Abstract
Objective
To evaluate the temporal stability of self-reported symptoms known to be associated with ovarian cancer.
Methods
This report is a longitudinal analysis of symptom reporting from 123 women who participated in the Seattle-based Ovarian Cancer Early Detection Study (OCEDS). The OCEDS population includes women at increased risk of ovarian cancer based on a family history of cancer or a BRCA I/II mutation. Data on symptoms were collected at two time points using a Symptoms Index that included abdominal pain, pelvic pain, feeling full quickly, inability to eat normally, abdominal bloating, and increased abdominal size.
Results
There was a median of 101 days between the two time points, with a range of 72–332 days. The median age of the women was 51, with a range of 32–79 years. Abdominal bloating was the most commonly reported symptom at both time points. The symptom least commonly reported at the two time points was inability to eat normally. The Symptoms Index was negative at both time points for 86% of all women and positive at both time points for 2% of all women. There were no statistically significant patterns of change for symptom reporting between time points.
Conclusions
The Symptoms Index and women’s report of abdominal pain, pelvic pain, feeling full quickly, unable to eat normally, abdominal bloating, increased abdominal size were stable between two time points in this sample. These findings provide evidence that longitudinal measurements of symptoms reporting by women in a screening study are likely to be reliable.
INTRODUCTION
Ovarian cancer has a higher fatality-to-case ratio than any other gynecological malignancy [1]. It was estimated that over 22,000 women would be diagnosed with ovarian cancer and 15,000 women would die from this disease in 2008 [2]. This high mortality rate is largely because the majority of women are not diagnosed until the disease is in an advanced, metastatic stage [1]. Fewer than 30% of the women who are diagnosed with advanced disease will survive five years past their diagnosis date [3]. On the contrary, women who are diagnosed when the disease is still confined to the ovaries have a five-year survival rate of 70–90% [3]. These statistics provide substantial motivation for the early detection of ovarian cancer.
Historically referred to as the “silent killer,” ovarian cancer was believed to have no symptoms until the disease had progressed to advanced stages. However, there is emerging evidence to suggest that women with ovarian cancer do experience symptoms, even when the disease is in an early stage [4–6]. As previously reported by Goff et al. [6], these symptoms include pelvic pain, abdominal pain, feeling full quickly, inability to eat normally, abdominal bloating, increased abdominal size, and urinary symptoms. Additional studies of ovarian cancer symptoms have supported these results and the presence of similar symptoms [7–12].
An ovarian cancer Symptom Index was developed in 2007 based on the results of a case-control study, in which the frequency, severity, and duration of symptoms were compared in 149 women with ovarian cancer and 233 controls [5]. The Symptom Index is considered to be positive if a woman reports that any of the following symptoms are new to her within the last year and occur more than 12 times per month: pelvic or abdominal pain, feeling full quickly, inability to eat normally, abdominal bloating, or increased abdominal size [5]. Using these criteria, the Symptom Index was found to have a sensitivity of 56.7% for early-stage disease and 79.5% for late-stage disease [5]. As reported by Andersen et al. [13], a composite marker, which is the combined use of the CA125 serum biomarker and the Symptom Index, has greater sensitivity for detecting ovarian cancer than CA125 alone, especially among early-stage cancers, which are commonly missed by CA125 [14]. The sensitivity of CA125 to detect early-stage disease increased to 80.6% for early-stage disease when used in combination with the Symptom Index and the specificity remained high at 83.5% [13]. These results highlight the potential clinical utility of using the Symptom Index in combination with other proven diagnostic tools to improve the detection of early-stage disease.
If symptom reporting is to be incorporated into screening programs for ovarian cancer, the temporal stability of the symptoms needs to be quantified and differences between subgroups of women need to be understood. Measuring the symptoms of ovarian cancer and utilizing the Symptom Index will not be a useful screening tool if there is significant variability of symptoms reporting with time. Therefore, our objective is to evaluate the stability and concordance of the specific symptoms that are included in the Symptom Index and to determine if any characteristics influence the positivity of the Symptom Index. Our sample is comprised of women who are at high-risk for ovarian cancer since they are the most likely to participate in a screening program for ovarian cancer.
