Abstract
Pancreatic β-cells are critical regulators of glucose homeostasis, and they vary dramatically in their glucose stimulated metabolic response and levels of insulin secretion. It is unclear whether these parameters are influenced by the developmental origin of individual β-cells. Using HOTcre, a Cre-based genetic switch that uses heat-induction to precisely control the temporal expression of transgenes, we labeled two populations of β-cells within the developing zebrafish pancreas. These populations originate in distinct pancreatic buds and exhibit gene expression profiles suggesting distinct functions during development. We find that the dorsal bud derived β-cells are quiescent and exhibit a marked decrease in insulin expression postembryonically. In contrast, ventral bud derived β-cells proliferate actively, and maintain high levels of insulin expression compared with dorsal bud derived β-cells. Therapeutic strategies to regulate β-cell proliferation and function are required to cure pathological states that result from excessive β-cell proliferation (e.g., insulinoma) or insufficient β-cell mass (e.g., diabetes mellitus). Our data reveal the existence of distinct populations of β-cells in vivo and should help develop better strategies to regulate β-cell differentiation and proliferation.
Keywords: zebrafish, pancreas, islet, insulin, lineage
Proliferation of β-cells is emerging as an important factor in the regulation of pancreatic β-cell mass. Under normal physiological conditions, proliferation of existing β-cells appears to be the predominant source of new β-cells in mouse (1, 2). Furthermore, inducing human β-cells to proliferate in vitro has been problematic. The most promising approaches to expand β-cell mass in vitro currently require de-differentiation to a mesenchymal cell-type (3). However, in certain models of pancreas injury, regenerating β-cells differentiate from endogenous progenitors (4). Thus, additional insight into the intrinsic and extrinsic regulation of β-cell proliferation will be critical for strategies to expand or restore β-cell mass.
Analysis of normal pancreatic development is one source of insight into the regulation of β-cell proliferation. The pancreas develops from a dorsal and a ventral bud of endodermal tissue that become morphologically distinct from the gut tube and fuses to generate the mature organ (5). In mouse, this initial budding is coincident with the appearance of pancreatic endocrine cells during the primary transition at E9.5 (6). These early endocrine cells lack some maturation markers and can coexpress multiple hormones including insulin at low levels (7). The function of these early endocrine cells is currently unclear, although it appears unlikely that they contribute to adult islets (8). Fully differentiated β-cells first appear during the secondary transition at around E13.5 in mouse (9). In zebrafish, the principal islet contains β-cells that differentiate from dispersed endodermal cells, which coalesce to form the dorsal pancreatic bud by 24-h post-fertilization (hpf) (10). Additional β-cells differentiate from ventral pancreatic bud derived tissues including the extra-pancreatic duct by 72 hpf (11, 12). It is not known whether the dorsal and ventral bud derived β-cells are also distinct in their function.
To analyze the developmental origins and proliferative potential of embryonic β-cells in zebrafish, we required a method for temporally and spatially restricted cell labeling. Transgene expression approaches in zebrafish currently rely on the ubiquitous heat-shock promoter for temporal control (13) or the GAL4/UAS system for spatial control (14). The major limitation of GAL4 based approaches is that control of gene expression is not binary; there is often basal, integration-site dependent, expression from the UAS element (15). For applications that require precise control of gene expression (e.g., lineage tracing, expression of functional transgenes), a system that contains a genetic switch is preferable.
The binary Cre/Lox system has been successfully established in zebrafish, initially using a heat-inducible Cre (16–18). In this study, we developed HOTcre, a bitransgenic system, which we used to restrict expression of a lineage marker to β-cells at different developmental time-points. This system utilizes a tissue specific Cre driver in combination with a heat-inducible reporter transgene to provide spatial and temporal control. Using this system, we analyzed the proliferative potential of dorsal and ventral bud derived β-cells in zebrafish. We find that dorsal bud derived β-cells are quiescent, while ventral bud derived β-cells proliferate in vivo with a doubling time similar to human β-cells in culture. In addition, we find significant gene expression differences between dorsal bud derived β-cells and ventral bud derived β-cells in zebrafish, suggesting that these cell populations have distinct functions during development.
Results and Discussion
The Principal Islet Contains Label-Retaining Endocrine Cells.
