Abstract
Eye-opening represents a turning point in the function of the visual cortex. Before eye-opening, the visual cortex is largely devoid of sensory inputs and neuronal activities are generated intrinsically. After eye-opening, the cortex starts to integrate visual information. Here we used in vivo two-photon calcium imaging to explore the developmental changes of the mouse visual cortex by analyzing the ongoing spontaneous activity. We found that before eye-opening, the activity of layer 2/3 neurons consists predominantly of slow wave oscillations. These waves were first detected at postnatal day 8 (P8). Their initial very low frequency (0.01 Hz) gradually increased during development to ≈0.5 Hz in adults. Before eye-opening, a large fraction of neurons (>75%) was active during each wave. One day after eye-opening, this dense mode of recruitment changed to a sparse mode with only 36% of active neurons per wave. This was followed by a progressive decrease during the following weeks, reaching 12% of active neurons per wave in adults. The possible role of visual experience for this process of sparsification was investigated by analyzing dark-reared mice. We found that sparsification also occurred in these mice, but that the switch from a dense to a sparse activity pattern was delayed by 3–4 days as compared with normally-reared mice. These results reveal a modulatory contribution of visual experience during the first days after eye-opening, but an overall dominating role of intrinsic factors. We propose that the transformation in network activity from dense to sparse is a prerequisite for the changed cortical function at eye-opening.
Keywords: calcium waves, cortical development, mouse, two-photon imaging, up-down states
During the first two postnatal weeks, important functional features such as retinotopic maps were shown to develop in the visual cortex of rodents (1, 2). This development is activity-dependent and takes place before eye-opening. Intrinsic activity patterns play a critical role in circuit refinement at this stage of development (1–3). Activity patterns known to be present in the rat visual cortex during the first postnatal week include bursts with spindle-shape field oscillations that are triggered by spontaneous retinal bursts (4). In the ferret, also before eye-opening, long-range correlated activity is generated by intrinsic circuits in the primary visual cortex and is modified by eye-specific connections from the retina via the lateral geniculate nucleus (5, 6).
Surprisingly, little is known about the spontaneous activity in the mouse visual cortex around eye-opening. An early form of cortical slow wave activity, the so-called early network oscillations (ENOs) (7), was detected in rodents as early as embryonic day 16 (8) and persists approximately until the end of the first postnatal week (7, 9). The ENOs share similarities with the waves that were originally described in the retina (10, 11) and in the spinal cord (12). The distinctive features of ENOs are their complete block by anesthetics in vivo (13), their strong sensitivity to AMPA receptor antagonists and their presence during the period at which GABAergic transmission is depolarizing (7, 14). Such waves of activity travel over long distances within the cortex, or even from the retina to the cortex (2, 4), and play a critical role in the accurate assembly of long-range neuronal connections (3). At the end of the first postnatal week (between postnatal days P5 and P9), GABA-driven cortical giant depolarizing potentials (GDPs) have been reported in slices of rat somatosensory cortex (9). Importantly, to our knowledge, nothing is known about the activity during the second postnatal week that just precedes eye-opening.
In adult mammals, slow wave activity was initially found in sleeping and in anesthetized animals in vivo (15). In some instances, slow wave activity can be detected in adults also in ex vivo preparations (16). The slow wave activity during early and late developmental stages shares similarities, but has also distinctive, stage-specific properties. The similarities include (i) the presence of depolarized “Up” states that can be associated with massive neuronal firing followed by relatively silent “Down” states, (ii) Ca2+ signaling during Up states and (iii) the long-range propagation of the waves involving large cortical areas (2, 7). The major difference is the 15- to 20-fold higher wave frequency in adults (0.5 Hz vs. ≈0.03 Hz in the cortex of neonates) (13, 15, 17).
An interesting feature of the slow waves recorded in the adult cortex is the sparse activation pattern with only 10% of cortical neurons firing during Up states (17). Such a sparseness of the spontaneous activity in the adult is an important prerequisite for sparse coding of sensory inputs, a general feature of information processing in different sensory systems, including the visual system (18–21). Spontaneous synchronous activity in the adult brain was found to strongly correlate with sensory processing as well as with motor planning and behavioral states (22–24). In the visual cortex of adult cats, it was shown that ongoing spontaneous activity contributes to the variability of sensory-evoked neuronal responses (25). Spontaneous activity also plays a critical role in the coordinated memory replay in the visual cortex and in the hippocampus, during slow wave sleep (26, 27). This process is believed to be of critical importance for memory consolidation (26, 27).
