Abstract
In 37 patients with ulcerative colitis (UC) and ileal pouch-anal anastomosis (IPAA) and 40 patients without IPAA fatigue was measured using the Short Form Gießen Subjective Complaints List GBB 24. Age, sex, disease activity (measured by the Colitis Activity Index CAI or the Pouch Disease Activity Index PDAI) and depressiveness (measured by the Hospital Anxiety and Depression Scale or the Center for Epidemiological Studies Depression Scale), active medical comorbidities and therapy by corticosteroids were tested for their predictive capacity regarding fatigue. One third of the patients indicated fatigue scores above the 95th percentile of the score of the general German population. The amount of fatigue in patients without IPAA was best predicted by the degree of depressiveness (corrected R2=0.30, β=0.57, p<0.001). The amount of fatigue in patients with IPAA was best predicted by the PDAI-score (corrected R2=0.20, β=1.35, p=0.03).
Keywords: fatigue, ulcerative colitis, ileal pouch-anal anastomosis, depressiveness, Inflammatory Bowel Disease Questionnaire
Abstract
Bei 37 Patienten mit Colitis ulcerosa (CU) und ileoanalem Pouch (IPAA) sowie 40 Patienten ohne IPAA wurde Fatigue mittels des Gießener Beschwerdebogens GBB 24 erfasst. Der prädiktive Wert von Alter, Geschlecht, Krankheitsaktivität (gemessen mittels des Colitis Activity Index CAI bzw. des Pouch Disease Activity Index PDAI), Depressivität (gemessen mittels der Deutschen Version der Krankenhaus Angst und Depression Skala HADS-D bzw. der Allgemeinen Depressionsskala ADS), aktiver medizinischer Komorbiditäten sowie aktueller Kortikosteroidtherapie wurden mittels einer multiplen Regressionsanalyse untersucht. Ein Drittel der Patienten gab Fatigue-Werte jenseits der 95%-Perzentile (im Vergleich zu einer repräsentativen Bevölkerungsstichprobe) an. Fatigue bei Patienten ohne IPAA wurde durch das Ausmaß der Depressivität (korrigiertes R2=0.30, β=0.57, p<0.001), bei Patienten mit IPAA durch den PDAI-Score (korrigiertes R2=0.20, β=1.35, p=0.03) vorhergesagt.
Introduction
Fatigue - defined as the subjective experience of reduced physical and/or mental energy/vitality - is a common symptom in the general population and in several somatic diseases such as chronic hepatitis C, multiple sclerosis, or in long-term survivors of cancer [1], [2], [3], [4], [5]. It is seen as well in patients affected by mental disorders such as depression [6]. "Fatigue" is a multidimensional construct with a cognitive, physical and mental/psychological dimension [2] which is nearly normally distributed in the general population [7]. Fatigue related to somatic diseases (chronic hepatitis C for example) had been reported to adversely affect functional capacity and work performance. Studies on its predictors in the general population and different diseases showed that fatigue is determined as well as by sociodemographic factors such as age und sex [7], psychological factors such as depressiveness [2], [3] and psychopharmacological treatment [6]. Although fatigue and depression are different constructs, there is a considerable overlap between them. To put it at its simpliest, a part of those who complain of fatigue will be depressed but nearly everyone with a major depression will also experience fatigue [2].
Fatigue has only recently gained the attention of gastroenterologists in quiescent inflammatory bowel disease (IBD). In a Dutch study more than 40% of IBD-patients in remission suffered from fatigue. 40% of the patients in remission suffered from a higher level of fatigue compared to age and sex matched healthy controls. No correlations could be found between fatigue, basal cortisol levels or other laboratory parameters. A correlation between fatigue and an impaired health related quality of life (HRQOL) was noted. The impact of fatigue on the social domains of HRQOL compared to other IBD-symptoms such as abdominal pain and diarrhea was not investigated. Patients with an ileostomy/ colostomy or an ileal pouch-anal anastomosis (IPAA) were not included in the study. The authors concluded that further research into the pathogenesis of fatigue in IBD is warranted [8].
