Abstract
WNK4 (with no lysine kinase 4) inhibits ROMK channel activity in the distal nephron by stimulating clathrin-dependent endocytosis, an effect attenuated by SGK1 (serum-glucocorticoids-induced kinase)-mediated phosphorylation. It has been suggested that increased ROMK activity because of SGK1-mediated inhibition of WNK4 plays a role in promoting renal K secretion in response to elevated serum K or high K (HK) intake. In contrast, intravascular volume depletion also increases SGK1 activity but fails to stimulate ROMK channels and K secretion. Because HK intake decreases Src family protein tyrosine kinase (PTK) activity an inhibitor of ROMK channels, it is possible that Src family PTK may modulate the effects of SGK1 on WNK4. Here, we show that c-Src prevents SGK1 from attenuating WNK4's inhibition of ROMK activity. This effect of c-Src was WNK4-dependent because c-Src had no effect on ROMK harboring mutation at the site of c-Src phosphorylation (R1Y337A) in the absence of WNK4. Moreover, expression c-Src diminished the SGK1-mediated increase in serine phosphorylation of WNK4, suggesting that c-Src enhances WNK4-mediated inhibition of ROMK channels by suppressing the SGK1-induced phosphorylation. This notion is also supported by the observation that c-Src was not able to modulate the interaction between SGK1 and WNK4 mutants (WNK4S1169A or WNK4S1169D) in which an SGK1-phosphorylation site (serine 1169) was mutated by alanine or aspartate. We conclude that c-Src inhibits SGK1-mediated phosphorylation hereby restoring the WNK4-mediated inhibition of ROMK channels thus suppressing K secretion.
Keywords: collecting duct, hyperkalemia, K secretion, Kir1.1
WNK4 is expressed in the connecting tubule (CNT) and cortical collecting duct (CCD) and plays an important role in the modulation of renal K secretion (1, 2). K secretion in the CNT and CCD takes place by K entering the cell through Na-K ATPase at the basolateral membrane and leaving the cell through apical K channels (3, 4). Two types of K channels, a Ca2+-activated big-conductance K (BK) and a small-conductance (ROMK or Kir1.1), are expressed in the apical membrane of the CNT and CCD and are responsible for K secretion (3, 5–9). It is generally accepted that ROMK channels are responsible for K secretion under normal dietary K intake, whereas both BK and ROMK channels are involved in mediating K secretion when the tubule flow rate is high or dietary K intake increases (10–12). Previous work demonstrated that WNK4 inhibited ROMK channels by stimulating its removal from the cell surface via clathrin-dependent endocytosis (13). However, this inhibitory effect is reversed by SGK1 phosphorylation of WNK4 on serine residue 1169 (14). Because a HK intake increases aldosterone release and SGK1 expression, it has been suggested that SGK1-mediated phosphorylation of WNK4 plays a key role in stimulating renal K secretion during hyperkalemia (2). Indeed, a HK intake augments the expression of ROMK channels in the apical membrane of the CNT and CCD (15–17).
Although a low Na intake increases aldosterone secretion and expression of SGK1 (18), this fails to stimulate ROMK channel activity in the CCD (19, 20), suggesting that under such conditions SGK1's inhibition of WNK4 is compromised. We have demonstrated that Src family PTK also plays an important role in regulating ROMK channels (21) and that the expression of c-Src is regulated by dietary K intake such that a HK intake suppresses whereas a low K intake increases c-Src expression (22). Therefore, the aim of the present study is to test the hypothesis that c-Src modulates the effect of SGK1 on WNK4.
