Abstract
A clear demonstration of the role of melatonin and its receptors in specific retinal functions is lacking. The present study investigated the distribution of MT1 receptors within the retina, and the scotopic and photopic electroretinograms (ERG) and retinal morphology in wild-type (WT) and MT1 receptor-deficient mice. MT1 receptor transcripts were localized in photoreceptor cells and in some inner retinal neurons. A diurnal rhythm in the dark-adapted ERG responses was observed in WT mice, with higher a- and b-wave amplitudes at night, but this rhythm was absent in mice lacking MT1 receptors. Injection of melatonin during the day decreased the scotopic response threshold and the amplitude of the a- and b-waves in the WT mice, but not in the MT1−/− mice. The effects of MT1 receptor deficiency on retinal morphology was investigated at three different ages (3, 12, and 18 months). No differences between MT1−/− and WT mice were observed at 3 months of age, whereas at 12 months MT1−/− mice have a significant reduction in the number of photoreceptor nuclei in the outer nuclear layer compared with WT controls. No differences were observed in the number of cells in inner nuclear layer or in ganglion cells at 12 months of age. At 18 months, the loss of photoreceptor nuclei in the outer nuclear layer was further accentuated and the number of ganglion cells was also significantly lower than that of controls. These data demonstrate the functional significance of melatonin and MT1 receptors in the mammalian retina and create the basis for future studies on the therapeutic use of melatonin in retinal degeneration.
Keywords: electroretinogram, neuroprotection, visual sensitivity, glaucoma
Melatonin plays an important role in many aspects of mammalian neurobiology by acting via two types of G protein-coupled receptors (MT1 and MT2) that are negatively coupled with adenylyl cyclase (1). Many investigations have shown that retinal photoreceptors of vertebrates synthesize melatonin under the direct control of a circadian clock (2, 3). In a few cases, the circadian pacemaker driving rhythmic melatonin synthesis has been localized to the photoreceptors themselves (2, 4, 5). In the retina melatonin levels are high during the night and low during the day and exposure to light during the night induces a rapid and dramatic decrease in the levels of melatonin (6, 7). Retinal melatonin synthesis is directly controlled by the circadian clock via E-box mediated transcription (8) and via the gating of the cAMP signaling cascade (9).
Several studies have reported that melatonin modulates retinal functions and accumulating experimental evidence indicates an involvement of this hormone in retinal pathologies (reviewed in refs. 10 and 11).
The role played by specific melatonin receptors in the retina is not well defined. Melatonin receptors are expressed by several retinal cell types, including the photoreceptors (12, 13), suggesting that melatonin receptors may be involved in the regulation of photoreceptor physiology. Indeed, previous studies have reported that melatonin influences the membrane conductance of dark adapted frog photoreceptors (14), acts directly on the rod photoreceptors to increase dark adaptation (15), and potentiates rod signals to ON type bipolar cells in fish retina (16). Melatonin is also involved in the modulation of the electroretinogram of the green iguana (17), chicken (18), and, possibly, man (19).
No previous study has investigated the functional roles of specific melatonin receptors in the mouse retina. This lack of data are because the vast majority of mouse strains do not produce melatonin (20) and many that do so carry a mutation that leads to the rapid degeneration of photoreceptors (e.g., C3H rd1). In the present study, we have crossed mice with targeted deletion of the MT1 melatonin receptor gene (MT1−/− mice) (21) onto the C3Hf+/+ background (20). The latter make melatonin but do not develop retinal degeneration (22, 23). We then investigated the effect of disruption of the MT1 gene on retinal structure and function.
Results
Distribution of MT1 Receptors mRNA in the Mouse Retina.
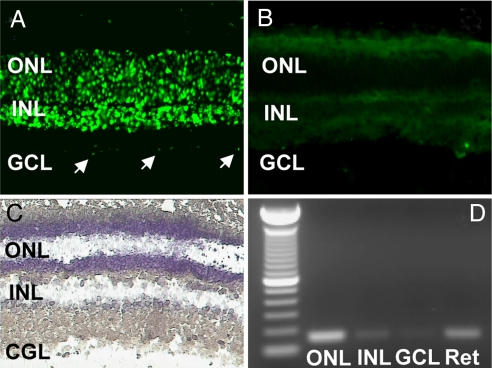
MT1 transcripts were detected in the outer nuclear layer (ONL), inner nuclear layer (INL), and ganglion cell layer (GCL) by fluorescent in situ hybridization. MT1 mRNA was abundant in the ONL, the retinal layer containing the nuclei of the photoreceptor cells, and the INL, where the cell bodies of horizontal, bipolar, amacrine, and Müller cells are located (Fig. 1A). In contrast, the MT1 transcript level was low in the GCL (Fig. 1A). No significant signal was detected in sections treated with the sense probe (Fig. 1B). To corroborate the results obtained by in situ hybridization with an independent technique, we performed RT-PCR with cells obtained from the various retinal layers using laser capture microdissection (LCM) (3, 8). A typical microdissection of a retinal section is shown in Fig. 1C. MT1 mRNA was amplified from cells captured in the ONL and INL; a faint band was also observed in GCL (Fig. 1D).
