Abstract
Uropathogenic Escherichia coli invade bladder epithelial cells (BECs) by direct entry into specialized cAMP regulated exocytic compartments. Remarkably, a significant number of these intracellular bacteria are subsequently expelled in a nonlytic and piecemeal fashion by infected BECs. Here, we report that expulsion of intracellular E. coli by infected BECs is initiated by the pattern recognition receptor, Toll-like receptor (TLR)4, after activation by LPS. Also, we reveal that caveolin-1, Rab27b, PKA, and MyRIP are components of the exocytic compartment, and that they form a complex involved in the exocytosis of bacteria. This capacity of TLR4 to mediate the expulsion of intracellular bacteria from infected cells represents a previously unrecognized function for this innate immune receptor.
Keywords: caveolin, exocytosis, signaling, urinary tract infection, uropathogenic Escherichia coli
During their lifetimes, 10–20% of American females will receive medical attention for a urinary tract infection (UTI), and ≈3% will experience more than one infection per year (1, 2). UTIs represent the second leading cause of physician visits in the U.S., costing the health care system over $2 billion per year (1, 2). Interestingly, when compared with other mucosal surfaces, the UT is difficult to colonize. Much of the resistance of the UT is attributable to the flushing actions of urine and to the impermeability of the epithelial lining. Because of their specialized role in storing urine, the apical surface of superficial bladder epithelial cells (BECs) is lined by scalloped-shaped plaques comprising a tightly interlaced latticework of proteins called uroplakins (3). These proteins are closely associated with a collection of lipids, sphingolipids, and cholesterol, often referred to as lipid rafts that cumulatively constitute a surface that is highly impregnable to urine, solutes, and potential pathogens (3).
Uropathogenic Escherichia coli (UPEC) are uniquely successful in overcoming the bladder defenses, accounting for >85% of all bladder infections. The singular success of UPEC in the UT is ascribed primarily to its capacity to penetrate and harbor within the superficial BECs (4). Several studies have reported that UPEC also assume distinct intracellular shapes and form “intracellular bacterial communities” (IBCs) in the cytoplasm of superficial epithelial cells of both rodent and human bladders (5–7). This ability of UPEC to successfully breach the mucosal barrier and colonize these cells represents a critical initiating event in the development of UTIs.
E. coli invasion of BECs has been reported to involve several components of lipid rafts such as caveolin-1, an integral membrane protein found in the inner leaflet of the lipid bilayer on many mammalian cells, and Rac1, a member of the Rho family of GTPases (8). A remarkable aspect of E. coli entry into BECs came from the recent finding that, after entry, the bacteria were harbored within specialized exocytic vesicles (9). These compartments (or fusiform vesicles) in BECs perform an important physiologic function by providing the necessary membranes required for bladder expansion. As urine volume increases, these vesicles fuse with the apical bladder surface in a cAMP-dependent manner allowing bladder expansion. That E. coli are harbored in fusiform compartments of infected BECs was confirmed by an immune microscopy, which revealed bacteria encased in membranes closely associated with Rab27b, a marker of fusiform vesicles (9).
Surprisingly, a significant number of intracellular E. coli, harbored within exocytic compartments of cultured BECs, were subsequently returned into the extracellular medium in piecemeal fashion (9). Seemingly, BECs have the innate capacity to sense and expel infecting bacteria. Here, we sought to examine the specificity of this host cell response and elucidate the underlying mechanism.
Results
Expulsion of E. coli from Infected Human BECs.
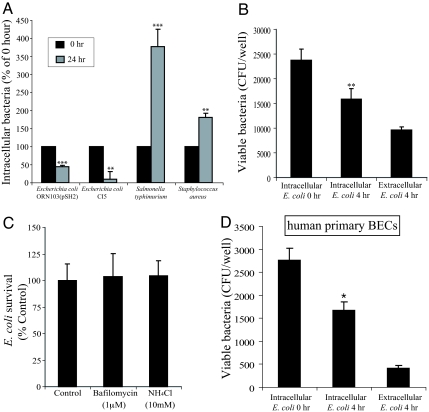
Our earlier studies have suggested that infected BECs have the capacity to spontaneously exocytose UPEC (9). We sought to examine whether this exocytic response of BECs was specific to E. coli, and if so, to identify the bacterial component(s) responsible. We infected the 5637 human BEC line with E. coli CI5, a previously described UTI isolate (12), as well as ORN103(pSH2), a K12 strain of E. coli expressing type 1 fimbriae, the critical bacterial component responsible for E. coli entry into BECs (10). To examine the specificity of the BEC response, we also used two other bacterial strains Salmonella typhimurium SL1344 and Staphyloccus aureus 54 in this assay. After 1 h of infection with each of the 4 strains of bacteria, a standard gentamicin protection assay was undertaken, and after 24 h, intracellular bacterial numbers assessed again. Whereas the numbers of intracellular S. typhimuium SL1344 and S. aureus 54 had markedly increased implying intracellular growth, the same could not be said of E. coli CI5 or E. coli ORN103(pSH2) (Fig. 1A). We found a ≈60% drop in intracellular E. coli numbers (Fig. 1A), suggesting active E. coli exocytosis by infected BECs, although we cannot rule out the possibility of intracellular bacterial killing. In any case, our findings suggest that this drop in intracellular numbers of E. coli is a unique phenomenon, because significant increases in intracellular S. typhimurium and S. aureus numbers were observed under the same incubation conditions (Fig. 1A).