Results
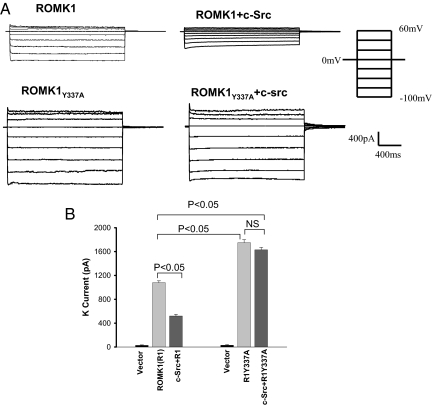
We have shown that c-Src inhibited ROMK1 channels in Xenopus oocytes (23). In the present study, we used the perforated whole-cell patch-clamp technique to examine the effect of c-Src on ROMK1 channels in HEK293T cells transfected with GFP-ROMK1 and c-Src. Twenty-four hours after transfection, we selected cells with green fluorescence, an indication of positive transfection, for experiments. K currents were measured before and after adding 0.5 mM BaCl2 to determine the Ba2+-sensitive K current. Because the endogenous Ba2+-sensitive K currents in cells transfected with empty vector were <2% of those in ROMK1 transfected cells, they were neglected. We also measured the cell capacitance, which varied between 24.5 to 26 pF. This value was used to normalize the measured K current to that of a cell with 25 pF capacitance. Fig. 1A shows a typical recording, demonstrating that K currents in cells transfected with GFP-ROMK1 were inward-rectifying and that expression of c-Src decreased K currents. Data summarized in Fig. 1B demonstrate that expression of c-Src significantly decreased K current from 1,080 ± 30 pA to 520 ± 30 pA (n = 6). We have demonstrated that tyrosine residue 337 in the c-terminus of ROMK1 is the c-Src phosphorylation site (24). We next examined the effect of c-Src on K current in HEK293T cells transfected with c-Src and GFP-ROMK1Y337A in which this tyrosine residue was mutated to alanine. As shown in Fig. 1A, it is apparent that K currents in cells transfected with ROMK1Y337A were significantly larger than those transfected with wild-type ROMK1. Moreover, expression of c-Src failed to decrease K currents in cells transfected with ROMK1Y337A. Data summarized in Fig. 1B show that K currents were 1,750 ± 50 pA with ROMK1Y337A and 1,630 ± 40 pA with ROMK1Y337A+c-Src.
Fig. 1.
c-Src inhibits ROMK1 but not RIY337A. (A) Effect of c-Src on K currents in HEK293T cells transfected with GFP-ROMK1 or GFP-ROMK1Y337A. The K currents were measured with perforated whole-cell patch-clamp from −100 to 60 mV at a step of 20 mV. (B) A bar graph summarizes the effect of c-Src on Ba2+-sensitive K currents measured with perforated whole-cell patch-clamp at −100 mV in HEK293T cells transfected with GFP-ROMK1 or GFP-ROMK1Y337A (R1Y337A). *, P < 0.05 was considered a significant difference from the control value.
We next examined the effect of WNK4 on K currents in cells transfected with either GFP-ROMK1 or GFP- ROMK1Y337A. We confirmed the finding reported by other investigators that WNK4 inhibited ROMK1 channels (13, 14) and decreased K current from 1,100 ± 30 to 560 ± 30 pA (n = 5) (Fig. 2). We found that coexpression of c-Src with WNK4 further reduced K currents in ROMK1-expressing cells to 300 ± 30 pA (n = 5), suggesting that the inhibitory effect of c-Src and WNK4 was additive. Expression of WNK4 also decreased K currents from 1,800 ± 25 to 820 ± 30 pA (n = 6) in cells transfected with GFP- ROMK1Y337A but coexpression of c-Src did not further reduce K currents (850 ± 30 pA) (n = 6), suggesting that WNK4 inhibits ROMK1Y337A channels by a c-Src-independent mechanism.
Fig. 2.
Effect of WNK4 on K currents in the presence or absence of c-Src in HEK293T cells transfected with GFP-ROMK1 or GFP-ROMK1Y337A (R1Y337A). The K currents were measured with perforated whole-cell patch-clamp at −100 mV in HEK293T cells transfected with GFP-ROMK1 or GFP-ROMK1Y337A.
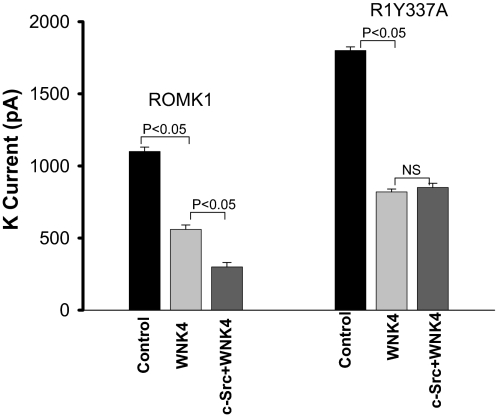
It has been shown that the inhibitory effect of WNK4 on ROMK channels is reversed by SGK1, via phosphorylation of WNK4 (14). We confirmed this effect of SGK1 on WNK4 and demonstrated that coexpression of SGK1 augmented K currents from 560 ± 30 pA (with WNK4 alone) to 1,030 ± 30 pA (with SGK1+WNK4), a value not significantly different from that seen in control expressing only ROMK1 (1,100 ± 35 pA, n = 6) (Fig. 3). Data summarized in Fig. 3 also show that SGK1 increases ROMK1 channel activity (K currents 1,700 ± 30 pA in cells transfected with SGK1+ROMK1) (n = 6). Interestingly, c-Src inhibited ROMK1 channels in the presence of SGK1, reducing K currents from 1,700 ± 30 pA to 750 ± 30 pA (n = 6). Most significantly, expression of c-Src also restored the inhibitory effect of WNK4 on ROMK1 channels in cells transfected with SGK1. Fig. 3 shows that K currents in cells transfected with SGK1+WNK4+c-Src were significantly lower (620 ± 20 pA) than in those with SGK1+WNK4 (1,030 ± 30 pA) but they were not different from those in cells transfected with WNK4 in the absence of SGK1 (560 ± 30 pA).