MATERIALS AND METHODS
Study Population
This report is a longitudinal analysis of symptom reporting from women who participated in the IRB-approved, Seattle-based Ovarian Cancer Early Detection Study (OCEDS). The OCEDS population includes women who are at increased risk of ovarian cancer due to a personal or family history of breast cancer, a family history of ovarian cancer, or the presence of a BRCAI/II mutation. Women must have met one of the following conditions to be eligible for the study: (1) the family included at least two ovarian or breast cancers among the subject or her first-degree or second degree relatives, (2) the woman is of Ashkenazi Jewish ethnicity with one first-degree or one second-degree relative with breast cancer or the subject is of Ashkenazi ancestry and has had breast cancer herself, (3) the woman has tested positive for a BRCA I or II mutation or (4) the woman has a first-degree or second-degree relative with a BRCA I or II mutation. This first condition could be satisfied by multiple primary cancers in the same person. In situations where breast cancer was used to meet this criterion, at least one breast cancer case must have been premenopausal at the time of diagnosis (age at diagnosis was <50 years if age at menopause was unknown). In situations where breast cancer was used to meet the second criterion, at least one breast cancer case must have been premenopausal at the time of diagnosis (same age assumption as first criteria). Women aged 30 years and older were eligible to enroll in the study. Women aged 25 years and older were eligible if they were known BRCA1 or II mutation carriers.
The ineligibility criteria have been previously described [15]. The current study included only the OCEDS participants who completed the questionnaire related to symptoms at two different screening appointments. All participants provide informed consent prior to enrollment. The objective of OCEDS is to evaluate screening using longitudinal CA125 levels and transvaginal sonography (TVS) for the early detection of ovarian cancer in women who are at high risk for the disease (as defined by the criterion above). The screening protocol involves obtaining baseline and quarterly CA125 measurements and an annual TVS. At each screening appointment, women also complete health status questionnaires and answer questions related to symptoms. The questionnaire was approximately 16-pages long and it was completely solely by the study participant. If a woman had an elevated CA125 level, she was given a follow-up TVS. All of the women included in this report were followed for ovarian cancer incidence through data linkage to the Puget Sound Surveillance Epidemiology and End Results (SEER) cancer registry for two years from the date of the symptom report that was used in our analyses.
Measuring the Symptoms
During the OCEDS screening visits, participants are asked to complete questions related to the presence or absence of the following specific symptoms: abdominal pain, pelvic pain, feeling full quickly, inability to eat normally, abdominal bloating, and increased abdominal size. Women reporting one or more of these symptoms are asked to report how many days per month they experienced each symptom (<1 day, 1–2 days, 3–6 days, 4–12 days, 13–18 days, ≥20 days) and the duration of the symptom (<1 month, 1–2 months, 3–4 months, 5–6 months, 7–9 months, 10–12 months, >12 months). As previously described by Goff et al. [5, 6] and Andersen et al. [13], women are considered to have a positive Symptom Index if any of the aforementioned six symptoms occurred >12 times per month and were present for less than one year.
Statistical Methods
STATA statistical software package [version 10.0, Stata Corporation, College State, TX] was used for these analyses. All statistical tests were two-sided and considered to be statistically significant at p≤0.05, unless otherwise stated. The characteristics of the study population (n=123) were assessed using descriptive statistics, which included the median and range for continuous variables and the frequency and percent for categorical variables.
We calculated the frequency and percent of women who self-reported each symptom at both time points for all women (n=123), women who reported they were still menstruating (n=41), women who reported their periods had stopped for more than three months (n=81), women who reported a personal history of breast cancer (n=41), women who reported at least one first-degree relative with ovarian cancer (n=55), and women who reported they were a BRCA I/II mutation carrier (n=17).
As part of this analysis, we calculated the percentage of women who had each symptom at any frequency or duration and the percentage of women for whom their symptom or symptoms met the criterion to be positive on the Symptom Index, as previously described. The percentage of women with a positive Symptom Index was also calculated. In addition, we assessed if there was an association between the women’s personal characteristics and the outcome of the Symptom Index using the rank-sum for continuous variables and the Fisher’s exact test for categorical variables.
To determine the level of stability and concordance for each symptom between the two time points, we calculated the number of women who reported a gain, loss, or no change in each symptom between the two time points (termed Time 1 and Time 2). A symptoms gain occurred when the symptom was absent at Time 1 but present at Time 2. A symptoms loss occurred when the symptom was present at Time 1 but absent at Time 2. No change in a symptom occurred if the symptom was present at both time points or absent at both time points. Each symptom and the Symptom Index were considered to be stable if there was no change in how they were reported between Time 1 and Time 2 for each woman. Since we had two reports of symptom data for each woman, the McNemar’s chi-square test was then used to test the null hypothesis that there is no difference in the presence or absence of each symptom and Time 1 and Time 2. Since there were a large number of comparisons made during this portion of our analyses, we used the Bonferroni methods of adjustment to reduce the likelihood of a false-positive result. The Bonferroni adjusted p-value is calculated by dividing alpha by the total number of comparisons. In this case our p-value of 0.05 was divided by 35 comparisons, resulting in adjusted p-value of 0.001. Therefore, results from the McNemar’s test were only deemed statistically significant if p-value ≤0.001.