Fig. 1.
E. coli exocytosis from infected BECs. (A) The 5637 BECs were infected with various bacteria for 1 h, after which a standard gentamicin protection assay was performed to quantify the number of intracellular bacteria (0 h). A significant decrease in intracellular E. coli ORN103(pSH2) and E. coli CI5 was seen at 24 h after the addition of gentamicin (24 h). Unlike E. coli ORN103(pSH2) and E. coli CI5, the numbers of intracellular S. typhimurium SL1344 and S. aureus 54 were significantly increased at 24 h after initial infection and after gentamicin treatment. (B) Intracellular and extracellular E. coli ORN103(pSH2) numbers in BECs at 0 and 4 h after gentamicin treatment; 5637 BECs were infected with 1000 MOI E. coli ORN103(pSH2). The sum of numbers of intracellular and extracellular E. coli at 4 h after gentamicin treatment was similar to intracellular bacteria numbers at 0 h, suggesting bacterial exocytosis from infected BECs. (C) Treatment of infected BECs with NH4Cl and Bafilomycin, which neutralize bactericidal activity within lysosomes, caused no change of numbers of intracellular bacteria, indicating the compartment harboring E. coli did not possess bactericidal activity. (D) Quantitative E. coli exocytosis assays were performed by using human primary BECs and a clinical isolate of UPEC strain E. coli CI5. Significant numbers of intracellular E. coli CI5 were expelled from infected primary BECs at 4 h after the gentamicin treatment. Bars represent the mean + SEM. *, P < 0.05; **, P < 0.03; ***, P < 0.001, relative to Intracellular E. coli titers at 0 h in A, B, and D.
We next sought to establish that the drop in the number of intracellular bacteria in E. coli infected BECs was attributable to expulsion of bacteria rather than loss of viability. For these and for most of the subsequent studies, we used E. coli ORN103(pSH2), because the genome of this strain is only a fraction of that found on UPEC strains, making it infinitely easier to pinpoint the genetic factor(s) responsible for reduced intracellular numbers. We infected multiple cultures of BECs with E. coli ORN103(pSH2) for 1 h followed by 30-min gentamicin treatment to eliminate extracellular bacteria. The intracellular bacterial numbers at this time (0 h) were assessed in one of the cultures. The other infected cultures were incubated in media containing the bacteriostatic agent trimethoprim (TMP)/sulfamethoxazole (SMZ) for 4 h. Thereafter, the intracellular as well as extracellular bacterial numbers were assessed. We found an appreciable drop in intracellular bacterial numbers at 4 h when compared with 0 h (Fig. 1B). Interestingly, the number of bacteria found in the extracellular medium corresponded with the drop in intracellular bacterial numbers (Fig. 1B), which is consistent with the notion that E. coli were exocytosed by BECs. To verify that the drop in intracellular E. coli numbers was not due to any loss of integrity of BECs, we compared lactose dehydrogenase (LDH) release from E. coli infected and uninfected BECs, and found minimal cellular damage in E. coli infected BECs (Fig. S1). Although the increase in intracellular numbers of S. typhimurium SL1344 and S. aureus 54 would suggest that BECs have limited capacity to kill intracellular bacteria (Fig. 1A), we examined the intracellular E. coli numbers in infected BECs after their exposure to bafilomycin or ammonium chloride, agents known to inhibit bactericidal activity of host cells (11, 12). We found no appreciable effect on intracellular bacterial numbers with either agent, confirming that the reduction in intracellular numbers of E. coli was not due to any bactericidal actions of BECs (Fig. 1C). Thus, these studies show that BECs have the unique ability to exocytose infecting E. coli in nonlytic fashion, but not S. typhiumurium or S. aureus.
