Abstract
Objectives: Currently there is a lack of information regarding the health-related quality of life (HRQoL) of healthy as well as chronically ill children on a European level. In 2 European projects questionnaires for the assessment of the HRQoL in children and adolescents have been developed: The KIDSCREEN project aims at a co-operative European development of a standardised screening instrument for children's HRQoL for the implementation in representative national and European health surveys. In the DISABKIDS project a chronic-generic questionnaire as well as modules for specific conditions (e.g. asthma) were developed for children with chronic conditions.
Methods: Both projects shared similar steps: 1. Development phase, in which the items were generated and tested, 2. Survey and Field phase, in which the modified questionnaires were tested with healthy and chronically ill children and adolescents in national representative surveys, and 3. Implementation phase in national health surveys or clinical studies.
Results: In the sister projects, the KIDSCREEN instrument was tested in 22,830 children and the DISABKIDS instrument in 1605 chronically ill children. The current paper describes the development and pilot-testing as well as psychometric results of the field tests of both studies.
Conclusion: The KIDSCREEN/DISABKIDS instruments make it possible to assess generic, chronic-generic and disease-specific aspects of quality of life in children and present an innovative approach to intercultural HRQoL assessment in health research.
Keywords: KIDSCREEN, DISABKIDS, health-related quality of life in children and adolescents, children with chronic condition
Abstract
Zielsetzung: Auf europäischer Ebene mangelt es an Angaben über die subjektive Gesundheit von gesunden und chronisch kranken Kindern, auch weil geeignete Messinstrumente fehlen. In zwei europäischen Forschungsprojekten wurden Fragebögen zur Erfassung der subjektiven Gesundheit von Kindern entwickelt: Im KIDSCREEN Projekt wurde ein populationsbasiertes Instrumentarium für Kinder und Jugendliche für die epidemiologische Forschung entwickelt. Für Kinder mit chronischen Erkrankungen wurden im DISABKIDS Projekt ein krankheitsbezogener Kernfragebogen und für einzelne Krankheitsbilder (z.B. Asthma) krankheitsspezifische Module erarbeitet.
Methodik: Beide Projekte kooperierten eng in der 1. Entwicklungsphase mit Zusammenstellung der Fragen und deren Prüfung in nationalen Pilottests, in der 2. Feldphase, in der die modifizierten Fragebögen an repräsentativen nationalen Stichproben bzw. an großen Gruppen gesunder und chronisch-kranker Kinder und Jugendlicher getestet wurden und in der 3. Implementationsphase, in der die Instrumente in Gesundheitssurveys und in klinischen Studien genutzt werden.
Ergebnisse: Im Rahmen dieser „Schwesterprojekte“ wurde das KIDSCREEN Instrumentarium an 22.830 Kindern geprüft, und die DISABKIDS Instrumente an 1605 chronisch kranken Kindern geprüft. Der vorliegende Artikel beschreibt die Entwicklungsschritte der beiden Instrumentarien sowie Ergebnisse aus der psychometrischen Testung der Instrumente in den Feldphasen.
Fazit: Die DISABKIDS/KIDSCREEN-Instrumentenfamilie ermöglicht die generische, krankheitsbezogene und krankheitsspezifische Erfassung der Lebensqualität von Kindern und stellt einen innovativen Weg der interkulturellen Messung von Lebensqualität in der Gesundheitsforschung dar.
Introduction
Within the last decades health-related quality of life (HRQoL) has become increasingly important in epidemiological research, e.g. in representative health surveys, as well as in clinical studies, e.g. clinical trials. The subjective information about health status is based on the individual’s perception of health that ought to include physical, psychological and social dimensions of well-being and functioning, as has already been claimed by the World Health Organisation (WHO) in 1948 [1]. That information, easily accessible by simply questioning a person, is very valuable as it can predict objectively measurable health-related outcomes and, therefore, represents a meaningful indicator for health [2].
Until now, instruments – applied for reporting health situation on both national and international levels, and as a criterion for evaluation in the clinical context – have usually been developed exclusively for adults. In striking contrast only a few European countries have developed questionnaires to assess the HRQoL of children and adolescents. These instruments either aimed at generic or disease-specific information.
With regard to the political and social development towards a united Europe, the description and analysis of children’s and adolescent’s health on a European level becomes more important. However, presently there is not sufficient data providing information about the subjective health of children and adolescents in different European countries. In addition, such data ought to examine the influence of socio-economic factors as well as further determinants of health (e.g. risk behaviour) on the subjective evaluation of children and adolescents. This would then allow an early identification of groups at risk. This information has not only yet to be gathered for healthy, but also for children and adolescents with a chronic disease or disability.
The measuring of HRQoL, especially for these chronically ill children, is of importance not only for describing their actual situation and their health care needs, but also for evaluating clinical interventions.
Acquisition of such data requires a simultaneous development of questionnaires to measure subjective quality of life for healthy, chronically ill and disabled children and adolescents on a European level. Two European research projects took on that task: the KIDSCREEN and the DISABKIDS project. The KIDSCREEN project aims at developing instruments (short and long versions) – in European collaborations – to measure HRQoL of healthy children and adolescents as well as testing and implementing these instruments in health surveys [3]. The DISABKIDS project, on the other hand, focuses on the development of disease-specific research instruments to measure the subjective health of children and adolescents with chronic illnesses [4]. Both research projects were funded by the EU within the 5th Framework-Program "Quality of Life and Management of Living Resources" of the European Union over a period of three years.