Fig. 3.
SGK1 abolishes the effect of WNK4 on ROMK channels whereas c-Src restores the inhibitory effect of WNK4. The Ba2+-sensitive K currents were measured with perforated whole-cell patch- clamp at −100 mV in HEK293T cells transfected with GFP-ROMK1 (control), GFP-ROMK1+WNK4, GFP-ROMK1+SGK1+WNK4, SGK1+GFP-ROMK1, c-Src+SGK+WNK4+GFP-ROMK1, and SGK1+c-Src+GFP-ROMK1, respectively.
The notion that c-Src enhanced the inhibitory effect of WNK4 on ROMK1 channels was further tested by examining the effect of c-Src on the effect of WNK4 on ROMK1Y337A. The advantage in using ROMK1Y337A is that c-Src has no effect on ROMK1Y337A in the absence of WNK4 (see Fig. 1).
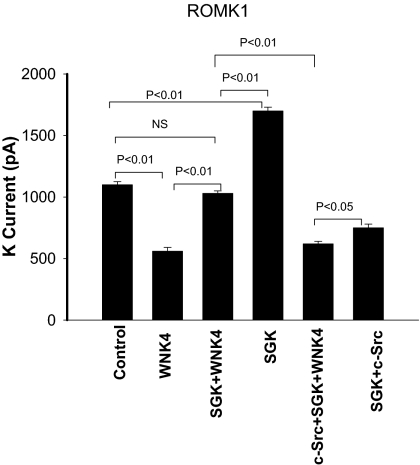
Data summarized in Fig. 4 show that expression of SGK1 not only significantly increased K currents in cells transfected with GFP- ROMK1Y337A from 1,800 ± 24 pA to 2,570 ± 80 pA (n = 6) but also abolished the inhibitory effect of WNK4 on ROMK channels: SGK1 increased K currents from 826 ± 20 pA in cells transfected with WNK4+GFP- ROMK1Y337A to 1,730 ± 70 pA, a value not different from the control. Also, c-Src alone had no effect on ROMK1Y337A because K currents in cells transfected with c-Src, SGK1 and ROMK1Y337A were similar (2,500 ± 80 pA) to those with SGK1+ ROMK1Y337A. However, expression of c-Src reversed the effect of SGK1 on WNK4 and restored the WNK4-mediated inhibition of K channels. In cells transfected with ROMK1Y337A+c-Src+WNK4+SGK1, K currents were 980 ± 40 pA (n = 7), a value not significantly different from those with WNK4, and significantly lower than those with WNK4+SGK1 (1,730 ± 70 pA). The results, therefore, strongly suggest that c-Src can inhibit ROMK1 channel activity not only through direct phosphorylation of ROMK1 channels but also through attenuation of the inhibitory effect of SGK1 on WNK4.
Fig. 4.
(A) SGK1 abolishes the effect of WNK4 on ROMK1Y337A (R1Y337A) channels whereas c-Src restores the inhibitory effect of WNK4. The Ba2+-sensitive K currents were measured with perforated whole-cell patch-clamp at −100 mV in HEK293T cells transfected with GFP-ROMK1Y337A(control), GFP-ROMK1Y337A+WNK4, GFP-ROMK1Y337A+SGK1+WNK4, SGK1+GFP-ROMK1Y337A, SGK1+c-Src+GFP-ROMK1Y337A, and c-Src+SGK+WNK4+GFP-ROMK1Y337A, respectively. (B) Western blot showing the expression of ROMK, WNKA, c-Src, and SGK1.