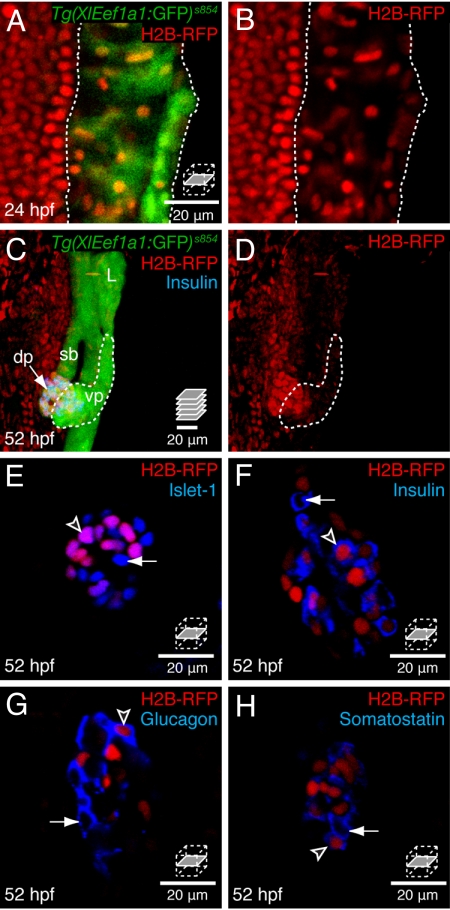
We hypothesized that dorsal and ventral bud derived β-cells might have different proliferative capacities and different functions. To examine the proliferation of pancreatic cells and their progenitors, we labeled all cells with an H2B-RFP fusion protein. The expression of a histone subunit (e.g., H2B) fused to a fluorescent reporter marks cell nuclei in dividing and nondividing cells (1). Furthermore, the fluorescent signal from H2B-XFP fusion proteins is very stable in quiescent cells but dilutes linearly with cell division in the absence of continued translation (1). We expressed H2B-RFP (19) ubiquitously by mRNA injection into one-cell Tg(XlEef1a1:GFP)s854 zebrafish embryos, which express GFP throughout the endoderm (20). At 24 hpf, the nuclear RFP signal appeared uniform throughout endodermal and nonendodermal nuclei (Fig. 1 A and B). After 24 hpf, RFP negative cells appeared, because of dilution and/or degradation of the H2B-RFP mRNA and protein (Fig. 1 C and D). By 34 hpf, pancreatic progenitors form morphologically distinguishable dorsal and ventral buds, which then undergo a morphogenetic fusion event which is complete by 52 hpf (11). To determine whether label-retaining cells were present in the embryonic pancreas, we examined H2B-RFP mRNA injected embryos undergoing pancreatic bud fusion. Most cells in the gut and other endodermal organs (liver, swim bladder, ventral pancreas) retained low levels of H2B-RFP (Fig. 1 C and D). However, a cluster of cells in the dorsal pancreatic bud clearly retained high levels of H2B-RFP (Fig. 1 C and D). All pancreatic label-retaining cells coexpressed the transcription factor Islet-1 (Fig. 1E), suggesting a pancreatic endocrine identity. Subsets of label-retaining cells expressed Insulin, Glucagon, or Somatostatin (see Fig. 1 F–H arrowheads). However, not all of the endocrine cells present at 52 hpf retained H2B-RFP (see Fig. 1 F–H arrows), and the source of these cells will be discussed below. We conclude that H2B-RFP mRNA injection at the one-cell stage specifically marks a subset of endocrine cells in the principal pancreatic islet.
Fig. 1.
The principal islet contains label-retaining endocrine cells. Embryos were labeled by injection of H2B-RFP mRNA at the one-cell stage. (A and B) Confocal sections through the endoderm at 24 hpf, and (C and D) confocal projections of endodermal tissue at 52 hpf, anterior to the top, dorsal to the left; L, liver, sb, swim bladder, dp, dorsal pancreas, vp, ventral pancreas. Dotted lines delineate Tg(XlEef1a1:GFP)s854 expressing endodermal tissue (A and B) or the ventral pancreatic bud (C and D). (E–H) Confocal sections through the principal islet. (A and B) All cell nuclei are initially labeled with H2B-RFP, including the GFP positive endodermal tissue (dotted outline). (C and D) At 52 hpf, the H2B-RFP label is retained in a dorsal cluster of endodermal cells, some of which coexpress Insulin (blue). Ventral pancreatic tissue (dotted outline) has diluted the H2B-RFP label. (E) All label-retaining endodermal cells coexpress the transcription factor Islet-1, arrowhead. (F–H) A subset of label-retaining endodermal cells express Insulin (F) Glucagon (G) or Somatostatin (H), arrowheads. (E–H) All islets at this stage also contain cells that are H2B-RFP negative but Islet-1 and hormone positive, arrows.
Label-Retaining β-Cells Are Derived from the Dorsal Pancreatic Bud.
