Abstract
Gap junction (GJ) channels assembled from connexin (Cx) proteins provide a structural basis for direct electrical and metabolic cell–cell communication. By combining fluorescence imaging and dual whole-cell voltage clamp methods, we demonstrate that in response to transjunctional voltage (Vj) Cx43/Cx45 heterotypic GJs exhibit both Vj-gating and dye transfer asymmetries. The later is affected by ionophoresis of charged fluorescent dyes and voltage-dependent gating. We demonstrate that small differences in resting (holding) potentials of communicating cells can fully block (at relative negativity on Cx45 side) or enhance (at relative positivity on Cx45 side) dye transfer. Similarly, series of high frequency Vj pulses resembling bursts of action potentials (APs) can fully block or increase the transjunctional flux (Jj) of dye depending on whether pulses are generated in the cell expressing Cx43 or Cx45, respectively. Asymmetry of Jj-Vj dependence is enhanced or reduced when ionophoresis and Vj-gating act synergistically or antagonistically, whereas single channel permeability (Pγ) remains unaffected. This modulation of intercellular signaling by Vj can play a crucial role in many aspects of intercellular communication in the adult, in embryonic development, and in tissue regeneration.
Keywords: connexin, intercellular permeability, voltage gating, dye transfer, fluorescent proteins
Gap junction (GJ) channels span the plasma membranes of adjacent cells and are formed by the docking of two hemichannels (connexons) oligomerized from connexin (Cx) proteins, which consist of 21 distinct isoforms (1). GJ channels formed from a single Cx isoform are called homotypic, whereas those formed between cells expressing different Cx isoforms are called heterotypic. GJs provide a direct pathway for cell-to-cell electrical signaling and metabolic communication, allowing the passage of small ions, amino acids, metabolites, tetraethylammonium and signaling molecules such as cAMP, IP3, siRNA and small peptides ((2–5) and reviewed in ref. 6).
Earlier studies have shown that heterotypic junctions in which a Cx45 is paired with Cx31, Cx40 or Cx43 exhibit a strong voltage-gating asymmetry and modulatable cell-to-cell electric signaling from nearly uni-directional to bidirectional (7–9). Cx45 is expressed in a variety of tissues, but most abundantly in cardiovascular and nervous systems (1, 10). Blood vessels express Cx37, Cx40, Cx43 and Cx45, with the most abundant expression of Cx37 and Cx40 in endothelial cells and Cx43 and Cx45 in smooth muscle cells (11, 12). Thus, heterotypic GJs containing Cx45 can be formed between smooth muscle cells as well as between smooth muscle and endothelial cells. Furthermore, Cx45 may form junctions with mCx30.2, Cx40 and Cx43 in the heart between cardiomyocytes or cardiomyocytes and fibroblasts (13), between neurons with mCx30.2 and Cx36 (14) and between astrocytes and neurons with Cx43 (15). Here, we show that the Vj initiated by small voltage steps or high frequency activity on one side of Cx43/Cx45 heterotypic junctions can substantially modulate metabolic communication and that these heterotypic junctions may act as voltage-sensitive regulatory valves for intercellular signaling.
Results
Voltage-Gating and Signaling Asymmetry.
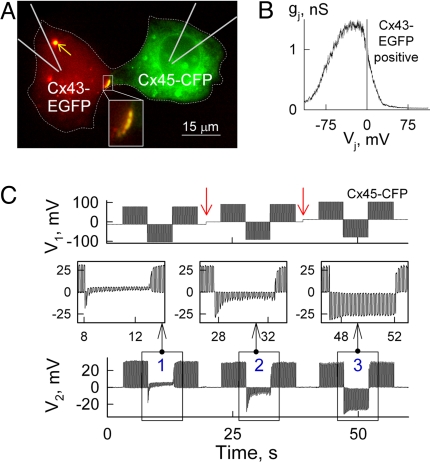
We examined voltage-gating and cell-to-cell signaling asymmetry using a dual whole cell voltage clamp method as previously described (9) between HeLa cells expressing Cx43 or Cx43-EGFP (cell-1) and those expressing Cx45, Cx45-CFP or Cx45-EGFP (cell-2) and forming heterotypic junctions. Fig. 1A shows a fluorescence image of a HeLaCx43-EGFP/HeLaCx45-CFP cell pair, in which the steady-state conductance (gj) dependence on transjunctional voltage (Vj), was recorded by applying slow (1 mV/s) Vj ramps to Cx43-expressing cell and measuring transjunctional current (Ij) (see Fig. 1B). The observed gj-Vj dependence had a peak of gj at Vj≈-25 mV and demonstrated strong Vj-gating asymmetry. The reduction of gj at positive Vjs is caused by the closure of Cx45 hemichannels that gate at relative negativity on their cytoplasmic side, whereas the reduction in gj for negative Vjs result from closure of Cx43 hemichannels that also gate at relative negativity but are less Vj sensitive than Cx45 hemichannels (7). It was proposed that the ≈3.5-fold lower conductance of the Cx45 hemichannel causes a higher fraction of Vj to drop across it resulting in an enhanced Vj-gating sensitivity of the Cx45 hemichannel and reduced Vj-gating sensitivity of the Cx43 hemichannel (9, 16). All 64 Cx43/Cx45 cell pairs examined demonstrated Vj-gating asymmetry comparable to that shown in Fig. 1B. Similar Vj-gating asymmetry was reported for Cx31/Cx45 (8) and Cx40/Cx45 (9) junctions.
Fig. 1.
