoxy)iodosyl]benzoate 514 revealed a pseudo-benziodoxole structure with three relatively weak intramolecular I•••O interactions. The dimethyl and diisopropyl esters of 2-iodoxyisophthalic acid were prepared by oxidation of the respective iodoarenes with dimethyldioxirane. Single crystal X-ray diffraction analysis of diisopropyl 2-iodoxyisophthalate 515 showed intramolecular I•••O interaction with the carbonyl oxygen of only one of the two carboxylic groups, while NMR spectra in solution indicated equivalency of both ester groups.96

The amides of 2-iodoxybenzoic acid (IBX-amides, 517) were prepared by the dioxirane oxidation of the appropriate derivatives of 2-iodobenzoic acid 516 (Scheme 175) in the form of stable, microcrystalline solids moderately soluble in dichloromethane and chloroform.94 This procedure (Scheme 175) can be used for the preparation of products 517 derived from numerous types of amino compounds, such as esters of α–amino acids, esters of β–amino acids, and (R)–1–phenylethylamine. Single crystal X-ray analysis of the phenylalanine derivative (517, R = (S)-CH(CH2Ph)CO2Me) revealed a close intramolecular contact of 2.571 Å between the hypervalent iodine center with the oxygen atom of the amido group within each molecule enforcing a planar geometry of the resulting five-membered ring, a geometry that is analogous to that observed for IBX and other benziodoxoles.94
Scheme 175.
2–Iodoxybenzamides 517 are useful oxidizing reagents towards alcohols with a reactivity pattern similar to IBX. A wide range of primary and secondary alcohols can be oxidized by these reagents to the respective carbonyl compounds in excellent yields under mild conditions in chloroform.94,943 Oxidative kinetic resolution of racemic sec-phenethyl alcohol using reagents 517 has showed very low enantioselectivity (1–6% ee).943
Lee and co-workers have synthesized the polymer-supported IBX-ester 518 and IBX-amides 519, 520 starting from the commercially available hydroxy or amino polystyrene in two steps.944 The oxidant resins 518–520 were prepared with loadings of 0.65–1.08 mmol/g, and were evaluated with a series of alcohol substrates. The polymer supported IBX-amide 520, exhibited particularly fast and efficient oxidative activities toward a series of alcohols under mild reaction conditions.944 IBX-amide resin 520 is also an efficient oxidant for oxidative bromination of activated aromatic compounds using tetraethylammonium bromide.945 Linclau and co-workers reported an improved synthesis of a solid-supported IBX-amide resins 521 and 522 using inexpensive and commercially available 2-iodobenzoic acid chloride and Merrifield resin.946 Oxidation of a range of alcohols to the corresponding carbonyl compounds can be accomplished using 1.2 equivalents of the resins 521 and 522. Recycling of the resin was also possible with minimal loss of activity after two reoxidations.946

Amides of 2-iodoxybenzenesulfonic acid 524 were prepared by the dioxirane oxidation of the corresponding 2-iodobenzenesulfamides 523 and isolated as stable, microcrystalline products (Scheme 176).947 Single crystal X-ray structures of 2-iodylbenzenesulfonamides 524 reveal a combination of intra- and intermolecular I•••O interactions leading to a unique heptacoordinated iodine(V) center in the alanine derivative 524 (R = (S)-CH(CH3)CO2Me).93
Scheme 176.
Likewise, esters of 2-iodoxybenzenesulfonic acid 526 were prepared by the dioxirane oxidation in dichloromethane of the respective monovalent iodine derivatives 525 (Scheme 177). These new pseudocyclic hypervalent iodine reagents can selectively oxidize benzyl alcohols to aldehydes, secondary amines to imines, and sulfides to sulfoxides.948
Scheme 177.
The soluble and stable IBX analogs having pseudo-benziodoxazine structure, N-(2-iodylphenyl)acylamides (NIPA) 528, were prepared in good yields by the oxidation of 2-iodoaniline derivatives 527 with 3,3-dimethyldioxirane under mild conditions (Scheme 178). X-Ray data on compounds 528 revealed a unique pseudo-benziodoxazine structure with intramolecular secondary I•••O (2.647 Å) bonding, which is the first reported example of a six-membered pseudo-cyclic scaffold for iodine(V). NIPA reagents 528 are able to selectively oxidize either alcohols or sulfides, with the reactivity depending largely on the substitution pattern on the amide group adjacent to the iodyl moiety.97 The synthesis of chiral NIPA reagents 529 and 530 has been carried out based on inexpensive and readily available (S)-proline.949 The evaluation of these compounds as stereoselective oxidizing reagents toward a racemic alcohol, meso-diol, and a sulfide was performed and moderate enantioselectivities of 29–41% were achieved. These preliminary results indicate that the NIPA scaffold is a promising structure for further elaboration of chiral iodine(V) oxidants.949
Scheme 178.
As a further expansion of this work, a polymer-supported version of N-(2-iodylphenyl)acylamides (NIPA resin) 531 has been prepared in three simple steps. The synthesis employs commercially available aminomethylated polystyrene and affords resin 531 with good loading of 0.70–0.80 mmol g−1. This convenient, recyclable reagent was shown to effect smooth and efficient oxidation of a broad variety of alcohols.950

2-Iodylphenol ethers 533 were prepared by the dioxirane oxidation of the corresponding 2-iodophenol ethers 532 (Scheme 179) and isolated as chemically stable, microcrystalline products.98 Single-crystal X-ray diffraction analysis of 1-iodyl-2-isopropoxybenzene and 1-iodyl-2-butoxybenzene revealed pseudo-polymeric arrangements in the solid state formed by intermolecular interactions between the IO2 groups of different molecules. 2-Iodylphenol ethers 533 can selectively oxidize sulfides to sulfoxides and alcohols to the respective aldehydes or ketones.98
Scheme 179.
The polymer-supported analogs of 2-iodylphenol ethers 534 and 535 based on the commercially available aminomethylated polystyrene or Merrifield resin have also been reported. These polymer-supported reagents effect clean and efficient conversion of a wide range of alcohols, including heteroatomic and unsaturated structures, to the corresponding carbonyl compounds. Recycling of the resins is possible with minimal loss of activity after several reoxidations.951

4.2. Iodine(V) Heterocycles
4.2.1. 2-Iodoxybenzoic Acid (IBX) and Analogs
4.2.1.1. Preparation, structure, and properties
The most important representative of pentavalent iodine heterocycles, 2-iodoxybenzoic acid (IBX, 537), was first prepared in 1893 by Hartman and Mayer.952 IBX has the structure of the cyclic benziodoxole oxide (1-hydroxy-1-oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one according to the IUPAC nomenclature), as determined by X-ray structural analysis.107,953,954 Most commonly IBX is prepared by the oxidation of 2-iodobenzoic acid with potassium bromate in an aqueous solution of sulfuric acid.955 IBX was reported to be explosive under excessive heating or impact, and Dess and Martin attributed the explosive properties of some samples to the presence of bromate impurities.106 A convenient procedure for the preparation of IBX 537 which involves oxidation of 2-iodobenzoic acid 536 with oxone (Scheme 180) was reported by Santagostino and co-workers.956 This protocol substantially reduced the amount of explosive impurities in the prepared IBX samples.
Scheme 180.
IBX samples, prepared by the oxidation of 2-iodobenzoic acid with potassium bromate, usually contain a mixture of the powder and the macrocrystalline forms. A detailed X-ray diffraction study of both forms of IBX was published by Stevenson and co-workers.107 It was also noticed that the powder form of IBX is more reactive in the reaction with acetic anhydride than the macrocrystalline form and thus is more useful as the Dess-Martin periodinane precursor. Treatment of the macrocrystalline IBX with aqueous sodium hydroxide and then with HCl can be used to convert it to the more reactive powder form.107
The theoretical and experimental study of the pKa value and proton affinity of IBX has been published by Williams and co-workers.957 Solution-phase acidity determinations were performed in both aqueous media and DMSO. In particular, the aqueous pKa value of 2.40 for IBX was obtained by using standard potentiometric titration methods. The relatively high acidity of IBX should be taken in consideration while using this important reagent in the oxidation of complex organic molecules. Very recently, O’Hair and co-authors reported the gas phase proton affinities of the anions of IBX (1300 ± 25 mol−1) and 2-iodosylbenzoic acid (1390 ± 10 kJ mol−1) using mass spectrometry-based experiments.958 The experimental results were supported by theoretical calculations, which yielded proton affinities of 1336 and 1392 kJ mol−1 for IBX− and IBA− respectively, at the B3LYP/aug-cc-PVDZ level of theory.
A nonexplosive formulation of IBX (SIBX), consisting of IBX, benzoic acid, and isophthalic acid, has been introduced by Quideau and co-workers.959 The synthetic utility of SIBX has been demonstrated on the reactions of hydroxylative phenol dearomatization,418,960,961 oxidation of sulfides into sulfoxides,962 oxidative demethylation of phenolic methyl aryl ethers,959 and other useful oxidative transformations.959
Several analogs of IBX have been reported in the literature. Vinod and co-workers have developed the water-soluble analogs of IBX, m-iodoxyphthalic acid (mIBX) 538963 and a similar derivative of terephthalic acid,964 which can oxidize benzylic and allylic alcohols to carbonyl compounds in water. Martin and co-workers first introduced bis(trifluoromethyl)benziodoxole oxides 539 and 540, which are stable and non-explosive oxidizing reagents soluble in a wide range of organic solvents.106,965 Wirth and co-workers have recently reported the preparation of the tetrafluoro IBX derivative (FIBX, 541), which is more soluble and has higher reactivity than its nonfluorinated counterpart.966 Moorthy and co-workers have developed o-methyl-substituted IBX (Me-IBX, 542), which is the first modified analog of IBX that oxidizes alcohols in common organic solvents at room temperature due to the hypervalent twisting-promoted rate enhancement.967

2-Iodoxybenzenesulfonic acid 545 (in a cyclic tautomeric form of 1-hydroxy-1H-1,2,3-benziodoxathiole 1,3,3-trioxide), a thia-analog of IBX and a powerful oxidizing reagent, was prepared by two different pathways: hydrolysis of the methyl ester of 2-iodylbenzenesulfonic acid 543 or direct oxidation of 2-iodobenzenesulfonic acid 544 (Scheme 181).104 The resulting 1-hydroxy-1H-1,2,3-benziodoxathiole 1,3,3-trioxide 545 was found to be thermally unstable and highly reactive towards organic solvents. The structure of its reductive decomposition product, 1-hydroxy-1H-1,2,3-benziodoxathiole 3,3-dioxide (the cyclic tautomeric form of 2-iodosylbenzenesulfonic acid), was established by single-crystal X-ray diffraction.104
Scheme 181.
Kawashima and co-workers reported the preparation and oxidative properties of aliphatic iodoxole oxide 547, which is the first example of this class of iodine(V) compounds. The tetracoordinate 1,2-iodoxetane 547 was prepared by the fluorination of a tricoordinate 1,2-iodoxetane 546 with xenon difluoride followed by hydrolysis (Scheme 182).968 Compound 547 oxidizes alcohols and sulfides to the corresponding carbonyl compounds and sulfoxides, respectively, in good yields under mild conditions.968
Scheme 182.
The preparation and oxidative reactivity of several polymer-supported analogs of IBX have been reported. Giannis and Mülbaier have developed the aminopropylsilica gel based reagent 548, which can oxidize various primary and secondary alcohols to the respective carbonyl compounds in excellent yields at room temperature in THF under heterogeneous conditions and can be regenerated by oxidation with oxone without any loss of activity.969 Rademann and coworkers prepared the polystyrene based polymeric analog of IBX 549, which was characterized by IR spectroscopy, elemental analysis, and MAS-NMR spectroscopy.970 Reagent 549 oxidizes various primary, secondary, benzylic, allylic, terpene alcohols, and the carbamate-protected aminoalcohols to afford the respective aldehydes or ketones in excellent yields, and it can be recycled by repeated oxidation after extensive washings. Lei and co-workers have developed a polymer-supported IBX derivative 550, which has the advantages of a simplified preparation method and a high oxidation activity of 1.5 mmol g−1.971 A conceptually different approach was used by Sutherland and co-workers for the preparation of the polystyrene based reagent 551; in this procedure the iodobenzoic acid moiety was introduced directly to the resin backbone by the iodination/oxidation sequence.972 Very recently, the preparation of functional organic-inorganic colloids modified by IBX 552 has been reported by Hatton and co-workers.973

4.2.1.2. Synthetic applications of IBX
IBX has attracted significant interest as a mild and selective oxidizing reagent. IBX is a particularly useful oxidant for the selective oxidation of alcohols to carbonyl compounds, even in complex molecules in the presence of other functional groups.974–976 Recently this oxidative methodology has been utilized in numerous syntheses, such as: the total synthesis of (+)-wailupemycin B,977 the total synthesis of (−)-decarbamoyloxysaxitoxin,978 the total synthesis of abyssomicin C and atrop-abyssomicin C,979 the stereoselective synthesis of pachastrissamine (jaspine B),980 the syntheses of (±)-pterocarpans and isoflavones,981 the total synthesis of (±)-nitidanin,982 the total synthesis of lagunamycin,983 the synthesis of (−)-agelastatin,984 the syntheses of heliannuols B and D,985 the synthesis of the C1-C15 fragment of dolabelide C,986 the total syntheses of (−)-subincanadines A and B,987 the synthesis of the spiro fused β-lactone-γ-lactam segment of oxazolomycin,988 the synthesis of marine sponge metabolite spiculoic acid A,989 the synthesis of optically pure highly functionalized tetrahydro-isoquinolines,990 the preparation of Fmoc-protected amino aldehydes from the corresponding alcohols,991 and the selective oxidation of hydroxyl-substituted organotrifluoroborates to the respective carbonyl compounds.992
The synthetic usefulness of IBX in general is significantly restricted by its low solubility in most organic solvents with the exception of DMSO. However, in several recent reports it has been shown that IBX can be used as an effective oxidant in other than DMSO solvents.993–996 More and Finney have found that primary and secondary alcohols can be oxidized into the corresponding aldehydes or ketones in excellent yields (90–100%) by heating a mixture of the alcohol and IBX in common organic solvents.993 All reaction by-products can be completely removed by filtration. This method was used for the efficient preparation of the ribosyl aldehyde 553 (Scheme 183), the key intermediate in the stereoselective synthesis of the core structure of the polyoxin and nikkomycin antibiotics.994
Scheme 183.
Kuhakarn and co-workers have recently found that IBX can be used for the oxidation of alcohols in a water/dichloromethane 1:1 mixture in the presence of tetrabutylammonium bromide.996
IBX is especially useful for the oxidation of 1,2-diols. Moorthy and co-workers have investigated the reactions of IBX with various vicinal diols and found that the oxidative cleavage of the C-C bond, as well as the previously known oxidation to α-ketols or α-diketones, can occur in these reactions.997 In DMSO solutions, IBX oxidatively cleaves strained and sterically hindered syn 1,2-diols, while the non-hindered secondary glycols are oxidized to α-ketols or α-diketones. The use of trifluoroacetic acid as a solvent leads to efficient oxidative fragmentation of 1,2-diols of all types.997 The oxidation of 1,2-diols using IBX in DMSO has been utilized for the synthesis of α-ketols977,998,999 or α-diketones.1000 For example, in the key step of the total synthesis of the streptomyces maritimus metabolite - wailupemycin B, IBX oxidation led to the desired hydroxyketone 554 without any cleavage of the glycol C-C bond (Scheme 184).977
Scheme 184.
An interesting IBX-mediated oxidation of primary alcohols or aldehydes to N-hydroxysuccinimide esters 555 was developed by Giannis and Schulze.1001 The generality of this procedure was demonstrated on a variety of aliphatic, allylic, and benzylic alcohols (Scheme 185).
Scheme 185.
Chen and co-workers reported a mild, efficient, and environmentally benign protocol for the oxidation of alcohols with IBX in the ionic liquid 1-butyl-3-methylimidazolium chloride and water.995 Stirring a solution of the alcohol and IBX in 1-butyl-3-methyl-imidazolium chloride followed by removal of water at room temperature and subsequent extraction with ether or ethyl acetate gives excellent yields (88–99%) of the corresponding carbonyl compounds. No overoxidation to acids was observed in the case of aldehyde products, and various functionalities such as methoxy and nitro groups, double bonds, and a furan ring could be tolerated. The oxidation of glycols under these conditions, depending of the amount of IBX used, affords α-ketols or α-diketones.995
Catalytic IBX-based procedures for the oxidation of alcohols have been reported by Giannis and Schulze,1002 Vinod and co-workers,1003 and by Page et al.1004 In particular, the oxidation of primary or secondary alcohols using catalytic amounts (20–30 mol%) of IBX or 2-iodobenzoic acid (IBA) in the presence of oxone as a stoichiometric oxidant in aqueous acetonitlile at 70 °C affords the corresponding carboxylic acids or ketones in 74–97% yield.1003 A further modification of this procedure employs tetraphenylphosphonium monoperoxysulfate as the oxidant in the presence of catalytic 2-iodobenzoic acid; in this case primary alcohols are oxidized to aldehydes without overoxidation to carboxylic acids.1004
IBX in DMF has been shown to be an excellent reagent for the oxidation of various phenols to o-quinones.1005 This procedure was used for the oxidation of phenol 556 to quinone 557 (Scheme 186), the key intermediate in the total synthesis of a novel cyclooxygenase inhibitor (±)-aiphanol.1006 The same protocol was recently utilized in the synthesis of (±)-brazilin, a tinctorial compound found in the alcoholic extracts of trees collectively referred to as Brazil wood, by Pettus et al.1007
Scheme 186.
Quideau and co-workers have recently utilized the non-explosive formulation of IBX (SIBX) in the total synthesis of the bissesquiterpene (+)-aquaticol by biomimetic oxidative dearomatization of the appropriate phenolic substrate via an orthoquinol intermediate.961
The practical value of IBX as a reagent was recently extended to a variety of other synthetically useful oxidative transformations, such as: the one-step synthesis of α,β-unsaturated carbonyl systems from saturated alcohols and carbonyl compounds,1008 the selective oxidation of the benzylic carbon,1009,1010 the oxidation of amines to imines1011,1012 and nitriles,1013–1017 the oxidative deprotection of dithianes1011 and 1,3-oxathiolanes,1018 the oxidation of indoles into 3-hydroxyoxindoles and isatins in the presence of InCl3 or CeCl3,1019,1020 the aromatization of 1,4-dihydropyridines,1021 the α-hydroxylation of the α-alkynyl carbonyl systems leading to the corresponding tertiary alcohols1022 or (Z)-enediones,1023 the synthesis of β-(hetero)aryl-α-nitro-α,β-enals,1024 the synthesis of quinoxaline derivatives from 1,2-diketones and o-phenylenediamines,1025 the oxidative cyclization of anilides and related compounds leading to various heterocyclic systems,1026 the generation of alkoxyamidyl radicals from the corresponding acylated alkoxyamines,1027 the preparation of nitrile oxides from aldoximes,1028 and various multicomponent oxidative transformations.1029–1032 Several specific examples of these reactions are discussed below.
Nicolaou and co-workers reported a one-pot procedure for the oxidation of alcohols, ketones, and aldehydes to the corresponding α,β-unsaturated species using IBX under mild conditions. For example, cycloalkanols 558 react with two equivalents of IBX in a 2:1 mixture of either fluorobenzene or toluene and DMSO at gentle heating to afford the corresponding α,β-unsaturated ketones 559 in good yields (Scheme 187).1008
Scheme 187.
IBX is an efficient and selective reagent for the oxidation of alkyl substituted aromatic compounds 560 at the benzylic position to the corresponding carbonyl derivatives 561 (Scheme 188). This reaction is quite general and can tolerate a variety of substituents within the aromatic ring. Overoxidation to the corresponding carboxylic acids is not observed even in the presence of electron-rich substituents.1009
Scheme 188.
Similar to the oxidation of alcohols, secondary amines 562 can be oxidized with IBX in DMSO to yield the corresponding imines 563 in good to excellent yields (Scheme 189).1011
Scheme 189.
A variety of new heterocycles 565 can be synthesized by the treatment of unsaturated aryl amides, carbamates, thiocarbamates, and ureas 564 with IBX (Scheme 190).1026,1033 The mechanism of this reaction has been investigated in detail.1034 On the basis of solvent effects and D-labeling studies, it was proposed that the IBX-mediated cyclization of anilides in THF involves an initial single electron transfer (SET) to a THF-IBX complex followed by deprotonation, radical cyclization, and concluding termination by hydrogen abstraction from THF.1034 A similar IBX-mediated cyclization was applied in the synthetic protocol for the stereoselective preparation of amino sugars.1035
Scheme 190.
Studer and Janza reported a method for the generation of alkoxyamidyl radicals starting from the corresponding acylated alkoxyamines using IBX as a single electron transfer (SET) oxidant. Stereoselective 5-exo and 6-exo reactions with these N-heteroatom-centered radicals lead to isoxazolidines and [1,2]oxazinanes (e.g. 566) (Scheme 191).1027
Scheme 191.
IBX has also been used for the preparation of the 3,5-disubstituted isoxazolines 567. SET oxidation of substituted aldoximes with IBX in dichloromethane produces the respective nitrile oxide which then undergoes 1,3-dipolar addition with an alkene component (Scheme 192).1028
Scheme 192.
A one-pot three-component synthesis of α-iminonitriles 568 by IBX/tetrabutylammonium bromide-mediated oxidative Strecker reaction (Scheme 193) was reported by Zhu, Masson and co-workers.1032 This methodology was applied to a two-step synthesis of indolizidine via a microwave-assisted intramolecular cycloaddition of α-iminonitrile.
Scheme 193.
The IBX-mediated oxidative Ugi-type multicomponent reaction of tetrahydroisoquinoline with isocyanides and carboxylic acids affords the N and C1 functionalized tetrahydroisoquinolines 569 in good to excellent yields.1031 Likewise, the three-component Passerini reaction of an alcohol, carboxylic acid, and an isonitrile in the presence of IBX affords the corresponding α-acyloxy carboxamides 570 in generally high yields (Scheme 194).1030
Scheme 194.
4.2.2. Dess-Martin Periodinane (DMP)
Dess-Martin periodinane (DMP, 572) was originally introduced in 19841036 and since then has emerged as the reagent of choice for the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively.22,59 DMP is best prepared by the reaction of IBX 571 with acetic anhydride in the presence of p-toluenesulfonic acid (Scheme 195).1037
Scheme 195.
Due to the mild reaction conditions (room temperature, absence of acidic or basic additives) and high chemoselectivity, DMP is especially suitable for the oxidation of alcohols containing sensitive functional groups, such as unsaturated moieties, amino groups, silyl ethers, phosphine oxides, sulfides, selenides, etc. In case of epimerization sensitive substrates, DMP allows clean oxidation with virtually no loss of enantiomeric excess. Thus, the oxidation of N-protected β-amino alcohols with DMP afforded the respective aldehydes with 99% ee and excellent chemical yields, while Swern oxidation gave unsatisfactory results (50–68% ee).1038 The DMP oxidation is accelerated by the addition of water to the reaction mixture immediately before or during the reaction.1039 Silyl ethers can be effectively used instead of alcohols in the DMP oxidations affording the corresponding carbonyl compounds in excellent yields.1040 The DMP oxidation of 1,2-diols generally cleaves the glycol C-C bond as illustrated by the synthesis of tricyclic enol ether 574 from diol 573 via tandem 1,2-diol cleavage-intramolecular cycloaddition (Scheme 196).1041
Scheme 196.
Because of the unique oxidizing properties and convenience of use, DMP is widely employed in the synthesis of biologically important natural products. Recently DMP has been used in the key oxidation steps of the following synthetic works: the preparation of 2-alkynyl acroleins,1042 the oxidation of α-diazo-β-hydroxyesters to α-diazo-β-ketoesters,1043 the scale-up syntheses of (−)-epicatechin-(4β,8)-(+)-catechin and (−)-epicatechin-3-O-galloyl-(4β,8)-(−)-epicatechin-3-O-gallate,1044 the synthesis of a potent anti-tumor therapeutic 7-Epi (+)-FR900482,1045 the formal total synthesis of (±)-platensimycin,1046 the total synthesis of several members of the vinca and tacaman classes of indole alkaloids,1047 the oxidation of the appropriately functionalized hydroxyporphyrins to chlorin-α-diones and bacteriochlorin-tetraones,1048 the synthesis of an N-mesityl substituted chiral imidazolium salt, the N-heterocyclic carbene precursor,1049 the synthesis of new lavendamycin analogues,1050 the synthetic studies towards the total synthesis of providencin,1051 the stereo-controlled synthesis of prelasalocid,1052 the total synthesis of (R,R,R)-α-tocopherol,1053 the stereoselective total syntheses of lycopodium alkaloids,1054 the synthetic studies towards bridgehead diprenyl-substituted bicyclol[3.3.1]nonane-2,9-diones,1055 the total synthesis of (−)-pseudolaric acid B,1056 the synthesis of azadirachtin,1057 the total synthesis of (±)-phomactin B2,1058 the stereoselective total synthesis of arenastatin A,1059 the stereoselective formal total synthesis of (+)-hyperaspine,1060 the asymmetric synthesis of salvinorin A,1061 the asymmetric syntheses of heliannuols B and D,985 the total synthesis of C16 analogs of (−)-dictyostatin,1062 the total synthesis of racemic clusianone and a formal synthesis of racemic garsubellin A,1063 the synthesis of 2,6-disubstituted dihydropyranones,1064 the enantioselective synthesis of hydrobenzofuranones,1065 the synthesis of di- and trisaccharide mimetics with non-glycosidic amino bridges,1066 the total synthesis of (4R,5S)-melithiazole C and (3R,4S)-cystothiazole E,1067 the synthesis of trifluoromethylated cyclodextrin derivatives,1068 the asymmetric total syntheses of ecteinascidin 597 and ecteinascidin 583,1069 the enantioselective total synthesis of (−)-erinacine B,1070 the synthesis of the C31-C67 fragment of amphidinol 3,1071 the total synthesis of (−)-himgaline,1072 the total synthesis of pseudolaric acid A,1073 and the total synthesis of (−)-sarain A.1074
The unique oxidizing properties of DMP can be illustrated by its application in the total synthesis of the CP-molecules, lead structures for cardiovascular and anticancer drugs, published by Nicolaou and co-workers.1075–1077 In this synthetic investigation, a hindered secondary alcohol 575 was oxidized with DMP to the stable diol 577 through intermediate hemiketal 576 (Scheme 197).
