Abstract
Scientific understanding of social pain—the hurt feelings resulting from social rejection, separation, or loss—has been facilitated by the hypothesis that such feelings arise, in part, from some of the same neural and neurochemical systems that generate the unpleasant feelings resulting from physical pain. Accordingly, in animals, the painkiller morphine not only alleviates the distress of physical pain, but also the distress of social separation. Because morphine acts on the μ-opioid receptor, we examined whether variation in the μ-opioid receptor gene (OPRM1), as measured by the functional A118G polymorphism, was associated with individual differences in rejection sensitivity. Participants (n = 122) completed a self-report inventory of dispositional sensitivity to social rejection and a subsample (n = 31) completed a functional MRI session in which they were rejected from an online ball-tossing game played with two supposed others. The A118G polymorphism was associated with dispositional sensitivity to rejection in the entire sample and in the fMRI subsample. Consistent with these results, G allele carriers showed greater reactivity to social rejection in neural regions previously shown to be involved in processing social pain as well as the unpleasantness of physical pain, particularly the dorsal anterior cingulate cortex (dACC) and anterior insula. Furthermore, dACC activity mediated the relationship between the A118G polymorphism and dispositional sensitivity to rejection, suggesting that this is a critical site for μ-opioid-related influence on social pain. Taken together, these data suggest that the A118G polymorphism specifically, and the μ-opioid receptor more generally, are involved in social pain in addition to physical pain.
Keywords: anterior cingulate, brain, exclusion, genetic, social pain
In common parlance, the phrase “I am hurt” can refer to the pain of a skinned knee or the pain of being rejected by a friend or lover. This overlap in the language used to describe the unpleasantness of physical and ‘social’ pain may not be purely coincidental. Accumulating evidence indicates that the hurt feelings resulting from broken social ties may arise from some of the same neural and neurochemical substrates that are involved in the unpleasant experience of physical pain.
Neurally, the affective or unpleasant component of physical pain tends to be associated with processing in various subregions of the anterior cingulate cortex (ACC) and anterior insula (1–3). Thus, lesions to the dorsal portion of the ACC (dACC) (4) or insula (5) result in patients reporting that they are no longer bothered by painful stimulation even though they can still perceive it. Similarly, neuroimaging studies have shown that the dACC tracks the affective component of pain (6) and is closely correlated with perceived pain unpleasantness (7–9). Analogous results have been found for the anterior insula, particularly with respect to chronic pain (10).
Notably, similar regions of the ACC and anterior insula are also involved in social pain processes. In monkeys, lesions to the ACC (that include both dorsal and ventral regions) eliminate the production of isolation calls, a form of distress vocalization intended to re-establish contact with members of the social group after social separation (11, 12). Conversely, stimulation of the ACC can trigger similar vocalizations (13, 14), suggesting a critical role for the ACC in the distress of being separated from others. Likewise, in humans, the distress of social rejection has been associated with activation of the dACC and anterior insula (15, 16), and greater feelings of social distress in response to social exclusion are directly related to greater dACC activity (17).
At the neurochemical level, physical pain and social pain share common substrates as well, in particular μ-opioid receptor (MOR) mediated signaling. Opiates, such as morphine, have well-documented pain-relieving effects (18) that appear to be mediated by the MOR. Thus, MOR knockout mice are unresponsive to the pain-relieving effects of morphine (19) and show altered baseline responses to multiple measures of physical pain (20).
With respect to social pain, low, nonsedative doses of morphine specifically reduce distress vocalizations made by infants when separated from their mother in multiple species, including monkeys (21), dogs (22), guinea pigs (23), rats (24), and chickens (25). The MOR would appear to be critical for these effects, as deletion of the MOR gene from mice reduces pups' distress during mother-infant separation (26). Similarly, in human adults, μ-opioid-related activity appears to signal the pain of social loss. In a positron emission tomography study (27), women exhibited decreased μ-opioid mediated neurotransmission when recalling the death of a loved one or the breakup of a romantic relationship, indicating that the MOR is involved in responding to the loss of connections to significant others.