Vj-gating and modulation of electrical signaling asymmetry. (A) Fluorescence image of a HeLaCx43-EGFP/HeLaCx45-CFP cell pair exhibiting a heterotypic junctional plaque (JP; see Inset). An internalized heterotypic JP is shown by the arrow. (B) gj-Vj dependence measured in the cell pair shown in A by applying slow (1 mV/s) Vj ramps, demonstrates voltage-gating asymmetry. (C) Modulation of intercellular electric signaling asymmetry in the cell pair shown in A. V1 and V2 traces show the voltage protocol applied to HeLaCx45-CFP (cell-1) and the electrotonic potentials measured in HeLaCx43-EGFP (cell-2), respectively. Red arrows indicate a 12 mV increase of the holding potential in cell-1 starting from −12 mV and going to 0 mV and then to 12 mV (Bottom Insets 1, 2 and 3, respectively).
Earlier, it was shown that Vj-gating asymmetry in Cx43/Cx45 junctions can cause asymmetry of electrical signal transfer, which can be effectively modulated by the difference in holding potentials between the cells (ΔVh) (7). Fig. 1C shows an experiment in which the Cx45-expressing cell (cell-1) was initially voltage clamped to −12 mV and repeated (5 Hz) 100 ms and 90 mV Vj pulses of positive and negative polarity were applied. Evoked coupling potentials were recorded in the Cx43-expressing cell (cell-2) maintained in current clamp mode. Positive pulses were effectively transferred to cell-2, whereas transfer of negative pulses was greatly attenuated. Transfer of negative pulses gradually decreased presumably due to gradual gj decrease. After increases in the Vh of cell-1 (from −12 to 0 and to +12 mV; see red arrows), responses in cell-2 progressively become more symmetric (see Insets 1, 2 and 3 in Fig. 1C). Therefore, cells demonstrate electrical signal transfer asymmetry, which can be modulated from virtually unidirectional to bidirectional by relatively small changes in ΔVh. Changes in the duration of the pulses from 5 to 100 ms, while maintaining interpulse interval equal to the duration of the pulse, resulted in similar signal transfer asymmetry that we observed in all 24 Cx43/Cx45 cell pairs examined. A similar phenomenon was reported in amphibian blastomeres exhibiting a small offset in resting potentials (17).
Dye Transfer Modulation by Transjunctional Voltage.
Earlier studies have documented that GJs are permeable to second messengers, such as Ca2+, cAMP and IP3 in a Cx type dependent manner (6). Compared with Cx32, Cx43 GJs demonstrate ≈15-fold higher permeability for metabolites such as glutamate, glutathione, ADP and AMP, and ≈10-fold lesser permeability to adenosine (18). Ionic forms of all the above-mentioned molecules are comparable in molecular mass and net charge with Alexa Fluor-350 (326 Da, z = −1) and Lucifer Yellow (443 Da, z = −2) used in our studies. We did not assess permeability to positively charged dyes because of their strong binding to nucleic acids.
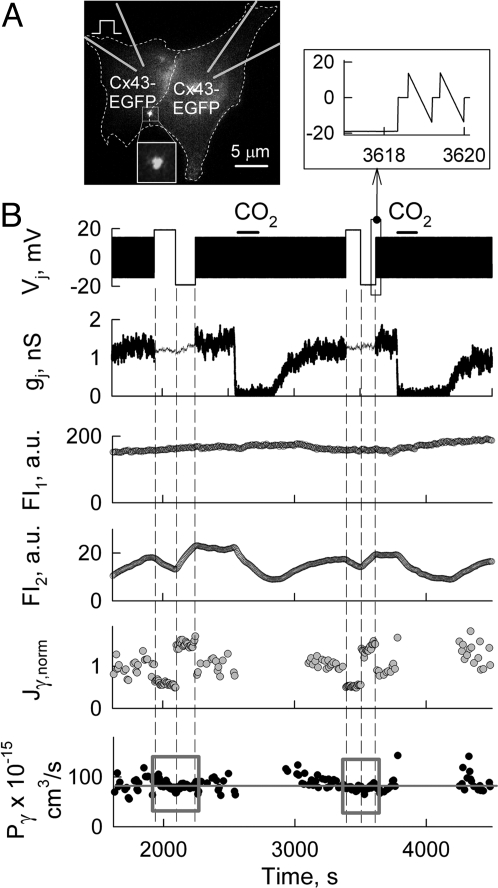
To determine the direct effect of transjuctional electrical field on the cell-to-cell transfer of dye molecules, we performed electrophysiological and fluorescence imaging studies in HeLaCx43-EGFP cell pairs (Fig. 2A), which exhibited almost no Vj-gating over ± 20 mV Vj steps. Cell-1 (Cx43-EGFP) was patched with the pipette containing Alexa Fluor-350 (AF350). After both cells were transferred to whole-cell voltage-clamp mode, fluorescence intensity (FI) of AF350 in cell-1 and cell-2 approached a steady state. Then CO2 was applied twice to block GJs. To measure Ij and gj, repeated small voltage ramps were applied to cell-1. In between repeated ramps, two series of Vj steps of ± 20 mV were applied to cell-1. The amplitude and duration of the steps were too small to induce Vj-gating and reduction in gj, whereas FI2 showed changes due to the direct effect of Vj on AF350 transfer. We and others assumed that when the concentration of dye is <1 mM, dye concentration (C) is directly proportional to FI, C = k(FI), where k is a constant (19, 20). The total transjunctional flux (Jj) of dye when both patch pipettes are in the whole-cell recording mode is determined by changes in the fluorescence intensity in cell-2 (ΔFI2) over the time interval (Δt) and dye leakage to patch pipette-2 so that,