Scheme 197.
The practical value of DMP as a reagent was recently extended to a variety of other synthetically useful oxidative transformations, such as: the synthesis of various polycyclic heterocycles via the oxidative cascade cyclization of anilides with pendant double bonds,1078 the oxidative aromatization of 1,4-dihydropyridines,1079 the one-pot oxidative allylation of Morita-Baylis-Hillman adducts with allyltrimethylsilane promoted by DMP/BF3•OEt2,1080 the DMP promoted oxidative coupling of Baylis-Hillman adducts with silyl enol ethers,1081 the synthesis of 2-amino-1,4-benzoquinone-4-phenylimides from anilines via DMP oxidation,1082 the α-bromination of 1,3-dicarbonyl compounds using DMP and tetraethylammonium bromide,1083 the decarboxylative bromination of α, β-unsaturated carboxylic acids with DMP and tetraethylammonium bromide,1084 the α-tosyloxylation of ketones using DMP and p-toluenesulfonic acid,1085 the solvent-free synthesis of 1-(p-toluenesulfonyloxy)-1,2-benziodoxol-3(1H)-one from DMP and p-toluenesulfonic acid and its subsequent utilitization for α-tosyloxylation of ketones,1086 the synthesis of 2-substituted benzothiazoles 579 via oxidative cyclization of thioformanilides 578 (Scheme 198),381 the synthesis of thioesters 582 from the corresponding aldehydes 580 and thiols 581 under mild conditions (Scheme 199),1087 and the synthesis of imides (e.g. 583), N-acyl vinylogous carbamates and ureas, and nitriles by the oxidation of amides and amines with DMP (Scheme 200).1088
Scheme 198.
Scheme 199.
Scheme 200.
5. Conclusions
The preceding survey of the recent developments in the chemistry of polyvalent iodine compounds reflects an active current interest in this highly versatile class of valuable reagents. From the practical point of view, especially important are the simplest, traditional reagents, such as (diacetoxyiodo)benzene and iodosylbenzene, which have been increasingly employed in organic synthesis. This growing interest in iodine(III) compounds is mainly due to their very useful oxidizing properties, combined with their benign environmental character and commercial availability.
There has been a major surge of activity in several areas of organic polyvalent iodine chemistry. These areas include the synthetic applications of IBX and similar oxidizing reagents based on the iodine(V) derivatives, the development and synthetic use of polymer-supported and recyclable polyvalent iodine reagents, structural studies of complexes and supramolecular assemblies of polyvalent iodine compounds, the catalytic applications of organoiodine compounds, and the transition metal catalyzed reactions of various hypervalent iodine reagents.
We hope and anticipate that this review will provide additional stimulus for the further development of the chemistry of polyvalent iodine compounds.
Scheme 150.
Scheme 172.
Acknowledgments
Our own work described here was supported by the National Science Foundation (NSF/CHE-0702734) at Minnesota and by the National Institute of Health [GM-57052] at Utah.
Biographies
 Viktor V. Zhdankin was born in Ekaterinburg, Russian Federation. His M.S. (1978), Ph.D. (1981), and Doctor of Chemical Sciences (1986) degrees were earned at Moscow State University in the research laboratories of Professor Nikolay S. Zefirov. He moved to the University of Utah in 1990, where he worked for three years as Instructor of organic chemistry and Research Associate. In 1993, he joined the faculty of the University of Minnesota Duluth, where he is currently a Professor of Chemistry. He has published over 200 scientific papers including 21 reviews and book chapters. His main research interests are in the fields of synthetic and mechanistic organic chemistry of hypervalent main-group elements (iodine, xenon, selenium, sulfur, and phosphorus) and organofluorine chemistry.
Viktor V. Zhdankin was born in Ekaterinburg, Russian Federation. His M.S. (1978), Ph.D. (1981), and Doctor of Chemical Sciences (1986) degrees were earned at Moscow State University in the research laboratories of Professor Nikolay S. Zefirov. He moved to the University of Utah in 1990, where he worked for three years as Instructor of organic chemistry and Research Associate. In 1993, he joined the faculty of the University of Minnesota Duluth, where he is currently a Professor of Chemistry. He has published over 200 scientific papers including 21 reviews and book chapters. His main research interests are in the fields of synthetic and mechanistic organic chemistry of hypervalent main-group elements (iodine, xenon, selenium, sulfur, and phosphorus) and organofluorine chemistry.
 Peter J. Stang is a Distinguished Professor of Chemistry at Utah where he has been since 1969. He is a member of the US National Academy of Sciences and a Fellow of the American Academy of Arts and Sciences as well as a foreign member of the Chinese Academy of Sciences and the Hungarian Academy of Sciences. His current research interest is self-assembly and supramolecular chemistry. He is the author or co-author of 430 scientific publications including seven monographs and two dozen review articles. Since January 2002 he is the Editor of the Journal of the American Chemical Society (JACS).
Peter J. Stang is a Distinguished Professor of Chemistry at Utah where he has been since 1969. He is a member of the US National Academy of Sciences and a Fellow of the American Academy of Arts and Sciences as well as a foreign member of the Chinese Academy of Sciences and the Hungarian Academy of Sciences. His current research interest is self-assembly and supramolecular chemistry. He is the author or co-author of 430 scientific publications including seven monographs and two dozen review articles. Since January 2002 he is the Editor of the Journal of the American Chemical Society (JACS).
Contributor Information
Viktor V. Zhdankin, Department of Chemistry and Biochemistry, University of Minnesota Duluth, Duluth, Minnesota 55812
Peter J. Stang, Department of Chemistry, 315 S 1400 E, Rm 2020, University of Utah, Salt Lake City, Utah 84112
References
- 1.Wirth T, editor. Top Curr Chem. Vol. 224. 2003. Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis; p. 2003. [Google Scholar]
- 2.Varvoglis A. The Organic Chemistry of Polycoordinated Iodine. VCH Publishers, Inc.; New York: 1992. [Google Scholar]
- 3.Varvoglis A. Hypervalent Iodine in Organic Synthesis. Academic Press; London: 1997. [Google Scholar]
- 4.Moriarty RM, Prakash O. Hypervalent Iodine in Organic Chemistry: Chemical Transformations. Wiley-Interscience; 2008. [Google Scholar]
- 5.Stang PJ, Zhdankin VV. Chem Rev. 1996;96:1123. doi: 10.1021/cr940424+. [DOI] [PubMed] [Google Scholar]
- 6.Zhdankin VV, Stang PJ. Chem Rev. 2002;102:2523. doi: 10.1021/cr010003+. [DOI] [PubMed] [Google Scholar]
- 7.Wirth T, Hirt UH. Synthesis. 1999:1271. [Google Scholar]
- 8.Stang PJ. J Org Chem. 2003;68:2997. doi: 10.1021/jo030022e. [DOI] [PubMed] [Google Scholar]
- 9.Moriarty RM. J Org Chem. 2005;70:2893. doi: 10.1021/jo050117b. [DOI] [PubMed] [Google Scholar]
- 10.Zhdankin VV. Science of Synthesis. 2007;31a:161. [Google Scholar]
- 11.Wirth T. Angew Chem, Int Ed. 2005;44:3656. doi: 10.1002/anie.200500115. [DOI] [PubMed] [Google Scholar]
- 12.Matveeva ED, Proskurnina MV, Zefirov NS. Heteroatom Chem. 2006;17:595. [Google Scholar]
- 13.Kitamura T, Fujiwara Y. Org Prep Proced Int. 1997;29:409. [Google Scholar]
- 14.Varvoglis A. Tetrahedron. 1997;53:1179. [Google Scholar]
- 15.Zhdankin VV, Stang PJ. In: Chemistry of Hypervalent Compounds. Akiba Ky., editor. VCH Publishers; New York: 1999. [Google Scholar]
- 16.Koser GF. In: Chemistry of Halides, Pseudo-Halides and Azides, Suppl D2. Patai S, Rappoport Z, editors. Wiley-Interscience; Chichester: 1995. [Google Scholar]
- 17.Zhdankin VV. Speciality Chemicals Magazine. 2002;22:38. [Google Scholar]
- 18.Deprez NR, Sanford MS. Inorg Chem. 2007;46:1924. doi: 10.1021/ic0620337. [DOI] [PubMed] [Google Scholar]
- 19.Ochiai M. Coord Chem Rev. 2006;250:2771. [Google Scholar]
- 20.Richardson RD, Wirth T. Angew Chem, Int Ed. 2006;45:4402. doi: 10.1002/anie.200601817. [DOI] [PubMed] [Google Scholar]
- 21.Silva LF., Jr Molecules. 2006;11:421. doi: 10.3390/11060421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladziata U, Zhdankin VV. ARKIVOC. 2006;ix:26. [Google Scholar]
- 23.Ladziata U, Zhdankin VV. Synlett. 2007:527. [Google Scholar]
- 24.Zhdankin VV. Curr Org Synth. 2005;2:121. [Google Scholar]
- 25.Koser GF. Adv Heterocycl Chem. 2004;86:225. [Google Scholar]
- 26.Tohma H, Kita Y. Adv Synth Catal. 2004;346:111. [Google Scholar]
- 27.Yoneda N. J Fluorine Chem. 2004;125:7. [Google Scholar]
- 28.Dauban P, Dodd RH. Synlett. 2003:1571. [Google Scholar]
- 29.Feldman KS. ARKIVOC. 2003;vi:179. [Google Scholar]
- 30.Togo H, Sakuratani K. Synlett. 2002:1966. [Google Scholar]
- 31.Moreno I, Tellitu I, Herrero MT, SanMartin R, Dominguez E. Curr Org Chem. 2002;6:1433. [Google Scholar]
- 32.Moriarty RM, Prakash O. Org React. 2001;57:327. [Google Scholar]
- 33.Morales-Rojas H, Moss RA. Chem Rev. 2002;102:2497. doi: 10.1021/cr9405462. [DOI] [PubMed] [Google Scholar]
- 34.Moore JD, Hanson PR. Chemtracts. 2002;15:74. [Google Scholar]
- 35.Togo H, Katohgi M. Synlett. 2001:565. [Google Scholar]
- 36.Ochiai M. J Organomet Chem. 2000;611:494. [Google Scholar]
- 37.Moriarty RM, Prakash O. Org React. 1999;54:273. [Google Scholar]
- 38.Ochiai M. In: Chemistry of Hypervalent Compounds. Akiba Ky., editor. VCH Publishers; New York: 1999. [Google Scholar]
- 39.Muraki T, Togo H, Yokoyama M. Rev Heteroatom Chem. 1997;17:213. [Google Scholar]
- 40.Moriarty RM, Prakash O. Adv Heterocycl Chem. 1998;69:1. [Google Scholar]
- 41.Koser GF. Aldrichimica Acta. 2001;34:89. [Google Scholar]
- 42.Zhdankin VV, Stang PJ. Tetrahedron. 1998;54:10927. [Google Scholar]
- 43.Grushin VV. Chem Soc Rev. 2000;29:315. [Google Scholar]
- 44.Umemoto T. Chem Rev. 1996;96:1757. doi: 10.1021/cr941149u. [DOI] [PubMed] [Google Scholar]
- 45.Zhdankin VV. Rev Heteroat Chem. 1997;17:133. [Google Scholar]
- 46.Pirkuliev NS, Brel VK, Zefirov NS. Russ Chem Rev. 2000;69:105. [Google Scholar]
- 47.Okuyama T. Acc Chem Res. 2002;35:12. doi: 10.1021/ar0100374. [DOI] [PubMed] [Google Scholar]
- 48.French AN, Bissmire S, Wirth T. Chem Soc Rev. 2004;33:354. doi: 10.1039/b310389g. [DOI] [PubMed] [Google Scholar]
- 49.Ochiai M. Chem Rec. 2007;7:12. doi: 10.1002/tcr.20104. [DOI] [PubMed] [Google Scholar]
- 50.Quideau S, Pouysegu L, Deffieux D. Curr Org Chem. 2004;8:113. [Google Scholar]
- 51.Ciufolini MA, Braun NA, Canesi S, Ousmer M, Chang J, Chai D. Synthesis. 2007:3759. [Google Scholar]
- 52.Kita Y, Fujioka H. Pure Appl Chem. 2007;79:701. [Google Scholar]
- 53.Rodriguez S, Wipf P. Synthesis. 2004:2767. [Google Scholar]
- 54.Okuyama T, Fujita M. Russ J Org Chem. 2005;41:1245. [Google Scholar]
- 55.Okuyama T, Fujita M. ACS Symp Ser. 2007;965:68. [Google Scholar]
- 56.Kirmse W. Eur J Org Chem. 2005:237. [Google Scholar]
- 57.Muller P, Allenbach YF, Chappellet S, Ghanem A. Synthesis. 2006:1689. [Google Scholar]
- 58.Muller P. Acc Chem Res. 2004;37:243. doi: 10.1021/ar0202619. [DOI] [PubMed] [Google Scholar]
- 59.Holsworth DD. In: Name Reactions for Functional Group Transformations. Li JJ, Corey EJ, editors. John Wiley & Sons, Inc.; Hoboken, N. J.: 2007. [Google Scholar]
- 60.Quideau S, Pouysegu L, Deffieux D. Synlett. 2008:467. [Google Scholar]
- 61.Frohn H-J, Hirschberg ME, Wenda A, Bardin VV. J Fluorine Chem. 2008;129:459. [Google Scholar]
- 62.Akiba Ky. In: Chemistry of Hypervalent Compounds. Akiba Ky., editor. VCH Publishers; New York: 1999. [Google Scholar]
- 63.Landrum GA, Goldberg N, Hoffmann R. J Chem Soc, Dalton Trans. 1997:3605. [Google Scholar]
- 64.Landrum GA, Goldberg N, Hoffmann R, Minyaev RM. New J Chem. 1998;22:883. [Google Scholar]
- 65.Kiprof P, Zhdankin V. ARKIVOC. 2003;vi:170. [Google Scholar]
- 66.Kiprof P. ARKIVOC. 2005;iv:19. [Google Scholar]
- 67.Ochiai M, Sueda T, Miyamoto K, Kiprof P, Zhdankin VV. Angew Chem, Int Ed. 2006;45:8203. doi: 10.1002/anie.200603055. [DOI] [PubMed] [Google Scholar]
- 68.Okuyama T, Yamataka H. Can J Chem. 1999;77:577. [Google Scholar]
- 69.Carroll MA, Martin-Santamaria S, Pike VW, Rzepa HS, Widdowson DA. J Chem Soc, Perkin Trans 2. 1999:2707. [Google Scholar]
- 70.Martin-Santamaria S, Carroll MA, Carroll CM, Carter CD, Rzepa HS, Widdowson DA, Pike VW. Chem Commun. 2000:649. [Google Scholar]
- 71.Martin-Santamaria S, Carroll MA, Pike VW, Rzepa HS, Widdowson DA. J Chem Soc, Perkin Trans 2. 2000:2158. [Google Scholar]
- 72.Su JT, Goddard WA., III J Am Chem Soc. 2005;127:14146. doi: 10.1021/ja054446x. [DOI] [PubMed] [Google Scholar]
- 73.Boucher M, Macikenas D, Ren T, Protasiewicz JD. J Am Chem Soc. 1997;119:9366. [Google Scholar]
- 74.Zhdankin VV, Arbit RM, Lynch BJ, Kiprof P, Young VG. J Org Chem. 1998;63:6590. [Google Scholar]
- 75.Hirt UH, Schuster MFH, French AN, Wiest OG, Wirth T. Eur J Org Chem. 2001:1569. [Google Scholar]
- 76.Pouysegu L, Chassaing S, Dejugnac D, Lamidey A-M, Miqueu K, Sotiropoulos J-M, Quideau S. Angew Chem, Int Ed. 2008;47:3552. doi: 10.1002/anie.200705816. [DOI] [PubMed] [Google Scholar]
- 77.Bakalbassis EG, Spyroudis S, Tsiotra E. J Org Chem. 2006;71:7060. doi: 10.1021/jo0610964. [DOI] [PubMed] [Google Scholar]
- 78.Ochiai M, Miyamoto K, Shiro M, Ozawa T, Yamaguchi K. J Am Chem Soc. 2003;125:13006. doi: 10.1021/ja0377899. [DOI] [PubMed] [Google Scholar]
- 79.Ochiai M, Miyamoto K, Suefuji T, Sakamoto S, Yamaguchi K, Shiro M. Angew Chem, Int Ed. 2003;42:2191. doi: 10.1002/anie.200250866. [DOI] [PubMed] [Google Scholar]
- 80.Ochiai M, Miyamoto K, Suefuji T, Shiro M, Sakamoto S, Yamaguchi K. Tetrahedron. 2003;59:10153. [Google Scholar]
- 81.Ochiai M, Miyamoto K, Yokota Y, Suefuji T, Shiro M. Angew Chem, Int Ed. 2004;44:75. doi: 10.1002/anie.200461375. [DOI] [PubMed] [Google Scholar]
- 82.Ochiai M, Suefuji T, Miyamoto K, Tada N, Goto S, Shiro M, Sakamoto S, Yamaguchi K. J Am Chem Soc. 2003;125:769. doi: 10.1021/ja0211205. [DOI] [PubMed] [Google Scholar]
- 83.Suefuji T, Shiro M, Yamaguchi K, Ochiai M. Heterocycles. 2006;67:391. [Google Scholar]
- 84.Ochiai M, Suefuji T, Miyamoto K, Shiro M. Chem Commun. 2003:1438. doi: 10.1039/b302579a. [DOI] [PubMed] [Google Scholar]
- 85.Zhdankin VV, Koposov AY, Yashin NV. Tetrahedron Lett. 2002;43:5735. [Google Scholar]
- 86.Zhdankin VV, Koposov AE, Smart JT, Tykwinski RR, McDonald R, Morales-Izquierdo A. J Am Chem Soc. 2001;123:4095. doi: 10.1021/ja0155276. [DOI] [PubMed] [Google Scholar]
- 87.Richter HW, Koser GF, Incarvito CD, Rheingold AL. Inorg Chem. 2007;46:5555. doi: 10.1021/ic0701716. [DOI] [PubMed] [Google Scholar]
- 88.Koposov AY, Netzel BC, Yusubov MS, Nemykin VN, Nazarenko AY, Zhdankin VV. Eur J Org Chem. 2007:4475. [Google Scholar]
- 89.Macikenas D, Skrzypczak-Jankun E, Protasiewicz JD. Angew Chem, Int Ed. 2000;39:2007. doi: 10.1002/1521-3773(20000602)39:11<2007::aid-anie2007>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 90.Macikenas D, Skrzypczak-Jankun E, Protasiewicz JD. J Am Chem Soc. 1999;121:7164. doi: 10.1002/1521-3773(20000602)39:11<2007::aid-anie2007>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 91.Meprathu BV, Protasiewicz JD. ARKIVOC. 2003;vi:83. [Google Scholar]
- 92.Meprathu BV, Justik MW, Protasiewicz JD. Tetrahedron Lett. 2005;46:5187. [Google Scholar]
- 93.Koposov AY, Nemykin VN, Zhdankin VV. New J Chem. 2005;29:998. [Google Scholar]
- 94.Zhdankin VV, Koposov AY, Netzel BC, Yashin NV, Rempel BP, Ferguson MJ, Tykwinski RR. Angew Chem, Int Ed. 2003;42:2194. doi: 10.1002/anie.200351018. [DOI] [PubMed] [Google Scholar]
- 95.Zhdankin VV, Litvinov DN, Koposov AY, Luu T, Ferguson MJ, McDonald R, Tykwinski RR. Chem Commun. 2004:106. doi: 10.1039/b312961f. [DOI] [PubMed] [Google Scholar]
- 96.Zhdankin VV, Koposov AY, Litvinov DN, Ferguson MJ, McDonald R, Luu T, Tykwinski RR. J Org Chem. 2005;70:6484. doi: 10.1021/jo051010r. [DOI] [PubMed] [Google Scholar]
- 97.Ladziata U, Koposov AY, Lo KY, Willging J, Nemykin VN, Zhdankin VV. Angew Chem, Int Ed. 2005;44:7127. doi: 10.1002/anie.200502707. [DOI] [PubMed] [Google Scholar]
- 98.Koposov AY, Karimov RR, Geraskin IM, Nemykin VN, Zhdankin VV. J Org Chem. 2006;71:8452. doi: 10.1021/jo0614947. [DOI] [PubMed] [Google Scholar]
- 99.Nikiforov VA, Karavan VS, Miltsov SA, Selivanov SI, Kolehmainen E, Wegelius E, Nissine M. ARKIVOC. 2003;vi:191. [Google Scholar]
- 100.Batchelor RJ, Birchall T, Sawyer JF. Inorg Chem. 1986;25:1415. [Google Scholar]
- 101.Zhdankin VV, Maydanovych O, Herschbach J, McDonald R, Tykwinski RR. J Am Chem Soc. 2002;124:11614. doi: 10.1021/ja0277780. [DOI] [PubMed] [Google Scholar]
- 102.Zhdankin VV, Koposov AY, Su LS, Boyarskikh VV, Netzel BC, Young VG. Org Lett. 2003;5:1583. doi: 10.1021/ol0344523. [DOI] [PubMed] [Google Scholar]
- 103.Yusubov MS, Funk TV, Chi K-W, Cha E-H, Kim GH, Kirschning A, Zhdankin VV. J Org Chem. 2008;73:295. doi: 10.1021/jo702112s. [DOI] [PubMed] [Google Scholar]
- 104.Koposov AY, Litvinov DN, Zhdankin VV, Ferguson MJ, McDonald R, Tykwinski RR. Eur J Org Chem. 2006:4791. doi: 10.1021/jo051010r. [DOI] [PubMed] [Google Scholar]
- 105.Dess DB, Wilson SR, Martin JC. J Am Chem Soc. 1993;115:2488. [Google Scholar]
- 106.Dess DB, Martin JC. J Am Chem Soc. 1991;113:7277. [Google Scholar]