In the present study, we investigated the specific features of the ongoing spontaneous activity during the postnatal development of the mouse visual cortex in vivo. We were particularly interested in the question of how eye-opening affects this ongoing activity. We used video-rate two-photon calcium imaging (28) of neural networks in vivo (29), an approach that allows the detection of action potential firing with a high fidelity and sensitivity (17).
Results
Slow Wave Activity in the Mouse Visual Cortex Before Eye-Opening.
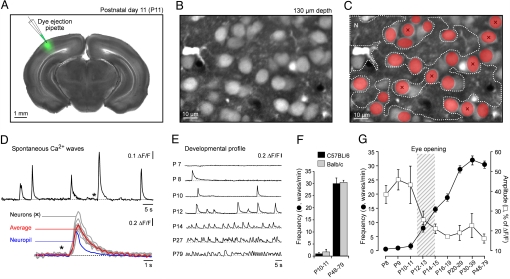
We stained neurons with the fluorescent Ca2+ indicator Oregon Green BAPTA-1 AM (OGB-1 AM) which was delivered through a patch pipette to the primary visual cortex of mice (Fig. 1A). Fig. 1B shows stained cortical layer 2/3 cells located ≈130 μm below the cortical surface of an 11-day-old (P11) C57BL/6 mouse. Continuous recordings of changes in Ca2+-dependent fluorescence from a region of interest covering the entire field of view revealed wave-like Ca2+ transients recurring at a slow rate (3 waves/min; Fig. 1 D). A more detailed analysis showed that the wave activity was present both in neuropil and in neurons (Fig. 1 C and D). Remarkably, a large fraction of the neurons participated in this spontaneous activity (Fig. 1C). A detailed developmental analysis revealed that Ca2+ waves were not detected before P7 in anesthetized mice (Fig. 1E). Ca2+ waves were first detected at P8, when they occurred at a low frequency (0.5 ± 0.1 waves/min). Over the next developmental days, the frequency gradually increased, whereas the amplitude decreased (Fig. 1 E–G). After the end of the fourth postnatal week, the Ca2+ waves reached a frequency of ≈30 waves/min (or 0.5 Hz), well within the range of the electrically-recorded Up-Down states or slow waves that were described originally by Steriade and colleagues (15). We performed experiments in nonpigmented BALB/c mice to relate the results to our earlier work performed in vitro (7) and in vivo (13) as well as in pigmented C57BL/6 mice, which exhibit a better visual detection, pattern discrimination and visual acuity (30, 31). The frequency of Ca2+ waves was at all developmental ages not significantly different in C57BL/6 and in BALB/c mice (Student's t test, P > 0.05, Fig. 1 F and G).
Fig. 1.
Spontaneous Ca2+ waves in the developing mouse visual cortex in vivo. (A) Combined fluorescence/transmitted light micrograph of a coronal brain slice from an 11-day-old C57BL/6 mouse taken after an in vivo experiment. The area stained in vivo with OGB-1 is shown in green. (B) An in vivo micrograph showing an area of layer 2/3 in the primary visual cortex where recordings illustrated in D were made. (C) Active cells (in red) during a representative Ca2+ wave at P11. (D) Upper: spontaneous Ca2+ waves, recorded from a region of interest covering the entire frame shown in B and C. Lower: cellular responses during the Ca2+ wave marked with an asterisk in the upper trace, in seven neurons (x) and in the neuropil (N) (regions of interest are indicated in C). The Average trace (red) is the mean of the seven neuronal responses. (E) Developmental profile of the spontaneous Ca2+ waves. Each trace is a recording from a large region of interest (e.g. D, upper trace), at different ages. (F) Frequencies of spontaneous Ca2+ waves in C57BL/6 and BALB/c mice before eye-opening (P10–11) and 6 weeks after eye-opening (P48–79), (C57BL/6 mice: 10 animals/P10–11, 5/P48–P79; BALB/c mice: 9/P10–11, 5/P48–P79). (G) Frequencies (filled circles) and amplitudes (open squares) of spontaneous Ca2+ waves at different ages. Data from C57BL/6 and BALB/c mice were pooled, because for each age-group there was no significant difference (Student's t test) between both strains (BALB/c mice: 5 animals/P8, 5/P9, 4/P10, 5/P11, 5/P12–P13, 5/P14–P15, 4/P16–P19, 5/P20–29, 4/P30–P39, 5/P48–P79; C57BL/6 mice: 5/P8–P9, 5/P10, 5/P11, 13/P12–P13, 13/P14–P15, 5/P16–P19, 10/P20–29, 5/P30–P39, 5/P48–P79). Here and below error bars represent standard error of the mean.