Because the above studies used immortalized human BECs, it was important to verify these observations employing primary human BECs and the clinical UPEC strain, CI5. We investigated whether freshly cultured human BECs from bladder biopsies would be able to expel intracellular UPEC. Primary BECs, cultured as described previously (13–15), exhibited the characteristic features of primary BECs, including expression of uroplakin-1a (a marker of asymmetrical unit membrane), ZO1 (the junctional complex protein), and cytokeratin, all of which are hallmarks of terminal differentiation in superficial BECs (Fig. S2). Primary BECs were infected with UPEC strain CI5 (16) and subjected to gentamicin treatment for 30 min. Fresh media containing bacteriostatic agents were then added to the infected cells. Four hours thereafter, intracellular and extracellular bacteria were assessed as described above. We found an appreciable (≈11%) decrease in intracellular UPEC by 4 h (Fig. 1D). As in the case of immortalized BECs, the extracellular bacteria population was comparable with the drop in the intracellular bacterial population by 4 h (Fig. 1D). We also sought to investigate the dynamics of bacterial exocytosis by primary BECs employing video microscopy. For these studies, we used a GFP-expressing UPEC CI5. To distinguish its extracellular location from intracellular UPEC CI5, we added into the slide chamber of infected BECs an Alexa Fluor 546-conjugated E. coli antibody, which binds only extracellular bacteria, because it does not penetrate the BECs. Only bacteria that failed to stain with this antibody were judged to be intracellular and were videotaped. In this video, UPEC CI5 were exocytosed by primary BECs 3 h and 32 min after gentamicin treatment (Movie S1). The exocytic event took ≈10 min, and as the bacteria are exocytosed, they shed many membrane vesicles. Together, our observations reveal that, after infection, primary human BECs are capable of exocytosing UPEC, and that this process is a dynamic and relatively rapid event.
Expulsion of E. coli from Infected BECs Is cAMP Dependent.
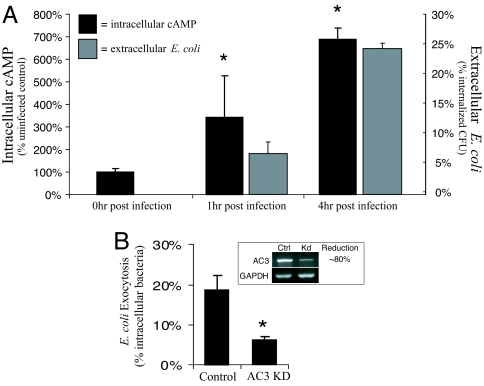
We hypothesize that a reason for why infected BECs exocytose E. coli and not other infecting bacteria is because E. coli are specifically harbored within fusiform compartments that are vesicles with distinct exocytic properties. Because regular exocytosis of fusiform vesicles in BECs depends on cAMP, bacterial expulsion from infected BECs would also be triggered by an increase in intracellular cAMP. To determine whether there was a relationship between intracellular cAMP levels and bacterial expulsion, we assayed intracellular cAMP levels in E. coli infected BECs (see black bars in Fig. 2A), as well as extracellular bacteria at 0, 1, and 4 h postinfection (see gray bars in Fig. 2A). We found that cAMP levels in the infected BECs had significantly increased over baseline levels by 1 h and were at markedly higher levels (600% of baseline levels) by 4 h (see black bars in Fig. 2A). When we assessed extracellular bacterial levels at the same time points (see gray bars in Fig. 2A), we found there was a good correlation between intracellular cAMP levels in BECs and expulsion of E. coli (see black and gray bars in Fig. 2A).
Fig. 2.
Expulsion of E. coli from infected BECs is cAMP dependent. (A) Intracellular cAMP levels were determined by a cAMP enzyme immunoassay either before or 1 or 4 h after E. coli ORN103(pSH2) infection of 5637 BECs. At the same time, the number of exocytosed E. coli ORN103(pSH2) was determined by sampling the extracellular media 1 and 4 h after gentamicin treatment. The increase in extracellular E. coli correlated to the increase in intracellular cAMP. (B) Control BECs and AC3 kd BECs were infected for 1h with E. coli ORN103(pSH2). After a 30-min gentamicin treatment, the E. coli ORN103(pSH2) infected BECs were incubated with a fresh medium containing bacteriostatic agents and D-mannose to prevent bacterial growth and reentry of bacteria, respectively. After 4 h, the medium was cultured for extracellular E. coli ORN103(pSH2), and revealed that AC3 kd BECs were significantly less effective in expelling intracellular E. coli ORN103(pSH2) than the control BECs. *, P < 0.05 by unpaired t test when compared with uninfected controls. Error bars represent SD (A) and SEM (B).
To demonstrate that bacterial expulsion requires cAMP, we used RNA interference techniques to knock down the enzyme responsible for cAMP production during E. coli infection of BECs, adenylyl cyclase (AC)3 (17). The production of AC3 mRNA was decreased in AC3 knockdown (kd) BECs by ≈80% (Fig. 2B Inset). We compared the ability of both sham transfected BECs and AC3 kd BECs with expel E. coli 4 h after infection. AC3 kd BECs were significantly less effective in expelling intracellular E. coli than control BECs (Fig. 2B). Together, these observations suggest that as predicted, expulsion of intracellular E. coli by infected BECs depends on intracellular cAMP levels.