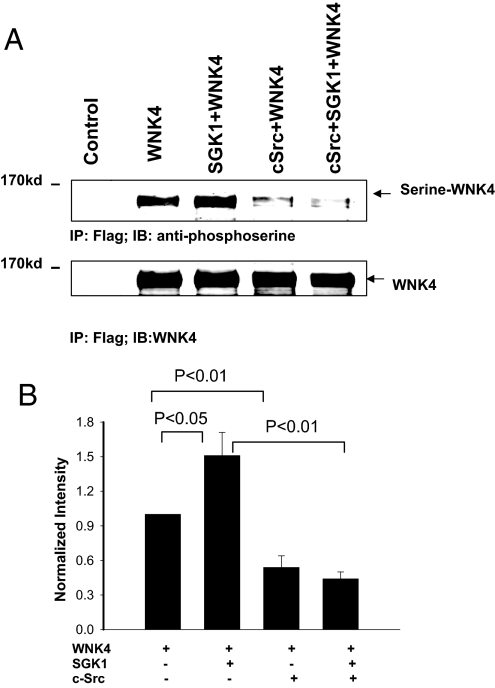
WNK4 has been shown to be a substrate of SGK1 at serine 1169 (ser1169) (14). If c-Src restores the inhibitory effect of WNK4 on ROMK1 by modulating the SGK1-mediated phosphorylation of WNK4, expression of c-Src should decrease the serine phosphorylation of WNK4. This notion was tested by examining serine phosphorylation of WNK4 in the presence and absence of c-Src. HEK293T cells were transfected with flag-tagged-WNK4 alone, WNK4+SGK1, WNK4+c-Src, and WNK4+SGK1+c-Src. Twenty-four hours after transfection, WNK4 proteins were harvested by immunoprecipitation and serine phosphorylation of WNK4 was measured by Western blotting with anti-phosphoserine antibodies (Fig. 5). The results show that SGK1 increased WNK4 phosphorylation by 50% (n = 4), an effect that was attenuated by coexpression with c-Src; interestingly, c-Src also reduced phosphorylation of WNK4 in the absence of SGK1. Although the basal level of WNK4 phosphorylation detected with serine phosphorylation antibody might include serine phosphorylation sites other than serine residue 1169, increased phosphorylation of WNK4 induced by SGK1 should include serine residue 1169, speculation that is supported by finding increased phosphorylation of WNK4 with SGK1
Fig. 5.
c-Src abolishes SGK1-mediated serine phosphotylation of WNK4. (A) Effect of SGK1 on serine phosphorylation in the presence or absence of c-Src in HEK293T cells transfected with empty vector (control), flag-tagged-WNK4 (WNK4), WNK4+SGK1, WNK4+c-Src, and WNK4+SGK1+c-Src. The Flag-tagged WNK4 was harvested through immunoprecipitation of cell lysates with Flag antibody. Serine phosphorylated WNK4 was detected with serine phosphorylation antibody. (B) Bar graph summary of results from four experiments.
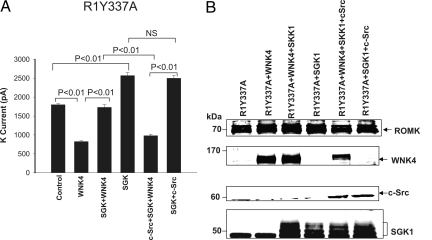
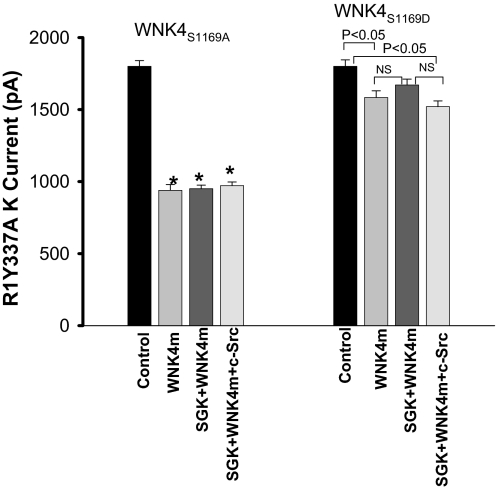
If the c-Src-induced restoration of WNK4-mediated inhibition of ROMK channels is due to modulation of WNK4 phosphorylation by SGK1, mutation of Ser-1169 of WNK4 should attenuate or abolish the effect of c-Src. We tested this hypothesis by examining the effect of c-Src on ROMK1Y337A in cells transfected with two WNK4 mutants, WNK4S1169A and WNK4S1169D, in which Ser-1169 was replaced by alanine (mimicking dephosphorylation) or aspartate (mimicking phopsphorylation). Results summarized in Fig. 6 demonstrate that WNK4S1169A inhibited ROMK1Y337A channels, from 1,780 ± 30 pA to 940 ± 40 pA (n = 5). Importantly, however, neither SGK1 nor c-Src+SGK1 altered the inhibitory effect of this WNK4 mutant on ROMK1Y337A channels (SGK1+WNK4S1169A, 950 ± 30 pA; SGK1+WNK4S1169A+c-Src, 970 ± 35 pA). Conversely, expression of WNK4S1169D had almost no inhibitory effect on ROMK1Y337A channels (1,600 ± 50 pA) (n = 5) compared with the control value (1,800 ± 50 pA), suggesting that WNK4S1169D mimicked the effect of the SGK1-phosphorylated WNK4. This lack of effect was not altered by expression of SGK1 (1,670 ± 40 pA) or by coexpression of c-Src and SGK1 (1,500 ± 40 pA, n = 5). These results demonstrate that the effects of SGK1 and c-SRC on WNK4's inhibitory effect on ROMK1 require serine at position 1169, and are consistent with modulated phosphorylation at this site determining the ability of WNK4 to inhibit ROMK1.
