Abstract
Studies were performed to induce cross-clade neutralizing antibodies (Abs) by testing various combinations of prime and boost constructs that focus the immune response on structurally conserved epitopes in the V3 loop of HIV-1 gp120. Rabbits were immunized with gp120 DNA containing a V3 loop characterized by the GPGR motif at its tip, and/or with gp120 DNA with a V3 loop carrying the GPGQ motif. Priming was followed by boosts with a V3-fusion proteins (V3-FPs) carrying the V3 sequence from a subtype B virus (GPGR motif), and/or with V3 sequences from subtypes A and C (GPGQ motif). The broadest and most consistent neutralizing responses were generated when using a clade C gp120 DNA prime and with the V3B-FP boost. Immune sera displayed neutralizing activity in three assays against pseudoviruses and primary isolates from subtypes A, AG, B, C, and D. Polyclonal Abs in the immune rabbit sera neutralized viruses that were not neutralized by pools of human anti-V3 monoclonal Abs. Greater than 80% of the neutralizing Abs were specific for V3, showing that the immune response could be focused on a neutralizing epitope and that vaccine-induced anti-V3 Abs have cross-clade neutralizing activity.
Keywords: HIV-1, Vaccine, Neutralizing antibodies, V3 loop, gp120, Pseudovirus, Immunization
Introduction
Although cell-mediated immunity (CMI) is crucial for control of established viral infections, neutralizing antibodies (Abs) are essential for reducing the infectivity of a virus inoculum and for neutralizing virions released during early rounds of replication (Plotkin, 2008; Robbins, Schneerson, and Szu, 1995). Humoral responses may also facilitate the development of effector and memory T cells which are needed to eliminate virus-infected cells and control the spread of infection (Haigwood et al., 1996; Yamamoto et al., 2007).
To protect against viral infections, vaccines have been developed using a multitude of methods: live attenuated viruses are used in vaccines against measles, mumps, rubella, polio, and influenza; inactivated viruses are also being used to immunize against polio and influenza. Currently, replicating and non-replicating viral vectors are being developed as the basis of various human vaccines, but none has yet has been licensed for use in humans. Other vaccine constructs include virus-like particles (VLPs), which are the basis of the vaccine against human papilloma virus, viral proteins, which are used in the hepatitis B vaccine, or DNA, which, by itself, has not yet proven effective as a vaccine construct (von Bubnoff, 2008). Several of these types of vaccine constructs have been tested in animals and humans as the basis of an HIV-1 vaccine. To date, none of these approaches has proven successful.
Because of the potential risk of infection with live attenuated or inactivated HIV, these are not currently being actively developed for human use, however, replicating and non-replicating viral vectors are being studied; most have been used in attempts to induce CMI and do not prevent infection or induce broadly neutralizing Abs in humans (Cox et al., 2008; Hanke et al., 2007). The use of VLPs, proteins, and DNA--the safest of the various modalities--is also being explored in attempts to induce CMI and/or the critical but elusive broadly neutralizing Ab response. To date, enveloped HIV-VLPs have induced anti-HIV neutralizing Abs to only a limited number of viruses (Buonaguro et al., 2007; Buonaguro et al., 2002; Crooks et al., 2007). HIV envelope glycoproteins, the only modality which has been tested in Phase III clinical trials, gives little CMI, inadequate neutralizing Ab responses, and have failed to protect (Gilbert et al., 2005). DNA, by itself, also appears to be inadequate in terms of induction of neutralizing Abs (Graham et al., 2006; Mulligan et al., 2006).
Various combinations of these different types of vaccine constructs have been used in prime/boost immunization protocols, e.g., recombinant canary pox + gp120 (Graham et al., 1998), DNA + recombinant modified vaccinia Ankara (Mulligan et al., 2006), and DNA + recombinant adenovirus vectors (Mascola et al., 2005). One of the most successful of the prime/boost protocols has been the use of DNA as a prime followed by protein as a boost. This regimen has proven to be more effective than the use of either DNA or protein immunogens alone and has been shown to induce both HIV-specific multifunctional T cell responses and/or Abs capable of neutralizing primary isolates (Bansal et al., 2008; Barnett et al., 1997; Beddows et al., 2005; Law et al., 2007; Lu et al., 1998; Richmond et al., 1998; Vaine et al., 2008; Zolla-Pazner et al., 2008). In these latter studies, the DNA and protein generally were derived from one or several subtype B strains or from multiple subtypes, and the neutralizing activity was narrow or capable of neutralizing isolates from various HIV subtypes only at modest titers.
In an attempt to induce anti-HIV neutralizing Abs of broader reactivity, we recently used the DNA prime/protein boost approach to focus the immune response on the V3 loop, a neutralizing epitope in gp120. The V3 loop was chosen to target from among the several known neutralizing envelope epitopes because, while exhibiting extreme sequence variation, it is a structurally conserved region of the virus envelope (Cardozo et al., 2007; Rosen et al., 2006; Sharon et al., 2003) and is required for interaction with the chemokine receptors CCR5 and CXCR4 which serve as coreceptors for HIV (Hill et al., 1997; Rizzuto et al., 1998; Trkola et al., 1996; Wang et al., 1999). Moreover, the V3 region of gp120 is known to induce Abs in humans that display broad neutralizing activity against strains within and between various clades (Binley et al., 2004; Gorny et al., 2004; Haynes et al., 2006; Krachmarov et al., 2005; Pantophlet et al., 2007). To target Abs to V3, we immunized rabbits with gp120 DNA from one or more HIV subtypes and boosted with recombinant fusion proteins bearing V3 loops of subtypes A, B and/or C (V3-FPs). Initial studies demonstrated that immune sera neutralized three of four primary isolates from subtypes B, CRF02_AG, and CRF011_cpx, and neutralized V3 chimeric pseudoviruses (psVs) carrying the consensus V3 sequences from subtypes A1, AG, B, CRF01_AE, and F (Zolla-Pazner et al., 2008). The study described below was undertaken to determine the extent of neutralizing activity against a much larger panel of primary isolates and psVs and to establish if different combinations of gp120 DNA priming constructs and different V3 sequences inserted into V3-FPs could induce broader neutralizing Ab responses.
Results
Immunogens and immunization protocols
In order to focus the immune response on the V3 region of gp120, rabbits were first immunized with gp120 DNA in order to prime memory T and B cells for V3 and other epitopes in this envelope glycoprotein. Animals were primed three times with codon-optimized gp120 DNA derived from subtype A or subtype C HIV env genes (Table 1). These genes were derived from primary isolate CA1 (an R5-tropic strain of CRF011_cpx carrying a subtype A Env) and from primary isolate 92BR025.9 (an R5-tropic strain of subtype C). The former was chosen because it is immunologically representative of a cluster of unrelated primary isolates from several strains (Nyambi et al., 2000) and carries a V3 loop which is characterized by the GPGR motif at the tip of the loop. The env gene of 92BR025.9 was chosen as a representative of subtype C and carries the GPGQ motif at the tip of the V3 loop (see sequences in footnote, Table 1).
Table 1.
Immunization Regimen*
| Immunizing Regimen | DNA prime† at weeks 0, 2, 4 | Protein boost† at weeks 10 & 14 |
|---|---|---|
| -/ABC | - - | V3A-, V3B-, & V3C-FP |
| AR/ABC | gp120/Clade A (GPGR) | V3A-, V3B-, & V3C-FP |
| CQ/ABC | gp120/Clade C (GPGQ) | V3A-, V3B- & V3C-FP |
| AR + CQ/ABC | gp120/Clade A (GPGR) & gp120/Clade C (GPGQ) | V3A-, V3B- & V3C-FP |
|
| ||
| CQ/A | gp120/Clade C (GPGQ) | V3A-FP |
| CQ/B | gp120/Clade C (GPGQ) | V3B-FP |
| CQ/C | gp120/Clade C (GPGQ) | V3C-FP |
| CQ/AC | gp120/Clade C (GPGQ) | V3A- & V3C-FP |
Two immunization experiments were performed. The groups shown above and below the dotted line were included in each of the experiments, respectively.