In light of this evidence connecting the MOR to both physical and social pain, individual differences in the intensity of physically and socially painful feelings may depend, in part, on naturally occurring variation in the μ-opioid receptor gene (OPRM1). Within OPRM1, there is a single nucleotide polymorphism (A118G) that leads to an amino acid change (N40D) in the MOR and has robust effects upon OPRM1 expression. In both postmortem and in vitro studies (28–30), the variant G allele is associated with reduced levels of MOR mRNA and protein relative to the A allele. Lower levels of MOR may underlie the reduced potency of opiates in G allele carriers (31–33), as carriers of the variant G allele require greater quantities of morphine to deal with postsurgical pain [(34–37), but see ref. 38] and cancer related pain (39, 40). It also appears that the greater experience of pain among G allele carriers elicits the increased morphine administration (34, 41, 42). These data, as well as evidence from experimental pain studies (43–45), indicate that the A118G polymorphism is involved in physical pain sensitivity and thus may be a good candidate for being involved in social pain sensitivity as well.
Although the potential relationship between the A118G polymorphism and social pain has not been examined in humans, rhesus monkeys possess a similar nonsynonymous polymorphism within OPRM1 that has been associated with social pain responses. Specifically, infant rhesus monkeys with the variant G allele displayed greater persistence of distress vocalizations following separation from the mother as well as greater preference for social contact with her upon reunion (46).
Based on these findings, we explored the potential influence of the A118G polymorphism on individual differences in sensitivity to one type of socially painful experience in humans, namely social rejection. We examined this in two ways. First, we assessed the relationship between the A118G polymorphism and individual differences in the self-reported dispositional tendency to be sensitive to social rejection in a sample of healthy young adults (n = 122). The sensitivity to social rejection scale assesses individual differences in the tendency to be fearful that social interactions will result in hurt feelings, criticism, and being a burden to others (47). Second, to examine the relationship between the A118G polymorphism and neural responses to social rejection, we scanned a subsample of these individuals (n = 31) as they played an online ball-tossing game, ‘Cyberball,’ (48) with two supposed other individuals who excluded them. The variant G allele was associated with higher levels of self-reported and neurally assessed rejection sensitivity, indicating that the MOR influences sensitivity to social pain in humans too.
Results
The A118G Polymorphism and Dispositional Sensitivity to Rejection.
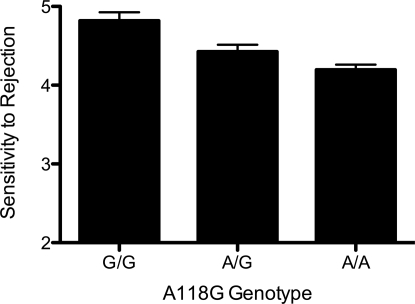
Genotype frequencies at the A118G locus did not deviate from Hardy-Weinberg equilibrium (n = 122, P = 0.74). Further analyses of the distribution of A118G alleles according to sex and ancestry are reported in the SI Text. To control for their potential influence upon the following neural and psychological results, sex, ancestry, and the sex by ancestry interaction terms were entered as covariates in all ANCOVAs.* Accordingly, a one-way ANCOVA revealed a significant relationship between the A118G polymorphism and self-reported dispositional sensitivity to rejection (Fig. 1; F (2, 116) = 3.19, P < 0.05). Post-test comparisons showed that individuals with the A/A genotype were significantly lower in dispositional sensitivity to rejection compared to individuals with the A/G genotype (t (115) = 2.13, P < 0.05) or the G/G genotype (t (76) = 2.46, P < 0.05).
Fig. 1.
Bar graph of the relationship between the A118G polymorphism and dispositional sensitivity to rejection (Total n = 122; A/A = 73; A/G = 44; G/G = 5).
The A118G Polymorphism and Neural Responses to Social Rejection.
A subset of participants (n = 31, 19 female) completed an fMRI scan in which they were told that they would be playing a virtual ball-tossing game over the internet with two other individuals; in reality, they played with a preset computer program. During one functional scan, they were included for the entire time, and during the other, they were excluded when they stopped receiving the ball (after 7 throws). In this fMRI subsample, one participant was homozygous for the variant G allele (G/G) and thus was grouped along with G/A individuals to create a two-level category consisting of G allele carriers and A allele homozygotes. As in the larger sample, G allele carriers in this subsample, relative to A allele homozygotes, reported significantly higher levels of dispositional sensitivity to rejection (F (1, 24) = 3.71, P < 0.05), controlling for sex, ancestry, and the sex by ancestry interaction.