At later developmental stages, Ca2+ transients recorded in vivo in the somata of neurons in the visual cortex result exclusively from neuronal firing (32), similarly to neurons in other cortical areas (17, 29). To test whether this is the case also at early stages of development we combined cell-attached and whole-cell recordings with Ca2+ imaging in vivo (Fig. 2A). Using a video rate two-photon imaging microscope (see Experimental Procedures), single action potential-evoked Ca2+ transients were reliably detected and the amplitude of Ca2+ transients correlated with the number of action potentials (R2 = 0.98 see Fig. 2 B and C) (17). The median number of action potentials per Ca2+ transient at P11-P12, before eye-opening, was found to be significantly higher (Kolmogorov-Smirnov test, Z = 5.331, P < 0.001) than that at P14–P16, after eye-opening (Fig. 2D). These results were in agreement with the larger amplitudes of Ca2+ waves recorded before P12 (see Fig. 1G).
Fig. 2.
Cellular properties of neuronal Ca2+ signals during slow wave activity. (A and B) Simultaneous recordings of spontaneous Ca2+ transients and underlying action potential firing in loose-seal cell-attached configuration (B) in a layer 2/3 neuron (A) in the primary visual cortex of a C57BL/6 15-day-old mouse. Spontaneously occurring action potentials (APs) are indicated in red. (C) Relation between the number of action potentials and the amplitude (ΔF/F) of the corresponding Ca2+ transient (P14-P16 C57BL/6 mice, seven cells as indicated). Dotted line indicates the linear least squares fit to the data. (D) Box-and-whisker plot (Left) and cumulative functions (Right) illustrating the distribution of number of action potentials per calcium transient at P11–P12 (5 cells) and P14–P16 (12 cells). The median number of action potentials per Ca2+ transient at P11–P12, before eye-opening, was significantly higher (Kolmogorov-Smirnov test, Z = 5.331, P < 0.001) than that at P14–P16, after eye-opening. (E) Simultaneous whole-cell patch-clamp recordings of electrical activity (Top) from a layer 2/3 cell within the field of view and Ca2+ recordings from the surrounding neuropil (Lower). Note that the Up-Down states are strictly associated with the Ca2+ wave activity in the neuropil. Mouse age was P17. (F) Histogram of the membrane potential values of the same neuron as in E.
The temporal relation between the electrical activity of single neurons and the Ca2+ waves was determined in experiments in which current clamp whole-cell recordings were combined with two-photon Ca2+ imaging. Fig. 2E illustrates an experiment performed in a P17-old mouse. Whole-cell recordings from a layer 2/3 neuron showed the highly characteristic Up-Down state-related membrane potential deflections (Fig. 2 E and F) known to occur during slow wave activity in the visual cortex as well as in other brain regions (17, 18, 24). At this developmental stage (P16–P18), ≈50% (47 ± 11%, n = 9 cells in 7 mice) of the Up-states in a given neuron were associated with action potential firing. During Down-states, single spikes were observed only occasionally, and spike bursts were virtually never encountered. The onset of the Up-states correlated closely with the onset of the Ca2+ waves recorded from the surrounding neuropil (Fig. 2E). These results, in agreement with previous findings in the motor and the somatosensory cortex (17), identify the neuropil Ca2+ waves as a reliable reporter for the Up-Down state activity.
Sparsification of Spontaneous Neuronal Activity After Eye-Opening.