V3 sequences contained in priming and boosting constructs are shown below. V3-FPs used for protein boosts were based on various V3 sequences fused to gp70 of MuLV. Variations in sequence from relevant consensus sequences are underlined, and the variation at the tip of the loop, position 18 (R/Q), is bolded.
| CA1 clade A1 env gp120 DNA prime (AR): | CTRPNNNTRKGIHIGPGRAIYATGDIIGDIRQAHC |
| 92BR025.9 clade C env gp120 DNA prime (CQ): | CTRPNNNTRKSIRIGPGQAFYATGEIIGDIRQAHC |
| V3A-FP derived from clade A strain 92UG037.08: | CTRPNNNTRKSVRIGPGQTFYATGDIIGDIRQAHC |
| V3B–FP derived from clade B strain JR-CSF: | CTRPSNNTRKSIHIGPGRAFYTTGEIIGDIRQAHC |
| V3C-FP derived from clade C strain 93IN904: | CTRPNNNTRKSIRIGPGQTFYATGDIIGDIRQAHC |
In order to specifically stimulate Abs to V3, rabbits were boosted twice with one or a combination of V3-FPs in which the V3 scaffold was a truncated form of Murine Leukemia Virus (MuLV) gp70; this triggers the proliferation and differentiation of the previously induced V3-specific memory B cells generated by the prime. The V3-FPs were constructed using a truncated form of MuLV gp70 with one of three V3 loops at its C-terminus as per previous work that had shown that such molecules present the V3 epitope in its immunologically correct conformation (Kayman et al., 1994). The three V3-FPs contained a V3 region from either a subtype A virus (V3A-FP), a subtype B virus (V3B-FP), or a subtype C virus (V3C-FP) (see V3 sequences in footnote, Table 1).
In the first set of experiments described here, the prime was varied and the boost was held constant. Three rabbits were immunized in each of four groups. Each group received a different priming immunization: either an empty vector, gp120 subtype A DNA bearing the GPGR V3 motif (AR), gp120 subtype C DNA bearing the GPGQ V3 motif (CQ), or a combination of AR and CQ DNAs. All animals in these four groups received the same multivalent boost consisting of V3A-FP, V3B-FP, and V3C-FP (see Table 1). Initial data derived using sera from these animals were previously published (Zolla-Pazner et al., 2008).
The next experiment consisted of an immunization regimen in which the prime was held constant and the boost varied. For this, five rabbits were immunized per group; each of the four groups was primed with the gp120 subtype C DNA bearing the GPGQ V3 motif (CQ). Boosting of each of the four groups consisted of administration of V3A-FP, V3B-FP, or V3C-FP, or a combination of V3A-FP and V3C-FP (Table 1).
ELISA results
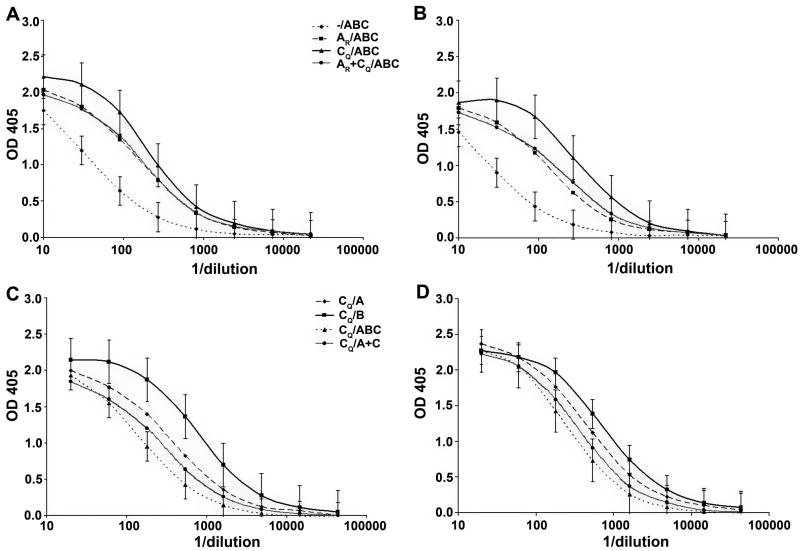
To test the ability of the different prime and boost regimens to induce anti-V3 Abs, the sera of all rabbits were tested for reactivity with a fusion protein consisting of the consensus sequences of the clade B or clade C V3 loop fused to the Fc fragment of rabbit Ig (V3-rFc) (Davis et al., 2008). The only common epitope between V3-rFc, the prime, and the boosts, is the V3 portion, insuring that the only Abs detected in this ELISA are specific for V3. The results are shown in Figure 1. In the top panels, we confirm previous data showing that the gp120 DNA prime significantly enhances the immune response to V3 epitopes (Wang et al., 2005; Zolla-Pazner et al., 2008). The data shown in the upper panels suggest that the CQ prime is stronger than either the AR prime or the AR + CQ prime, but the differences are not statistically significant between these groups. In experiments where the CQ prime was held constant. and different V3-FPs were used for boosts (lower panels), the V3B-FP induces the strongest and most consistent response.
Figure 1.
Titration of V3 binding Abs in the sera of immunized rabbits. Rabbit sera were tested in ELISA for reactivity against V3B-rFc (A and C) or V3C-rFc (B and D). The curves are based on the average OD values (+/- SD) at each dilution from assays run in duplicate with sera from individual rabbits in each group. The immunization regimen for each group is defined in Table 1. Data from animals receiving different primes and the triple protein boost of V3A- V3B- and V3C-gp70 are shown in panels A and B; data from animals receiving the same prime (CQ) and different boosts are shown in panels C and D.
