Abstract
Eczema vaccinatum (EV) is a complication of smallpox vaccination occurring in patients with atopic dermatitis. In affected individuals, vaccinia virus (VV) spreads through the skin, resulting in large primary lesions and satellite lesions, and infects internal organs. BALB/c mice inoculated with VV at sites of Th2-biased allergic skin inflammation elicited by epicutaneous ovalbumin (OVA) sensitization exhibited larger primary lesions that were erosive, more satellite lesions, and higher viral loads in skin and internal organs than mice inoculated in saline-exposed skin, unsensitized skin, or skin sites with Th1-dominant inflammation. VV inoculation in OVA-sensitized skin induced marked local expression of IL-17 transcripts and massive neutrophil infiltration compared to VV inoculation in saline-exposed skin. Treatment with anti-IL-17 decreased the size of primary lesions, numbers of satellite lesions, and viral loads. Addition of IL-17 promoted VV replication in skin explants. These results suggest that IL-17 may be a potential therapeutic target in EV.
Keywords: atopic dermatitis, eczema vaccinatum
Vaccinia virus (VV) belongs to the orthopoxvirus genus of the Poxviridae family, which includes variola virus, the agent that causes smallpox, and is a potent vaccine against smallpox (1). Smallpox was eradicated in 1980 and vaccination ceased in the U.S. in 1972 and in the rest of the world in 1979 (1). Recent concern that VV might be used as a bioterror weapon has led to selective vaccination of military personnel and to contingency plans for mass vaccination. A major concern is that patients with atopic dermatitis (AD), which affects 15–20% of children in industrialized countries, are susceptible to eczema vaccinatum (EV), a life-threatening complication in which VV disseminates in the skin, resulting in satellite skin lesions, and spreads to internal organs (2). Although smallpox vaccination is contraindicated in AD patients, they are at risk for EV following contact with recently immunized individuals (3).
IL-4 and IL-10 promote VV spread (4, 5). In contrast, IFN-γ (6), IgG antibody (7), CD8+ cytotoxic T cells (8), NK cells (9), and the cathelicidin family of antimicrobial peptides (AMPs) (10) are thought to contribute to VV containment. It has been suggested that IL-4 driven down-regulation of AMPs in AD skin lesions predisposes to EV (10). IL-17 is expressed in acute skin lesions of patients with AD and in their peripheral blood CD4+ T cells (11, 12). There is controversy as to whether IL-17 promotes or attenuates VV infection (13, 14).
We have previously shown that epicutaneous (EC) sensitization of BALB/c mice with ovalbumin (OVA) results in skin inflammation with similarities to AD skin lesions, characterized by infiltration with CD4+ cells and eosinophils and by increased expression of mRNA for Th2 cytokines and IL-17, but not IFN-γ (15, 16). In this study, we show that inoculation of VV in OVA-sensitized skin results in larger primary lesions, more satellite lesions, and higher viral loads and markedly higher local expression of IL-17 than VV inoculation in saline-exposed skin or skin with Th1-dominant inflammation. We show that IL-17 promotes viral dissemination in this model.
Results
VV Inoculation at Sites of Allergic Skin Inflammation Results in Large Erosive Primary Lesions, Satellite Skin Lesions, and Increased Viral Spread.
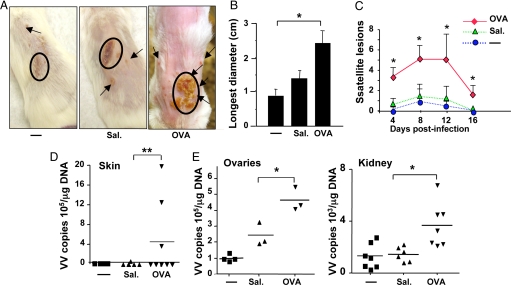
Eight days after infection, BALB/c mice inoculated with VV in OVA-sensitized skin developed significantly larger primary lesions that were erosive and pustular, as well as significantly higher numbers of satellite lesions than controls inoculated with VV in saline-exposed or unsensitized skin (Fig. 1A–C). VV genomes were significantly higher in the inoculated skin, ovaries, and kidneys of OVA-sensitized mice than in controls (Fig. 1D and E). When VV was inoculated distantly (>3 cm away) from the EC sensitization site, there was no difference between OVA-sensitized mice and controls (data not shown), indicating that EC sensitization with OVA did not cause a general impairment in the ability to contain VV.