- 107.Stevenson PJ, Treacy AB, Nieuwenhuyzen M. J Chem Soc, Perkin Trans 2. 1997:589. [Google Scholar]
- 108.Hiller A, Patt JT, Steinbach J. Magn Reson Chem. 2006;44:955. doi: 10.1002/mrc.1875. [DOI] [PubMed] [Google Scholar]
- 109.Cerioni G, Uccheddu G. Tetrahedron Lett. 2004;45:505. [Google Scholar]
- 110.Mocci F, Uccheddu G, Frongia A, Cerioni G. J Org Chem. 2007;72:4163. doi: 10.1021/jo070111h. [DOI] [PubMed] [Google Scholar]
- 111.Richter HW, Cherry BR, Zook TD, Koser GF. J Am Chem Soc. 1997;119:9614. [Google Scholar]
- 112.Silva LF, Lopes NP. Tetrahedron Lett. 2005;46:6023. [Google Scholar]
- 113.Saltzman H, Sharefkin JG. Org Synth Coll Vol V. 1973:658. [Google Scholar]
- 114.Gao Y, Jiao X, Fan W, Shen J-K, Shi Q, Basolo F. J Coord Chem. 1993;29:349. [Google Scholar]
- 115.Lucas HJ, Kennedy ER, Formo MW. Org Synth Coll Vol III. 1955:483. [Google Scholar]
- 116.Sawaguchi M, Ayuba S, Hara S. Synthesis. 2002:1802. [Google Scholar]
- 117.Schardt BC, Hill CL. Inorg Chem. 1983;22:1563. [Google Scholar]
- 118.McQuaid KM, Pettus TRR. Synlett. 2004:2403. doi: 10.1055/s-2004-832814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carmalt CJ, Crossley JG, Knight JG, Lightfoot P, Martin A, Muldowney MP, Norman NC, Orpen AG. J Chem Soc, Chem Commun. 1994:2367. [Google Scholar]
- 120.Barea G, Maseras F, Lledos A. New J Chem. 2003;27:811. [Google Scholar]
- 121.Koposov AY, Netzel BC, Yusubov Mekhman S, Nemykin VN, Nazarenko AY, Zhdankin VV. Eur J Org Chem. 2007:4475. [Google Scholar]
- 122.Ochiai M, Miyamoto K, Yokota Y, Suefuji T, Shiro M. Angew Chem, Int Ed. 2005;44:75. doi: 10.1002/anie.200461375. [DOI] [PubMed] [Google Scholar]
- 123.Tohma H, Takizawa S, Maegawa T, Kita Y. Angew Chem, Int Ed. 2000;39:1306. doi: 10.1002/(sici)1521-3773(20000403)39:7<1306::aid-anie1306>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 124.Tohma H, Maegawa T, Takizawa S, Kita Y. Adv Synth Catal. 2002;344:328. [Google Scholar]
- 125.Tohma H, Takizawa S, Watanabe H, Kita Y. Tetrahedron Lett. 1998;39:4547. [Google Scholar]
- 126.Moriarty RM, Duncan MP, Prakash O. J Chem Soc, Perkin Trans 1. 1987:1781. [Google Scholar]
- 127.Moriarty RM, Rani N, Condeiu C, Duncan MP, Prakash O. Synth Commun. 1997;27:3273. [Google Scholar]
- 128.Francisco CG, Herrera AJ, Suarez E. J Org Chem. 2002;67:7439. doi: 10.1021/jo026004z. [DOI] [PubMed] [Google Scholar]
- 129.Francisco CG, Freire R, Gonzalez CC, Leon EI, Riesco-Fagundo C, Suarez E. J Org Chem. 2001;66:1861. doi: 10.1021/jo0057452. [DOI] [PubMed] [Google Scholar]
- 130.Francisco CG, Herrera AJ, Suarez E. J Org Chem. 2003;68:1012. doi: 10.1021/jo026314h. [DOI] [PubMed] [Google Scholar]
- 131.Huang W-J, Singh OV, Chen C-H, Chiou S-Y, Lee S-S. Helv Chim Acta. 2002;85:1069. [Google Scholar]
- 132.Tada N, Miyamoto K, Ochiai M. Chem Pharm Bull. 2004;52:1143. doi: 10.1248/cpb.52.1143. [DOI] [PubMed] [Google Scholar]
- 133.Ueno M, Nabana T, Togo H. J Org Chem. 2003;68:6424. doi: 10.1021/jo030045t. [DOI] [PubMed] [Google Scholar]
- 134.Fujita M, Lee HJ, Sugimura T, Okuyama T. Chem Commun. 2007:1139. doi: 10.1039/b615888a. [DOI] [PubMed] [Google Scholar]
- 135.Miyamoto K, Tada N, Ochiai M. J Am Chem Soc. 2007;129:2772. doi: 10.1021/ja070179e. [DOI] [PubMed] [Google Scholar]
- 136.Miyamoto K, Hirobe M, Saito M, Shiro M, Ochiai M. Org Lett. 2007;9:1995. doi: 10.1021/ol0706105. [DOI] [PubMed] [Google Scholar]
- 137.Ono T, Henderson P. Tetrahedron Lett. 2002;43:7961. [Google Scholar]
- 138.Lee S, MacMillan DWC. Tetrahedron. 2006;62:11413. [Google Scholar]
- 139.Yusubov MS, Gilmkhanova MP, Zhdankin VV, Kirschning A. Synlett. 2007:563. [Google Scholar]
- 140.Kirschning A, Yusubov MS, Yusubova RY, Chi K-W, Park JY. Beilstein J Org Chem. 2007;3:19. doi: 10.1186/1860-5397-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Simonneaux G, Tagliatesta P. J Porphyrins Phthalocyanines. 2004;8:1166. [Google Scholar]
- 142.Groves JT. J Porphyrins Phthalocyanines. 2000;4:350. [Google Scholar]
- 143.Rose E, Andrioletti B, Zrig S, Quelquejeu-Etheve M. Chem Soc Rev. 2005;34:573. doi: 10.1039/b405679p. [DOI] [PubMed] [Google Scholar]
- 144.Bernadou J, Meunier B. Adv Synth Catal. 2004;346:171. [Google Scholar]
- 145.Vinhado FS, Martins PR, Iamamoto Y. Curr Top Catal. 2002;3:199. [Google Scholar]
- 146.Kang M-J, Song WJ, Han A-R, Choi YS, Jang HG, Nam W. J Org Chem. 2007;72:6301. doi: 10.1021/jo070557y. [DOI] [PubMed] [Google Scholar]
- 147.de Visser SP, Oh K, Han A-R, Nam W. Inorg Chem. 2007;46:4632. doi: 10.1021/ic700462h. [DOI] [PubMed] [Google Scholar]
- 148.Silva GdF, Carvalho da Silva D, Guimaraes AS, do Nascimento E, Reboucas JS, Peres de Araujo M, Dai de Carvalho MEM, Idemori YM. J Mol Cat A. 2007;266:274. [Google Scholar]
- 149.Song WJ, Seo MS, DeBeer George S, Ohta T, Song R, Kang M-J, Tosha T, Kitagawa T, Solomon EI, Nam W. J Am Chem Soc. 2007;129:1268. doi: 10.1021/ja066460v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lindsay Smith JR, Iamamoto Y, Vinhado FS. J Mol Cat A. 2006;252:23. [Google Scholar]
- 151.Miyachi H, Nagatsu Y. Chem Pharm Bull. 2002;50:1137. doi: 10.1248/cpb.50.1137. [DOI] [PubMed] [Google Scholar]
- 152.Murakami Y, Konishi K. J Am Chem Soc. 2007;129:14401. doi: 10.1021/ja075051b. [DOI] [PubMed] [Google Scholar]
- 153.Santos MMC, Silva AMS, Cavaleiro JAS, Levai A, Ptonay T. Eur J Org Chem. 2007:2877. [Google Scholar]
- 154.Babakhania R, Bahadoran F, Safari N. J Porphyrins Phthalocyanines. 2007;11:95. [Google Scholar]
- 155.Pouralimardan O, Chamayou A-C, Janiak C, Hosseini-Monfared H. Inorg Chim Acta. 2007;360:1599. [Google Scholar]
- 156.Santos CMM, Silva AMS, Cavaleiro JAS, Patonay T, Levai A. J Heterocycl Chem. 2006;43:1319. [Google Scholar]
- 157.Collman JP, Zeng L, Wang HJH, Lei A, Brauman JI. Eur J Org Chem. 2006:2707. [Google Scholar]
- 158.Suh Y, Seo MS, Kim KM, Kim YS, Jang HG, Tosha T, Kitagawa T, Kim J, Nam W. J Inorg Biochem. 2006;100:627. doi: 10.1016/j.jinorgbio.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 159.Das P, Kuzniarska-Biernacka I, Silva AR, Carvalho AP, Pires J, Freire C. J Mol Cat A. 2006;248:135. [Google Scholar]
- 160.Park S-E, Song WJ, Ryu YO, Lim MH, Song R, Kim KM, Nam W. J Inorg Biochem. 2005;99:424. doi: 10.1016/j.jinorgbio.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 161.Collman JP, Zeng L, Brauman JI. Inorg Chem. 2004;43:2672. doi: 10.1021/ic035360c. [DOI] [PubMed] [Google Scholar]
- 162.Rosa Silva A, Freire C, de Castro B. New J Chem. 2004;28:253. [Google Scholar]
- 163.Wang SH, Mandimutsira BS, Todd R, Ramdhanie B, Fox JP, Goldberg DP. J Am Chem Soc. 2004;126:18. doi: 10.1021/ja038951a. [DOI] [PubMed] [Google Scholar]
- 164.Silva AR, Vital J, Figueiredo JL, Freire C, de Castro B. New J Chem. 2003;27:1511. [Google Scholar]
- 165.Zolezzi S, Spodine E, Decinti A. Polyhedron. 2003;22:1653. [Google Scholar]
- 166.Davoras EM, Coutsolelos AG. J Inorg Biochem. 2003;94:161. doi: 10.1016/s0162-0134(02)00610-4. [DOI] [PubMed] [Google Scholar]
- 167.Poriel C, Ferrand Y, Le Maux P, Rault-Berthelot J, Simonneaux G. Tetrahedron Lett. 2003;44:1759. [Google Scholar]
- 168.Liu H-Y, Lai T-S, Yeung L-L, Chang CK. Org Lett. 2003;5:617. doi: 10.1021/ol027111i. [DOI] [PubMed] [Google Scholar]
- 169.Jitsukawa K, Shiozaki H, Masuda H. Tetrahedron Lett. 2002;43:1491. [Google Scholar]
- 170.Yang ZW, Kang QX, Quan F, Lei ZQ. J Mol Cat A. 2007;261:190. [Google Scholar]
- 171.Vatele J-M. Synlett. 2006:2055. [Google Scholar]
- 172.Murahashi S-I, Noji S, Hirabayashi T, Komiya N. Synlett. 2004:1739. [Google Scholar]
- 173.Wolckenhauer SA, Devlin AS, Du Bois J. Org Lett. 2007;9:4363. doi: 10.1021/ol701950d. [DOI] [PubMed] [Google Scholar]
- 174.Ferrand Y, Daviaud R, Le Maux P, Simonneaux G. Tetrahedron: Asymmetry. 2006;17:952. [Google Scholar]
- 175.Bryliakov KP, Talsi EP. Chem Eur J. 2007;13:8045. doi: 10.1002/chem.200700566. [DOI] [PubMed] [Google Scholar]
- 176.Zupan M, Pollak A. J Fluorine Chem. 1976;7:445. [Google Scholar]
- 177.Gregorcic A, Zupan M. Bull Chem Soc Jpn. 1977;50:517. [Google Scholar]
- 178.Abo-Amer A, Frohn H-J, Steinberg C, Westphal U. J Fluorine Chem. 2006;127:1311. [Google Scholar]
- 179.Sheremetev AB, Dmitriev DE, Konkina SM. Russ Chem Bull. 2004;53:1130. [Google Scholar]
- 180.Bailly E, Barthen P, Breuer W, Frohn HJ, Giesen M, Helber J, Henkel G, Priwitzer A. Z Anorg Allg Chem. 2000;626:1406. [Google Scholar]
- 181.Padelidakis V, Tyrra W, Naumann D. J Fluorine Chem. 1999;99:9. [Google Scholar]
- 182.Frohn HJ, Bardin VV. J Fluorine Chem. 2005;126:1036. [Google Scholar]
- 183.Ye C, Twamley B, Shreeve JM. Org Lett. 2005;7:3961. doi: 10.1021/ol051446t. [DOI] [PubMed] [Google Scholar]
- 184.Kasumov TM, Pirguliyev NS, Brel VK, Grishin YK, Zefirov NS, Stang PJ. Tetrahedron. 1997;53:13139. [Google Scholar]
- 185.Pirkuliyev NS, Brel’ VK, Zhdankin VV, Zefirov NS. Russ J Org Chem. 2002;38:1224. [Google Scholar]
- 186.Fuchigami T, Fujita T. J Org Chem. 1994;59:7190. [Google Scholar]
- 187.Hara S, Hatakeyama T, Chen S-Q, Ishi-i K, Yoshida M, Sawaguchi M, Fukuhara T, Yoneda N. J Fluorine Chem. 1998;87:189. [Google Scholar]
- 188.Carpenter WR. J Org Chem. 1966;31:2688. [Google Scholar]
- 189.Arrica MA, Wirth T. Eur J Org Chem. 2005:395. [Google Scholar]
- 190.Minkwitz R, Berkei M. Inorg Chem. 1998;37:5247. [Google Scholar]
- 191.Choo J, Kim S, Joo H, Kwon Y. THEOCHEM. 2002;587:1. [Google Scholar]
- 192.Porter CW. PhD Thesis. The University of Akron; 1996. Structural analyses using x-ray crystallography ((difluoroiodo)toluene, hydroxymethylbenziodoxathiole) [Google Scholar]
- 193.Hara S, Sekiguchi M, Ohmori A, Fukuhara T, Yoneda N. J Chem Soc, Chem Commun. 1996:1899. [Google Scholar]
- 194.Yoshida M, Fujikawa K, Sato S, Hara S. ARKIVOC. 2003;vi:36. [Google Scholar]
- 195.Sato S, Yoshida M, Hara S. Synthesis. 2005:2602. [Google Scholar]
- 196.Motherwell WB, Greaney MF, Tocher DA. J Chem Soc, Perkin Trans 1. 2002:2809. [Google Scholar]
- 197.Motherwell WB, Greaney MF, Edmunds JJ, Steed JW. J Chem Soc, Perkin Trans 1. 2002:2816. [Google Scholar]
- 198.Inagaki T, Nakamura Y, Sawaguchi M, Yoneda N, Ayuba S, Hara S. Tetrahedron Lett. 2003;44:4117. [Google Scholar]
- 199.Furrow ME, Myers AG. J Am Chem Soc. 2004;126:12222. doi: 10.1021/ja0459779. [DOI] [PubMed] [Google Scholar]
- 200.Hara S, Nakahigashi J, Ishi-i K, Sawaguchi M, Sakai H, Fukuhara T, Yoneda N. Synlett. 1998:495. [Google Scholar]
- 201.Hara S, Nagahigashi J, Ishi-i K, Fukuhara T, Yoneda N. Tetrahedron Lett. 1998;39:2589. [Google Scholar]
- 202.Conte P, Panunzi B, Tingoli M. Tetrahedron Lett. 2005;47:273. [Google Scholar]
- 203.Panunzi B, Picardi A, Tingoli M. Synlett. 2004:2339. [Google Scholar]
- 204.Ochiai M, Hirobe M, Yoshimura A, Nishi Y, Miyamoto K, Shiro M. Org Lett. 2007;9:3335. doi: 10.1021/ol071345q. [DOI] [PubMed] [Google Scholar]
- 205.Yoshida M, Osafune K, Hara S. Synthesis. 2007:1542. [Google Scholar]
- 206.Yoshida M, Kawakami K, Hara S. Synthesis. 2004:2821. [Google Scholar]
- 207.Frohn H-J, Bardin VV. Z Anorg Allg Chem. 2008;634:82. [Google Scholar]
- 208.Frohn H-J, Wenda A, Floerke U. Z Anorg Allg Chem. 2008;634:764. [Google Scholar]
- 209.Lucas HJ, Kennedy ER. Org Synth Coll Vol III. 1955:482. [Google Scholar]
- 210.Zanka A, Takeuchi H, Kubota A. Org Process Res Dev. 1998;2:270. [Google Scholar]
- 211.Yusubov MS, Drygunova LA, Zhdankin VV. Synthesis. 2004:2289. [Google Scholar]
- 212.Obeid N, Skulski L. Molecules. 2001;6:869. [Google Scholar]
- 213.Baranowski A, Plachta D, Skulski L, Klimaszewska M. J Chem Res, Synop. 2000:435. [Google Scholar]
- 214.Zielinska A, Skulski L. Tetrahedron Lett. 2004;45:1087. [Google Scholar]
- 215.Zhao X-F, Zhang C. Synthesis. 2007:551. [Google Scholar]
- 216.Lulinski P, Obeid N, Skulski L. Bull Chem Soc Jpn. 2001;74:2433. [Google Scholar]
- 217.Zefirov NS, Safronov SO, Kaznacheev AA, Zhdankin VV. Zh Org Khim. 1989;25:1807. [Google Scholar]
- 218.Salamant W, Hulme C. Tetrahedron Lett. 2006;47:605. [Google Scholar]
- 219.Archer EM, van Schalkwyk TGD. Acta Cryst. 1953;6:88. [Google Scholar]
- 220.Bekoe DA, Hulme R. Nature. 1956;177:1230. [Google Scholar]
- 221.Carey JV, Chaloner PA, Hitchcock PB, Neugebauer T, Seddon KR. J Chem Res, Synop. 1996:358. [Google Scholar]
- 222.Mishra AK, Olmstead MM, Ellison JJ, Power PP. Inorg Chem. 1995;34:3210. [Google Scholar]
- 223.Protasiewicz JD. J Chem Soc, Chem Commun. 1995:1115. [Google Scholar]
- 224.Grebe J, Geiseler G, Harms K, Dehnicke K. Z Naturforsch, B: Chem Sci. 1999;54:140. [Google Scholar]
- 225.Montanari V, DesMarteau DD, Pennington WT. J Mol Str. 2000;550–551:337. [Google Scholar]
- 226.Masson S, Thuillier A. Bull Soc Chim Fr. 1969:4368. [Google Scholar]
- 227.Yusubov MS, Yusubova RJ, Filimonov VD, Chi K-W. Synth Commun. 2004;34:443. [Google Scholar]
- 228.Yusubov MS, Drygunova LA, Tkachev AV, Zhdankin VV. ARKIVOC. 2005;iv:179. [Google Scholar]
- 229.Ibrahim H, Kleinbeck F, Togni A. Helv Chim Acta. 2004;87:605. [Google Scholar]
- 230.Jin L-M, Yin J-J, Chen L, Guo C-C, Chen Q-Y. Synlett. 2005:2893. [Google Scholar]
- 231.Telvekar VN. Synth Commun. 2007;37:2647. [Google Scholar]
- 232.Benjahad A, Guillemont J, Andries K, Nguyen CH, Grierson DS. Bioorg Med Chem Lett. 2003;13:4309. doi: 10.1016/j.bmcl.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 233.Benjahad A, Oumouch S, Guillemont J, Pasquier E, Mabire D, Andries K, Nguyen CH, Grierson DS. Bioorg Med Chem Lett. 2007;17:712. doi: 10.1016/j.bmcl.2006.10.082. [DOI] [PubMed] [Google Scholar]
- 234.Prakash O, Kaur H, Batra H, Rani N, Singh SP, Moriarty RM. J Org Chem. 2001;66:2019. doi: 10.1021/jo001504i. [DOI] [PubMed] [Google Scholar]
- 235.Prakash O, Sharma V, Batra H, Moriarty RM. Tetrahedron Lett. 2001;42:553. [Google Scholar]
- 236.Kita Y, Takeda Y, Okuno T, Egi M, Iio K, Kawaguchi K-I, Akai S. Chem Pharm Bull. 1997;45:1887. [Google Scholar]
- 237.Prakash O, Kaur H, Pundeer R, Dhillon RS, Singh SP. Synth Commun. 2003;33:4037. [Google Scholar]
- 238.Li X-Q, Zhao X-F, Zhang C. Synthesis. 2008:2589. [Google Scholar]
- 239.Cook TR, Esswein AJ, Nocera DG. J Am Chem Soc. 2007;129:10094. doi: 10.1021/ja073908z. [DOI] [PubMed] [Google Scholar]
- 240.Cotton FA, Koshevoy IO, Lahuerta P, Murillo CA, Sanau M, Ubeda MA, Zhao Q. J Am Chem Soc. 2006;128:13674. doi: 10.1021/ja0656595. [DOI] [PubMed] [Google Scholar]
- 241.Whitfield SR, Sanford MS. J Am Chem Soc. 2007;129:15142. doi: 10.1021/ja077866q. [DOI] [PubMed] [Google Scholar]
- 242.Khusniyarov MM, Harms K, Sundermeyer J. J Fluorine Chem. 2006;127:200. [Google Scholar]
- 243.Hayton TW, Legzdins P, Patrick BO. Inorg Chem. 2002;41:5388. doi: 10.1021/ic020340g. [DOI] [PubMed] [Google Scholar]
- 244.Bastian M, Morales D, Poli R, Richard P, Sitzmann H. J Organomet Chem. 2002;654:109. [Google Scholar]
- 245.Kalyani D, Sanford MS. J Am Chem Soc. 2008;130:2150. doi: 10.1021/ja0782798. [DOI] [PubMed] [Google Scholar]
- 246.Kalyani D, Dick AR, Anani WQ, Sanford MS. Tetrahedron. 2006;62:11483. doi: 10.1021/ol060747f. [DOI] [PubMed] [Google Scholar]
- 247.Sharefkin JG, Saltzman H. Org Synth Coll Vol V. 1973:660. [Google Scholar]
- 248.Ficht S, Mulbaier M, Giannis A. Tetrahedron. 2001;57:4863. [Google Scholar]
- 249.Chen F-E, Xie B, Zhang P, Zhao J-F, Wang H, Zhao L. Synlett. 2007:619. [Google Scholar]
- 250.Shang Y, But TYS, Togo H, Toy PH. Synlett. 2007:67. [Google Scholar]
- 251.Qian W, Jin E, Bao W, Zhang Y. Angew Chem, Int Ed. 2005;44:952. doi: 10.1002/anie.200461889. [DOI] [PubMed] [Google Scholar]
- 252.Handy ST, Okello M. J Org Chem. 2005;70:2874. doi: 10.1021/jo047807k. [DOI] [PubMed] [Google Scholar]
- 253.Zhdankin VV, Scheuller MC, Stang PJ. Tetrahedron Lett. 1993;34:6853. [Google Scholar]
- 254.Stang PJ, Zhdankin VV. J Am Chem Soc. 1993;115:9808. [Google Scholar]
- 255.Gallop PM, Paz MA, Fluckiger R, Stang PJ, Zhdankin VV, Tykwinski RR. J Am Chem Soc. 1993;115:11702. [Google Scholar]
- 256.Kazmierczak P, Skulski L, Kraszkiewicz L. Molecules. 2001;6:881. [Google Scholar]
- 257.Lee BC, Lee KC, Lee H, Mach RH, Katzenellenbogen JA. Bioconjugate Chem. 2007;18:514. doi: 10.1021/bc060191g. [DOI] [PubMed] [Google Scholar]
- 258.Ross TL, Ermert J, Hocke C, Coenen HH. J Am Chem Soc. 2007;129:8018. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]
- 259.Zielinska A, Skulski L. Molecules. 2002;7:806. [Google Scholar]
- 260.Tohma H, Maruyama A, Maeda A, Maegawa T, Dohi T, Shiro M, Morita T, Kita Y. Angew Chem, Int Ed. 2004;43:3595. doi: 10.1002/anie.200454234. [DOI] [PubMed] [Google Scholar]
- 261.Dohi T, Maruyama A, Yoshimura M, Morimoto K, Tohma H, Shiro M, Kita Y. Chem Commun. 2005:2205. doi: 10.1039/b501475a. [DOI] [PubMed] [Google Scholar]
- 262.Dohi T, Morimoto K, Takenaga N, Maruyama A, Kita Y. Chem Pharm Bull. 2006;54:1608. doi: 10.1248/cpb.54.1608. [DOI] [PubMed] [Google Scholar]
- 263.Dohi T, Morimoto K, Takenaga N, Goto A, Maruyama A, Kiyono Y, Tohma H, Kita Y. J Org Chem. 2007;72:109. doi: 10.1021/jo061820i. [DOI] [PubMed] [Google Scholar]
- 264.Moroda A, Togo H. Tetrahedron. 2006;62:12408. [Google Scholar]
- 265.Hossain MD, Kitamura T. Bull Chem Soc Jpn. 2006;79:142. [Google Scholar]
- 266.Hossain D, Kitamura T. Synthesis. 2005:1932. [Google Scholar]
- 267.Page TK, Wirth T. Synthesis. 2006:3153. [Google Scholar]
- 268.McKillop A, Kemp D. Tetrahedron. 1989;45:3299. [Google Scholar]
