Abstract
Background
There is considerable research examining differences in adolescent and adult sensitivity and tolerance to ethanol related behavioral phenotypes. However, the available published data has almost exclusively assessed these behaviors in outbred rats. The present study was conducted using the alcohol preferring inbred mouse strain C57BL/6J (B6) and the alcohol non-preferring inbred mouse strain DBA/2J (D2) to determine if differences in the sedative and ataxic effects of ethanol exist between adolescents and adults, and to determine whether there are any genetic influences involved therein.
Methods
Adolescent and adult mice of each sex and genotype were given intraperitoneal (i.p.) injections of ethanol (1.5, 1.75 or 4.0 g/kg) or saline and assessed for the loss of righting reflex (LORR) or hind footslips on the balance beam apparatus. These animals were then tested for the development of tolerance to these behaviors on subsequent days.
Results
Despite evident pharmacokinetic differences, D2 adults were found to be relatively more sensitive than their adolescent D2 counterparts in addition to B6 animals of both age groups. Furthermore, although adult animals appeared to develop significantly greater degrees of tolerance to ethanol-induced hypnosis compared to adolescents, these effects are likely in part related to differences in ethanol absorption/metabolism across time. Taking into account pharmacokinetic differences and the overall poor performance of male adults, adolescent animals were found to be equally if not more sensitive to the motor incoordinating (ataxic) effects of ethanol. Overall, tolerance to these effects varied by age and genotype but appeared to be related to changes in ethanol pharmacokinetics rather than strict behavioral sensitivity.
Conclusion
The current work suggests that adolescent B6 and D2 inbred mice exhibit ontogenetic differences in sensitivity to ethanol’s hypnotic and ataxic effects. Importantly, in some cases age differences emerge as a function of differential ethanol pharmacokinetics. These results extend the current literature examining this critical developmental period in mice and illustrate the benefits of comparing ethanol related developmental differences in different genetic mouse populations.
Keywords: Adolescent, Alcohol, Tolerance, Mice, Loss of Righting Reflex, Ataxia
Alcohol (ethanol) use often begins during the teenage years, a sensitive period during which a number of neurobiological, hormonal, and behavioral changes occur. A recent survey suggests that approximately 30% of high school seniors have had five or more drinks in a row (i.e., have engaged in binge drinking) during the previous two weeks (Johnston et al., 2002). Moreover, clinical reports indicate that adolescents frequently exhibit the symptoms of alcohol dependence. For example, some adolescents have difficulty controlling their alcohol intake and display signs of tolerance (Hawkins et al., 1997; Spear, 2000; Stewart and Brown, 1995). Such alcohol use during adolescence may have profound effects on brain development/maturation, and may even influence the propensity to use the drug in later life. Additional research is clearly needed to better understand how alcohol’s actions during this critical developmental period differ from its actions during adulthood.
The development of useful animal models is crucial if we are to understand the complex interaction between alcohol sensitivity and the profound changes that occur during adolescence. Although human adolescents undergo developmental changes unparalleled in lower organisms, other mammalian species (including rodents) also experience a developmental period similar to adolescence (Spear, 2000). These changes have been reviewed for both rats (Spear, 2004) and mice (Laviola et al., 2003). With regard to ethanol, a number of studies have demonstrated that adolescent rats exhibit an altered behavioral sensitivity to the compound compared to their adult counterparts (see Spear and Varlinskaya, 2004 for review). Adolescent rats have been documented to display reduced sensitivity to the sedative/hypnotic (Silveri and Spear, 1998; Silveri and Spear, 1999), motor impairing or ataxic (Silveri and Spear, 2001), hypothermic (Silveri and Spear, 2000), and anxiolytic (Varlinskaya and Spear, 2002) effects of ethanol. Adolescent rats have demonstrated increased sensitivity to still other ethanol-related behavioral traits. Adolescent rats are more sensitive to the amnestic (Markwiese et al., 1998) and social faciliatory (Varlinskaya and Spear, 2002) actions of ethanol. Moreover, recent evidence suggests that adolescent rats may develop greater acute (within session) ethanol tolerance compared to their adult counterparts (Silveri and Spear, 1998; Varlinskaya and Spear, 2006). Taken together, the above evidence indicates that rodents might provide a powerful model system for investigating the neurobiological underpinnings of ethanol’s actions during the critical developmental period of adolescence.
