Abstract
G protein-coupled receptor kinases (GRKs) specifically phosphorylate activated G protein-coupled receptors. While the X-ray crystal structures of several GRKs have been solved, the mechanism of GRK interaction with GPCRs is currently unknown. To further characterize the role of the GRK2 amino terminus in receptor interaction and phosphorylation, we generated a series of point mutations within the first 10 amino acids of GRK2 and tested their ability to phosphorylate receptor and non-receptor substrates. Although all mutants showed some impairment in receptor phosphorylation, three of the mutants, D3K, L4A and D10A, were the most severely affected. Using the β2-adrenergic receptor and rhodopsin as receptor substrates and tubulin as a non-receptor substrate, we demonstrated that the kinase activity towards the receptors was severely decreased in the mutants, while they fully retained their ability to phosphorylate tubulin. Moreover, the amino terminal mutants were able to bind to the receptor but, in contrast to wild-type GRK2, were not activated by receptor binding. A synthetic peptide containing residues 1–14 of GRK2 served as a non-competitive inhibitor of receptor phosphorylation by GRK2, while a comparable peptide from GRK5 had no effect on GRK2 activity. Secondary structure prediction and circular dichroism suggest that the GRK2 amino terminal peptide forms an amphipathic alpha helix. Taken together, we propose a mechanism whereby the extreme amino terminus of GRK2 forms an intramolecular interaction that selectively enhances the catalytic activity of the kinase towards receptor substrates.
G protein-coupled receptors (GPCRs2) are a large class of plasma membrane proteins that respond to a wide variety of stimuli including hormones, odorants, peptides and lipids (1). Agonist binding transduces the signal into the cell by promoting receptor interaction with heterotrimeric G proteins, which subsequently activate effectors such as adenylyl cyclase, phosphodiesterases, phosphatidylinositol 3-kinase and various ion channels. Cellular responsiveness to external stimuli is tightly regulated. In GPCR-mediated signaling, the waning of receptor responsiveness to agonist is a key regulatory mechanism known as desensitization (2). Additional regulatory processes such as receptor endocytosis and downregulation limit the cellular response to continued stimuli by reducing the number of receptors on the cell surface (3,4). Some of these events are regulated by a family of serine/threonine protein kinases called GPCR kinases (GRKs) that specifically phosphorylate agonist occupied receptors (5,6). Receptor phosphorylation by GRKs promotes the binding of arrestins, which effectively uncouple the receptor from G protein and terminate signaling (7).
The seven mammalian members of the GRK family can be divided into three subfamilies based on their structural differences: i) GRK1 and GRK7; ii) GRK2 and GRK3; and iii) GRK4, GRK5 and GRK6. All GRKs share a common topological structure that includes an N-terminal regulator of G protein signaling homology (RH) domain, a central kinase catalytic domain, and a C-terminal membrane targeting domain. GRK2 is the most extensively characterized member of the family and is ubiquitously expressed in mammals. GRK2 phosphorylates a variety of GPCRs and can be activated by numerous signaling molecules including activated GPCRs, Gβγ subunits, and phospholipids (8–12).
The X-ray crystal structures of GRK2 (13), GRK2/Gβ1γ2(13,14), GRK2/Gβ1γ2/Gαq (15), GRK6 (16) and GRK1 (17) have provided significant insight into GRK function. Interestingly, the crystal structure of GRK2 reveals that the N-terminal RH domain, central catalytic domain, and C-terminal pleckstrin homology domain form an equilateral triangle that is ~80 Å on a side. The RH domain contacts both the kinase and PH domains and consists of two discontinuous regions with the characteristic nine-helix bundle in the N-terminal region and two additional helices following the kinase domain. The interaction surface of GRK2 with Gαq primarily resides on the α5 and α6 helices of the RH domain (15,18). The kinase domain is most similar to that of PKA, PKB and PDK1 but appears to be in an inactive conformation in the crystal structure. This inactive conformation is also observed in the structure of GRK6 even when bound to AMPPNP and GRK1 when bound to ATP (16,17). Interestingly, the structure of the RH and kinase domain cores of GRKs appear to have similarities to the inactive structure of Src with the α10 helix of the RH domain potentially functioning to regulate GRK activation (14,16). Similarly, the interface between the α4-α5 loop of the RH domain and the αJ helix of the kinase large lobe might also modulate GRK activity by influencing the orientation of the large and small kinase lobes. It is worth noting that the crystal structures of GRK2 and GRK6 reveal that the extreme amino-terminus as well as the nucleotide gate in the kinase domain are disordered (14,16), although residues 5–30 are observed in GRK1˙(Mg2+)2˙ATP (17). The nucleotide gate is predicted to make contacts with the active site cleft, promoting the active conformation of the kinase, while the extreme amino-terminus is required for efficient receptor phosphorylation and may be involved in the structural transition from the inactive to the active state of the kinase (19,20).
We and others have previously shown that GRK2 can interact with several domains in the receptor (21–23), however, the sites on GRK2 that are critical for receptor interaction remain poorly defined. Previous studies have suggested that the GRK amino-terminus is required for efficient receptor phosphorylation. For example, antibodies specifically targeting the amino terminus of GRK1 blocked phosphorylation of light activated rhodopsin, but did not disrupt phosphorylation of a peptide substrate (24). In addition, amino-terminal truncation of GRK1, GRK2 and GRK5 resulted in the loss of receptor phosphorylation although the GRK1 mutant was still able to bind to the receptor (19,20). Studies from Ferguson and co-workers implicated a role for the GRK2 RH domain in binding the metabotropic glutamate receptor and reported that mutation of Asp-527 in GRK2 disrupted receptor binding (25). In another study, a role for the GRK1 amino-terminal 15 residues in receptor phosphorylation was implicated via an ability to bind to recoverin and sterically block GRK1 interaction with rhodopsin (26). Taken together, these studies suggest a regulatory role for the GRK amino terminus in receptor phosphorylation.
Recent studies have also started to identify some of the GRK-specific residues within the catalytic domain that contribute to receptor phosphorylation. For example, a V477D mutation in the AGC kinase C-tail of GRK2 was defective in receptor phosphorylation and receptor-mediated activation although it also had reduced activity against non-receptor substrates (27). Similarly, Huang et al. identified a number of residues in the GRK1 catalytic domain including Phe-190, Lys-191, Leu-212, Tyr-274, Val-476 and Val-484 that when mutated were defective in receptor phosphorylation and also had decreased activity against peptide substrates (28). Interestingly, the GRK1-K191A mutant also had a >10-fold increase in the Km for rhodopsin phosphorylation suggesting an important role for this residue in receptor binding. Since similar catalytic defects were observed with an N-terminal deletion, the authors proposed a model whereby the amino terminus interacts with a surface on the GRK1 catalytic domain that stabilizes the closed active conformation of the kinase (28).