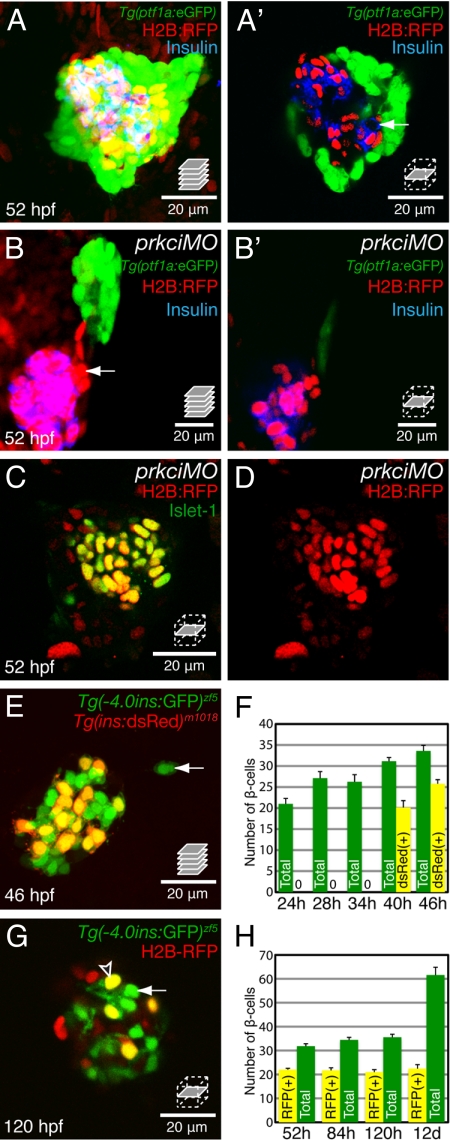
Since most cells in the ventral pancreatic bud retained low levels of H2B-RFP during bud fusion (Fig. 1 C and D), we hypothesized that the label-retaining β-cells derived from the dorsal bud. To test this hypothesis, we used the Tg(ptf1a:eGFP)jh1 line (21) to specifically mark the ventral bud in combination with protein kinase C iota (prkci) morpholino (MO) knockdown (22), which causes defects in pancreatic bud fusion (11). Wild-type islets at 52 hpf contain a mixture of label-retaining and label-diluted β-cells (Fig. 2 A and A′). Analysis of Tg(ptf1a:eGFP)jh1 embryos coinjected with H2B-RFP mRNA and prkci MO revealed that the cluster of label-retaining β-cells was clearly separate from Tg(ptf1a:eGFP)jh1-expressing tissue (Fig. 2 B and B′), indicating that these cells derived exclusively from the dorsal pancreatic bud. We designate these label-retaining β-cells as dorsal bud derived β-cells (DBCs). Because insulin expression was not detected in the GFP positive ventral bud tissue in Tg(ptf1a:eGFP)jh1 embryos injected with prkci MO (Fig. 2B), we hypothesized that prkci function might be required for the differentiation of this tissue. To test this hypothesis, we further examined endocrine differentiation in prkci morphants. Unlike what is observed in wild-type (Fig. 1E), all of the Islet-1 positive endocrine cells retained the H2B-RFP label in prkci morphants at 52 hpf (Fig. 2 C and D), further suggesting that prkci is required for β-cell differentiation in the H2B-RFP label diluted ventral pancreas. This block is not absolute, as β-cells are clearly present in ventral bud derived tissues of prkci mutants at 72 hpf (11, and Fig. S1). To examine β-cell differentiation before 52 hpf, we analyzed Tg(−4.0ins:GFP)zf5; Tg(ins:dsRed)m1018 double transgenics which express GFP and dsRed under the control of the insulin promoter (23, 24) (Fig. 2 E and F). The delay between the maturation of the GFP and the dsRed chromophores was 18–22h (Fig. 2F, compare green bars at 24 and 28 hpf with yellow bars at 40 and 46 hpf), which permitted the identification of recently differentiated β-cells. GFP only positive β-cells were observed outside the principal islet by 46 hpf (arrow, Fig. 2E), indicating that β-cell neogenesis first occurs in the ventral bud by this stage. Based on our analysis of prkci morphants, we predicted that ventral bud derived β-cells (VBCs), which form outside the principal islet, would not retain the H2B-RFP label after mRNA injection. Indeed, we showed that β-cells that differentiate near the extra pancreatic duct do not retain the H2B-RFP label (Fig. S1). Altogether, these data indicate that DBCs retain the H2B-RFP label, while VBCs do not.
Fig. 2.