Fig. 1.
Localization of MT1 transcripts in the retina determined by in situ hybridization using a fluorescein-labeled probe. (A) The antisense probe detected a clear signal in the outer nuclear layer (ONL) and inner retinal layer (INL); a faint signal was also present in some ganglion cells (GCL, arrows). (B) No signal was detected using the sense probe. (C) Laser capture microdissection (LCM) of individual cell layers from the mouse retina. (D) RT-PCR generated amplicons of MT1 receptor mRNA in the outer nuclear layer (ONL), in the inner nuclear layer (INL), and in the ganglion cell layer (GCL) collected with LCM. The RT-PCR products had the predicted size (150 bp).
Dark-Adapted Electroretinogram.
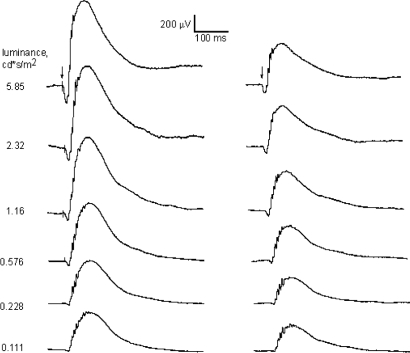
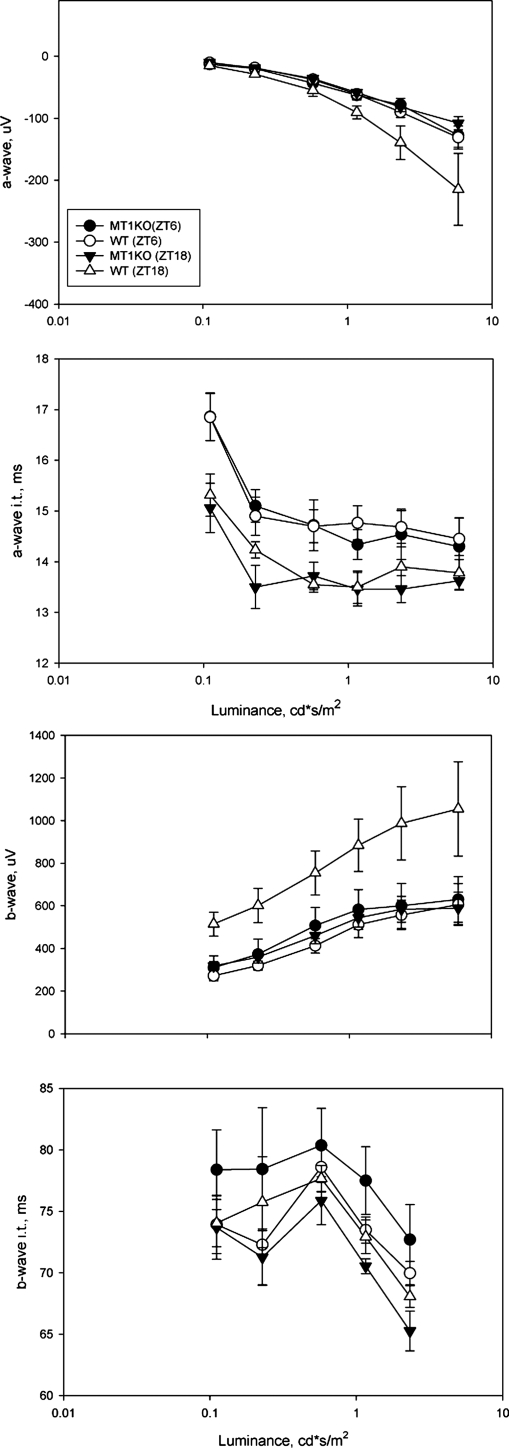
The electroretinogram (ERG) is a powerful tool to noninvasively study the response of the retina to a flash of light. The major components of the ERG are the a-wave and the b-wave, which represent the activation of the photoreceptors and the depolarizing second order neurons (on bipolar cells) respectively. The amplitudes and implicit times of the a-wave and b-wave are used to quantify the retinal light response (24, 25). Fig. 2 shows typical traces for the dark-adapted ERG recorded with our experimental protocol under different luminance levels. At the lowest luminance levels this ERG reflects the activity of the rod pathway, whereas at the higher intensities, both the cone and the rod systems may contribute to the response. Consistent with previous reports (24), the ERG waveform showed a clear dependence on the luminance levels; the amplitude of the a- and b-wave significantly increased with the increase in the luminance levels, whereas the implicit times decreased. In C3H/f+/+ wild-type mice, the amplitudes of the a- and b-wave were significantly greater at midnight than at midday (two-way ANOVA, P < 0.001) (Fig. 3), but not in C3H f+/+ MT1−/− mice (two-way ANOVA, P > 0.1, Fig. 3). The implicit times of the a- and b-wave also showed a clear dependence on the time of the day (shorter implicit time at midnight than at midday), but the genotype did not significantly affect the implicit time (two-way ANOVA, P > 0.05) (Fig. 3).