The purpose of the present study was to extend previous findings of possible determinants of fatigue in IBD-outpatients in remission or with slight disease activity because somatic causes of fatigue such as severe anemia can be excluded in these patients. Because IBD-patients in remission and with slight disease activity are able to work, the relationship of fatigue with social functioning is of particular interest. The aims of our study thus were
- to compare the subjective burden of fatigue in UC-patients with the general German population by a validated self-report questionnaire,
- to compare the relative predictive value of fatigue on physical and social domains of HRQOL to that of bowel symptoms,
- to assess possible disease-related (disease activity, medication), psychological (depressiveness) and sociodemographic (age, sex) factors predicting fatigue in patients with quiescent and mild ulcerative colitis (UC) with and without IPAA.
Material and methods
Patients
The study was a secondary analysis of data on HRQOL gathered in studies validating the German version of the Inflammatory Bowel Disease Questionnaire (IBDQ) in patients with ulcerative colitis and ileal pouch-anal anastomosis (IPAA) and restorative proctocolectomy [9] and the German version of the Short - IBDQ (SIBDQ-D) in patients with UC and Crohn's disease (CD) [10]. Because in the study of Minderhoud and coworkers CD- and UC-patients differed in their levels of fatigue (8) we analysed only data of the UC-patients of the SIBDQ-D sample.
UC-patients with IPAA: Participants for this study were recruited in two ways. Members of the working group IPAA of the German Morbus Crohn/Colitis ulcerosa Foundation (DCCV), and patients with IPAA and UC who had been operated in the department of general surgery or had been treated in the medical IBD-outpatient department of the Universitätskliniken des Saarlandes (Germany) from January 1993 to December 2002 were contacted by a letter inviting them to take part in a study on HRQOL and pouchitis. The address lists of the DCCV included not only patients with IPAA but also IBD-patients without IPAA and relatives. Due to German laws on data protection a selective addressing of IPAA-patients was not possible. The patients of the DCCV had been operated on other surgical centers than the university of the Saarland. Inclusion criteria were age >18 years, UC in history confirmed by biopsy and an ability to read German. Patients who showed interest to take part in the study were contacted by a second letter scheduling the examination which included physical examination, endoscopy of the pouch and a set of questionnaires The questionnaires were to be answered 8 days before the examination.
UC-patients without IPAA: Consecutive outpatients of the gastroenterological department of the Virchow-Klinikum Charité in the Humboldt-University of Berlin were asked during regular outpatients visits to take part in the study. Inclusion criteria were age ≥18 years, IBD in history confirmed by biopsy and an ability to read German. The current medication and symptoms and the the medical history were assessed in an interview.
Disease activity scores and questionnaires
Disease activity in IPAA-patients was measured by the Pouchitis Activity Index (PDAI) [11]. To calculate the clinical subscore of the PDAI, patients answered the medical questionnaire of the German competence network "Chronic inflammatory bowel disease". It includes questions regarding the history of the IBD, current symptoms, current extraintestinal manifestations, present medication and also questions regarding IPAA [9]. In IPAA-patients endoscopy scores were calculated by endoscopists not aware of the patients' clinical history. No patient had an anastomotic stricture, pouch outlet obstruction, perianal abscess or external fistula. A single pathologist blinded to the clinical presentation and clinical findings assessed pouch biopsies for the grade of inflammation based on the criteria of Sandborn. Pouchitis was diagnosed if the Pouchitis Activity Index (PDAI) was ≥7 [11]. Disease activity was measured in UC by the Colitis Activity Index (CAI) [12]. Quiescent disease was defined by a CAI between 0-3 and slight disease activity by a CAI between 4-6. The clinical and laboratory data necessary to calculate the CAI were gathered from medical records.