- 269.Ochiai M, Takaoka Y, Masaki Y, Nagao Y, Shiro M. J Am Chem Soc. 1990;112:5677. [Google Scholar]
- 270.Ochiai M, Oshima K, Ito T, Masaki Y, Shiro M. Tetrahedron Lett. 1991;32:1327. [Google Scholar]
- 271.Togo H, Nabana T, Yamaguchi K. J Org Chem. 2000;65:8391. doi: 10.1021/jo001186n. [DOI] [PubMed] [Google Scholar]
- 272.Daub KS, Habermann B, Hahn T, Teich L, Eger K. Eur J Org Chem. 2004:894. [Google Scholar]
- 273.Rocaboy C, Gladysz JA. Chem Eur J. 2003;9:88. doi: 10.1002/chem.200390034. [DOI] [PubMed] [Google Scholar]
- 274.Fujita M, Okuno S, Lee HJ, Sugimura T, Okuyama T. Tetrahedron Lett. 2007;48:8691. [Google Scholar]
- 275.Hossain MD, Kitamura T. J Org Chem. 2005;70:6984. doi: 10.1021/jo050927n. [DOI] [PubMed] [Google Scholar]
- 276.Hossain MD, Kitamura T. Tetrahedron Lett. 2006;47:7889. [Google Scholar]
- 277.Stang PJ, Boehshar M, Wingert H, Kitamura T. J Am Chem Soc. 1988;110:3272. [Google Scholar]
- 278.Sutherland A, Vederas JC. Chem Commun. 2002:224. doi: 10.1039/b109343f. [DOI] [PubMed] [Google Scholar]
- 279.Ray DG, III, Koser GF. J Org Chem. 1992;57:1607. [Google Scholar]
- 280.Koposov AY, Boyarskikh VV, Zhdankin VV. Org Lett. 2004;6:3613. doi: 10.1021/ol0484714. [DOI] [PubMed] [Google Scholar]
- 281.Merkushev EB, Novikov AN, Makarchenko SS, Moskal’chuk AS, Glushkova VV, Kogai TI, Polyakova LG. J Org Chem USSR (Engl Trans) 1975;11:1246. [Google Scholar]
- 282.Das JP, Roy UK, Roy S. Organometallics. 2005;24:6136. [Google Scholar]
- 283.Sheremetev AB, Konkina SM. Mendeleev Commun. 2003:277. [Google Scholar]
- 284.Spyroudis S, Varvoglis A. Synthesis. 1975:445. [Google Scholar]
- 285.Togo H, Aoki M, Yokoyama M. Tetrahedron. 1993;49:8241. [Google Scholar]
- 286.Alcock NW, Harrison WD, Howes C. J Chem Soc, Dalton Trans. 1984:1709. [Google Scholar]
- 287.De Mico A, Margarita R, Parlanti L, Vescovi A, Piancatelli G. J Org Chem. 1997;62:6974. [Google Scholar]
- 288.Piancatelli G, Leonelli F, Do N, Ragan J. Org Synth. 2006;83:18. [Google Scholar]
- 289.Pozzi G, Quici S, Shepperson I. Tetrahedron Lett. 2002;43:6141. [Google Scholar]
- 290.Vatele J-M. Tetrahedron Lett. 2006;47:715. [Google Scholar]
- 291.Vugts DJ, Veum L, al-Mafraji K, Lemmens R, Schmitz RF, de Kanter FJJ, Groen MB, Hanefeld U, Orru RVA. Eur J Org Chem. 2006:1672. [Google Scholar]
- 292.Herrerias CI, Zhang TY, Li C-J. Tetrahedron Lett. 2006;47:13. [Google Scholar]
- 293.But TYS, Tashino Y, Togo H, Toy PH. Org Biomol Chem. 2005;3:970. doi: 10.1039/b500965k. [DOI] [PubMed] [Google Scholar]
- 294.Pozzi G, Cavazzini M, Quici S, Benaglia M, Dell’Anna G. Org Lett. 2004;6:441. doi: 10.1021/ol036398w. [DOI] [PubMed] [Google Scholar]
- 295.Holczknecht O, Cavazzini M, Quici S, Shepperson I, Pozzi G. Adv Synth Catal. 2005;347:677. [Google Scholar]
- 296.Pozzi G, Cavazzini M, Holczknecht O, Quici S, Shepperson I. Tetrahedron Lett. 2004;45:4249. [Google Scholar]
- 297.Qian W, Jin E, Bao W, Zhang Y. Tetrahedron. 2006;62:556. [Google Scholar]
- 298.Hansen TM, Florence GJ, Lugo-Mas P, Chen J, Abrams JN, Forsyth CJ. Tetrahedron Lett. 2002;44:57. [Google Scholar]
- 299.Li Y, Hale KJ. Org Lett. 2007;9:1267. doi: 10.1021/ol0700862. [DOI] [PubMed] [Google Scholar]
- 300.Tohma H, Maegawa T, Kita Y. Synlett. 2003:723. [Google Scholar]
- 301.Karade NN, Shirodkar SG, Dhoot BM, Waghmare PB. J Chem Res. 2005:274. [Google Scholar]
- 302.Karade NN, Tiwari GB, Huple DB. Synlett. 2005:2039. [Google Scholar]
- 303.Karade NN, Budhewar VH, Katkar AN, Tiwari GB. ARKIVOC. 2006;xi:162. [Google Scholar]
- 304.Kansara A, Sharma PK, Banerji KK. J Chem Res. 2004:581. [Google Scholar]
- 305.Huang S, Wang F, Gan L, Yuan G, Zhou J, Zhang S. Org Lett. 2006;8:277. doi: 10.1021/ol052602z. [DOI] [PubMed] [Google Scholar]
- 306.Moriarty RM, Hu H. Tetrahedron Lett. 1981;22:2747. [Google Scholar]
- 307.Moriarty RM, Prakash O, Karalis P, Prakash I. Tetrahedron Lett. 1984;25:4745. [Google Scholar]
- 308.Moriarty RM, Prakash O. J Org Chem. 1985;50:151. [Google Scholar]
- 309.Moriarty RM, Prakash O, Thachet CT, Musallam HA. Heterocycles. 1985;23:633. [Google Scholar]
- 310.Prakash O, Saini N, Tanwar MP, Moriarty RM. Contemp Org Synth. 1995;2:121. [Google Scholar]
- 311.Ochiai M, Takeuchi Y, Katayama T, Sueda T, Miyamoto K. J Am Chem Soc. 2005;127:12244. doi: 10.1021/ja0542800. [DOI] [PubMed] [Google Scholar]
- 312.Rao W, Chan PWH. Tetrahedron Lett. 2007;48:3789. [Google Scholar]
- 313.Celik M, Alp C, Coskun B, Gueltekin MS, Balci M. Tetrahedron Lett. 2006;47:3659. [Google Scholar]
- 314.Yusubov MS, Zholobova GA, Filimonova IL, Chi K-W. Russ Chem Bull. 2004;53:1735. [Google Scholar]
- 315.Alvarez HM, Barbosa DP, Fricks AT, Aranda DAG, Valdes RH, Antunes OAC. Org Process Res Dev. 2006;10:941. [Google Scholar]
- 316.Yu L, Chen B, Huang X. Tetrahedron Lett. 2007;48:925. [Google Scholar]
- 317.Mironov YV, Sherman AA, Nifantiev NE. Tetrahedron Lett. 2004;45:9107. [Google Scholar]
- 318.Shi M, Wang B-Y, Li J. Eur J Org Chem. 2005:759. [Google Scholar]
- 319.Tellitu I, Dominguez E. Tetrahedron. 2008;64:2465. [Google Scholar]
- 320.Tingoli M, Tiecco M, Chianelli D, Balducci R, Temperini A. J Org Chem. 1991;56:6809. [Google Scholar]
- 321.Tingoli M, Tiecco M, Testaferri L, Balducci R. Synlett. 1993:211. [Google Scholar]
- 322.Tingoli M, Tiecco M, Testaferri L, Temperini A. J Chem Soc, Chem Commun. 1994:1883. doi: 10.1021/jo960866g. [DOI] [PubMed] [Google Scholar]
- 323.Tingoli M, Tiecco M, Testaferri L, Temperini A. Synth Commun. 1998;28:1769. [Google Scholar]
- 324.Kirschning A, Plumeier C, Rose L. J Chem Soc, Chem Commun. 1998:33. [Google Scholar]
- 325.Hashem A, Jung A, Ries M, Kirschning A. Synlett. 1998:195. [Google Scholar]
- 326.Kirschning A, Kunst E, Ries M, Rose L, Schoenberger A, Wartchow R. ARKIVOC. 2003;vi:145. [Google Scholar]
- 327.Sourkouni-Argirusi G, Kirschning A. Org Lett. 2000;2:3781. doi: 10.1021/ol006483t. [DOI] [PubMed] [Google Scholar]
- 328.Kirschning A, Monenschein H, Wittenberg R. Angew Chem, Int Ed. 2001;40:650. [PubMed] [Google Scholar]
- 329.Merkushev EB. Synthesis. 1988:923. [Google Scholar]
- 330.Panunzi B, Rotiroti L, Tingoli M. Tetrahedron Lett. 2003;44:8753. [Google Scholar]
- 331.Yan J, Li J, Cheng D. Synlett. 2007:2442. [Google Scholar]
- 332.Karade NN, Tiwari GB, Huple DB, Siddiqui TAJ. J Chem Res. 2006:366. [Google Scholar]
- 333.Yusubov MS, Yusubova RY, Kirschning A, Park JY, Chi K-W. Tetrahedron Lett. 2008;49:1506. [Google Scholar]
- 334.Cheng D-P, Chen Z-C, Zheng Q-G. Synth Commun. 2003;33:2671. [Google Scholar]
- 335.Karade NN, Tiwari GB, Shirodkar SG, Dhoot BM. Synth Commun. 2005;35:1197. [Google Scholar]
- 336.Catir M, Kilic H. Synlett. 2004:2151. [Google Scholar]
- 337.Itoh N, Sakamoto T, Miyazawa E, Kikugawa Y. J Org Chem. 2002;67:7424. doi: 10.1021/jo0260847. [DOI] [PubMed] [Google Scholar]
- 338.Prakash O, Pundeer R, Kaur H. Synthesis. 2003:2768. [Google Scholar]
- 339.Prakash O, Kaur H, Sharma V, Bhardwaj V, Pundeer R. Tetrahedron Lett. 2004;45:9065. [Google Scholar]
- 340.Tellitu I, Urrejola A, Serna S, Moreno I, Herrero MT, Dominguez E, SanMartin R, Correa A. Eur J Org Chem. 2007:437. doi: 10.1021/jo062320s. [DOI] [PubMed] [Google Scholar]
- 341.Herrero MT, Tellitu I, Dominguez E, Hernandez S, Moreno I, SanMartin R. Tetrahedron. 2002;58:8581. [Google Scholar]
- 342.Herrero MT, Tellitu I, Dominguez E, Moreno I, SanMartin R. Tetrahedron Lett. 2002;43:8273. [Google Scholar]
- 343.Correa A, Tellitu I, Dominguez E, Moreno I, SanMartin R. J Org Chem. 2005;70:2256. doi: 10.1021/jo047872u. [DOI] [PubMed] [Google Scholar]
- 344.Churruca F, SanMartin R, Tellitu I, Dominguez E. Eur J Org Chem. 2005:2481. [Google Scholar]
- 345.Serna S, Tellitu I, Dominguez E, Moreno I, SanMartin R. Tetrahedron. 2004;60:6533. [Google Scholar]
- 346.Churruca F, SanMartin R, Carril M, Urtiaga MK, Solans X, Tellitu I, Dominguez E. J Org Chem. 2005;70:3178. doi: 10.1021/jo0501036. [DOI] [PubMed] [Google Scholar]
- 347.Herrero MT, Tellitu I, Hernandez S, Dominguez E, Moreno I, SanMartin R. ARKIVOC. 2002;v:31. [Google Scholar]
- 348.Serna S, Tellitu I, Dominguez E, Moreno I, SanMartin R. Tetrahedron Lett. 2003;44:3483. [Google Scholar]
- 349.Correa A, Tellitu I, Dominguez E, SanMartin R. J Org Chem. 2006;71:8316. doi: 10.1021/jo061486q. [DOI] [PubMed] [Google Scholar]
- 350.Serna S, Tellitu I, Dominguez E, Moreno I, SanMartin R. Org Lett. 2005;7:3073. doi: 10.1021/ol0510623. [DOI] [PubMed] [Google Scholar]
- 351.Tellitu I, Serna S, Herrero MT, Moreno I, Dominguez E, SanMartin R. J Org Chem. 2007;72:1526. doi: 10.1021/jo062320s. [DOI] [PubMed] [Google Scholar]
- 352.Correa A, Tellitu I, Dominguez E, SanMartin R. J Org Chem. 2006;71:3501. doi: 10.1021/jo060070+. [DOI] [PubMed] [Google Scholar]
- 353.Correa A, Tellitu I, Dominguez E, SanMartin R. Tetrahedron. 2006;62:11100. [Google Scholar]
- 354.Huang J, Liang Y, Pan W, Yang Y, Dong D. Org Lett. 2007;9:5345. doi: 10.1021/ol702362n. [DOI] [PubMed] [Google Scholar]
- 355.Fan R, Wen F, Qin L, Pu D, Wang B. Tetrahedron Lett. 2007;48:7444. [Google Scholar]
- 356.Huang X, Shao N, Palani A, Aslanian R. Tetrahedron Lett. 2007;48:1967. [Google Scholar]
- 357.Mamaeva EA, Bakibaev AA. Tetrahedron. 2003;59:7521. [Google Scholar]
- 358.Cheng D-P, Chen Z-C. Synth Commun. 2002;32:2155. [Google Scholar]
- 359.Wardrop DJ, Zhang W, Landrie CL. Tetrahedron Lett. 2004;45:4229. [Google Scholar]
- 360.Wardrop DJ, Burge MS. Chem Commun. 2004:1230. doi: 10.1039/b403081h. [DOI] [PubMed] [Google Scholar]
- 361.Wardrop DJ, Landrie CL, Ortiz JA. Synlett. 2003:1352. [Google Scholar]
- 362.Wardrop DJ, Burge MS, Zhang W, Ortiz JA. Tetrahedron Lett. 2003;44:2587. [Google Scholar]
- 363.Wardrop DJ, Zhang W. Org Lett. 2001;3:2353. doi: 10.1021/ol0161514. [DOI] [PubMed] [Google Scholar]
- 364.Wardrop DJ, Basak A. Org Lett. 2001;3:1053. doi: 10.1021/ol015626o. [DOI] [PubMed] [Google Scholar]
- 365.Wardrop DJ, Burge MS. J Org Chem. 2005;70:10271. doi: 10.1021/jo051252r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kikugawa Y, Nagashima A, Sakamoto T, Miyazawa E, Shiiya M. J Org Chem. 2003;68:6739. doi: 10.1021/jo0347009. [DOI] [PubMed] [Google Scholar]
- 367.Mogilaiah K, Babu HR, Reddy NV. Synth Commun. 2002;32:2377. [Google Scholar]
- 368.Prakash O, Bhardwaj V, Kumar R, Tyagi P, Aneja KR. Eur J Med Chem. 2004;39:1073. doi: 10.1016/j.ejmech.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 369.Prakash O, Kumar R, Sharma D, Naithani R, Kumar R. Heteroatom Chem. 2006;17:653. [Google Scholar]
- 370.Prakash O, Kumar R, Kumar R, Tyagi P, Kuhad RC. Eur J Med Chem. 2007;42:868. doi: 10.1016/j.ejmech.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 371.Liu S-j, Zhang J-z, Tian G-r, Liu P. Synth Commun. 2005;35:1753. [Google Scholar]
- 372.Zhang X, Gan L, Huang S, Shi Y. J Org Chem. 2004;69:5800. doi: 10.1021/jo0493368. [DOI] [PubMed] [Google Scholar]
- 373.Rao VS, Sekhar KC. Synth Commun. 2004;34:2153. [Google Scholar]
- 374.Shang Z. Synth Commun. 2006;36:2927. [Google Scholar]
- 375.Somogyi L. J Heterocycl Chem. 2007;44:1235. [Google Scholar]
- 376.Kumar D, Chandra Sekhar KVG, Dhillon H, Rao VS, Varma RS. Green Chem. 2004;6:156. [Google Scholar]
- 377.Aggarwal R, Sumran G. Synth Commun. 2006;36:1873. [Google Scholar]
- 378.Huang H-Y, Hou R-S, Wang H-M, Chen L-C. Heterocycles. 2005;65:1881. [Google Scholar]
- 379.Das B, Holla H, Mahender G, Venkateswarlu K, Bandgar BP. Synthesis. 2005:1572. [Google Scholar]
- 380.Du Y, Liu R, Linn G, Zhao K. Org Lett. 2006;8:5919. doi: 10.1021/ol062288o. [DOI] [PubMed] [Google Scholar]
- 381.Bose DS, Idrees M. J Org Chem. 2006;71:8261. doi: 10.1021/jo0609374. [DOI] [PubMed] [Google Scholar]
- 382.Aggarwal R, Sumran G, Saini A, Singh SP. Tetrahedron Lett. 2006;47:4969. [Google Scholar]
- 383.Mogilaiah K, Rani JU, Sakram B, Reddy NV. J Heterocycl Chem. 2006;43:485. [Google Scholar]
- 384.Shang Z, Reiner J, Chang J, Zhao K. Tetrahedron Lett. 2005;46:2701. [Google Scholar]
- 385.Karade NN, Tiwari GB, Gampawar SV. Synlett. 2007:1921. [Google Scholar]
- 386.Prakash O, Pannu K. ARKIVOC. 2007;xiii:28. [Google Scholar]
- 387.Du L-H, Wang Y-G. Synthesis. 2007:675. [Google Scholar]
- 388.Das B, Srinivas Y, Holla H, Krishnaiah M, Narender R. Chem Lett. 2007;36:1270. [Google Scholar]
- 389.Liu L-P, Lu J-M, Shi M. Org Lett. 2007;9:1303. doi: 10.1021/ol070178r. [DOI] [PubMed] [Google Scholar]
- 390.Huang X, Shao N, Palani A, Aslanian R, Buevich A, Seidel-Dugan C, Huryk R. Tetrahedron Lett. 2008;49:3592. [Google Scholar]
- 391.Moroda A, Togo H. Synthesis. 2008:1257. [Google Scholar]
- 392.Karade NN, Shirodkar SG, Patil MN, Potrekar RA, Karade HN. Tetrahedron Lett. 2003;44:6729. [Google Scholar]
- 393.Boye AC, Meyer D, Ingison CK, French AN, Wirth T. Org Lett. 2003;5:2157. doi: 10.1021/ol034616f. [DOI] [PubMed] [Google Scholar]
- 394.Biland AS, Altermann S, Wirth T. ARKIVOC. 2003;vi:164. [Google Scholar]
- 395.Fujioka H, Matsuda S, Horai M, Fujii E, Morishita M, Nishiguchi N, Hata K, Kita Y. Chem Eur J. 2007;13:5238. doi: 10.1002/chem.200601341. [DOI] [PubMed] [Google Scholar]
- 396.Kita Y, Matsuda S, Fujii E, Horai M, Hata K, Fujioka H. Angew Chem, Int Ed. 2005;44:5857. doi: 10.1002/anie.200501686. [DOI] [PubMed] [Google Scholar]
- 397.Kita Y, Matsuda S, Fujii E, Kitagaki S, Inoguchi R, Hata K, Fujioka H. Heterocycles. 2005;66:309. [Google Scholar]
- 398.Chanu A, Safir I, Basak R, Chiaroni A, Arseniyadis S. Eur J Org Chem. 2007:4305. doi: 10.1021/ol070207y. [DOI] [PubMed] [Google Scholar]
- 399.Iglesias-Arteaga MA, Velazquez-Huerta GA. Tetrahedron Lett. 2005;46:6897. [Google Scholar]
- 400.Iglesias-Arteaga MA, Arcos-Ramos RO. Tetrahedron Lett. 2006;47:8029. [Google Scholar]
- 401.Iglesias-Arteaga MA, Arcos-Ramos RO, Mendez-Stivalet JM. Tetrahedron Lett. 2007;48:7485. [Google Scholar]
- 402.Liu H, Tan C-H. Tetrahedron Lett. 2007;48:8220. [Google Scholar]
- 403.Harayama Y, Yoshida M, Kamimura D, Kita Y. Chem Commun. 2005:1764. doi: 10.1039/b418212j. [DOI] [PubMed] [Google Scholar]
- 404.Harayama Y, Yoshida M, Kamimura D, Wada Y, Kita Y. Chem Eur J. 2006;12:4893. doi: 10.1002/chem.200501635. [DOI] [PubMed] [Google Scholar]
- 405.Li X, Xu Z, DiMauro EF, Kozlowski MC. Tetrahedron Lett. 2002;43:3747. [Google Scholar]
- 406.Abo T, Sawaguchi M, Senboku H, Hara S. Molecules. 2005;10:183. doi: 10.3390/10010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Zhang L-h, Kauffman GS, Pesti JA, Yin J. J Org Chem. 1997;62:6918. [Google Scholar]
- 408.Zhang L-h, Chung JC, Costello TD, Valvis I, Ma P, Kauffman S, Ward R. J Org Chem. 1997;62:2466. doi: 10.1021/jo9612537. [DOI] [PubMed] [Google Scholar]
- 409.Prakash O, Batra H, Kaur H, Sharma PK, Sharma V, Singh SP, Moriarty RM. Synthesis. 2001:541. [Google Scholar]
- 410.Song H, Chen W, Wang Y, Qin Y. Synth Commun. 2005;35:2735. [Google Scholar]
- 411.Davis MC, Stasko D, Chapman RD. Synth Commun. 2003;33:2677. [Google Scholar]
- 412.Berkessel A, Glaubitz K, Lex J. Eur J Org Chem. 2002:2948. [Google Scholar]
- 413.Pelter A, Elgendy SMA. J Chem Soc, Perkin Trans 1. 1993:1891. [Google Scholar]
- 414.Kurti L, Herczegh P, Visy J, Simonyi M, Antus S, Pelter A. J Chem Soc, Perkin Trans 1. 1999:379. [Google Scholar]
- 415.McKillop A, McLaren L, Taylor RJK. J Chem Soc, Perkin Trans 1. 1994:2047. [Google Scholar]
- 416.Mitchell AS, Russell RA. Tetrahedron Lett. 1993;34:545. [Google Scholar]
- 417.Lion CJ, Vasselin DA, Schwalbe CH, Matthews CS, Stevens MFG, Westwell AD. Org Biomol Chem. 2005;3:3996. doi: 10.1039/b510240e. [DOI] [PubMed] [Google Scholar]
- 418.Quideau S, Pouysegu L, Deffieux D, Ozanne A, Gagnepain J, Fabre I, Oxoby M. ARKIVOC. 2003;vi:106. [Google Scholar]
- 419.Karam O, Martin A, Jouannetaud M-P, Jacquesy J-C. Tetrahedron Lett. 1999;40:4183. [Google Scholar]
- 420.Wipf P, Kim Y, Fritch PC. J Org Chem. 1993;58:7195. [Google Scholar]
- 421.Quideau S, Looney MA, Pouysegu L, Ham S, Birney DM. Tetrahedron Lett. 1999;40:615. [Google Scholar]
- 422.Kita Y, Tohma H, Kikuchi K, Inagaki M, Yakura T. J Org Chem. 1991;56:435. [Google Scholar]
- 423.Murakata M, Yamada K, Hoshino O. Chem Commun. 1994:443. [Google Scholar]
- 424.Kita Y, Tohma H, Inagaki M, Hatanaka K, Yakura T. J Am Chem Soc. 1992;114:2175. [Google Scholar]
- 425.Berard D, Giroux M-A, Racicot L, Sabot C, Canesi S. Tetrahedron. 2008;64:7537. [Google Scholar]
- 426.Basset L, Martin-Mingot A, Jouannetaud M-P, Jacquesy J-C. Tetrahedron Lett. 2008;49:1551. [Google Scholar]
- 427.Karam O, Martin-Mingot A, Jouannetaud M-P, Jacquesy J-C, Cousson A. Tetrahedron. 2004;60:6629. [Google Scholar]
- 428.Roy H, Sarkar M, Mal D. Synth Commun. 2005;35:2183. [Google Scholar]
- 429.Dey S, Mal D. Tetrahedron Lett. 2005;46:5483. [Google Scholar]
- 430.Venkateswarlu R, Kamakshi C, Subhash PV, Moinuddin SGA, Reddy DRS, Ward RS, Pelter A, Gelbrich T, Hursthouse MB, Coles SJ, Light ME. Tetrahedron. 2006;62:4463. [Google Scholar]
- 431.Felpin F-X. Tetrahedron Lett. 2007;48:409. [Google Scholar]
- 432.Quideau S, Looney MA, Pouysegu L. Org Lett. 1999;1:1651. [Google Scholar]
- 433.Quideau S, Pouysegu L, Oxoby M, Looney MA. Tetrahedron. 2001;57:319. [Google Scholar]
- 434.Lebrasseur N, Fan G-J, Quideau S. ARKIVOC. 2004;xiii:5. [Google Scholar]
- 435.Lebrasseur N, Fan G-J, Oxoby M, Looney MA, Quideau S. Tetrahedron. 2005;61:1551. [Google Scholar]
- 436.Braun NA, Ousmer M, Bray JD, Bouchu D, Peters K, Peters E-M, Ciufolini MA. J Org Chem. 2000;65:4397. doi: 10.1021/jo000341v. [DOI] [PubMed] [Google Scholar]