RESULTS
None of the women in OCEDS had developed ovarian cancer at the time of our analyses. Table 1 summarizes the characteristics of the study population. Table 2 summarizes the frequency and percentage of women who reported each symptom at Time 1 and Time 2 for the entire population and separately by each personal characteristic. Abdominal bloating and pelvic pain were the two most commonly reported symptoms for all women and in the majority of the sub-groups evaluated. Among women with a personal history of breast cancer and those who reported having a BRCA I/II mutation, pelvic pain and feeling full quickly were also reported with equal frequency.
Table 1.
Summary of the study population (n=123).
| Personal Characteristics | Statistic |
|---|---|
| Age at Time 1, median (range) | 51 (32 – 78) |
| Age at Time 2, median (range) | 51 (32 – 79) |
| Days Between Time 1 and Time 2, median (range) | 101 (72 – 332) |
| Caucasian, n (%) | 115 (93%) |
| BRCA I/II mutation carrier, n (%) | 17 (14%) |
| Menstruation Status | |
| Still menstruating | 41 (33%) |
| Periods have stopped for more than 3 months | 81 (66%) |
| Unknown | 1 (1%) |
| Personal and Familial Cancer History | |
| Personal history of breast cancer, n (%) | 41 (33%) |
| First-degree relative with ovarian cancer, n (%) | 55 (45%) |
Table 2.
Number of women who reported each symptom at Time 1 and Time 2.
| All Women (n=123) |
Still Menstruating (n=41) |
Periods Have Stopped >3 Months (n=81) |
Personal History of Breast Cancer (n=41) |
1° Relative with Ovarian Cancer (n=55) |
BRCAI/II Mutation Carrier (n=17) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | Time 1 n (%) |
Time 2 n (%) |
Time 1 n (%) |
Time 2 n (%) |
Time1 n (%) |
Time 2 n (%) |
Time 1 n (%) |
Time 2 n (%) |
Time 1 n (%) |
Time 2 n (%) |
Time 1 n (%) |
Time 2 n (%) |
| Abdominal Pain | ||||||||||||
| Had Symptom at Any Frequency or Duration | 22 (18) | 31 (25) | 11 (27) | 13 (32) | 11 (14) | 18 (22) | 5 (12) | 8 (20) | 9 (16) | 12 (22) | 2 (12) | 4 (24) |
| Symptom met Index Positive Criterion* | 2/22=11% | 1/31=3% | 0% | 0% | 2/11=18% | 1/18=6% | 1/5=20% | 0% | 1/9=11% | 1/12=8% | 0% | 0% |
| Pelvic Pain | ||||||||||||
| Had Symptom at Any Frequency or Duration | 31 (25) | 28 (23) | 14 (34) | 11 (27) | 17 (21) | 17 (21) | 8 (20) | 9 (22) | 13 (24) | 6 (11) | 3 (18) | 2 (12) |
| Symptom met Index Positive Criterion* | 1/31=3% | 3/28=11% | 0% | 0% | 1/17=16% | 3/17=18% | 1/8=13% | 2/9=22% | 0% | 1/6=17% | 0% | 0% |
| Feeling Full Quickly | ||||||||||||
| Had Symptom at Any Frequency or Duration | 16 (13) | 19 (15) | 5 (12) | 5 (12) | 10 (12) | 14 (17) | 8 (20) | 9 (22) | 5 (9) | 10 (18) | 3 (18) | 2 (12) |
| Symptom met Index Positive Criterion* | 5/16=31% | 2/19=11% | 2/5=40% | 0% | 3/10=30% | 2/14=14% | 2/8=25% | 2/9=22% | 2/5=40% | 1/10=10% | 1/3=33% | 0% |
| Unable to Eat Normally | ||||||||||||
| Had Symptom at Any Frequency or Duration | 5 (4) | 6 (5) | 0 (0) | 0 (0) | 5 (6) | 6 (7) | 2 (5) | 1 (2) | 1 (2) | 2 (4) | 1 (6) | 1 (6) |
| Symptom met Index Positive Criterion* | 0% | 2/6=33% | 0% | 0% | 0% | 2/6=33% | 0% | 1/1=100% | 0% | 0% | 0% | 0% |
| Abdominal Bloating | ||||||||||||
| Had Symptom at Any Frequency or Duration | 35 (28) | 44 (36) | 19 (46) | 21 (51) | 15 (19) | 22 (27) | 11 (27) | 14 (34) | 11 (20) | 17 (31) | 5 (29) | 5 (29) |
| Symptom met Index Positive Criterion* | 3/35=9% | 3/44=7% | 0% | 0% | 2/15=13% | 3/22=14% | 0% | 2/14=14% | 2/11=18% | 1/17=6% | 1/5=20% | 0% |
| Increased Abdominal Size | ||||||||||||
| Had Symptom at Any Frequency or Duration | 18 (15) | 29 (24) | 7 (17) | 13 (32) | 10 (12) | 15 (19) | 9 (22) | 5 (9) | 7 (13) | 3 (18) | 3 (18) | 4 (24) |