The subscale "Fatigue" of the Short Form Giessen Subjective Complaints List GSCL was used to measure fatigue. With 24 items on a 5-point Likert scale ranging from 0 (no suffering from this symptom) to 4 (severe suffering from this symptom) bodily symptoms can be assessed in 4 subscales (fatigue, dyspeptic symptoms, musculoskeletal symptoms, cardio respiratory symptoms and a total score). Within the subscales scores range from 0-24 and in the total score from 0-144 with 0 indicating no suffering from bodily symptoms and 24, 144 respectively severe suffering from bodily symptoms. The GSCL has been developed and validated in German and proved to be reliable and valid [13]. We compared the fatigue-scores of the GSCL in the study sample with a representative sample (as to the distribution of age, sex and education) of the German population (N=1943 people) which was recruited 1994 by an independent service of surveys, methods and analyses [13].To allow comparisons with the study of Minderhoud and coworkers [8] we defined (exceptional) fatigue by a GSCL- fatigue score >10 (95 percentile in the representative sample of the German population).
HRQOL was measured in IPAA-patients by the German version of the IBDQ [9], [14] and in UC-patients by the German version of the SIBDQ [10], [15]. The (S)IBDQ is an IBD-specific questionnaire containing 32 (10) items with a graded response rate ranging from 1 (worst) to 7 (best) and a possible total score of 32-224 (10-70). The 32 (10) items can be arranged in four dimensional scores: Bowel symptoms 10 (3) items, systemic symptoms 5 (2) items (including fatigue), emotional well-being 12 (3) items and social functioning 5 (2) items [7], [16]. The Canadian (S)IBDQ has been translated to German by a forward-backward procedure and has undergone rigorous psychometric testing proving a good (sufficient) reliability and validity [9], [10]. To avoid interferences of symptoms with functioning the subscale "Social" of the (S)IBDQ not including somatic symptoms was used as dependent variable to assess the impact of fatigue compared to intestinal symptoms on the social domain of HRQOL. Additionally in IPAA-patients HRQOL was measured by the German version of the Medical Outcome Study Short-Form 36 (SF-36 Items). The SF-36 is a reliable and valid instrument measuring all domains of the health status. It covers 4 domains in the area of physical health (physical functioning, role limitation - physical, bodily pain and general health) and 4 domains in the area of mental health (role limitation - emotional, vitality, mental health and social functioning). Two comprehensive indexes of HRQOL can also be computed (physical component summary and mental component summary). The responses to the questions in each domain are added to provide eight scores between 0 and 100 with higher scores reflecting better HRQOL. The mean of the two comprehensive scores is 50 with a standard error of the mean (SEM) of 10 [16]. The SF-36 has been translated by a forward-backward procedure into German and the translated version proved to be reliable and valid. Data from a representative sample of the German population as well as from medically ill and mentally disordered populations are available [17]. To avoid interferences of symptoms with functioning the subscales "Physical and social functioning" of the SF-36 not including somatic symptoms were used as dependent variable to assess the impact of fatigue compared to intestinal symptoms on the physical and social functioning.
Psychological distress was measured by the Allgemeine Depressionskala (ADS) in UC-patients without IPAA and by the German version of the Hospital Anxiety and Depression Scale (HADS-D) in UC-patients with IPAA. The ADS is a German version of the Center for Epidemiological Studies Depression Scale (CES-D) [18], [19]. With 15 items (Short Form ADS-K) on a 4-point Likert scale ranging from 0 (rarely of the time) to 3 (most of the time) emotional, somatic and cognitive symptoms of depressive mood can be assessed. Respondents who score >17 on the total score are regarded as having a symptom severity of depression indicative of a probable psychological disorder. The ADS has been translated by forward-backward procedure into German and proved to be reliable and valid. Normative data of the German version ADS-(K) from a general German population as well as from large international medically ill populations are available [19]. The Hospital Anxiety and Depression Scale HADS-D was specifically designed for the assessment of anxiety and depression in patients with physical illness. With 7 items each on a 4-point Likert scale ranging from 0 (not present) to 3 (always present) a subscale score for the 2 subscales anxiety and depression can be calculated. Respondents who score ≥ 11 on either subscale are regarded as having a symptom severity of depression or anxiety indicative of a probable psychological disorder (i.e. clinical depression or anxiety disorder) [20]. The HADS-D has been translated by forward-backward procedure into German and proved to be reliable and valid [21]. Normative data of the validated German version HADS-D from a general German population as well as from large international medically ill populations are available [21], [22].