Fig. 2.
Representative trace of dark-adapted ERG in C3H/f+/+ (Left) and C3H/f+/+MT1−/− (Right) at the seven different luminance levels recorded at night (ZT18). A clear reduction in the amplitude of the a- and b-waves is present in C3H/f+/+MT1−/−.
Fig. 3.
Quantification of dark-adapted ERG responses to flashes of light recorded in the middle of the day (ZT6) and in the middle of the night (ZT18). Mice (3–4 months old) were dark-adapted for at least 30 min before the recordings were performed. Data are presented as mean ± SEM; n = 4–6 for each time point and genotype. Two-way ANOVA indicated a statistically significant contribution of the time of day for the a-wave (P < 0.05) and b-wave (P < 0.001) amplitudes and for genotype (P < 0.005, a-wave; P < 0.001, b-wave). The implicit time of the a-wave and b-wave was also affected by the time of the day (P < 0.001 in both cases), but not by the genotype (P > 0.1 and P > 0.1, respectively).
Effects of Melatonin Injection on the Dark-Adapted ERGs.
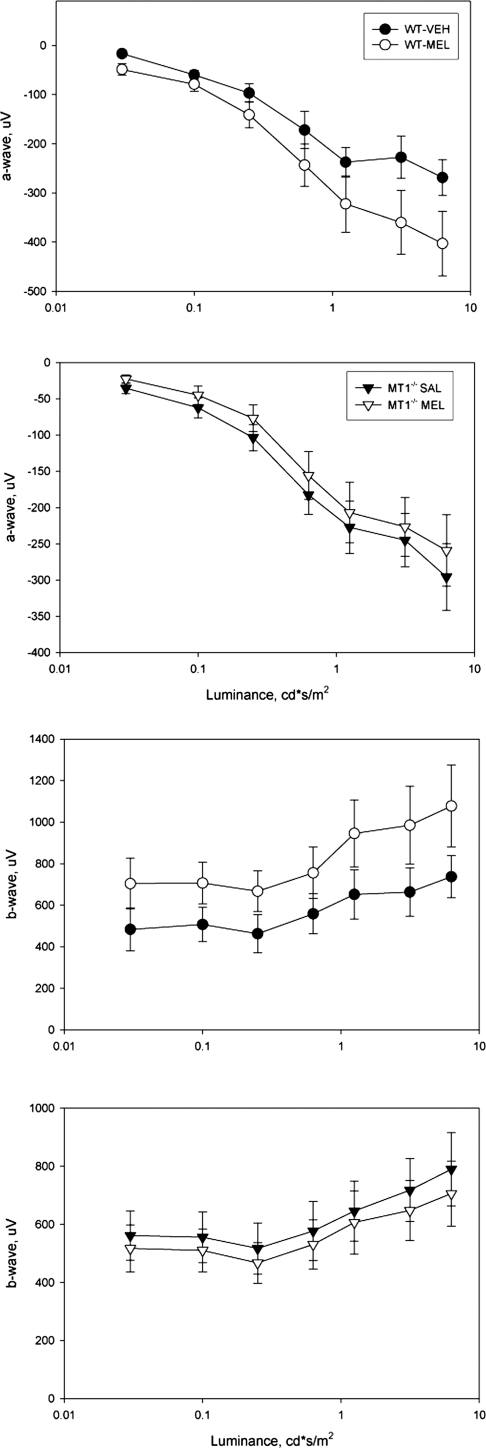
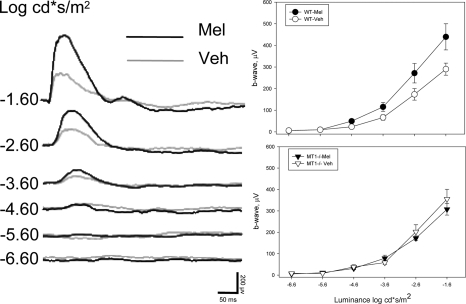
We tested whether i.p. injection of melatonin (1 mg/kg) during the day (ZT6) would affect the dark-adapted ERG. C3H/f+/+ and C3H/f+/+MT1−/− mice were injected with melatonin and dark adapted for 1 h before recording the ERG. We found that melatonin injected during the daytime significantly increased the amplitude of the a- and b-wave in C3H/f+/+ mice compared with saline injected control mice (two-way ANOVA, P < 0.01) (Fig. 4). Melatonin injection did not produce any changes in the ERG of MT1−/− mice (two-way ANOVA, P > 0.05, Fig. 4). The implicit times were not affected by melatonin in either genotype (two-way ANOVA, P > 0.05 in all cases). We then tested whether the scotopic threshold response was also affected by melatonin injection using a protocol similar to those used in previous studies (24, 25). As observed above, melatonin injection in C3H/f+/+ significantly enhanced the amplitude of the b-wave with respect to the values measured in vehicle injected mice (two-way ANOVA, P < 0.001, Fig. 5). C3H/f+/+ mice injected with melatonin had a lower scotopic response threshold (−4.6 log cd*s/m2) than vehicle injected (−3.6 log cd*s/m2). Melatonin injection did not affect the scotopic response threshold in C3H/f+/+MT1−/− (two-way ANOVA, P > 0.1) (Fig. 5) further suggesting that the effect of melatonin on the modulation of visual sensitivity is mediated by MT1 receptors. Similar data were obtained when the experiment was performed in the middle of the night (ZT18, two-way ANOVA, P < 0.05) (Fig. S1).