where vol2 is the volume of cell-2 and Pp is the permeability characterizing dye leakage from cell-2 to pipette-2. We assumed that dye concentration in pipette-2 is equal to zero due to its relatively high volume compared with the cell volume. Permeability is determined as the ratio of the flux to the driving force, which involves both the concentration gradient and the electric field for a charged molecule. To calculate the total junctional permeability (Pj) of GJs, we used a modified Goldman-Hodgkin-Katz (GHK) equation (21) describing electrodiffusion,
where z is the net charge of the dye molecule, F is Faraday's constant, R is the gas constant, T is the absolute temperature, and FI1 and FI2 are the fluorescence intensities in cell-1 (dye-donor) and cell-2 (dye-recipient), respectively. Pp was calculated from the dynamics of FI2 decay after gj was blocked by CO2 or alkanols and using equation Pp = −vol2(ΔFI2/Δt)/FI2. Typically, the decay of FI2 was close to exponential (see FI2 traces in Fig. 2B, Fig. 3 and Fig. 6). Pp depends mainly on the size of the open patch at the very tip of the patch pipette, and in this experiment, Pp was ≈1.3 × 10−11 cm3/s. The single channel flux (Jγ) or permeability (Pγ) can be found by dividing Jj or Pj by the number of functional channels, Nf = gj/γ, where γ is the single channel conductance, i.e., Jγ = Jj(γ/gj) and Pγ = Pj(γ/gj). See (SI Appendix) for more details about all equations used in this study. In accordance with an earlier report of Verselis et al. (2) the measured value of Pj at Vj≈0 mV (Pj,0) would be as follows:
In the experiment shown in Fig. 2B, Jγ of negatively charged AF350 (calculated using Eq. 1 divided by Nf and normalized with Jγ0 measured just before the first positive Vj step, Jγ,norm) is modulated during Vj steps. Fig. 2B shows that positive Vj steps caused a ≈60% reduction, whereas negative Vj steps caused a ≈30% increase in Jγ,norm. Despite changes in Jγ,norm, Pγ (calculated using Eq. 2 divided by Nf) values boxed into gray squares remained constant during Vj steps (Fig. 2B). This suggests that the GHK equation used is applicable to describe permeability through GJs for at least Vjs of ≈±20 mV. During repeated Vj ramps of small amplitude, Pγ was calculated using Eq. 3 divided by Nf. Fig. 2B show that on average Pγ = 82.6 ± 4.8 × 10−15 cm3/s (n = 4), which is close to the Pγ previously reported for Cx43-EGFP (20). In summary, in all five experiments we obtained data similar to those shown in Fig. 2B demonstrating that dye transfer can be accelerated or decelerated by ionophoresis, whereas Pγ remains unaffected in the absence of Vj-gating.
Fig. 2.
Dye transfer modulation by ionophoretic effect of Vj in the absence of Vj-gating. (A) Fluorescence image of a HeLaCx43-EGFP cell pair exhibiting a single JP (see Inset). (B) Simultaneous electrophysiological and fluorescence imaging recordings in the cell pair shown in A. The Vj trace shows the voltage protocol applied to cell-1 loaded with AF350 (see diagram in A). Repeated Vj ramps of ± 15 mV applied in cell-1 were used to measure Ij in between Vj steps of ± 20 mV (see expanded traces in the Top-Right Inset). FI1 and FI2 traces show dynamics of dye fluorescence in cell-1 and cell-2, respectively. Jγ,norm and Pγ traces show single channel flux normalized to the control value, and single channel permeability, respectively. On average, at Vj≈0 mV, Pγ = ≈83 ± 5 × 10−15 cm3/s (gray line). Two consecutive applications of CO2 (horizontal bars) were used to block GJs and calculate averaged Pp (Pp = 1.3 × 10−11 cm3/s).
Fig. 3.
Dye transfer modulation by Vj steps. Electrophysiological and fluorescence imaging recordings in a HeLaCx43-EGFP/HeLaCx45-CFP cell pair. The Vj trace shows the voltage protocol applied to the Cx43-EGFP expressing cell-2. The Cx43-CFP expressing cell-1 was loaded with AF350 (see Top-Right diagram). Repeated Vj ramps of ± 25 mV applied before and after voltage steps of ± 80 mV were used to measure gj (Top-Left Inset). FI1 and FI2 traces show the dynamics of AF350 fluorescence in cell-1 and cell-2, respectively. CO2 application (horizontal bar) was used to block GJs and calculate Pp (Bottom-Right Inset). The Jj,norm. trace shows the normalized total junctional flux. The asterisks indicate times when slow decay of Jj,norm was caused mainly by a reduction in the difference between FI1 and FI2. Δ indicates moment of patch opening in cell-1.
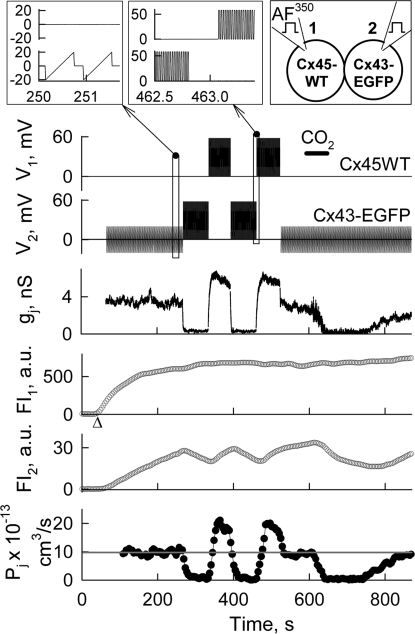
Fig. 6.