Statistical analysis
Up to one missing item of each scale of the questionnaires was replaced by the "individual" median of the items subscale (with the exception of the SIBDQ-D). If more than one item was missing the respective questionnaire was excluded form further analysis. All data were analysed using Winstat for Excel (Version 2001.1, R.Fitch Software, Staufen, Germany). Data derived from descriptive statistical analysis are presented in the form of percentages for categoric variables and for continuous data as mean ± one standard deviation (SD) and range and/or as median and interquartile ranges (25-75 percentiles). Most variables are not normally distributed. To allow comparisons with the results of other studies both mean and median are presented in the tables. Comparisons of variables between groups were performed by parametric (student's t-test) for comparisons with the data of the general population (because only mean and SD were available) and non-parametric tests (Mann-Whitney U test, Chi-Square test) for comparisons between the different groups of patients with UC. The level of significance was set at α<0.05 (two-sided) in case of single comparisons. Effect sizes were calculated by the method of Cohen [23].
In patients with IPAA stepwise multiple regression analyses with p in and out =0.05 were performed to assess the impact of fatigue (IBDQ Item 2) compared to other intestinal symptoms of IBD (Number of stools -IBDQ Item 1; Liquidity of stools - IBDQ Item 5; Abdominal cramps - IBDQ Item 9; Abdominal pain - IBDQ Item 13; Passing gas - IBDQ Item 17; Bloating - IBDQ Item 20; Rectal bleeding - IBDQ Item 22; Going to bathroom despite empty bowels - IBDQ Item 24; Soiling underwear - IBDQ Item 26) in the IBDQ subscales "Social" and the SF-36 subscales "Physical Functioning", "Physical Role Limitation" and "Social Functioning". In patients without IPAA the predictive value of fatigue (SIBDQ Item 1) compared to other intestinal symptoms of IBD (Abdominal pain - SIBDQ Item 4; Passing gas - SIBDQ Item 6; Going to bathroom despite empty bowels - SIBDQ Item 9) was tested with the SIBDQ subscale "Social" (SIBDQ Items 2 and 3) as dependent variables.
Stepwise multiple regression analyses with p in and out =0.05 were performed to identify variables predicting high scores on the subscale "Fatigue" of the GSCL. The following seven independent variables were entered into regression analysis for IPAA-patients:
- Medical variables of the disease: PDAI (because it seems to be rather arbitrary to distinguish silent from active pouchitis by a cut-off at seven points in the PDAI and because the intercorrelations of the clinical, endoscopic and histological score of the PDAI are nearly zero [24], we entered the PDAI as ordinal data); psychopharmacological treatment (dichotomous); number of medical comorbidities (ordinal data); current extraintestinal manifestations (dichotomous);
- Depressiveness: Total Score of the subscale "Depression" of the HADS- D (ordinal data);
- Sociodemographic variables: Age (ordinal data) and sex (dichotomous).
The following six independent variables were entered into regression analysis for UC-patients without IPAA:
- Medical variables of the bowel disease: CAI (ordinal data); treatment by corticosteroids (dichotomous); number of medical comorbidities (ordinal data)
- Depressiveness: Total Score of the ADS-K (ordinal data);
- Sociodemographic variables: Age (ordinal data) and sex.