Fig. 1.
Response of BALB/c mice to VV inoculation at sites of OVA sensitization. (A) Primary and satellite lesions in BALB/c mice inoculated with VV in unsensitized (-), saline-exposed (Sal.), and OVA-sensitized skin. (B) Largest diameter of primary lesion (n = 4–5 per group). (C) Number of satellite lesions (n = 5–12 per group). (D) Viral load in inoculated skin. VV genomes were detected in OVA-sensitized skin from seven of eight mice, but not in skin from five saline-exposed mice. (E) Viral load in internal organs (n = 3–7 per group). Values and error bars represent mean ± SD. *, P < 0.05; **, P < 0.01.
OVA-sensitized skin of C57BL/6 mice exhibits less marked eosinophil infiltration than OVA-sensitized skin of BALB/c mice and up-regulates expression of IFN-γ, in addition to Th2 cytokines (17). C57BL/6 mice inoculated with VV in OVA-sensitized skin developed significantly larger primary lesions and higher viral loads in skin, ovaries, and kidneys than controls (Fig. S1). However, primary lesions were smaller than in BALB/c mice, and no satellite skin lesions were observed, possibly because of the local IFN-γ response of C57BL/6 mice. These results suggest that increased susceptibility to VV in mice inoculated at sites of allergic skin inflammation is not strain-specific.
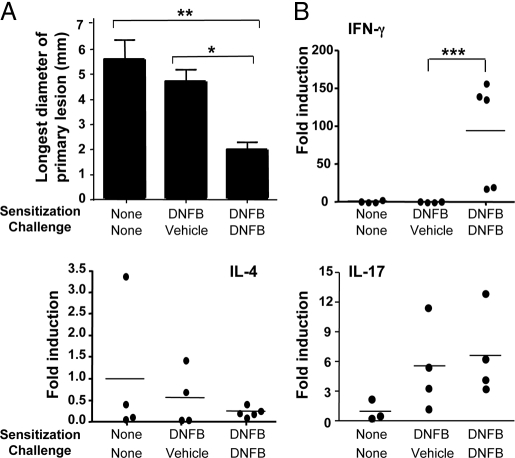
BALB/c mice sensitized on abdominal skin with the hapten dinitrofluorobenzene (DNFB) and challenged 5 days later on back skin with hapten develop Th1-dominated skin inflammation with a striking increase in IFN-γ, but not IL-4 or IL-13 mRNA expression (18). BALB/c mice inoculated with VV at DNFB-challenged skin sites developed significantly smaller primary lesions than controls (Fig. 2A), with no detectable viral genomes. Furthermore, they did not exhibit satellite lesions, and viral loads in internal organs were comparable to controls (data not shown). These results suggest that VV inoculation into sites of Th2-dominated skin inflammation is selectively associated with increased susceptibility to the virus.
Fig. 2.
Response of BALB/c mice to VV inoculation at sites of DNFB hapten challenge. (A) Longest diameter of primary lesion. (B) Cytokine mRNA expression (n = 4–5 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.005.
VV Inoculation in OVA-Sensitized Skin Elicits a Mixed, Modestly Th2 Skewed, Th Response.
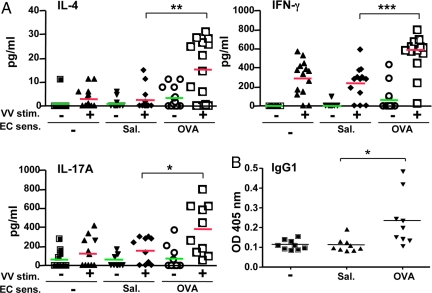
Splenocytes from mice inoculated with VV via OVA-sensitized skin secreted ≈5-fold more IL-4 and ≈2-fold more IFN-γ and IL-17 in response to VV than splenocytes from control mice (Fig. 3A). This resulted in a modest Th2 skewing with a ≈2.5-fold decrease in the IFN-γ:IL-4 ratio. Accordingly, VV-specific serum IgG1 levels were significantly higher in mice inoculated with VV in OVA-sensitized skin than in control mice (Fig. 3B). VV-specific IgG2a levels were comparable (data not shown).