- 437.Shigehisa H, Takayama J, Honda T. Tetrahedron Lett. 2006;47:7301. [Google Scholar]
- 438.Quideau S, Lebon M, Lamidey A-M. Org Lett. 2002;4:3975. doi: 10.1021/ol026855t. [DOI] [PubMed] [Google Scholar]
- 439.Wipf P, Spencer SR. J Am Chem Soc. 2005;127:225. doi: 10.1021/ja044280k. [DOI] [PubMed] [Google Scholar]
- 440.Canesi S, Bouchu D, Ciufolini MA. Angew Chem, Int Ed. 2004;43:4336. doi: 10.1002/anie.200460178. [DOI] [PubMed] [Google Scholar]
- 441.Canesi S, Belmont P, Bouchu D, Rousset L, Ciufolini MA. Tetrahedron Lett. 2002;43:5193. [Google Scholar]
- 442.Nicolaou KC, Edmonds DJ, Li A, Tria GS. Angew Chem, Int Ed. 2007;46:3942. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]
- 443.Tohma H, Harayama Y, Hashizume M, Iwata M, Kiyono Y, Egi M, Kita Y. J Am Chem Soc. 2003;125:11235. doi: 10.1021/ja0365330. [DOI] [PubMed] [Google Scholar]
- 444.Baxendale IR, Ley SV, Nessi M, Piutti C. Tetrahedron. 2002;58:6285. [Google Scholar]
- 445.Pouysegu L, Avellan A-V, Quideau S. J Org Chem. 2002;67:3425. doi: 10.1021/jo020010d. [DOI] [PubMed] [Google Scholar]
- 446.Quideau S, Pouysegu L, Avellan AV, Whelligan DK, Looney MA. Tetrahedron Lett. 2001;42:7393. [Google Scholar]
- 447.Van De Water RW, Hoarau C, Pettus TRR. Tetrahedron Lett. 2003;44:5109. [Google Scholar]
- 448.Vo NT, Pace RDM, O’Hara F, Gaunt MJ. J Am Chem Soc. 2008;130:404. doi: 10.1021/ja077457u. [DOI] [PubMed] [Google Scholar]
- 449.Baxendale IR, Deeley J, Griffiths-Jones CM, Ley SV, Saaby S, Tranmer GK. Chem Commun. 2006:2566. doi: 10.1039/b600382f. [DOI] [PubMed] [Google Scholar]
- 450.Baxendale IR, Lee A-L, Ley SV. J Chem Soc, Perkin Trans 1. 2002:1850. [Google Scholar]
- 451.Moisan L, Wagner M, Comesse S, Doris E. Tetrahedron Lett. 2006;47:9093. [Google Scholar]
- 452.Dohi T, Maruyama A, Yoshimura M, Morimoto K, Tohma H, Kita Y. Angew Chem, Int Ed. 2005;44:6193. doi: 10.1002/anie.200501688. [DOI] [PubMed] [Google Scholar]
- 453.Dohi T, Maruyama A, Minamitsuji Y, Takenaga N, Kita Y. Chem Commun. 2007:1224. doi: 10.1039/b616510a. [DOI] [PubMed] [Google Scholar]
- 454.Yakura T, Konishi T. Synlett. 2007:765. [Google Scholar]
- 455.Dohi T, Maruyama A, Takenage N, Senami K, Minamitsuji Y, Fujioka H, Caemmerer S, Kita Y. Angew Chem, Int Ed. 2008;47:3787. doi: 10.1002/anie.200800464. [DOI] [PubMed] [Google Scholar]
- 456.Gagnepain J, Mereau R, Dejugnac D, Leger J-M, Castet F, Deffieux D, Pouysegu L, Quideau S. Tetrahedron. 2007;63:6493. [Google Scholar]
- 457.Lai C-H, Lin P-Y, Peddinti RK, Liao C-C. Synlett. 2002:1520. [Google Scholar]
- 458.Cook SP, Danishefsky SJ. Org Lett. 2006;8:5693. doi: 10.1021/ol062067i. [DOI] [PubMed] [Google Scholar]
- 459.Berube A, Drutu I, Wood JL. Org Lett. 2006;8:5421. doi: 10.1021/ol061737h. [DOI] [PubMed] [Google Scholar]
- 460.Chittimalla SK, Liao C-C. Synlett. 2002:565. [Google Scholar]
- 461.Hou H-F, Peddinti RK, Liao C-C. Org Lett. 2002;4:2477. doi: 10.1021/ol020087o. [DOI] [PubMed] [Google Scholar]
- 462.Boldron C, Aromi G, Challa G, Gamez P, Reedijk J. Chem Commun. 2005:5808. doi: 10.1039/b510378a. [DOI] [PubMed] [Google Scholar]
- 463.Hata K, Hamamoto H, Shiozaki Y, Caemmerer SB, Kita Y. Tetrahedron. 2007;63:4052. [Google Scholar]
- 464.Kita Y, Tohma H, Hatanaka K, Takada T, Fujita S, Mitoh S, Sakurai H, Oka S. J Am Chem Soc. 1994;116:3684. [Google Scholar]
- 465.Takada T, Arisawa M, Gyoten M, Hamada R, Tohma H, Kita Y. J Org Chem. 1998;63:7698. [Google Scholar]
- 466.Hamamoto H, Anilkumar G, Tohma H, Kita Y. Chem Commun. 2002:450. doi: 10.1039/b111178g. [DOI] [PubMed] [Google Scholar]
- 467.Hamamoto H, Anilkumar G, Tohma H, Kita Y. Chem Eur J. 2002;8:5377. doi: 10.1002/1521-3765(20021202)8:23<5377::AID-CHEM5377>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 468.Pingaew R, Ruchirawat S. Synlett. 2007:2363. [Google Scholar]
- 469.Taylor SR, Ung AT, Pyne SG, Skelton BW, White AH. Tetrahedron. 2007;63:11377. [Google Scholar]
- 470.Moreno I, Tellitu I, Dominguez E, SanMartin R. Eur J Org Chem. 2002:2126. doi: 10.1021/jo047872u. [DOI] [PubMed] [Google Scholar]
- 471.Dohi T, Ito M, Morimoto K, Iwata M, Kita Y. Angew Chem, Int Ed. 2008;47:1301. doi: 10.1002/anie.200704495. [DOI] [PubMed] [Google Scholar]
- 472.Besong G, Jarowicki K, Kocienski PJ, Sliwinski E, Boyle FT. Org Biomol Chem. 2006;4:2193. doi: 10.1039/b603857c. [DOI] [PubMed] [Google Scholar]
- 473.Hata K, Hamamoto H, Shiozaki Y, Kita Y. Chem Commun. 2005:2465. doi: 10.1039/b501792k. [DOI] [PubMed] [Google Scholar]
- 474.Hamamoto H, Shiozaki Y, Hata K, Tohma H, Kita Y. Chem Pharm Bull. 2004;52:1231. doi: 10.1248/cpb.52.1231. [DOI] [PubMed] [Google Scholar]
- 475.Huang W-J, Singh OV, Chen C-H, Lee S-S. Helv Chim Acta. 2004;87:167. [Google Scholar]
- 476.Kita Y, Egi M, Okajima A, Ohtsubo M, Takada T, Tohma H. J Chem Soc, Chem Commun. 1996:1491. [Google Scholar]
- 477.Kita Y, Egi M, Ohtsubo M, Saiki T, Takada T, Tohma H. J Chem Soc, Chem Commun. 1996:2225. [Google Scholar]
- 478.Hamamoto H, Hata K, Nambu H, Shiozaki Y, Tohma H, Kita Y. Tetrahedron Lett. 2004;45:2293. [Google Scholar]
- 479.Hamamoto H, Shiozaki Y, Nambu H, Hata K, Tohma H, Kita Y. Chem Eur J. 2004;10:4977. doi: 10.1002/chem.200400358. [DOI] [PubMed] [Google Scholar]
- 480.Dohi T, Minamitsuji Y, Maruyama A, Hirose S, Kita Y. Org Lett. 2008;10:3559. doi: 10.1021/ol801321f. [DOI] [PubMed] [Google Scholar]
- 481.Tohma H, Iwata M, Maegawa T, Kita Y. Tetrahedron Lett. 2002;43:9241. [Google Scholar]
- 482.Mirk D, Willner A, Froehlich R, Waldvogel SR. Adv Synth Catal. 2004;346:675. [Google Scholar]
- 483.Jean A, Cantat J, Berard D, Bouchu D, Canesi S. Org Lett. 2007;9:2553. doi: 10.1021/ol070941h. [DOI] [PubMed] [Google Scholar]
- 484.Dohi T, Morimoto K, Kiyono Y, Maruyama A, Tohma H, Kita Y. Chem Commun. 2005:2930. doi: 10.1039/b503058g. [DOI] [PubMed] [Google Scholar]
- 485.Tohma H, Iwata M, Maegawa T, Kiyono Y, Maruyama A, Kita Y. Org Biomol Chem. 2003;1:1647. doi: 10.1039/b302462h. [DOI] [PubMed] [Google Scholar]
- 486.Dohi T, Morimoto K, Maruyama A, Kita Y. Org Lett. 2006;8:2007. doi: 10.1021/ol060333m. [DOI] [PubMed] [Google Scholar]
- 487.Francisco CG, Gonzalez Martin C, Suarez E. J Org Chem. 1998;63:2099. [Google Scholar]
- 488.Dorta RL, Martin A, Salazar JA, Suarez E, Prange T. J Org Chem. 1998;63:2251. doi: 10.1021/jo980834o. [DOI] [PubMed] [Google Scholar]
- 489.Francisco CG, Gonzalez Martin C, Suarez E. J Org Chem. 1998;63:8092. [Google Scholar]
- 490.Francisco CG, Gonzalez CC, Paz NR, Suarez E. Org Lett. 2003;5:4171. doi: 10.1021/ol035611l. [DOI] [PubMed] [Google Scholar]
- 491.Alonso-Cruz CR, Kennedy AR, Rodriguez MS, Suarez E. Org Lett. 2003;5:3729. doi: 10.1021/ol035435g. [DOI] [PubMed] [Google Scholar]
- 492.Alonso-Cruz CR, Kennedy AR, Rodriguez MS, Suarez E. Tetrahedron Lett. 2007;48:7207. [Google Scholar]
- 493.Alonso-Cruz CR, Leon EI, Ortiz-Lopez FJ, Rodriguez MS, Suarez E. Tetrahedron Lett. 2005;46:5265. [Google Scholar]
- 494.Francisco CG, Herrera AJ, Kennedy AR, Melian D, Suarez E. Ang Chem, Int Ed. 2002;41:856. doi: 10.1002/1521-3773(20020301)41:5<856::aid-anie856>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 495.Francisco CG, Freire R, Herrera AJ, Perez-Martin I, Suarez E. Tetrahedron. 2007;63:8910. [Google Scholar]
- 496.Francisco CG, Freire R, Herrera AJ, Perez-Martin I, Suarez E. Org Lett. 2002;4:1959. doi: 10.1021/ol025981u. [DOI] [PubMed] [Google Scholar]
- 497.Martin A, Quintanal LM, Suarez E. Tetrahedron Lett. 2007;48:5507. [Google Scholar]
- 498.Boto A, Hernandez D, Hernandez R. Org Lett. 2007;9:1721. doi: 10.1021/ol070412d. [DOI] [PubMed] [Google Scholar]
- 499.Boto A, Hernandez D, Hernandez R, Suarez E. Org Lett. 2004;6:3785. doi: 10.1021/ol048439+. [DOI] [PubMed] [Google Scholar]
- 500.Chen J-M, Huang X. Synthesis. 2004:2459. [Google Scholar]
- 501.Antunes CSA, Bietti M, Lanzalunga O, Salamone M. J Org Chem. 2004;69:5281. doi: 10.1021/jo049524y. [DOI] [PubMed] [Google Scholar]
- 502.Boto A, Hernandez R, Montoya A, Suarez E. Tetrahedron Lett. 2002;43:8269. [Google Scholar]
- 503.Boto A, Gallardo JA, Hernandez R, Saavedra CJ. Tetrahedron Lett. 2005;46:7807. [Google Scholar]
- 504.Boto A, Hernandez R, De Leon Y, Murguia JR, Rodriguez-Afonso A. Tetrahedron Lett. 2004;45:6841. [Google Scholar]
- 505.Iglesias-Arteaga MA, Avila-Ortiz CG, Juaristi E. Tetrahedron Lett. 2002;43:5297. [Google Scholar]
- 506.Dohi T, Takenaga N, Goto A, Maruyama A, Kita Y. Org Lett. 2007;9:3129. doi: 10.1021/ol071315n. [DOI] [PubMed] [Google Scholar]
- 507.Barluenga J, Gonzalez-Bobes F, Gonzalez JM. Angew Chem, Int Ed. 2002;41:2556. doi: 10.1002/1521-3773(20020715)41:14<2556::AID-ANIE2556>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 508.Pradhan TK, Hassner A. Synthesis. 2007:3361. [Google Scholar]
- 509.Martin A, Perez-Martin I, Suarez E. Org Lett. 2005;7:2027. doi: 10.1021/ol050526u. [DOI] [PubMed] [Google Scholar]
- 510.Francisco CG, Herrera AJ, Martin A, Perez-Martin I, Suarez E. Tetrahedron Lett. 2007;48:6384. [Google Scholar]
- 511.Fan R, Pu D, Wen F, Wu J. J Org Chem. 2007;72:8994. doi: 10.1021/jo7016982. [DOI] [PubMed] [Google Scholar]
- 512.Momiyama N, Yamamoto Y, Yamamoto H. J Am Chem Soc. 2007;129:1190. doi: 10.1021/ja066037m. [DOI] [PubMed] [Google Scholar]
- 513.Singh CB, Ghosh H, Murru S, Patel BK. J Org Chem. 2008;73:2924. doi: 10.1021/jo702628g. [DOI] [PubMed] [Google Scholar]
- 514.But TYS, Toy PH. J Am Chem Soc. 2006;128:9636. doi: 10.1021/ja063141v. [DOI] [PubMed] [Google Scholar]
- 515.Kansara A, Sharma PK, Banerji KK. J Chem Res. 2004:315. [Google Scholar]
- 516.ter Wiel MKJ, Vicario J, Davey SG, Meetsma A, Feringa BL. Org Biomol Chem. 2005;3:28. doi: 10.1039/b414959a. [DOI] [PubMed] [Google Scholar]
- 517.Shang Z, Reiner J, Zhao K. Synth Commun. 2006;36:1529. [Google Scholar]
- 518.Zhutov EV, Skornyakov YV, Proskurina MV, Zefirov NS. Russ J Org Chem. 2003;39:1672. [Google Scholar]
- 519.Tohma H, Maegawa T, Kita Y. ARKIVOC. 2003;vi:62. [Google Scholar]
- 520.Burghardt TE. J Sulfur Chem. 2005;26:411. [Google Scholar]
- 521.Wu Y, Shen X, Yang Y-Q, Hu Q, Huang J-H. J Org Chem. 2004;69:3857. doi: 10.1021/jo049971d. [DOI] [PubMed] [Google Scholar]
- 522.Wu Y, Shen X, Yang Y-Q, Hu Q, Huang J-H. Tetrahedron Lett. 2004;45:199. [Google Scholar]
- 523.Fleming FF, Funk L, Altundas R, Tu Y. J Org Chem. 2001;66:6502. doi: 10.1021/jo0157829. [DOI] [PubMed] [Google Scholar]
- 524.Shi X-X, Wu Q-Q. Synth Commun. 2000;30:4081. [Google Scholar]
- 525.Makowiec S, Rachon J. Heteroatom Chem. 2003;14:352. [Google Scholar]
- 526.Young KJH, Mironov OA, Periana RA. Organometallics. 2007;26:2137. [Google Scholar]
- 527.Li Z, Xia C-G. J Mol Catal A. 2004;214:95. [Google Scholar]
- 528.In J-H, Park S-E, Song R, Nam W. Inorg Chim Acta. 2003;343:373. [Google Scholar]
- 529.Yusubov MS, Chi K-W, Park JY, Karimov R, Zhdankin VV. Tetrahedron Lett. 2006;47:6305. [Google Scholar]
- 530.Iwasa S, Morita K, Tajima K, Fakhruddin A, Nishiyama H. Chem Lett. 2002:284. [Google Scholar]
- 531.Miyamura H, Akiyama R, Ishida T, Matsubara R, Takeuchi M, Kobayashi S. Tetrahedron. 2005;61:12177. [Google Scholar]
- 532.Sun W, Wang H, Xia C, Li J, Zhao P. Angew Chem, Int Ed. 2003;42:1042. doi: 10.1002/anie.200390268. [DOI] [PubMed] [Google Scholar]
- 533.Li Z, Tang ZH, Hu XX, Xia CG. Chem Eur J. 2005;11:1210. doi: 10.1002/chem.200400818. [DOI] [PubMed] [Google Scholar]
- 534.Karimipour GR, Shadegan HA, Ahmadpour R. J Chem Res. 2007:252. [Google Scholar]
- 535.Adam W, Hajra S, Herderich M, Saha-Moeller CR. Org Lett. 2000;2:2773. doi: 10.1021/ol000142y. [DOI] [PubMed] [Google Scholar]
- 536.Kunst E, Gallier F, Dujardin G, Yusubov MS, Kirschning A. Org Lett. 2007;9:5199. doi: 10.1021/ol702319p. [DOI] [PubMed] [Google Scholar]
- 537.Provins L, Murahashi S-I. ARKIVOC. 2007;x:107. [Google Scholar]
- 538.Dick AR, Hull KL, Sanford MS. J Am Chem Soc. 2004;126:2300. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]
- 539.Alexanian EJ, Lee C, Sorensen EJ. J Am Chem Soc. 2005;127:7690. doi: 10.1021/ja051406k. [DOI] [PubMed] [Google Scholar]
- 540.Desai LV, Hull KL, Sanford MS. J Am Chem Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 541.Kalyani D, Sanford MS. Org Lett. 2005;7:4149. doi: 10.1021/ol051486x. [DOI] [PubMed] [Google Scholar]
- 542.Kalyani D, Dick AR, Anani WQ, Sanford MS. Org Lett. 2006;8:2523. doi: 10.1021/ol060747f. [DOI] [PubMed] [Google Scholar]
- 543.Wang D-H, Hao X-S, Wu D-F, Yu J-Q. Org Lett. 2006;8:3387. doi: 10.1021/ol061384m. [DOI] [PubMed] [Google Scholar]
- 544.Welbes LL, Lyons TW, Cychosz KA, Sanford MS. J Am Chem Soc. 2007;129:5836. doi: 10.1021/ja071204j. [DOI] [PubMed] [Google Scholar]
- 545.Desai LV, Sanford MS. Angew Chem, Int Ed. 2007;46:5737. doi: 10.1002/anie.200701454. [DOI] [PubMed] [Google Scholar]
- 546.Liu G, Stahl SS. J Am Chem Soc. 2006;128:7179. doi: 10.1021/ja061706h. [DOI] [PubMed] [Google Scholar]
- 547.Streuff J, Hoevelmann CH, Nieger M, Muniz K. J Am Chem Soc. 2005;127:14586. doi: 10.1021/ja055190y. [DOI] [PubMed] [Google Scholar]
- 548.Kalberer EW, Whitfield SR, Sanford MS. J Mol Catal A. 2006;251:108. [Google Scholar]
- 549.Dick AR, Kampf JW, Sanford MS. Organometallics. 2005;24:482. [Google Scholar]
- 550.Muniz K, Hoevelmann CH, Streuff J. J Am Chem Soc. 2008;130:763. doi: 10.1021/ja075041a. [DOI] [PubMed] [Google Scholar]
- 551.Giri R, Chen X, Yu J-Q. Angew Chem, Int Ed. 2005;44:2112. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 552.Daugulis O, Zaitsev VG. Angew Chem, Int Ed. 2005;44:4046. doi: 10.1002/anie.200500589. [DOI] [PubMed] [Google Scholar]
- 553.Dick AR, Kampf JW, Sanford MS. J Am Chem Soc. 2005;127:12790. doi: 10.1021/ja0541940. [DOI] [PubMed] [Google Scholar]
- 554.Yan J, Wu J, Jin H. J Organomet Chem. 2007;692:3636. [Google Scholar]
- 555.Yan J, Lin F, Yang Z. Synthesis. 2007:1301. [Google Scholar]
- 556.Zefirov NS, Sorokin VD, Zhdankin VV, Koz’min AS. Russ J Org Chem. 1986;22:450. [Google Scholar]
- 557.Bassindale AR, Katampe I, Taylor PG. Can J Chem. 2000;78:1479. [Google Scholar]
- 558.Robinson RI, Woodward S. Tetrahedron Lett. 2003;44:1655. [Google Scholar]
- 559.Koser GF, Wettach RH. J Org Chem. 1977;42:1476. [Google Scholar]
- 560.Nabana T, Togo H. J Org Chem. 2002;67:4362. doi: 10.1021/jo0200670. [DOI] [PubMed] [Google Scholar]
- 561.Yamamoto Y, Togo H. Synlett. 2005:2486. [Google Scholar]
- 562.Hatzigrigoriou E, Varvoglis A, Bakola-Christianopoulou M. J Org Chem. 1990;55:315. [Google Scholar]
- 563.Yusubov MS, Wirth T. Org Lett. 2005;7:519. doi: 10.1021/ol047363e. [DOI] [PubMed] [Google Scholar]
- 564.Abe S, Sakuratani K, Togo H. Synlett. 2001:22. doi: 10.1021/jo010333u. [DOI] [PubMed] [Google Scholar]
- 565.Abe S, Sakuratani K, Togo H. J Org Chem. 2001;66:6174. doi: 10.1021/jo010333u. [DOI] [PubMed] [Google Scholar]
- 566.Zefirov NS, Zhdankin VV, Dan’kov YV, Sorokin VD, Semerikov VN, Koz’min AS, Caple R, Berglund BA. Tetrahedron Lett. 1986;27:3971. [Google Scholar]
- 567.Hembre RT, Scott CP, Norton JR. J Org Chem. 1987;52:3650. [Google Scholar]
- 568.Zhdankin VV, Crittell CM, Stang PJ. Tetrahedron Lett. 1990;31:4821. [Google Scholar]
- 569.Kitamura T, Inoue D, Wakimoto I, Nakamura T, Katsuno R, Fujiwara Y. Tetrahedron. 2004;60:8855. [Google Scholar]
- 570.Koser GF, Wettach RH, Troup JM, Frenz BA. J Org Chem. 1976;41:3609. [Google Scholar]
- 571.Wessig P, Muhling O. Helv Chim Acta. 2003;86:865. [Google Scholar]
- 572.Kumar D, Sundaree S, Rao VS. Synth Commun. 2006;36:1893. [Google Scholar]
- 573.Prakash O, Pannu K, Prakash R, Batra A. Molecules. 2006;11:523. doi: 10.3390/11070523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Kumar D, Sundaree S, Rao VS, Varma RS. Tetrahedron Lett. 2006;47:4197. [Google Scholar]
- 575.Kumar D, Sundaree MS, Patel G, Rao VS, Varma RS. Tetrahedron Lett. 2006;47:8239. [Google Scholar]
- 576.Xie Y-Y, Chen Z-C. Synth Commun. 2002;32:1875. [Google Scholar]
- 577.Wan D-B, Chen J-M. J Chem Res. 2006:32. [Google Scholar]
- 578.Prakash O, Batra A, Chaudhri V, Prakash R. Tetrahedron Lett. 2005;46:2877. [Google Scholar]
- 579.Lee JC, Ku CH. Synlett. 2002:1679. [Google Scholar]
- 580.Xie Y-Y. Synth Commun. 2005;35:1741. [Google Scholar]
- 581.Handy ST, Okello M. Synlett. 2002:489. [Google Scholar]
- 582.Aggarwal R, Sumran G. Synth Commun. 2006;36:875. [Google Scholar]
- 583.Aggarwal R, Pundeer R, Kumar V, Chaudhri V, Singh SP, Prakash O. Synth Commun. 2004;34:2659. [Google Scholar]
- 584.Lee JC, Seo J-W, Baek JW. Synth Commun. 2007;37:2159. [Google Scholar]
- 585.Lee JC, Choi HJ, Lee YC. Tetrahedron Lett. 2003;44:123. [Google Scholar]
- 586.Kawano Y, Togo H. Synlett. 2008:217. [Google Scholar]
- 587.Muthukrishnan M, Patil PS, More SV, Joshi RA. Mendeleev Commun. 2005:100. [Google Scholar]
- 588.Kawamura Y, Maruyama M, Tokuoka T, Tsukayama M. Synthesis. 2002:2490. [Google Scholar]
- 589.Patonay T, Levai A, Riman E, Varma RS. ARKIVOC. 2004;vii:183. [Google Scholar]
- 590.Karade NN, Gampawar SV, Kondre JM, Shinde SV. Tetrahedron Lett. 2008;49:4402. [Google Scholar]
- 591.Li M, Zhao G, Wen L, Cao W, Zhang S, Yang H. J Heterocycl Chem. 2005;42:209. [Google Scholar]
- 592.Li M, Zhao G, Wen L, Yang H. Synth Commun. 2005;35:493. [Google Scholar]
- 593.Hou RS, Wang HM, Huang HY, Chen LC. Org Prep Proced Int. 2004;36:491. [Google Scholar]