Fig. 6.
Effect of WNK4 mutants (WNK4m), WNK4S1169A, or WNK4S1169D, on K currents in the presence or absence of SGK1 or c-Src+SGK1. The Ba2+-sensitive K currents were measured with perforated whole-cell-patch clamp at −100 mV in HEK293T cells transfected with GFP-ROMK1Y337A (R1Y337A)(control), GFP-ROMK1Y337A+WNK4m, GFP-ROMK1Y337A +SGK1+WNK4m, and SGK1+GFP-ROMK1Y337A+WNK4m+c-Src, respectively. *, significant difference from the control.
Discussion
The present study provides evidence that c-Src modulates WNK4-mediated inhibition of ROMK channels in the presence of SGK1. ROMK channels play an important role in mediating K secretion in the CNT and CCD (25) and their activity is regulated by several factors including Src-family PTK, SGK1, and WNK4 (14, 23, 26). We have demonstrated that the Src family PTK phosphorylates ROMK1 channel at tyrosine residue 337 (24) thereby facilitating internalization of ROMK channels (27). Moreover, low K intake stimulates Src family PTK and increases tyrosine phosphorylation of ROMK channels (28), thereby inhibiting ROMK channels and K secretion in the CCD.
In addition to c-Src, WNK4 is also expressed in the CNT and CCD and has been shown to inhibit ROMK channels (13, 14). The importance of WNK4 in regulating renal K secretion is demonstrated in patients with WNK4 mutation exhibiting familial hyperkalemic hypertension resulting from increased Na absorption and diminished K secretion in the distal nephron (2, 13). The inhibitory effect of WNK4 on ROMK channels depends on stimulating clathrin-mediated endocytosis because deletion of tyrosine-based clathrin binding motif of ROMK channels abolishes the effect of WNK4 (13). It has been also demonstrated that intersectin, a scaffold protein containing two Eps15 homology domains, is required for the WNK4-mediated and clathrin-dependent endocytosis of ROMK channels (29).
Whereas both WNK4 and c-Src inhibit ROMK channels, SGK1 has been reported to stimulate ROMK1 channels in Xenopus oocytes by facilitating the phosphorylation of ROMK channels at serine residue 44 thereby enhancing the export of ROMK channels from the ER (26, 30). However, serine residue 44 of ROMK1 is also a putative phosphorylation site for protein kinase A (31). Therefore, it is not clear whether PKA or SGK1 is responsible for the phosphorylation of ROMK1 in the native tubule. SGK1 also suppresses the effect of WNK4 on ROMK channels through the phosphorylation of WNK4 at serine residue 1169 (14). Thus, SGK1 and WNK4 interaction should play an important role in stimulating renal K secretion during hyperkalemia which is expected to increase SGK1 activity.
In the present study, we provide several lines of evidence to suggest that Src family PTK is involved in modulating the interaction between SGK1 and WNK4. First, the expression of c-Src antagonized the effect of SGK1 on WNK4 and restored the inhibitory effect of WNK4 on ROMK channels. The inhibitory effect of c-Src on ROMK channels was not due to directly stimulating tyrosine phosphorylation of ROMK channels because the c-Src-induced restoration of WNK4-mediated inhibition was also observed in ROMK1 mutant (ROMK1Y337A) that was not sensitive to c-Src in the absence of WNK4 (see Fig. 1). Second, in the presence of c-Src, SGK1 was not able to abolish WNK4-mediated inhibition of ROMK channels. Third, the expression of c-Src attenuated SGK1-mediated serine phosphorylation of WNK4, suggesting that c-Src plays an important role in modulating the effect of SGK1 on WNK4 phosphorylation.
Genetic, biochemical and animal model studies have implicated WNK4 in regulation of the balance between renal salt reabsorption and K secretion (2). It has been proposed that alternative states of WNK4 promote either K secretion (by increasing ENaC activity and ROMK channel activity) or Na-Cl reabsorption by increasing activity of the thiazide-sensitive Na-Cl cotransporter along with ENaC and paracellular Cl- permeability while inhibiting ROMK activity. These alternative states could correspond to the physiologic states of hyperkalemia and intravascular volume depletion, respectively. In the former state, aldosterone levels are elevated but angiotensin II levels are not whereas both are elevated in the latter. Aldosterone increases expression of SGK1, which phosphorylates WNK4 at S1169, abrogating WNK4's inhibition of ROMK. If SGK1 expression is also increased by volume depletion, why doesn't this state result in increased ROMK channel activity? We have shown that c-Src prevents SGK1 from inhibiting WNK4's inhibition of ROMK activity, a function that would be consistent with preserving inhibition of ROMK1 in the setting of volume depletion. Consistent with this speculation, there is evidence that c-Src activity is increased by signaling through the angiotensin II receptor (32). These findings provide a mechanism by which SGK1 action can be modulated to distinguish the physiologic response to hyperkalemia and volume depletion.