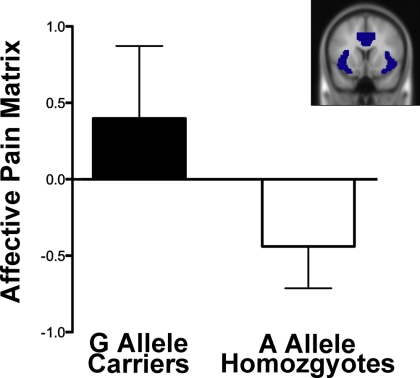
To examine the hypothesis that the A118G polymorphism would be associated with differential neural responses in regions known to be involved in processing social pain as well as the affective component of physical pain, we used a region of interest (ROI) based approach focusing on the dACC and anterior insula, the combination of which we refer to here as the affective pain matrix. Consistent with the hypothesis, activity within the affective pain matrix ROI was significantly greater in G allele carriers than A allele homozygotes (Fig. 2; F (1, 24) = 4.71, P < 0.05) during exclusion relative to inclusion (controlling for sex, ancestry, and the sex by ancestry interaction). When this matrix was subdivided into its constituent parts, both the dACC ROI (F (1, 24) = 5.78, P < 0.05) and the left anterior insula ROI (F (1, 24) = 3.04 < 0.05) showed significantly greater activity in G allele carriers than A allele homozygotes; the right anterior insula ROI did not (F (1, 24) = 1.8, n.s.).
Fig. 2.
Mean parameter estimates for the affective pain matrix ROI according to A118G genotype (F (1, 24) = 4.71, P < 0.05). (Inset) Shaded regions denote ROI location.
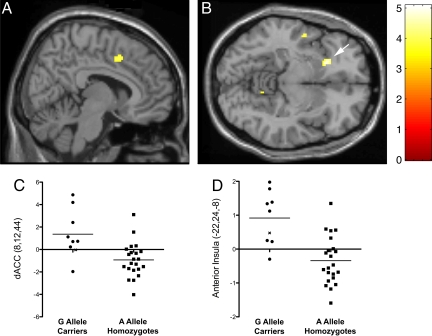
To obtain a more detailed picture of the specific areas where neural reactivity differed as a function of the A118G polymorphism, a whole-brain analysis was conducted controlling for sex, ancestry, and the sex by ancestry interaction. Consistent with the ROI analyses, the whole-brain analysis revealed significantly more activity in G allele carriers compared to A allele homozygotes in both the dACC (Fig. 3A; 8, 12, 44; t = 4.06, P < 0.001) and the left anterior insula (Fig. 3B; −22, 24, −8; t = 5.07, P < 0.001, global maximum). In addition, there was greater activation in G allele carriers than A allele homozygotes in several other regions known to have a relatively high concentration of MOR (49), such as the superior temporal gyrus, inferior parietal lobule, and cerebellum (see Table 1 for a complete list of activations). In the reverse comparison, there were no areas showing greater response to exclusion versus inclusion amongst A allele homozygotes, relative to G allele carriers.
Fig. 3.
Sagittal (A; dACC) and axial (B; anterior insula, denoted by arrow) sections of neural activations during social exclusion vs. inclusion that showed significantly greater activity (P < 0.001, 20 voxel extent) for G allele carriers than A allele homozygotes. (C) Parameter estimates from the dACC (8,12,44; t (24) = 4.06, P < 0.001); (D) Parameter estimates from the left anterior insula (−22,24,–8; t (24) = 5.07, P < 0.001). x denotes G allele homozygote.
Table 1.
Neural activations during social exclusion vs. inclusion that showed significantly more activity for G allele carriers than A allele homozygotes (controlling for sex, ancestry, and the sex by ancestry interaction) at P < 0.001, 20 voxel extent threshold
| Region | Side | Brodmann area | MNI Coordinate | t | k (voxels) | r (SR) |
|---|---|---|---|---|---|---|
| Paralimbic cortices | ||||||
| Anterior insula | L | −22 24 −8 | 5.07 | 81 | 0.33* | |
| Dorsal anterior cingulate cortex | R | 32 | 8 12 44 | 4.06 | 73 | 0.34* |
| Frontal lobe | ||||||
| Supplementary motor area | L | 6 | −20 6 60 | 3.92 | 34 | 0.45* |
| Supplementary motor area | R | 6 | 16 6 60 | 4.07 | 27 | 0.34* |
| Temporal lobe | ||||||
| Superior temporal gyrus | L | 22 | −52 −4 −10 | 4.16 | 23 | 0.33* |
| Superior temporal gyrus | R | 21 | 54 −30 2 | 4.13 | 37 | 0.25 |
| Parietal lobe | ||||||
| Primary somatosensory cortex | L | 3 | −40 −22 46 | 4.41 | 79 | 0.24 |
| Inferior parietal lobule | R | 40 | 62 −50 36 | 4.17 | 24 | 0.33* |
| Occipital lobe and cerebellum | ||||||
| Cuneus | R | 19 | 20 −76 28 | 3.97 | 30 | 0.29 |
| Cerebellum | R | 12 −52 −10 | 3.97 | 22 | 0.29 |
The far right column is the correlation coefficient (r) for the correlation of the activation cluster with Mehrabian's Sensitivity to Rejection scale (SR; *, P < 0.05).