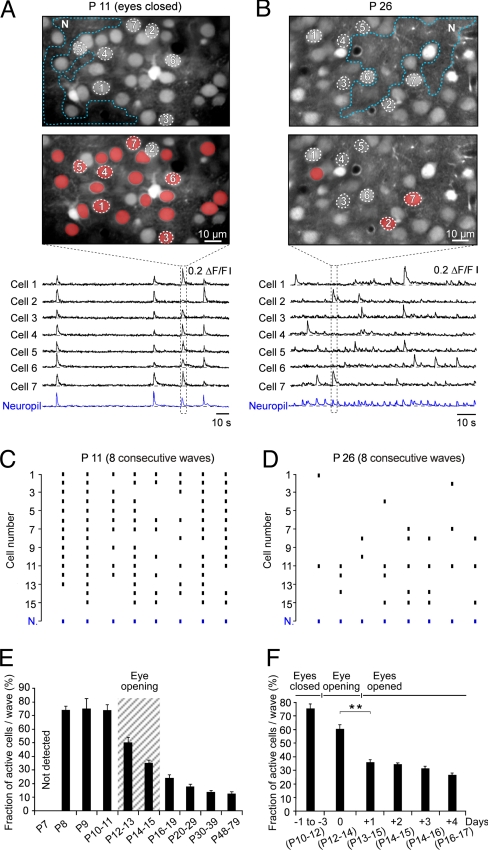
Before eye-opening, a striking feature of the spontaneous activity was the fact that most neurons participated in each wave with a Ca2+ transient (Fig. 3A, C, and E). Thus, at P8–P11, the average fraction of active neurons per wave was 74.5 ± 4.6%. This dense activation pattern is in striking contrast to the sparse neuronal activity that is found in adults (Fig. 3B, D, and E). Closer inspection of the developmental profile (Fig. 3F) showed that on the day of eye-opening, a process of sparsification was initiated leading to a strong decrease in the fraction of active cells per wave from 59.8 ± 3.6% at the day of eye-opening to 36.0 ± 1.7% only 1 day later. This sparsification was nearly completed within 1 week after eye-opening and reached a level of ≈12% of active cells per wave in adults (Fig. 3E, fraction of active cells per wave was 17.5 ± 1.9% at P20–29 and 12.3 ± 1.7% at P48–79). As previously reported for the adult somatosensory and frontal cortices (17, 33), we found that at all developmental ages ≈10% of the visual cortex neurons were ‘silent’ and did not display any calcium transient during our periods of recording (> 3 min). The other neurons were active at characteristic age-dependent frequencies (from 1.1 ± 0.19 waves/min at P10 to 4.05 ± 0.49 waves/min at P48–60).
Fig. 3.
Sparsification of spontaneous neuronal activity after eye-opening. (A and B) Recordings from layer 2/3 in the visual cortex of a P11 (A) and a P26 C57BL/6 mouse (B). The two images in the top row indicate the regions of interest. The two images in the middle row display active cells (in red) during a representative Ca2+ wave (dotted lines) at P11 and P26, respectively. Bottom row: wave-associated Ca2+ transients in individual neurons. Note the pronounced increase in the frequency of neuropil wave activity at P26. (C and D) Dot plot representation of cellular responses during eight consecutive Ca2+ waves at P11 and P26, respectively (same experiments as those shown in A and B). (E) Fraction of cells active during a given Ca2+ wave as a function of postnatal age. Data obtained from both C57BL/6 and BALB/c mice were pooled, because for each age-group there was no significant difference (Student's t test) between both strains (same experiments as those shown in Fig. 1G). (F) Fraction of active cells per Ca2+ wave in relation to the day of eye-opening. The day of eye-opening (P12–14) is indicated in the graph as day 0. All results were obtained in C57BL/6 mice [eyes closed (-1 to −3); 6 animals; day of eye-opening: 6 animals; eyes opened (+1 through + 4 days): 6, 5, 4, and 4 animals, respectively). The corresponding postnatal ages of the animals are indicated for each group in parenthesis. The asterisks indicate significance (Kolmogorov-Smirnov test, P < 0.005).
Role of Visual Experience.