Neutralizing activity in immune rabbit sera against V3 chimeric pseudoviruses
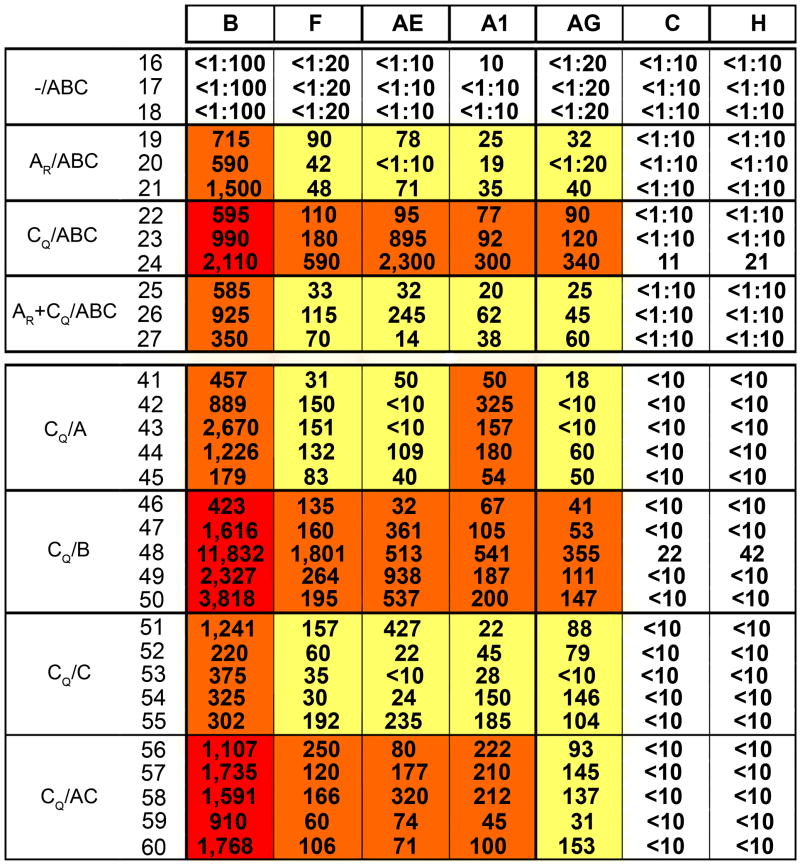
To assess the range of V3 diversity recognized by anti-V3 Abs induced in the immunized rabbits, the neutralizing activity of the immune sera was first tested against a panel of V3 chimeric psVs which were constructed using the env gene of SF162, a neutralization-sensitive virus from subtype B in which the V3 loop is highly accessible to Abs (Krachmarov et al., 2006). In these V3 chimeric psVs, the V3 loop of SF162 was replaced with the consensus V3 sequences from subtypes A1, B, C, F, H, CRF01_AE (E), and CRF02_AG (AG) (see footnote, Figure 2). Given the neutralization sensitivity of psVs carrying the SF162 Env, the 90% geometric mean neutralizing titers (GMT90) were assessed. The data confirm previous studies (Wang et al., 2005; Zolla-Pazner et al., 2008) showing that without a gp120 DNA prime, the V3-FPs do not induce a strong or broad neutralizing Ab response (see group -/ABC, Figure 2). All other groups which received either the AR and/or CQ DNA primes and protein boosts mounted immune responses that include Abs capable of recognizing and mediating 90% neutralization of psVs carrying the V3 loops of subtypes A1, AG, B, F and E (Figure 2). The groups that were primed with AR or AR + CQ had weak responses to non-B V3 chimeric psVs. The strongest responses to B and non-B V3 chimeric psVs were induced by groups primed with CQ and boosted with V3B-FP (CQ/B), V3A- and V3C-FPs (CQ/AC), or with all three V3-FPs (CQ/ABC). Although 90% neutralization was not achieved against V3 chimeric psVs carrying the consensus V3 sequences of subtypes C or H, the same groups that gave the strongest responses to other psVs (CQ/B, CQ/AC and CQ/ABC) all achieved 50% neutralization of V3C and V3H psVs (GMT50 values of 1:76-88; GMT50 values of 1:20-86, respectively). Interestingly, the group that received the AR + CQ prime displayed a generally weaker response than any of the groups that received the CQ prime alone. This may be because only 18 ug of each DNA prime per dose was administered to the AR + CQ group whereas animals receiving only the CQ prime received 36 ug per dose. Thus, using the gp120 DNA prime/V3-FP boost regimen, Abs were induced that are capable of recognizing the V3 loops from multiple viral subtypes, and the CQ prime gave the broadest and strongest responses.
Figure 2.
Reciprocal 90% neutrlizing titers (NT90) of immune rabbit sera vs. V3 chimeric pseudoviruses. Values represent NT90s for serum from each individual rabbit vs. each V3 chimeric psV, while colored boxes are coded to show the 90% geometric mean titer (GMT90) for each rabbit group vs. each V3 chimeric psV: red boxes denote GMT90 >1:1000; orange boxes, titers 1:100 – 999; yellow boxes, titers 1:10 - 99, and white boxes, no neutralizing activity. Columns show neutralizing titers against V3 chimeric SF162 pseudoviruses carrying the following consensus sequences of subtypes B, F, AE, A1, AG, C and H:
| B | C T R P N N N T R K S I H I G P G R A F Y T T G E I I G D I R Q A H C |
| F | - - - - - - - - - - - - H - - - - Q - - - A - - E - - - - - - K - - - |
| AE | - - - - S - - - - T - - T - - - - Q V - - R - - D - - - - - - K - Y - |
| A1 | - - - - - - - - - - - - R - - - - Q - - - A - - D - - - - - - - - - - |
| AG | - - - - - - - - - - - V R - - - - Q T - - A - - D - - - - - - - - - - |
| C | - - - - - - - - - - - - R - - - - Q T - - A - - D - - - - - - - - - - |
| H | - - - - - - - - - - - - H L - - - Q - - - A - - D - - - - - - - - - - |
The V3 chimeric psV carrying the consensus sequence of V3 from subtype B was the most sensitive to neutralization regardless of whether the prime or the boost contained a V3 with the GPGR motif of subtype B (Figure 2). This may imply that: a) the subtype B V3 loop bears structural or physicochemical characteristics that allow it to best adapt to the combining site of the induced V3 Abs, b) the backbone of SF162 accommodates a clade B V3 loop better than the V3 loops of other subtypes, or c) other unidentified factors enhance the relative neutralization sensitivity of the V3B sequence. In contrast, the clade C-bearing chimeric psV162 is quite resistant, a phenomenon that may be related to the relative inflexibility of the clade C V3 region (T. Cardozo, personal communication).
Neutralizing activity in immune rabbit sera against primary isolates
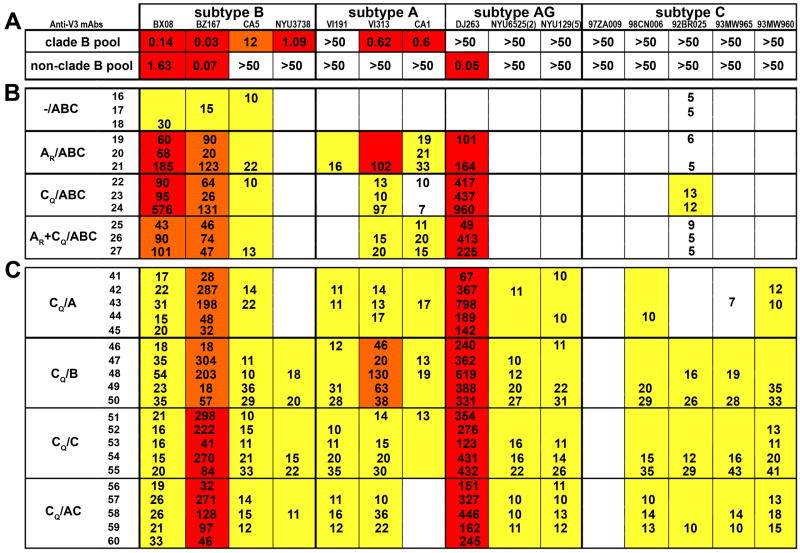
It is well documented in studies of HIV and other viruses that the potency of neutralizing activity detected in immune sera depends on the assay and the conditions used (Baldinotti et al., 1994; Dimmock, 1993; Polonis et al., 2008; Vaine et al., 2008; Zolla-Pazner, 1996). Therefore, additional neutralizing assays were used to interrogate the activity of the rabbit immune sera. The second assay used assessed the neutralizing activity of the rabbit immune sera against primary isolates rather than psVs. For this, a panel of 15 primary isolates was selected from viruses of subtypes A, AG, B and C in order to represent (a) a spectrum of viral subtypes, and (b) a range of neutralization sensitivities based on each virus's sensitivity to neutralization by a pool of anti-V3 monoclonal Abs (mAbs) derived from subjects infected with subtype B or non-B viruses (see Methods). Thus, the 15 primary isolates were tested for their ability to be neutralized by mAbs pools at concentrations ≤50 ug/ml. As shown in Figure 3A, two of these viruses (from subtype B) were sensitive to both clade B-derived and non-clade B-derived pools of anti-V3 mAbs with ND50 values of <2 ug mAb/ml. Five viruses (from subtypes B, A and AG) were sensitive to only one of the two mAb pools with a 50% neutralizing dose (ND50) between 0.05 and 12 ug/ml, and eight viruses (from subtypes A, AG, and C) were not neutralized by either pool when tested at 50 ug mAb/ml.