Methods
The projects, both closely co-operating, passed comparable phases in the design of the questionnaires. In the Development phase extensive literature research, questioning of experts (Delphi method) [5] and European-wide focus groups with children [6] have been carried out in order to identify dimensions and items of quality of life, that have thereafter been compiled into a questionnaire. Following international guidelines the questionnaires were then translated into the languages of the participating countries and subsequently tested and modified in a pilot study in each of these countries. In the Field Test phase the questionnaires were applied in national representative health surveys with children and adolescents (KIDSCREEN) [7] or administered in groups of children and adolescents with chronic diseases or disabilities (DISABKIDS) to examine their psychometric characteristics.
In the Implementation phase the KIDSCREEN questionnaire has been included in representative health surveys and health reporting on national level [8]. The DISABKIDS instrument has been evaluated by including it in clinical studies in the participating countries. At the same time user-friendly instructions and manuals have been published and made available online for both the public and experts.
The projects aimed at developing a system of instruments to measure quality of life in order to provide healthy children and adolescents, and those with chronic diseases as well as their parents and families with the possibility to express their needs.
What is special about these projects is the inter-cultural perspective, the modular system to gain quality of life data as well as the combination of specific and generic aspects spanning a wide range of age from the parents’ as well as the children’s point of view.
The specific project goal was the development of a standardised questionnaire to assess the quality of life for children and adolescents with and without chronic health limitations. The collaboration with several countries and, therefore, languages in the development allows multi-national European studies of quality of life, including the patients’ perspective by considering their needs.
Conceptually both projects are based on a definition of quality of life that focuses on mental, social and physical components. Furthermore, the KIDSCREEN project contributes to health reporting, whilst the DISABKIDS project makes a contribution for improving disease-related research, specifically in clinical studies with chronically ill children (chronic-generic module) and even more so by including specific modules for special diseases (disease-specific modules).
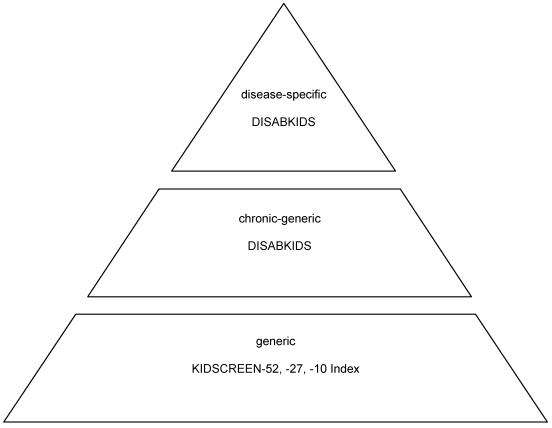
In summary, what makes the whole system so valuable, is the possibility to apply generic (KIDSCREEN) as well as disease-specific (chronic-generic, DISABKIDS) modules that can easily be combined. That way, all levels of quality of life can be measured, from generic and chronic-generic to disease-specific by using different methods for different age groups as well as from different perspectives (cf. Figure 1).
Figure 1. Structure of the KIDSCREEN/DISABKIDS family of instruments.
A similar system of modular assessment for adults is the FACIT (Functional Assessment of Chronic Illness Therapy) [9]. This family of instruments encompasses a) generic CORE questionnaires, b) Cancer-Specific Measures, c) Cancer-Specific Symptom Indices, d) Treatment-Specific Measures, e) Symptom-Specific Measures and f) non Cancer-Specific Measures. Different studies have confirmed the validity and usability of that modular system (http://www.facit.org/).
Results
The European KIDSCREEN project
Development Phase
The KIDSCREEN project (Screening for and Promotion of HRQoL in Children and Adolescents – a European Public Health Perspective) [3], aimed at developing a standardised and population-based instrument (short and long versions) for epidemiological research to measure subjective health of children and adolescents between 8-18 years of age as well as their families. At the same time a parent version is being supplied, in which parents rate their children’s quality of life. Applying that instrument in representative national as well as European health surveys should enable an identification of risk groups with possibilities for early interventions.
The KIDSCREEN project started in January 2001 in collaboration with seven European countries: Austria, Germany, France, the Netherlands, Spain, Switzerland and Great Britain. In the meantime three candidates for the European Union – the Czech Republic, Hungary and Poland – as well as Greece, Ireland and Sweden joined the project.
In the development phase of the questionnaire the theoretical concept, the theoretically relevant dimensions and the items were drawn from literature research, extensive interviewing of experts on a European level (Delphi method) [5] as well as European-wide focus groups with children.
Focus groups are discussion rounds with a half-structured interview guideline, in which perception and attitudes of children and adolescents towards relevant aspects of health and disease are being discussed.
These discussions have been recorded and transliterated. With regard to the principles of qualitative research, items for the questionnaires were drawn and formulated on the basis of the children’s contributions to the discussion [6]. This preliminary questionnaire was then – according to international guidelines – translated into the respective languages of the participating countries. All individual items were translated forward and backward, followed by a harmonisation session in which the items were further discussed by the relevant countries. This approach ensures the consistency of all relevant criteria, namely conceptual clarity, colloquial wording and significant meaning.
The questionnaire was tested in pilot studies in schools with about 300 to 400 children and adolescents and their parents in each country (N=3019) in combination with several other questionnaires about determinants of health (socio-demographic data, health status, use of the health care system).
Extensive psychometric analyses that applied methods of the classical test theory as well as the item response theory resulted in a pilot questionnaire with 52 items in 10 dimensions with a solid reliability and acceptance of the questionnaire [7].