A118G-Related Neural Activity and Dispositional Sensitivity to Rejection.
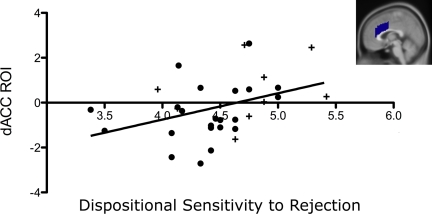
To examine the relationship between A118G-related neural activity during this acute rejection episode and dispositional sensitivity to rejection, participants' dispositional sensitivity to rejection scores were correlated with neural activity in the previously described ROIs (controlling for sex, ancestry, and the sex by ancestry interaction). Although dispositional sensitivity to rejection was not significantly associated with overall activity in the affective pain matrix ROI (r = 0.23, n.s.), it was significantly associated with activity in the dACC ROI (Fig. 4; r = 0.34, P < 0.05). There was no relationship between dispositional sensitivity to rejection and neural activity in either the left (r = 0.2, n.s.) or right (r = −.01, n.s.) anterior insula ROIs.
Fig. 4.
Correlation of the dACC ROI with dispositional sensitivity to rejection (r = 0.34, P < 0.05); + denotes G allele carriers; • denotes A allele homozygotes. (Inset) Sagittal section of ROI.
In exploratory analyses, we also examined whether dispositional sensitivity to rejection was associated with neural activity from suprathreshold clusters obtained from the aforementioned whole-brain analysis comparing G allele carriers to A allele homozygotes. Both the dACC (r = 0.34, P < 0.05) and the left anterior insula (r = 0.33, P < 0.05) clusters were significantly correlated with dispositional sensitivity to rejection, as were several others (see Table 1).
Mediation Analyses.
Based on inter-relationships between the A118G polymorphism, neural activity in the dACC ROI, and dispositional sensitivity to rejection, we next sought to determine if activity in the dACC ROI mediated the relationship between the A118G polymorphism and dispositional sensitivity to rejection. Using a bootstrapping approach to test the significance of the indirect pathway (50), the mediated path was found to be significant [standardized indirect effect = 0.11, P < 0.05, 95% CI = (0.28, 0.02)], indicating that the dACC may be an area where the A118G polymorphism influences sensitivity to rejection.
We also performed exploratory mediation analyses on the dACC and left anterior insula clusters derived from the whole-brain analysis. Although this test of mediation was not independent of the gene-brain relationship (because the clusters of activation were determined based on the A118G polymorphism), results were consistent with those from the ROI analyses. Thus, the mediated path approached marginal significance for the dACC activation [8, 12, 44; standardized indirect effect = 0.11, P = 0.099, 95% CI = (−0.02, 0.36)] but not for the left anterior insula activation [−22, 24, −8;. standardized indirect effect = 0.097, P = n.s., 95% CI = (−0.15, 0.36)].
Discussion
In this study, the A118G polymorphism of the OPRM1 gene was associated with individual differences in sensitivity to social rejection. This effect was found using two different methods: self-reports of dispositional sensitivity to rejection and fMRI measurement of neural responses during an actual experience of social rejection. The neural areas that showed differential activation between carriers of the G allele and individuals homozygous for the A allele are consistent with prior data from studies of the neural circuitry involved in social pain, the neural circuitry involved in the affective component of physical pain, and data on the anatomy and pharmacology of the opioid system.
The association of the A118G polymorphism with differential neural activity in the dACC and anterior insula is consistent with prior work showing these areas to be the principal sites of activation during a social rejection experience (17) as well as during the viewing of social rejection related stimuli (15). Hence, these data indicate that the A118G polymorphism is associated with the acute neural response to social rejection.
In addition to acute responses, the A118G polymorphism was also associated with the trait-like tendency to be more concerned about rejection. That dACC activity statistically mediated the relationship between the A118G polymorphism and self-reported dispositional sensitivity to rejection suggests that this is a critical neural region where this polymorphism may influence reactivity to rejection. In light of prior work showing dACC activity to be correlated with the distress of social rejection (11, 12), it is possible that greater reactivity in such circuits underlies the development of a dispositional proclivity to be apprehensive of social situations that could potentially result in rejection.