Although much is known about the behavioral actions of ethanol in adolescent rats, little work has been done in adolescent mice despite the fact that mice have been one of the most widely used animal models in ethanol research for decades. Inbred mouse strains have been particularly well studied. An inbred strain is a population of mice in which members have been brother-sister mated for 20 generations or more, effectively rendering all mice within the strain genetically identical. Differences among the members of an inbred strain are likely due to environmental influences, whereas differences between inbred strains are likely due to genetic differences (assuming environmental parameters have been rigorously controlled).
The C57BL/6J (B6) and DBA/2J (D2) inbred mouse strains differ widely in their relative behavioral sensitivities to ethanol. For example, in the earliest of these studies, McClearn and Rodgers (1959) demonstrated that whereas B6 mice will readily consume ethanol in a standard two-bottle choice paradigm, D2 mice avoid the solution. Since then, the relative sensitivity of these two inbred strains has been shown to exhibit widely different sensitivities on a number of other ethanol-related behavioral traits, including ethanol’s locomotor (Phillips et al., 1995), hangover (Metten and Crabbe, 1994), hypnotic (Crabbe et al., 2006), ataxic (Crabbe et al., 2003), and reinforcing actions (Cunningham et al., 1992). Thus, the use of inbred mouse strains provides a means by which to rigorously control for genetic differences in ethanol sensitivity. Moreover, because the ethanol behavioral sensitivities of B6 and D2 adults are so extensively characterized, examination of adolescent sensitivity in these strains will provide a useful picture of how ethanol behavioral sensitivity during adolescence relates to that of adulthood.
Several labs have recently published reports describing ethanol behavioral sensitivity in adolescent and adult B6 (Hefner and Holmes, 2007) or D2 (Stevenson et al., 2007) inbred mice, but neither has compared the two, or examined tolerance to these actions. Indeed, a number of ethanol-related behaviors may undergo tolerance. Three different types of tolerance have been described. Whereas acute or within session tolerance is that which develops over a single ethanol exposure, rapid tolerance develops within 24 hours after first exposure and chronic tolerance develops with repeated ethanol exposures over days (Kalant, 1993). Although each of these forms of tolerance has been investigated in adolescent rats (Silveri and Spear; 1998, 1999; Ristuccia and Spear, 2004, 2005; Varlinskaya and Spear, 2006, 2007; Spear and Varlinskaya, 2005), they have not been thoroughly investigated with respect to hypnosis or ataxia in mice. The goal of the present study was to investigate sensitivity as well as rapid and chronic tolerance to the sedative and motor impairing effects of ethanol in adolescent and adult B6 and D2 inbred mice. Based on the available adult mouse sensitivity literature we hypothesized that D2 mice of both age groups would display greater sensitivity to the hypnotic (Crabbe et al., 2006) and motor impairing (Crabbe et al., 2003) actions of ethanol than B6 animals of the same age. Furthermore, based on the available adolescent mouse (Hefner and Holmes, 2007) and rat (previously mentioned) literature we predicted that adolescent animals would exhibit reduced sensitivity to both behavioral actions of ethanol and that the development of tolerance to these actions would also be greater.
Materials and Methods
Animals
Male and female B6 and D2 mice bred in our colony at the Binghamton University animal facility were used in each experiment. Breeders were originally purchased from Jackson Laboratory (Bar Harbor, ME) and care was taken to avoid breeding past 2 generations from these founder mice. Whereas adolescent and adult animals aged 30 ± 3 (PD 30 ± 3) and 60 ± 3 (PD 60 ± 3) respectively, were used for LORR and metabolism experiments (1 and 2), adolescent and adult animals age 33 ± 3 (PD 30 ± 3) and 60 ± 3 (PD 60 ± 3) respectively, were used for the balance beam experiments (3 and 4). B6 and D2 adolescent animals for experiments 1 and 2 weighed 12.15 ± 0.03 and 12.00 ± 0.03 g respectively, whereas adult B6 and D2 animals weighed 20.65 ± 0.03 and 21.00 ± 0.04 g respectively. For experiments 3 and 4, B6 and D2 adolescents weighed 17.32 ± 0.08 and 15.67 ± 0.06 g respectively, and B6 and D2 adults weighed 20.90 ± 0.09 and 21.70 ± 0.10 respectively. Same-sex animals were group housed 2–5 per cage in standard, clear, polycarbonate shoebox mouse cages and had ad lib access to standard rodent chow and tap water except during experimental procedures and behavioral testing. Up to three litters were sometimes represented in a cage, at times making it difficult to know which mouse belonged to which original litter. Thus, although we generally avoided inclusion of more than one same-sex littermate in a particular experimental group, this undoubtedly occurred from time to time. Although experiments were sometimes repeated using different cohorts of animals to increase group sizes, every attempt was made to balance the cohorts across genotype, sex, and age. Vivarium lighting was maintained on a 12/12 hour cycle with the lights turning on at 7:00 AM and the temperature and humidity were held at approximately 21°C and 50%, respectively. All of the procedures were approved by the Binghamton University Institutional Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Ethanol Administration
200 proof Ethanol was purchased from Pharmco, Inc (Brookfield, CT) and diluted in sterile 0.9% physiological saline. Ethanol was administered by intraperitoneal (i.p.) injection in a volume of 0.2 ml per 10 g of body weight at doses of 1.5, 1.75 and 4.0 g/kg.