Ethics
All patients gave their voluntary informed consent after full written explanation of the aims of the study, including considerations regarding ethics and data protection and the anonymous deposition of the questionnaires apart from the medical charts. The study was approved by the ethics committee of the board of physicians of the Saarland (Rec. 08/03) and the medical faculty of the Humboldt-University of Berlin (Rec. 107/99).
Results
To estimate the enrollment rate of IPAA-patients we supposed that 70 of the 280 members of the working group IPAA of the DCCV were IPAA-patients, the others being either members prior to surgical intervention or relatives. 45 of these 70 were interested in getting further information regarding the study. From 18 of these 70 patients (25.7%) a complete data set (endoscopy, questionnaires) was available. 34 of 61 (55.7%) patients of the university of the Saarland were interested in getting further information regarding the study. From 19 of these 34 patients (55.8%) a complete data set (endoscopy, questionnaires) was available. Altogether the estimated enrollment rate was 37/131 (28.9%). None of the included patients had to be excluded due to more than one missing item in any subscale of the questionnaires.
Out of the 142 consecutive patients with IBD (UC and CD) approached 125 (86.8%) agreed to participate in the study and completed questionnaires. 90/142 data sets were available for analysis (60.6%). 44 (48.9%) of the patients were diagnosed with ulcerative colitis (UC) and 46 (51.1%) with Crohn's disease (CD). Data of the patients with CD are not presented in this paper. Four UC-patients had to be excluded due to more than one missing item in at least one subscale of the questionnaires. Sociodemographic and medical data of the patients are presented in Table 1 (Tab. 1).
Table 1. Sociodemographic and medical data of the study population .
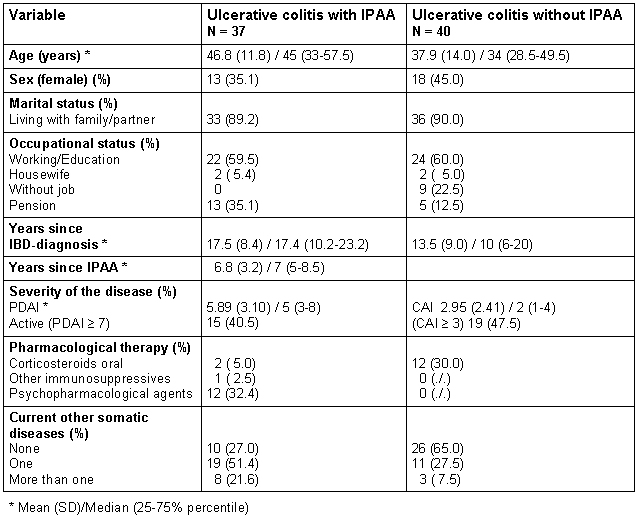
The fatigue-mean values ± one SD of the general German population and the study groups of UC-patients are presented in Figure 1 (Fig. 1). The median (25%-75% percentile; range) of the scale fatigue of the GSCL was 6.5 (2-12.25, 0-20) in patients with IPAA and 7 (3-13;0-22) in patients without IPAA. Only the mean ± one SD of the general German population (4.01±4.25) is available. Both groups of UC-patients complained about a higher degree of fatigue than the general German population (t=-7.32, a posteriori effect size =1.25 [with IPAA]; t=-5.07 , a posteriori effect size =0.92 [without IPAA]; both p's <0.001). 28.9% of the patients with IPAA and 33.3% of the patients without IPAA were above the 95% percentile of the score from the general German population. There were no significant differences in the level of fatigue or in the percentage of patients above the 95% percentile of the General German population between patients with and without IPAA (Chi2=0.13; p=0.71) The impact of fatigue in the physical and social functioning measured by the IBDQ-D and the SF-36 in patients with IPAA is shown in Table 2 (Tab. 2).
Figure 1. Fatigue measured by the Giessen Subjective Complaints List GSCL in the general German population and in patients with ulcerative colitis with and without ileal pouch-anal anastomosis (IPAA).