In this study, we further delineate the role of the amino terminus of GRK2 in receptor phosphorylation and propose that the GRK2 N-terminal region forms an intramolecular interaction that regulates kinase activity toward receptor substrates. To test this, we generated a series of amino terminal GRK2 mutants and found that three of the mutants (D3K, L4A, and D10A) were severely defective in both receptor-promoted activation and receptor phosphorylation. Interestingly, these mutants were fully capable of binding to the β2-adrenergic receptor (β2AR) and phosphorylating a non-receptor substrate with activities comparable to wild-type GRK2. In addition, we show that a peptide composed of the first 14 residues of GRK2 forms an amphipathic alpha helix and that this peptide inhibits receptor phosphorylation and enhances GRK2 binding to phospholipids. These studies provide insight into the mechanism of GRK2 activation by GPCRs and suggest that the amino terminus of GRK2 forms an intramolecular interaction that modulates the catalytic activity of the kinase toward receptor substrates.
MATERIAL AND METHODS
Plasmid construction
Amino terminal mutants generated by PCR were digested with EcoRI and KpnI, purified and inserted into EcoRI/KpnI digested pcDNA3-GRK2. DNA sequences were verified by dideoxy chain sequence analysis. D3K, L4A and D10A were also cloned into pFastBac vector for expression in Sf9 insect cells.
GRK2 expression and purification
GRK2 and amino terminal mutants were overexpressed and purified from Sf9 insect cells as previously detailed (29). Briefly, cells were harvested by low speed centrifugation 48 hr after infection and the pellet was homogenized in 20 mM Hepes, pH 7.2, 250 mM NaCl, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 0.2 mg/ml benzamidine, and 0.02% Triton X-100 followed by high speed centrifugation. The supernatant was diluted and applied to an SP-Sepharose column and the column was washed and eluted with a 50–300 mM NaCl gradient. Peak fractions were pooled, loaded onto a heparin-Sepharose CL-6B column, and eluted with a 100–600 mM NaCl gradient. Peak fractions were pooled, aliquoted, and stored at −80°C. Protein purity was determined by SDS-PAGE and Coomassie blue staining.
GRK2 Substrate Phosphorylation
GRK2 (20 nM) was incubated at 30°C for the indicated time with substrate (1–2 µM light-activated rhodopsin (Rho*), 50 nM β2AR in mixed micelles in the absence or presence of 10 µM isoproterenol, or 0.1–5 µM tubulin) in 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, and 1–2 µCi [γ32P] ATP in a final volume of 20 µl. Reactions were stopped by the addition of 5 µl of SDS sample buffer and incubation at room temperature for 30 min. Samples were then electrophoresed on a 10% polyacrylamide gel, the gel was dried and autoradiographed, and 32P labeled proteins were excised and counted by scintillation.
Receptor binding assay
A tagged human β2AR was expressed in Sf9 insect cells and purified as previously described (30). Purified phosphatidylinositol (PI) was resuspended in 20 mM Tris-HCl, pH 7.4 and 2 mM EDTA to a final concentration of 10 mg/ml and vesicles were formed by sonicating the sample on ice three times for 1 min. Purified β2AR (10 pmol) in 10 µl of buffer containing 20 mM Hepes, pH 7.5, 100 mM NaCl, 1 mM EDTA, 100 µM FLAG peptide, 15% glycerol and 0.1% dodecyl maltoside was added to PI vesicles (2 µl of 10 mg/ml) and incubated on ice for 20 min to allow for insertion of the receptor into the phospholipid/detergent mixed micelles as previously described (9). This preparation of β2AR was used in both receptor binding and phosphorylation assays. GRK2 (20 nM) was added to the β2AR/PI mixture in a buffer containing 20 mM Tris-HCl, pH 7.5 and 50 mM NaCl and then incubated at room temperature for 15 min. The total volume of the incubations was 40 µl and the final concentrations of all key components in the assay were 20 mM Tris-HCl, pH 7.5, 60 mM NaCl, 0.25 µM β2AR, 20 nM GRK2, 0.5 mg/ml PI and 0.025% dodecyl maltoside. The sample was centrifuged at 100,000 × g for 20 min and equal portions of supernatant and pellet were resuspended in sample buffer. Equal volumes of the supernatant and pellet fractions were run on SDS-PAGE, transferred to nitrocellulose and detected by immunoblotting using a GRK2/3 monoclonal antibody (Upstate Biotechnology) at a 1:10,000 dilution.
Activation of GRK2 by rhodopsin
The peptide RRRASAAASAA was synthesized by the solid state Merrifield method on an Applied Biosystems automated synthesizer and purified by reverse phase high performance liquid chromatography. Activation assays (20 µl) were carried out by incubating 1 mM synthetic peptide with 0.2 µM GRK2 and 2 µM Rho* for 10 min at 30°C in a buffer containing 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, and 1–2 µCi [γ32P] ATP. Control incubations were performed in the absence of the peptide. Reactions were quenched by the addition of trichloroacetic acid to a final concentration of 15% and then centrifuged for 10 min at 14,000 × g to remove phosphorylated rhodopsin. Supernatants (10 µl) were spotted onto P81 paper and washed six times with 75 mM phosphoric acid. GRK2 activity was defined as the amount of phosphate incorporated into the peptide.
Synthesis of GRK peptides and peptide inhibition studies
The Wt GRK2 peptide corresponds to the first 14 residues of GRK2 (MADLEAVLADVYSL) while the Pro peptide has a proline at position 7 in place of valine (MADLEAPLADVYSL). The GRK5 peptide corresponds to the first 13 residues of GRK5 (MELENIVANTLLK). Peptides were synthesized by the solid state Merrifield method and purified by reverse phase high performance liquid chromatography. Various concentrations of peptide (0–200 µM) were tested in phosphorylation and binding assays and kinetic studies used 1–15 µM rhodopsin in the presence or absence of 60 µM GRK2 peptide.