Identification of a central role for the ACC and MOR in social pain is consistent with a study by Zubieta et al. (22) that used positron emission tomography to measure MOR availability. They found that μ-opioid neurotransmission within the ACC was negatively correlated with self-reported negative affect during the recollection of the death of a loved one or the ending of a romantic relationship. The involvement of the μ-opioid system within the ACC in both the painful feelings arising from the severance of a prior social bond as well as the severing of a potential social bond, as seen here, suggests that the MOR is involved in multiple facets of the social pain experience.
The association of the A118G polymorphism with differential activation in the ACC and anterior insula is also consistent with the anatomical distribution of the MOR. The ACC and the anterior insula are the cortical areas with the highest concentrations of the MOR (51–54). Not surprisingly, pharmacological administration of MOR ligands also has robust effects upon neural activity in these areas (55, 56). Thus, the pattern of activation associated with the A118G polymorphism in the current study closely corresponds to the pattern of neural responses seen following MOR stimulation.
Although the relationship between the A118G polymorphism and social pain has not been examined previously in humans, the association of variation in the OPRM1 gene with rejection sensitivity is consistent with data from the monkey associating variation in the same portion of this gene (OPRM1 C77G) to a similar social pain construct, separation distress in infancy (46). In that study, carriers of the rare allele, 77G, exhibited more prolonged distress following separation from their mother. Although the precise cellular effects of these variants are not clear yet, the similar behavioral findings across species point to a role for the OPRM1 gene in social pain.
As with any genetic association study, these data should be considered suggestive until replicated. Furthermore, given recent recommendations that imaging genetic studies should have sample sizes of approximately 60 subjects (57), we note that one of the limitations of this study is the relatively small sample size, particularly in light of the heterogeneity of the sample. Yet, the corroborative results across the two methodologies used here, as well as analogous results in the rhesus monkey, would seem to indicate that OPRM1 is indeed involved in social pain related processes. Thus, in concert with prior research, it appears that at multiple biological levels, including the neurochemical, neuroanatomical, and now genetic, feeling hurt physically shares more than just linguistic commonality with feeling hurt socially.
Materials and Methods
Sample.
Participants responded to advertisements posted at the University of California, Los Angeles campus offering $60 for participation in a study of psychological responses to stress. Prospective participants with the following conditions were excluded: serious physical or mental health problems; current treatment from a mental health professional; and current use of mental health related medication (e.g., selective serotonin reuptake inhibitors). The full sample consisted of 128 participants (35% European-American, 43% Asian American, 12% Hispanic, 5% mixed, and 4% African-American; three participants declined to report their ethnicity). For analyses, non-European-American and non-Asian American participants were grouped into an “other” ancestry category, which represented 21% of the sample.
All participants from this larger sample (n = 128) were then re-contacted to see if they were interested in being part of a neuroimaging study. Participants (n = 33) who agreed to be part of a neuroimaging study and met the additional criteria of being right-handed and not being claustrophobic or having metal in their bodies (dental fillings were allowed) participated in the neuroimaging session. Genotypes of these individuals were not known before the scanning session and hence participants were not selected based on genetic criteria. Participants (median age = 21 + 3.06) received an additional $20 for completing the neuroimaging procedures. One participant was omitted from the MRI analyses due to being an extreme outlier (> 3 standard deviations) on neural activity and another participant was omitted due to having previously participated in the Cyberball task. Thus, the final neuroimaging sample consisted of 31 participants (19 female; 26% European-American, 45% Asian, and 28% “other” that consisted of 16% Hispanic, 6% African-American, and 6% “mixed”). Although some data from this sample has been reported previously (16), relationships with the A118G polymorphism and dispositional sensitivity to rejection have not been reported previously. The UCLA Human Subjects Protection Committee approved all experimental procedures, and informed consent was obtained from all participants.
Self-Report Assessments.
As part of the initial pre-MRI session, participants completed a standard battery of self-report inventories including the Mehrabian Sensitivity to Rejection Scale (47). Participants responded to the 24 items on a 7-point agreement-disagreement scale (Cronbach's alpha = 0.74). Items were designed to assess several interrelated components of sensitivity to rejection, including being easily hurt by negative feedback from others and fearing such feedback (e.g., “I am very sensitive to any signs that a person might not want to talk to me”); a reluctance to impose on others (e.g., “If I ask someone to go someplace with me and they refuse, I am hesitant to ask them again”); and avoidance of expressing sentiments that can be criticized (e.g., “I am cautious about expressing my opinions until I know people quite well”). As test re-test reliability has been shown to be 0.92 over a 4-week interval (47), this measure is presumed to reflect a trait-like dispositional tendency to be apprehensive of social interactions with others due to fear of rejection.