Figure 3.
Neutralizing activity of immune rabbit sera against primary isolates. (A) The 50% neutralizing doses (ND50, ug/ml) are shown for anti-V3 mAb pools composed of mAbs from clade B and from non-clade B-infected individuals against each of 15 primary isolates from subtypes B, A, AG and C. Each mAb pool contained five anti-V3 mAbs. Red boxes denote ND50 <2 ug/ml; orange boxes, ND50 between 10-20 ug/ml, white boxes, ND50 >50 ug/ml. (B and C) For each rabbit/virus combination, the reciprocal NT50 value is shown. Where no value is listed, 50% neutralization was not achieved at the lowest dilution tested, either 1:5 or 1:10. The prime and boost delivered to each group of animals is shown as per designations specified in Table 1. The strength of the response by each group of rabbits to each virus is denoted by color: where reciprocal GMT50 values for each immunization group/virus are >80, the box is shaded red; GMT50 values between 40 and 80 are shown in orange, between 10 and 39 are shown in yellow, and those <10 are shown in white.
The GMT50 values of sera from responding animals in each group are shown in Figures 3B and 3C vs. each of the 15 viruses; the presence of demonstrable neutralizing Abs in the serum of each animal is also shown. Given that percent neutralization of immune serum is based on pre-bleeds of the same animal (see Methods), and positive neutralization was based on achieving 50% neutralization with a reproducible dose-response curves for individual sera vs. each virus (see Figure 4), 50% neutralization titers (NT50) for individual sera and GMT50 values for each group ≥1:10 are highly significant and reproducible.
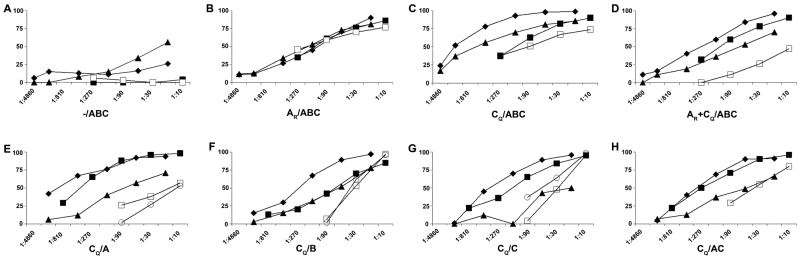
Figure 4.
Dose-response neutralization curves of individual immune rabbit sera. The serum from one rabbit from each group listed in Table 1 is shown in each panel. Neutralization curves are shown for viruses DJ263 (subtype AG) (◆), BZ167 (subtype B) (■); BX08 (subtype B) (▲); VI313 (subtype A) (□), and 93MW960 (subtype C) (○). Serum dilutions are shown on the x-axis and percent neutralization is shown on the y-axis. Percent neutralization is based on the activity in the immune sera vs. the corresponding animal's pre-immune serum at the same dilution. Data shown are from one of two or more experiments. The sera used were from the following rabbits (as identified in Figures 2 and 3): Panel A = rabbit 18; B = rabbit 21; C = rabbit 24; D = rabbit 26; E = rabbit 43; F = rabbit 50; G = rabbit 55; H = rabbit 58.
The results confirm the requirement for the DNA prime and show again that the broadest responses are induced with the CQ prime. While the CQ prime with the triple protein boost gave less breadth, the immune sera from the groups receiving the CQ prime and either the V3B-FP, or V3C-FP, or V3A- and V3C-FP boosts neutralized 61%, 63% and 55% of the serum/virus combinations compared to 38% and 36% neutralization of serum/virus combinations neutralized by animals primed with AR or AR + CQ gp120 (Fig. 2) (p = 0.016, Fisher's exact test). Notably, sera from the groups receiving the CQ prime and either the V3B-FP, V3C-FP, or V3A- and V3C-FP boosts neutralized 13-14 of the 15 primary isolates from subtypes B, A, AG and C (Fig. 2). These neutralized viruses included those which were sensitive, moderately resistant, or completely resistant to neutralization by anti-V3 mAb pools. Further quantitative information on the serum neutralizing activity is provided by the dose-response curves shown in Figure 4 against primary isolates tested with the serum from the rabbit in each group that gave the strongest response.
To determine whether the neutralizing Abs induced by these immunogens were directed against V3 epitopes, peptides representing the V3 sequence of subtype B virus BaL and that of the subtype C V3 consensus were used to block neutralizing activity in sera against subtype B virus BZ167 or CRF02_AG virus DJ263. For all sera tested, where neutralization of these two viruses was demonstrable, a minimum of 78% of neutralizing activity could be blocked by at least one of the two peptides. In most cases, both peptides blocked >50% of the neutralizing activity. These results are shown in Figure 5 where the inhibition of serum neutralizing activity in the presence of either the BaL V3 peptide or the consensus C V3 peptide is calculated based on the neutralizing activity in the presence of a scrambled V3 peptide. The BaL and consensus C V3 peptides were approximately equivalent in their ability to block neutralization of BZ167. Differential blocking of DJ263 neutralizing activity was noted by these two peptides, but no clear pattern was discernable. Since a minimum of 78% of neutralizing activity is blocked by at least one of the two peptides and since it is highly probable that some overlap exists in the ability of the two peptides to block the neutralizing Abs, it is not possible to calculate the additive effects of the two peptides used in these experiments, but the data indicate that the vast majority of the neutralizing activity was directed against V3.
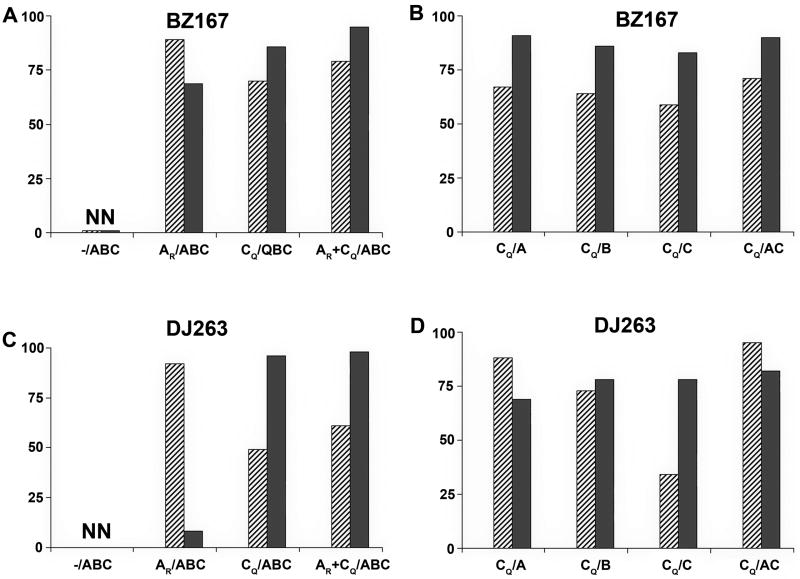
Figure 5.
Ability of V3 peptides to block serum neutralizing activity. Subtype B virus BZ167 (panels A and B) or CRF02_AG virus DJ263 (panels C and D) were tested for their ability to be neutralized by sera diluted 1:20 or 1:60, respectively. Serum from each rabbit in each group was pre-incubated with 180 ug/ml of the subtype B BaL V3 peptide (hatched bars) or the subtype C consensus V3 peptide (gray bars). The mean percent inhibition of neutralization by each peptide assessed for animals in each group is shown on the y-axis and is based on neutralization of the same serum in the presence of 180 ug/ml of a scrambled V3 peptide. Data are from one of two experiments. Groups are designated as per Table 1. NN, non-neutralizing.