| Symptom met Index Positive Criterion* | 5/18=28% | 2/29=10% | 7/7=100% | 13/13=100% | 4/10=40% | 3/15=20% | 1/9=11% | 2/5=40% | 2/7=29% | 2/3 (67%) | 3/3=100% | 0% |
| Symptom Index** | ||||||||||||
| Positive | 10 (8) | 10 (8) | 2 (5) | 0 (0) | 7 (9) | 10 (12) | 4 (10) | 5 (12) | 4 (7) | 5 (9) | 2 (12) | 0 (0) |
| Negative | 113 (92) | 113 (92) | 39 (95) | 41 (100) | 74 (91) | 71 (88) | 37 (90) | 36 (88) | 51 (93) | 50 (91) | 15 (88) | 17 (100) |
The symptom was index positive if the frequency of the symptom was > 12 times per month and the duration of the symptom was < 1 year. The percentage was calculated using the total number women who had the symptom as the denominator.
A woman was considered to have a positive Symptom Index if the frequency of the symptom was > 12 times per month and the duration of the symptom was < 1 year.
Table 2 also summarizes the number of women who met the Index positive criterion. Of the 123 women in our study population, 10 (8%) had a positive Symptom Index at each time point. Feeling full quickly and inability to eat normally were the two most commonly reported symptoms to meet the Symptom Index positive criteria for all women. The highest percentage of women with a positive Symptom Index included those who reported a personal history of breast cancer (10% at Time 1 and 12% at Time 2). The lowest percentage of women with a positive Symptom Index included those who reported that they were still menstruating (5% at Time 1 and 0% at Time 2).
Table 3 reports the association of the women’s personal characteristics and the classification of the Symptom Index. There were no statistically significant associations between age, personal history of breast cancer, first-degree relative with ovarian cancer, or documented BRCA I/II mutation and Symptom Index status at either time point.
Table 3.
Personal characteristics of the women by Symptom Index status at Time 1 and Time 2.
| Characteristics | Time 1 | Time 2 | ||||
|---|---|---|---|---|---|---|
| Symptom Index Positive* (n=10) |
Symptom Index Negative (n=113) |
p-value** | Symptom Index Positive* (n=10) |
Symptom Index Negative (n=113) |
p-value** | |
| Age, median (range) | 48 (35–65) | 51 (32 – 78) | 0.42 | 49 (35–65) | 52 (32 – 79) | 0.42 |
| Still Menstruating, n (%) | 2 (20) | 39 (35) | 0.72 | 0 (0) | 41 (36) | 0.02 |
| Periods have Stopped >3 Months, n (%) | 7 (70) | 74 (65) | 10 (100) | 71 (63) | ||
| Personal History of Breast Cancer, n (%) | 4 (40) | 37 (33) | 0.74 | 5 (50) | 36 (32) | 0.27 |
| 1 ° Relative with Ovarian Cancer, n (%) | 4 (50) | 51 (45) | 0.98 | 5 (50) | 50 (44) | 0.48 |
| BRCAI/II Mutation Carrier, n (%) | 2 (20) | 15 (13) | 0.98 | 0 (0) | 17 (15) | 0.47 |
A woman was considered to have a positive Symptom Index if the frequency of the symptom was > 12 times per month and the duration of the symptom was < 1 year.
p-value obtained by the Rank-sum for continuous variable and the Fisher’s exact for categorical variables.
Table 4 summarizes the number of women who reported a gain, loss or no change in their symptoms between Time 1 and Time 2. As noted in the Methods, since there were a large number of comparisons made during this portion of our analyses, the Bonferroni methods of adjustment were used to reduce the likelihood of a false-positive result. Therefore, the results for this portion of our analyses were deemed statistically significant if p≤0.001. Under these criteria, there were no statistically significant patterns of symptoms reporting between the two time points. These results indicate there were no substantial changes or differences in the number of women who reported each symptom at one time point versus the other time point.
Table 4.