The 10 dimensions include Physical Well-Being, Psychological Well-Being, Moods & Emotions, Self-Perception, Autonomy, Parent Relation & Home Life, Peers & Social Support, School Environment, Bullying, Financial Resources, as well as perceived financial resources. Depending on the item wording, answers are to be given on a 5-point frequency- (never-seldom-sometimes-often-always) or intensity scale (not at all-slightly-moderately-very-extremely). In addition to the long version (KIDSCREEN-52) two further versions have been developed: a 27 item short version, covering 5 dimensions (KIDSCREEN-27) as well as a 10 item index version (KIDSCREEN-10 Index). The results of the pilot study revealed that the questionnaire allowed the identification of risk groups [6], [10].
Children and adolescents that rate the family’s wealth as low, also rate their subjective health significantly lower compared to children and adolescents that state a high wealth level of their family. According to the results, the KIDSCREEN pilot questionnaire not only satisfyingly complies to the test’s theoretical criteria of quality (Cronbach’s alpha = 0.77-0.88), but is also practicable and allows to differentiate between risk groups and, hence, meets the requirements of the project.
Field Test Phase
Alongside with further concepts of health determinants, the generic KIDSCREEN-52-questionnaire for children and adolescents between 8 to 18 years of age was tested in a representative survey, administered via paper-pencil questionnaire or by a telephone interview to over 22,830 children and adolescents all over Europe. The aim was not only to perform psychometric analysis but also to conduct national and European-wide intercultural comparisons.
The KIDSCREEN-52 questionnaire was tested in 13 European countries. Six of these countries used computer-assisted telephone interviews to draw their sample and then sent out the questionnaires (AT, DE, CH, ES, FR, NL). Five countries applied school-based sample draws and data collection (EL, HU, IE, PL, SE). Great Britain combined both methods of telephone and school sampling, whilst the Czech Republic randomly drew their sample on both communal and household levels. In addition to the KIDSCREEN-52 all children and parents completed further surveys, such as the Pediatric Quality of Life Questionnaire (PedsQoL) [11], a HRQoL measure consisting of 23 items in 4 scales (Physical, Emotional, Social, and School) and the Satisfaction domain of the Child Health and Illness Profile-Adolescent Edition (CHIP-AE), a generic measure of health status [12]. In addition, the Youth Quality of Life Instrument - Surveillance version (YQOL-S), a 13-item self-administered generic quality of life (QoL) questionnaire designed to assess quality of life among adolescents [13], was administered. Furthermore, the Children with Special Health Care Needs Screener (CSHCN) was included to identify children with impaired health conditions. The CSHCN contains five sequences of questions: each main question is followed by two additional questions pertaining to the presence and duration of any health conditions. The five questions address the use of or need for prescription medication; the use of or need for medical, mental health or educational services; functional limitations; use of and need for specialized therapies (for example occupational therapy, physiotherapy, speech therapy); and treatment or counseling for emotional or developmental problems [14], [15].
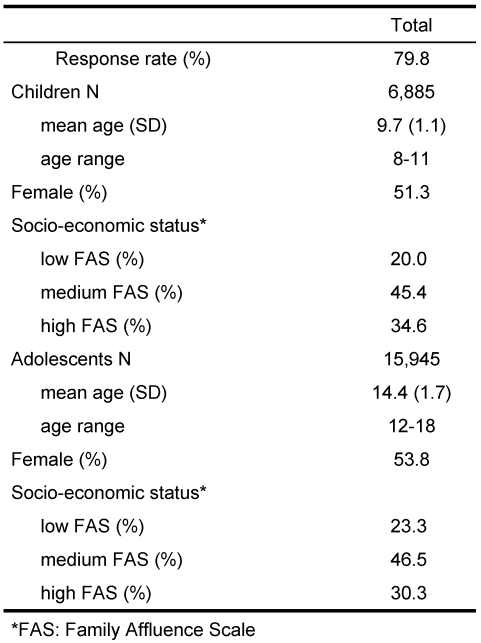
Table 1 displays the socio-demographic information about the 22,830 children and adolescents.
Table 1. KIDSCREEN-52: Sample characteristics .
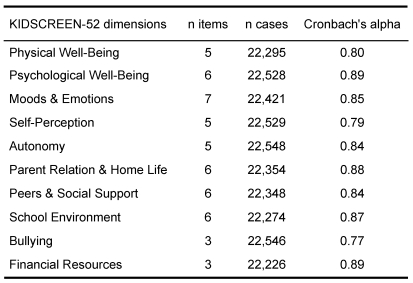
Statistical analyses mainly focused on first results about reliability and validity for the complete data set. The psychometric analyses revealed good to excellent Cronbach’s alpha for all ten subscales of the KIDSCREEN (cf. Table 2).
Table 2. KIDSCREEN-52: Reliability (Cronbach's alpha).
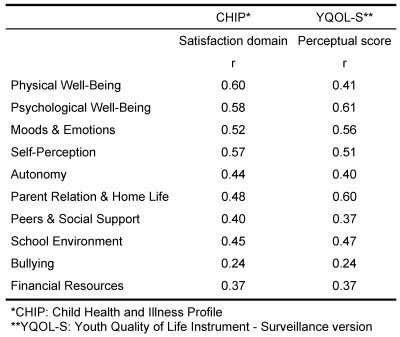
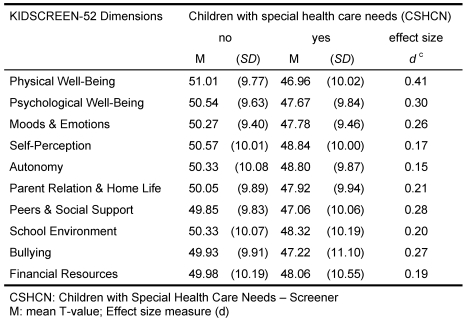
Tests for convergent validity of the KIDSCREEN showed moderate correlations with the subscales of the YQOL [13] and of the CHIP-AE [12] (cf. Table 3). The KIDSCREEN-52 is able to differentiate between children with and without health problems. Children and adolescents that were rated chronically ill by their parents on a screening instrument (Children with Special Health Care Needs CSHCN-Screener) [14], [15], ascribed themselves a lower HRQoL than healthy children and adolescents (cf. Table 4). Thus the known-groups validity of the instrument could be demonstrated.