Label-retaining β-cells are derived from the dorsal pancreatic bud. Embryos were injected at the one-cell stage with H2B-RFP mRNA (A, A′, and G) or H2B-RFP mRNA and prkci MO (B, B′, C, and D). (A and B) Confocal projections of 52 hpf Tg(ptf1a:GFP)jh1 pancreata and (A′ and B′) confocal sections from each projection. (C and D) Confocal sections through the principal islet at 52 hpf. (E) Confocal projection of an islet coexpressing Tg(−4.0ins:GFP)zf5 and Tg(insulin:dsRed)m1018 at 46 hpf. (F) Quantification of Tg(ins:dsRed)m1018 expressing, and total β-cells per embryo from 24 to 46 hpf, expressed as mean ± SEM (See Table S1). (G) Confocal section through a Tg(−4.0ins:GFP)zf5 positive islet. (H) Quantification of H2B-RFP label-retaining, and total β-cells per embryo from 52 hpf to 12 dpf, expressed as mean ± SEM (see Table S1). (A and A′) β-cells that do not retain the H2B:RFP label appear by 52 hpf. (B and B′) Insulin expression is restricted to H2B-RFP positive cells in prkci morphants. (B) None of the label-retaining β-cells colocalized with Tg(ptf1a:GFP)jh1 expression, indicating that these cells derive from the dorsal bud. Some label-retaining cells do not express Insulin (arrow) and are likely other endocrine cell types. (C and D) All Islet-1 positive endocrine cells retained the H2B-RFP label when bud fusion was inhibited. (E) Recently differentiated β-cells only express GFP (green, arrow). (F) β-cells that differentiated by 28 hpf (green bars) coexpress dsRed at 46 hpf (yellow bars). (G) At 120 hpf, some β-cells (yellow, arrowhead) retain the H2B-RFP label while others (green, arrow) do not. (H) Yellow bars: The number of H2B-RFP label-retaining β-cells does not change from 52 hpf to 12 dpf. Green bars: The total number of β-cells increases over this period.
Dorsal Bud-Derived β-Cells Do Not Contribute to the Expansion of β-Cell Mass.
Next, we asked whether the DBCs observed at 52 hpf retain the H2B-RFP label over time. To focus our analysis on the β-cell mass, we injected H2B-RFP mRNA into Tg(−4.0ins:GFP)zf5 embryos. This method clearly labeled two types of β-cells at 120 hpf: a population that coexpressed Tg(−4.0ins:GFP)zf5 and H2B-RFP (Fig. 2 G arrowhead), and another population that only expressed Tg(−4.0ins:GFP)zf5 (Fig. 2 G and H arrow; Fig. S1). The total number of β-cells increased during the first twelve days of development (Fig. 2H, green bars). However, quantification of the two populations of β-cells at different developmental time-points revealed a constant number of label-retaining β-cells throughout development (Fig. 2H, yellow bars, and Table S1). This result suggests that the label-retaining β-cells do not proliferate, and that the increase in β-cells reflects de novo differentiation, and possibly replication, of β-cells from H2B-RFP negative precursors. These hypotheses will be tested below.
HOTcre, a Cre-Based Genetic Switch for Tissue and Stage-Specific Cell Labeling.
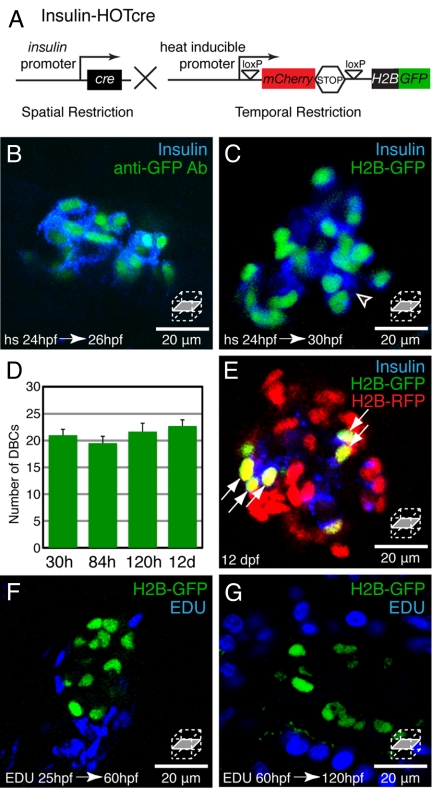
Since the ventral pancreatic bud does not retain the H2B-RFP label (Figs. 1 C and D and 2 B and B′ and Fig. S1), the endocrine cells that are H2B-RFP negative at 52 hpf (Fig. 1 G and H arrows) are likely to be ventral bud derived cells. To follow VBCs over time, we required a method for tissue and stage-specific expression of an H2B fusion protein. The available Cre-ER based methods label cells in a time-specific manner by the addition of tamoxifen (25). Unfortunately, Cre-ER based approaches often result in incomplete labeling of the target tissue. For example, RIP-CreER incompletely labels β-cells (<30%) and there is a significant delay (days to weeks) between tamoxifen addition and peak Cre activity (2). To investigate the rapid development and differentiation of zebrafish β-cells, we developed a system for Heat-inducible Over-expression of Transgenes in a cre restricted pattern, HOTcre (Fig. 3A). We used the hsp70l promoter (13) to control the temporal expression of a H2B-GFP reporter, which is only expressed in cells that have undergone a Cre mediated excision event. We placed the Cre recombinase under the control of the zebrafish insulin promoter (23). The crystallin alpha A eye specific promoter (26) was used to drive Venus or Cerulean expression to facilitate maintenance of the Tg(ins:Cre; cryaa:Venus)s924 and Tg(hsp70l:loxP-mCherry-STOP-loxP-H2B-GFP; cryaa:Cerulean)s923 lines (Fig. 3A). We will refer to these double transgenics as Insulin-HOTcre animals. Following Insulin-HOTcre mediated labeling at 24 hpf with a 30 min heat-shock induction, all Insulin positive cells also expressed H2B-GFP at 26 hpf (Fig. 3B, n = 5 islets). By 30 hpf, rare Insulin-positive cells were H2B-GFP negative (arrowhead, Fig. 3C). These β-cells had thus differentiated after the heat-shock. We conclude that the Insulin-HOTcre system efficiently labels β-cells and can be used to distinguish recently differentiated β-cells from older β-cells.