Pattern of symptoms reporting* between Time 1 and Time 2.
| Symptom | No Change: Symptoms Absent n (%) |
No Change: Symptoms Present n (%) |
Symptom Gain n (%) |
Symptom Loss n (%) |
p-value† |
|---|---|---|---|---|---|
| All Women (n=123) | |||||
| Symptom Index γ | 106 (86) | 3 (2) | 7 (6) | 7 (6) | 0.99 |
| Abdominal pain | 86 (70) | 16 (13) | 15 (12) | 6 (5) | 0.08 |
| Pelvic Pain | 81 (66) | 17 (14) | 11 (9) | 14 (11) | 0.69 |
| Feeling Full Quickly | 98 (80) | 10 (8) | 9 (7) | 6 (5) | 0.61 |
| Unable to Eat Normally | 116 (94) | 4 (3) | 2 (2) | 1 (1) | 0.99 |
| Abdominal Bloating | 74 (60) | 30 (24) | 14 (11) | 5 (4) | 0.06 |
| Increased Abdominal Size | 89 (72) | 13 (11) | 16 (13) | 5 (4) | 0.03 |
| Women Still Menstruating (n=41) | |||||
| Symptom Index γ | 39 (95) | 0 (0) | 0 (0) | 2 (5) | 0.16 |
| Abdominal pain | 23 (56) | 6 (15) | 7 (17) | 5 (12) | 0.56 |
| Pelvic Pain | 23 (56) | 7 (17) | 4 (10) | 7 (17) | 0.37 |
| Feeling Full Quickly | 34 (83) | 3 (7) | 2 (5) | 2 (5) | 0.99 |
| Unable to Eat Normally | 41 (100) | 0 (0) | 0 (0) | 0 (0) | -- |
| Abdominal Bloating | 16 (39) | 15 (37) | 6 (15) | 4 (10) | 0.53 |
| Increased Abdominal Size | 26 (63) | 5 (12) | 8 (20) | 2 (5) | 0.06 |
| Periods Stopped >3 Months (n=81) | |||||
| Symptom Index γ | 67 (82) | 3 (4) | 7 (9) | 4 (5) | 0.37 |
| Abdominal pain | 62 (77) | 10 (12) | 8 (10) | 1 (1) | 0.02 |
| Pelvic Pain | 57 (70) | 10 (12) | 7 (9) | 7 (9) | 0.99 |
| Feeling Full Quickly | 64 (79) | 7 (9) | 7 (9) | 3 (4) | 0.21 |
| Unable to Eat Normally | 74 (91) | 4 (5) | 2 (2) | 1 (1) | 0.56 |
| Abdominal Bloating | 58 (72) | 14 (17) | 8 (10) | 1 (1) | 0.02 |
| Increased Abdominal Size | 63 (78) | 7 (9) | 8 (10) | 3 (4) | 0.13 |
| Personal History of Breast Cancer (n=41) | |||||
| Symptom Index γ | 33 (81) | 1 (2) | 4 (10) | 3 (7) | 0.71 |
| Abdominal pain | 31 (76) | 3 (7) | 5 (12) | 2 (5) | 0.26 |
| Pelvic Pain | 28 (68) | 4 (10) | 5 (12) | 4 (10) | 0.74 |
| Feeling Full Quickly | 30 (73) | 6 (15) | 3 (7) | 2 (5) | 0.65 |
| Unable to Eat Normally | 39 (95) | 1 (2) | 0 (0) | 1 (2) | 0.32 |
| Abdominal Bloating | 26 (63) | 10 (24) | 4 (10) | 1 (2) | 0.18 |
| Increased Abdominal Size | 30 (73) | 4 (10) | 5 (12) | 2 (5) | 0.26 |
| First-degree Relative with Ovarian Cancer (n=55) | |||||
| Symptom Index γ | 48 (87) | 2 (4) | 3 (5) | 2 (4) | 0.65 |
| Abdominal pain | 41 (75) | 7 (13) | 5 (9) | 2 (4) | 0.26 |
| Pelvic Pain | 41 (75) | 5 (9) | 1 (2) | 8 (15) | 0.02 |
| Feeling Full Quickly | 43 (78) | 3 (5) | 7 (13) | 2 (4) | 0.10 |
| Unable to Eat Normally | 53 (96) | 1 (2) | 1 (2) | 0 (0) | 0.32 |
| Abdominal Bloating | 37 (67) | 10 (18) | 7 (13) | 1 (2) | 0.03 |
| Increased Abdominal Size | 46 (84) | 3 (5) | 4 (7) | 2 (4) | 0.41 |
Symptom gain = absent at Time 1, present at Time 2, Symptom loss= present at Time 1, absent at Time 2, No Change: Symptoms Present = present at both time points, No Change: Symptoms Absent = absent at both time points
p-values were obtained from the McNemar’s X2 test.
A woman was considered to have a positive Symptom Index if any of the 6 symptoms occurred > 12 times per month but were present for < 1 year.