Table 3. KIDSCREEN-52: Convergent Validity.
Table 4. KIDSCREEN-52: Known Groups Validity .
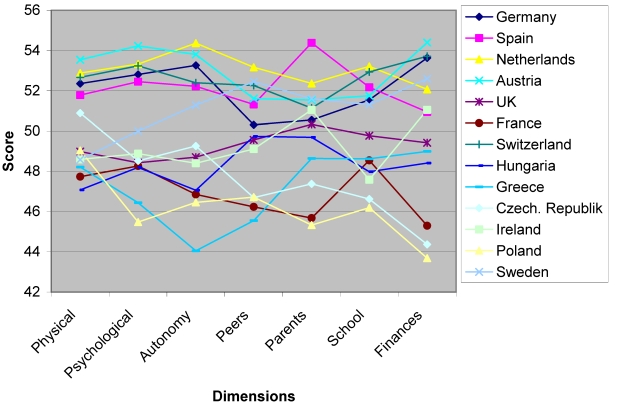
The differentiation of the KIDSCREEN across the countries was confirmed in the field test: applying data profiles across nations (statistically controlling for age & gender by including these aspects as covariates in the ANCOVA and adjusting the results for their mean level/prevalence) revealed differences in the overall evaluation of quality of life. The intercultural comparison indicated that children and adolescents of all participating countries very similarly rate their quality of life on different dimensions, except for French children and adolescents (cf. Figure 2).
Figure 2. KIDSCREEN-52: Country differences in different dimensions.
Currently, further analyses of socio-demographic and psychological determinants of quality of life on a national and international level are being undertaken.
The results of the field test contribute to the development of a theoretical framework model of subjective health by showing the influence of several determinants. Such a model could provide the theoretical base to identify risk groups as a practical application as well as suggestions for prevention and intervention programs. A set of European and national reference data will be supplied.
In the implementation phase the questionnaire, a user’s guide and manuals will be made available for the public via modern communication technology. The inclusion into the health reporting is also one goal. Specific Information about the KIDSCREEN questionnaires and their analysis can be obtained online (http://www.kidscreen.de) as well as in the recent published KIDSCREEN manual including a CD-ROM [16].
The European DISABKIDS project
Development phase
The DISABKIDS project (Quality of Life in Children and Adolescents with Disabilities and their Families – Assessing Patient Views and Patient Needs for Comprehensive Care) was coordinated by the study centre, based at the University Hospital Hamburg-Eppendorf, in collaboration with seven European countries (Germany, Austria, France, Great Britain, Greece, the Netherlands and Sweden). The project sample consisted of children and adolescents in the age groups 4-7 years, 8-12 years and 13-16 years with asthma, atopic dermatitis, diabetes mellitus, cystic fibrosis, epilepsy, juvenile chronic arthritis and cerebral palsy [4], [17].
One of the main project goals was to develop a disease-spanning questionnaire that allows a comparison of children and adolescents with different diseases and disabilities. Therefore, disease-specific modules were designed to allow the comparison within a disease condition. A chronic-generic module was designed to examine the interventions for specific diseases and across all disease groups.
As carried out for the KIDSCREEN project, the phase of developing items and dimensions for DISABKIDS applied a composition of methods such as questioning of experts, literature research and focus groups consisting of children with specific chronic diseases and their parents in particular. Here, the focus was not only placed upon the children’s quality of life, but also upon the strains and health care needs of the family. The results from the focus groups revealed a classification of the children’s statements into three categories: (1) generic, (2) disease-related and (3) disease-specific statements about quality of life. Further statements were identified as related to disease coping, health care needs, satisfaction with medical care as well as family’s distress by the diseases.
In the DISABKIDS project these steps include first of all a literature research and secondly focus groups with three to six children in all participating countries, classified by age, type and severity of the disease, and socio-demographic information, respectively. Based on the statements issued from the focus groups, items for the questionnaire were created.
The development of items was based on the collection of 3700 statements from the focus groups. 119 chronic-generic and 21 disease-specific items for each module were finally selected. For the next step all of the items were translated forward and backward. In a harmonisation session between the different countries an agreement was reached to guarantee the relevant criteria, conceptual clarity, colloquial wording and significant meaning.
For the pilot study a total of 360 children and adolescents between 8 to 16 years with the above mentioned diseases, as well as their parents were questioned. An additional 147 children between 4 to 7 years were tested with an age-specific version that used smiley items. Comprehensive psychometric analyses resulted in the final questionnaire with six dimensions and 53 items: Emotions, Physical Well-Being, Medication, Social Exclusion, Social Inclusion and Independence [18]. Furthermore, seven short disease-specific instruments with each 1-2 subscales were developed, e.g. for asthma. The psychometric results led to a revision of the questionnaire that was then applied in the field test with 1606 children in the age groups 4-7, 8-12 and 13-16 years, respectively.
Field Test Phase
Methodically the DISABKIDS project was characterized by including children with asthma in all participating countries. In addition, each country further included one of the following groups into their testing: cystic fibrosis, epilepsy, cerebral palsy, diabetes mellitus, juvenile arthritis as well as atopic dermatitis [19].