Circular Dichroism
The circular dichroism (CD) spectra of the GRK2 Wt and Pro peptides were determined on a Jasco-810 spectrapolarimeter at 4°C in the range of 200–280 nm using cuvettes with a pathlength of 1.0 cm. The acquisition parameters were 1 nm/min with a 4 s response and a 1 nm bandwidth. Peptides were diluted in phosphate buffered saline solution, pH 7.0 to 16 µM with various amounts of trifluoroethanol (TFE). Molar ellipticities were determined using the formula [θ] = [θ]obs (MRW)/10lc, where [θ]obs is the observed ellipticity in millidegrees, MRW is the mean residue weight, l is the cell path length in centimeters, and C is the peptide concentration in mg/ml. The relative helix content was deduced according to Chen et al. (31) as the percentage of helix = [θ]222/[θ]max 222(1-k/n) (in deg cm2 dmol−1), where [θ]222 is the observed mean residue ellipticity at 222 nm, [θ]max222 is the theoretical mean residue ellipticity for a helix of infinite length, n is the number of residues, and k is a wavelength-dependent constant (2.57 for 222 nm).
RESULTS AND DISCUSSION
The amino terminal domain of GRK2 is important for receptor phosphorylation
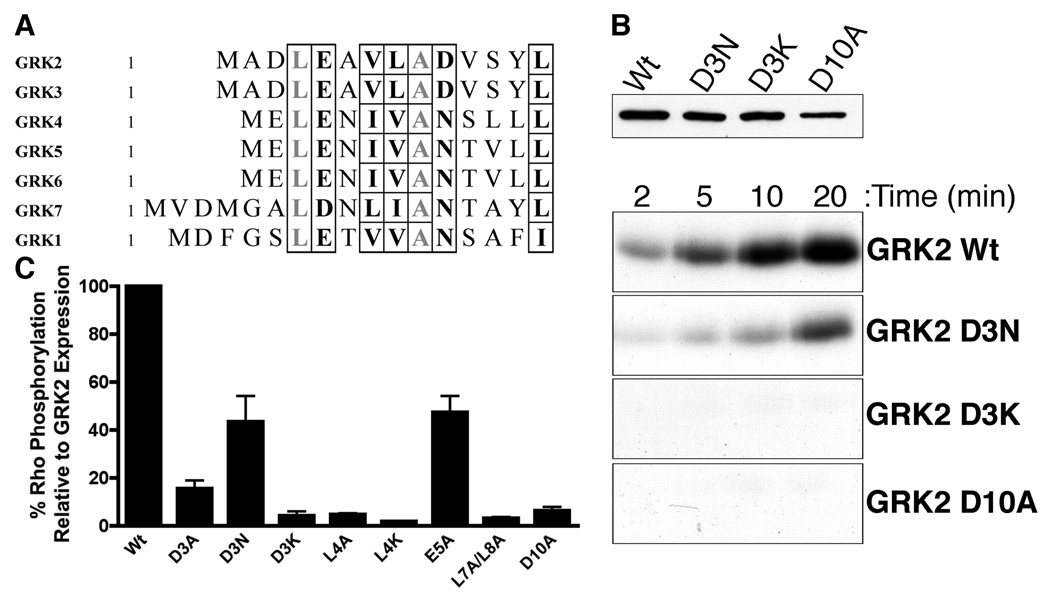
The amino terminus of GRKs has been implicated to play an important role in receptor phosphorylation. A previous study used a yeast bioassay to screen for GRK mutations that disrupt receptor phosphorylation and found multiple mutations within the first 10 residues of GRK5 (20) while another study showed that a conserved glutamic acid in GRK1 (Glu-7) and GRK2 (Glu-5) were critical for receptor phosphorylation (19). The first fourteen residues in the GRKs exhibit significant sequence conservation with an overall similarity of 50% between all GRKs, 100% within the GRK2 subfamily (GRK2 and GRK3), and 86% within the GRK4 subfamily (GRK4, GRK5 and GRK6) (Fig. 1A). To gain a better understanding of how the amino-terminus mediates receptor phosphorylation, we made several point mutations within the first 10 amino acids of GRK2. The mutants were expressed in COS-1 cells and then screened for the ability to phosphorylate rhodopsin. The results obtained for three mutants, D3N, D3K and D10A, are shown in Figure 1B. The mutants were expressed to comparable levels in COS-1 cells, although D10A was somewhat lower, and D3K and D10A were found to be completely defective in their ability to phosphorylate Rho* compared to wildtype GRK2 (Fig. 1B). Five additional point mutations in GRK2 were also generated and their ability to phosphorylate Rho* was assessed. All of the mutants tested exhibited a significant defect in mediating receptor phosphorylation (Fig. 1C). Interestingly, mutation of Asp-3 to asparagine resulted in an ~60% decrease in receptor phosphorylation while mutation to either a neutral amino acid (alanine) or an opposite charged residue (lysine) resulted in 85% and 95% reduced phosphorylation, respectively. This suggests that both the side chain and negative charge of Asp-3 contribute to mediating GPCR phosphorylation. Leu-4 mutations also had ~95% reduced activity when mutated to either alanine or lysine. The E5A mutant exhibited an ~50% decrease in Rho* phosphorylation compared to wild-type GRK2, while the V7A/L8A and D10A mutants were decreased by ~95%.
Figure 1. The first ten amino acids are critical for receptor phosphorylation.
(A) Sequence alignment of the amino terminal region of GRK1-7. Highlighted in grey are residues that are identical and in black are residues that are conserved within the extreme amino-terminus. (B). GRK2 mutants were expressed in COS-1 cells and lysates were used to phosphorylate Rho*. COS-1 cell lysates were separated by SDS-PAGE, transferred to nitrocellulose and immunoblotted for GRK2 expression. Phosphorylation reactions were performed in a total volume of 20 µl containing 3 µg of COS-1 cell lysate, 2 µM ROS, 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, ~1–2 µCi [γ32P] ATP. The reactions were exposed to light to activate rhodopsin, incubated at 30°C for 2–20 min and then analyzed by SDS-PAGE and autoradiography. (C) Rhodopsin phosphorylation by GRK2 point mutants were performed as described above and analyzed at the 10 min time point. After autoradiography, bands were excised and counted by scintillation. Data shown are the mean ± SD of 3 experiments. The amount of phosphorylation by each mutant relative to the wildtype was adjusted based on the expression of the constructs as assessed using an Odysessy Infrared Imaging System (expression varied <2-fold).