E. coli Expulsion from Infected BECs Is Initiated When Toll-Like Receptor (TLR)4 Interacts with Bacterial LPS.
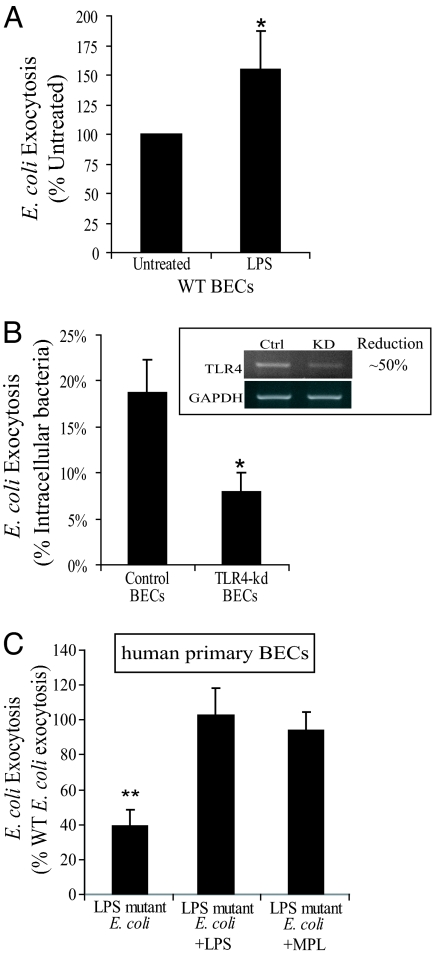
Next, we sought to identify the bacterial component(s) responsible for triggering E. coli expulsion by BECs. We have previously reported that after exposure to E. coli or purified LPS, TLR4 on BECs initiates a distinct signaling response involving the activation of AC3 and culminating in a powerful cAMP response (17). It is conceivable that LPS is the bacterial component triggering cAMP mediated E. coli expulsion from BECs, and that TLR4 is the critical cellular component initiating these events. To examine these possibilities, we added ultrapurified soluble E. coli LPS to 5637 BECs already infected with E. coli ORN103(pSH2) to see whether this agent would accelerate bacterial expulsion. Due to the possibility of lipoprotein contamination, we used ultra purified LPS in this study (E. coli 055:B5 LPS; Sigma). Treatment of BECs with LPS resulted in a significant increase in E. coli expulsion (Fig. 3A). To further confirm that the LPS on E. coli was the primary bacterial determinant responsible for activating BECs, we sought to demonstrate the involvement of TLR4, the cognate receptor for LPS. We again used RNA interference to generate 5637 BEC transfectants whose TLR4 mRNA levels were reduced by ≈50% when compared with sham transfected BECs (Fig. 3B Inset). As predicted, when compared with control BECs, bacterial expulsion from TLR4 kd BECs was markedly decreased by ≈50%, which is consistent with the decrease in TLR4 mRNA levels (Fig. 3B).
Fig. 3.
E. coli expulsion from infected BECs is initiated when TLR4 interacts with bacterial LPS. (A) WT BECs were infected for 1h with E. coli ORN103(pSH2). When cells were treated with gentamicin, 100 μg/mL LPS was added to the culture medium to examine the effect on bacterial exocytosis, when indicated. After a 30-min gentamicin and LPS treatment, infected BECs were incubated with fresh media containing bacteriostatic agents and D-mannose. After 4 h, the medium was cultured for extracellular E. coli. Treatment of BECs with LPS resulted in a significant increase in E. coli ORN103(pSH2) exocytosis compared with untreated BECs (Untreated). (B) Exocytosis assays were performed using control and TLR4 kd BECs as described above except LPS treatment. TLR4 kd BECs were significantly less effective in expelling intracellular E. coli than the control BECs. *, P < 0.05 by unpaired t test when compared with controls. Error bars represent SEM. (C) Human primary BECs were used for exocytosis assays. Primary BECs were infected with either WT E. coli (E. coli W3110) or LPS mutant E. coli (a msbB mutant E. coli MLK1067). When indicated, LPS or MPL were added to the media as described before. **, P < 0.0005 by unpaired t test when compared with WT E. coli infected BECs. Error bars represent SD.
To further implicate TLR4 in the expulsion of E. coli from infected BECs, we compared the exocytosis of WT E. coli W3110 and its LPS modified derivative E. coli MLK1067 from primary human BECs, prepared and cultured as described above. E. coli MLK1067 contains a mutation in the msbB gene such that its LPS has limited ability to activate TLR4 (18). We found that the level of expulsion of the msbB mutant by infected human primary BECs was markedly lower than the level observed with the WT E. coli W3110, which was reversed by pretreatment of human primary BECs with soluble E. coli LPS or the nontoxic LPS derivative monophosphoryl lipid (MPL)A (Fig. 3C). Together, TLR4 modulates bacterial exocytosis from infected BECs, and is likely mediated by the same LPS-initiated signaling pathway that have been reported to increase intracellular cAMP within BECs (17).
E. coli Exocytosis by BECs Depends on Rab27b and Caveolin-1.
To understand how TLR4 and cAMP were modulating bacterial exocytosis in infected BECs, we sought to identify components associated with the bacteria-harboring compartment that were essential for bacterial exocytosis. A recent study has shown that, after entry into BECs, E. coli became encased in membranes highly enriched in Rab27b, a marker of fusiform vesicles (9). Rab27 mediates the regulated delivery and/or targeting of fusiform vesicles to the apical plasma membrane of BECs during bladder expansion (19). Of the two Rab27 isoforms identified, human BECs express only the b isoform (19). It has been reported that fusion of Rab27b enriched fusiform vesicles into the plasma membrane is a preceding event in the entry of E. coli into BECs (9), and that subsequently intracellular E. coli are encased in Rab27b enriched membranes (9). Caveolin-1, a component of the lipid and cholesterol enriched lipid microdomains, is a scaffolding and signaling molecule also found in membranes encasing intracellular E. coli in BECs (4). Caveolin-1 is linked to the mobilization of cellular cytoskeleton promoting bacterial uptake by BECs (4).