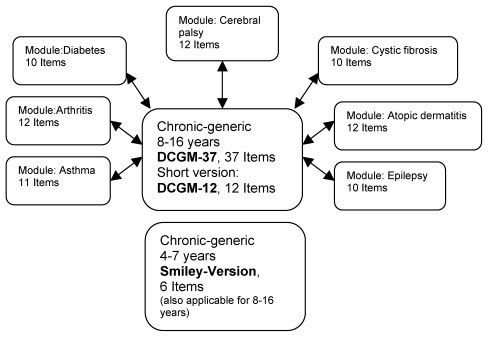
The DISABKIDS instruments are a group of different questionnaire versions whose core piece is the 37-item DISABKIDS Chronic-Generic Module (DCGM-37). This chronic-generic version in its self-report and proxy form is also available as a short version with 12 items for age groups of 8-16 years. Furthermore, a smiley version to assess self-reported quality of life in young children (4-6 years) was developed (as a self-reporting version as well as a version for proxy report). These 6 item tools could also be carried on into the age groups of 6-18 years.
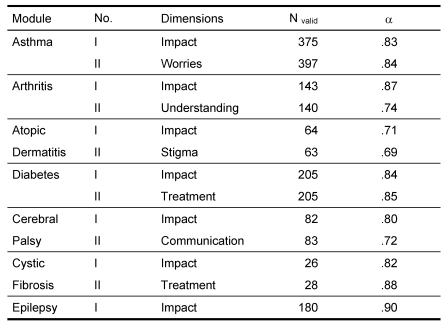
The following disease-specific modules are also available: asthma (11 items), juvenile chronic arthritis (12 items), atopic dermatitis (12 items), diabetes mellitus (10 items), cerebral palsy (12 items), cystic fibrosis (10 items), and epilepsy (10 items). An overview of all instruments is given in Figure 3.
Figure 3. The DISABKIDS Instrument .
Of all different questionnaires the 37-item DISABKIDS chronic-generic module will further be explicated below.
This instrument is available as a self-reporting version (child-version) and as a parent version (proxy-version), in the languages German, English, Dutch, French, Greek and Swedish. The questionnaire can be applied as a computer-assisted version as well as a paper-pencil version.
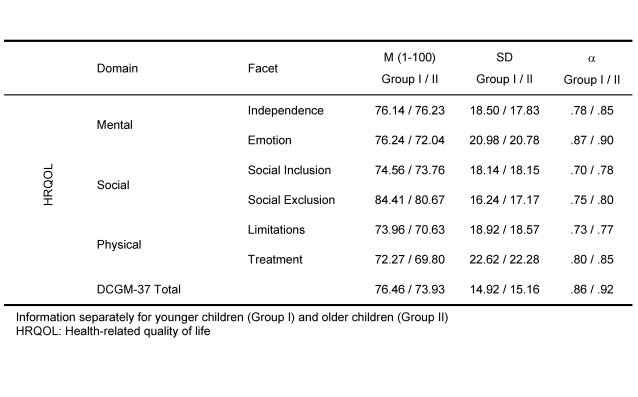
The DISABKIDS chronic-generic module (DCGM-37) consists of 37 Likert-scaled items assigned to six dimensions (Independence, Emotion, Social inclusion, Social exclusion, Limitation, and Treatment).
Pilot test results denoted satisfactory internal consistencies of all scales, ranging from α=.79 (Social Inclusion) to α=.90 (Emotion) [18]. The sub-scales of these six dimensions of the DCGM-37 can be combined to produce a general score for HRQoL – the DCGM-37 Total Score. Furthermore, all sub-scales are associated with three domains, namely mental, social and physical health. All of the subscales of the DISABKIDS DCGM-37 consist of six items, except for the emotion scale containing seven items. On the mental level independence, confidence regarding the future and emotions constitute emotion-related concerns and problems due to the disease. On the social level the social inclusion refers to the understanding by others, positive social relationships, whereas the social exclusion dimension is represented by the stigma and the feeling of being excluded. The physical level summarises functional limitations, treatment perspectives and the perceived influence of treatment options because of the disease.
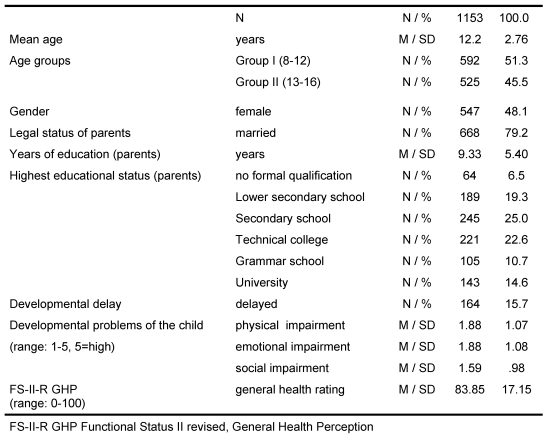
All psychometric results of the DISABKIDS field test are presented for groups of children between 8-16 years (n=1128), young children not included (4-7 years, n=453) (cf. Table 5).
Table 5. DISABKIDS: Sample characteristics.
The group of children with asthma is the biggest (405), followed by children with diabetes (204), epilepsy (181) and arthritis (148).
Table 6 gives an overview of reliabilities of the DGCM-37 children self-report scales showing values above .70, acceptable for group comparison.
Table 6. DISABKIDS DCGM-37: Score characteristics and reliability (Cronbach's alpha) .