Genotyping.
DNA was obtained with the Orasure oral specimen collection device (Orasure Technologies) and extracted using the Puregene DNA purification kit (Gentra Systems). All samples were whole genome amplified using a GenomiPhi V2 DNA Amplification Kit (GE Healthcare) according to the manufacturer's instructions.
The A118G polymorphism (rs1799971) was genotyped using a 5′ nuclease assay to discriminate between the two alleles (Taqman SNP Genotyping Assay C_8950074_1_, Applied Biosystems Inc.). Polymerase chain reactions were performed using 5-μL reaction volumes in 384-well plates with 5 ng of DNA. The standard protocol provided with the kit was followed. End point reads of fluorescence levels were obtained with an ABI 7900HT Sequence Detection System. For quality control, each sample was regenotyped and demonstrated complete concordance (125 of 128 participants were successfully genotyped). Tests of Hardy-Weinberg equilibrium for the entire sample and each ancestral category were conducted using the software program Haploview v3.32 (http://www.broad.mit.edu/mpg/haploview/) (58). χ2 tests were used to assess allele distributions (2n). For association of the A118G polymorphism with self-reported dispositional sensitivity to rejection, a one-way analysis of covariance was used with an additive model. Sex, ancestry, and the interaction between the two were included as covariates (because three participants declined to report ancestry, the final sample consisted of 122 participants). Because there were three levels of ancestry (European-American, Asian, and other), two dummy variables were created (i.e., Asian = 1, all others = 0; and European-American = 1, all others = 0). The interaction terms were generated by multiplying each of these variables by gender (coded 0 or 1).
fMRI Paradigm.
Participants were scanned while completing the Cyberball social exclusion task, in a manner similar to previous work (17). Participants were told that they would be playing a virtual ball-tossing game with two other individuals who were also in fMRI scanners. In reality, however, there were no other players; participants played with a preset computer program. On a computer screen displayed through fMRI compatible goggles, participants saw cartoon images representing the other players, as well as a cartoon image of their own ‘hand’ that they controlled using a button-box. After 9 s, one cartoon player started the game by throwing the ball to either the other cartoon player or the participant. The participant could return the ball to one of the players by pressing one of two keys on a button box. The Cyberball program was set for 60 throws per game, with the computer players waiting 0.5–3.0 seconds (determined randomly) before making a throw to heighten the sense that the participant was actually playing with other individuals.
During the task, participants completed two scans. In the first scan (inclusion), participants played with the two other players for the entire scanning period, with each virtual player throwing the ball to the participant on approximately 50% of the throws. In the second scan (exclusion), participants received the ball for a total of seven throws and were then excluded for the rest of the scan when the two players stopped throwing the ball to the participant (60–90 s). At the conclusion of the experiment, participants were fully debriefed concerning the true nature of the study as well as the reason for the use of deception in psychological research.
fMRI Data Acquisition and Processing.
Data were acquired on a Siemens Allegra 3T head-only scanner. Head movements were restrained with foam padding and surgical tape placed across each participant's forehead. For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo; TR = 5,000 ms; TE = 33 ms; matrix size 128 × 128; 36 axial slices; FOV = 20-cm; 3-mm thick, skip 1-mm) was acquired coplanar with the functional scans. Two functional scans were acquired (echo planar T2*-weighted gradient-echo, TR = 3,000 ms, TE = 25 ms, flip angle = 90°, matrix size 64 × 64, 36 axial slices, FOV = 20-cm; 3-mm thick, skip 1-mm), each lasting 2 min and 30 s.
The imaging data were analyzed using SPM'99 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, U.K.). Images for each participant were realigned to correct for head motion using a six-parameter affine ‘rigid-body’ transformation, normalized (12-parameter affine transformation) into a standard stereotactic space, and smoothed with an 8-mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio.