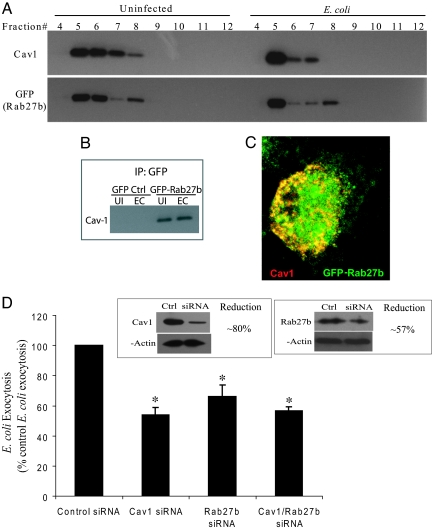
Because both Rab27b and caveolin-1 appear to be enriched in the membranes encasing intracellular E. coli, we investigated whether Rab27b and caveolin-1 were in any way colocalized and/or coassociated. Because caveolin-1 is typically found in cellular lipid raft microdomains, we investigated whether Rab27b was found in lipid raft fractions of BECs, and whether infection affected the location of either protein. To facilitate detection of Rab27b in BECs, we stably transfected 5637 BECs with GFP-Rab27b. We purified lipid raft fractions from 5637 BEC homogenates before and after exposure to E. coli ORN103(pSH2). After sucrose density gradient centrifugation, various BEC fractions were subjected to SDS/PAGE followed by Western blotting employing as probes antibodies to caveolin-1 or GFP. The lipid raft fractions were the light buoyant fractions (5–8), where the majority of caveolin-1 was detected. Interestingly, GFP-Rab27b also appeared to colocalize in the same fractions as caveolin-1 in uninfected as well as infected BECs (Fig. 4A). There also appeared to an appreciable shift in the location of the both caveolin-1 and Rab27b into the more buoyant fractions after E. coli infection (Fig. 4A). These observations reveal that caveolin-1 and Rab27b have similar distributions in BECs. To investigate whether caveolin-1 and Rab27b are physically interacting with each other, we attempted to immunoprecipitate caveolin-1 with GFP-specific antibodies in both uninfected and BECs infected with E. coli. As shown in Fig. 4B, GFP-specific antibodies precipitated caveolin-1 in uninfected and infected GFP-Rab27b BECs, but not in GFP BECs (Fig. 4B). Consistent with this finding, shown in Fig. 4C is a confocal image of a GFP-Rab27b BEC probed with antibodies to caveolin-1 revealing similar cellular distribution of Rab27b and caveolin-1. Together, under steady state conditions, Rab27b are complexed with caveolin-1 in lipid raft domains of BECs, and these interactions are sustained after E. coli uptake.
Fig. 4.
E. coli exocytosis by BECs depends on Rab27b and caveolin-1. (A) Cell extracts from uninfected (Uninfected) and E. coli ORN103(pSH2)-infected (E. coli) BECs were fractionated on a sucrose gradient. Rab27b and caveolin-1 were distributed in fractions 5 to 8 in uninfected BECs, and after infection, the majority of these proteins had shifted into fraction 5. (B) A pull-down assay showing specific coassociation between Rab27b and caveolin-1. The assays using GFP-Rab27b expressing BECs (GFP-Rab27b), but not control cells expressing only GFP (GFP Ctrl) detected caveolin-1 as a binding partner. UI, uninfected; EC, E. coli infected. (C) Indirect immunofluorescence showed colocalization between caveolin-1 (red) and Rab27b (green). (D) Rab27b and/or caveolin-1 expression levels in regular BECs were reduced by using gene specific siRNAs. Compared with levels in sham siRNA transfected cells (Ctrl), protein expression levels of Rab27b and caveolin-1 were reduced by ≈57 and ≈80% in kd cells (siRNA), respectively (Inset). A significant decrease in bacterial exocytosis in caveolin-1 kd BECs (Cav1 siRNA), in Rab27b kd BECs (Rab27b siRNA), and in double kd BECs (Cav1/Rab27b siRNA) was observed compared with controls. *, P < 0.03 by unpaired t test when compared with negative control siRNA transfected BECs. Error bars represent SEM.