We also closely evaluated which specific symptom represented a symptoms gain (absent at Time 1, but present at Time 2), a symptoms loss (present at Time 1, but absent at Time 2), no change in the presence of the symptoms (present at Time 1 and Time 2), and no change in the absence of the symptoms (absent at Time 1 and Time 2). Abdominal bloating was the most commonly reported symptom that was present at both time points. Inability to eat normally was the symptom least commonly reported symptom at both time points. Pelvic pain and abdominal pain were the symptoms most commonly reported to change between time points (i.e.: to be classified as a symptom gain or loss). In general, all subgroups of women reported some fluctuation in pelvic or abdominal pain between the two time points. However, these symptoms rarely occurred with enough frequency to result in a positive Symptom Index and thus appear to have little impact on the Symptom Index results overall.
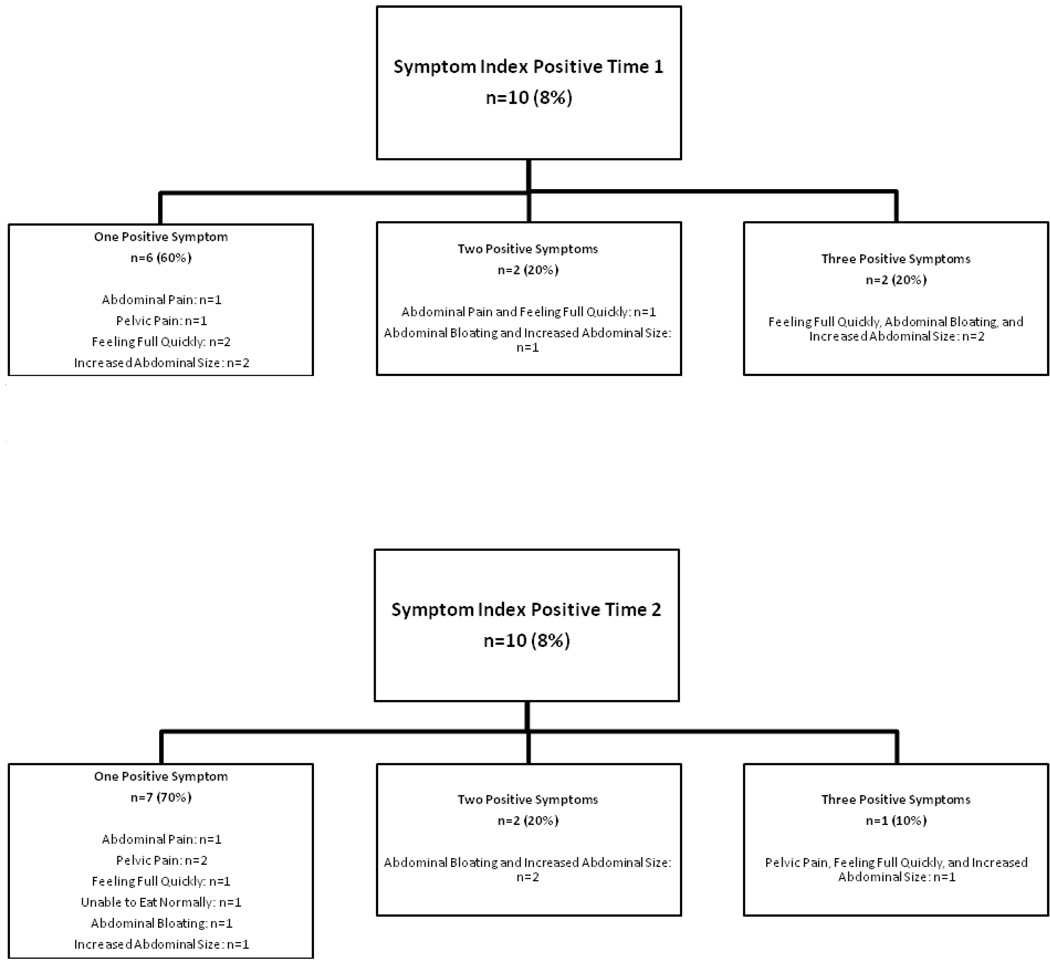
Figure 1 illustrates the number of women who had one, two, or three symptoms that met the criteria to be Symptom Index positive. It is interesting to note that both women who had three symptoms that met the criterion reported feeling full quickly, abdominal bloating, and increased abdominal size. No women had more than three symptoms that met the criterion at either Time 1 or Time 2.
Figure 1.
Number of women who had one, two or three symptoms that met the criterion to be Symptom Index positive.