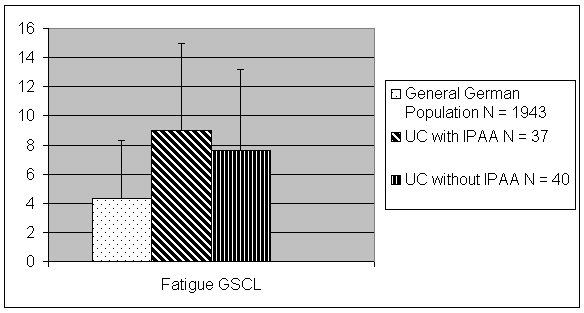
Table 2. Multiple stepwise regression analysing the predictive value of fatigue on the domains of physical and social functioning of health related quality of life in patients with ulcerative colitis and ileal pouch-anal anastomosis, comparing it to other gastrointestinal symptoms (measured by isolated items of the German version of the Inflammatory Bowel Disease Questionnaire IBDQ-D).
Independently from gastrointestinal symptoms, fatigue had a small, but significant additional predictive value for the physical functioning and the social functioning as measured by the SF-36 and the IBDQ-D. In patients without IPAA social functioning in the SIBDQ-D (items No 2 and 3) was predicted by abdominal pain (item No 4) (corrected R2=0.40, β=0.68, p=0.0007), "having to go to bathroom despite empty bowels" (item No 9) (corrected R2=0.55, β=0.62, p=0.002) and fatigue (item No 1) (corrected R2=0.61, β=0.45, p=0.01).
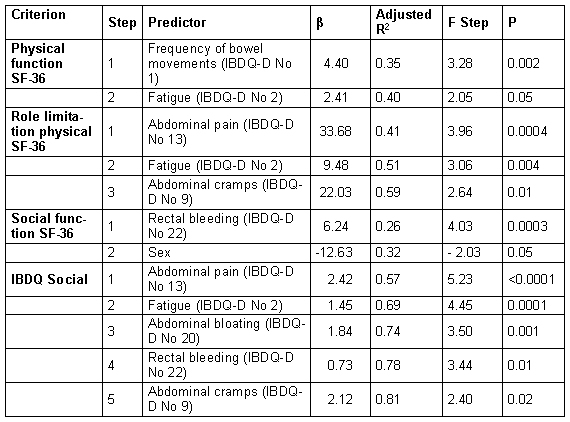
Using multiple stepwise regression analyses the amount of fatigue in IPAA-patients measured by the GSCL was predicted by the PDAI (corrected R2=0.20, β=1.35, p=0.03) and in patients without IPAA by the ADS- total score (corrected R2=0.30, β=0.57, p=0.0005), whereas the other variables had no significant predictive value (see Table 3 (Tab. 3)).
Table 3. Multiple stepwise regression identifying predictors of fatigue (measured by the Giessen Subjective Complaints list GSCL) in patients with ulcerative colitis with and without and ileal pouch-anal anastomosis.
Discussion
In this exploratory study the impact of fatigue on HRQOL in patients with ulcerative colitis was assessed and compared with the effect of bowel symptoms on HRQOL. Potential biopsychosocial predictors of fatigue in patients with UC were tested for. The main results of our study can be summarized as follows:
- Approximately one third of the UC-patients suffered from a level of fatigue higher than the one in the general population.
- Fatigue was experienced to be more disabling by IPAA-patients than most of the bowel symptoms.
- The level of disease activity could predict fatigue in IPAA-patients, depressiveness predicted fatigue-scores in patients without IPAA.
The 30% of fatigue-scores in individual patients surpassing the 95-percentile of the German norm in our study are lower than the 40% seen in the study of Minderhoud and coworkers [8]. The use of different instruments for the assessment of fatigue and different criteria for selecting the study-groups might account for that. In the German norm-sample nobody was excluded because of a given disease, whereas in the Dutch study only explicitly healthy people were taken as controls. In a recent study Björnsson and coworkers demonstrated a higher level of fatigue in Swedish IBD-patients compared to age- and sex-matched controls from the general population too without using percentiles [25].