- 594.Lee JC, Park H-J, Park JY. Tetrahedron Lett. 2002;43:5661. [Google Scholar]
- 595.Xie YY, Chen ZC, Zheng QG. J Chem Res (S) 2002:618. [Google Scholar]
- 596.Xie Y-Y, Chen Z-C, Zheng Q-G. Synthesis. 2002:1505. [Google Scholar]
- 597.Hou R-S, Wang H-M, Lin Y-C, Chen L-C. Heterocycles. 2005;65:649. [Google Scholar]
- 598.Richardson RD, Page TK, Altermann S, Paradine SM, French AN, Wirth T. Synlett. 2007:538. [Google Scholar]
- 599.Yamamoto Y, Togo H. Synlett. 2006:798. [Google Scholar]
- 600.Akiike J, Yamamoto Y, Togo H. Synlett. 2007:2168. [Google Scholar]
- 601.Yamamoto Y, Kawano Y, Toy PH, Togo H. Tetrahedron. 2007;63:4680. [Google Scholar]
- 602.Justik MW, Koser GF. Tetrahedron Lett. 2004;45:6159. [Google Scholar]
- 603.Hossain MM, Tokuoka T, Yamashita K, Kawamura Y, Tsukayama M. Synth Commun. 2006;36:1201. [Google Scholar]
- 604.Silva LF, Jr, Siqueira FA, Pedrozo EC, Vieira FYM, Doriguetto AC. Org Lett. 2007;9:1433. doi: 10.1021/ol070027o. [DOI] [PubMed] [Google Scholar]
- 605.Prakash O, Kumar A, Sadana AK, Singh SP. Synthesis. 2006:21. [Google Scholar]
- 606.Justik MW, Koser GF. Molecules. 2005;10:217. doi: 10.3390/10010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Silva LF, Jr, Vasconcelos RS, Nogueira MA. Org Lett. 2008;10:1017. doi: 10.1021/ol800048f. [DOI] [PubMed] [Google Scholar]
- 608.Koser GF, Telu S, Laali KK. Tetrahedron Lett. 2006;47:7011. [Google Scholar]
- 609.Comins DL, Kuethe JT, Miller TM, Fevrier FC, Brooks CA. J Org Chem. 2005;70:5221. doi: 10.1021/jo050559n. [DOI] [PubMed] [Google Scholar]
- 610.Chen J-M, Huang X. Synthesis. 2004:1577. [Google Scholar]
- 611.Miyazawa E, Sakamoto T, Kikugawa Y. J Org Chem. 2003;68:5429. doi: 10.1021/jo034318w. [DOI] [PubMed] [Google Scholar]
- 612.Misu Y, Togo H. Org Biomol Chem. 2003;1:1342. doi: 10.1039/b301330h. [DOI] [PubMed] [Google Scholar]
- 613.Walker SJ, Hart DJ. Tetrahedron Lett. 2007;48:6214. [Google Scholar]
- 614.Lazbin IM, Koser GF. J Org Chem. 1986;51:2669. [Google Scholar]
- 615.Vasudevan A, Koser GF. J Org Chem. 1988;53:5158. [Google Scholar]
- 616.Moriarty RM, Khosrowshahi JS, Awasthi AK, Penmasta R. Synth Commun. 1988;18:1179. [Google Scholar]
- 617.Liu SJ, Zhang JZ, Tian GR, Liu P. Synth Commun. 2005;36:823. [Google Scholar]
- 618.Lee JC, Lee JY, Lee SJ. Tetrahedron Lett. 2004;45:4939. [Google Scholar]
- 619.Sridhar PR, Prabhu KR, Chandrasekaran S. Eur J Org Chem. 2004:4809. doi: 10.1021/jo0266947. [DOI] [PubMed] [Google Scholar]
- 620.Lee J, Lee J. Synth Commun. 2006;36:1071. [Google Scholar]
- 621.Yan J, Zhou Z, Zhu M. Tetrahedron Lett. 2005;46:8173. [Google Scholar]
- 622.Yan J. J Chem Res. 2006:459. [Google Scholar]
- 623.Yan J, Zhu M, Zhou Z. Eur J Org Chem. 2006:2060. [Google Scholar]
- 624.Hadjiarapoglou L, Spyroudis S, Varvoglis A. Synthesis. 1983:207. [Google Scholar]
- 625.Papadopoulou M, Varvoglis A. J Chem Res (S) 1983:66. [Google Scholar]
- 626.Papadopoulou M, Varvoglis A. J Chem Res (S) 1984:166. [Google Scholar]
- 627.Zhdankin VV, Krasutsky AP, Kuehl CJ, Simonsen AJ, Woodward JK, Mismash B, Bolz JT. J Am Chem Soc. 1996;118:5192. [Google Scholar]
- 628.Magnus P, Lacour J, Evans PA, Roe MB, Hulme C. J Am Chem Soc. 1996;118:3406. [Google Scholar]
- 629.Magnus P, Lacour J, Weber W. J Am Chem Soc. 1993;115:9347. [Google Scholar]
- 630.Magnus P, Lacour J, Weber W. Synthesis. 1998:547. [Google Scholar]
- 631.Pedersen CM, Marinescu LG, Bols M. Org Biomol Chem. 2005;3:816. doi: 10.1039/b500037h. [DOI] [PubMed] [Google Scholar]
- 632.Chung R, Yu E, Incarvito CD, Austin DJ. Org Lett. 2004;6:3881. doi: 10.1021/ol0490532. [DOI] [PubMed] [Google Scholar]
- 633.Kirschning A, Abul Hashem M, Monenschein H, Rose L, Schoening K-U. J Org Chem. 1999;64:6522. [Google Scholar]
- 634.Chen J-M, Huang X. Synth Commun. 2004;34:1745. [Google Scholar]
- 635.Umemoto T, Kuriu Y, Shuyama H, Miyano O, Nakayama S. J Fluorine Chem. 1986;31:37. [Google Scholar]
- 636.Umemoto T, Kuriu Y, Shuyama H, Miyano O, Nakayama S. J Fluorine Chem. 1982;20:695. [Google Scholar]
- 637.Tesevic V, Gladysz JA. Green Chem. 2005;7:833. [Google Scholar]
- 638.Tesevic V, Gladysz JA. J Org Chem. 2006;71:7433. doi: 10.1021/jo0612067. [DOI] [PubMed] [Google Scholar]
- 639.Yagupol’skii LM, Maletina II, Kondratenko NV, Orda VV. Synthesis. 1978:835. [Google Scholar]
- 640.Kuehl CJ, Bolz JT, Zhdankin VV. Synthesis. 1995:312. [Google Scholar]
- 641.Zhdankin VV, Kuehl C. Tetrahedron Lett. 1994;35:1809. [Google Scholar]
- 642.Umemoto T, Goto Y. Bull Chem Soc Jpn. 1987;60:3307. [Google Scholar]
- 643.Zhdankin VV, Kuehl CJ, Simonsen AJ. Tetrahedron Lett. 1995;36:2203. [Google Scholar]
- 644.Zhdankin VV, Kuehl CJ, Simonsen AJ. J Org Chem. 1996;61:8272. doi: 10.1021/jo961336n. [DOI] [PubMed] [Google Scholar]
- 645.Zhdankin VV, Erickson SA, Hanson KJ. J Am Chem Soc. 1997;119:4775. [Google Scholar]
- 646.Minkwitz R, Berkei M. Inorg Chem. 1999;38:5041. doi: 10.1021/ic990441n. [DOI] [PubMed] [Google Scholar]
- 647.Minkwitz R, Berkei M. Inorg Chem. 2001;40:36. doi: 10.1021/ic000439s. [DOI] [PubMed] [Google Scholar]
- 648.Minkwitz R, Berkei M. Z Naturforsch, B: Chem Sci. 2000;55:718. [Google Scholar]
- 649.Minkwitz R, Berkei M. Z Anorg Allg Chem. 2000;626:2325. [Google Scholar]
- 650.Minkwitz R, Berkei M, Ludwig R. Eur J Inorg Chem. 2000:2387. doi: 10.1021/ic000525s. [DOI] [PubMed] [Google Scholar]
- 651.DesMarteau DD, Montanari V. J Chem Soc, Chem Commun. 1998:2241. [Google Scholar]
- 652.Meyer V, Wachter W. Ber. 1892;25:2632. [Google Scholar]
- 653.Willgerodt C. J Prakt Chem. 1894;49:466. [Google Scholar]
- 654.Wolf W, Steinberg L. J Chem Soc, Chem Commun. 1965:449. [Google Scholar]
- 655.Zhdankin VV, Arbit RM, McSherry M, Mismash B, Young VG. J Am Chem Soc. 1997;119:7408. [Google Scholar]
- 656.Amey RL, Martin JC. J Org Chem. 1979;44:1779. [Google Scholar]
- 657.Ochiai M, Masaki Y, Shiro M. J Org Chem. 1991;56:5511. [Google Scholar]
- 658.Shefter E, Wolf W. J Pharm Sci. 1965;54:104. doi: 10.1002/jps.2600540124. [DOI] [PubMed] [Google Scholar]
- 659.Etter MC. J Am Chem Soc. 1976;98:5326. [Google Scholar]
- 660.Etter MC. J Am Chem Soc. 1976;98:5331. [Google Scholar]
- 661.Gougoutas JZ, Lessinger L. J Solid State Chem. 1974;9:155. [Google Scholar]
- 662.Ochiai M, Ito T, Masaki Y, Shiro M. J Am Chem Soc. 1992;114:6269. [Google Scholar]
- 663.Ochiai M, Ito T, Shiro M. J Chem Soc, Chem Commun. 1993:218. [Google Scholar]
- 664.Zhdankin VV, Kuehl CJ, Arif AM, Stang PJ. Mendeleev Commun. 1996:50. [Google Scholar]
- 665.Akai S, Okuno T, Takada T, Tohma H, Kita Y. Heterocycles. 1996;42:47. [Google Scholar]
- 666.Braddock DC, Cansell G, Hermitage SA, White AJP. Chem Commun. 2006:1442. doi: 10.1039/b600455e. [DOI] [PubMed] [Google Scholar]
- 667.Eisenberger P, Gischig S, Togni A. Chem Eur J. 2006;12:2579. doi: 10.1002/chem.200501052. [DOI] [PubMed] [Google Scholar]
- 668.Kieltsch I, Eisenberger P, Togni A. Angew Chem, Int Ed. 2007;46:754. doi: 10.1002/anie.200603497. [DOI] [PubMed] [Google Scholar]
- 669.Koser GF. In: The Chemistry of Functional Groups, Suppl D: Chem Halides, Pseudo-Halides, Azides. Patai S, Rappoport Z, editors. Wiley-Interscience; Chichester: 1983. [Google Scholar]
- 670.Sueda T, Fukuda S, Ochiai M. Org Lett. 2001;3:2387. doi: 10.1021/ol016202x. [DOI] [PubMed] [Google Scholar]
- 671.Ochiai M, Ito T, Takahashi H, Nakanishi A, Toyonari M, Sueda T, Goto S, Shiro M. J Am Chem Soc. 1996;118:7716. [Google Scholar]
- 672.Dolenc D, Plesnicar B. J Am Chem Soc. 1997;119:2628. [Google Scholar]
- 673.Ochiai M, Nakanishi A, Ito T. J Org Chem. 1997;62:4253. doi: 10.1021/jo970081q. [DOI] [PubMed] [Google Scholar]
- 674.Ochiai M, Sueda T. Tetrahedron Lett. 2004;45:3557. [Google Scholar]
- 675.Krasutsky AP, Kuehl CJ, Zhdankin VV. Synlett. 1995:1081. [Google Scholar]
- 676.Zhdankin VV, Kuehl CJ, Krasutsky AP, Formaneck MS, Bolz JT. Tetrahedron Lett. 1994;35:9677. [Google Scholar]
- 677.Zhdankin VV, Kuehl CJ, Krasutsky AP, Bolz JT, Mismash B, Woodward JK, Simonsen AJ. Tetrahedron Lett. 1995;36:7975. [Google Scholar]
- 678.Sueda T, Takeuchi Y, Suefuji T, Ochiai M. Molecules. 2005;10:195. doi: 10.3390/10010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Eisenberger P, Kieltsch I, Armanino N, Togni A. Chem Commun. 2008:1575. doi: 10.1039/b801424h. [DOI] [PubMed] [Google Scholar]
- 680.Zhang W, Zhu J, Hu J. Tetrahedron Lett. 2008;49:5006. [Google Scholar]
- 681.Okuyama T, Takino T, Sueda T, Ochiai M. J Am Chem Soc. 1995;117:3360. [Google Scholar]
- 682.Shirai A, Kubo H, Takahashi E. J Photopolym Sci Technol. 2002;15:29. [Google Scholar]
- 683.VanderHart DL, Prabhu VM, Lin EK. Chem Mater. 2004;16:3074. [Google Scholar]
- 684.Slegt M, Minne F, Zuilhof H, Overkleeft HS, Lodder G. Eur J Org Chem. 2007:5353. doi: 10.1021/jo0518957. [DOI] [PubMed] [Google Scholar]
- 685.Tasdelen MA, Kumbaraci V, Jockusch S, Turro NJ, Talinli N, Yagci Y. Macromolecules. 2008;41:295. [Google Scholar]
- 686.Goldstein EJC, Citron DM, Warren Y, Merriam CV, Tyrrell K, Fernandez H, Radhakrishnan U, Stang PJ, Conrads G. Antimicrobial Agents and Chemotherapy. 2004;48:2766. doi: 10.1128/AAC.48.7.2766-2770.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Montanari V, Kumar K. J Am Chem Soc. 2004;126:9528. doi: 10.1021/ja0479033. [DOI] [PubMed] [Google Scholar]
- 688.DesMarteau DD, Montanari V. Chem Lett. 2000:1052. [Google Scholar]
- 689.DesMarteau DD, Montanari V. J Fluorine Chem. 2001;109:19. [Google Scholar]
- 690.Montanari V, Kumar K. J Fluorine Chem. 2006;127:565. [Google Scholar]
- 691.Montanari V, Kumar K. Eur J Org Chem. 2006:874. [Google Scholar]
- 692.Liu CY, Li H, Meng AG. Acta Crystallogr, Sect E: Struct Rep. 2007;E63:o3647. doi: 10.1107/S1600536807010689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.DesMarteau DD, Pennington WT, Montanari V, Thomas BH. J Fluorine Chem. 2003;122:57. [Google Scholar]
- 694.Kaafarani BR, Gu H, Pinkerton AA, Neckers DC. J Chem Soc, Dalton Trans. 2002:2318. [Google Scholar]
- 695.Bykowski D, McDonald R, Hinkle RJ, Tykwinski RR. J Org Chem. 2002;67:2798. doi: 10.1021/jo015910t. [DOI] [PubMed] [Google Scholar]
- 696.Ochiai M, Toyonari M, Sueda T, Kitagawa Y. Tetrahedron Lett. 1996;37:8421. [Google Scholar]
- 697.Chen D-W, Ochiai M. J Org Chem. 1999;64:6804. doi: 10.1021/jo990809y. [DOI] [PubMed] [Google Scholar]
- 698.Kalyani D, Deprez NR, Desai LV, Sanford MS. J Am Chem Soc. 2005;127:7330. doi: 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]
- 699.Ochiai M, Ito T, Takaoka Y, Masaki Y. J Am Chem Soc. 1991;113:1319. [Google Scholar]
- 700.Helber J, Frohn H-J, Klose A, Scholten T. ARKIVOC. 2003;vi:71. [Google Scholar]
- 701.Dohi T, Ito M, Morimoto K, Minamitsuji Y, Takenaga N, Kita Y. Chem Commun. 2007:4152. doi: 10.1039/b708802g. [DOI] [PubMed] [Google Scholar]
- 702.Koser GF, Wettach RH. J Org Chem. 1980;45:1542. [Google Scholar]
- 703.Carman CS, Koser GF. J Org Chem. 1983;48:2534. [Google Scholar]
- 704.Pike VW, Butt F, Shah A, Widdowson DA. J Chem Soc, Perkin Trans 1. 1999:245. [Google Scholar]
- 705.Zhang M-R, Kumata K, Suzuki K. Tetrahedron Lett. 2007;48:8632. [Google Scholar]
- 706.Carroll MA, Pike VW, Widdowson DA. Tetrahedron Lett. 2000;41:5393. [Google Scholar]
- 707.Kitamura T, Matsuyuki J, Nagata K, Furuki R, Taniguchi H. Synthesis. 1992:945. [Google Scholar]
- 708.Shah A, Pike VW, Widdowson DA. J Chem Soc, Perkin Trans 1. 1997:2463. [Google Scholar]
- 709.Hossain MD, Kitamura T. Tetrahedron. 2006;62:6955. [Google Scholar]
- 710.Hossain MD, Ikegami Y, Kitamura T. J Org Chem. 2006;71:9903. doi: 10.1021/jo061889q. [DOI] [PubMed] [Google Scholar]
- 711.Hossain MD, Kitamura T. Bull Chem Soc Jpn. 2007;80:2213. [Google Scholar]
- 712.Bielawski M, Zhu M, Olofsson B. Adv Synth Catal. 2007;349:2610. [Google Scholar]
- 713.Bielawski M, Olofsson B. J Chem Soc, Chem Commun. 2007:2521. doi: 10.1039/b701864a. [DOI] [PubMed] [Google Scholar]
- 714.Zhu M, Jalalian N, Olofsson B. Synlett. 2008:592. [Google Scholar]
- 715.Bielawski M, Aili D, Olofsson B. J Org Chem. 2008;73:4602. doi: 10.1021/jo8004974. [DOI] [PubMed] [Google Scholar]
- 716.Kraszkiewicz L, Skulski L. Synthesis. 2008:2373. [Google Scholar]
- 717.Stang PJ, Tykwinski R, Zhdankin V. J Heterocycl Chem. 1992;29:815. [Google Scholar]
- 718.Stang PJ, Zhdankin VV, Tykwinski R, Zefirov NS. Tetrahedron Lett. 1992;33:1419. [Google Scholar]
- 719.Radhakrishnan U, Stang PJ. J Org Chem. 2003;68:9209. doi: 10.1021/jo030246x. [DOI] [PubMed] [Google Scholar]
- 720.Stang PJ, Olenyuk B, Chen K. Synthesis. 1995:937. [Google Scholar]
- 721.Aggarwal VK, Olofsson B. Angew Chem, Int Ed. 2005;44:5516. doi: 10.1002/anie.200501745. [DOI] [PubMed] [Google Scholar]
- 722.Beringer FM, Nathan RA. J Org Chem. 1970;35:2095. doi: 10.1021/jo00826a001. [DOI] [PubMed] [Google Scholar]
- 723.Beringer FM, Nathan RA. J Org Chem. 1969;34:685. [Google Scholar]
- 724.Stang PJ, Chen K. J Am Chem Soc. 1995;117:1667. [Google Scholar]
- 725.Wang F-Y, Chen Z-C, Zheng Q-G. J Chem Res (S) 2003:620. [Google Scholar]
- 726.Wang F-Y, Chen Z-C, Zheng Q-G. J Chem Res (S) 2003:810. [Google Scholar]
- 727.Wang F-Y, Chen Z-C, Zheng Q-G. J Chem Res. 2004:206. [Google Scholar]
- 728.Carroll MA, Wood RA. Tetrahedron. 2007;63:11349. [Google Scholar]
- 729.Yan J, Hu W, Rao G. Synthesis. 2006:943. [Google Scholar]
- 730.Xue Z, Yang D, Wang C. J Organomet Chem. 2006;691:247. [Google Scholar]
- 731.Fujita M, Mishima E, Okuyama T. J Phys Org Chem. 2007;20:241. [Google Scholar]
- 732.Ermert J, Hocke C, Ludwig T, Gail R, Coenen HH. J Labelled Compd Radiopharm. 2004;47:429. [Google Scholar]
- 733.Oh CH, Kim JS, Jung HH. J Org Chem. 1999;64:1338. [Google Scholar]
- 734.Ochiai M, Kitagawa Y, Takayama N, Takaoka Y, Shiro M. J Am Chem Soc. 1999;121:9233. [Google Scholar]
- 735.Ozanne-Beaudenon A, Quideau S. Angew Chem, Int Ed. 2005;44:7065. doi: 10.1002/anie.200501638. [DOI] [PubMed] [Google Scholar]
- 736.Quideau S, Pouysegu L, Ozanne A, Gagnepain J. Molecules. 2005;10:201. doi: 10.3390/10010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Marsh G, Stenutz R, Bergman A. Eur J Org Chem. 2003:2566. [Google Scholar]
- 738.Couladouros EA, Moutsos VI, Pitsinos EN. ARKIVOC. 2003;xv:92. [Google Scholar]
- 739.Ryan JH, Stang PJ. Tetrahedron Lett. 1997;38:5061. [Google Scholar]
- 740.Zhang B-X, Nuka T, Fujiwara Y, Yamaji T, Hou Z, Kitamura T. Heterocycles. 2004;64:199. [Google Scholar]
- 741.Zhou T, Chen Z-C. J Chem Res. 2004:404. [Google Scholar]
- 742.Zhou T, Li T-C, Chen Z-C. Helv Chim Acta. 2005;88:290. [Google Scholar]
- 743.Kang S-K, Lee H-W, Jang S-B, Ho P-S. J Org Chem. 1996;61:4720. doi: 10.1021/jo960195m. [DOI] [PubMed] [Google Scholar]
- 744.Bumagin NA, Tsarev DA. Tetrahedron Lett. 1998;39:8155. [Google Scholar]
- 745.Al-Qahtani MH, Pike VW. J Chem Soc, Perkin Trans 1. 2000:1033. [Google Scholar]
- 746.Kang S-K, Yamaguchi T, Ho P-S, Kim W-Y, Yoon S-K. Tetrahedron Lett. 1997;38:1947. [Google Scholar]
- 747.Kang S-K, Choi S-C, Baik T-G. Synth Commun. 1999;29:2493. [Google Scholar]
- 748.Kang S-K, Ryu H-C, Kim J-W. Synth Commun. 2001;31:1021. [Google Scholar]
- 749.Kang S-K, Yamaguchi T, Ho P-S, Kim W-Y, Ryu H-C. J Chem Soc, Perkin Trans 1. 1998:841. [Google Scholar]
- 750.Kang S-K, Lee H-W, Jang S-B, Kim T-H, Pyun S-J. J Org Chem. 1996;61:2604. doi: 10.1021/jo951923t. [DOI] [PubMed] [Google Scholar]
- 751.Kang S-K, Baik T-G, Hur Y. Tetrahedron. 1999;55:6863. [Google Scholar]
- 752.Kang S-K, Ha Y-H, Yang H-Y. J Chem Res (S) 2002:282. [Google Scholar]
- 753.Wang L, Chen Z-C. Synth Commun. 2000;30:3607. [Google Scholar]
- 754.Liang Y, Luo S, Liu C, Wu X, Ma Y. Tetrahedron. 2000;56:2961. [Google Scholar]
- 755.Zhu M, Song Y, Cao Y. Synthesis. 2007:853. [Google Scholar]
- 756.Radhakrishnan U, Stang PJ. Org Lett. 2001;3:859. doi: 10.1021/ol015555t. [DOI] [PubMed] [Google Scholar]
- 757.Becht J-M, Le Drian C. Org Lett. 2008;10:3161. doi: 10.1021/ol8011293. [DOI] [PubMed] [Google Scholar]
- 758.Deprez NR, Kalyani D, Krause A, Sanford MS. J Am Chem Soc. 2006;128:4972. doi: 10.1021/ja060809x. [DOI] [PubMed] [Google Scholar]
- 759.Canty AJ, Patel J, Rodemann T, Ryan JH, Skelton BW, White AH. Organometallics. 2004;23:3466. [Google Scholar]
- 760.Kitamura T, Yamane M, Inoue K, Todaka M, Fukatsu N, Meng Z, Fujiwara Y. J Am Chem Soc. 1999;121:11674. [Google Scholar]
- 761.Kitamura T, Meng Z, Fujiwara Y. Tetrahedron Lett. 2000;41:6611. [Google Scholar]
- 762.Kitamura T, Wasai K, Todaka M, Fujiwara Y. Synlett. 1999:731. [Google Scholar]
- 763.Kitamura T, Aoki Y, Isshiki S, Wasai K, Fujiwara Y. Tetrahedron Lett. 2006;47:1709. [Google Scholar]
- 764.Kitamura T, Abe T, Fujiwara Y, Yamaji T. Synthesis. 2003:213. [Google Scholar]
- 765.Xue J, Huang X. Synth Commun. 2007;37:2179. [Google Scholar]
- 766.Kamila S, Koh B, Biehl ER. Synth Commun. 2006;36:3493. [Google Scholar]
- 767.Thiemann T, Fujii H, Ohira D, Arima K, Li Y, Mataka S. New J Chem. 2003;27:1377. [Google Scholar]
- 768.Xue J, Yang Y, Huang X. Synlett. 2007:1533. [Google Scholar]
- 769.Rao UN, Sathunuru R, Maguire JA, Biehl E. J Heterocycl Chem. 2004;41:13. [Google Scholar]
- 770.Chen Y-L, Hau C-K, Wang H, He H, Wong M-S, Lee AWM. J Org Chem. 2006;71:3512. doi: 10.1021/jo060099d. [DOI] [PubMed] [Google Scholar]
- 771.Lee T, Jeon J, Song KH, Jung I, Baik C, Park K-M, Lee SS, Kang SO, Ko J. J Chem Soc, Dalton Trans. 2004:933. doi: 10.1039/b315205g. [DOI] [PubMed] [Google Scholar]