Neutralizing activity against pseudoviruses expressing the Envs of patient-derived isolates
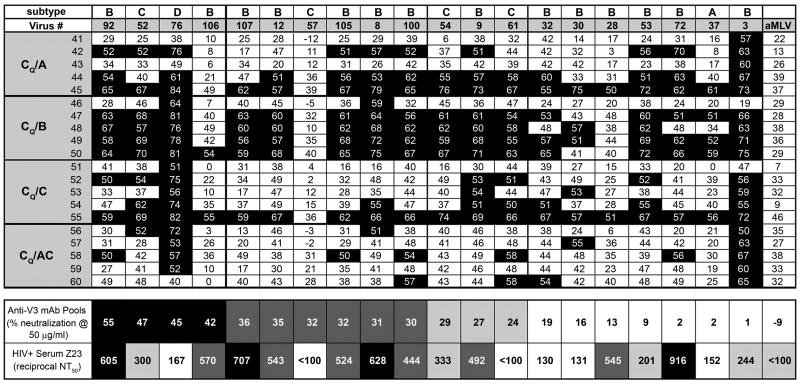
Using a third neutralization assay, 57 psVs were initially tested with four pools of human anti-V3 mAbs derived from cells of subjects infected with either subtypes AG, B, C, G, or H. Four mAb pools were tested, and the activity of each pool was assessed at a final total concentration of 50 ug mAbs/ml. Simultaneously, an HIV+ plasma specimen with broadly neutralizing activity was titrated between dilutions of 1:10-1:40,000. This plasma, Z23, had previously been chosen from among 30 plasmas as showing the broadest neutralizing activity against a panel of viruses. The 57 psVs were constructed from the viral Envs derived from patients infected with subtypes A, B, C or D. Twenty of these psVs were selected for further study on the basis of their neutralization sensitivity or resistance and on the diversity of their origins from subjects infected acutely or chronically by different routes and by different viral subtypes. Table 2 shows the characteristics of the patients and viruses from which these 20 psVs were constructed. Data generated with the 20 psVs are presented in Figure 6 where they are ranked in order of their sensitivity to the pools of anti-V3 mAbs (from left to right). Thus, psV 92 was the most sensitive since its average level of neutralization by the four pools of anti-V3 mAbs when tested at 50 ug/ml was 55%. Pseudovirus 3 was the least sensitive to neutralization, being neutralized at an average level of 1% by the four pools of anti-V3 mAbs at 50 ug/ml. For comparison, psVs carrying the Envs of SF162, JR-CSF and NL43 were neutralized at average levels of 100%, 65%, and 72%, respectively, by the four anti-V3 mAb pools at 50 ug/ml (data not shown) and the negative control aMLV virus was not neutralized by any of the pools. Thirteen of the 20 psVs were neutralized to a significant level by the pools of anti-V3 mAbs as defined on the basis of the 95% confidence limit established with a pool of irrelevant human mAbs.
Table 2.
Characteristics of Patients and Viruses from which Pseudoviruses were Constructed
| Virus Name | Subtype | Nature of Infection* | Infection Status¶ |
|---|---|---|---|
| MB-SZP-3 | B | heterosexual | acute |
| MB-SZP-8 | B | heterosexual | acute |
| MB- SZP-9 | B | heterosexual | acute |
| MB-SZP-12 | B | heterosexual | acute |
| MB-SZP-28 | B | MSM | acute |
| MB- SZP-30 | B | MSM | acute |
| MB-SZP-32 | A | unknown | unknown |
| MB-SZP-37 | A | unknown | unknown |
| MB- SZP-52 | C | unknown | unknown |
| MB-SZP-53 | C | unknown | unknown |
| MB-SZP-54 | C | unknown | unknown |
| MB- SZP-57 | C | unknown | unknown |
| MB-SZP-61 | C | unknown | unknown |
| MB- SZP-72 | D | unknown | unknown |
| MB-SZP-76 | D | unknown | unknown |
| MB-SZP-92 | B | unknown | Progressor |
| MB- SZP-100 | B | unknown | Progressor |
| MB-SZP-105 | B | unknown | LTNP |
| MB-SZP-106 | B | unknown | LTNP |
| MB- SZP-107 | B | unknown | LTNP |
MSM: men who have sex with men
LTNP: Long-term non-progressors (also defined as “viremic controllers” who are untreated and asymptomatic subjects infected for >7 years with viral loads between 50-200)
Figure 6.
Neutralizing activity of rabbit immune sera, anti-V3 mAb pools, and a broadly neutralizing HIV+ serum against pseudoviruses expressing the Envs of patient-derived isolates. Twenty pseudoviruses carrying the Envs from clade A, B, C or D viruses were tested against immune sera from rabbits immunized with gp120 DNA from clade C and V3-FPs, as per Table 1. The percent neutralization displayed by rabbit immune sera diluted 1:10 is shown against the 20 psVs as well as the negative control aMLV psV. While analysis of pre-bleed sera indicates that neutralization of ≥40% is statistically significant (p>0.05), the more conservative convention is used in this figure where only serum/psV combinations achieving 50% neutralization are denoted by black squares. The same viruses were tested for neutralization by four anti-V3 mAb pools under conditions in which the final concentration of each pool of four mAbs was 50 ug mAbs/ml. The average percent neutralization by the four pools is shown, and viruses are listed, left to right, in terms of most to least sensitive to anti-V3 mAb neutralization. Positive neutralization (defined in the text) is demarcated by gray and black boxes. The same viruses were also tested for neutralization sensitivity to an HIV+ serum, Z23. The 50% reciprocal neutralization titers (NT50) against each pseudovirus are shown. Positive neutralization (defined in the text) is demarcated by gray and black boxes.
Figure 6 also shows the reciprocal NT50 values at which each of the 20 psVs were neutralized by HIV+ plasma Z23. Based on its reactivity with the aMuLV negative virus control, a positive neutralization titer for Z23 is >1:200, accounting for its rather high background activity, and thus 14/20 psVs were neutralized by Z23 with ND50 values reaching 1:916 (Figure 6). For comparison, the NT50 values for Z23 against the psVs carrying the Envs of SF162, JR-CSF and NL43 were 1:21251, 1:3532 and 1:360, respectively. Interestingly, there were psVs that were neutralized well by Z23 and poorly by the anti-V3 mAb pools (psV 72) and vice versa (psV 76), confirming that the anti-V3 mAb pools and the HIV+ serum contain Abs of different specificities, and that individual psVs vary in their sensitivities to these different Ab specificities.
Using these 20 psVs, neutralizing activity was assessed by titration of coded specimens consisting of the individual rabbit immune sera drawn two weeks after the second boost; sera tested came from the rabbits primed with CQ and boosted with V3A-FP, V3B-FP, V3C-FP, or V3A- and V3C-FPs (see Table 1). Negative controls consisted of pools of pre-bleed sera containing equal quantities of serum drawn prior to immunization from the animals in each experimental group tested against the 20 psVs, aMLV, and psVs carrying the Envs of JR-CSF, NL43 and SF162. In total, 120 assays were done with combinations of pre-bleed pools and the various psVs. Analysis of the 95% confidence limit with the results of these negative control experiments indicate that significant neutralization was achieved when neutralization levels of ≥40% was achieved. In Figure 6, the more stringent convention of 50% neutralization is used to denote positive neutralization.
Notably, sera from the groups receiving the CQ prime and the V3B-FP boost (CQ/B) neutralized 18 of the 20 psVs tested with sera diluted 1:10, and four of the five animals neutralized 13 psVs from subtypes B, C and D. (Figure 6). The proportion of viruses neutralized by sera from this CQ/B group (i.e., positive serum/virus combinations tested for each group) was 66% whereas the proportion of serum/psV combinations neutralized by the other three groups ranged from 20-43%. When the neutralizing potencies of sera in each group were compared by the Kruskal-Wallis analysis with Dunnett's multiple comparison test the CQ/B group was found to give significantly stronger responses than the other three groups (p<0.001) while there was no difference among the other three groups.