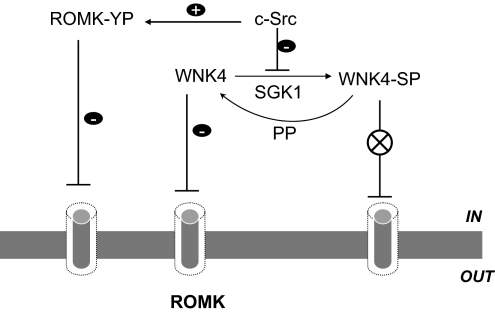
Fig. 7 is a cell scheme illustrating the possible mechanisms by which c-Src and SGK1 modulate WNK4 effect in principal cell. In the absence of c-Src, a high SGK1 activity is expected to stimulate ROMK channels and K secretion through increasing WNK4 phosphorylation and abolishing the inhibitory effect of WNK4. Such mechanism could play a role in stimulating renal K secretion in response to a HK intake which decreases c-Src activity (22). Thus, an increased expression of SGK1 and decreased expression of Src family PTK should work in concert to stimulate the ROMK channel activity during a HK intake. Therefore, suppressing WNK4-mediated inhibition of ROMK channels is an important mechanism by which a HK intake stimulates ROMK channels and K secretion. During low K intake, which increases the expression and activity of Src family PTK (28), PTK inhibits ROMK channels in the CCD by two mechanisms: i) c-Src directly inhibits ROMK channels by stimulation of tyrosine phosphorylation; ii) c-Src diminishes the effect of SGK1 on WNK4 and shifts WNK4 to a nonphosphorylated state thereby inhibiting ROMK channels.
Fig. 7.
A cell scheme illustrating the possible mechanism by which c-Src modulates the interaction between WNK4 and SGK1 and inhibits ROMK channels in the CCD. Abbreviations: ROMK-YP, tyrosine phosphorylated ROMK; WNK4-SP, serine phosphorylated WNK4; PP, protein phosphatase. Arrow and a line with bar mean stimulation and inhibition, respectively. A circle with a cross indicates blockade.
Fig. 7 also illustrates that the interaction among c-Src, SGK1 and WNK4 plays a role in preventing K waste during Na-restriction which has been shown to stimulate ENaC but has no significant effect on ROMK channels in the CCD (20). Low Na intake increases not only SGK1 activity but also a high c-Src activity because low Na intake increases the renin-angiotensin II pathway which may stimulate c-Src activity (32). The presence of c-Src opposes the effect of SGK1 on WNK4 phosphorylation thereby keeping WNK4 in a nonphosphorylated mode that restores the inhibitory effect on ROMK channels. Therefore, although low Na stimulates SGK1 activity in the distal nephron, SGK1 fails to increase apical K channels in the CCD. Finally, the model also illustrates the importance of aldosterone and SGK1 in modulating renal K secretion. Increased aldosterone secretion and SGK1 expression stimulate both Na-K-ATPase and ENaC (33, 34), leading to increasing the driving force for K secretion across the apical membrane. Moreover, SGK1 plays a key role in switching WNK4 from nonphosphorylated to phosphorylated state which suppresses the WNK4-mediated inhibition of ROMK channels. Therefore, in the absence of aldosterone or SGK1 suppressing c-Src activity by a HK intake would not be sufficient to stimulate ROMK channels in the CCD.
In addition to WNK4, WNK1 has also been shown to inhibit ROMK channels in the CCD (35). However, high K intake increases the expression of a kidney specific splice form of WNK1 (KS-WNK1), in which an alternative 5′exon replaces the first four exons of WNK1 (36). Because KS-WNK1 does not block ROMK channels and antagonizes the inhibitory effect of WNK1 (37, 38), an increase in KS-WNK1 expression should inhibit the effect of WNK1 on ROMK channels and indirectly stimulate ROMK channels in the CCD during high K intake. We conclude that the Src family PTK plays a significant role in modulating the effect of WNK4 on ROMK channels in the presence of SGK1: SGK1 reverses the inhibitory effect of WNK4 on ROMK channels during a low PTK activity but SGK1-induced inhibition of WNK4 effect is diminished in the presence of high PTK activity.
Materials and Methods
Cell Culture and Transient Transfection.