In view of the interaction between Rab27b and caveolin-1, we investigated their contribution to bacterial exocytosis from BECs. We reduced Rab27b and/or caveolin-1 expression levels in regular BECs by using gene specific siRNAs. Compared with levels in sham siRNA transfected cells, protein expression levels of Rab27b and caveolin-1 were reduced by ≈57 and ≈80% in kd cells, respectively (Fig. 4D Inset). We observed a significant decrease in bacterial exocytosis in caveolin-1 kd BECs and in Rab27b kd BECs compared with controls (Fig. 4D). However, when we knocked down both Rab27b and caveolin-1, we did not observed any additive effect in the inhibition of E. coli expulsion (Fig. 4D). Together, Rab27b and caveolin-1, two molecules previously reported to be enriched in the membrane encasing intracellular E. coli, appear to be coupled even in uninfected BECs and each of these molecules contribute to bacterial exocytosis.
E. coli Expulsion by BECs Requires MyRIP Activity.
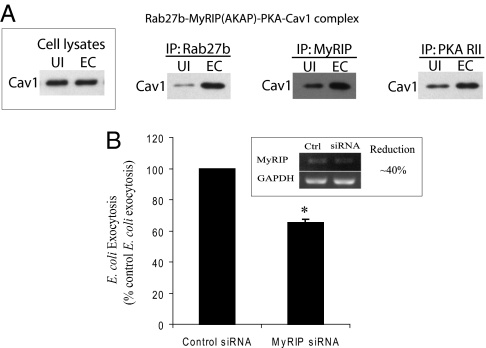
To connect TLR4-initiated and cAMP-mediated signaling with bacterial expulsion mediated by Rab27b-caveolin-1 complexes, we searched for known intermediates of TLR4/cAMP signaling that could potentially interact with either Rab27b or caveolin-1. MyRIP (Slac2c) is a candidate of interest, because it is a scaffolding protein that has previously been shown to tether PKA, a well known downstream effector of the TLR4/cAMP pathway, to exocytic compartments by directly binding with Rab27b (20). Interestingly, MyRIP (Slac2c) has previously been shown to mediate exocytic activities such as melanosome and insulin exocytosis and through its interaction with Rab27b (21, 22). Conceivably, MyRIP serves as the link between the TLR4/cAMP signaling and the Rab27b/caveolin-1 complexes found localized on the fusiform vesicle membranes encapsulating bacteria. To see whether MyRIP associates with the Rab27b/caveolin-1 complexes in BECs, we performed a pull-down assay using Rab27-, MyRIP-, and PKA RII-specific antibodies. Control immunoblots demonstrated that equal levels of caveolin-1 protein were expressed in the cell extracts before and after infection (Fig. 5A Inset). Interestingly, all these signaling molecules were coassociated with caveolin-1 in BECs even before infection and appeared even more so after infection with E. coli 103(pSH2) (Fig. 5A). These interactions are highly specific, because when a pull-down assay was performed with a specific antibody to lysosomal marker Lamp1, no band was detected when probed for caveolin-1 on a Western blotting. To further demonstrate the functional contribution of MyRIP in E. coli expulsion by BECs, we examined the effects of knocking down MyRIP expression employing a gene specific siRNA on E. coli expulsion. MyRIP mRNA levels were reduced by ≈40% in MyRIP kd BECs (Fig. 5B Inset), and levels of E. coli exocytosis were significantly dropped by 40% (Fig. 5B). Together, MyRIP has a key role in modulating E. coli exocytosis by serving as a critical conduit for signaling between TLR4/cAMP pathway and the exocytic compartment housing intracellular bacteria.
Fig. 5.
E. coli expulsion by BECs requires MyRIP activity. (A) A pull-down assay was performed using Rab27-, MyRIP-, and PKA RII-specific antibodies. A control immunoblot revealed that equal levels of caveolin-1 protein were expressed in the cell extracts before and after infection (Inset). All these signaling molecules were coassociated with caveolin-1 in BECs even before infection (UI) and after infection (EC), the coassociation became markedly more increased. (B) An E. coli exocytosis assay was performed to examine effects of MyRIP on bacteria expulsion. MyRIP mRNA levels were reduced by ≈40% in kd BECs (Inset), and levels of E. coli exocytosis were significantly dropped by 40%. *, P < 0.002 by unpaired t test when compared with control siRNA transfected BECs. Error bars represent SEM.
Discussion
Here, we report that BECs have the capacity to expel intracellular bacteria, and that the critical sensory molecule responsible for bacterial expulsion is TLR4. Evidence implicating TLR4 comes from the observations that treatment of BECs with soluble LPS shows higher levels of E. coli expulsion compared with untreated BECs, and that knocking down TLR4 expression in BECs markedly reduces E. coli expulsion compared with controls. Also, the level of exocytosis of WT E. coli is markedly higher than an LPS mutant that fails to activate TLR4, and when its failure is rescued by LPS or MPL, the levels of exocytosis of LPS mutant E. coli are returned to the levels seen in exocytosis of WT E. coli (Fig. 3). TLR4-mediated expulsion of E. coli by infected BECs is possible because cAMP is a major byproduct of TLR4 signaling (17), and because the intracellular bacteria are harbored within specialized exocytic compartments that are primed to exocytose when intracellular cAMP levels become elevated (9). The inability of BECs to expel intracellular S. typhimurium and S. aureus could be related at least in part to the fact that these bacteria are not housed in exocytic compartments, but in compartments that resemble traditional endocytic compartments (23, 24).