Characterization of GRK2 amino terminal mutants
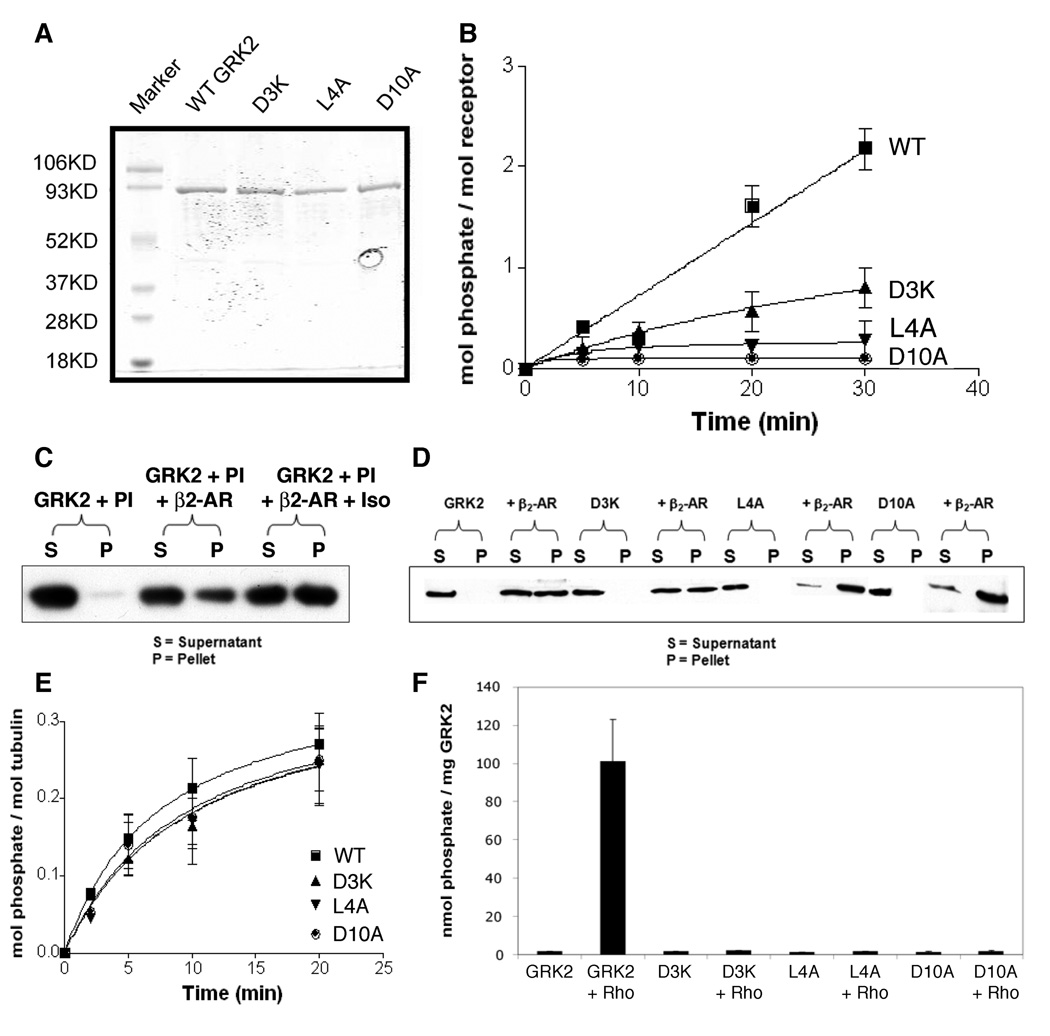
The β2AR is readily phosphophorylated by GRK2 and has been extensively used as a model receptor for studying GRKs (32). To further characterize the mutants with the largest defect in receptor phosphorylation, we expressed GRK2-D3K, -L4A and -D10A in Sf9 insect cells, purified them to near homogeneity (Fig. 2A) and assessed their ability to phosphorylate purified β2AR in the presence of the agonist isoproterenol. All three mutants had significantly reduced activity compared to wild-type GRK2 with L4A and D10A having ~90–95% lower activity and D3K ~60% lower activity (Fig. 2B).
Figure 2. GRK2 mutants are specifically defective in receptor phosphorylation.
(A) SDS-PAGE of wild-type and mutant GRK2 expressed and purified from Sf9 insect cells. Wild-type GRK2 and the GRK2 mutants D3K, L4A and D10A were cloned and expressed using the Bac to Bac expression system in Sf9 insect cells and then purified as described in Materials and Methods. One µg of each purified protein was run on SDS-PAGE and stained with Coomassie blue. (B) Activity of the mutants compared with wild-type GRK2. Phosphorylation reactions were performed in a total volume of 20 µl containing 50 nM purified β2AR in mixed micelles, 20 nM wild-type or mutant GRK2, 10 µM isoproterenol, 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, ~1 –2 µCi [γ32P] ATP. The reactions were incubated at 30°C for the times indicated and analyzed by SDS-PAGE and autoradiography. Bands were excised and counted by scintillation. Shown are the mean ± SD from 3 experiments. (C) 20 nM wildtype GRK2 was incubated with mixed micelles, 0.25 µM of β2AR in mixed micelles, or β2AR in mixed micelles in the presence of isoproterenol. Reactions were centrifuged at 100,000 × g for 15 min. Pellets and supernatants were separated and equal amounts from each fraction were run on SDS-PAGE and immunoblotted for GRK2 using a monoclonal GRK2/3 antibody. Data are representative of 3–5 independent experiments. (D) GRK2 mutants bind to β2AR. 20 nM of wildtype and GRK2 mutants were incubated with mixed micelles with or without β2AR and processed as described above. (E) Phosphorylation reactions were performed in a total volume of 20 µl containing 0.5 µM tubulin, 20 nM wild-type or mutant GRK2, 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, ~1–2 µCi [γ32P] ATP. The reactions were incubated at 30°C for the times indicated, analyzed by SDS-PAGE and autoradiography, and the bands were excised and counted by scintillation. The graph shows the mean + SD of 3 experiments. (F) Activation of wild-type or mutant GRK2 by rhodopsin. The synthetic peptide RRRASAAASAA (1 mM) was incubated with 0.2 µM wild-type or mutant GRK2 and 2 µM rhodopsin in a total volume of 20 µl in 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, ~1–2 µCi [γ32P] ATP. Reactions were exposed to light to activate rhodopsin, incubated at 30°C for 10 min, quenched by the addition of 15% trichloroacetic acid and centrifuged for 10 min at 14,000 × g. Supernatants (10 µl) were spotted on P-81 paper and washed six times with 75 mM phosphoric acid. Peptide phosphorylation was assessed by scintillation counting. Data are the mean ± SD from 3 experiments performed in duplicate.