- 772.Ochiai M, Sumi K, Takaoka Y, Kunishima M, Nagao Y, Shiro M, Fujita E. Tetrahedron. 1988;44:4095. [Google Scholar]
- 773.Ochiai M, Sueda T, Noda R, Shiro M. J Org Chem. 1999;64:8563. [Google Scholar]
- 774.Ochiai M, Toyonari M, Nagaoka T, Chen D-W, Kida M. Tetrahedron Lett. 1997;38:6709. [Google Scholar]
- 775.Fujita M, Lee HJ, Okuyama T. Org Lett. 2006;8:1399. doi: 10.1021/ol0601850. [DOI] [PubMed] [Google Scholar]
- 776.Hinkle RJ, Thomas DB. J Org Chem. 1997;62:7534. [Google Scholar]
- 777.Pirguliyev NS, Brel VK, Akhmedov NG, Zefirov NS. Synthesis. 2000:81. [Google Scholar]
- 778.Yoshida M, Hara S. Org Lett. 2003;5:573. doi: 10.1021/ol027512y. [DOI] [PubMed] [Google Scholar]
- 779.Yoshida M, Komata A, Hara S. Tetrahedron. 2006;62:8636. [Google Scholar]
- 780.Hinkle RJ, Stang PJ. Synthesis. 1994:313. [Google Scholar]
- 781.McNeil AJ, Hinkle RJ, Rouse EA, Thomas QA, Thomas DB. J Org Chem. 2001;66:5556. doi: 10.1021/jo015746+. [DOI] [PubMed] [Google Scholar]
- 782.Chen J-M, Huang X. Synlett. 2004:552. [Google Scholar]
- 783.Zhang P, Chen Z. J Chem Res (S) 2003:570. [Google Scholar]
- 784.Okuyama T, Fujita M. Acc Chem Res. 2005;38:679. doi: 10.1021/ar040293r. [DOI] [PubMed] [Google Scholar]
- 785.Fujita M, Kim WH, Sakanishi Y, Fujiwara K, Hirayama S, Okuyama T, Ohki Y, Tatsumi K, Yoshioka Y. J Am Chem Soc. 2004;126:7548. doi: 10.1021/ja0496672. [DOI] [PubMed] [Google Scholar]
- 786.Fujita M, Sakanishi Y, Nishii M, Yamataka H, Okuyama T. J Org Chem. 2002;67:8130. doi: 10.1021/jo020398c. [DOI] [PubMed] [Google Scholar]
- 787.Fujita M, Kim WH, Fujiwara K, Okuyama T. J Org Chem. 2005;70:480. doi: 10.1021/jo049218k. [DOI] [PubMed] [Google Scholar]
- 788.Hinkle RJ, Mikowski AM. ARKIVOC. 2003;vi:201. [Google Scholar]
- 789.Fujita M, Sakanishi Y, Nishii M, Okuyama T. J Org Chem. 2002;67:8138. doi: 10.1021/jo0203995. [DOI] [PubMed] [Google Scholar]
- 790.Slegt M, Gronheid R, Van der Vlugt D, Ochiai M, Okuyama T, Zuilhof H, Overkleeft HS, Lodder G. J Org Chem. 2006;71:2227. doi: 10.1021/jo0518957. [DOI] [PubMed] [Google Scholar]
- 791.Okuyama T, Lodder G. Adv Phys Org Chem. 2002;37:1. [Google Scholar]
- 792.Fujita M, Sakanishi Y, Kim WH, Okuyama T. Chem Lett. 2002:908. [Google Scholar]
- 793.Fujita M, Ihara K, Kim WH, Okuyama T. Bull Chem Soc Jpn. 2003;76:1849. [Google Scholar]
- 794.Yan J, Jin H, Chen Z. J Chem Res. 2007:233. [Google Scholar]
- 795.Zhang P-F, Chen Z-C. J Chem Res (S) 2002:388. [Google Scholar]
- 796.Guan T, Yoshida M, Hara S. J Org Chem. 2007;72:9617. doi: 10.1021/jo701784t. [DOI] [PubMed] [Google Scholar]
- 797.Hara S, Guan T, Yoshida M. Org Lett. 2006;8:2639. doi: 10.1021/ol0608707. [DOI] [PubMed] [Google Scholar]
- 798.Ochiai M, Shu T, Nagaoka T, Kitagawa Y. J Org Chem. 1997;62:2130. doi: 10.1021/jo962007y. [DOI] [PubMed] [Google Scholar]
- 799.Kang S-K, Yamaguchi T, Kim T-H, Ho P-S. J Org Chem. 1996;61:9082. [Google Scholar]
- 800.Huang X, Xu X-H. J Chem Soc, Perkin Trans 1. 1998:3321. [Google Scholar]
- 801.Kang S-K, Yoon S-K, Kim Y-M. Org Lett. 2001;3:2697. doi: 10.1021/ol0162825. [DOI] [PubMed] [Google Scholar]
- 802.Thielges S, Bisseret P, Eustache J. Org Lett. 2005;7:681. doi: 10.1021/ol047516y. [DOI] [PubMed] [Google Scholar]
- 803.Moriarty RM, Epa WR, Awasthi AK. J Am Chem Soc. 1991;113:6315. [Google Scholar]
- 804.Yoshida M, Hara S, Fukuhara T, Yoneda N. Tetrahedron Lett. 2000;41:3887. [Google Scholar]
- 805.Yoshida M, Yoshikawa S, Fukuhara T, Yoneda N, Hara S. Tetrahedron. 2001;57:7143. [Google Scholar]
- 806.Yoshida M, Komata A, Hara S. J Fluorine Chem. 2004;125:527. [Google Scholar]
- 807.Guan T, Takemura K, Senboku H, Yoshida M, Hara S. Tetrahedron Lett. 2007;49:76. [Google Scholar]
- 808.Ochiai M, Uemura K, Masaki Y. J Am Chem Soc. 1993;115:2528. [Google Scholar]
- 809.Ochiai M, Kunishima M, Tani S, Nagao Y. J Am Chem Soc. 1991;113:3135. [Google Scholar]
- 810.Feldman KS. In: Strategies and Tactics in Organic Synthesis. Harmata M, editor. Vol. 4 Elsevier; London: 2004. [Google Scholar]
- 811.Ochiai M, Kunishima M, Sumi K, Nagao Y, Fujita E, Arimoto M, Yamaguchi H. Tetrahedron Lett. 1985;26:4501. [Google Scholar]
- 812.Rebrovic L, Koser GF. J Org Chem. 1984;49:4700. [Google Scholar]
- 813.Koumbis AE, Kyzas CM, Savva A, Varvoglis A. Molecules. 2005;10:1340. doi: 10.3390/10101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Yoshida M, Nishimura N, Hara S. Chem Commun. 2002:1014. doi: 10.1039/b200659f. [DOI] [PubMed] [Google Scholar]
- 815.Stang PJ, Surber BW, Chen ZC, Roberts KA, Anderson AG. J Am Chem Soc. 1987;109:228. [Google Scholar]
- 816.Kitamura T, Lee CH, Taniguchi H, Matsumoto M, Sano Y. J Org Chem. 1994;59:8053. [Google Scholar]
- 817.Stang PJ, Williamson BL, Zhdankin VV. J Am Chem Soc. 1991;113:5870. [Google Scholar]
- 818.Feldman KS, Bruendl MM, Schildknegt K, Bohnstedt AC. J Org Chem. 1996;61:5440. [Google Scholar]
- 819.Feldman KS, Saunders JC, Wrobleski ML. J Org Chem. 2002;67:7096. doi: 10.1021/jo026287v. [DOI] [PubMed] [Google Scholar]
- 820.Feldman KS, Perkins AL, Masters KM. J Org Chem. 2004;69:7928. doi: 10.1021/jo0487911. [DOI] [PubMed] [Google Scholar]
- 821.Wardrop DJ, Fritz J. Org Lett. 2006;8:3659. doi: 10.1021/ol0609053. [DOI] [PubMed] [Google Scholar]
- 822.Bachi MD, Bar-Ner N, Crittell CM, Stang PJ, Williamson BL. J Org Chem. 1991;56:3912. [Google Scholar]
- 823.Stang PJ, Arif AM, Crittell CM. Angew Chem, Int Ed. 1990;29:287. [Google Scholar]
- 824.Ochiai M, Ito T, Takaoka Y, Masaki Y, Kunishima M, Tani S, Nagao Y. J Chem Soc, Chem Commun. 1990:118. [Google Scholar]
- 825.Stang PJ, Murch P. Synthesis. 1997:1378. [Google Scholar]
- 826.Zhang J-L, Chen Z-C. Synth Commun. 1997;27:3757. [Google Scholar]
- 827.Zhang J-L, Chen Z-C. Synth Commun. 1997;27:3881. [Google Scholar]
- 828.Zhang J-L, Chen Z-C. Synth Commun. 1998;28:175. [Google Scholar]
- 829.Kitamura T, Tashi N, Tsuda K, Chen H, Fujiwara Y. Heterocycles. 2000;52:303. [Google Scholar]
- 830.Kerwin SM, Nadipuram A. Synlett. 2004:1404. [Google Scholar]
- 831.Witulski B, Stengel T. Angew Chem, Int Ed. 1999;38:2426. [PubMed] [Google Scholar]
- 832.Hashmi ASK, Salathe R, Frey W. Synlett. 2007:1763. [Google Scholar]
- 833.Martinez-Esperon MF, Rodriguez D, Castedo L, Saa C. Org Lett. 2005;7:2213. doi: 10.1021/ol050609a. [DOI] [PubMed] [Google Scholar]
- 834.Bykowski D, McDonald R, Tykwinski RR. ARKIVOC. 2003;vi:21. [Google Scholar]
- 835.Canty AJ, Rodemann T, Skelton BW, White AH. Organometallics. 2006;25:3996. [Google Scholar]
- 836.Chaudhuri PD, Guo R, Malinakova HC. J Organomet Chem. 2008;693:567. [Google Scholar]
- 837.Canty AJ, Rodemann T. Inorg Chem Commun. 2003;6:1382. [Google Scholar]
- 838.Canty AJ, Watson RP, Karpiniec SS, Rodemann T, Gardiner MG, Jones RC. Organometallics. 2008;27:3203. [Google Scholar]
- 839.Kitamura T, Lee CH, Taniguchi Y, Fujiwara Y, Matsumoto M, Sano Y. J Am Chem Soc. 1997;119:619. [Google Scholar]
- 840.Yang D-Y, He J, Miao S. Synth Commun. 2003;33:2695. [Google Scholar]
- 841.Yu C-M, Kweon J-H, Ho P-S, Kang S-C, Lee GY. Synlett. 2005:2631. [Google Scholar]
- 842.Liu Z, Chen Z-C, Zheng Q-G. J Chem Res (S) 2003:715. [Google Scholar]
- 843.Liu Z, Chen Z-C, Zheng Q-G. J Heteterocycl Chem. 2003;40:909. [Google Scholar]
- 844.Liu Z, Chen Z-C, Zheng Q-G. Synth Commun. 2004;34:361. [Google Scholar]
- 845.Miyamoto K, Nishi Y, Ochiai M. Angew Chem, Int Ed. 2005;44:6896. doi: 10.1002/anie.200502438. [DOI] [PubMed] [Google Scholar]
- 846.Wipf P, Venkatraman S. J Org Chem. 1996;61:8004. doi: 10.1021/jo961681c. [DOI] [PubMed] [Google Scholar]
- 847.Gudriniece E, Neiland O, Vanags G. Zh Obshch Khim. 1957;27:2737. [Google Scholar]
- 848.Hatjiarapoglou L, Varvoglis A, Alcock NW, Pike GA. J Chem Soc, Perkin Trans 1. 1988:2839. [Google Scholar]
- 849.Mueller P, Fernandez D. Helv Chim Acta. 1995;78:947. [Google Scholar]
- 850.Hadjiarapoglou L, Spyroudis S, Varvoglis A. J Am Chem Soc. 1985;107:7178. [Google Scholar]
- 851.Alcock NW, Bozopoulos AP, Hatzigrigoriou E, Varvoglis A. Acta Crystallogr, Sect C: Cryst Struct Commun. 1990;C46:1300. [Google Scholar]
- 852.Moriarty RM, Prakash I, Prakash O, Freeman WA. J Am Chem Soc. 1984;106:6082. [Google Scholar]
- 853.Matveeva ED, Podrugina TA, Grishin YK, Tkachev VV, Zhdankin VV, Aldoshin SM, Zefirov NS. Russ J Org Chem. 2003;39:536. [Google Scholar]
- 854.Huang Z, Yu X, Huang X. J Org Chem. 2002;67:8261. doi: 10.1021/jo026077i. [DOI] [PubMed] [Google Scholar]
- 855.Yang R, Dai L, Chen C. J Chem Soc, Chem Commun. 1992:1487. [Google Scholar]
- 856.Zhu S-Z. Heteroatom Chem. 1994;5:9. [Google Scholar]
- 857.Ochiai M, Tada N, Okada T, Sota A, Miyamoto K. J Am Chem Soc. 2008;130:2118. doi: 10.1021/ja074624h. [DOI] [PubMed] [Google Scholar]
- 858.Ochiai M, Okada T, Tada N, Yoshimura A. Org Lett. 2008;10:1425. doi: 10.1021/ol800211x. [DOI] [PubMed] [Google Scholar]
- 859.Moriarty RM, Tyagi S, Ivanov D, Constantinescu M. J Am Chem Soc. 2008;130:7564. doi: 10.1021/ja802735f. [DOI] [PubMed] [Google Scholar]
- 860.Ochiai M, Kitagawa Y, Yamamoto S. J Am Chem Soc. 1997;119:11598. [Google Scholar]
- 861.Ochiai M, Tuchimoto Y, Higashiura N. Org Lett. 2004;6:1505. doi: 10.1021/ol0495669. [DOI] [PubMed] [Google Scholar]
- 862.Ochiai M, Nishitani J, Nishi Y. J Org Chem. 2002;67:4407. doi: 10.1021/jo0107711. [DOI] [PubMed] [Google Scholar]
- 863.Bonge HT, Hansen T. Synlett. 2007:55. [Google Scholar]
- 864.Bonge HT, Hansen T. Tetrahedron Lett. 2008;49:57. [Google Scholar]
- 865.Moreau B, Charette AB. J Am Chem Soc. 2005;127:18014. doi: 10.1021/ja056192l. [DOI] [PubMed] [Google Scholar]
- 866.Wurz RP, Charette AB. Org Lett. 2003;5:2327. doi: 10.1021/ol034672g. [DOI] [PubMed] [Google Scholar]
- 867.Telu S, Durmus S, Koser GF. Tetrahedron Lett. 2007;48:1863. [Google Scholar]
- 868.Gogonas EP, Nyxas I, Hadjiarapoglou LP. Synlett. 2004:2563. [Google Scholar]
- 869.Malamidou-Xenikaki E, Spyroudis S, Tsanakopoulou M. J Org Chem. 2003;68:5627. doi: 10.1021/jo0343679. [DOI] [PubMed] [Google Scholar]
- 870.Spagou K, Malamidou-Xenikaki E, Spyroudis S. Molecules. 2005;10:226. doi: 10.3390/10010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Koulouri S, Malamidou-Xenikaki E, Spyroudis S, Tsanakopoulou M. J Org Chem. 2005;70:8780. doi: 10.1021/jo051151t. [DOI] [PubMed] [Google Scholar]
- 872.Huang X-C, Liu Y-L, Liang Y, Pi S-F, Wang F, Li J-H. Org Lett. 2008;10:1525. doi: 10.1021/ol800051k. [DOI] [PubMed] [Google Scholar]
- 873.Gololobov YG, Golding IR, Galkina MA, Lokshin BV, Garbuzova IA, Petrovskii PV, Starikova ZA, Averkiev BB. Russ Chem Bull. 2006;55:883. [Google Scholar]
- 874.Bakalbassis EG, Spyroudis S, Tsipis CA. Eur J Org Chem. 2008:1783. [Google Scholar]
- 875.Zhdankin VV, Maydanovych O, Herschbach J, Bruno J, Matveeva ED, Zefirov NS. J Org Chem. 2003;68:1018. doi: 10.1021/jo026604y. [DOI] [PubMed] [Google Scholar]
- 876.Zhdankin VV, Maydanovych O, Herschbach J, Bruno J, Matveeva ED, Zefirov NS. Tetrahedron Lett. 2002;43:2359. [Google Scholar]
- 877.Huang Z-Z, Yu X-C, Huang X. Tetrahedron Lett. 2002;43:6823. [Google Scholar]
- 878.Matveeva ED, Podrugina TA, Grishin YK, Pavlova AS, Zefirov NS. Russ J Org Chem. 2007;43:201. [Google Scholar]
- 879.Deng G. J Chem Res (S) 2002:558. [Google Scholar]
- 880.Batsila C, Gogonas EP, Kostakis G, Hadjiarapoglou LP. Org Lett. 2003;5:1511. doi: 10.1021/ol0343008. [DOI] [PubMed] [Google Scholar]
- 881.Adam W, Gogonas EP, Hadjiarapoglou LP. Tetrahedron. 2003;59:7929. [Google Scholar]
- 882.Adam W, Gogonas EP, Hadjiarapoglou LP. Eur J Org Chem. 2003:1064. doi: 10.1021/jo035362e. [DOI] [PubMed] [Google Scholar]
- 883.Lee YR, Yoon SH, Seo Y, Kim BS. Synthesis. 2004:2787. [Google Scholar]
- 884.Batsila C, Kostakis G, Hadjiarapoglou LP. Tetrahedron Lett. 2002;43:5997. [Google Scholar]
- 885.Lee YR, Yoon SH. Synth Commun. 2006;36:1941. [Google Scholar]
- 886.Lee YR, Jung YU. J Chem Soc, Perkin Trans 1. 2002:1309. [Google Scholar]
- 887.Murphy GK, West FG. Org Lett. 2006;8:4359. doi: 10.1021/ol061772o. [DOI] [PubMed] [Google Scholar]
- 888.Adam W, Gogonas EP, Hadjiarapoglou LP. J Org Chem. 2003;68:9155. doi: 10.1021/jo035362e. [DOI] [PubMed] [Google Scholar]
- 889.Adam W, Gogonas EP, Hadjiarapoglou LP. Synlett. 2003:1165. doi: 10.1021/jo035362e. [DOI] [PubMed] [Google Scholar]
- 890.Adam W, Gogonas EP, Nyxas IA, Hadjiarapoglou LP. Synthesis. 2007:3211. [Google Scholar]
- 891.Adam W, Bosio SG, Gogonas EP, Hadjiarapoglou LP. Synthesis. 2002:2084. [Google Scholar]
- 892.Ghanem A, Lacrampe F, Schurig V. Helv Chim Acta. 2005;88:216. [Google Scholar]
- 893.Mueller P, Allenbach YF, Ferri M, Bernardinelli G. ARKIVOC. 2003;vii:80. [Google Scholar]
- 894.Muller P, Bolea C. Helv Chim Acta. 2002;85:483. [Google Scholar]
- 895.Zhu Q, Wu J, Fathi R, Yang Z. Org Lett. 2002;4:3333. doi: 10.1021/ol020159b. [DOI] [PubMed] [Google Scholar]
- 896.Yamada Y, Yamamoto T, Okawara M. Chem Lett. 1975:361. [Google Scholar]
- 897.Sodergren MJ, Alonso DA, Bedekar AV, Andersson PG. Tetrahedron Lett. 1997;38:6897. [Google Scholar]
- 898.Cicero RL, Zhao D, Protasiewicz JD. Inorg Chem. 1996;35:275. doi: 10.1021/ic951031b. [DOI] [PubMed] [Google Scholar]
- 899.Abramovitch RA, Bailey TD, Takaya T, Uma V. J Org Chem. 1974;39:340. [Google Scholar]
- 900.Mansuy D, Mahy JP, Dureault A, Bedi G, Battioni P. J Chem Soc, Chem Commun. 1984:1161. [Google Scholar]
- 901.Protasiewicz JD. Acta Crystallogr, Sect C: Cryst Struct Commun. 1996;C52:1570. [Google Scholar]
- 902.Li J, Liang J-L, Chan PWH, Che C-M. Tetrahedron Lett. 2004;45:2685. [Google Scholar]
- 903.Liang Y, Jiao L, Wang Y, Chen Y, Ma L, Xu J, Zhang S, Yu Z-X. Org Lett. 2006;8:5877. doi: 10.1021/ol062504t. [DOI] [PubMed] [Google Scholar]
- 904.Richardson RD, Desaize M, Wirth T. Chem Eur J. 2007;13:6745. doi: 10.1002/chem.200700306. [DOI] [PubMed] [Google Scholar]
- 905.Chang JWW, Chan PWH. Angew Chem, Int Ed. 2008;47:1138. [Google Scholar]
- 906.Avenier F, Goure E, Dubourdeaux P, Seneque O, Oddou J-L, Pecaut J, Chardon-Noblat S, Deronzier A, Latour J-M. Angew Chem, Int Ed. 2008;47:715. doi: 10.1002/anie.200703580. [DOI] [PubMed] [Google Scholar]
- 907.Li Z, Capretto DA, Rahaman RO, He C. J Am Chem Soc. 2007;129:12058. doi: 10.1021/ja0724137. [DOI] [PubMed] [Google Scholar]
- 908.Li Z, Capretto DA, Rahaman R, He C. Angew Chem, Int Ed. 2007;46:5184. doi: 10.1002/anie.200700760. [DOI] [PubMed] [Google Scholar]
- 909.Cui Y, He C. Angew Chem, Int Ed. 2004;43:4210. doi: 10.1002/anie.200454243. [DOI] [PubMed] [Google Scholar]
- 910.He L, Yu J, Zhang J, Yu X-Q. Org Lett. 2007;9:2277. doi: 10.1021/ol070537i. [DOI] [PubMed] [Google Scholar]
- 911.Fiori KW, Du Bois J. J Am Chem Soc. 2007;129:562. doi: 10.1021/ja0650450. [DOI] [PubMed] [Google Scholar]
- 912.Fructos MR, Trofimenko S, Diaz-Requejo MM, Perez PJ. J Am Chem Soc. 2006;128:11784. doi: 10.1021/ja0627850. [DOI] [PubMed] [Google Scholar]
- 913.Caselli A, Gallo E, Ragaini F, Oppezzo A, Cenini S. J Organomet Chem. 2005;690:2142. [Google Scholar]
- 914.He L, Chan PWH, Tsui W-M, Yu W-Y, Che C-M. Org Lett. 2004;6:2405. doi: 10.1021/ol049232j. [DOI] [PubMed] [Google Scholar]
- 915.Jensen MP, Mehn MP, Que L., Jr Angew Chem, Int Ed. 2003;42:4357. doi: 10.1002/anie.200351605. [DOI] [PubMed] [Google Scholar]
- 916.Kohmura Y, Katsuki T. Tetrahedron Lett. 2001;42:3339. [Google Scholar]
- 917.Evans DA, Bilodeau MT, Faul MM. J Am Chem Soc. 1994;116:2742. [Google Scholar]
- 918.Li Z, Quan RW, Jacobsen EN. J Am Chem Soc. 1995;117:5889. [Google Scholar]
- 919.Li Z, Conser KR, Jacobsen EN. J Am Chem Soc. 1993;115:5326. [Google Scholar]
- 920.Ma L, Du D-M, Xu J. Chirality. 2006;18:575. doi: 10.1002/chir.20282. [DOI] [PubMed] [Google Scholar]
- 921.Wang X, Ding K. Chem Eur J. 2006;12:4568. doi: 10.1002/chem.200501109. [DOI] [PubMed] [Google Scholar]
- 922.Ma L, Du D-M, Xu J. J Org Chem. 2005;70:10155. doi: 10.1021/jo051765y. [DOI] [PubMed] [Google Scholar]
- 923.Gullick J, Taylor S, Ryan D, McMorn P, Coogan M, Bethell D, Bulman Page PC, Hancock FE, King F, Hutchings GJ. Chem Commun. 2003:2808. doi: 10.1039/b309507j. [DOI] [PubMed] [Google Scholar]
- 924.Gillespie KM, Sanders CJ, O’Shaughnessy P, Westmoreland I, Thickitt CP, Scott P. J Org Chem. 2002;67:3450. doi: 10.1021/jo025515i. [DOI] [PubMed] [Google Scholar]
- 925.Anada M, Tanaka M, Washio T, Yamawaki M, Abe T, Hashimoto S. Org Lett. 2007;9:4559. doi: 10.1021/ol702019b. [DOI] [PubMed] [Google Scholar]
- 926.Tanaka M, Kurosaki Y, Washio T, Anada M, Hashimoto S. Tetrahedron Lett. 2007;48:8799. [Google Scholar]
- 927.Liang J-L, Yuan S-X, Chan PWH, Che C-M. Org Lett. 2002;4:4507. doi: 10.1021/ol0270475. [DOI] [PubMed] [Google Scholar]
- 928.Okamura H, Bolm C. Org Lett. 2004;6:1305. doi: 10.1021/ol049715n. [DOI] [PubMed] [Google Scholar]
- 929.Knapp S, Yu Y. Org Lett. 2007;9:1359. doi: 10.1021/ol0702472. [DOI] [PubMed] [Google Scholar]
- 930.Guthikonda K, Wehn PM, Caliando BJ, Du Bois J. Tetrahedron. 2006;62:11331. [Google Scholar]
- 931.Dick AR, Remy MS, Kampf JW, Sanford MS. Organometallics. 2007;26:1365. [Google Scholar]
- 932.Mancheno OG, Bolm C. Org Lett. 2006;8:2349. doi: 10.1021/ol060640s. [DOI] [PubMed] [Google Scholar]
- 933.Mancheno OG, Bolm C. Chem Eur J. 2007;13:6674. [Google Scholar]
- 934.Leca D, Song K, Amatore M, Fensterbank L, Lacote E, Malacria M. Chem Eur J. 2004;10:906. doi: 10.1002/chem.200305525. [DOI] [PubMed] [Google Scholar]