Fig. 3.
VV-specific Th1 and Th2 cytokine and antibody production. (A) Cytokine secretion by splenocytes following VV stimulation using uninfected (-) or VV infected (+) A20 cells (n = 10–14 per group). (B) Serum VV-specific IgG1 antibody titers (n = 9 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
CD8+ cells are important in containing VV (8). H-2Kk/VV peptide pentamers for use in BALB/c mice are not available, but H-2Kb/VV TSYKFESV pentamers for use in C57BL/6 mice are. The frequencies of CD8+pentamer+ and of pentamer+IFN-γ+ cells were comparable in spleens of C57BL/6 mice inoculated with VV via OVA-sensitized skin and controls (Fig. S2 A and B). The numbers of splenic DX5+ NK cells and the level of NK cell cytotoxic activity against YAC-1 target cells were also comparable in the two groups (Fig. S2C and data not shown).
Selectively Increased IL-17 Expression in Sites of Allergic Skin Inflammation Inoculated with VV.
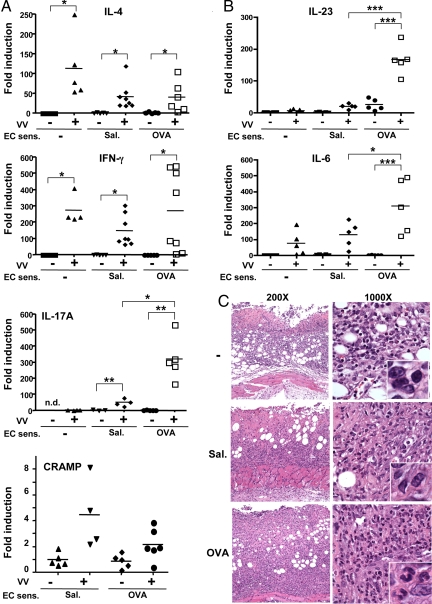
We previously showed that IL-4 and IL-17 expression is up-regulated and CRAMP expression is down-regulated in OVA-sensitized skin of BALB/c mice (16, 19). Eight days after cessation of EC sensitization, expression of IL-4, IL-17, and CRAMP in OVA-sensitized skin was comparable to that in control skin sites (Fig. 4A), suggesting that the changes in expression of these cytokines in OVA-sensitized skin are dependent on persistent antigen exposure. Following VV inoculation, expression of IL-4 and IL-10, which promote VV spread, and of IFN-γ and CRAMP, which inhibit it, was comparable in VV-inoculated OVA-sensitized, saline-exposed, and unsensitized skin (Fig. 4A and (Fig. S3). In contrast, VV inoculation in OVA-sensitized EC-sensitized skin strongly up-regulated IL-17A expression to significantly higher levels than VV inoculation in saline-exposed or unsensitized skin. VV inoculation in OVA-sensitized skin also strongly and significantly up-regulated mRNA expression of the Th17 promoting cytokines IL-6 and IL-23 compared to inoculation in saline-exposed or unsensitized skin (Fig. 4B). IL-17A causes neutrophil accumulation by inducing the expression of neutrophil attracting CXC chemokines (20, 21). VV-inoculated unsensitized and saline-exposed control sites showed cutaneous and s.c. infiltration with mononuclear cells with a minority of neutrophils. In contrast, VV-inoculated OVA-sensitized sites exhibited a predominantly neutrophilic infiltrate, with a minority of mononuclear cells (Fig. 4C).
Fig. 4.
Cytokine expression and histology of VV-infected skin. (A) Cytokine expression as fold induction relative to uninfected unsensitized skin. IL-17A mRNA levels were normalized to VV-infected unsensitized skin, as IL-17 mRNA was not detectable (n.d.) in uninfected unsensitized skin (n = 4–8 per group). (B) Expression of IL-23 and IL-6 (n = 5 per group). (C) Representative photomicrographs of vaccinia inoculation sites at 200× and 1,000×; inset, 5× photo enlargement of 1,000×. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
VV inoculation of DNFB-challenged skin of DNFB-sensitized mice caused strong up-regulation (≈90-fold) of IFN-γ, but not IL-4 or IL-13, mRNA expression compared to VV inoculation of vehicle-challenged skin from these mice or of skin from unsensitized mice (Fig. 2B and data not shown). There was only modest (≈6-fold) and comparable up-regulation of IL-17 expression in VV-inoculated hapten and vehicle-challenged skin sites of DNFB-sensitized mice relative to VV-inoculated skin of unsensitized mice (Fig. 2B). These results suggested that VV infection and Th2-dominated skin inflammation synergize to drive a vigorous IL-17 response to the virus in inoculated skin.