In contrast to findings in the general German population [8], [13], age and sex had no influence on fatigue in our study. An age-related increase of fatigue in the general population starts with the age of 40 years and increases every decade [13]. Because most of our patients were between 30 and 50 years of age we might have missed to demonstrate an effect of age on fatigue in UC-patients. We did not find any differences between male and female patients neither in the level of fatigue nor in the level of depressiveness (data not presented). We were able to confirm our hypothesis on the predictive value of depressiveness on fatigue only in UC-patients without IPAA. Perhaps this finding is due to the fact that the rate of IPAA-patients taking psychopharmacological agents (35.3%) was higher than the one in the group without IPAA (6.5%), with a possible effect of a reduction of clinical depressiveness in our IPAA-patients. Yet we did not differentiate in the questionnaire or in the clinical interview between different types of psychopharmacological agents. The Swedish study also found depressiveness as a predictor of fatigue in patients with primary sclerosing cholangitis with and without IBD [25]. Only in IPAA-patients disease activity could significantly predict fatigue. This could be due to the different weight of subjectively experienced symptoms in the CAI and PDAI. One third of the score of the PDAI is defined by (four) rather subjective symptoms [11] whereas the CAI is composed by one such symptom and six objective findings [12]. If in the IPAA-patients only the endoscopic or histological scores of the PDAI would be entered into multiple regression analyses instead of the total score, we would have failed to demonstrate any predictive value of the PDAI for the subscales of the IBDQ-D. Therefore our data confirm the findings of Minderhoud and coworkers [8] that fatigue in UC-patients is (mainly) independent from objective disease measures in quiescent disease. Our findings lead to the assumption that this is true also for patients with mild disease activity.
Some limitations of our study must be considered: The total enrollment rate (37% for the whole sample) was rather low. Especially in the IPAA-sample a selection bias for motivated and active patients is possible. If this assumption were true, selection biases due to restriction of patients of tertiary centers would be counterbalanced . Nevertheless our data might not be representative for all patients with UC. We retrospectively compared data from two different studies that used partially different clinical and psychometric instruments. Any prospective study could provide results more reliably. Yet data exchange of two German groups working on HRQOL in IBD seemed to be appropriate for the explorative aims of the study. Although the investigators collecting the questionnaires were different (gastroenterologists in IPAA-patients, psychologists in the other group) we do not see a substantial risk of a measurement bias because in both settings standardized instructions for the patients to fill out the questionnaires were used. It would be more appropriate to compare the patients with Crohn's disease and ulcerative colitis and both IBD-diseases with subgroups of the general population which are matched for age, sex and job situation. Therefore the robustness of our exploratory findings must be tested by bigger sample sizes. Especially the role of disease activity taking into account other possible determinants of fatigue such as the course of the IBD (stable remission, rare or frequent flare ups and their former severity, chronic active disease), of somatic and psychiatric comorbidities and of pharmacological therapy (e.g. corticosteroids, antidepressants) deserves further research.
In sum, we could not only demonstrate a high prevalence of fatigue in patients with quiescent and slight UC- disease activity but also a high relevance of fatigue for HRQOL. Fatigue research in gastroenterology should be continued.
Acknowledgements
This investigation is part of the Competence Network IBD, sponsored by the German Ministry of Education and Research (BMBF D 20.00415).