Dye transfer modulation by bursts of + 60 mV pulses 10 ms in duration repeated at 50 Hz frequency (Top-Middle Inset), and applied alternately to cell-1 and cell-2 of a HeLaCx43-EGFP/HeLaCx45WT cell pair (Top-Right diagram). V1 and V2 traces show voltage protocols applied in cell-1 (loaded with AF350) and cell-2, respectively. Repeated Vj ramps of ± 20 mV applied in cell-2 (Top-Left Inset) were used to measured Ij and calculate the gj trace. FI1 and FI2 traces show dynamics of dye fluorescence in cell-1 and cell-2, respectively. The Pj trace shows the total junctional permeability. On average, at Vj = 0 mV, Pj = ≈9.9 × 10−13 cm3/s (gray line). During series of pulses, Pj was calculated using Eq. 2 at Vj = 21 mV, i.e., ≈35% of 60 mV pulses. CO2 application (horizontal bar) was used to block GJs and calculate Pp. Δ indicates moment of patch opening in cell-1.
Dye Transfer Modulation in the Presence of Vj-Gating.
Because we showed that Cx43/Cx45 heterotypic junctions exhibit Vj-gating and electric cell-to-cell signaling asymmetries (Fig. 1B), we hypothesized that these asymmetries should cause an asymmetry of Jj-Vj and Pj-Vj dependencies. To address this, we examined the effect of Vj on dye transfer in HeLa cells forming Cx43/Cx45 heterotypic GJs.
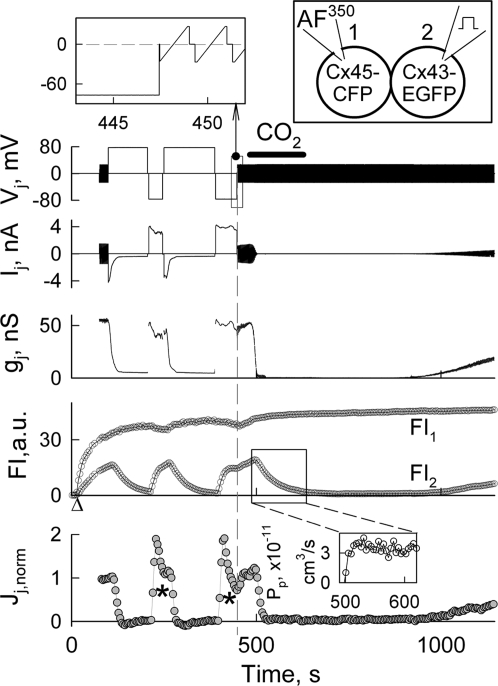
Fig. 3 shows an example of combined electrophysiological and fluorescence imaging recordings in a Cx43-EGFP/Cx45-CFP cell pair (see diagram). Initially, the patch was open in cell-2. After opening the patch in cell-1, FI started rising in cell-1 and with slower kinetics in cell-2. Repeated ramps of ± 25 mV were used to measure gj before and after voltage steps of ± 80 mV were applied to cell-2. Initially gj was ≈55 nS, but it decayed rapidly after applications of positive voltage steps to Cx43-EGFP cell reaching a steady state at gj = ≈5 nS (see gj trace). During negative voltage steps, gj recovered quickly followed by a ≈20% decay. These gj changes are in agreement with the Vj-gating asymmetry shown in Fig. 1B. CO2 application for ≈2 min induced transient uncoupling. From FI2 changes shortly after CO2 application, we found that Pp = 3.8·10−11 cm3/s. Jj was calculated using Eq. 1 and normalized with Jjo measured just before the first positive Vj step (see Jj,norm trace). During positive Vj steps, Jj,norm declined to zero even though cells remained coupled with gj = ≈5 nS. Jj,norm recovered rapidly during voltage steps of negative polarity. The decay of Jj,norm to zero during positive Vj steps occurred despite the fact that positive voltage applied to the Cx43-EGFP expressing cell should increase the transfer of negatively charged AF350 molecules, suggesting that positive Vj steps drove the channels to a non-permeable substate. During negative Vj steps, the slow decrease in Jj,norm (see asterisks) is presumably due to the reduction in the difference between FI1 and FI2. Similar data were obtained in seven other cell pairs forming Cx43-EGFP/Cx45-CFP and Cx43-EGFP/Cx45WT GJs. All collected data show that dye flux through Cx43/Cx45 heterotypic GJs can be modulated effectively by Vj-gating. As seen in Fig. 3, gj does not reach a zero level at positive Vj steps. This is caused by the so called residual conductance resulting from the inability of the fast gating mechanism to close the GJ channel fully (22). Earlier, we and others reported that GJ channels closed to the residual state become impermeable to AF350, Lucifer yellow (LY) and cAMP, while remaining permeable to small ions, major charge carriers for electrical cell–cell coupling (23, 24). In concert with those reports, in Fig. 3, Jj,norm reached a zero level despite the fact that gj is still ≈5 nS. We did not calculate Pγ for this experiment because an estimation of the numbers of functional channels can be under evaluated due to an effect of series resistance on gj measurements at high gjs (≈60 nS) (25). We evaluated Pγ for Cx43/Cx45 heterotypic GJ channel from the experiments shown in Figs. 4 and 6 exhibiting relatively low gjs (see below).