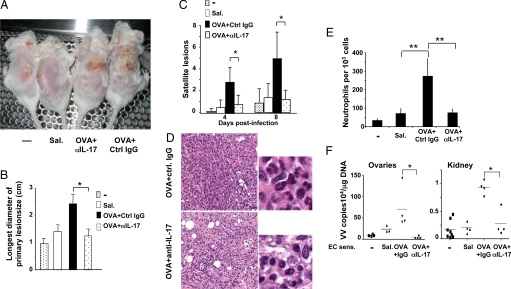
Treatment with Anti-IL-17 Reverses the Skin Pathology and Inhibits Viral Spread in Mice Inoculated with VV at Sites of Allergic Skin Inflammation.
OVA-sensitized mice were injected i.v. with a neutralizing monoclonal anti-IL-17 mAb, or IgG isotype control, on days −1, 0, and + 2 of VV inoculation. Anti-IL-17, but not control IgG, caused a significant decrease in the size of the primary lesions, numbers of neutrophils infiltrating these lesions, and numbers of satellite lesions to levels comparable to those in VV-inoculated saline-exposed skin (Fig. 5A–E). It also reduced viral loads in the ovary and kidney to levels comparable to those in control mice (Fig. 5F). Anti-IL-17 treatment had no significant effect on the production of IFN-γ by VV-stimulated splenocytes, IFN-γ expression in inoculated skin, NK cell activity, or IgG1 and IgG2a antibody responses to VV (Fig. S4 A and B and data not shown), but increased IL-4 mRNA expression in VV-infected skin, consistent with the inhibitory effect of IL-17 on Th2 cytokine expression (22). Anti-IL-17 treatment decreased CRAMP mRNA expression, but not significantly (Fig. S4C).
Fig. 5.
Effect of anti-IL-17 treatment of mice inoculated with VV in OVA-sensitized skin. (A) Primary lesions 8 days postinfection. (B) Size of the largest diameter of the primary lesion (n = 5–8 mice per group). (C) Number of satellite lesions (n = 5–8 mice per group). (D) Skin histology (400×, H&E stain). (E) Numbers of neutrophils in skin per 1,000 cells counted (n = 4 mice per group). (F) Viral load in internal organs. *, P < 0.05; **, P < 0.01.
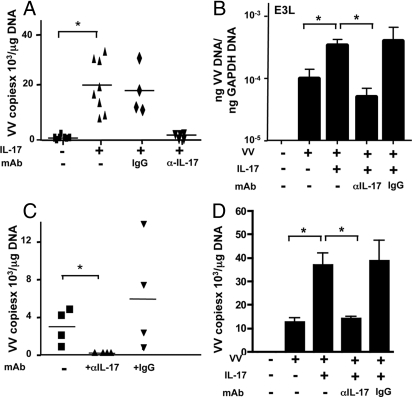
IL-17 Promotes Viral Recovery in VV-Inoculated Skin Explants.
We tested the possibility that local expression of IL-17 promotes VV replication in skin. Very few viral genomes could be detected 2 days after in vitro inoculation of unsensitized mouse skin explants from BALB/c mice. Addition of IL-17 resulted in significantly increased recovery of viral genomes (Fig. 6A). IL-17 caused a significant increase in expression of the VV-encoded gene E3L (Fig. 6B), a gene expressed within 2 h of viral entry and involved in the early phase of VV replication (23). Both IL-17-mediated increased VV genome recovery and increased E3L expression were inhibited by anti-IL-17 mAb, but not control IgG. VV genome recovery was significantly higher in explants of OVA-sensitized skin compared to unsensitized skin (VV copies/μg DNA 3,175 ± 950, n = 4 vs. 740 ± 326, n = 7, P < 0.05). Addition of anti-IL-17 mAb, but not control IgG, to explants of OVA-sensitized skin drastically inhibited VV genome recovery (Fig. 6C). These findings suggest that IL-17 promotes VV recovery in infected skin.