To determine whether sparsification requires visual experience we examined mice that were reared from birth in the dark. In these mice, sparsification also occurred in the absence of visual experience. The activity patterns recorded before eye-opening (P11) as well as those recorded 2 (P20–29) or more than 7 weeks (P48–79) after eye-opening, were similar in normally and in dark-reared mice (Fig. 4F and Fig. S1). Specifically, the number of active cells per wave was virtually identical at these later developmental stages (Fig. 4F). However, the inspection of the activity patterns recorded 1, 2, and 3 days after eye-opening revealed a strong difference between normally and dark reared mice (Fig. 4 A–E). Fig. 4 A–D shows an example of activity patterns recorded in mice of the same age (P15) with the same number of days after eye-opening (+3 days) but one reared in normal conditions and the other reared in the dark. The fraction of active cells per wave was markedly higher (1.8 times higher) in dark-reared than in normally-reared mice (Fig. 4 E and F). Thus, in dark-reared animals, the switch from a dense activity pattern to a sparse one occurred 4–5 days after eye-opening instead of occurring 1 day after eye-opening as in normally-reared mice. These results indicate that visual experience is not required for the process of sparsification, but has a strong modulatory effect at the onset of vision.
Fig. 4.
Sparsification is modulated by visual experience. (A and B) Wave-associated Ca2+ transients in individual layer 2/3 neurons in a control (A) and in a dark-reared (B) 15-day-old mouse, 3 days after eye-opening. The two images in the top row indicate the regions of interest. Bottom row: wave-associated Ca2+ transients in individual neurons. Active cells during a representative Ca2+ wave (indicated by dotted lines in the bottom row) are marked in red. (C and D) Dot plot representation of cellular responses during consecutive waves in a control (C) and in a dark-reared (D) 15-day-old mouse (same experiments as in A and B). (E and F) Fraction of active cells per Ca2+ wave in relation to the day of eye-opening. The day of eye-opening is indicated as day 0, the days before and after eye-opening are indicated by −1 to −3 and by + 1, +2, and so on, respectively. (E) Each circle indicates the percentage obtained in one animal. (F) Mean values of the fraction of active cells per Ca2+ wave in control and dark reared mice. All results were obtained in C57BL/6 mice. Normally reared mice (NR), same number of animals as in Fig. 3E—(F). Dark reared (DR) mice, eyes closed (-1 to −3): 5 animals; day of eye-opening: 5 animals; eyes opened (+1 through + 3 days): 5, 4, and 7 animals, respectively; eyes opened (+4–5 through + 43–75 days): 5, 5, 7, and 10 animals, respectively). The asterisks indicate significance in F (Kolmogorov-Smirnov test, P < 0.01). The corresponding postnatal ages of the animals are indicated for each group in parenthesis.
Discussion
By using two-photon calcium imaging of neural networks in vivo, we identified in this study two distinct modes of network activity of layer 2/3 neurons in the mouse primary visual cortex. A dense mode is present before eye-opening, when the cortex generates and receives largely intrinsic signals, and a sparse mode develops after eye-opening, when the cortex processes both intrinsic and visually driven signals. This sparsification process also occurs in dark-reared animals, indicating a critical role of intrinsic factors for this process. However, we find that the process of sparsification is modulated by visual experience during the first 4 days after eye-opening.
This study reveals the presence of a wave-like network activity at P8–P9, at a time point which is ≈4–7 days before eye-opening. These waves recur at very slow rates in the subdelta range and are produced by simultaneously active neurons. We show that this slow wave activity is present in the visual cortex during a period (from P8–9 to eye-opening) that corresponds to stage III of the retinal waves (2). As previously shown during the first postnatal week for stage II retinal waves (4), it is possible that stage III retinal waves also trigger the slow waves at the cortical level during the second postnatal week. It has been suggested that spontaneous activity is involved in the wiring of the distinct pattern of connectivity in the cortex (1, 2). In the mammalian visual system, as in the developing Xenopus retino-tectal system (34), spontaneous activity, through activation of NMDA receptors, may regulate axonal growth and, thereby, the pattern of innervation territories in the cortex. Such early forms of patterned spontaneous activities promote the establishment of the coarse connectivity and of sensory maps in the visual system (1, 35, 36).