The neutralizing activity in the sera of rabbits immunized with CQ/B was broader than that displayed by the anti-V3 mAb pools as shown in Figure 6 where the psVs are ranked in order of neutralization sensitivity to the anti-V3 mAb pools (left to right). The rabbit immune sera from the CQ/B group neutralized 6 of 7 psVs that were not neutralized by the mAb pools. Sera from this group of animals also neutralized 8 of 9 psVs whose neutralization sensitivity to anti-V3 mAb pools was intermediate, and neutralized all of the four psVs that were sensitive to anti-V3 neutralization. Thus, in four of five of the rabbits in this group, immunization induced neutralizing Abs of greater breadth than that shown by pools of human anti-V3 mAbs.
Discussion
An immunization protocol that focuses the immune response on the structurally-conserved V3 epitope induced Abs in rabbits that were capable of neutralizing psVs and primary isolates constructed or isolated from subjects infected with HIV subtypes A, AG, B, C, and D. These psVs and primary isolates were derived from patients who were acutely or chronically infected by heterosexual or homosexual exposure. The neutralizing activity was assessed in three different assays, run in three independent laboratories, and was shown by peptide blocking experiments to be directed against V3 epitopes. The immunization regimen that gave the most consistent and broadest responses when assessed in all three assays was that in which the animals were primed with subtype C gp120 DNA and boosted with V3B-FP; the breadth of this response was greater than that displayed by pools of human anti-V3 mAbs.
Given the variation in results achieved with different neutralization assays and the challenges of standardizing a single assay for use in several labs, the immune rabbit sera generated by the DNA prime/protein boost protocols used here were tested against three different groups of viruses and psVs in three different assays in three different labs: (a) Clonal V3 chimeric psVs carrying the V3 sequences of various subtypes presented in the context of the neutralization-sensitive SF162 backbone were tested using the CD4+CCR5+ U87 line as target cells. (b) Primary isolates grown in PBMCs were tested using the engineered CD4+CCR5+ TZM-bl line as target cells. And (c) psVs constructed with an uncloned panel of primary envelopes derived from the plasma of HIV+ individuals were tested using the CD4+CCR5+CXCR4+ U87 line as target cells. The latter assay was run on coded samples. The data from all of these assay systems were consistent in showing that priming with DNA containing a V3 sequence characterized by the GPGQ motif at the tip was a better prime than a DNA prime containing a V3 sequence carrying the GPGR motif. The immunization regimen that gave the strongest and/or broadest neutralizing Ab responses utilized the clade C gp120 DNA prime together with boosts of the V3B-FP. Various combinations of V3A-,V3B-, and/or V3C-FPs also induced cross-clade neutralizing Abs, although the level and breadth of positive results from animals receiving the combined V3-scaffold protein boosts were less reliably demonstrated in all of the neutralization assays.
The demonstration that the CQ/B immunized group gave the highest ELISA titers and the most consistent cross-clade neutralizing Ab response reflects tenets in the classic immunologic literature relevant to the induction of cross-reactive Abs. Thus, priming with immunogen A and boosting with immunogen B is known to induce Abs reactive with both immunogens (Richards et al., 1975). The data presented above are also consistent with previously published data that suggest that immunizing with constructs from more than one clade of HIV induces broader immune responses than immunizing with constructs from a single clade (Burke et al., 2009; Seaman et al., 2005; Wang et al., 2006; Zolla-Pazner et al., 2008). However, the data presented indicate that some combinations, e.g., CQ/B, are better than others. Determining which prime and boost will give the optimal qualitative response, i. e., Abs with the broadest neutralizing activity, can be address rationally and informed by both structural and immunologic studies of monoclonal Abs. However, it is noteworthy that in the experiments described above the group receiving the multivalent prime (see Table 1) did not give as strong or broad a neutralizing response as the groups primed with the clade C gp120 alone. This quantitative effect of dose in multivalent vaccines needs further study.
The level of neutralizing Abs achieved in these experiments varied from 1:10-1:2400 depending on the assay system and the virus or psV that was employed. V3 chimeric psVs carrying the Env of the neutralization-sensitive SF162 virus were, not surprisingly, the most sensitive (Figure 2). Primary isolates known to be neutralization sensitive, e.g., BX08, BZ167 and DJ263, gave the next highest titers, with titers often >1:100 (Figures 3 and 4). When more resistant viruses and psVs (those often referred to as “Tier 2”) were tested, detectable GMT50 values for neutralization Ab ranged from 1:10 to 1:102 (Figures 3 and 6). Many of the primary isolates were in early passage after isolation (NYU3738, NYU6525, NY129), and had been grown in PBMCs. Since such viruses are more resistant than psVs when tested in the TZM assay, these primary isolate neutralizing titers, which were calculated on the basis of 2-3 titration experiments each run in triplicate against a multiclade virus panel, are especially noteworthy. The titers against “Tier 2” viruses and psVs are quantitatively comparable or stronger, and qualitatively broader, than those recently reported by others (Burke et al., 2009; Law et al., 2007; Mascola et al., 2005; Morner et al., 2009; Seaman et al., 2005; Vaine et al., 2008; Wu et al., 2006).
Protective levels of vaccine-induced serum neutralizing Abs against polio have been reported at 1:4 (Plotkin, 2008) and ≥1:20 (Wicker et al., 2007). Similarly, vaccine-induced serum hemagglutinin inhibitory Ab titers ≥1:40 are considered to be the protective threshold for influenza (Hannoun, Megas, and Piercy, 2004). The relevance of these values to protective Ab titers against HIV is open to question. However, challenge with various strains of SHIV in passively immunized macaques demonstrates that serum neutralizing titers of 1:1 (neat serum) to 1:38 can protect against infection (Hessell et al., 2009; Mascola et al., 2000; Shibata et al., 1999). Such low Ab titers that confer protection suggest that previous conclusions about the need for high Ab titers (Baba et al., 2000; Parren and Burton, 2001; Parren et al., 2001) may be unwarranted. It is noteworthy, however, that these data are derived from experiments in which the challenging “animal infectious doses” used induced infection in most or all control animals. In contrast, infectious doses for human heterosexual transmission per contact is on the order of one in 500 (Dunkle et al., 2008; Gray et al., 2001). Given the requirements of experimental design in animal studies to insure statistically meaningful results, the infectious doses used in animal models appear to be orders of magnitude larger than those occurring in humans. Thus, the level of Abs needed to protect humans may be much less than that needed to protect animals that are infected in most experimental models, and it is probable that the level of Abs required to protect against natural human infection will not be established until protection is achieved with a vaccine for humans. Therefore, it is impossible to know whether the relatively low vaccine-induced cross-clade neutralizing Ab titers which have been reported by us and others would be protective. However, now that considerable breadth has been achieved, additional experiments using alternative adjuvants, vaccine formulations and delivery systems should significantly increase Ab levels, and thus achieve the desired goal of inducing neutralizing Abs with a breadth and potency capable of preventing infection with HIV.