Experiment 1
The goal of experiment 1 was to assess sensitivity and tolerance to the hypnotic effects of ethanol. On day 1, animals received i.p. injections of 4 g/kg ethanol and were immediately placed in classic V-shaped troughs and assessed for loss of righting reflex (LORR) as previously described (McClearn and Kakihana, 1981; see Crabbe 2006). Briefly, LORR was defined as the time immediately following injection when the animal was unable to right itself onto all four paws. The duration of LORR was defined as the time from loss of the righting reflex to the time of recovery of the righting reflex (ability to right twice in 60 seconds). Immediately upon recovery of the righting reflex retro-orbital sinus bloods were collected to determine blood ethanol concentration (BEC; see detailed methods below). On day 2 half of the animals were treated identically to day 1; duration of LORR was monitored after which blood was collected for comparison to day 1. On days 2–4 the remaining animals received once daily ethanol injections (4 g/kg) and were returned to their home cages (LORR was not assessed). On day 5 animals received a final ethanol injection (4 g/kg) and duration of LORR was assessed and retro-orbital sinus bloods were sampled in identical fashion as the second day of experiment 1 above.
Experiment 2
Possible age and genotype differences in ethanol pharmacokinetics following the 4.0 g/kg dose were assessed in experiment 2. Retro-orbital sinus bloods were sampled following i.p. injections on days 1 and 5 at predetermined time intervals. The same mice were used throughout to reduce the number of animals required and to allow for within subjects comparisons across time. However, two separate cohorts of animals were tested which allowed us to remove blood volumes of ≤ 1% body weight per animal per day and provided adequate time for recovery of blood volumes (Hoff, 2000). The first cohort were sampled after acute administration at the 20, 40 and 80 minute time intervals which roughly corresponded to the times at which individual groups of animals regained the righting reflex on day 1 (see experiment 1 results). The second cohort was tested after 240 minutes on days 1 and 5 to evaluate overall differences in metabolism as a function of relative exposure.
Experiment 3
Experiment 3 was undertaken to examine sensitivity and tolerance to the motor incoordinating or ataxic actions of ethanol using the balance beam apparatus (see Moore et al. 2007, for details). The adult balance beam apparatus consisted of a 122 cm long by 2 cm wide by 4 cm tall wood block placed on top of two ring stands measuring 48 cm high. The balance beam apparatus was situated on top of a table so that the wood block was a total of 130 cm off the floor. Based on unpublished work from our lab and literature indicating crown-rump and gait differences between adolescents and adults (Doremus et al., 2003, 2004, 2006; Varlinskaya and Spear, 2002, 2006, 2007), a second balance beam was constructed that was scaled to ¾ of the size of the adult beam (91.5 cm long by 1.5 cm wide by 3 cm tall wood block) and placed on top of the same ring stands and table described above.
Mice were trained to walk the length of the beam immediately prior to ethanol challenge. During training, mice were encouraged to walk the beam by gently nudging their hind quarters with the eraser end of a pencil. Preliminary data indicated that such training was sufficient to ensure that mice would rapidly traverse the beam without the aid of the eraser following ethanol challenge (data not shown). Immediately after all mice were trained, mice were systematically dosed with ethanol (1.5 g/kg) and returned to the home cage. Hind foot slips were recorded on the balance beam 10 minutes later; immediately after this testing retro-orbital sinus blood samples were taken for determination of BEC. This procedure was repeated on day 2 to look for the development of rapid tolerance. However, on this day only half of the animals were tested on the balance beam to determine if this additional exposure to the apparatus was sufficient to elicit significant learned tolerance upon subsequent testing (on day 5). On days 3 and 4 all animals were given ethanol injections (1.5 g/kg) and returned to the home cage. On Day 5 all animals were again injected with ethanol (1.5 g/kg) and tested on the balance beam to assess the development of chronic tolerance.