CONCLUSIONS
Until recently, ovarian cancer was thought to be relatively asymptomatic. However, numerous studies have found that the majority of women with ovarian cancer will report a common set of non-specific symptoms prior to diagnosis. A systematic review of 24 studies from multiple institutions also found that symptoms that are associated with the diagnosis of ovarian cancer [16]. Within this review, the proportion of women who reported they were asymptomatic prior to diagnosis was 5–10% for studies that obtained data directly from patients, 20% when hospital records were used to collect the data, and 7% when general practice notes were used [16]. Given this summary of information, can we still really believe this disease is silent?
To our knowledge, this is the first evaluation of temporal stability for ovarian cancer symptoms. The results of our analyses showed that the Symptom Index is stable within a population of women who do not have ovarian cancer. Reports of abdominal pain, pelvic pain, feeling full quickly, inability to eat normally, abdominal bloating, and increased abdominal size appear to be stable in this population when measured at quarterly screening visits. In addition, our results suggested that there are personal patterns of experiencing most of these symptoms, which do not appear to change dramatically over time (even among women who are still menstruating). Our results also showed no association between the women’s personal characteristics and the outcome of the Symptom Index. There was a significant association between menstruation status and the outcome of the Symptom Index at Time 2 (p=0.02); however, this was likely caused by the disproportionate number of Symptom Index positive women who reported that their periods had stopped for more than three months versus the number of Symptom Index positive women who reported that they were still menstruating (100% vs. 0%, respectively).
Although the small number of index positive women in the sample precludes statistical analyses comparing women with positive index results, this co-occurrence of two or more frequent symptoms that are new to a woman in a substantial proportion of those with a positive index result based on one symptom suggests that these co-occurrences may be more frequent that would be expected due to random variation. This phenomena may be worthy of additional study. These findings provide evidence that longitudinal measurements of symptoms in a screening study are likely to be reliable within each woman. Longitudinal algorithms that utilize previous levels of a serum biomarker within a woman have been shown to be more robust than using single threshold rules for blood-based screening programs [17, 18]. This strategy is implicit in the Symptom index decision-rule based on its effectiveness in cross-sectional data, but longitudinal changes in symptom reporting have not been studied. These results suggest that temporal changes in the symptoms now found to be associated with ovarian cancer is modest.
CA125 is currently used as the first-line screen for ovarian cancer, with referral to transvaginal ultrasound for women in need of further screening. However, CA125 is known to have inadequate sensitivity for early stage disease [14] and the results of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial showed that its positive predictive value at the prevalence screen was only 3.7% for invasive tumors [19]. It is plausible that the Symptom Index could contribute to a more comprehensive first-line screen to identify women in need of further testing for ovarian cancer. Anderson et al. [13] reported that when the Index was used in combination with CA125, 80.6% of the early-stage and 95.1% of the late-stage cancers were identified, with a specificity of 83.5% [13].
The sample in the current report included women who are at high-risk for ovarian cancer based on family history of cancer or BRCA I/II. It is plausible that women with this classification have a higher sensitivity to changes in their body than average-risk women. The strength of our study is that we used a validated Symptom Index for data ascertainment [5]. In addition, our data were collected prospectively. The limitations of our study included the relatively small sample size and the potential for recall bias with self-reported data. In addition, the overall generalizability of our results may be limited only to women who are high-risk for ovarian cancer. Future research on the stability of these symptoms in the general population is warranted and is currently underway.
The objective of this study was to evaluate the temporal stability of the Symptom Index and the specific symptoms that comprise the Index. Our results support the stability of ovarian cancer symptoms and the Symptom Index in this population. Understanding the stability of this diagnostic tool may provide confidence to researchers and clinicians that the results from the SI are reliable. From a research methods perspective, understanding the reliability and validity of a tool are at the foundation of understanding its utility. The validity of the SI had been previously explored and described. These findings contribute to the growing body of evidence that illustrates the viability of using symptoms as a tool for ovarian cancer diagnosis. Since the cost of the Symptom Index is low (both in terms of cost and time burden on the patient), its implementation in the clinic is likely to be feasible. Future studies are needed to assess the clinical utility of using Symptom Index scores and symptoms information.
ACKNOWLEDGMENTS
This project was supported by a grant from the Marsha Rivkin Center for Ovarian Cancer Research (Seattle, WA) and by National Institutes of Health/National Cancer Institute grant P50 CA83636 to N. Urban (“Pacific Ovarian Cancer Research Consortium: Specialized Program of Research Excellence in Ovarian Cancer.”). This project was also partially supported by grant number R21NR010571 from the National Institute of Nursing Research. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. We thank the Fred Hutchinson Cancer Research Center staff, Marcia Gaul, Vandana Oza and Kristi Schurman, for their administrative support, and Shelly Hager for her software programming support of this study. We would also like to thank the Canary Foundation for contributing to support of the TOR laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST STATEMENT
None of the authors have a financial or personal relationship with other people or organizations that could inappropriately influence or bias this work.