Expulsion of intracellular E. coli by infected BECs represents a previously undescribed function for TLR4. In the UT, TLR4 is largely known for initiating the rapid and vigorous neutrophil responses to E. coli and other Gram-negative bacterial infections. On sensing LPS on infecting bacteria, TLR4 molecules on BECs and other uroepithelial cells trigger the release of inflammatory cytokines including neutrophil chemoattractants (17, 25, 26). Although these recruited phagocytes are effective in clearing extracellular pathogens, they are largely ineffective in eliminating bacteria hidden within superficial BECs. Thus, TLR4 mediated expulsion of intracellular bacteria could be viewed as a mechanism to improve efficiency of neutrophil mediated bacterial clearance.
Although cAMP-dependent exocytosis of fusiform vesicles into the apical plasma membrane of superficial BECs is a normal physiological event, relatively little is known regarding the molecular machinery involved. From tracking the exocytosis of E. coli harbored in fusiform vesicles of BECs, we have uncovered the essential role of several new components in the signaling machinery leading to exocytosis of fusiform vesicles. First, we have identified the immune surveillance molecule, TLR4, on the plasma membrane of BECs as an activator of AC3 mediated production of intracellular cAMP. Second, we have revealed the critical role had by the scaffolding protein, MyRIP (Slac2c). This scaffolding protein was found bound to proteins comprising the fusiform vesicle membrane. We believe that MyRIP (Slac2c) serves to sequester PKA, a major product of cAMP signaling, to confine its phosphorylating activities to proteins in the immediate vicinity of the fusiform vesicle. PKA has previously been shown to promote vesicle fusion by phosphorylating various regulators of membrane–membrane fusion events such as t-SNAREs (27, 28). Third, bound to MyRIP (Slac2c) is Rab27b (a small GTPase) that we have shown to be important in bacterial exocytosis. Rab27b is believed to mediate regulated delivery of fusiform vesicles to the plasma membrane (19). Last, caveolin-1 is another protein found in the membrane of fusiform vesicles coassociated with MyRIP (Slac2c) and Rab27b, and shown to be essential for fusiform vesicle exocytosis. Although most studies to date have suggested an endocytic role for caveolin-1 (4), there are certain indications for an exocytic role for this protein. For example, on phosphorylation caveolin-1 has been shown to promote trafficking and fusion of caveolin-1 enriched vesicles with the plasma membrane (29). Thus, our studies have provided valuable information regarding the progression of signaling events leading to the exocytosis of fusiform vesicles. A schematic representation of the signaling interactions triggered by TLR4 leading to the exocytosis of E. coli from infected BECs is shown in Fig. S3.
Our findings of TLR mediated bacterial expulsion from infected BECs, together with recent reports of TLR mediated rapid cytokine responses (17) and inhibition of bacterial invasion (16), reveal a multifaceted response initiated by TLR4, which is directed at preventing bladder infections. That UTIs occur despite these antimicrobial actions of BECs argues that UPEC strains must have evolved effective mechanisms to counter or evade these activities. After bladder infections by UPEC, Hultgren and coworkers (6) have reported outgrowth of distinct morphological types, including filamentous and self-aggregated forms within the BECs. We did not observe any of these distinct morphological types of E. coli in our in vitro culture systems, presumably because they are only triggered by the stress associated with survival in vivo (6). Nevertheless, this morphological plasticity exhibited by UPEC within BECs could be an adaptive measure to avoid expulsion from the host cell.
Last, our findings point to a previously undescribed strategy for the treatment of UTI, especially in cases that are refractive to antibiotic treatment. Conceivably, UTIs may be reduced by employing compounds BECs such as TLR4 ligands and activators of cAMP that promote exocytosis of intracellular vesicle encased bacteria from BECs. Indeed, we have demonstrated recently that treating infected mice with forskolin, a potent elevator of intracellular cAMP, can significantly reduce UTIs (9). The possibility of expelling intracellular bacteria in nonlytic fashion as a form of treatment may have broad therapeutic implications, because most pathogens tend to seek intracellular refuge in at least one stage of the infectious process.
Experimental Procedures
For bacterial strains and cell lines, we used E. coli ORN103(pSH2) (chloramphenicol; 100 μg/mL) (10), E. coli CI5 (10, 30, 31), E. coli CI5 expressing GFP (ampicillin; 100 μg/mL) (this study), E. coli W3110 (18), E. coli MLK1067 (chloramphenicol; 100 μg/mL) (18), S. typhimurium SL1344, and Staphyloccocus aureus 54 (16).
The human BEC line 5637 (ATCC HTB-9), 5637 AC3 kd cells (17), and 5637 TLR4 kd cells (16) were grown in supplemented RPMI MEDIUM 1640 (Sigma) (16). Human primary BECs were maintained in keratinocyte-SFM, containing recombinant epidermal growth factor and bovine pituitary extract (Invitrogen). All experiments were performed using 5–7 passaged human primary BECs.