Fig. 4.
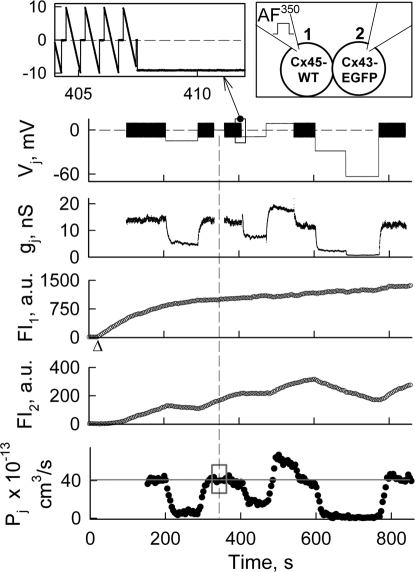
Dye transfer modulation by small Vjs. Electrophysiological and fluorescence imaging recordings in a HeLaCx43-EGFP/HeLaCx45WT cell pair. Vj trace shows the voltage protocol applied to the Cx45-expressing cell-1 (loaded with AF350, see Top-Right diagram). Repeated Vj ramps of ± 10 mV (Top-Left Inset) were used to measure gj in between Vj steps. FI1 and FI2 are fluorescence intensities measured in cell-1 and cell-2, respectively. The Pj trace shows the total junctional permeability. On average, during repeated small amplitude Vj ramps, Pj = ≈39.6 × 10−13 cm3/s (gray line). Δ indicates moment of patch opening in cell-1.
What Is the Minimal Vj That Can Affect Dye Transfer?
To answer this question, we examined the transfer of AF350 by applying relatively small Vj steps. Fig. 4 shows an experiment in which consecutive Vj steps of −14, −9, +9, −30, and −60 mV elicited Vj-gating and modulation of Pj (calculated using Eq. 2). During repeated Vj ramps we assumed that Vj = 0 mV, and Pj was calculated using Eq. 3. In this experiment, we used cells expressing Cx45WT (see diagram) instead of Cx45-CFP to show that CFP does not change the asymmetry of Pj-Vj dependencies. Repeated ±10 mV Vj ramps were applied in between Vj steps to measure gj, which was ≈14 nS. After ≈330 s, Vj ramps were not applied for ≈30 s to verify that they did not change Pj (see gray square on Pj trace). During these 30 s we calculated Pj using Eq. 3. During all consecutive Vj steps of negative polarity (−14, −9, −30, and −60 mV), gj decreased but some residual conductance still remained with gjs of ≈4, 8, 2, and 0.3 nS, respectively. At the same time, Pj decreased ≈80, 55, 95 and 100%, respectively. During a Vj step of +9 mV, gj and Pj increased ≈30%. From Pj and gj measurements at the beginning of the record and assuming that for Cx43/Cx45 channel γ = 55 pS (7), we found that Pγ,Cx43/Cx45 = Pj(γ/gj) = ≈15 × 10−15 cm3/s, which is in good agreement with earlier estimates of Pγ,Cx43/Cx45 at Vj ≈0 mV (20).
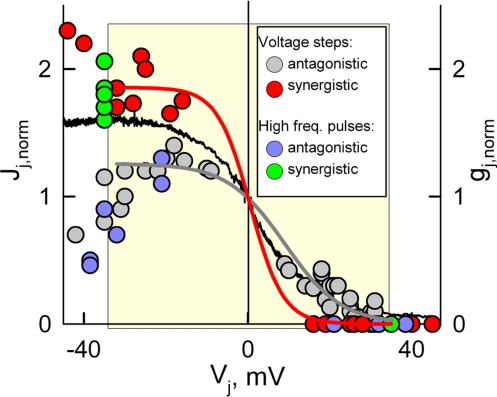
Thus, our data show that in Cx43/Cx45 junctions, Vjs as low as ≈±10 mV can substantially modulate transfer of metabolites comparable in size with the dyes used (≈400 Da). This modulation of charged molecules can be amplified or reduced depending on whether Vj-gating and ionophoresis act synergistically or antagonistically. If a Cx43-expressing cell is loaded with AF350 and subjected to positive or negative Vj steps up to ≈30 mV, then gj should be reduced or increased and Vj should decelerate or accelerate transfer of AF350, respectively. Thus, at both Vj polarities, Vj-gating and ionophoresis should act on dye transfer synergistically. On the contrary, if a Cx45-expressing cell is loaded with AF350, then Vj-gating and ionophoresis should affect dye transfer antagonistically. Data summarized from 24 cell pairs in Fig. 5 show the synergistic and antagonistic normalized Jj-Vj dependencies observed when cells expressing Cx43 or Cx45, respectively, were loaded with AF350; data were normalized in respect to Jj at Vj≈0 mV. Red (synergistic) and gray (antagonistic) circles indicate experimental data in which Vj steps of negative or positive polarity were applied to either cell of the pair. Red and gray curves show fitting of the data encompassed in the yellow square and shown in red and gray circles, respectively, to a sigmoidal formula, Jj = a/(1+exp(-(Vj-Vo)/b)). The major goal of this fitting was to evaluate the difference in steepness of Jj changes (ΔJj/ΔVj) at Vjs around Vj = 0 mV. We found that coefficient b determining ΔJj/ΔVj was equal to −4.2 ± 0.5 mV and −6.9 ± 0.6 mV for synergistic and antagonistic Jj-Vj dependence, respectively. Consequently, on average, ΔJj/ΔVj was equal to ∼−0.09 and −0.03 normalized units of Jj per mV for synergistic and antagonistic dependencies, respectively. Therefore, Vjs as small as ≈10 mV around Vj = 0 mV can cause substantial changes in Jj and these changes are ≈3-fold bigger under synergistic versus antagonistic action of ionophoresis and Vj-gating. In Fig. 5, the black line shows the normalized gj-Vj dependence averaged from five gj-Vj plots. Synergistic Jj-Vj dependence was more whereas antagonistic one was less steep than gj-Vj dependence at Vj≈0 mV.