Fig. 4.
Quantification of dark-adapted ERG responses to flashes (0.11–5.85 cd*s/m2) of light recorded after 1 h of dark adaptation and i.p. injection of melatonin (1 mg/kg) or vehicle in the middle of the day (ZT6). Data are presented as mean ± SEM; n = 4–6 for each time point and genotype. In C3H/f+/+ mice melatonin injection induced a significant increase in the amplitude of the a-wave and b-wave (two-way ANOVA, P < 0.001 in both cases). In C3H/f+/+MT1−/− mice melatonin injection did not affect the amplitude of the a-wave or b-wave (two-way ANOVA, P > 0.1, a-wave; and P > 0.1, b-wave). Mice were 3–4 months old at the time of the experiment.
Fig. 5.
Representative traces of responses in 3- to 4-month-old C3H/f+/+ mice injected with melatonin or vehicle (Left). Quantification of the dark-adapted response of the b-wave to flashes of light (−6.6 to −1.6 log cd*s/m2; 0.11 5 cd*s/m2) recorded after 1 h of dark adaptation and intraperitoneal injection of melatonin (1 mg/kg) or vehicle in the middle of the day (Right). Data are presented as mean ± SEM; n = 5–8 for each time point and genotype. In C3H/f+/+ mice, melatonin injection induced a significant reduction in the scotopic threshold response and increased the amplitude of the b-wave (two-way ANOVA, P < 0.001). In C3H/f+/+MT1−/− mice, melatonin injection did not affect the scotopic threshold response or the amplitude of the b-wave (two-way ANOVA, P > 0.1).
Effect of Melatonin Injection on the Photopic ERGs.
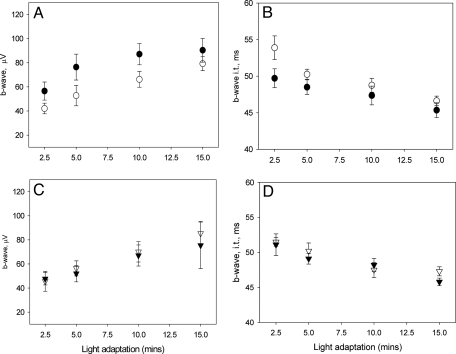
The effects of melatonin injection on the photopic ERGs were investigated using a protocol similar to that used by Cameron et al. (24). ERGs from C3H/f+/+ and MT1−/− mice were recorded after 2.5, 5, 10, and 15 min of rod saturating background light exposure. In C3H/f+/+ mice, melatonin injection increased the amplitude of the b-wave whereas no significant effect was observed in C3H/f+/+MT1−/− (two-way ANOVA, P < 0.001 and P > 0.1 respectively, Fig. 6). The implicit time of the b-wave was slightly but significantly reduced in C3H/f+/+ (two-way ANOVA, P < 0.05) (Fig. 6).
Fig. 6.
Quantification of light-adapted ERG responses to flashes of light recorded after 1 h of dark adaptation and i.p. injection of melatonin (1 mg/kg) in the middle of the day (ZT6). Data are presented as mean ± SEM; n = 4–8 for each time point and genotype. In C3H/f+/+ mice (A and B) melatonin injection induced a significant increase in the amplitude of the b-wave (two-way ANOVA, P < 0.001 in both cases) and a reduction in the implicit time (two-way ANOVA, P < 0.05). In C3H/f+/+MT1−/− mice (C and D) melatonin injection did not affect the amplitude of the b-wave or the implicit time (two-way ANOVA, P > 0.1, b-wave; P > 0.1, implicit time). Mice were 3–4 months old at the time of the experiment.
Melatonin injection at ZT 18 did not affect the photopic ERGs (b-wave amplitude and implicit time) in C3H/f+/+ or in C3H/f+/+MT1−/− (two-way ANOVA, P > 0.1) (Fig. S2).