- 935.Archibald SJ, Boa AN, Pesa N. Chem Commun. 2003:1736. [Google Scholar]
- 936.Jiang Y, Zhou G-C, He G-L, He L, Li J-L, Zheng S-L. Synthesis. 2007:1459. [Google Scholar]
- 937.Jain SL, Sharma VB, Sain B. Tetrahedron Lett. 2003;44:4385. [Google Scholar]
- 938.Wirth T. Angew Chem, Int Ed. 2001;40:2812. doi: 10.1002/1521-3773(20010803)40:15<2812::aid-anie2812>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 939.Kraszkiewicz L, Skulski L. ARKIVOC. 2003;vi:120. [Google Scholar]
- 940.Koposov AY, Karimov RR, Pronin AA, Skrupskaya T, Yusubov MS, Zhdankin VV. J Org Chem. 2006;71:9912. doi: 10.1021/jo062073s. [DOI] [PubMed] [Google Scholar]
- 941.Alcock NW, Sawyer JF. J Chem Soc, Dalton Trans. 1980:115. [Google Scholar]
- 942.Koposov AY, Zhdankin VV. Synthesis. 2005:22. [Google Scholar]
- 943.Kuhakarn C, Kittigowittana K, Pohmakotr M, Reutrakul V. ARKIVOC. 2005;i:143. [Google Scholar]
- 944.Chung W-J, Kim D-K, Lee Y-S. Tetrahedron Lett. 2003;44:9251. [Google Scholar]
- 945.Kim D-K, Chung W-J, Lee Y-S. Synlett. 2005:279. [Google Scholar]
- 946.Lecarpentier P, Crosignani S, Linclau B. Molecular Diversity. 2005;9:341. doi: 10.1007/s11030-005-8105-2. [DOI] [PubMed] [Google Scholar]
- 947.Koposov AY, Litvinov DN, Zhdankin VV. Tetrahedron Lett. 2004;45:2719. [Google Scholar]
- 948.Zhdankin V, Goncharenko RN, Litvinov DN, Koposov AY. ARKIVOC. 2005;iv:8. [Google Scholar]
- 949.Ladziata U, Carlson J, Zhdankin VV. Tetrahedron Lett. 2006;47:6301. [Google Scholar]
- 950.Ladziata U, Willging J, Zhdankin VV. Org Lett. 2006;8:167. doi: 10.1021/ol052684r. [DOI] [PubMed] [Google Scholar]
- 951.Karimov RR, Kazhkenov Z-GM, Modjewski MJ, Peterson EM, Zhdankin VV. J Org Chem. 2007;72:8149. doi: 10.1021/jo7015746. [DOI] [PubMed] [Google Scholar]
- 952.Hartman C, Mayer V. Chem Ber. 1893;26:1727. [Google Scholar]
- 953.Gougoutas JZ. Cryst Struct Comm. 1981;10:489. [Google Scholar]
- 954.Katritzky AR, Savage GP, Palenik GJ, Qian K, Zhang Z, Durst HD. J Chem Soc, Perkin Trans 2. 1990:1657. [Google Scholar]
- 955.Boeckman RK, Shao P, Mullins JJ. Org Synth. 2000;77:141. [Google Scholar]
- 956.Frigerio M, Santagostino M, Sputore S. J Org Chem. 1999;64:4537. [Google Scholar]
- 957.Gallen MJ, Goumont R, Clark T, Terrier F, Williams CM. Angew Chem, Int Ed. 2006;45:2929. doi: 10.1002/anie.200504156. [DOI] [PubMed] [Google Scholar]
- 958.Waters T, Boulton J, Clark T, Gallen MJ, Williams CM, O’Hair RAJ. Org Biomol Chem. 2008;6:2530. doi: 10.1039/b803452d. [DOI] [PubMed] [Google Scholar]
- 959.Ozanne A, Pouysegu L, Depernet D, Francois B, Quideau S. Org Lett. 2003;5:2903. doi: 10.1021/ol0349965. [DOI] [PubMed] [Google Scholar]
- 960.Lebrasseur N, Gagnepain J, Ozanne-Beaudenon A, Leger J-M, Quideau S. J Org Chem. 2007;72:6280. doi: 10.1021/jo0708893. [DOI] [PubMed] [Google Scholar]
- 961.Gagnepain J, Castet F, Quideau S. Ang Chem Int Ed. 2007;46:1533. doi: 10.1002/anie.200604610. [DOI] [PubMed] [Google Scholar]
- 962.Ozanne-Beaudenon A, Quideau S. Tetrahedron Lett. 2006;47:5869. [Google Scholar]
- 963.Thottumkara AP, Vinod TK. Tetrahedron Lett. 2002;43:569. [Google Scholar]
- 964.Kommreddy A, Bowsher MS, Gunna MR, Botha K, Vinod TK. Tetrahedron Lett. 2008;49:4378. [Google Scholar]
- 965.Stickley SH, Martin JC. Tetrahedron Lett. 1995;36:9117. [Google Scholar]
- 966.Richardson RD, Zayed JM, Altermann S, Smith D, Wirth T. Angew Chem, Int Ed. 2007;46:6529. doi: 10.1002/anie.200702313. [DOI] [PubMed] [Google Scholar]
- 967.Moorthy JN, Singhal N, Senapati K. Tetrahedron Lett. 2008;49:80. [Google Scholar]
- 968.Kano N, Ohashi M, Hoshiba K, Kawashima T. Tetrahedron Lett. 2004;45:8173. [Google Scholar]
- 969.Muelbaier M, Giannis A. Angew Chem, Int Ed. 2001;40:4393. doi: 10.1002/1521-3773(20011203)40:23<4393::aid-anie4393>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 970.Sorg G, Mengei A, Jung G, Rademann J. Angew Chem, Int Ed. 2001;40:4395. doi: 10.1002/1521-3773(20011203)40:23<4395::aid-anie4395>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 971.Lei ZQ, Ma HC, Zhang Z, Yang YX. React Funct Polym. 2006;66:840. [Google Scholar]
- 972.Lei Z, Denecker C, Jegasothy S, Sherrington DC, Slater NKH, Sutherland AJ. Tetrahedron Lett. 2003;44:1635. [Google Scholar]
- 973.Bromberg L, Zhang H, Hatton TA. Chem Mater. 2008;20:2001. [Google Scholar]
- 974.Frigerio M, Santagostino M. Tetrahedron Lett. 1994;35:8019. [Google Scholar]
- 975.Paintner FF, Allmendinger L, Bauschke G. Synthesis. 2001:2113. [Google Scholar]
- 976.Martin C, Macintosh N, Lamb N, Fallis AG. Org Lett. 2001;3:1021. [PubMed] [Google Scholar]
- 977.Kirsch S, Bach T. Angew Chem, Int Ed. 2003;42:4685. doi: 10.1002/anie.200351455. [DOI] [PubMed] [Google Scholar]
- 978.Iwamoto O, Koshino H, Hashizume D, Nagasawa K. Angew Chem, Int Ed. 2007;46:8625. doi: 10.1002/anie.200703326. [DOI] [PubMed] [Google Scholar]
- 979.Nicolaou KC, Harrison ST. Angew Chem, Int Ed. 2006;45:3256. doi: 10.1002/anie.200601116. [DOI] [PubMed] [Google Scholar]
- 980.Venkatesan K, Srinivasan KV. Tetrahedron: Asymmetry. 2008;19:209. [Google Scholar]
- 981.Skouta R, Li C-J. Tetrahedron Lett. 2007;48:8343. [Google Scholar]
- 982.Kuboki A, Yamamoto T, Taira M, Arishige T, Ohira S. Tetrahedron Lett. 2007;48:771. [Google Scholar]
- 983.Hosokawa S, Kuroda S, Imamura K, Tatsuta K. Tetrahedron Lett. 2006;47:6183. [Google Scholar]
- 984.Ichikawa Y, Yamaoka T, Nakano K, Kotsuki H. Org Lett. 2007;9:2989. doi: 10.1021/ol0709735. [DOI] [PubMed] [Google Scholar]
- 985.Zhang J, Wang X, Wang W, Quan W, She X, Pan X. Tetrahedron. 2007;63:6990. [Google Scholar]
- 986.Vincent A, Prunet J. Synlett. 2006:2269. [Google Scholar]
- 987.Suzuki K, Takayama H. Org Lett. 2006;8:4605. doi: 10.1021/ol061908i. [DOI] [PubMed] [Google Scholar]
- 988.Mohapatra DK, Mondal D, Gonnade RG, Chorghade MS, Gurjar MK. Tetrahedron Lett. 2006;47:6031. [Google Scholar]
- 989.Kirkham JED, Lee V, Baldwin JE. Chem Commun. 2006:2863. doi: 10.1039/b607035c. [DOI] [PubMed] [Google Scholar]
- 990.Kaluza Z, Mostowicz D, Dolega G, Wojcik R. Tetrahedron. 2008;64:2321. [Google Scholar]
- 991.Chen JJ, Aduda V. Synth Commun. 2007;37:3493. [Google Scholar]
- 992.Molander GA, Petrillo DE. J Am Chem Soc. 2006;128:9634. doi: 10.1021/ja062974i. [DOI] [PubMed] [Google Scholar]
- 993.More JD, Finney NS. Org Lett. 2002;4:3001. doi: 10.1021/ol026427n. [DOI] [PubMed] [Google Scholar]
- 994.More JD, Finney NS. Synlett. 2003:1307. [Google Scholar]
- 995.Liu Z, Chen Z-C, Zheng Q-G. Org Lett. 2003;5:3321. doi: 10.1021/ol0351549. [DOI] [PubMed] [Google Scholar]
- 996.Kuhakarn C, Kittigowittana K, Ghabkham P, Pohmakotr M, Reutrakul V. Synth Commun. 2006;36:2887. [Google Scholar]
- 997.Moorthy JN, Singhal N, Senapati K. Org Biomol Chem. 2007;5:767. doi: 10.1039/b618135j. [DOI] [PubMed] [Google Scholar]
- 998.Corey EJ, Palani A. Tetrahedron Lett. 1995;36:3485. [Google Scholar]
- 999.Corey EJ, Palani A. Tetrahedron Lett. 1995;36:7945. [Google Scholar]
- 1000.De Munari S, Frigerio M, Santagostino M. J Org Chem. 1996;61:9272. [Google Scholar]
- 1001.Schulze A, Giannis A. Adv Synth Catal. 2004;346:252. [Google Scholar]
- 1002.Schulze A, Giannis A. Synthesis. 2006:257. [Google Scholar]
- 1003.Thottumkara AP, Bowsher MS, Vinod TK. Org Lett. 2005;7:2933. doi: 10.1021/ol050875o. [DOI] [PubMed] [Google Scholar]
- 1004.Page PCB, Appleby LF, Buckley BR, Allin SM, McKenzie MJ. Synlett. 2007:1565. [Google Scholar]
- 1005.Magdziak D, Rodriguez AA, Van De Water RW, Pettus TRR. Org Lett. 2002;4:285. doi: 10.1021/ol017068j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006.Kuboki A, Yamamoto T, Ohira S. Chem Lett. 2003;32:420. [Google Scholar]
- 1007.Huang Y, Zhang J, Pettus TRR. Org Lett. 2005;7:5841. doi: 10.1021/o1023749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1008.Nicolaou KC, Montagnon T, Baran PS, Zhong YL. J Am Chem Soc. 2002;124:2245. doi: 10.1021/ja012127+. [DOI] [PubMed] [Google Scholar]
- 1009.Nicolaou KC, Baran PS, Zhong Y-L. J Am Chem Soc. 2001;123:3183. doi: 10.1021/ja004218x. [DOI] [PubMed] [Google Scholar]
- 1010.Moorthy JN, Singhal N, Senapati K. Tetrahedron Lett. 2006;47:1757. [Google Scholar]
- 1011.Nicolaou KC, Mathison CJN, Montagnon T. J Am Chem Soc. 2004;126:5192. doi: 10.1021/ja0400382. [DOI] [PubMed] [Google Scholar]
- 1012.Ma HC, Jiang XZ. Synthesis. 2007:412. [Google Scholar]
- 1013.Chaudhari KH, Mahajan US, Bhalerao DS, Akamanchi KG. Synlett. 2007:2815. doi: 10.1021/jo0619074. [DOI] [PubMed] [Google Scholar]
- 1014.Bhalerao DS, Mahajan US, Chaudhari KH, Akamanchi KG. J Org Chem. 2007;72:662. doi: 10.1021/jo0619074. [DOI] [PubMed] [Google Scholar]
- 1015.Arote ND, Bhalerao DS, Akamanchi KG. Tetrahedron Lett. 2007;48:3651. [Google Scholar]
- 1016.Chiampanichayakul S, Pohmakotr M, Reutrakul V, Jaipetch T, Kuhakarn C. Synthesis. 2008:2045. [Google Scholar]
- 1017.Anwar HF, Hansen TV. Tetrahedron Lett. 2008;49:4443. [Google Scholar]
- 1018.Reddy MS, Narender M, Mahesh A, Nageswar YVD, Rao KR. Synth Commun. 2006;36:3771. [Google Scholar]
- 1019.Yadav JS, Reddy BVS, Reddy CS, Krishna AD. Synthesis. 2007:693. [Google Scholar]
- 1020.Yadav JS, Reddy BVS, Reddy CS, Krishna AD. Tetrahedron Lett. 2007;48:2029. [Google Scholar]
- 1021.Yadav JS, Reddy BVS, Basak AK, Baishya G, Narsaiah AV. Synthesis. 2006:451. [Google Scholar]
- 1022.Kirsch SF. J Org Chem. 2005;70:10210. doi: 10.1021/jo051898j. [DOI] [PubMed] [Google Scholar]
- 1023.Crone B, Kirsch SF. Chem Commun. 2006:764. doi: 10.1039/b515838a. [DOI] [PubMed] [Google Scholar]
- 1024.Martinez-Bescos P, Cagide-Fagin F, Roa LF, Ortiz-Lara JC, Kierus K, Ozores-Viturro L, Fernandez-Gonzalez M, Alonso R. J Org Chem. 2008;73:3745. doi: 10.1021/jo702731b. [DOI] [PubMed] [Google Scholar]
- 1025.Heravi MM, Bakhtiari K, Tehrani MH, Javadi NM, Oskooie HA. ARKIVOC. 2006;xvi:16. [Google Scholar]
- 1026.Nicolaou KC, Baran PS, Zhong YL, Barluenga S, Hunt KW, Kranich R, Vega JA. J Am Chem Soc. 2002;124:2233. doi: 10.1021/ja012126h. [DOI] [PubMed] [Google Scholar]
- 1027.Janza B, Studer A. J Org Chem. 2005;70:6991. doi: 10.1021/jo0509399. [DOI] [PubMed] [Google Scholar]
- 1028.Das B, Holla H, Mahender G, Banerjee J, Reddy MR. Tetrahedron Lett. 2004;45:7347. [Google Scholar]
- 1029.Yadav JS, Reddy BVS, Singh AP, Basak AK. Tetrahedron Lett. 2007;48:4169. [Google Scholar]
- 1030.Ngouansavanh T, Zhu J. Angew Chem, Int Ed. 2006;45:3495. doi: 10.1002/anie.200600588. [DOI] [PubMed] [Google Scholar]
- 1031.Ngouansavanh T, Zhu J. Angew Chem, Int Ed. 2007;46:5775. doi: 10.1002/anie.200701603. [DOI] [PubMed] [Google Scholar]
- 1032.Fontaine P, Chiaroni A, Masson G, Zhu J. Org Lett. 2008;10:1509. doi: 10.1021/ol800199b. [DOI] [PubMed] [Google Scholar]
- 1033.Nicolaou KC, Zhong Y-L, Baran PS. Angew Chem, Int Ed. 2000;39:622. [PubMed] [Google Scholar]
- 1034.Nicolaou KC, Baran PS, Kranich R, Zhong Y-L, Sugita K, Zou N. Angew Chem, Int Ed. 2001;40:202. doi: 10.1002/1521-3773(20010105)40:1<202::AID-ANIE202>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 1035.Nicolaou KC, Baran PS, Zhong Y-L, Vega JA. Angew Chem, Int Ed. 2000;39:2525. [PubMed] [Google Scholar]
- 1036.Dess DB, Martin JC. J Org Chem. 1983;48:4155. [Google Scholar]
- 1037.Ireland RE, Liu L. J Org Chem. 1993;58:2899. [Google Scholar]
- 1038.Myers AG, Zhong B, Movassaghi M, Kung DW, Lanman BA, Kwon S. Tetrahedron Lett. 2000;41:1359. doi: 10.1021/ol006427s. [DOI] [PubMed] [Google Scholar]
- 1039.Meyer SD, Schreiber SL. J Org Chem. 1994;59:7549. [Google Scholar]
- 1040.Deng G, Xu B, Liu C. Tetrahedron. 2005;61:5818. [Google Scholar]
- 1041.Candela Lena JI, Martin Hernando JI, Rico Ferreira MdR, Altinel E, Arseniyadis S. Synlett. 2001:597. [Google Scholar]
- 1042.Thongsornkleeb C, Danheiser RL. J Org Chem. 2005;70:2364. doi: 10.1021/jo047869a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Li P, Majireck MM, Korboukh I, Weinreb SM. Tetrahedron Lett. 2008;49:3162. doi: 10.1016/j.tetlet.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1044.Sharma PK, Kolchinski A, Shea HA, Nair JJ, Gou Y, Romanczyk LJ, Jr, Schmitz HH. Org Process Res Dev. 2007;11:422. [Google Scholar]
- 1045.Trost BM, O’Boyle BM. Org Lett. 2008;10:1369. doi: 10.1021/ol800127a. [DOI] [PubMed] [Google Scholar]
- 1046.Nicolaou KC, Tang Y, Wang J. Chem Commun. 2007:1922. doi: 10.1039/b704589a. [DOI] [PubMed] [Google Scholar]
- 1047.England DB, Padwa A. J Org Chem. 2008;73:2792. doi: 10.1021/jo8001003. [DOI] [PubMed] [Google Scholar]
- 1048.Zhang W, Wicks MN, Burn PL. Org Biomol Chem. 2008;6:879. doi: 10.1039/b718542a. [DOI] [PubMed] [Google Scholar]
- 1049.Struble JR, Kaeobamrung J, Bode JW. Org Lett. 2008;10:957. doi: 10.1021/ol800006m. [DOI] [PubMed] [Google Scholar]
- 1050.Nourry A, Legoupy S, Huet F. Tetrahedron. 2008;64:2241. [Google Scholar]
- 1051.Schweizer E, Gaich T, Brecker L, Mulzer J. Synthesis. 2007:3807. [Google Scholar]
- 1052.Migita A, Shichijo Y, Oguri H, Watanabe M, Tokiwano T, Oikawa H. Tetrahedron Lett. 2008;49:1021. [Google Scholar]
- 1053.Rein C, Demel P, Outten RA, Netscher T, Breit B. Angew Chem, Int Ed. 2007;46:8670. doi: 10.1002/anie.200703268. [DOI] [PubMed] [Google Scholar]
- 1054.Kozaka T, Miyakoshi N, Mukai C. J Org Chem. 2007;72:10147. doi: 10.1021/jo702136b. [DOI] [PubMed] [Google Scholar]
- 1055.Pouplin T, Tolon B, Nuhant P, Delpech B, Marazano C. Eur J Org Chem. 2007:5117. [Google Scholar]
- 1056.Trost BM, Waser J, Meyer A. J Am Chem Soc. 2007;129:14556. doi: 10.1021/ja076165q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057.Veitch GE, Beckmann E, Burke BJ, Boyer A, Ayats C, Ley SV. Angew Chem, Int Ed. 2007;46:7633. doi: 10.1002/anie.200703028. [DOI] [PubMed] [Google Scholar]
- 1058.Huang J, Wu C, Wulff WD. J Am Chem Soc. 2007;129:13366. doi: 10.1021/ja074275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Kotoku N, Narumi F, Kato T, Yamaguchi M, Kobayashi M. Tetrahedron Lett. 2007;48:7147. [Google Scholar]
- 1060.Krishna PR, Sreeshailam A. Tetrahedron Lett. 2007;48:6924. [Google Scholar]
- 1061.Scheerer JR, Lawrence JF, Wang GC, Evans DA. J Am Chem Soc. 2007;129:8968. doi: 10.1021/ja073590a. [DOI] [PubMed] [Google Scholar]
- 1062.Jung W-H, Harrison C, Shin Y, Fournier J-H, Balachandran R, Raccor BS, Sikorski RP, Vogt A, Curran DP, Day BW. J Med Chem. 2007;50:2951. doi: 10.1021/jm061385k. [DOI] [PubMed] [Google Scholar]
- 1063.Ahmad NM, Rodeschini V, Simpkins NS, Ward SE, Blake AJ. J Org Chem. 2007;72:4803. doi: 10.1021/jo070388h. [DOI] [PubMed] [Google Scholar]
- 1064.Wang H, Shuhler BJ, Xian M. J Org Chem. 2007;72:4280. doi: 10.1021/jo070346t. [DOI] [PubMed] [Google Scholar]
- 1065.Liu Q, Rovis T. Org Process Res Dev. 2007;11:598. doi: 10.1021/op600278f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1066.Neumann J, Weingarten S, Thiem J. Eur J Org Chem. 2007:1130. [Google Scholar]
- 1067.Takayama H, Kato K, Kimura M, Akita H. Heterocycles. 2007;71:75. [Google Scholar]
- 1068.Bjerre J, Fenger TH, Marinescu LG, Bols M. Eur J Org Chem. 2007:704. [Google Scholar]
- 1069.Chen J, Chen X, Willot M, Zhu J. Angew Chem, Int Ed. 2006;45:8028. doi: 10.1002/anie.200603179. [DOI] [PubMed] [Google Scholar]
- 1070.Watanabe H, Takano M, Umino A, Ito T, Ishikawa H, Nakada M. Org Lett. 2007;9:359. doi: 10.1021/ol0628816. [DOI] [PubMed] [Google Scholar]
- 1071.de Vicente J, Huckins JR, Rychnovsky SD. Angew Chem, Int Ed. 2006;45:7258. doi: 10.1002/anie.200602742. [DOI] [PubMed] [Google Scholar]
- 1072.Shah U, Chackalamannil S, Ganguly AK, Chelliah M, Kolotuchin S, Buevich A, McPhail A. J Am Chem Soc. 2006;128:12654. doi: 10.1021/ja065198n. [DOI] [PubMed] [Google Scholar]
- 1073.Geng Z, Chen B, Chiu P. Angew Chem, Int Ed. 2006;45:6197. doi: 10.1002/anie.200602056. [DOI] [PubMed] [Google Scholar]
- 1074.Garg NK, Hiebert S, Overman LE. Angew Chem, Int Ed. 2006;45:2912. doi: 10.1002/anie.200600417. [DOI] [PubMed] [Google Scholar]
- 1075.Nicolaou KC, Zhong YL, Baran PS, Jung J, Choi HS, Yoon WH. J Am Chem Soc. 2002;124:2202. doi: 10.1021/ja0120126. [DOI] [PubMed] [Google Scholar]
- 1076.Nicolaou KC, Jung J, Yoon WH, Fong KC, Choi HS, He Y, Zhong YL, Baran PS. J Am Chem Soc. 2002;124:2183. doi: 10.1021/ja012010l. [DOI] [PubMed] [Google Scholar]
- 1077.Nicolaou KC, Baran PS, Zhong YL, Fong KC, Choi HS. J Am Chem Soc. 2002;124:2190. doi: 10.1021/ja012011d. [DOI] [PubMed] [Google Scholar]
- 1078.Nicolaou KC, Baran PS, Zhong YL, Sugita K. J Am Chem Soc. 2002;124:2212. doi: 10.1021/ja012124x. [DOI] [PubMed] [Google Scholar]
- 1079.Karade NN, Gampawar SV, Kondre JM, Shinde SV. ARKIVOC. 2008;xii:9. [Google Scholar]
- 1080.Yadav JS, Reddy BVS, Singh AP, Basak AK. Synthesis. 2008:469. [Google Scholar]
- 1081.Yadav JS, Reddy BVS, Mandal SS, Basak AK, Madavi C, Kunwar AC. Synlett. 2008:1175. [Google Scholar]
- 1082.Ma HC, Jiang XZ. Synlett. 2007:1679. [Google Scholar]
- 1083.Salgaonkar PD, Shukla VG, Akamanchi KG. Synth Commun. 2007;37:275. [Google Scholar]
- 1084.Telvekar VN, Arote ND, Herlekar OP. Synlett. 2005:2495. [Google Scholar]
- 1085.Mahajan US, Akamanchi KG. Synlett. 2008:987. [Google Scholar]
- 1086.Karade NN, Tiwari GB, Shinde SV, Gampawar SV, Kondre JM. Tetrahedron Lett. 2008;49:3441. [Google Scholar]
- 1087.Bandgar SB, Bandgar BP, Korbad BL, Sawant SS. Tetrahedron Lett. 2007;48:1287. [Google Scholar]
- 1088.Nicolaou KC, Mathison CJN. Angew Chem, Int Ed. 2005;44:5992. doi: 10.1002/anie.200501853. [DOI] [PubMed] [Google Scholar]