Fig. 5.
Summarized data of Jj-Vj for AF350 measurements in 25 Cx43/Cx45 cell pairs. Data were normalized to Jj at Vj = 0 mV. Red (synergistic) and gray (antagonistic) circles indicate experimental data in which Vj steps of negative or positive polarity were applied to either cell of the cell pair. Red and gray curves show fitting of the data encompassed in the yellow square and shown in red and gray circles, respectively, by a sigmoidal equation. Green (synergistic) and blue (antagonistic) filled circles indicate experimental data in which high frequency bursts of pulses of positive polarity were applied to either cell of the cell pair (Vj was positive when the Cx43 cell was stimulated and negative when the Cx45 cell was stimulated, see Fig. 6). The data presented show that Jj dependence on Vj in experiments with synergistic action of ionophoresis and Vj-gating (red and green filled circles) is steeper around Vj = 0 mV than in experiments with antagonistic action of ionophoresis and Vj-gating (gray and blue filled circles). The black line shows normalized gj-Vj plot, averaged from five experiments.
Dye Transfer Modulation by Vj Steps Repeated with High Frequency.
Here, we examined whether dye transfer through heterotypic junctions can be modulated by series of Vj pulses resembling bursts of action potentials (APs) similar to those shown in Fig. 1C. In these experiments, we used only positive pulses because APs generated by excitable cells are generally positive, and we examined Pj during stimulation of either cell expressing Cx45 or Cx43. Fig. 6 shows recordings of voltage (V1 and V2) and FI (FI1 and FI2) and Pj. Cell-1 expressing Cx45WT was loaded with AF350 (see diagram). Initially, repeated small ramps were applied in cell-2 to measure gj, which was ≈3.5 nS. In response to repeated (50 Hz) pulses of 60 mV in amplitude and 10 ms in duration applied to cell-2, gj decayed and over a ≈4 s period reached a steady state of ≈0.2 nS. Subsequently, when a burst of pulses was applied to cell-1, gj increased to ≈6.5 nS followed by decay over ≈5 s to ≈5.5 nS, suggesting that more GJ channels were open during this period of stimulation. To find Pp, cells were fully uncoupled with a short application of CO2. During small Vj ramps, Pj was calculated using Eq. 3. During Vj pulses, Pj was calculated using Eq. 2. Assuming a linear relationship between Pj and gj (Pj = Pj,0(gj/gj,0), were gj,0 is the conductance at control conditions), then by examining Pjs at different Vjs, we found that Pj increases 1.9-fold at Vj = 21 mV, i.e., ≈35% of 60 mV pulses (see Pjs shown in filled circles).
Single channel permeability estimates in this experiment using the same procedure as we did for data shown in Fig. 4 resulted to Pγ,Cx43/Cx45 = ≈14 × 10−15 cm3/s, which is close to values obtained from Fig. 4 and reported earlier for Vj ≈0 mV (20).
Thus, high frequency stimulation of the Cx43-EGFP expressing cell blocked AF350 transfer whereas stimulation of the Cx45 expressing cell increased both gj and Pj equally if Vj was equal 35% of the amplitude of pulses. Green (synergistic) and blue (antagonistic) circles in Fig. 5 indicate experimental data in which a high frequency burst of pulses of positive polarity were applied to either cell of the cell pair (Vj positive when Cx43 cell was stimulated and v.v.). Data shown in green and blue circles were not included in the fitting process but, in general, they show that application of Vj steps or high frequency bursts of pulses result in similar effects on Jj.
In summary, these data show that dye transfer of AF350 in Cx43/Cx45 GJs can be enhanced or reduced depending whether APs first arrive in the cell expressing Cx43 or Cx45. Similar data were obtained in five other Cx43/Cx45 cell pairs by using AF350. Comparable results were obtained in Cx43-EGFP/Cx45-CFP cell pairs using LY instead of AF350 (Fig. S1).
Discussion
We have demonstrated that Vj can increase or fully block cell–cell transfer of dye molecules comparable in size and net charge with many metabolites and signaling molecules indicating that the data apply to metabolic cell–cell communication and intercellular signaling. We found that Vj steps as small as ≈10 mV as well as series of pulses of high frequency resembling bursts of APs can modulate to a large extent metabolic communication through GJs exhibiting Vj-gating asymmetry.
Transfer of charged molecules is affected by ionophoresis and Vj-gating. Ionophoresis affects cell–cell transfer of charged metabolites independent of connexin type and whether GJ channels are homotypic, heterotypic or heteromeric. Our data show that the modified GHK equation used predicts Pγ for negatively charged AF350 at Vjs up to at least ≈±20 mV (Fig. 2). This may not necessarily be true for larger molecules or higher Vjs due to: (i) breakdown of ionic independence, (ii) electrostatic interaction with the channel's wall, etc.