Effect MT1 Removal on Retinal Circuitry.
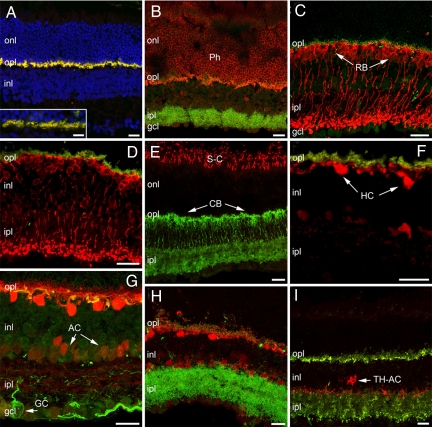
No major alterations in retinal morphology were evident between C3H/f+/+ and C3H/f+/+MT1−/− at 3 months of age. Major retinal cell types, including rods and cones (Fig. 7A, B, and E), horizontal cells (Fig. 7 F and G), rod and cone bipolar cells (Fig. 7 C and D), various types of amacrine cells (Fig. 7 F and G), such as TH-positive amacrine cells (Fig. 7I), and ganglion cells (Fig. 7G), and Müller glia and astrocytes (Fig. 7 A and B), were visualized with specific antibodies (Table S1) and appeared normal in morphology, number, pattern of stratification, and overall architecture, conforming to published literature (26). Synaptic markers, and namely mGluR6, PSD95, Bassoon, Kinesin II and synaptophysin were normally distributed in the two plexiform layers of both genotypes (Fig. 7 A–D, F, H, and I). Local signs of focal rearrangements (i.e., formation of rosettes, a common finding in retinal reorganization) or signs of glial reactivity in Muller cells and astrocytes, also frequently reported in a variety of mutants and KO mice (27) were absolutely undetected in the strains under use.
Fig. 7.
Immunofluorescence staining of vertical retinal sections from C3H/f+/+MT1−/− mice at 3 months, showing a normal complement of cell types and retinal lamination. (A) TOTO-3 nuclear staining (blue) shows normal retinal layering. mGluR6 (red) and PSD95 (green-yellow) are normally distributed in the outer plexiform layer (opl), as also shown at higher magnification (Inset). (B) Recoverin staining (red) of photoreceptor (Ph) outer segments and cell bodies. Bassoon staining (green) shows the characteristic pattern of semicircular, punctate structures in the opl, associated to photoreceptor synaptic ribbons. (C) Protein kinase Cα antibodies (red) show rod bipolar cells (RB) with a normal complement of kinesin II-positive puncta (green) decorating their dendritic tips. (D) RB cells (labeled red by PKCα antibodies are postsynaptic to rod spherules, labeled green by PSD95. (E) Antibody against S-cone opsin (red) reveals a normal pattern of cones (dorsal retinal quadrant); Goα (green) shows the morphology of ON-bipolar cells, including cone bipolars (CB). (F) Calbindin staining (red) of horizontal cells (HCs). A normal complement of amacrine cells (AC) are also more weakly stained. PSD95-positive photoreceptor terminals in the opl (green) are appropriately apposed to horizontal cell dendrites. (G) Calbindin staining of HC cell bodies (red) combined with associated to neurofilament staining of their axonal arborizations (green). Neurofilament labels ganglion cells in their entirety. One large-size ganglion cell is indicated (GC). (H) Calbindin staining (red) associated to bassoon show that dendrites of HCs are appropriately decorated by ribbons at their presynaptic sites in the opl. Some dendritic sprouting, also observed in the C3H/f+/+ retina, is visible in the opl. (I) Antibodies against tyrosine hydroxylase show a population of dopaminergic amacrine cells (TH-ACs). Their typical pattern of stratification, mostly visible in sublamina 1 of the ipl and in the opl, is indistinguishable from the C3H/f+/+ counterpart. Synaptophysin vesicle staining (green) shows a normal distribution in photoreceptor terminals in the opl and throughout the ipl, as expected. (Scale bars, 20 μm.)
Effect of MT1 Removal on Retinal Cell Viability During Aging.