Fig. 6.
IL-17 promotes VV recovery in skin explants. (A) VV genomes in VV inoculated skin explants. (B) Real-time RT-PCR analysis of E3L gene mRNA expression (n = 5). (C) Effect of anti-IL-17 mAb on the recovery of VV genomes from inoculated explants of OVA-sensitized skin. (D) VV genomes in BMDC culture. *, P < 0.05.
Addition of IL-17 had no significant effect on VV replication in the mouse keratinocyte cell line PAM212 or in mouse fibroblasts (data not shown), but significantly increased viral replication in mouse bone marrow-derived DCs (BMDCs) (Fig. 6D). This was specifically inhibited by anti-IL-17 mAb, but not control IgG.
Discussion
We demonstrate that IL-17 plays an important role in the progression of the primary lesion, formation of satellite lesions, and increase in viral load in mice inoculated with VV at sites of allergic skin inflammation.
Large erosive primary lesions, increased numbers of satellite lesions, and increased viral loads in inoculated skin and systemic viral spread are consistent with EV. In a previous study, inoculation in OVA-sensitized skin of a thymidine kinase deficient (TK-) WR strain caused small non-erosive primary lesions, very few satellite lesions and no increase in VV loads compared to inoculation in saline-exposed skin (19). This suggests that the development of EV-like features in our model, which uses a TK+ WR strain, is related to virulence. EV features did not develop after inoculation of VV in skin sites distant from the site of antigen sensitization demonstrating that they were not due to a systemic impairment of the immune response in OVA-sensitized mice. More importantly, EV-like features did not develop when VV was inoculated at sites of Th1-dominated skin inflammation.
Inoculation of VV in OVA-sensitized skin of BALB/c mice elicited a mixed systemic Th response evidenced by secretion of IL-4, IFN-γ, and IL-17 by splenocytes stimulated with VV-infected APCs. Splenocytes from mice inoculated in OVA-sensitized skin secreted higher amounts of all three cytokines than splenocytes from controls inoculated in saline-exposed or unsensitized skin. However, while the increases in IFN-γ and IL-17 secretion were comparable (≈2-fold each), there was a relatively greater increase in IL-4 secretion (≈5-fold), indicating modest Th2 skewing and consistent with the higher serum IgG1 anti-VV levels in these mice. We previously observed Th2 skewing in mice inoculated with the TK- WR strain in OVA-sensitized skin (19). The increased cytokine production across the board in mice inoculated in OVA-sensitized skin could be due to stronger antigenic stimulation resulting from increased antigenic load in the skin and possibly to increased VV access through inflamed skin. The relatively greater increase in IL-4 production might be explained by the observation that DCs from DLN of OVA-sensitized, but not saline-exposed, skin drive IL-4 production in naive T cells (data not shown).
Expression of IL-4 and IL-10, which promote VV spread, and IFN-γ and CRAMP, which inhibit it, was comparable in VV-inoculated OVA-sensitized, saline-exposed, and unsensitized skin. In contrast, there was a striking local up-regulation of IL-17 mRNA expression in response to VV inoculation at sites of allergic inflammation, but not at sites of Th1-dominated inflammation. Strong expression of IL-17 in VV-infected OVA-sensitized skin was associated with a predominantly neutrophilic infiltrate and a purulent exudate, consistent with the known ability of IL-17 to induce neutrophil chemoattractants (20, 21), and with the tissue destructive character of Th17-dominated inflammation (24). There was markedly less IL-17 expression in VV-inoculated saline-exposed skin and very little in VV-inoculated unsensitized skin, and lymphocytes were the predominant infiltrating cells in these sites. The higher IL-17 mRNA expression in the skin of saline-sensitized mice compared to the skin of unsensitized mice, both before and after VV inoculation, may explain the bigger size of the primary lesions and the greater number of satellite lesions observed in the former. VV inoculation induced significantly higher levels of the Th17-promoting cytokines IL-6 and IL-23 in OVA-sensitized skin compared to saline-exposed or unsensitized skin. Increased local IL-6 and IL-23 expression would amplify IL-17 production by skin homing Th17 cells and explain the higher expression of IL-17 in VV-inoculated OVA-sensitized skin.