For a long time, scientists working in the gap junction field have been asking, why there is a need for voltage-dependent gating of connexins expressed in non-excitable tissues such as hepatocytes, astrocytes and epithelial cells. Although these cells do not generate action potentials that can lead to large Vjs between adjacent cells and trigger Vj-gating, we demonstrate that even relatively small differences in the resting potential between adjacent cells (≈10 mV) can substantially modulate transfer of metabolites and this effect is augmented in heterotypic gap junctions. Can such Vjs be physiologically relevant? The resting potential (VR) varies among different cell types in broad ranges exceeding tens of mV under normal conditions, and even more under pathological conditions when cells lose their electrochemical gradients. It has been shown that astrocytes, which are well coupled through gap junctions, exhibit a wide range of VRs from −22 to −82 mV, and exhibit spontaneous changes of their VRs under different physiological conditions (26). Numerous studies have shown that hypoxia, ischemia, hyperkalemia or similar conditions that damage energetic and ionic homeostasis result in alteration of VRs. Depending upon the severity of ischemic or hypoxic conditions, changes in VR can be far greater than 10 mV (27). Thus, even in cells that have the same VR under normal conditions, changes in their network profile or local ischemia can form differences in VR (ΔVR) and consequently modulate metabolic communication by ionophoresis in all types of GJs and, in addition, by Vj-gating if cells are coupled through heterotypic GJs. It is important to note that actual ΔVR depend on the intrinsic VRs of communicating cells, coupling strength and their input resistance. We assume that ΔVRs even <10 mV can be effective because they can be long-lasting. If ΔVR were positioned on a gj-Vj plot of Cx43/Cx45 heterotypic junction as the working point (WP), then changing ΔVR would move the WP along the Vj axis and cause substantial changes in gj and Jj (see Fig. S2).
As reported, GJ channels operated by the fast gating mechanism close to the residual state that remains permeant for small ions but restricts permeation of more complex molecules such as AF350, LY and cAMP (22, 24). Therefore, Pj-Vj dependence can exhibit even higher asymmetry than gj-Vj dependence and Vj can modulate metabolic communication more effectively than gj. This asymmetry can be enhanced by synergistic action of ionophoresis and Vj-gating (Fig. 5) to the degree that ΔVRs as small as 5–10 mV can substantially modulate metabolic communication. For more details about theoretically predicted changes of Jj, versus Vj see SI Appendix and Fig. S3.
Vj-gating under physiological conditions can also take place in electrically excitable tissues during the spread of excitation. It was shown that Vj arising on the front of excitation spread can dynamically reduce gj and may play a role in the development of cardiac arrhythmias (28). Electrical activity during cardiac arrhythmias resembles bursts of APs similar to those in Fig. 1C that may cause profound changes of gj and Pj. Similar instances can occur between neurons that express or coexpress mCx30.2, Cx36 and Cx45 (14). Dynamic changes in gj can also occur when only one of the coupled cells is excitable, as at GJs between neurons and astrocytes, between endothelium and smooth muscle cells in blood vessels, etc. All these systems coexpress Cx45 in parallel with Cx31, Cx36, Cx40, Cx43 and/or Cx47 that can form heterotypic junctions exhibiting Vj-gating asymmetry. In the heart, fibroblasts that are not excitable express Cx45 (29) and are coupled with cardiomyocytes preferentially expressing Cx43. Fibroblasts, exhibiting relatively small VR, will be more depolarized than cardiomyocytes during the repolarisation phase. Thus, most of Cx43/Cx45 GJs should open and cells should be able to exchange metabolites. During APs when the membrane potential of cardiomyocytes becomes positive, Cx43/Cx45 channels should close resulting in the reduction of gj, Pj and the “sink” effect of the fibroblasts' network on the excitation of cardiomyocytes thereby enhancing the safety factor for the spread of excitation in the syncytial network of cardiomyocytes. During the rest of the cardiac cycle, gj should increase to a degree that fibroblasts may help cardiomyocytes to restore their energetic and ionic balance.
In summary, we demonstrate that long-lasting Vjs of small amplitude or series of Vj pulses resembling bursts of APs can modulate dye transfer with high efficacy suggesting that heterotypic GJs may act as voltage-dependent regulatory valves for intercellular signaling. Movie S1 demonstrates modulation of dye transfer by voltage steps over 20 min of time-lapse imaging. The regulation of metabolic communication and intercellular signaling by Vj can play a crucial role in many aspects of normal physiology, embryonic development, and tissue regeneration. In addition, the role of GJs can be substantially enhanced under pathological conditions when intercellular gradients of nutrients and signaling molecules are increased. Thus, we describe a general phenomenon of the modulation of intercellular electrical and chemical signaling by voltage that may have a broad impact on cellular and tissue function.
Methods
SI Appendix includes details of the experimental procedures and equations. Experiments were performed on HeLa cells transfected with wild type Cx43 and Cx45 and their fusion forms with color variants of green fluorescent proteins (EGFP or CFP) tagged to the C terminus. Junctional conductance (gj) was measured using the dual whole-cell voltage clamp method (9). For dye transfer studies, a given fluorescent dye was introduced into cell-1 of a cell pair through a patch pipette in whole-cell voltage clamp mode and the fluorescence intensity of dye was measured in cell-1 and cell-2. Dyes used include (molecular mass of the fluorescent ion, valence): Alexa Fluor-350 (AF350) (326, −1) and Lucifer yellow (LY) (443, −2) (Invitrogen, Eugene, OR). For calculation of the total junctional permeability (Pj) we used the Goldman-Hodgkin-Katz (GHK) equation (21) already used earlier for Pj studies by Verselis et al. (2). We modified this equation allowing for Pj evaluation during combined fluorescence imaging and gj recordings when there is constant dye leakage from cell-2 to the patch pipette-2.