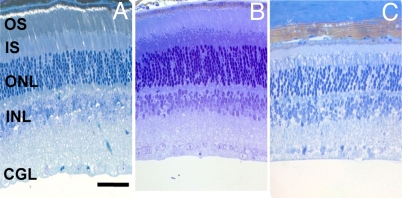
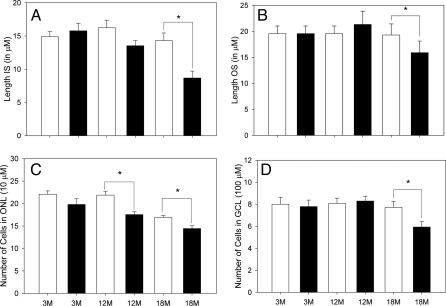
A detailed morphometric investigation of the retina was performed using light microscopy in C3H/f+/+ and the C3H/f+/+MT1−/− mice at three different ages (Figs. 8 and 9). The statistical analysis indicated a significant effect of the age and strain for many of the parameters examined (two-way ANOVA, P < 0.05). No significant differences were observed at 3 months of age between the C3H/f+/+ and C3Hf+/+MT1−/− in any of the morphometric parameters investigated (two-way ANOVA, P > 0.1 in all cases) (Fig. 9). At 12 months of age, we observed a significant reduction in the number of photoreceptor cells (Holm-Sidak test, P < 0.05) in C3H/f+/+MT1−/− with respect to C3H/f+/+ at the same age. All other parameters were not significantly different (two-way ANOVA, P > 0.1 in all cases). At 18 months of age, the differences between the C3H/f+/+ and the C3Hf+/+MT1−/− retinas were quite remarkable. The length of the inner segments (IS), outer segments (OS), and the number of photoreceptor cells and cells in the GCL of C3Hf+/+MT1−/− retinas were significantly reduced with respect to the values measured at 3 months of age and with respect to the values in age matched C3H/f+/+ mice (Holm-Sidak test, P < 0.05 in all cases). No significant differences were observed in the number of cells in the inner nuclear layer (two-way ANOVA, P > 0.1) (Fig. S3).
Fig. 8.
Photomicrographs of retinas from C3H/f+/+MT1−/− mice. Mice were 3 (A), 12 (B), and 18 (C) months of age. (Scale bars, 50 μm.)
Fig. 9.
Morphometric analysis of retinas of C3H/f+/+ (white bars) and C3H/f+/+MT1−/− (black bars) at the three different ages. In panels A–D are shown the results obtained by measuring the length of the rod outer segment (OS) and inner segment (IS), counting the total number of photoreceptor nuclei (ONL) and ganglion cells in the central superior retina. A significant change in the length of the OS, IS, and in the number of cells in the ONL and GCL is present between C3H/f+/+ and C3H/f+/+MT1−/− at 18 months of age. In both genotypes, the number of cells in the ONL showed a significant reduction with aging (two-way ANOVA, P < 0.05). Each bar, the mean ± SEM; n = 4–6. *, P < 0.05 (Holm-Sidak test).
Discussion
The data obtained in the current study indicate that MT1 receptors are widely distributed within the murine retina (Fig. 1). The distribution of MT1 mRNA that we have observed is consistent with data reported in the literature in other mammalian species (12, 13).
The fact that MT1 transcripts are localized on photoreceptors may suggest that melatonin is involved in the modulation of visual function, and consistent with this hypothesis, our data indicate that melatonin and MT1 receptors are involved in the modulation of visual sensitivity. In C3H/f+/+ mice, the dark-adapted ERG had significantly larger amplitudes of the a- and b-waves at night (when melatonin levels are high) than during the middle of the day (Fig. 3). This diurnal difference was not present in the C3H/f+/+MT1−/− animals, suggesting that the observed increase in the amplitude is due to the action of melatonin via MT1 melatonin receptors. Although it is possible that the difference in the amplitude of the a-wave and b-waves measured in the middle of the day and in the middle of the night in C3H/f+/+ mice may be due in part to the difference in the length of the dark adaptation (30 min vs. 6 h), we believe that this contribution is negligible since dark adaptation processes are largely complete within 30 min (28). This conclusion is supported by the analysis of melatonin injections on ERG responses after a short period of dark adaptation at midday.
Administration of exogenous melatonin (1 mg/kg) during the daytime increased the amplitude of the a- and b-wave to levels similar to those observed during the night in C3H/f+/+ but not in MT1−/− mice, providing direct evidence that melatonin modulates ERG responses of the mouse retina (Figs. 3 and 4). Additional evidence that melatonin increases the light sensitivity of the retina comes from the observation that melatonin injection lowers the scotopic threshold (Fig. 5). These results are consistent with previous reports in the green iguana, chicken, and man (17–19) and expand those findings by demonstrating that melatonin acts via MT1 receptors. Our data also indicate that melatonin can affect the amplitude of the photopic ERG response in an MT1 receptor-dependent manner (Fig. 6). It is not known whether these effects on ERG responses are due to the action of melatonin on photoreceptors, inner retinal circuitry, or both. However, the enhanced a-wave amplitudes in dark adapted mice after melatonin injection implicate a photoreceptor site of action for at least part of the response.
A previous study has reported that MT1 receptor mRNA expression in the rat retina peaked at embryonic day 16 and then decreased reaching the adult level at postnatal day 14, suggesting these receptors may have a role in retinal development (29). Our data do not support such a conclusion since in young (3-month-old) C3H/f+/+MT1−/− the pattern of stratification and overall architecture of the retina is similar to that of age matched C3H/f+/+ and conforming to published literature (26).