The design was modeled using a boxcar function convolved with a canonical hemodynamic response function. For each participant, periods of inclusion and exclusion were modeled as epochs based on the length of that participant's inclusion and exclusion episodes (these varied slightly between participants due to the random delay assigned to the virtual players when throwing the ball and the participant's own reaction time to return the throw). Thus, neural activity during the inclusion and exclusion episodes was an average of the sustained neural activity that occurred during each of those episodes. After the task was modeled for each participant, planned comparisons were computed as linear contrasts to investigate neural activity during the exclusion compared to the inclusion episode. Random effects analyses of the group were computed using the contrast images generated for each participant. All coordinates are reported in Montreal Neurological Institute (MNI) format.
fMRI Analyses.
To examine the relationship between the A118G polymorphism and neural responses to social exclusion, two types of analyses were performed: (i) structural ROI analyses, based on specific anatomical hypotheses and (ii) whole-brain analyses to further explore the specific neural regions involved in differential neural responses to exclusion as a function of the A118G polymorphism.
For the ROI analyses, we created a structural ROI that included the conjunction of regions known to be involved in social pain as well as the affective component of physical pain, namely the dACC and bilateral anterior insula (which we refer to here as the affective pain matrix). The ROIs were constructed in PickAtlas (59) using the automated anatomical atlas [AAL; (60) for further details see SI Text].
The Marsbar toolbox (http://marsbar.sourceforge.net) was then used to extract mean parameter estimates (that model the amplitude of the BOLD response during exclusion vs. inclusion) averaged across all voxels in the ROI. Standard statistical software (SPSS 14.0) was used to conduct one-way ANCOVAs with sex, ancestry, and the sex by ancestry interaction as covariates to assess differences in neural activation as a function of genotype (P < 0.05). To determine if the neural regions associated with the A118G polymorphism were also related to dispositional sensitivity to rejection, the mean parameter estimates from these ROIs were correlated with scores on the dispositional sensitivity to rejection scale using partial correlations to control for sex, ancestry, and the sex by ancestry interaction. Analyses were conducted for the affective pain matrix ROI and then for each constituent ROI separately. Based on a priori hypotheses, one-tailed tests were used for all analyses in the neuroimaging subsample.
For the whole brain analysis, a one-way ANCOVA (with sex, ancestry, and sex by ancestry interaction as covariates) was performed in SPM contrasting G allele carriers to A allele homozygotes during the contrast: exclusion vs. inclusion. A significance threshold of P < 0.001 (uncorrected for multiple comparisons) was used with a cluster size threshold of 20 voxels (61). To correlate these activations with dispositional sensitivity to rejection, parameter estimates were extracted from the suprathreshold clusters and analyses were performed in SPSS.
Mediation Analyses.
Statistical mediation was assessed according to the criteria established by Shrout and Bolger [2002; (50)] using a bootstrapping procedure implemented through Amos 15.0 software (SPSS Inc.) to determine 95% confidence intervals and significance tests of mediated paths.
Supplementary Material
Acknowledgments.
We thank Matthew D. Lieberman, Ph.D., Elliot T. Berkman, Richard B. Slatcher, Ph.D., the UCLA Brain Mapping Center, and the UCLA Genotyping Core, particularly Sugandha Dandekar, for their assistance. This research was supported by a National Institute of Mental Health postdoctoral fellowship to B.M.W. as part of the UCLA Health Psychology Program, National Institute of Aging Grant AG030309, National Institute of Mental Health Grant R21MH07152, and a grant from the Harry Frank Guggenheim Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812612106/DCSupplemental.
The subsequently reported results did not change appreciably when covariates were not included in the analyses.