HEK293T cells were purchased from the American Type Culture Collection and grown in Dulbecco's modified Eagle medium (DMEM; Invitrogen) supplemented with 10% FBS (Invitrogen) in 5% CO2 and 95% air at 37 °C. The cells were transfected at 50–70% confluence with the cDNA using Lipofectamine 2000 transfection reagent (Invitrogen) with the magnet-assisted lipofection enhancer (IBA) as described by the manufacturers. After transfection the cells were incubated for an additional 24 h before use.
Electrophysiology Experiment.
Within 24 h after transfection, the cells were treated with trypsin-containing medium (Tryple Ecpresscare) (Gibco) for 10 min to detach the cells. The cell suspension (0.2 mL in volume) was carefully removed to a 5 × 5-mm cover glass coated with polylysin followed by additional incubation for 30 min to allow the cells to adhere to the cover glass. The cover glass was transferred to a chamber (1 mL) mounted on the stage of a Nikon inverted microscope. For the perforated whole-cell patch-clamp experiments, the cells were incubated with a bath solution containing 138 mM KCl, 0.5 mM MgCl2, 1.5 mM CaCl2, and 10 mM Hepes (pH 7.4). The experiments were performed at room temperature. Fluorescence signal (an indication of positive transfection) was detected with an intensified video imaging system including SIT 68 camera (Long Island Industries). Borosilicate glass (1.7-mm OD) was used to make the patch-clamp pipettes that were pulled with a Narishege electrode puller. The pipette had a resistance of 2–4 MΩ when filled with 138 mM KCl. The tip of the pipette was filled with pipette solution containing 138 mM KCl, 4 mM MgCl2, 1 mM CaCl2, 1 mM EGTA, and 5 mM Hepes (pH 7.4). Then, the pipette was then back-filled with amphotericin B (2 μg/0.1 mL) containing pipette solution. After forming a high resistance seal (>2 GΩ), the membrane capacitance was monitored until the whole-cell patch configuration was formed. The cell membrane capacitance was measured and compensated. The K current was measured by an Axon 200A patch-clamp amplifier. The current was low-pass filtered at 1 KHz and digitized by an Axon interface (Digidata 1200) and data were stored in an IBM-compatible computer and were analyzed using the pClamp software system 7 (Axon).
Preparation of Protein Samples.
The cells were placed in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 1% Nonidet P-40 (pH 8.0), and protease inhibitor mixture (1%) (Sigma) was added to the lysis buffer. The tissues were then homogenized and allowed to sit on ice for an additional 30 min. The sample was subjected to centrifugation at 13,000 rpm for 8 min at 4 °C, and protein concentrations were measured in duplicate using a Bio-Rad Dc protein assay kit.
Immunoprecipitation and Western Blot Analysis.
The corresponding antibody was added to the protein samples (500 μg) harvested from cell cultures with a ratio of 4 μg/1 mg protein. The mixture was gently rotated at 4 °C overnight, followed by incubation with 25 μL protein A/G plus agarose (Santa Cruz) for an additional 2 h at 4 °C. The tube containing the mixture was centrifuged at 3000 rpm and the agarose bead pellets were mixed with 25 μL 2× SDS sample buffer containing 4% SDS, 100 mM Tris·HCl (pH 6.8), 20% glycerol, 200 mM DTT, and 0.2% bromophenol blue. After boiling the sample for 5 min, the proteins were resolved by electrophoresis on 8% SDS-polyacrylamide gels followed by transferring them to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS), rinsed and washed with 0.05% Tween20-TBS buffer. The membranes were washed 3 times with PBS and scanned by Odyssey infrared imaging system (LI-COR) at a wave-length of 680 or 800 nM.
Experimental Materials and Statistics.
Anti-phosphoserine was obtained from Millipore. Antibodies to WNK4 and Flag were purchased from Abcam and Sigma, respectively. The data are presented as mean ± SEM. We used a one-way ANOVA test to determine the statistical significance. P <0.05 was considered to be significant.
Acknowledgments.