Treatment of BALB/c mice inoculated with VV via OVA-sensitized skin with anti-IL-17 reduced the size of the primary lesions, the number of satellite lesions, and the viral load in internal organs to levels comparable to those in control mice inoculated in saline-exposed skin. Treatment with anti-IL-17 caused no changes in Th1 or Th2 cytokine production by splenocytes, NK cell activity, IL-4, IFN-γ, or CRAMP expression in the skin that would favor VV containment. IL-17 directly enhanced VV replication in mouse skin explants, as evidenced by increased recovery of VV genomes and expression of the replication associated viral gene E3L. Furthermore, neutralization of IL-17 inhibited VV replication in explanted OVA-sensitized skin, which expresses IL-17 mRNA, probably derived from infiltrating T cells, as it contains very few, if any neutrophils (16). Recently IL-17 was shown to promote replication of Theiler's murine encephalomyelitis virus by enhancing the survival of virus-infected cells and blocking target cell destruction by cytotoxic T cells (25). IL-17 promoted VV replication in mouse BMDCs, but not fibroblasts or the keratinocyte cell line PAM212, suggesting that increased viral replication in skin DCs may contribute to the promoting effect of IL-17 on VV replication in skin explants. We cannot rule out additional direct effects of IL-17 on VV replication on other skin cells such as Langerhans cells. It is also possible that IL-17 might induce the production of cytokines/factors by DCs, or other skin cells, that promote viral replication in a paracrine manner.
Two previous studies explored the role of IL-17 in VV immunity and reached opposite conclusions. In these, mice were infected i.p. with VV expressing IL-17 (VV-IL-17). In one study, VV-IL-17 was more virulent than WT virus (13), while in the other it was less virulent (14). The reason for the discrepancy is not clear. Unlike VV inoculation in the skin, which mimics human vaccination, i.p. injection of VV does not cause the development of skin lesions and leads to death at higher doses of VV. Moreover, in mice infected with VV-IL-17, the tissue distribution and level of expression of IL-17 are unlikely to reflect those elicited by vaccinia immunization.
In our study, the relative expression of IL-17 to the protective cytokine IFN-γ was markedly higher in OVA-sensitized skin inoculated with OVA compared to saline-exposed skin inoculated with VV. In contrast, the ratio of production of these two cytokines by splenocytes was comparable in mice inoculated with VV in OVA-sensitized skin and controls. This selective increase in cutaneous IL-17 expression may explain the observation that the major effects of VV inoculation at sites of allergic skin inflammation were observed at the site of inoculation while viral loads in internal organs were only modestly (≈2–3-fold) increased. Since IL-17 is expressed in human AD skin lesions (11, 12), therapies that target IL-17 might be useful in limiting VV dissemination in patients with AD.
Materials and Methods
Mice.
Mice were purchased from Jackson Laboratories and kept in a pathogen-free environment. All procedures performed on the mice were in accordance with the Animal Care and Use Committee of the Children's Hospital.
Vaccinia Virus.
Vaccinia Virus Western Reserve strain (VR-1354) was obtained from American Type Culture Collection and expanded in monkey CV1 cells as previously described (19).
EC Sensitization, Hapten Challenge, and VV Inoculation.
EC sensitization with OVA, sensitization with DNFB, and VV inoculation of 4- to 6-week-old female mice were performed as previously described (18, 19). Skin was examined 8 days after inoculation.
Quantitative PCR Analysis of VV Genomes.
Tissue samples were immediately frozen and stored at −80 °C. Quantification of VV genomes was performed as previously described (19). Viral genome measurements by PCR correlated with viral titers measurements by plaque assay.
In Vitro Cytokine Production and VV-Specific IgG2a and IgG1 Antibody ELISA.
These assays were performed as described previously (19), except that VR-1354 virus was used to infect A20 cells and coat ELISA plates.
Analysis of mRNA Expression for Cytokines and CRAMP in Skin.