References
- 1.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 2.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Foltz EL, White LE., Jr Pain ”relief“ by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 5.Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: A sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- 6.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 7.Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 8.Peyron R, et al. Haemodynamic brain responses to acute pain in humans: Sensory and attentional networks. Brain. 1999;122:1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 9.Tolle TR, et al. Region-specific encoding of sensory and affective components of pain in the human brain: A positron emission tomography correlation analysis. Ann Neurol. 1999;45:40–47. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Schreckenberger M, et al. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64:1175–1183. doi: 10.1212/01.WNL.0000156353.17305.52. [DOI] [PubMed] [Google Scholar]
- 11.MacLean PD, Newman JD. Role of midline frontolimbic cortex in production of the isolation call of squirrel monkeys. Brain Res. 1988;450:111–123. doi: 10.1016/0006-8993(88)91550-8. [DOI] [PubMed] [Google Scholar]
- 12.Hadland KA, Rushworth MFS, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41:919–931. doi: 10.1016/s0028-3932(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 13.Smith WK. The functional significance of the rostral cingular cortex as revealed by its responses to electrical stimulation. J Neurolphysiol. 1945;8:241–255. [Google Scholar]
- 14.Robinson BW. Vocalization evoked from forebrain in Macaca mulatta. Physiol Behav. 1967;2:345–354. [Google Scholar]
- 15.Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007;19:945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 18.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 19.Sora I, et al. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 21.Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- 22.Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: Opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- 23.Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- 24.Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- 25.Warnick JE, McCurdy CR, Sufka KJ. Opioid receptor function in social attachment in young domestic fowl. Behav Brain Res. 2005;160:277–285. doi: 10.1016/j.bbr.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 27.Zubieta JK, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 29.Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 30.Kroslak T, et al. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 31.Lotsch J, Geisslinger G. Relevance of frequent mu-opioid receptor polymorphisms for opioid activity in healthy volunteers. Pharmacogenomics J. 2006;6:200–210. doi: 10.1038/sj.tpj.6500362. [DOI] [PubMed] [Google Scholar]
- 32.Oertel BG, Schmidt R, Schneider A, Geisslinger G, Lotsch J. The mu-opioid receptor gene polymorphism 118A>G depletes alfentanil-induced analgesia and protects against respiratory depression in homozygous carriers. Pharmacogenet Genomics. 2006;16:625–636. doi: 10.1097/01.fpc.0000220566.90466.a2. [DOI] [PubMed] [Google Scholar]
- 33.Romberg RR, et al. Polymorphism of mu-opioid receptor gene (OPRM1:c. 118A>G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology. 2005;102:522–530. doi: 10.1097/00000542-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Sia AT, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- 35.Coulbault L, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79:316–324. doi: 10.1016/j.clpt.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Chou WY, et al. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 37.Chou WY, et al. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105:334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Landau R, Kern C, Columb MO, Smiley RM, Blouin JL. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14. doi: 10.1016/j.pain.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83:559–566. doi: 10.1038/sj.clpt.6100385. [DOI] [PubMed] [Google Scholar]
- 40.Reyes-Gibby CC, et al. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130:25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klepstad P, et al. The 118 A > G polymorphism in the human mu-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48:1232–1239. doi: 10.1111/j.1399-6576.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 42.Tan EC, et al. Ethnic differences in pain perception and patient-controlled analgesia usage for postoperative pain. J Pain. 2008;9:849–855. doi: 10.1016/j.jpain.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Bruehl S, Chung OY, Burns JW. The mu opioid receptor A118G gene polymorphism moderates effects of trait anger-out on acute pain sensitivity. Pain. 2008;139:406–415. doi: 10.1016/j.pain.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fillingim RB, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Lotsch J, Stuck B, Hummel T. The human mu-opioid receptor gene polymorphism 118A > G decreases cortical activation in response to specific nociceptive stimulation. Behav Neurosci. 2006;120:1218–1224. doi: 10.1037/0735-7044.120.6.1218. [DOI] [PubMed] [Google Scholar]
- 46.Barr CS, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehrabian A. Evidence bearing on the affiliative tendency (MAFF) and sensitivity to rejection (MSR) scales. Curr Psychol. 1994;13:97–116. [Google Scholar]
- 48.Williams KD, Cheung CK, Choi W. Cyberostracism: Effects of being ignored over the Internet. J Pers Soc Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- 49.Wise SP, Herkenham M. Opiate receptor distribution in the cerebral cortex of the Rhesus monkey. Science. 1982;218:387–389. doi: 10.1126/science.6289441. [DOI] [PubMed] [Google Scholar]
- 50.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 51.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 52.Wamsley JK, Zarbin MA, Young WS, 3rd, Kuhar MJ. Distribution of opiate receptors in the monkey brain: An autoradiographic study. Neuroscience. 1982;7:595–613. doi: 10.1016/0306-4522(82)90066-5. [DOI] [PubMed] [Google Scholar]
- 53.Pilapil C, Welner S, Magnan J, Gauthier S, Quirion R. Autoradiographic distribution of multiple classes of opioid receptor binding sites in human forebrain. Brain Res Bull. 1987;19:611–615. doi: 10.1016/0361-9230(87)90080-3. [DOI] [PubMed] [Google Scholar]
- 54.Baumgartner U, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30:692–699. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 55.Leppa M, et al. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage. 2006;31:661–669. doi: 10.1016/j.neuroimage.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Wise RG, et al. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage. 2002;16:999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- 57.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. doi: 10.1038/mp.2009.36. in press. [DOI] [PubMed] [Google Scholar]
- 58.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 59.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 60.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 61.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.