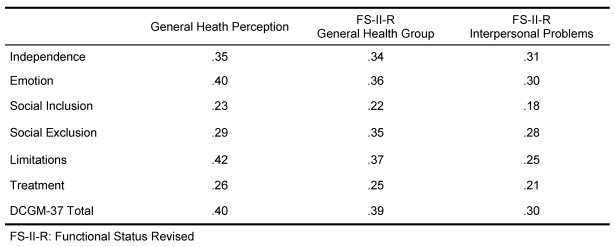
Correlations of the DISABKIDS instruments with other questionnaires indicate the convergent validity, considering that the correlations with the functional health status as well as the General Health and Interpersonal Functioning Index are high (cf. FS-II-R GHP; Table 7).
Table 7. DISABKIDS: Convergent Validity Pearson correlation coefficients.
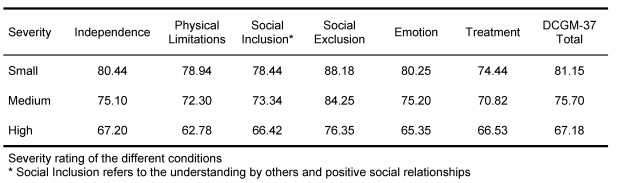
Measured differences in mean scale-levels between diseases and their severity degrees showed the known group validity. The grouping of children according to three severity degrees (mild, moderate, severe) proves the ability of the DISABKIDS chronic-generic module to differentiate these severity degrees from one another (cf. Table 8).
Table 8. DISABKIDS: Known Groups Validity (Severity rating of disease) .
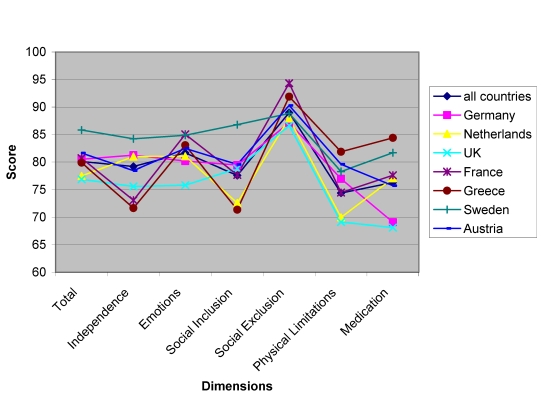
The instrument also showed cross-cultural validity [20]. Figure 4 shows cross-cultural differences in the DGCM-37 for children with asthma.
Figure 4. DISABKIDS: Country differences in DCGM-37 dimensions, asthma sub-sample, 8-16 years.
The analysis of the disease-specific modules also revealed appropriate reliabilities (cf. Table 9).
Table 9. DISABKIDS: Reliability disease-specific modules .
Specific information about the DISABKIDS questionnaires and their analysis can be obtained online (http://www.disabkids.de) as well as in the recent published DISABKIDS Manual including a CD-ROM [21].
Discussion
The special strength of the KIDSCREEN project is the representative data collection and the inclusion of socio-demographic and psycho-social determinants. Within the DISABKIDS study a particular focus was placed upon the implementation in the clinical-pediatric setting. This required not only alignment of the HRQoL instruments towards chronic diseases, but also the precise measurement of clinical severity degrees and of the impact regarding the care and support situation in order to identify the influence of the factors pertaining to the quality of life. Therefore, methods assessing severity degrees of the diseases in different languages were developed and applied, for clinical staff as well as the parents. Clinical parameters assigning the severity degrees were specifically developed for each of the diseases. Furthermore, generic indicators were also applied.
In addition, the care and support requirements of the families were measured. Since existing instruments for quality management only insufficiently consider the perspective of families with children with special care and support requirements [22], it is of prime importance to further analyse these in particular. With regard to existing health services, the perceived quality of treatment processes as well as the evaluation of the treatment results is of interest.
The instrument contained the following components (1) care and support requirements, (2) access to supply, (3) claiming care and support services (medical and non-medical), (4) evaluation of the care and support quality, and (5) the satisfaction with the care and support.
Altogether, the international project perspective in the DISABKIDS project allows a comparison of the quality of life of children with chronic diseases. Additional information is available about the perceived care and support requirements as well as the perceived treatment quality against the background of very different national health systems.
Within the DISABKIDS study an instrument to assess the children’s and adolescent’s coping with the disease was developed in the pilot study and tested in the field study. This approach was applied to add further information to the well-discussed relation between quality of life, coping and supply (Coping with Disease CODI) [23]. An essential question of the field test was concerned with the verification as to what extent the KIDSCREEN generic instrument, the chronic-generic module and the disease-specific modules can identify children’s quality of life. Therefore, both projects each included the questionnaire from the other study in the field test to allow examination of disease-specific and generic aspects of children’s health within both studies.
Conclusions
Questionnaires and data that describe differences between subjective health of children and adolescents across several European countries and that examine their causes are still insufficient. The analysis of determinants that influence health is of great importance for the identification of risk groups. Furthermore, it can serve as a source for prevention programs and interventions. Although there are several questionnaires that have been translated from one language into another and are internationally available, such as the KINDL [24] or the PEDSQOL [11], these questionnaires are mainly based on a so-called sequential development.
Simultaneous developments (i.e. concurrent and cooperative international efforts) to assess the quality of life in children and adolescents had yet not been carried out, which highlights why the sister projects DISABKIDS/KIDSCREEN present a special improvement. The specific challenges of intercultural development of questionnaires have recently been recognised [25], and will be targeted in future studies The European KIDSCREEN project, which developed generic questionnaires to assess quality of life by children and adolescents as well as their parents, can help answering these questions on a population level.
Results of the pilot study have already revealed differences in the health-related quality of life in children and adolescents from different nations and identified risk groups. After implementing the surveys in the field test final, questionnaire versions (KIDSCREEN-52, -27 und -10 Index) as well as differentiated results on a European level are finally available.