Histological Analysis.
Multiple 4-μm sections were stained with hematoxylin and eosin (H&E). The number of neutrophils among 1,000 cells was counted, blinded from four independent samples examined.
Anti-IL-17 Treatment.
At days −1, 0, and +2 of VV inoculation, mice received intravenously 100 μg neutralizing IgG anti-mouse IL-17 mAb (clone 50104) or IgG control mAb (R&D Systems) in 100 μL.
VV Replication in Skin Explants.
Skin explants were prepared, cultured, and inoculated with VV as previously described (10). IL-17 (250 ng/mL; R&D Systems) was added alone or together with 50 ng/mL anti-IL-17 mAb or IgG isotype control (R&D Systems). After 48 h, the explants were extensively washed. DNA was analyzed for viral genomes (19). RNA was isolated, and 1 μg RNA was reverse-transcribed, and real-time RT-PCR was performed for E3L and GAPDH as previously described (10).
DC Generation.
DCs from BALB/c WT mice were generated as described (26). Briefly, bone marrow was isolated from the long bones of the hind legs of BALB/c WT mice. Cells (2 × 106) were plated in 100-mm dishes in complete RPMI medium 1640 (Invitrogen) supplemented with 20 ng/mL GM-CSF (Peprotech). On day 6, cells were cultured in the presence and absence of IL-17 and/or anti-IL-17 for 24 h before stimulation with VV. Cells were then infected with 0.1 pfu/cell VV for 24 h. After incubation, cells were washed with media to remove the remaining VV, then VV genomes were quantified.
Statistical Analysis.
Significance of differences between the means was analyzed by using nonparametric Mann-Whitney U test.
Supplementary Material
Acknowledgments.
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) under Contract HHSN266200400030C.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904021106/DCSupplemental.
References
- 1.Moore ZS, Seward JF, Lane JM. Smallpox. Lancet. 2006;367:425–435. doi: 10.1016/S0140-6736(06)68143-9. [DOI] [PubMed] [Google Scholar]
- 2.Engler RJ, Kenner J, Leung DY. Smallpox vaccination: Risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357–365. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 3.Wollenberg A, Engler R. Smallpox, vaccination and adverse reactions to smallpox vaccine. Curr Opin Allergy Clin Immunol. 2004;4:271–275. doi: 10.1097/01.all.0000136758.66442.28. [DOI] [PubMed] [Google Scholar]
- 4.Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Den Broek M, et al. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-gamma and nitric oxide synthetase 2. J Immunol. 2000;164:371–378. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez JC, Tapia E, Esteban M. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J Gen Virol. 2002;83:1059–1067. doi: 10.1099/0022-1317-83-5-1059. [DOI] [PubMed] [Google Scholar]
- 8.Binder D, Kundig TM. Antiviral protection by CD8+ versus CD4+ T cells. CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent IL. J Immunol. 1991;146:4301–4307. [PubMed] [Google Scholar]
- 9.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell MD, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Toda M, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111:875–881. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 12.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 13.Patera AC, Pesnicak L, Bertin J, Cohen JI. Interleukin 17 modulates the immune response to vaccinia virus infection. Virology. 2002;299:56–63. doi: 10.1006/viro.2002.1400. [DOI] [PubMed] [Google Scholar]
- 14.Kohyama S, et al. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J Immunol. 2007;179:3917–3925. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- 15.Spergel JM, et al. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effendy I, Loffler H, Maibach HI. Epidermal cytokines in murine cutaneous irritant responses. J Appl Toxicol. 2000;20:335–341. doi: 10.1002/1099-1263(200007/08)20:4<335::aid-jat698>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Scott JE, et al. Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J Allergy Clin Immunol. 2007;120:1382–1388. doi: 10.1016/j.jaci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prause O, Laan M, Lotvall J, Linden A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol. 2003;462:193–198. doi: 10.1016/s0014-2999(03)01341-4. [DOI] [PubMed] [Google Scholar]
- 22.Schnyder-Candrian S, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amegadzie BY, Ahn BY, Moss B. Identification, sequence, and expression of the gene encoding a Mr 35,000 subunit of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1991;266:13712–13718. [PubMed] [Google Scholar]
- 24.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonysamy MA, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.