Experimental evidence suggests a possible influence of melatonin in photoreceptor degeneration. In albino rats, administration of exogenous melatonin increases light-induced photoreceptor degeneration (30) and intraocular injection of luzindole, a melatonin receptor antagonist, decreases light damage (25). Although melatonin appears to enhance light damage, it may have neuroprotective effects in other models of photoreceptor degeneration. In mice carrying a genetic defect that causes the degeneration of the rod photoreceptors (rds/rds), daily injections of melatonin significantly delayed photoreceptors loss, suggesting that melatonin could be of potential use for treatment of this form of retinal degeneration (31). Our data indicate that melatonin via MT1 receptors may indeed increase photoreceptor viability during aging (Figs. 8 and 9).
A role for melatonin in protecting against the development of glaucoma and in lowering the intraocular pressure has been also proposed (10–11). Indeed, our data showing that in C3H/f+/+MT1−/− mice have a significant reduction in the number of ganglion cells (Figs. 8 and 9) with respect to age matched C3H/f+/+ mice reinforce the idea that melatonin acting via MT1 receptors protects ganglion cells from degeneration.
Melatonin may also protect photoreceptors and ganglion cells during aging by acting as antioxidant as well via its action on MT1 receptors. Indeed, previous studies have shown a possible role for retinal melatonin as a free radical scavenger within photoreceptors. In isolated photoreceptors from frog retina, melatonin was approximately100 times more potent than vitamin E in inhibiting light-induced oxidative processes (32) and melatonin can reduce the lipid peroxidation induced by nitric oxide in rat retinal homogenates (33). However, supraphysiological concentrations of melatonin may be needed for the antioxidant actions, and MT1 receptor activation appears to play a dominant role in maintaining cell viability. Indeed recent clinical data indicate that melatonin levels are significantly lower in patients affected by age-related macular degeneration (AMD) thus suggesting a possible role of melatonin in the etiology of AMD (34). Our data indicate that activation of MT1 receptors via specific agonists may provide a tool to treat and possibly prevent AMD. The mechanisms whereby MT1 receptor activation delays age-related retinal cell death are yet to be determined.
In conclusion, our data indicate that melatonin, via MT1 receptors, plays an important role in the modulation of retinal function at night, optimizing the performance of the visual system in this nocturnal animal. Because both a-wave and b-wave amplitudes are affected, this modulation may be initiated at the level of the photoreceptor cells, consistent with the expression of MT1 mRNA by these primary sensory neurons. This fine tuning of the visual system performance can provide a clear evolutionary advantage for a nocturnal animal under natural conditions. Our study also indicates that melatonin and MT1 receptors increase photoreceptor and ganglion cell viability during aging. These data provide direct experimental evidence in support of the functional significance of melatonin and MT1 receptors in the mammalian retina. They also form the basis for future studies on the therapeutic use of melatonin in retinal diseases associated with decreased retinal sensitivity to light and degeneration of retinal cells, such as that in age-related macular degeneration or glaucoma.
Methods
Animals.
C3H MT1−/− knock-out mice homozygous for the rd1 mutation, generously donated by Drs. Reppert and Weaver (University of Massachusetts Medical School), were back-crossed with C3H/f+/+ mice in which the rd1 mutation has been removed to produce C3H/f+/+MT1−/−.
In Situ Hybridization and Immunocytochemistry (ICCH).
Details about the procedures used for in situ hybridization and ICCH are described in detail in published work (26, 27). For additional details, see SI Methods and Table S1.
Laser Capture Microdissection (LCM) of Retinal Layers and RT-PCR Analysis.
Tissue preparation and RT-PCR were performed as described (3, 9).
Electroretinogram (ERG).
Dark adapted electroretinogram was performed as described in previously published work (24, 25) and detailed in SI.
Retinal Morphology.
Mice were euthanized, and before eye nucleation the superior cornea was marked with a hot needle.
Supporting Information.
For more methods see SI Text.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants NS 43459 and NS 60659 (to G.T.), EY004864 (to P.M.I), EY12654 (to E.S), the Core Grant for Vision Research P30EY006360 to Emory University, and Research to Prevent Blindness. We also thank the Keck Genomic Center at Morehouse School of Medicine for the use of their facilities and the Georgia Research Alliance for providing funds to purchase the electroretinogram equipment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904400106/DCSupplemental.