Experiment 4
Sensitivity and tolerance to the motor incoordinating actions of a higher dose of ethanol (1.75 g/kg) were assessed in experiment 4 using the balance beam apparatus. The only difference between this experiment and experiment 3 was the ethanol dose.
Blood Ethanol Concentration
After each animal regained its righting reflex (experiment 1), at predetermined time intervals after injection (experiment 2), or immediately after performance on the balance beam apparatus (experiment 3 and 4) 25 µl retro-orbital sinus bloods were collected for determination of BEC. Blood was spun down in 0.5mL micro-centrifuge tubes and plasma was withdrawn and stored at −80°C for later analysis. Samples were processed using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA) and recorded as mg/dL (mg %).
Statistical Analysis
Marked failure was observed in the ability of some mice (particularly adolescents) to lose the righting reflex within 10 min of injection. Ponomarev and Crabbe (2002) have attributed the inability of adult mice to loose the righting response to the failure of some intraperitoneal injections to effectively deliver ethanol solution to the peritoneal cavity. However, it is not immediately clear whether these instances were actually due to incomplete ethanol injection, relative insensitivity to ethanol upon initial exposure on day 1, or tolerance to repeated ethanol exposure on days 2 or 5. Nevertheless, we were left with no alternative other than to exclude such mice from the statistical analysis. In fact, although we attempted to evaluate differences between groups with an additional lower dose (3.5 g/kg), over 50% of adolescents failed to lose the righting reflex compared to about 13% of adults. Therefore, as the remaining adolescents were not representative of the true population these data had to be eliminated from statistical analysis. This was not the case for the 4.0 g/kg dose where attrition was distributed relatively equally across groups and time. The interested reader is referred to Table 1 for a detailed list of such animals, as well as animals that could not be included in some analyses due to difficulties sampling or assaying blood samples.
Table 1.
Attrition Over Days
| 4.0 Rapid Tolerance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Age | Sex | Original N | failed LORR (Day1) |
BEC error (Day1) |
N (Day1) |
failed LORR (Day2) |
BEC error (Day2) |
N (Day2) |
||
| C57BL/6J | Adol | Both | 23 | 2 | * | 21 | 1 | * | 20 | ||
| Male | 13 | 1 | * | 12 | * | * | 12 | ||||
| Female | 10 | 1 | * | 9 | 1 | * | 8 | ||||
| Adult | Both | 20 | * | 1 | 19 | * | * | 19 | |||
| Male | 10 | * | * | 10 | * | * | 10 | ||||
| Female | 10 | * | 1 | 9 | * | * | 9 | ||||
| DBA/2J | Adol | Both | 20 | * | 1 | 19 | 2 | * | 17 | ||
| Male | 10 | * | 1 | 9 | 1 | * | 8 | ||||
| Female | 10 | * | * | 10 | 1 | * | 9 | ||||
| Adult | Both | 20 | * | * | 20 | * | 1 | 19 | |||
| Male | 10 | * | * | 10 | * | * | 10 | ||||
| Female | 10 | * | * | 10 | * | 1 | 9 | ||||
| 4.0 Chronic Tolerance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Age | Sex | Original N | failed LORR (Day1) |
BEC error (Day1) |
N (Day1) |
failed LORR (Day5) |
BEC error (Day5) |
N (Day5) |
|
| C57BL/6J | Adol | Both | 41 | 4 | 1 | 36 | 2 † | 3 | 29 | |
| Male | 20 | 1 | * | 19 | 1 | 1 | 16 | |||
| Female | 21 | 3 | 1 | 17 | 1 | 2 | 13 | |||
| Adult | Both | 36 | * | 4 | 32 | 3 | 8 | 21 | ||
| Male | 17 | * | 2 | 15 | 3 | 1 | 11 | |||
| Female | 19 | * | 2 | 17 | * | 7 | 10 | |||
| DBA/2J | Adol | Both | 27 | 4 | 2 | 21 | * | 1 | 20 | |
| Male | 13 | 2 | 1 | 10 | * | 1 | 9 | |||
| Female | 14 | 2 | 1 | 11 | * | * | 11 | |||
| Adult | Both | 28 | * | 4 | 24 | 2 | 3 | 19 | ||
| Male | 13 | * | 2 | 11 | 2 | * | 9 | |||
| Female | 15 | * | 2 | 13 | * | 3 | 10 | |||
Between days two and five 1 female and 1 male adolescent B6 had to be euthanised due to poor appearance
To asses differences in hypnotic sensitivity (duration and BEC at regain), three-way analyses of variance (ANOVA) with genotype, sex, and age as the between groups factors were performed. Rapid and chronic tolerance to ethanol’s hypnotic actions were calculated as change in duration or BEC from day 1, and analyzed by three-way ANOVA with genotype, sex, and age as the between groups factors. Because results indicated no main effects of sex or any other sex interactions, data were collapsed on this factor for graphical presentation. Subsequent Newman-Keuls post-hoc tests were run where appropriate for all experiments. Results were considered significant at P<0.05.