To address whether the defect in receptor phosphorylation was due to impaired receptor binding, we developed a GRK2/β2AR binding assay. Purified β2AR in phosphatidylinositol-detergent mixed micelles was incubated with purified GRK2. Samples were subjected to highspeed centrifugation and equal volumes of supernatant and pellet fractions were immunoblotted for GRK2. In the absence of β2AR, the supernatant fraction contained the vast majority of the GRK2 (Fig. 2C). Addition of β2AR resulted in a significant increase of wild-type GRK2 in the pellet fraction, demonstrating that GRK2 is able to directly bind to the receptor. Activation of the receptor with the agonist isoproterenol had little effect on the ability of GRK2 to interact with the receptor with either no change or an ~2-fold increase in binding (Fig. 2C and data not shown). Thus, GRK2 appears to be able to bind to the β2AR in the absense of agonist, perhaps reflecting the high concentration of receptor (0.25 µM) used in the binding assay. The D3K, L4A and D10A GRK2 mutants were also able to effectively bind to the β2AR. Interestingly, the L4A and D10A mutants demonstrated enhanced association with the pellet fraction compared to wild-type GRK2 (Fig. 2D). We also used a GST construct containing the third intracellular loop of the α2A-adrenergic receptor (α2aAR) to test binding of the purified GRK2 mutants, since this construct was previously shown to directly bind GRK2 (22). The GRK2 mutants were all able to bind to the α2aAR third loop as well as wild-type GRK2 (data not shown). These data suggest that the GRK2 mutants are able to efficiently bind to GPCRs, however, they are impaired in receptor phosphorylation. Thus, the observed defect in receptor phosphorylation does not appear to be caused by deficient receptor binding.
We next tested the kinase activity of these mutants using the non-receptor substrate tubulin (33). All mutants were able to phosphorylate tubulin comparable to wild-type GRK2, demonstrating that the mutants are catalytically active and selectively deficient in receptor phosphorylation (Fig. 2E). Tubulin provides a good control for these studies since it is a bona fide GRK substrate that reflects both functional binding and catalytic activity of the kinase (33,34). In fact, the Km for the phosphorylation of tubulin by GRK2 (0.4 µM) is comparable to that observed for GRK2 phosphorylation of the β2AR (0.25 µM), and in striking contrast to the Km observed for peptide substrates (0.2–2 mM) (29,33). However, tubulin is not a particularly good substrate for GRK2 (Vmax = 17–26 nmol/min/mg) compared to the β2AR (1–2 µmol/min/mg) and, in fact, is comparable to a good peptide substrate (11 nmol/min/mg) (29,33). This suggests that tubulin likely contains domains that bind GRKs with an affinity comparable to a GPCR but that this binding does not promote closure and activation of the catalytic domain.
GRK activity can be modulated by interaction with activated receptors. For example, GRK2 is activated upon binding to agonist-occupied β2AR or Rho* (35). To test whether the GRK2 amino terminal mutants are activated by rhodopsin, we assayed the ability of the mutants to phosphorylate the peptide substrate RRRASAAASAA (35). This assay showed that light activated rhodopsin was able to activate wild-type GRK2 ~100 fold whereas it had minimal effect on the D3K, L4A and D10A mutants (Fig. 2F). Taken together, these data suggest that despite being capable of binding to the receptor, the GRK2 mutants are not activated by receptor binding and are deficient in phosphorylating receptors.
Residues 1–14 of GRK2 form an amphipathic helix
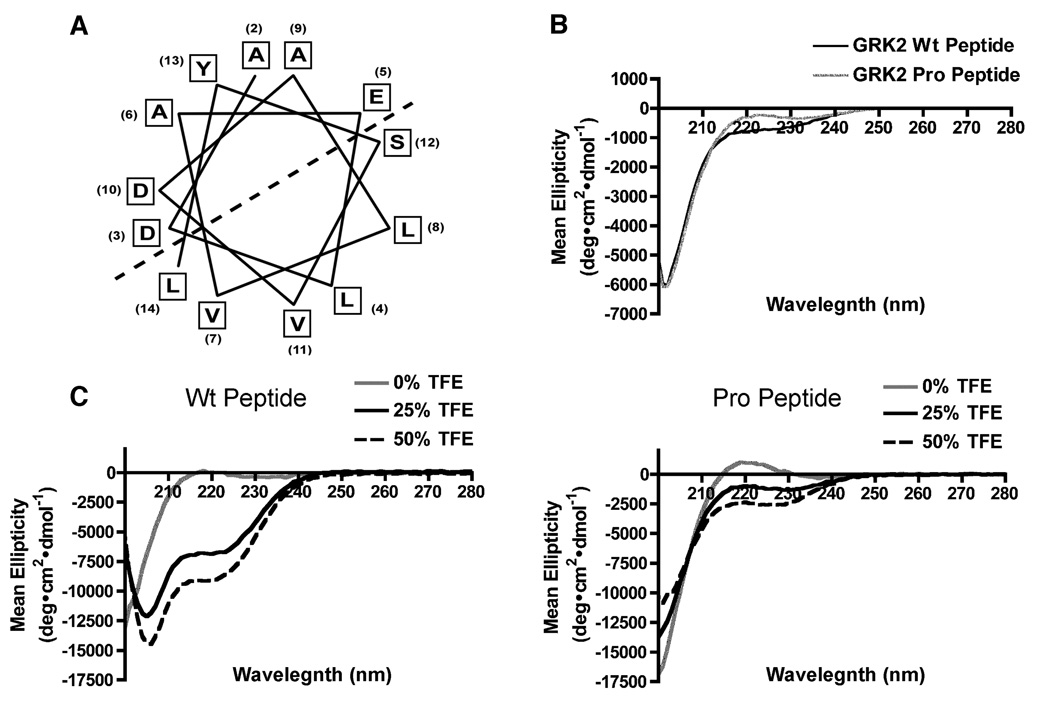
The X-ray crystal structure of the inactive form of GRK2 has been solved, revealing an equilateral triangle formed by the RH, kinase and pleckstrin homology domains (13,14). While the first 29 residues of GRK2 were not observed in the X-ray structure (13,14), analysis of residues 2–14 of GRK2 using secondary structure prediction algorithms suggest an amphipathic alpha helix, where one side is rich in hydrophobic residues and the other side rich in polar residues (Fig. 3A). The amino terminal regions of GRK1 and GRK5 have also been predicted to be alpha helices (20,26). The ability of this region to form an alpha helix was examined by circular dichroism using GRK2 peptides corresponding to the first 14 residues of GRK2 (Wt Peptide) or the first 14 residues with proline in place of valine at position 7 (Pro Peptide). Since the Wt and Pro peptides exhibited minimal helical formation in solution (Fig. 3B), trifluoroethanol (TFE), a solvent known to promote and stabilize alpha helix formation (36,37), was added to the peptides. The addition of 25% TFE to the Wt peptide induced an alpha helix, as characterized by two minimums displayed at 208 nm and 222 nM (Fig. 3C). Increasing the TFE concentration to 50% increased alpha helix formation of the peptide to ~44% helicity, while no helix formation was observed for the Pro peptide in the presence of TFE. Thus, the GRK2 N-terminal 14 amino acid region has a propensity to form an alpha helix.