References
- 1.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 2.Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Fukuhara C, Wessel JH, Iuvone PM, Tosini G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004;315:197–201. doi: 10.1007/s00441-003-0822-1. [DOI] [PubMed] [Google Scholar]
- 4.Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: A mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–100. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuhara C, Dirden JC, Tosini G. Photic regulation of melatonin in rat retina and the role of proteasomal proteolysis. Neuroreport. 2001;12:3833–3837. doi: 10.1097/00001756-200112040-00046. [DOI] [PubMed] [Google Scholar]
- 7.Iuvone PM, et al. Retinal melatonin production: Role of proteasomal proteolysis in the circadian and photic control of arylalkylamine N-acetyltransferase. Invest Ophthalmol Vis Sci. 2002;43:564–572. [PubMed] [Google Scholar]
- 8.Chen W, Baler R. The rat arylalkylamine N- acetyltransferase E-box: Different use in a master vs. slave oscillator. Mol Brain Res. 2000;81:43–50. doi: 10.1016/s0169-328x(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 9.Fukuhara C, et al. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iuvone PM, et al. Circadian clocks, clock-controlled genes and melatonin biosynthesis in the retina. Prog Ret Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Wiechmann AF, Summers JA. Circadian Rhythms in the eye: The physiological significance of melatonin receptors in ocular tissue. Prog Ret Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Fujieda H, et al. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: Immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci. 2000;17:63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- 13.Scher J, Wankiewicz E, Brown GM, Fujieda H. MT(1) melatonin receptor in the human retina: Expression and localization. Invest Ophthalmol Vis Sci. 2002;43:889–897. [PubMed] [Google Scholar]
- 14.Cosci B, Longoni B, Marchiafava PL. Melatonin induces membrane conductance changes in isolated retinal rod receptor cells. Life Sci. 1997;60:1885–1889. doi: 10.1016/s0024-3205(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 15.Wiechmann AF, Vrieze MJ, Dighe R, Hu Y. Direct modulation of rod photoreceptor responsiveness through a Mel1c melatonin receptor in transgenic Xenopus Laevis retina. Invest Ophthal Vis Sci. 2003;44:4522–4531. doi: 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- 16.Ping Y, Huang H, Zhang XJ, Yang XL. Melatonin potentiates rod signals to ON type bipolar cells in fish retina. J Physiol. 2008;586:2683–2694. doi: 10.1113/jphysiol.2008.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda-Anaya M, Bartell PA, Menaker M. Circadian rhythm of iguana electroretinogram: The role of dopamine and melatonin. J Biol Rhythms. 2002;17:526–538. doi: 10.1177/0748730402238235. [DOI] [PubMed] [Google Scholar]
- 18.Peters JL, Cassone VM. Melatonin regulates circadian electroretinogram rhythms in a dose- and time-dependent fashion. J Pineal Res. 2005;38:209–215. doi: 10.1111/j.1600-079X.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 19.Rufiange M, Dumont M, Lachappelle P. Correlating retinal function with melatonin secretion in subject with an early or late circadian phase. Invest Ophthalmol Vis Sci. 2002;43:2491–2499. [PubMed] [Google Scholar]
- 20.Tosini G, Menaker M. The clock in the mouse retina: Melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 22.Lupi D, et al. Transgenic ablation of rod photoreceptors alters the circadian phenotype of mice. Neurosci. 1999;89:363–374. doi: 10.1016/s0306-4522(98)00353-4. [DOI] [PubMed] [Google Scholar]
- 23.Lucas RJ, et al. Regulation of mammalian circadian behavior by nonrod, noncone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 24.Cameron MA, et al. Electroretinography of wild type and cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008;23:489–501. doi: 10.1177/0748730408325874. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara T, Sieving PA, Iuvone PM, Bush RA. The melatonin antagonist luzindole protects retinal photoreceptors from light damage in the rat. Invest Ophthalmol Vis Sci. 1998;39:2458–2465. [PubMed] [Google Scholar]
- 26.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damiani D, et al. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–4887. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamb TD, Pugh EN. Phototransduction, dark adaptation, and rhodopsin regeneration. Invest Ophthal Vis Sci. 2006;47:5138–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- 29.Fujieda H, Scher J, Lukita-Atmadja W, Brown GM. Gene regulation of melatonin and dopamine receptors during eye development. Neuroscience. 2003;120:301–307. doi: 10.1016/s0306-4522(03)00298-7. [DOI] [PubMed] [Google Scholar]
- 30.Wiechmann AF, O'Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 1992;33:1894–1902. [PubMed] [Google Scholar]
- 31.Liang FQ, et al. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport. 2001;12:1011–1014. doi: 10.1097/00001756-200104170-00029. [DOI] [PubMed] [Google Scholar]
- 32.Marchiafava PL, Longoni B. Melatonin as an antioxidant in retinal photoreceptors. J Pineal Res. 1999;26:184–189. doi: 10.1111/j.1600-079x.1999.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 33.Siu AW, Reiter RJ, To CH. Pineal indolamines and vitamin E reduce nitric oxide-induced lipid peroxidation in rat retinal homogenates. J Pineal Res. 1999;27:122–128. doi: 10.1111/j.1600-079x.1999.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 34.Perez VR, et al. Melatonin levels in age-related macular degeneration patients as measured by urinary 6-sulfatoxymelatonin level. Arvo Abstract. 2009:736. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.