What still needs to be obtained, is information about HRQoL of children and adolescents with chronic diseases and disabilities. Such data, supplemented by information about coping on the level of individual resources, information about severity degrees of the diseases and information about supply requirements of the families on the level of structural resources, is required in order to optimise the supply offers in Europe.
The DISABKIDS project provides such data. Within this project an instrument was developed that contains a disease-specific core questionnaire (as long, short and smiley version), disease-specific modules as well as questionnaires assessing severity degrees, supply requirements of the families as well as coping strategies [23].
The field test indicates that the questionnaires, producing satisfying psychometric results, are being accepted by the families. After implementing the questionnaires in longitudinal studies, it will be possible to not only examine the sensitivity of changes of the DISABKIDS instrument, but also the influence of therapeutic interventions.
Hence, the KIDSCREEN and DISABKIDS projects, funded by the European Union, are the first of their kind to have simultaneously developed and tested a modular designed, flexible system for the assessment of quality of life of children and adolescents with chronic diseases – on an intercultural level.
Our results reveal that test-theoretical characteristics of reliability and validity for both instruments are satisfying. Furthermore, combining the DISABKIDS instruments with the KIDSCREEN instrument, that represents a generic method to measure quality of life in children and adolescents, provides an interesting opportunity to assess generic, diseases-generic as well as disease-specific aspects of quality of life.
The similarity in implementation, methodological development and the careful consideration of the intercultural perspective between both projects during item construction and data analyses, represent an innovative contribution to health assessment.
In the course of increasing internationalisation of research and the development of chronic diseases of children and adolescents (on a national level often with prevalence rates too low to allow for providing a reference to international study results) the DISABKIDS instrument offers interesting opportunities to assess quality of life in different countries.
On the other hand, the KIDSCREEN provides a generic instrument that can be applied in multinational epidemiological and clinical studies.
What remains is the hope that offering instruments to measure the quality of life on an international level will increase the chance of including it as an outcome in clinical studies or surveys. Preliminary results of the two projects described here, lay the necessary foundation for application of these instruments in the future by showing their benefits and potential. Hence, it would be highly desirable and important to apply these instruments in research and clinical settings in the time to come.
Notes
Conflicts of interest
None declared.
Acknowledgement
This paper has been written by the members of the German study centres and the European KIDSCREEN* und DISABKIDS** Groups.
The KIDSCREEN Project (Projectnumber QLG4-CT-2000-00751, Co-ordinator: Ulrike Ravens-Sieberer) and the DISABKIDS Project (Projectnumber QLG5-CT-2000-00716, Co-ordinator Monika Bullinger) have been funded by the European Commision.
*European KIDSCREEN Group: Thomas Abel, Jordi Alonso, Anna Aszman, Clare Atherton, Pascal Auquier, Marta Aymerich, Monika Bauer, Silvina Berra, Corinna Bisegger, Jeanet Bruil, Stef van Buuren, Bernhard Cloetta, Ladislav Czemy, Agnes Czimbalmos, Symone Detmar, Christina Dimitrakaki, Wolfgang Duer, Michael Erhart, Claudia Farley, Eimear Flannery, Kristina Fuerth, Angela Gosch, Curt Hagquist, Michael Herdman, Jennifer Nickel, Jean Kilroe, Bärbel-Maria Kurth, Joanna Mazur, Ewa Mierzejewska, Delphine Orbicini, Katy Phillips, Mick Power, Luis Rajmil, Ulrike Ravens-Sieberer, Michael Rigby, Stephane Robitail, Ursula von Rueden, Marie-Claude Simeoni, Cristian Tebé, Alan Tennant, Yannis Tountas, Eric Veripps, John Ware and Liz Waters
**European DISABKIDS Gruppe: Claire Atherton, Rolanda Baars, Monika Bullinger, John Eric Chaplin, David Debensason, Elpis Hatziagorou, Peter Hoare, Paraskevi Karagianni, Hendrik Koopman, Holger Mühlan, Esther Müller-Godeffroy, Corinna Petersen, Mick Power, Michael Quittan, Silke Schmidt, Othmar Schuhfried, Marie-Claude Simeonie, John Tsanakas, Ute Thyen, Athanasios Vidalis.