REFERENCES
- 1.Holschneider CH, Berek J. Ovarian Cancer: Epidemiology, Biology and Prognostic Factors. Seminars in Surgical Oncology. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Rubin SC, Thomas GM, Robboy SJ. Epithelial ovarian cancer. In: Hoskins WJ, Perez CA, Young R, Barakat R, et al., editors. Principles and Practice of Gynecologic Oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 895–987. [Google Scholar]
- 4.Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian Carcinoma Diagnosis: Results of a National Ovarian Cancer Survey. Cancer. 2000;89:2068–2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Goff BA, Mandel LS, Drescher CW, Urban N, Gough S, Schurman KM, Patras J, Mahoney BS, Andersen MR. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221–227. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 6.Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. Jama. 2004;291:2705–2712. doi: 10.1001/jama.291.22.2705. [DOI] [PubMed] [Google Scholar]
- 7.Koldjeski D, Kirkpatrick MK, Swanson M, Everett L, Brown S. Ovarian cancer: early symptom patterns. Oncol Nurs Forum. 2003;30:927–933. doi: 10.1188/03.ONF.927-933. [DOI] [PubMed] [Google Scholar]
- 8.Lataifeh I, Marsden DE, Robertson G, Gebski V, Hacker NF. Presenting symptoms of epithelial ovarian cancer. Aust N Z J Obstet Gynaecol. 2005;45:211–214. doi: 10.1111/j.1479-828X.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 9.Olson SH, Mignone L, Nakraseive C, Caputo TA, Barakat RR, Harlap S. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212–217. doi: 10.1016/s0029-7844(01)01457-0. [DOI] [PubMed] [Google Scholar]
- 10.Ryerson AB, Eheman C, Burton J, McCall N, Blackman D, Subramanian S, Richardson LC. Symptoms, diagnosis, and time to key diagnositc procedures among older U.S. women with ovarian cancer. Obstet Gynecol. 2007;109:1053–1061. doi: 10.1097/01.AOG.0000260392.70365.5e. [DOI] [PubMed] [Google Scholar]
- 11.Vine MF, Ness RB, Calingaert B, Schildkraut JM, Berchuck A. Types and duration of symptoms prior to diagnosis of invasive or borderline ovarian tumor. Gynecol Oncol. 2001;83:466–471. doi: 10.1006/gyno.2001.6411. [DOI] [PubMed] [Google Scholar]
- 12.Wikborn C, Pettersson F, Silfversward C, Moberg PJ. Symptoms and diagnostic difficulties in ovarian epithelial cancer. Int J Gynaecol Obstet. 1993;42:261–264. doi: 10.1016/0020-7292(93)90222-i. [DOI] [PubMed] [Google Scholar]
- 13.Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, Paley P, Urban N. Combining a symptoms index with CA 125 to improve detection of ovarian cancer. Cancer. 2008;113:484–489. doi: 10.1002/cncr.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban N, McIntosh MW, Andersen M, Karlan BY. Ovarian cancer screening. Hematol Oncol Clin North Am. 2003;17:989–1005. doi: 10.1016/s0889-8588(03)00063-7. ix. [DOI] [PubMed] [Google Scholar]
- 15.Lowe KA, Shah C, Wallace E, Anderson G, Paley P, McIntosh M, Andersen MR, Scholler N, Bergan L, Thorpe J, Urban N, Drescher CW. Effects of personal characteristics on serum CA125, mesothelin, and HE4 levels in healthy postmenopausal women at high-risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2480–2487. doi: 10.1158/1055-9965.EPI-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankhead CR, Kehoe ST, Austoker J. Symptoms associated with ovarian cancer : a systematic review. BJOG. 2005;112:857–865. doi: 10.1111/j.1471-0528.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 17.McIntosh MW, Urban N. A parametric empirical Bayes method for cancer screening using longitudinal observations of a biomarker. Biostatistics. 2003;4:27–40. doi: 10.1093/biostatistics/4.1.27. [DOI] [PubMed] [Google Scholar]
- 18.McIntosh MW, Urban N, Karlan B. Generating Longitudinal Screening Algorithms Using Novel Biomarkers for Disease. Cancer Epidemiol Biomarkers Prev. 2002;11:159–166. [PubMed] [Google Scholar]
- 19.Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, Hartge P, Fagerstrom RM, Ragard LR, Chia D, Izmirlian G, Fouad M, Johnson CC, Gohagan JK. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–1639. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]