References
- 1.Kroenke K, Mangelsdorf D. Common symptoms in ambulatory care: incidence, evaluation, therapy and outcome. Am J Med. 1989;86:262–265. doi: 10.1016/0002-9343(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 2.Wessely S, Pariante C. Fatigue, depression and chronic hepatitis C infection. Psychol Medicine. 2002;32:1–10. doi: 10.1017/s0033291701004615. [DOI] [PubMed] [Google Scholar]
- 3.Hassoun Z, Willems B, Deslaurires J, Nguyen BN, Huet PM. Assessment of fatigue in patients with chronic hepatitis C using the fatigue impact scale. Dig Dis Sci. 2002;27:2674–2681. doi: 10.1023/a:1021040702370. [DOI] [PubMed] [Google Scholar]
- 4.Fossa SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21:1249–1254. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 5.Flachendecker P, Kumpfel T, Kallmann B. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002;8:523–526. doi: 10.1191/1352458502ms839oa. [DOI] [PubMed] [Google Scholar]
- 6.Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64 Suppl 14:30–34. [PubMed] [Google Scholar]
- 7.Schwarz R. Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26:140–144. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 8.Minderhoud IM, Oldenburg B, van Dam PS, van Berge Henegouwen GP. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. Am J Gastroenterol. 2003;98:1088–1093. doi: 10.1111/j.1572-0241.2003.07414.x. [DOI] [PubMed] [Google Scholar]
- 9.Häuser W, Dietz N, Grandt D, Steder-Neukamm U, Janke KH, Stallmach A. Validation of a German Version of the Inflammatory Bowel Disease Questionnaire IBDQ-D in patients with ileal pouch anal anastomosis for ulcerative colitis. Z Gastroenterol. 2004;42:131–140. doi: 10.1055/s-2004-812835. [DOI] [PubMed] [Google Scholar]
- 10.Rose M, Fliege H, Hildebrandt M, Körber J, Arck P, Dignass A, Klapp B. Validation of the German version of the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) Z Gastroenterol. 2000;38:277–286. doi: 10.1055/s-2000-14868. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Tremaine WJ, Batts KP. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–415. doi: 10.1016/s0025-6196(12)61634-6. [DOI] [PubMed] [Google Scholar]
- 12.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. Brit J Med. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brähler E, Schumacher J, Brähler C. First standardisation of the Short version of the Giessen Subjective Complaints List GBB-24 in re-unified Germany. Psychother Med Psychol. 2000;50:14–21. doi: 10.1055/s-2000-13233. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt G, Mitchell A, Irvine EJ, Singer G, Williams N, Goodacre R, Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–810. [PubMed] [Google Scholar]
- 15.Irvine EJ, Zhou Q, Thompson K CCRPT Investigators. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 16.Ware JE., Jr . SF-36 Health Survey. Manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 17.Bullinger M, Kirchberger I. SF 36 Health Survey. Handbook. Göttingen: Hogrefe; 1998. [Google Scholar]
- 18.Radloff LS. The CES-D Scale. A self-report depression-scale for research in the general population. Appl Psychol Measurement. 1997;1:385–392. [Google Scholar]
- 19.Hautzinger M, Bailer M. Allgemeine Depressionsskala ADS. Weinheim: Beltz; 1995. [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann C, Buss U, Snaith RP. HADS-D: Hospital Anxiety and Depressions Scale - D German Version. Bern: Hans Huber; 1995. [Google Scholar]
- 22.Hinz A, Schwarz R. Anxiety and depression in the general population. Standardised values of the hospital anxiety and depression scale. Psychother Psychosom med Psychol. 2001;51:193–200. doi: 10.1055/s-2001-13279. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioural sciences. New York: Academic Press; 1988. [Google Scholar]
- 24.Shen B, Achkar JP, Lashner BA, Ormby AH, Remzi FH, Bevins CL, Brzezinski A, Petras RE, Fazio VW. Endoscopic and histological evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001;121:261–267. doi: 10.1053/gast.2001.26290. [DOI] [PubMed] [Google Scholar]
- 25.Björnsson E, Simren M, Olsson R, Chapman RW. Fatigue in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2004;39:961–968. doi: 10.1080/00365520410003434. [DOI] [PubMed] [Google Scholar]