Fig. 3.
Dorsal bud derived β-cells (DBCs) are quiescent. (A) Insulin-HOTcre, Heat-inducible Overexpression of Transgenes in an insulin: Cre restricted domain. Expression of Cre in β-cells excises the mCherrySTOP cassette and permits heat-inducible expression of H2B-GFP. (B–G) Insulin-HOTcre embryos were heat-shocked at 24 hpf. (B and C) Confocal sections through an islet stained for Insulin and GFP at 26 hpf (B) or Insulin at 30 hpf (C). (D) Quantification of H2B-GFP positive cells per embryo after 24 hpf heat-shock from 30 hpf to 12 dpf, mean ± SEM (Table S1). (E) Insulin-HOTcre embryos were injected with H2B-RFP mRNA at the one-cell stage, and heat-shocked at 24 hpf. Confocal section of the principal islet stained for Insulin at 12 dpf. The mCherry heat-shock induction marker is degraded by 12 dpf (See Fig. S2A). (F and G) Embryos were labeled by pericardial EDU injection at 25 (F) or 60 (G) hpf. Confocal sections through islets at 60 (F) or 120 (G) hpf. (B) All Insulin-positive cells coexpress H2B-GFP at 26 hpf. (C) β-cells that differentiated after the heat-shock at 24 hpf express Insulin but do not express H2B-GFP (arrowhead). (D and E) The number of H2B-GFP positive cells after a 24 hpf heat-shock does not significantly change during development (D). Furthermore, all H2B-GFP-positive cells labeled after heat-shock at 24 hpf, also retain the H2B-RFP label (arrows, E). (F and G) Many cells within the pancreas divide during embryonic and larval development (blue nuclei). However, β-cells labeled by Insulin-HOTcre induction at 24 hpf did not exhibit EDU incorporation (n = 5 islets for each condition).
To further validate these new reagents, we quantified the number of cells that retained the H2B-GFP label in Insulin-HOTcre animals following heat-shock at 24 hpf (Fig. 3D). The number of H2B-GFP positive cells at multiple time-points after the heat-shock was constant and indistinguishable from the number of label-retaining β-cells identified by H2B-RFP mRNA injection (compare Figs. 2H and 3D). To determine whether the identical subset of β-cells was labeled by both methods, we injected H2B-RFP mRNA into Insulin-HOTcre embryos and heat-shocked them at 24 hpf. At 12 days-post-fertilization (dpf), all H2B-GFP-positive cells were also H2B-RFP positive (see arrows, Fig. 3E). We conclude that Insulin-HOTcre is a robust method for labeling β-cells.
Dorsal Bud-Derived β-Cells Are Quiescent.
The number of DBCs remains static during at least the first twelve days of development (Figs. 2H and 3D). The DBCs could be quiescent, or a balance of cell proliferation and cell death could maintain a stable population size. To determine whether a subpopulation of DBCs progresses through the cell-cycle, we pulsed embryos with the thymidine analog EDU (27). Injection of EDU into the yolk at 25 hpf or pericardial injection of EDU at 60 hpf labeled cells throughout the embryo. However, when Insulin-HOTcre embryos were heat-shocked at 24 hpf and pulsed from 25 to 60 hpf or 60 to 120 hpf, none of the H2B-GFP positive β-cells incorporated EDU (n = 5 animals for each experiment; Fig. 3 F and G). Therefore, proliferation of β-cells formed by 24 hpf appears to contribute very little, if at all, to the β-cell population.
Ventral Bud-Derived β-Cells Proliferate.