While a yet-to-be-defined minimal Ab titer is of critical significance for protection against infection, induction of Abs with broad reactivity is an absolute requirement for any HIV vaccine candidate; consequently, the aim of the experiments described here was to increase the breadth of the neutralizing Ab response. One of the variables examined to accomplish this was the nature of the prime. Although the composition of gp120 molecules from clades B and C are known to differ by ∼30%, one crucial difference which is especially relevant to the induction of anti-V3 Abs occurs at the tip of the V3 loop, with the GPGR motif characterizing most clade B viruses and the GPGQ “tip type” occurring in most clade C viruses. This R/Q distinction has been shown to have considerable antigenic significance in that the R/Q mutation can alter neutralization sensitivity (Zolla-Pazner et al., 2004). Similarly the R/Q difference between B and non-B clades affects to the breadth of anti-V3 Abs produced by HIV-infected individuals (Brown et al., 2008; Gorny et al., 2004; Gorny et al., 2006; Krachmarov et al., 2006; Krachmarov et al., 2005). This R/Q difference appears to have contributed to the broader anti-V3 Ab response induced by the clade C gp120 DNA prime. This conclusion is supported by the finding that mAbs that engage the R residue in the GPGR motif at the tip of the V3 are quite restricted in their neutralizing activity for viruses carrying the GPGR tip type (Binley et al., 2004; Stanfield et al., 2004; Zolla-Pazner et al., 2004). In contrast, anti-V3 mAbs where the R/Q residue at the tip of V3 does not interact with the Ab surface are able to neutralize both B and no-B clade viruses ((Stanfield et al., 2006) and unpublished data). The data suggest that the CQ prime establishes memory for anti-V3 Abs that are independent of the V3 tip type, and that, in the context of broadly reactive anti-V3 memory cells, the V3-FP boosts activate those memory B cells that are maximally cross-reactive and capable of binding to both GPGR-containing and GPGQ-containing V3 peptides and of neutralizing viruses from many subtypes regardless of the motif at the tip of the V3 loop.
As shown in Figure 3, the rabbits receiving the CQ prime and any one of the V3-FPs produced Abs that could neutralize primary isolates that were not neutralized by the anti-V3 mAbs pools. Thus, the polyclonal Abs induced with a multi-clade, V3-focused immunization protocol showed broader neutralizing activity than cocktails of various anti-V3 monoclonal Abs. In neutralization assays for polyclonal Abs against recombinant psVs constructed from the Envs of patients infected acutely or chronically with various subtypes (Figure 6), the group of rabbits immunized with CQ/B gave the broadest response: 66% of the 100 serum/psV combinations were neutralized by the sera from the five animals in this group, and four of the five rabbits in this group neutralized psVs from clades B, C and D. In contrast, several of the psVs neutralized by the immune rabbit sera were neutralized poorly or were entirely resistant to anti-V3 mAbs pools or to a broadly neutralizing HIV+ human serum used as a positive control. Moreover, four of five immune sera from the CQ/B group neutralized primary isolates and psVs from subtypes A, AG, B, C and D in the various neutralization assays performed in different labs (Figure 2, Figures 3 and 6). Taking all of the data together, immunization with the CQ/B regimen was shown to be capable of inducing the most broad, cross-clade neutralizing Ab response.
Methods
Use of codon-optimized HIVenv DNA vaccine constructs
Codon-optimized env genes from HIV CRF011_cpx primary isolate CA1 and clade C primary isolate 92BR025 were prepared as described previously (Zolla-Pazner et al., 2008) and were chemically synthesized by Geneart (Regensburg, Germany). The DNA vaccine plasmids were prepared from Escherichia coli (HB101 strain) with a Mega purification kit (Qiagen, Valencia, CA) for in vivo animal immunization studies.
Protein immunogens
The V3-FPs contain a 45-amino acid domain encompassing the V3 sequences of either JR-CSF (clade B), 92UG037.08 (clade A) or 93IN904 (clade C) (see footnote of Table 1). In addition to the V3 sequences, these constructs included the five N-terminal and five C-terminal amino acid residues that bracket V3, including the two terminal N-linked glycosylation sites. The clade A and C proteins also contained a synthetic TH epitope N-terminal to the V3, as previously described (Kayman et al., 1994; Krachmarov et al., 2005). The insert is joined to the C-terminus of a 263 amino acid fragment of MuLV gp70, the V3-FPs were synthesized in CHO cells, and all fusion proteins were purified on Ni2+-nitrilotriacetic acid resin (NTA Superflow; Qiagen, Valencia, CA) as described (Kayman et al., 1994; Krachmarov et al., 2001).
Immunization protocol
Female New Zealand White rabbits 6–8 weeks old (with a body weight of ∼2 kg) were purchased from Millbrook Farm (Amherst, MA) and housed in the animal facility managed by the Department of Animal Medicine at the University of Massachusetts Medical School in accordance with an IACUC-approved protocol. Groups of rabbits received three DNA immunizations at weeks 0, 2, and 4 using a Bio-Rad Helios gene gun (Bio-Rad Laboratories, Hercules, CA). The gp120 DNA vaccine plasmids or the negative control pJW4303 vector plasmid was coated onto 1.0 μm gold beads at a ratio of 2 μg of DNA per mg of gold. Each gene gun shot delivered 1 μg of DNA to a total of 36 non-overlapping sites on the shaved abdominal skin of each rabbit at each of the three priming immunizations. The animals then received two boosts with one or more of the V3-FPs at weeks 10 and 14 (Table 1). A total of 100 μg/per injection of the V3-FP(s) was administered intramuscularly with IFA. Blood was collected prior to immunization and two weeks after each immunization.
ELISA assay
To determine the presence of anti-V3 Abs in immune rabbit sera, ELISA binding studies were performed using in U-bottom, 96-well polyvinyl chloride plates (Falcon, Becton Dickinson). The V3B- or V3C-rFC fusion proteins (Davis et al., 2008) were adsorbed at 2 μg/ml to the wells for 2 h in 50 μl of carbonate buffer (pH=9.6) at 37°C, followed by blocking with 100 μl/well of 2.5% dry milk in phosphate-buffered saline (PBS) for 1 h. Serial three-fold dilutions of rabbit sera were prepared in 2.5% dry milk/PBS and, after 2 h incubation at 37°C, bound serum Abs were detected with goat anti-rabbit Fab-specific, alkaline phosphatase-conjugated secondary Abs (Zymed). Finally, the substrate, p-nitrophenyl phosphate (Sigma) in 10% diethanolamine, pH=9.8, was added, and plates were read at 405 nm.
Neutralization assays
Neutralization of primary isolates
JC53-BL cells (also termed TZM-bl cells) expressing CD4 and CCR5 and containing the Tat-responsive reporter genes for firefly luciferase and Escherichia coli β-galactosidase under the regulatory control of the HIV long terminal repeat were obtained from the NIH AIDS Research and Reference Reagent Program (NIH AIDS RRRP catalog no. 8129). These cells were used to measure neutralizing activity of heat-inactivated rabbit sera against primary isolates as previously described (Zolla-Pazner et al., 2008) on the basis of the reduction in the luc reporter gene expression after a single round of virus infection. Briefly, 200 TCID50 of virus, produced in PHA-stimulated peripheral blood mononuclear cells, was incubated with various dilutions of test serum or anti-V3 mAb pools before addition to TZM-bl cells in the presence of 1 μM indinavir sulfate (NIH AIDS RRRP, catalog no. 8145). Virus production was assessed after 48 h of incubation. The human anti-V3 mAb pools used in these experiments each contained five mAbs from clade B-infected individuals from the US (clade B pool) or five mAbs from non-clade B-infected individuals from Cameroon and India (non-clade B pool). For peptide inhibition studies, a V3 peptide representing either the V3 of the BaL2 strain (TRPNNNTRKSIHIGPGRAFYTTG), consensus C (NNTRKSIRIGPGQTFYATGDIIG), or a scrambled V3 peptide (IGPGRATRPNNNFYTTGTRKSIH) was incubated for 30 min at a final concentration of 180 μg/ml with rabbit serum prior to its 1 h incubation with 200 TCID50 of virus in culture medium. For all experiments, background controls contained cells only, while the virus controls contained cells plus virus. The percent neutralization for immune rabbit sera was calculated relative to the effect of pre-immune serum from the same rabbit. Thus, for all dilutions of sera, the percent neutralization was calculated based on the RLU in the presence of immune serum from a given animal divided by the RLU in the presence of the same dilution of pre-immune serum from the same animal. NT50 values for sera or ND50 values for mAb pools were determined from the linear portion of the titration curves using the method of Least Squares. Pairwise statistical comparison of differences in the proportion of viruses neutralized by sera in different immunization groups was made by Fisher's exact test.