Figure 3. The first 14 amino acids form an amphipathic alpha helix.
(A) Secondary structure prediction programs revealed that the first 14 amino acids of GRK2 form an amphipathic alpha helix, where one face is rich in polar residues and the other rich in hydrophobic residues as delineated by the dashed line. (B) Representative CD spectra of the GRK2 Wt (black line) and Pro (grey line) amino terminal peptides were determined in the range of 200–280 nm at 4°C using a spectrapolarimeter. (C) Increasing concentrations of the monohydric alcohol TFE increased the helicity of the GRK2 peptide. 0% TFE shown in gray represents a random coil, 25% TFE (solid black) has ~20% helicity and at 50% TFE (dashed black), there is ~44% helicity.
We hypothesize that the hydrophobic face of the helix, which includes three leucines, 2 valines and a serine in GRK2, might be involved in phospholipid binding. The charged face of the helix contains two aspartic acids, one glutamic acid, three alanines and a tyrosine (Tyr) at position 13 that can be phosphorylated by c-Src (38) and would likely contribute to the polarity of the helix. Since Asp-3 and Asp-10 on the polar side of the amphipathic helix do not appear to be involved in receptor binding (Fig. 2B), this region might be involved in an intramolecular interaction in the kinase. One region that might interact with the polar face of the N-terminus is the surface of the catalytic domain recently identified to be critical for receptor phosphorylation (28). This region includes a number of basic residues with Arg-195 being particularly important in GRK2 function (28).
A GRK2 amino terminal peptide inhibits receptor phosphorylation
In order to further characterize the GRK2 amino terminal peptide, we tested whether it could affect GRK2-mediated receptor phosphorylation. We found that the GRK2 peptide effectively inhibited isoproterenol-stimulated β2AR phosphorylation with an IC50 of ~50 µM (Fig. 4A). We also used Rho* as a substrate and found a similar pattern of inhibition by the peptide (Fig. 4B). In contrast, the peptide had no effect on GRK2 phosphorylation of the non-receptor substrate tubulin (Fig. 4C). Kinetic studies revealed that the peptide inhibited receptor phosphorylation non-competitively, with no change in the Km and a decrease in Vmax (Fig. 4D). These data suggest that the GRK2 amino terminal peptide may be binding to the kinase itself, since it does not appear to compete for kinase binding to the receptor.
Figure 4. The GRK2 amino terminal peptide inhibits β2AR phosphorylation.
(A) Increasing amounts of the GRK2 peptide were used in a phosphorylation reaction containing 50 nM purified β2AR in mixed micelles and 10 µM isoproterenol. The reaction also contained 20 nM GRK2, 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, ~1–2 µCi [γ32P] ATP. The reactions were incubated at 30°C for 10 min and analyzed by SDS-PAGE and autoradiography. Shown are the mean + SD from 3 experiments. (B) Phosphorylation reactions were performed as above using 2 µM light-activated rhodopsin as the substrate. (C) Phosphorylation reactions were performed in a total volume of 20 µl containing 0.5 µM tubulin, 20 nM GRK2, 0–100 µM GRK2 peptide, 20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 5 mM MgCl2, 0.2 mM ATP, ~1–2 µCi [γ32P] ATP. The reactions were incubated at 30°C for 10 min and analyzed by SDS-PAGE and autoradiography. Bands were excised and counted by scintillation. Shown are the mean ± SD from 3 experiments. (D) Non-competitive inhibition by the GRK2 amino terminal peptide. Phosphorylation of 1–15 µM rhodopsin was performed in the absence (solid line) or presence (dashed line) of 60 µM GRK2 peptide using conditions as described in panel A. Data were plotted using Graphpad Prism and are the mean + SD of three independent experiments. (E) The GRK2 peptide specifically inhibits GRK2 phosphorylation of β2AR. A synthetic peptide of residues 1–14 of GRK5 was generated. Phosphorylation reactions were performed as described above in the absence or presence of either 200 µM of the GRK2 or GRK5 amino terminal peptide. The mean ± SD from three experiments is shown. (F) The GRK2 peptide enhances GRK2 binding to phosphatidylinositol. Binding assays were performed as described in Materials and Methods with the addition of increasing concentrations of the GRK2 peptide (0–200 µM). In the top panel, reactions included purified GRK2, β2AR, and phosphatidylinositol. The middle panel contains GRK2 and phosphatidylinositol in the absence of β2AR and the bottom panel serves as a control with only GRK2 present. Data shown are representative of 3 independent experiments.
Our previous work using a GRK5 amino terminal peptide showed that it inhibited rhodopsin phosphorylation by GRK5 with an IC50 of 20 µM but had no effect on GRK2-mediated phosphorylation of rhodopsin (20). Here, we verified that the GRK5 peptide had no significant effect on GRK2-mediated phosphorylation of the β2AR while the GRK2 peptide effectively inhibited β2AR phosphorylation (Fig. 4E). Taken together, these results suggest that the amino terminal GRK peptides are specific for their respective kinases lending further support for this region of the kinase being involved in an intramolecular interaction.
Interestingly, addition of the GRK2 peptide to the GRK2/β2AR binding assay led to an increase in the amount of GRK2 in the pellet fraction, with ~90% in the pellet at 100 µM peptide (Fig. 4F, top panel). To test whether the GRK2 peptide enhances GRK2 interaction with phosphatidylinositol, the peptide was added to GRK2 and purified phosphatidylinositol in the absence of the receptor (Fig. 4F, middle panel). At 50 µM peptide, there was significant association of GRK2 with the pellet fraction suggesting that the GRK2 amino terminal peptide enhanced GRK2 binding to phospholipid even in the absence of receptor. When combined with previous findings demonstrating that the GRK5 amino terminal peptide may directly bind to phospholipids (20), our studies suggest an important role for the amino terminus of GRKs in membrane binding.