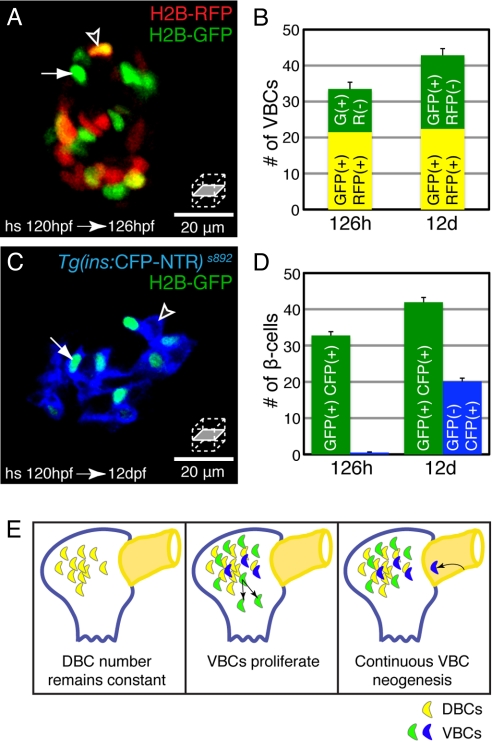
To determine whether VBCs can proliferate, we used a combination of H2B-RFP mRNA injection and the Insulin-HOTcre system to specifically label VBCs at 120 hpf. Previous studies have shown that H2B-GFP labeled cells can be followed for at least three rounds of cell-division before the label is diluted (1). A 30-min heat-shock at 120 hpf labeled all β-cells with H2B-GFP (Fig. 4A). DBCs coexpressed H2B-RFP (Fig. 4A, arrowhead), while VBCs did not (Fig. 4A, arrow). The number of VBCs approximately doubled between 126 hpf (11 ± 1.5) and 12 dpf (19.4 ± 1.8)(Fig. 4B and Table S1), which corresponds to an average cell-cycle length of 9.2 days. This number is similar to the doubling time of primary human β-cells in culture (7 days) (3). We conclude that proliferation of VBCs contributes to the expansion of the β-cell mass.
Fig. 4.
The population of ventral bud derived β-cells (VBCs) expands by proliferation and neogenesis. (A and B) Insulin-HOTcre embryos were injected with H2B-RFP mRNA at the one-cell stage and heat-shocked at 120 hpf. (A) Confocal section of an islet at 126 hpf. (B) Quantification of VBCs per embryo at 126 hpf and 12 dpf, mean ± SEM (Table S1). (C and D) Insulin-HOTcre; Tg(ins:CFP-NTR)s892 embryos heat-shocked at 120 hpf. (C) Confocal section of an islet at 12 dpf. (D) Quantification of β-cells per embryo that had differentiated before the heat-shock (green bars) and those that differentiated after the heat-shock (blue bars), mean ± SEM (Table S1). (A and B). Some cells (DBCs) express both H2B-RFP and H2B-GFP (arrowhead); other cells (VBCs) express only the H2B-GFP label (arrow). The number of VBCs increased between 126 hpf and 12 dpf, reflecting division of existing VBCs. (C and D) β-cells marked by Insulin-HOTcre labeling at 120 hpf express H2B-GFP and CFP (arrow), whereas β-cells that differentiated after the heat-shock only express CFP (arrowhead). β-cell neogenesis contributes a significant proportion of β-cells during larval development (blue bars). (E) β-cells include both DBCs (yellow) and VBCs (green and blue) and the population of VBCs expands through a combination of proliferation (green) and neogenesis from the extra-pancreatic duct (blue), and later, intrapancreatic ducts (data not shown).
β-Cells Continuously Differentiate During Development.
To dissect the relative contribution of proliferation and de novo differentiation to the pool of β-cells, we examined Insulin-HOTcre labeled β-cells in the Tg(ins:CFP-NTR)s892 background (28). Directly following Insulin-HOTcre induction at 120 hpf, all β-cells were double positive for CFP and H2B-GFP (Fig. S2). Tg(ins:CFP-NTR)s892 continuously labels all Insulin-expressing cells, while Insulin-HOTcre marks β-cells that were present at a defined developmental stage, and their direct descendants. Therefore, β-cells that differentiated de novo after heat-shock at 120 hpf only expressed CFP (Fig. 4C, arrowhead). de novo differentiation contributed 20 ± 2.4 VBCs per embryo between 126 hpf and 12 dpf (Fig. 4D and Table S1), while proliferation of existing β-cells contributed 8.4 ± 2.3 cells over the same period (Fig. 4B). Therefore, from 126 hpf to 12 dpf, β-cell neogenesis makes a 2.4-fold greater contribution to the β-cell mass compared with proliferation of existing β-cells. To summarize, at least until 12 dpf, new β-cells in zebrafish derive from two sources: replication of existing VBCs and de novo differentiation from duct-associated precursors that have never expressed Insulin (Fig. 4E).
Dynamic Regulation of insulin Expression During Zebrafish Development.