Neutralization of V3 chimeric pseudoviruses (psVs)
The infectious pseudotyped viruses were generated by co-transfection of 293 cells with an env expression vector and with the complementing vector pNL4-3.Luc.R-E- (NIH AIDS RRRP, catalog no. 3418, donated by Dr. Nathaniel Landau) (Connor et al., 1995; He et al., 1995). The env expression vectors for chimeric forms of SF162 env with various consensus V3 sequences were generated by introducing the modifications sequentially by QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA), as described (Krachmarov et al., 2006). The V3 sequences inserted in place of the SF162 V3 are shown in the footnote of Figure 2. Only the sequences inside the V3 cysteines were changed. Neutralizing activity was determined as previously described (Krachmarov et al., 2001; Zolla-Pazner et al., 2008) with a single-cycle infectivity assay using U87 cells expressing CCR5 and CD4 as target cells. GMT90 values of immune sera were determined by interpolation from neutralization curves and are averages of at least two independent assays; they were calculated by determining the serum dilution at which RLUs were reduced 90% compared to control wells containing virus alone. GMT90 values are calculated from two to three assays of sera from each individual rabbit.
Neutralization of viral pseudotypes expressing the Envs of patient-derived isolates
Env genes were extracted from viruses in patients' plasma, amplified and used for preparation of psVs as previously described (Schweighardt et al., 2007). Subsequently, the PhenoSense™ HIV neutralization assay was run by Monogram Biosciences, Inc. on coded rabbit sera. This assay, a single round recombinant assay, was used to measure neutralization against 20 psVs as previously described (Richman et al., 2003; Schweighardt et al., 2007). Briefly, U87 cells expressing CD4, CCR5 and CXCR4 were incubated with psV stocks which had been pre-incubated with serial dilutions of mAb pools or heat-inactivated rabbit immune sera. The ability of sera and mAbs to neutralize virus was assessed by measuring luciferase activity 72 h after psV inoculation in comparison to a control infection with virus pseudotyped with the Env of MuLV (aMuLV). Four anti-V3 mAb pools were tested for their ability to neutralize these 20 psVs. Each pool contained four mAbs and was assessed at a final concentration of 50 ug/ml; thus, each of the mAbs in each pool was present at a final concentration of 12.5 ug/ml. The data from these latter experiments are expressed as the average percent neutralization of the given psV by the four pools tested at a final concentration of 50 ug mAbs/ml. The mAb pools were assembled to include human anti-V3 mAbs from non-clade B-infected individuals from Cameroon; from clade B-infected individuals from the US, from non-B-infected individuals from Cameroon and India, or from non-B and B-infected individuals from the US and Cameroon. Pre-immune and immune rabbit sera and a broadly neutralizing human HIV+ serum specimen (Z23) were titrated to assess the presence of neutralizing activity. Data were analyzed by the Kruskal-Wallis statistic using Dunnett's multiple comparison test making without matching and making no assumption for Gaussian distribution.
Acknowledgments
The authors wish to thank Drs. Arthur Nadas and Catarina Hioe for performing the statistical analyses and insightful discussions of the data. This work was supported by grants from the Bill and Melinda Gates Foundation, the National Institutes of Health (AI 36085 and AI 27742), and research funds from the Department of Veterans Affairs.
Contributor Information
Sandra Cohen, Email: Sandra.Sharpe-Cohen@med.nyu.edu.
Abraham Pinter, Email: pinterab@umdnj.edu.
Chavdar Krachmarov, Email: krachmcp@umdnj.edu.
Terri Wrin, Email: twrin@MonogramBio.com.
Shixia Wang, Email: Shixia.Wang@umassmed.edu.
Shan Lu, Email: shan.lu@umassmed.edu.
References
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6(2):200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Davis KL, Bibollet-Ruche F, Li H, Decker JM, Kutsch O, Morris L, Salomon A, Pinter A, Hoxie JA, Hahn BH, Kwong PD, Shaw GM. Human immunodeficiency virus type 2 (HIV-2)/HIV-1 envelope chimeras detect high titers of broadly reactive HIV-1 V3-specific antibodies in human plasma. J Virol. 2008;83(3):1240–59. doi: 10.1128/JVI.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103(12):133–8. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5(5) doi: 10.1371/journal.ppat.1000433. e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachmarov C, Honnen WJ, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J Virol. 2006;80(14):7127–35. doi: 10.1128/JVI.02619-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Sambor A, Beaudry K, Santra S, Welcher B, Louder MK, Vancott TC, Huang Y, Chakrabarti BK, Kong WP, Yang ZY, Xu L, Montefiori DC, Nabel GJ, Letvin NL. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J Virol. 2005;79(2):771–9. doi: 10.1128/JVI.79.2.771-779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- Nyambi PN, Nadas A, Mbah HA, Burda S, Williams C, Gorny MK, Zolla-Pazner S. Immunoreactivity of intact virions of human immunodeficiency virus type 1 (HIV-1) reveals the existence of fewer HIV-1 immunotypes than genotypes. J Virol. 2000;74(22):10670–80. doi: 10.1128/jvi.74.22.10670-10680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75(17):8340–7. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- Richards FF, Konigsberg WH, Rosenstein RW, Varga JM. On the specificity of antibodies. Science. 1975;187(4172):130–7. doi: 10.1126/science.46122. [DOI] [PubMed] [Google Scholar]
- Schweighardt B, Liu Y, Huang W, Chappey C, Lie YS, Petropoulos CJ, Wrin T. Development of an HIV-1 Reference Panel of Subtype B Envelope Clones Isolated From the Plasma of Recently Infected Individuals. J Acquir Immune Defic Syndr. 2007;46(1):1–11. doi: 10.1097/QAI.0b013e318074eb5a. [DOI] [PubMed] [Google Scholar]
- Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5(2):204–10. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- Stanfield RL, Gorny MK, Zolla-Pazner S, Wilson IA. Crystal structures of HIV-1 neutralizing antibody 2219 in complex with three different V3 peptides reveal a new binding mode for HIV-1 cross-reactivity. J Virol. 2006;80(12):6093–105. doi: 10.1128/JVI.00205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bubnoff A. The great barrier: Understanding mucosal immune responses is critical to developing effective AIDs vaccines, but progress has been slow. IAV Report. 2008 March-April;:10–14. [Google Scholar]
- Wicker S, Rabenau HF, Gottschalk R, Doerr HW, Allwinn R. Seroprevalence of vaccine preventable and blood transmissible viral infections (measles, mumps, rubella, polio, HBV, HCV and HIV) in medical students. Med Microbiol Immunol. 2007;196(3):145–50. doi: 10.1007/s00430-007-0036-3. [DOI] [PubMed] [Google Scholar]
- Zolla-Pazner S, Cohen SS, Krachmarov C, Wang S, Pinter A, Lu S. Focusing the immune response on the V3 loop, a neutralizing epitope of the HIV-1 gp120 envelope. Virology. 2008;372(2):233–46. doi: 10.1016/j.virol.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Zolla-Pazner S, Zhong P, Revesz K, Volsky B, Williams C, Nyambi P, Gorny MK. The Cross-Clade Neutralizing Activity of a Human Monoclonal Antibody Is Determined by the GPGR V3 Motif of HIV Type 1. AIDS Res Hum Rertroviruses. 2004;20(11):1254–58. doi: 10.1089/aid.2004.20.1254. [DOI] [PubMed] [Google Scholar]