Detailed methods and materials are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments.
We thank Alison Hofmann [Department of Pediatrics, Duke University Medical Center (DUMC)] for critical reading of this manuscript and Christian Raetz (Department of Biochemistry, DUMC) for the gift of the msbB mutant and corresponding WT E. coli. This work was supported by the National Institutes of Health Grants AI050021, DK077159, DK077307, DK62863, and DK050814.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900527106/DCSupplemental.
References
- 1.Andriole VT, Patterson TF. Epidemiology, natural history, and management of urinary tract infections in pregnancy. Med Clin North Am. 1991;75:359–373. doi: 10.1016/s0025-7125(16)30459-x. [DOI] [PubMed] [Google Scholar]
- 2.Patton JP, Nash DB, Abrutyn E. Urinary tract infection: Economic considerations. Med Clin North Am. 1991;75:495–513. doi: 10.1016/s0025-7125(16)30466-7. [DOI] [PubMed] [Google Scholar]
- 3.Apodaca G. The uroepithelium: Not just a passive barrier. Traffic. 2004;5:117–128. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem. 2004;279:18944–18951. doi: 10.1074/jbc.M400769200. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GG, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 6.Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol. 2008;6:162–168. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- 7.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. Rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- 9.Bishop BL, et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 10.Orndorff PE, Falkow S. Identification and characterization of a gene product that regulates type 1 piliation in Escherichia coli. J Bacteriol. 1984;160:61–66. doi: 10.1128/jb.160.1.61-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bidani A, et al. Bactericidal activity of alveolar macrophages is suppressed by V-ATPase inhibition. Lung. 2000;178:91–104. doi: 10.1007/s004080000012. [DOI] [PubMed] [Google Scholar]
- 12.Malaviya R, et al. Mast cell phagocytosis of FimH-expressing enterobacteria. J Immunol. 1994;152:1907–1914. [PubMed] [Google Scholar]
- 13.Cilento BG, Freeman MR, Schneck FX, Retik AB, Atala A. Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro. J Urol. 1994;152:665–670. doi: 10.1016/s0022-5347(17)32676-9. [DOI] [PubMed] [Google Scholar]
- 14.Gupta K, et al. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol. 2007;177:2357–2360. doi: 10.1016/j.juro.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross WR, Eardley I, Leese HJ, Southgate J. A biomimetic tissue from cultured normal human urothelial cells: Analysis of physiological function. Am J Physiol Renal Physiol. 2005;289:F459–F468. doi: 10.1152/ajprenal.00040.2005. [DOI] [PubMed] [Google Scholar]
- 16.Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4 initiated and cAMP mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–298. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, et al. A Novel TLR4-Mediated Signaling Pathway Leading to IL-6 Responses in Human Bladder Epithelial Cells. PLoS Pathog. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clementz T, Zhou Z, Raetz CR. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J Biol Chem. 1997;272:10353–10360. doi: 10.1074/jbc.272.16.10353. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, et al. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci USA. 2003;100:14012–14017. doi: 10.1073/pnas.2436350100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goehring A, Pedroja B, Hinke S, Langeberg L, Scott J. MyRIP anchors protein kinase A to the exocyst complex. J Biol Chem. 2007;282:33155–33167. doi: 10.1074/jbc.M705167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda M, Kuroda T. Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem. 2002;277:43096–43103. doi: 10.1074/jbc.M203862200. [DOI] [PubMed] [Google Scholar]
- 22.Waselle L, et al. Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell. 2003;14:4103–4113. doi: 10.1091/mbc.E03-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauck C, Ohlsen K. Sticky connections: Extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr Opin Microbiol. 2006;9:5–11. doi: 10.1016/j.mib.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Mills S, Finlay B. Comparison of Salmonella typhi and Salmonella typhimurium invasion, intracellular growth and localization in cultured human epithelial cells. Microb Pathog. 1994;17:409–423. doi: 10.1006/mpat.1994.1086. [DOI] [PubMed] [Google Scholar]
- 25.Haraoka M, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 26.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 27.Foster L, et al. Binary interactions of the SNARE proteins syntaxin-4, SNAP23, and VAMP-2 and their regulation by phosphorylation. Biochemistry. 1998;37:11089–11096. doi: 10.1021/bi980253t. [DOI] [PubMed] [Google Scholar]
- 28.Risinger C, Bennett M. Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J Neurochem. 1999;72:614–624. doi: 10.1046/j.1471-4159.1999.0720614.x. [DOI] [PubMed] [Google Scholar]
- 29.Nomura R, Fujimoto T. Tyrosine-phosphorylated caveolin-1: Immunolocalization and molecular characterization. Mol Biol Cell. 1999;10:975–986. doi: 10.1091/mbc.10.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham SN, et al. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary D-mannose receptors. Infect Immun. 1985;48:625–628. doi: 10.1128/iai.48.3.625-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thankavel K, et al. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental UTI. J Clin Invest. 1997;100:1123–1136. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.