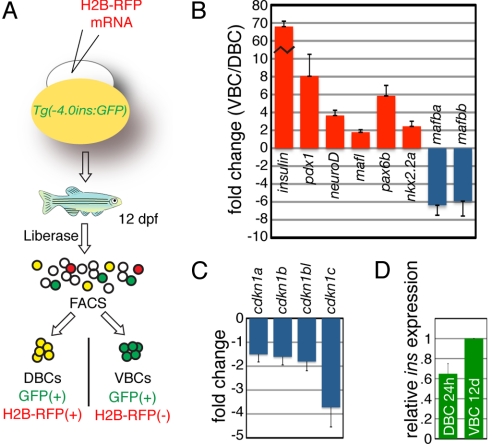
The β-cell gene expression profile changes markedly during embryonic development (29). Since DBCs and VBCs in zebrafish have different proliferative potentials and origins, we speculated that they might have different functions. We used FACS analysis to isolate DBCs and VBCs at 12 dpf based on their differential retention of the H2B-RFP label (Fig. 5A). Both DBC and VBC samples were enriched for insulin mRNA (1.9 fold and 130 fold respectively) and had undetectable levels of ptf1a mRNA, a transcript restricted to exocrine cells in the pancreas (30). Since there was a striking differential expression of insulin mRNA in DBCs versus VBCs at 12 dpf (69-fold), we focused our analysis on transcriptional regulators of insulin expression (Fig. 5B). Zebrafish homologs of Pdx-1, NeuroD1, and MafA, which are largely responsible for the glucose responsive regulation of mammalian Insulin expression (31), were up-regulated in VBCs versus DBCs (pdx1 8.1-fold, neurod 3.7-fold, and mafl 1.8-fold). In addition, pax6 and nkx2.2, which encode transcription factors that directly bind and activate the insulin promoter (32, 33) were also expressed at higher levels in VBCs versus DBCs (pax6b 5.9-fold and nkx2.2a 2.4-fold). Conversely, at least in mouse, the transcription factor MafB is expressed in early embryonic β-cells and down-regulated in mature β-cells (34). The zebrafish genome contains two MafB homologs, both of which were expressed at higher levels in DBCs versus VBCs (mafba 6.4-fold and mafbb 5.9-fold). Taken together, at 12 dpf, VBCs express markers of β-cells that are actively transcribing insulin, while DBCs appear to have down-regulated the insulin expression program.
Fig. 5.
Dynamic regulation of insulin expression during zebrafish development. (A) FACS sorting strategy. Tg(−4.0ins:GFP)zf5 embryos were injected with H2B-RFP mRNA at the one-cell stage and dissociated into a single-cell suspension at 12 dpf. DBCs and VBCs were isolated from the cell suspension by FACS. (B–D) Relative levels (VBC/DBC) by real-time RT-PCR of genes (at least four independent reactions, expressed as mean ± SEM). All values were normalized to β-actin levels. Primer sequences and raw Ct values are listed in Table S2 and Table S3. (B) Insulin, and transcription factor genes implicated in Insulin expression are expressed at higher levels in VBCs (red bars). MafB homologs are expressed at lower levels in VBCs relative to DBCs (blue bars). (C) Genes encoding cell-cycle inhibitors are expressed at higher levels in DBCs (blue bars). (D) Recently differentiated (24 hpf) DBCs express Insulin at a level similar to mature (12 dpf) VBCs.
Since DBCs appeared to be quiescent in our proliferation studies, we also examined the expression of cell-cycle inhibitors in these cells. We focused on the cyclin-dependent kinase inhibitors (CKIs) since all of the mammalian family members are expressed in mouse β-cells (35). Four of the five zebrafish CKIs were detectable in the FACS samples, and all four were expressed at higher levels in the DBCs as compared with the VBCs at 12 dpf (range 1.5-fold to 3.7-fold, Fig. 5C). The accumulation of cell-cycle inhibitors in DBCs likely inhibits their proliferation. What might be the function of early embryonic β-cells that do not proliferate? One possibility is that Insulin is required very early in development to ensure stable glucose levels for developing organs (36). Another, nonexclusive possibility is that the initial cluster of β-cells and other dorsal bud derived endocrine cell types are required to recruit and organize the later differentiating endocrine cells. To determine whether DBCs express high levels of insulin early in development, we isolated β-cells from the Tg(−4.0ins:GFP)zf5 transgenic line at 24 hpf, a time-point when only DBCs are present. DBCs isolated at 24 hpf express insulin at a level comparable to VBCs isolated at 12 dpf (Fig. 5D). High levels of insulin expression may be required at this early developmental time-point to regulate glucose levels or as a growth factor.
Conclusions
We developed methods to selectively label different populations of β-cells in zebrafish. H2B-RFP label retention allows one to distinguish DBCs from VBCs. Although all endocrine cells in the dorsal bud derived tissue appear to retain the H2B-RFP label (Fig. 2C), it is possible that this tissue also contains rare H2B-RFP negative endocrine precursors. We further found that DBCs were quiescent while VBCs proliferate. Finally, we introduced a method, HOTcre, which allows the differential labeling of newly differentiated cells, and which will also allow the tissue specific overexpression of transgenes in a time controlled manner.