A remarkable aspect of the cortical slow wave activity before eye-opening is the fact that the majority of layer 2/3 neurons is engaged in each wave of activity. Such a dense activation pattern had not been observed before in vivo and is in remarkable contrast to the sparse activity found at later stages of development. Our analysis of mice at defined days before and after eye-opening revealed that the process of sparsification is strongly accelerated at the day of eye-opening. Irrespective of the biological age of the mouse—P12, P13, or P14—there is a sudden drop in the fraction of active cells per wave, from 60% at the day of eye-opening to 36% one day later (Fig. 3F). This suggests that eye-opening directly affects the process of sparsification. A role of visual experience is further supported by the observation that during the first 3 days after eye-opening the fraction of active cells per wave is significantly higher in dark-reared mice compared with normally-reared animals (Fig. 4 E and F). However, the finding that sparsification eventually develops in animals that were reared in the dark indicates that intrinsic factors play a decisive role in the process of sparsification. In these animals, the delayed decrease in the percentage of active cells per wave at 4–5 days after eye-opening (from 55.6 ± 3.4% to 32.2 ± 3.4%, Fig. 4F) is as sudden as that observed in control animals at 1 day after eye-opening (from 59.8 ± 3.6% to 36.0 ± 1.7%, Fig. 4F). This similarity suggests that the sudden reduction is part of an intrinsic developmental program that, in normal conditions, is just triggered by visual stimuli. The period of eye-opening is characterized not only by a switch in the cortical firing mode, but also by a change in retinal firing.
In normally-reared mice, retinal waves begin to break down at P15, just after eye-opening. By P21, waves are not present any more, and the activity pattern is comparable with that observed in adults (37). Although the precise, day-by-day changes of the pattern of spontaneous retinal activity between P15 and P21 are unknown, it is remarkable that the retina reaches, as the cortex, at ≈1 week after eye-opening a level of maturation that is similar to that found in adults (37). In addition, in dark-reared mice a similar time course for the disappearance of retinal waves was observed. Remarkably, at P15, dark-reared retinas occasionally showed abnormally long periods of relative inactivity, not seen in controls (37). In view of the evidence that during the first postnatal week retinal waves trigger the activity in the visual cortex (4), it remains to be determined whether the changes of spontaneous retinal activity around eye-opening contribute to the sparsification of cortical activity.
Further support for the decisive role of intrinsic factors comes from the finding that the same level of sparseness is eventually reached in mice with and without visual experience (for example at P57–89, 9.6 ± 0.9% of active cells per wave in control mice and 12.0 ± 1.2% in dark reared mice, no significant difference between both groups). It seems that these unknown intrinsic factors exert their action not only in the visual cortex, but also in other cortical areas, at least for layer 2/3 neurons. We have obtained evidence for such a switch from dense to sparse activity also in the auditory cortex (Fig. S2), whereas others have shown sparse neuronal activity at late developmental stages in the somatosensory (17) and the motor cortex (17, 38).
In conclusion, we demonstrate that the activity of the visual cortex undergoes profound functional changes at the onset of vision. In the present work, we investigated the properties of the circuitry by analyzing the spontaneous slow wave activity. The observed sparse signaling may be of direct significance for information processing during slow-wave sleep in the visual cortex (26, 27). It is likely, however, that the developmental changes of the circuitry are also essential for the function of the visual cortex during signal processing in behaving animals. We propose that the rapid development of visual performance during the first 8–10 days after eye-opening [see for example (39, 40)] is based, at least in part, on the sparsification of layer 2/3 neurons spontaneous activity in the primary visual cortex.
Experimental Procedures
Animals.
All experimental procedures were performed in accordance with institutional animal welfare guidelines and were approved by the state government of Bavaria, Germany. Litters of C57BL/6 (7- to 89-day-old) and BALB/c mice (7- to 65-day-old) were kept under 12 h/12 h light/dark cycles except for dark-rearing experiments. The day of birth (P0) was accurately ascertained as well as the day of eye-opening. For this, the eyes were checked four times per day (at 8 AM, 1 PM, 6 PM, 8–9 PM) from the age of P10 on, and the eyes were considered opened as soon as we observed the initial break in the membrane sealing the eyelids. For dark-reared mice, the same procedure was performed with the help of infrared viewers.
Surgical Procedures.