This work was supported by National Institutes of Health Grants DK54983 (to W.H.W.) and DK081594 (to Q.L.) and American Heart Association Grant SDG:0830389N (to Q.L.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Subramanya AR, Yang CL, McCormick JA, Ellison DH. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int. 2006;70:630–634. doi: 10.1038/sj.ki.5001634. [DOI] [PubMed] [Google Scholar]
- 2.Kahle KT, Ring AM, Lifton RP. Mol Physiology of the WNK Kinases. Ann Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 3.Palmer LG. Potassium secretion and the regulation of distal nephron K channels. Am J Physiol. 1999;277:F821–F825. doi: 10.1152/ajprenal.1999.277.6.F821. [DOI] [PubMed] [Google Scholar]
- 4.Giebisch G. Renal potassium transport: Mechanisms and regulation. Am J Physiol Renal Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 5.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol. 1989;256:F143–F151. doi: 10.1152/ajprenal.1989.256.1.F143. [DOI] [PubMed] [Google Scholar]
- 6.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol. 2004;287:F1030–F1037. doi: 10.1152/ajprenal.00169.2004. [DOI] [PubMed] [Google Scholar]
- 7.Frindt G, Palmer LG. Ca-activated K channels in apical membrane of mammalian CCT, and their role in K secretion. Am J Physiol. 1987;252:F458–F467. doi: 10.1152/ajprenal.1987.252.3.F458. [DOI] [PubMed] [Google Scholar]
- 8.Ho K. The ROMK-cystic fibrosis transmembrane conductance regulator connection: New insights into the relationship between ROMK and cystic fibrosis transmembrane conductance regulator channels. Curr Opin Nephrol Hypertens. 1998;7:49–58. doi: 10.1097/00041552-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol. 1997;272:F397–F404. doi: 10.1152/ajprenal.1997.272.3.F397. [DOI] [PubMed] [Google Scholar]
- 10.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 11.Satlin LM. Developmental regulation of expression of renal potassium secretory channels. Curr Opin Nephrol Hypertens. 2004;13:445–450. doi: 10.1097/01.mnh.0000133979.17311.21. [DOI] [PubMed] [Google Scholar]
- 12.Bailey MA, et al. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter's syndrome and in adaptation to a high K diet. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 13.Kahle KT, et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 14.Ring AM, et al. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frindt G, Shah A, Edvinsson J, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol. 2009;296:F347–F354. doi: 10.1152/ajprenal.90527.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol. 1999;277:F805–F812. doi: 10.1152/ajprenal.1999.277.5.F805. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Schwab A, Giebisch G. Regulation of small-conductance K channel in apical membrane of rat cortical collecting tubule. Am J Physiol. 1990;259:F494–F502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- 18.Bhargava A, et al. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology. 2001;142:1587–1594. doi: 10.1210/endo.142.4.8095. [DOI] [PubMed] [Google Scholar]
- 19.Palmer LG, Frindt G. Regulation of apical membrane Na and K channels in rat renal collecting tubules by aldosterone. Seminars Nephrol. 1992;12:37–43. [PubMed] [Google Scholar]
- 20.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol. 1994;104:693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang WH. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channels. Am J Physiol Renal Physiol. 2000;278:F165–F171. doi: 10.1152/ajprenal.2000.278.1.F165. [DOI] [PubMed] [Google Scholar]
- 23.Moral Z, et al. Regulation of ROMK1 channels by protein-tyrosine kinase and -tyrosine phosphatase. J Biol Chem. 2001;276:7156–7163. doi: 10.1074/jbc.M008671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin DH, et al. K depletion increases the protein tyrosine-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol. 2002;283:F671–F677. doi: 10.1152/ajprenal.00160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo D, et al. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem. 2003;278:23066–23075. doi: 10.1074/jbc.M212301200. [DOI] [PubMed] [Google Scholar]
- 27.Lin DH, et al. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol. 2004;286:F881–F892. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Effect of dietary K intake on the apical small-conductance K channel in the CCD: Role of protein tyrosine kinase. Am J Physiol Renal Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 29.He G, Wang HR, Huang SK, Huang C-L. Intersectin links WNK kinase to endocytosis of ROMK1. J Clin Invest. 2007;117:1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connell AD, et al. Phosphorylation-regulated endoplasmic reticulum retention signal in the renal outer-medullary K+ channel (ROMK) Proc Natl Acad Sci USA. 2005;102:9954–9959. doi: 10.1073/pnas.0504332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho K, et al. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 32.Ohtsu H, et al. Angiotensin II signal transduction through small GTP-binding proteins: Mechanism and significance in vascular smooth muscle cells. Hypertension. 2006;48:534–540. doi: 10.1161/01.HYP.0000237975.90870.eb. [DOI] [PubMed] [Google Scholar]
- 33.Palmer LG, Antonian L, Frindt G. Regulation the the Na-K pump of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993;102:43–57. doi: 10.1085/jgp.102.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossier BC, Canessa CM, Schild L, Horisberger J-D. Epithelial sodium channels. Curr Opin Nephrol Hypertens. 1994;3:487–496. doi: 10.1097/00041552-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Cope G, et al. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol. 2006;17:1867–1874. doi: 10.1681/ASN.2005111224. [DOI] [PubMed] [Google Scholar]
- 36.O'Reilly M, Marshall E, Speirs HJL, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol. 2003;14:2447–2456. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- 37.Wade JB, et al. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA. 2006;103:8558–8563. doi: 10.1073/pnas.0603109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA. 2006;103:1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]