Based on the data generated using these tools, we propose that DBCs may constitute a specialized cell-type that releases Insulin to support growth during embryonic development. In contrast, VBCs may have the capacity to differentiate into fully functional β-cells. Similar specialization may explain the presence of primary and secondary transition β-cells during mouse development. The last differentiation steps of human embryonic stem cells into functional β-cells currently requires unknown factors that can be provided by in vivo maturation (37). Our results suggest that further analysis of DBC and VBC development will help identify molecular factors critical for the differentiation, and proliferation, of mature, functional β-cells in other organisms including humans.
Experimental Procedures
H2B-RFP mRNA Injections and Analysis.
50pg of in vitro transcribed H2B-RFP mRNA (mMessage mMachine, Ambion) was injected per embryo. To establish the threshold for identifying label-retaining endocrine cells, the brightness and contrast of confocal images was adjusted to minimize saturation of the positive cells minimize background signal from the surrounding exocrine tissue.
DNA Constructs and Transgenic lines.
See SI Text.
Supplementary Material
Acknowledgments.
We thank Ana Ayala and Milagritos Alva for expert help with the fish; Dave Pilgrim, Chester Chamberlain, Bruce Adams, Philipp Gut, and Stephanie Hesselson for critical reading of the manuscript; Takeshi Miyatsuka and other members of the German laboratory for helpful discussions. This work was supported by a Larry L. Hillblom Foundation postdoctoral fellowship (D.H.), Juvenile Diabetes Research Foundation (JDRF) postdoctoral fellowship (R.M.A.), National Institutes of Health Grant DK075032, and the Packard Foundation (D.Y.R.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906348106/DCSupplemental.
References
- 1.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 3.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Wessells NK, Cohen JH. Early Pancreas Organogenesis: Morphogenesis, Tissue Interactions, and Mass Effects. Dev Biol. 1967;15:237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]
- 6.Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- 7.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 8.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 9.Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- 10.Biemar F, et al. Pancreas development in zebrafish: Early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- 11.Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 12.Dong PD, et al. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- 13.Halloran MC, et al. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- 14.Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 15.McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: A synthesis of time and space. Trends Genet. 2004;20:384–391. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Langenau DM, et al. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thummel R, et al. Cre-mediated site-specific recombination in zebrafish embryos. Dev Dyn. 2005;233:1366–1377. doi: 10.1002/dvdy.20475. [DOI] [PubMed] [Google Scholar]
- 18.Le X, et al. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci USA. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Megason SG, Fraser SE. Digitizing life at the level of the cell: High-performance laser-scanning microscopy and image analysis for in toto imaging of development. Mech Dev. 2003;120:1407–1420. doi: 10.1016/j.mod.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Field HA, Ober EA, Roeser T, Stainier DY. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol. 2003;253:279–290. doi: 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 21.Godinho L, et al. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- 22.Peterson RT, Mably JD, Chen JN, Fishman MC. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr Biol. 2001;11:1481–1491. doi: 10.1016/s0960-9822(01)00482-1. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Vogel SS, Liu N, Melton DA, Lin S. Analysis of pancreatic development in living transgenic zebrafish embryos. Mol Cell Endocrinol. 2001;177:117–124. doi: 10.1016/s0303-7207(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 24.Shin CH, et al. Multiple roles for Med12 in vertebrate endoderm development. Dev Biol. 2008;317:467–479. doi: 10.1016/j.ydbio.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- 26.Kurita R, et al. Suppression of lens growth by alphaA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Dev Biol. 2003;255:113–127. doi: 10.1016/s0012-1606(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 27.Chehrehasa F, Meedeniya AC, Dwyer P, Abrahamsen G, Mackay-Sim A. EdU, a new thymidine analogue for labeling proliferating cells in the nervous system. J Neurosci Methods. 2008;177:122–130. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Curado S, et al. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 29.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zecchin E, et al. Evolutionary conserved role of ptf1a in the specification of exocrine pancreatic fates. Dev Biol. 2004;268:174–184. doi: 10.1016/j.ydbio.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J. 2008;415:1–10. doi: 10.1042/BJ20081029. [DOI] [PubMed] [Google Scholar]
- 32.Sander M, et al. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 33.Cissell MA, Zhao L, Sussel L, Henderson E, Stein R. Transcription factor occupancy of the insulin gene in vivo. Evidence for direct regulation by Nkx2.2. J Biol Chem. 2003;278:751–756. doi: 10.1074/jbc.M205905200. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura W, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- 36.Madsen OD. Pancreas phylogeny and ontogeny in relation to a ‘pancreatic stem cell’. C R Biol. 2007;330:534–537. doi: 10.1016/j.crvi.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.