Pharmacokinetic differences were assessed by four-way repeated measures ANOVAs with genotype, sex, and age as the between groups factors and time bin or day as the within subjects factor. In both cohorts for these studies, data were collapsed on sex and genotype for graphical presentation. Results were considered significant at P<0.05.
Differences in sensitivity to ethanol’s ataxic effects (performance as assessed by hind footslips and corresponding BEC) were analyzed by three-way repeated measures ANOVA with genotype, sex, and age as the between groups factors. Rapid and chronic tolerance to ethanol’s ataxic actions were calculated as change in performance or BEC from day 1, and analyzed by three-way ANOVA with genotype, sex, and age as the between groups factors. Subsequent Newman-Keuls post-hoc tests were run where appropriate. The level of significance was set at P<.05.
Results
Experiment 1 – Sensitivity
Sensitivity to the hypnotic actions of ethanol following the 4.0 g/kg dose are shown in Figure 1. Although sex was not a significant factor in the analyses and therefore not represented graphically, the interested reader is referred to Table 2 for hypnotic sensitivity data broken down by this factor.
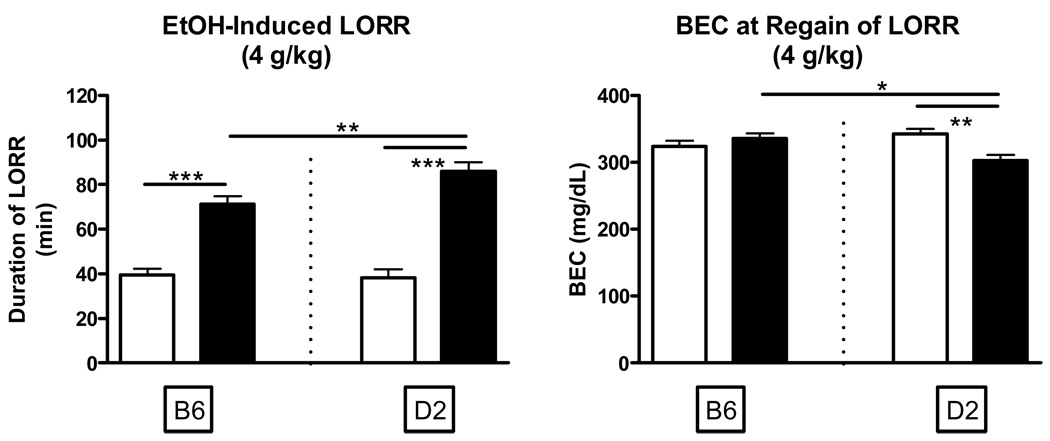
Figure 1.
Sensitivity to ethanol-induced LORR (4.0 and 3.5 g/kg) in adolescent and adult C57BL/6J and DBA2/J mice. A. Blood ethanol concentrations after regaining the righting reflex (4.0 g/kg). B. Duration of LORR in adolescent and adult mice (4.0 g/kg). C. Blood ethanol concentrations after regaining the righting reflex (3.5 g/kg). D. Duration of LORR in adolescent and adult mice (3.5 g/kg).
Table 2.