Surgery was performed in accordance with institutional animal welfare guidelines as described previously (29). Briefly, the mice were placed onto a warming plate (38°C) and anesthetized by inhalation of 1.5% isoflurane (Curamed) in pure O2. The depth of anesthesia was assessed by monitoring the tail-pinch reflex and the respiration rate. After removing the skin, the skull was gently thinned under a dissecting microscope using dental drills. The custom-made recording chamber (41) was then glued to the skull with cyanoacrylic glue (UHU). The mouse was transferred into the set-up, placed onto a warming plate (38°C) and continuously supplied with 0.9–1.1% isoflurane in pure O2. The recording chamber was perfused with warm (37°C) extracellular perfusion saline containing (in mM): 125 NaCl, 4.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, and 20 glucose, pH 7.4, when bubbled with 95% O2 and 5% CO2. For two-photon imaging of cortical neurons a small craniotomy (≈1 mm) was performed above an area devoid of big blood vessels using a thin (30 G) injection needle.
In Vivo Ca2+ Imaging of Network Activity.
The position of the primary visual cortex was located according to brain atlas coordinates (Bregma −3 to −4.5 mm, 2–3 mm lateral to the midline for P18–P65 (42)). In younger animals, the position of the primary visual cortex (0–1 mm anterior to the lambda suture, 1.5–3 mm lateral to the midline) was identified according to the Golgi atlas of the postnatal mouse brain (43). In all experiments the correct location of the imaged neurons was confirmed in vivo by their responses to light flashes and/or posthoc by imaging of the stained brain area (e.g., Fig. 1A). Similar procedures were used to identify the location of the primary auditory cortex (Bregma −2.2 to −3.6 mm, 4–4.5 mm lateral to the midline) (42).
The brain area of interest was stained in vivo with the fluorescent Ca2+ indicator dye Oregon Green BAPTA-1 (OGB-1) (29). Two-photon imaging was performed with a custom-build video-rate two-photon microscope based on a resonance scanner (44) and a mode-locked femtosecond pulse laser, operating at 710–920 nm wavelength (MaiTai, Spectra Physics). The scanner was mounted on an upright microscope (BX61WI, Olympus) equipped with water-immersion objectives (60×, 1.0 NA Nikon, or 40×, 0.8 NA Olympus). Because isoflurane is known to act differently in rodents of different ages, we adjusted its concentration in each experiment such that the animals were continuously breathing at a rate of 90–110 per minute. In general, we started recordings at 1.1% isoflurane in pure O2 at P17–P65 (1.1–1.2% at P7–P16) and gradually decreased its concentration to levels at which the breathing rate was constant, just above the threshold for awakening.
Cell-Attached and Whole-Cell Patch Clamp Recordings in Vivo.
Loose-seal cell-attached and whole-cell patch clamp recordings were performed using an EPC-9 patch clamp amplifier (HEKA) as described in (17, 18). All data were filtered at 3–10 kHz and digitized at 5–20 kHz. The pipette solution for loose-seal cell-attached recordings contained (in mM): 150 NaCl, 2.5 KCl, 10 HEPES, 2 Ca2Cl, 1 MgCl2, and 20 glucose, pH 7.4. The pipette solution for whole-cell patch clamp recordings contained (in mM): 148 K-Gluconate, 10 Hepes, 10 NaCl, 0.5 MgCl2, 4 Mg-ATP, 0.4 Na-GTP, and 0.05 Alexa Fluor 594, pH 7.3.
Image Analysis.
The image analysis was performed off-line in two steps. First, the ImageJ software (http://rsb.info.nih.gov/ij/) was used for drawing regions of interest (ROIs) around cell bodies and around a large area of cell-free neuropil. Astrocytes were excluded from the analysis based on their selective staining by sulforhodamine 101 (45, 46), brighter appearance after staining with OGB1-AM (17) and their specific morphology with clearly visible processes (see for example on the left edge of Fig. 1B). The presence of glial processes was assessed by inspecting 3D-stacks (30 μm below and upper the imaged plane) obtained routinely at the end of each experiment. Fluorescence values from all ROIs were extracted. In the next step, custom made routines of the Igor Pro software (Wavemetrics) were used for the detection of wave-associated Ca2+ transients in individual neurons and in the neuropil. Background fluorescence, measured in blood vessel lumen, was subtracted from all signals. All signals were expressed as relative fluorescence changes (dF/F) after background subtraction. All dF/F traces were smoothed with a binomial filter (time window = 0.3 s). Each smoothed trace was subtracted from the original dF/F trace, resulting in the ‘baseline noise’ trace. Calcium transients were automatically detected with a template-matching algorithm, taking into account their sharp rise. They were accepted as signals if their amplitude was three times larger than the standard deviation of the corresponding baseline noise values. After the automatic analysis, all traces were carefully inspected again to correct for false positive or negative signals.