Proposed role of the amino terminus of GRK2 in receptor phosphorylation
In this study, we have shown that mutations in the amino terminus of GRK2 do not affect the catalytic activity or the ability of the kinase to bind to receptor, however, these mutations effectively inhibit GRK2 activation and receptor phosphorylation (Fig. 1 and Fig. 2). We also demonstrated that a peptide containing the first 14 residues of GRK2 forms an amphipathic alpha helix, inhibits GRK2-mediated receptor phosphorylation, and promotes GRK2 binding to phosphatidylinositol vesicles (Fig. 3 and Fig. 4). The amino terminal region of GRK2 appears to be flexible and unable to anchor to a specific site while the enzyme is in its inactive state as shown in the crystal structure (13,14). In light of these findings, we propose a novel regulatory mechanism whereby receptor binding regulates the ability of the amphipathic amino terminal alpha helix of GRK2 to anchor GRK2 on the inner leaflet of the cytoplasmic membrane, with the hydrophobic side binding to phospholipids and the polar side forming an intramolecular interaction with GRK2. This intramolecular interaction anchors the flexible amino terminal region, promoting a conformational change in the catalytic domain, resulting in an active kinase. We further hypothesize that the interaction with the amino terminal region takes place within the kinase domain of GRK2. This hypothesis is supported by the recent studies identifying a surface on the kinase domain that appears to be critical for receptor phosphorylation (28). In these studies, mutation of Lys-191 in GRK1 or Lys-195 in GRK2 results in an ~1000-fold decrease in kcat/Km. The GRK1-K191A mutant also has a >10-fold increase in the Km for rhodopsin phosphorylation suggesting an important role for this residue in receptor binding. The GRK1˙(Mg2+)2˙ATP structure is also informative since residues 12–23 of the N-terminus appear to be alpha helical (17) while residues 4–16 form an amphipathic alpha helix when an N-terminal GRK1 peptide is bound to recoverin (39).
The GRK1, GRK2 and GRK6 crystal structures reveal that there is a bipartite interaction between the RH and kinase domains (14,16,17). This interaction suggests several roles for the RH domain in terms of kinase activation. First, the RH domain could play a role in stabilizing the small lobe of the kinase domain in its active state through stabilizing the hydrophobic motif. Second, the RH domain could act to bridge the small and large lobes of the kinase domain. The crystal structure reveals that there is an extensive interface between the kinase domain and the α10 helix of the RH domain, mainly through salt bridges and hydrogen bonds (14,16). It is possible that binding of the amino terminal region to the kinase domain could cause a conformational change thereby bringing the small and large lobes together. This conformation would form the active state of the kinase, which could then phosphorylate its receptor substrate. Other studies have implicated such intramolecular interactions in regulating kinase activity. For example, in the Src-family kinases, the SH2 domain interacts with a phosphorylated tyrosine residue in the regulatory domain of the kinase thereby stabilizing the inactive conformation of the kinase.
In conclusion, we propose a novel mechanism for the activation of GRK2, in which the polar face of the alpha helical amino terminus is involved in an intramolecular interaction with the kinase domain. We hypothesize that this interaction is regulated by kinase binding to an activated GPCR and that this causes a conformational change that activates the kinase and enhances its ability to bind to phospholipids and phosphorylate receptor. While this proposed mechanism would likely regulate all GRKs, co-crystallization of a GPCR/GRK complex may be needed to more fully understand the conformation of an activated GRK.
ACKNOWLEDGEMENTS
We thank RoseAnn Stracquatanio for technical assistance, Drs. Kirby Steger and Michael Root for their expertise in circular dichroism, and Dr. Brian Kobilka for supplying purified β2AR.
Footnotes
This work was supported by National Institutes of Health grants R01GM44944 (to JLB) and T32DK07705 (to BLB).
The abbreviations used are: α2AAR, α2A-adrenergic receptor; β2AR, β2-adrenergic receptor; CD, circular dichroism; GPCR, G protein-coupled receptor; GRK, G protein-coupled receptor kinase; RH domain, regulator of G protein-signaling homology domain; Rho*, light-activated rhodopsin; TFE, trifluoroethanol.
REFERENCES
- 1.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 2.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 4.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 5.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 6.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 7.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 8.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 9.Onorato JJ, Gillis ME, Liu Y, Benovic JL, Ruoho AE. The β-adrenergic receptor kinase (GRK2) is regulated by phospholipids. J Biol Chem. 1995;270:21346–21353. doi: 10.1074/jbc.270.36.21346. [DOI] [PubMed] [Google Scholar]
- 10.Carman CV, Barak LS, Chen C, Liu-Chen LY, Onorato JJ, Kennedy SP, Caron MG, Benovic JL. Mutational analysis of Gβγ and phospholipid interaction with G protein-coupled receptor kinase 2. J Biol Chem. 2000;275:10443–10452. doi: 10.1074/jbc.275.14.10443. [DOI] [PubMed] [Google Scholar]
- 11.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Lodowski DT, Barnhill JF, Pyskadlo RM, Ghirlando R, Sterne-Marr R, Tesmer JJ. The role of Gβγ and domain interfaces in the activation of G protein-coupled receptor kinase 2. Biochemistry. 2005;44:6958–6970. doi: 10.1021/bi050119q. [DOI] [PubMed] [Google Scholar]
- 14.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 15.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 16.Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. The structure of G protein-coupled receptor kinase (GRK)-6 defines a second lineage of GRKs. J Biol Chem. 2006;281:16785–16793. doi: 10.1074/jbc.M601327200. [DOI] [PubMed] [Google Scholar]