Supplementary Material
Acknowledgments.
We thank Dr. Vytautas K. Verselis and Dr. Mindaugas Rackauskas for discussions and help at an initial stage of this project, Dr. Michael V. L. Bennett for helpful comments and remarks, and Angele Bukauskiene for excellent technical assistance. This work was supported by National Institutes of Health Grants RO1 NS036706 and RO1HL084464 (to F.F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901923106/DCSupplemental.
References
- 1.Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 2.Verselis V, White RL, Spray DC, Bennett MVL. Gap junctional conductance and permeability are linearly related. Science. 1986;234:461–464. doi: 10.1126/science.3489990. [DOI] [PubMed] [Google Scholar]
- 3.Neijssen J, et al. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 4.Valiunas V, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedner P, et al. Selective Permeability of Different Connexin Channels to the Second Messenger Cyclic AMP. J Biol Chem. 2006;28:6673–6681. doi: 10.1074/jbc.M511235200. [DOI] [PubMed] [Google Scholar]
- 6.Harris AL. Emerging issues of connexin channels: Biophysics fills the gap. Q Rev Biophys. 2001;34:325–427. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 7.Bukauskas FF, Bukauskiene A, Verselis VK, Bennett MVL. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: Properties of fast and slow gating mechanisms. Proc Natl Acad Sci USA. 2002;99:7113–7118. doi: 10.1073/pnas.032062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams CK, et al. Properties of human connexin 31, which is implicated in hereditary dermatological disease and deafness. Proc Natl Acad Sci USA. 2006;103:5213–5218. doi: 10.1073/pnas.0511091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rackauskas M, et al. Gating properties of heterotypic gap junction channels formed of connexins 40, 43 and 45. Biophys J. 2007;92:1952–1965. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreuzberg MM, Willecke K, Bukauskas F. Connexin-Mediated Cardiac Impulse Propagation: Connexin 30.2 Slows Atrioventricular Conduction in Mouse Heart. Trends in Cardiovasc Med. 2006;16:266–272. doi: 10.1016/j.tcm.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Simard JM. Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am J Physiol Heart Circulatory Physiol. 2001;281(5):H1890–H1898. doi: 10.1152/ajpheart.2001.281.5.H1890. [DOI] [PubMed] [Google Scholar]
- 13.Kreuzberg MM, et al. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005;96:1169–1177. doi: 10.1161/01.RES.0000169271.33675.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreuzberg MM, et al. Expression of connexin30.2 in interneurons of the central nervous system in the mouse. Mol Cell Neurosci. 2008;37:119–134. doi: 10.1016/j.mcn.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Rozental R, et al. Gap junction-mediated bidirectional signaling between human fetal hippocampal neurons and astrocytes. Dev Neurosci. 2001;23(6):420–431. doi: 10.1159/000048729. [DOI] [PubMed] [Google Scholar]
- 16.Paulauskas N, Pranevicius M, Pranevicius H, Bukauskas FF. A four-state model of contingent gating of gap junction channels containing two “fast” gates sensitive to transjunctional voltage. Biophys J. 2009;96:3936–3948. doi: 10.1016/j.bpj.2009.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris AL, Spray DC, Bennett MVL. Control of intercellular communication by voltage dependence of gap junctional conductance. J Neurosci. 1983;3:79–100. doi: 10.1523/JNEUROSCI.03-01-00079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Ek-Vitorin JF, Burt JM. Quantification of gap junction selectivity. Am J Physiol Cell Physiol. 2005;289:C1535–C1546. doi: 10.1152/ajpcell.00182.2005. [DOI] [PubMed] [Google Scholar]
- 20.Rackauskas M, Verselis VK, Bukauskas FF. Permeability of homotypic and heterotypic gap junction channels formed of cardiac connexins mCx30.2, Cx40, Cx43, and Cx45. Am J Physiol Heart Circ Physiol. 2007;293(3):H1729–H1736. doi: 10.1152/ajpheart.00234.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 22.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bukauskas FF, Bukauskiene A, Verselis VK. Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol. 2002;119:171–186. doi: 10.1085/jgp.119.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Y, Dahl G. Function of the voltage gate of gap junction channels: Selective exclusion of molecules. Proc Natl Acad Sci USA. 2002;99:697–702. doi: 10.1073/pnas.022324499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilders R, Jongsma HJ. Limitations of the dual voltage clamp method in assaying conductance and kinetics of gap junction channels. Biophys J. 1992;63:942–953. doi: 10.1016/S0006-3495(92)81664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKhann GM, 2nd RDA, Janigro D. Heterogeneity of astrocyte resting membrane potentials and intercellular coupling revealed by whole-cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J Neurosci. 1997;17:6850–6863. doi: 10.1523/JNEUROSCI.17-18-06850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyllienmark L, Brismar T. Effect of hypoxia on membrane potential and resting conductance in rat hippocampal neurons. Neuroscience. 1999;91:511–517. doi: 10.1016/s0306-4522(98)00650-2. [DOI] [PubMed] [Google Scholar]
- 28.Lin X, Gemel J, Beyer EC, Veenstra RD. Dynamic model for ventricular junctional conductance during the cardiac action potential. Am J Physiol Heart Circ Physiol. 2005;288:H1113–H1123. doi: 10.1152/ajpheart.00882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.