Supplementary Material
Acknowledgments.
We thank Jia Lou for her help with the figures. This work was supported by grants of the Deutsche Forschungsgemeinschaft (SFB 596, GA 654/1–1, HO 2156/2–1) and the Bundesministerium für Bildung und Forschung (NGFN-2). M.N. was supported by a fellowship from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907660106/DCSupplemental.
References
- 1.Cang J, et al. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Bendito G, Molnar Z. Thalamocortical development: How are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- 4.Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu C, Weliky M. Spontaneous activity in developing ferret visual cortex in vivo. J Neurosci. 2001;21:8906–8914. doi: 10.1523/JNEUROSCI.21-22-08906.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu C, Weliky M. Relationship of correlated spontaneous activity to functional ocular dominance columns in the developing visual cortex. Neuron. 2002;35:1123–1134. doi: 10.1016/s0896-6273(02)00867-x. [DOI] [PubMed] [Google Scholar]
- 7.Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- 8.Corlew R, Bosma MM, Moody WJ. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J Physiol. 2004;560:377–390. doi: 10.1113/jphysiol.2004.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allene C, et al. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 2008;28:12851–12863. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- 11.Feller MB, Butts DA, Aaron HL, Rokhsar DS, Shatz CJ. Dynamic processes shape spatiotemporal properties of retinal waves. Neuron. 1997;19:293–306. doi: 10.1016/s0896-6273(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 12.Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8:988–990. doi: 10.1038/nn1502. [DOI] [PubMed] [Google Scholar]
- 14.Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 17.Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci USA. 2005;102:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson J, Lampl I, Reichova I, Carandini M, Ferster D. Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat Neurosci. 2000;3:617–621. doi: 10.1038/75797. [DOI] [PubMed] [Google Scholar]
- 19.Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science. 2000;287:1273–1276. doi: 10.1126/science.287.5456.1273. [DOI] [PubMed] [Google Scholar]
- 22.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert CD, Sigman M. Brain states: Top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 26.Aton SJ, et al. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 28.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 29.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkema GW, Drager UC. Impaired visual thresholds in hypopigmented animals. Vis Neurosci. 1991;6:577–585. doi: 10.1017/s095252380000256x. [DOI] [PubMed] [Google Scholar]
- 31.Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- 33.Busche MA, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 34.Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- 35.Albert MV, Schnabel A, Field DJ. Innate visual learning through spontaneous activity patterns. PLoS Comput Biol. 2008;4:e1000137. doi: 10.1371/journal.pcbi.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feller MB, Scanziani M. A precritical period for plasticity in visual cortex. Curr Opin Neurobiol. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Demas J, Eglen SJ, Wong RO. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci. 2003;23:2851–2860. doi: 10.1523/JNEUROSCI.23-07-02851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- 39.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 40.Cancedda L, et al. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garaschuk O, Milos RI, Konnerth A. Targeted bulk-loading of fluorescent indicators for two-photon brain imaging in vivo. Nat Protoc. 2006;1:380–386. doi: 10.1038/nprot.2006.58. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, Calif.: Academic; 2001. [Google Scholar]
- 43.Valverde F. Golgi Atlas of the Postnatal Mouse Brain. Wien New York: Springer-Verlag; 1998. [Google Scholar]
- 44.Leybaert L, de Meyer A, Mabilde C, Sanderson MJ. A simple and practical method to acquire geometrically correct images with resonant scanning-based line scanning in a custom-built video-rate laser scanning microscope. J Microsc. 2005;219:133–140. doi: 10.1111/j.1365-2818.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 45.Garaschuk O, et al. Optical monitoring of brain function in vivo: From neurons to networks. Pflugers Arch. 2006;453:385–396. doi: 10.1007/s00424-006-0150-x. [DOI] [PubMed] [Google Scholar]
- 46.Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1:31–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.