- 17.Singh P, Wang B, Maeda T, Palczewski K, Tesmer JJ. Structures of rhodopsin kinase in different ligand states reveal key elements involved in G protein-coupled receptor kinase activation. J Biol Chem. 2008;283:14053–14062. doi: 10.1074/jbc.M708974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne-Marr R, Tesmer JJ, Day PW, Stracquatanio RP, Cilente JA, O'Connor KE, Pronin AN, Benovic JL, Wedegaertner PB. G protein-coupled receptor kinase 2/Gαq/11 interaction. A novel surface on a regulator of G protein signaling homology domain for binding Gα subunits. J Biol Chem. 2003;278:6050–6058. doi: 10.1074/jbc.M208787200. [DOI] [PubMed] [Google Scholar]
- 19.Yu QM, Cheng ZJ, Gan XQ, Bao GB, Li L, Pei G. The amino terminus with a conserved glutamic acid of G protein-coupled receptor kinases is indispensable for their ability to phosphorylate photoactivated rhodopsin. J Neurochem. 1999;73:1222–1227. doi: 10.1046/j.1471-4159.1999.0731222.x. [DOI] [PubMed] [Google Scholar]
- 20.Noble B, Kallal LA, Pausch MH, Benovic JL. Development of a yeast bioassay to characterize G protein-coupled receptor kinases. Identification of an NH2-terminal region essential for receptor phosphorylation. J Biol Chem. 2003;278:47466–47476. doi: 10.1074/jbc.M308257200. [DOI] [PubMed] [Google Scholar]
- 21.Benovic JL, Onorato J, Lohse MJ, Dohlman HG, Staniszewski C, Caron MG, Lefkowitz RJ. Synthetic peptides of the hamster β2-adrenoceptor as substrates and inhibitors of the β-adrenoceptor kinase. Br J Clin Pharmacol. 1990;1 30 Suppl doi: 10.1111/j.1365-2125.1990.tb05462.x. 3S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pao CS, Benovic JL. Structure/function analysis of α2A–adrenergic receptor interaction with G protein-coupled receptor kinase 2. J Biol Chem. 2005;280:11052–11058. doi: 10.1074/jbc.M412996200. [DOI] [PubMed] [Google Scholar]
- 23.Dhami GK, Babwah AV, Sterne-Marr R, Ferguson SS. Phosphorylation-independent regulation of metabotropic glutamate receptor 1 signaling requires G protein-coupled receptor kinase 2 binding to the second intracellular loop. J Biol Chem. 2005;280:24420–24427. doi: 10.1074/jbc.M501650200. [DOI] [PubMed] [Google Scholar]
- 24.Palczewski K, Buczylko J, Lebioda L, Crabb JW, Polans AS. Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J Biol Chem. 1993;268:6004–6013. [PubMed] [Google Scholar]
- 25.Dhami GK, Dale LB, Anborgh PH, O'Connor-Halligan KE, Sterne-Marr R, Ferguson SS. G protein-coupled receptor kinase 2 regulator of G protein signaling homology domain binds to both metabotropic glutamate receptor 1a and Gαq to attenuate signaling. J Biol Chem. 2004;279:16614–16620. doi: 10.1074/jbc.M314090200. [DOI] [PubMed] [Google Scholar]
- 26.Higgins MK, Oprian DD, Schertler GF. Recoverin binds exclusively to an amphipathic peptide at the N terminus of rhodopsin kinase, inhibiting rhodopsin phosphorylation without affecting catalytic activity of the kinase. J Biol Chem. 2006;281:19426–19432. doi: 10.1074/jbc.M602203200. [DOI] [PubMed] [Google Scholar]
- 27.Sterne-Marr R, Leahey PA, Bresee JE, Dickson HM, Ho W, Ragusa MJ, Donnelly RM, Amie SM, Krywy JA, Brookins-Danz ED, Orakwue SC, Carr MJ, Yoshino-Koh K, Li Q, Tesmer JJ. GRK2 activation by receptors: role of the kinase large lobe and carboxyl-terminal tail. Biochemistry. 2009;48:4285–4293. doi: 10.1021/bi900151g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CC, Yoshino-Koh K, Tesmer JJ. A surface of the kinase domain critical for the allosteric activation of G protein-coupled receptor kinases. J Biol Chem. 2009;284:17206–17215. doi: 10.1074/jbc.M809544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CM, Dion SB, Onorato JJ, Benovic JL. Expression and characterization of two β-adrenergic receptor kinase isoforms using the baculovirus expression system. Receptor. 1993;3:39–55. [PubMed] [Google Scholar]
- 30.Kobilka BK. Amino and carboxyl terminal modifications to facilitate the production and purification of a G protein-coupled receptor. Anal Biochem. 1995;231:269–271. doi: 10.1006/abio.1995.1533. [DOI] [PubMed] [Google Scholar]
- 31.Chen YH, Yang JT, Chau KH. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 32.Benovic JL, DeBlasi A, Stone WC, Caron MG, Lefkowitz RJ. β-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989;246:235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- 33.Carman CV, Som T, Kim CM, Benovic JL. Binding and phosphorylation of tubulin by G protein-coupled receptor kinases. J Biol Chem. 1998;273:20308–20316. doi: 10.1074/jbc.273.32.20308. [DOI] [PubMed] [Google Scholar]
- 34.Pitcher JA, Hall RA, Daaka Y, Zhang J, Ferguson SS, Hester S, Miller S, Caron MG, Lefkowitz RJ, Barak LS. The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J Biol Chem. 1998;273:12316–12324. doi: 10.1074/jbc.273.20.12316. [DOI] [PubMed] [Google Scholar]
- 35.Chen CY, Dion SB, Kim CM, Benovic JL. β-adrenergic receptor kinase. Agonist-dependent receptor binding promotes kinase activation. J Biol Chem. 1993;268:7825–7831. [PubMed] [Google Scholar]
- 36.Zhang YX, Yan SL, Zhou HM. Inactivation and conformational changes of aminoacyclase in trifluoroethanol solutions. J Protein Chem. 1996;15:631–637. doi: 10.1007/BF01886745. [DOI] [PubMed] [Google Scholar]
- 37.Segawa S, Fukuno T, Fujiwara K, Noda Y. Local structures in unfolded lysozyme and correlation with secondary structures in the native conformation: helix-forming or -breaking propensity of peptide segments. Biopolymers. 1991;31:497–509. doi: 10.1002/bip.360310505. [DOI] [PubMed] [Google Scholar]
- 38.Mariggio S, Garcia-Hoz C, Sarnago S, De Blasi A, Mayor F, Jr, Ribas C. Tyrosine phosphorylation of G-protein-coupled-receptor kinase 2 (GRK2) by c-Src modulates its interaction with Gαq. Cell Signal. 2006;18:2004–2012. doi: 10.1016/j.cellsig.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Ames JB, Levay K, Wingard JN, Lusin JD, Slepak VZ. Structural basis for calcium-induced inhibition of rhodopsin kinase by recoverin. J Biol Chem. 281:37237–37245. doi: 10.1074/jbc.M606913200. [DOI] [PubMed] [Google Scholar]