References
- 1.WHO. Constitution of the World Health Organization. Genf: WHO; 1948. [Google Scholar]
- 2.Ravens-Sieberer U. Lebensqualitätsansätze in der Pädiatrie. In: Ravens-Sieberer U, Cieza A, editors. Lebensqualität und Gesundheitsökonomie in der Medizin. Landsberg: ecomed; 2000. pp. 277–292. [Google Scholar]
- 3.Ravens-Sieberer U, Gosch A, Abel T, Auquier P, Bellach BM, Bruil J, Dur W, Power M, Rajmil L European KIDSCREEN Group. Quality of life in children and adolescents: a European public health perspective. Soz Praventivmed. 2001;46(5):294–302. doi: 10.1007/BF01321080. [DOI] [PubMed] [Google Scholar]
- 4.Bullinger M, Schmidt S, Petersen C. Assessing quality of life of children with chronic health conditions and disabilities: a European approach. Int J Rehabil Res. 2002;25(3):197–206. doi: 10.1097/00004356-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Herdman M, Rajmil L, Ravens-Sieberer U, Bullinger M, Power M, Alonso J European Kidscreen and Disabkids groups. Expert consensus in the development of a European health-related quality of life measure for children and adolescents: a Delphi study. Acta Pediatr. 2002;91(12):1385–1390. doi: 10.1111/j.1651-2227.2002.tb02838.x. [DOI] [PubMed] [Google Scholar]
- 6.Detmar SB, Bruil J, Ravens-Sieberer U, Gosch A, Bisegger C European KIDSCREEN group. The Use of Focus Groups in the Development of the KIDSCREEN HRQL Questionnaire. Qual Life Res. 2006;15(8):1345–1353. doi: 10.1007/s11136-006-0022-z. [DOI] [PubMed] [Google Scholar]
- 7.Ravens-Sieberer U, Gosch A, Rajmil L, Erhart M, Bruil J, Duer W, Auquier P, Power M, Abel T, Czemy L, Mazur J, Czimbalmos A, Tountas Y, Hagquist C, Kilroe J European KIDSCREEN group. The KIDSCREEN-52 Quality of life measure for children and adolescents: Development and first results from a European survey. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):353–364. doi: 10.1586/14737167.5.3.353. [DOI] [PubMed] [Google Scholar]
- 8.Bettge S, Ravens-Sieberer U. Seelische Gesundheit von Kindern und Jugendlichen in Deutschland - die Bella-Studie. Psychomedizin. 2005;17(4):214–222. [Google Scholar]
- 9.Cella DF, Tulsky DS, Gray G, Sarafian B, Lloyd S, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, Eckberg K, Purl S, Blendowski C, Goodman M, Barnicle M, Stewart I, McHale M, Bonomi P, Kaplan E, Taylor S, Thomas C, Harris J. The Functional Assessment of Cancer Therapy (FACT) scale: Development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 10.Von Rueden U, Gosch A, Rajmil L, Bisegger C, Ravens-Sieberer U European KIDSCREEN group. Socio-economic determinants of health-related quality of life in childhood and adolescence: results from a European study. J Epidemiol Community Health. 2006;60:130–135. doi: 10.1136/jech.2005.039792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Starfield B, Riley A, Ensminger M, Green B, Ryan S, Kim-Harris S, Johnston D, Vogel K. Manual for the Child Health and Illness Profile-Adolescent Edition (CHIP-AETM) Baltimore, MD: The Johns Hopkins University; 1994/1997/2000. [Google Scholar]
- 13.Patrick DL, Edwards TC, Topolski TD. Adolescent quality of life, part II: Initial validation of a new instrument. J Adolesc. 2002;25(3):287–300. doi: 10.1006/jado.2002.0471. [DOI] [PubMed] [Google Scholar]
- 14.Bethell CD, Read D, Stein REK, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002;2:38–48. doi: 10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Bethell CD, Read D, Neff J, Blumberg SJ, Stein REK, Sharp V, Newacheck PW. Comparison of the Children With Special Health Care Needs Screener to Questionnaire for Identifying Children With Chronic Conditions. Ambul Pediatr. 2002;2:49–57. doi: 10.1367/1539-4409(2002)002<0049:cotcws>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.KIDSCREEN Group Europe. The KIDSCREEN questionnaires. Lengerich: Papst; 2006. [Google Scholar]
- 17.Baars RM, Atherton CI, Koopman HI, Bullinger M, Power M DISABKIDS group. The European DISABKIDS project: development of seven condition-specific modules to measure health-related quality of life in children and adolescents. Health Qual Life Outcomes. 2005;13(3):70. doi: 10.1186/1477-7525-3-70. Available from: http://www.hqlo.com/content/3/1/70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen C, Schmidt S, Power M, Bullinger M. Development and pilot-testing of a health-related quality of life chronic generic module for children and adolescents with chronic health conditions: A European perspective. Qual Life Res. 2005;14(4):1065–1077. doi: 10.1007/s11136-004-2575-z. [DOI] [PubMed] [Google Scholar]
- 19.Simeoni MC, Schmidt S, Mühlan H, Debensason D, Bullinger M DISABKIDS Group. Field testing of a European quality of life instrument for children and adolescents with chronic conditions: the 37-item DISBKIDS Chronic Generic Module. Qual Life Res. 2007;16(5):881–893. doi: 10.1007/s11136-007-9188-2. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt S, Debensason D, Mühlan H, Petersen C, Power M, Simeoni MC, Bullinger M. The DISABKIDS generic quality of life instrument showed cross-cultural validity. J Clin Epidemiol. 2006;59(6):587–598. doi: 10.1016/j.jclinepi.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 21.DISABKIDS Group Europe. The DISABKIDS questionnaires. Lengerich: Pabst; 2006. [Google Scholar]
- 22.Kuhlthau K, Ferris TG, Beal AC, Gortmaker SL, Perrin JM. Who cares for medicaid enrolled children with chronic conditions? Paediatrics. 2001;108(4):906–912. doi: 10.1542/peds.108.4.906. [DOI] [PubMed] [Google Scholar]
- 23.Petersen C, Schmidt S, Bullinger M. Brief Report: Development and Pilot-Testing of a Coping Questionnaire for Children and Adolescents with Chronic Health Conditions. J Pediatr Psychol. 2004;29(8):335–340. doi: 10.1093/jpepsy/jsh066. [DOI] [PubMed] [Google Scholar]
- 24.Ravens-Sieberer U. Der Kindl-R Fragebogen zur Erfassung der gesundheitsbezogenen Lebensqualität bei Kindern und Jugendlichen - Revidierte Form. In: Schumacher J, Klaiberg A, Brähler E, editors. Diagnostische Verfahren zu Lebensqualität und Wohlbefinden. Göttingen: Hogrefe; 2003. pp. 184–188. [Google Scholar]
- 25.Schmidt S. Cross-cultural analyses of components and determinants of health-related quality of life. Hamburg: Hamburg University, Medical School; 2005. [Google Scholar]