Hypnotic Sensitivity
| 4.0 g/kg Sensitivity | ||||||
|---|---|---|---|---|---|---|
| Genotype | Age | Sex | N | LORR (min) | BEC (mg/dL) | |
| C57BL/6J | Adol | Male | 31 | 39 ± 4 | 311 ± 11 | |
| Female | 26 | 39 ± 4 | 339 ± 11 | |||
| Adult | Male | 25 | 64 ± 6 | 333 ± 12 | ||
| Female | 26 | 77 ± 3 | 337 ± 10 | |||
| DBA/2J | Adol | Male | 19 | 31 ± 4 | 350 ± 7 | |
| Female | 21 | 44 ± 4 | 334 ± 13 | |||
| Adult | Male | 21 | 85 ± 7 | 311 ± 9 | ||
| Female | 23 | 86 ± 4 | 293 ± 14 | |||
Lower sensitivity to ethanol’s hypnotic actions was generally defined as recovery of the righting response at a relative higher BEC. The overall three-way analysis of BEC at the 4.0 g/kg dose indicated a significant age*genotype interaction [F(1, 188)=9.41 P<.01] with adult D2 animals displaying significantly lower BECs than both D2 adolescents (P<.05) and B6 adults (P<.01) at recovery (Figure 1A). Analysis of corresponding duration of LORR data indicated a significant main effect of age [F(1, 188)=124.19 P<.001] and a significant age*genotype interaction [F(1, 188)=4.94 P<.05]. Post-hoc tests confirmed that both B6 (P<.001) and D2 (P<.001) adolescents exhibited significantly shorter durations of LORR than adults of the same genotype, and that adult D2 animals had significantly longer LORR sleep times than B6 adults (P<.01; Figure 1B). Taken together, the combined BEC and behavioral data are consistent with the notion that D2 adolescents are less sensitive than D2 adults, and that D2 adults are more sensitive than B6 adults at the 4.0 g/kg dose. Moreover, the present data suggest that suggest that B6 adolescents possess faster rates of absorption and/or metabolism than B6 adults.
Experiment 1 - Tolerance
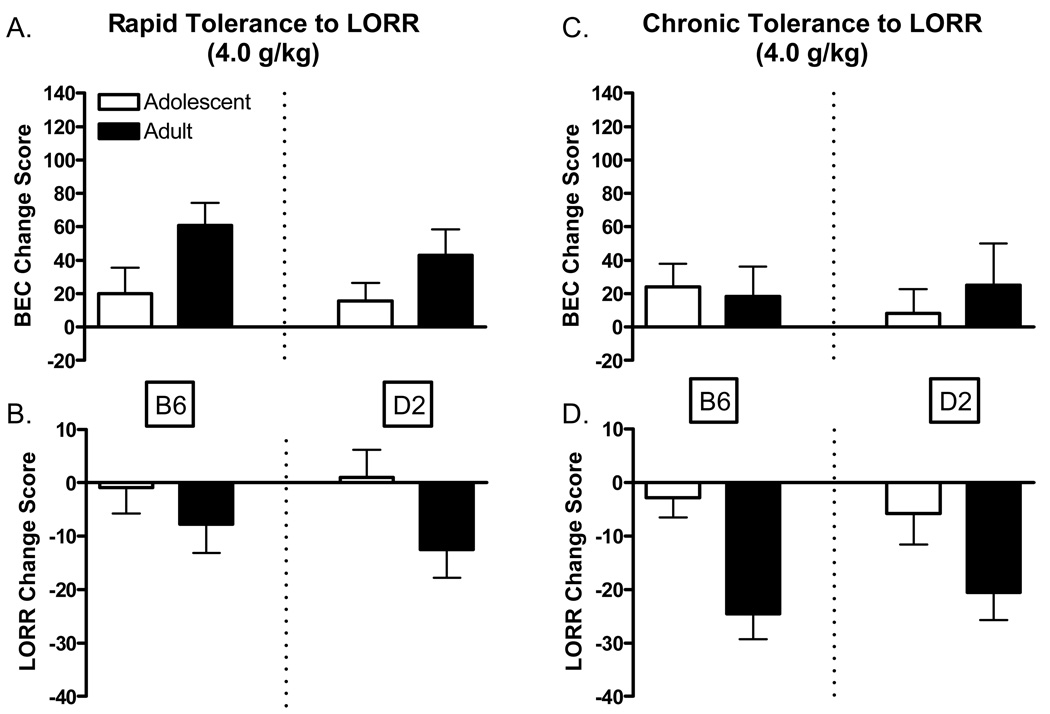
Differences in the development of rapid and chronic tolerance to the hypnotic actions of ethanol can be seen in Figure 2. Change scores were calculated by subtracting day one values from those on day two (day2 – day1 = change score) for both the BEC at regain (mg/dL) and corresponding LORR duration (min). Negative BEC change scores (although none were apparent) indicate decreased BEC at regain from day one, whereas positive values indicate increased BEC at regain. Negative LORR change scores indicate decreased sleep time, whereas positive values indicate longer sleep times compared to day one. All possible scenarios involving relationships between the directions of these change scores are further elaborated in the discussion, although regain of the righting response at a higher BEC on either days 2 or 5 were generally taken to indicate the development of rapid or chronic tolerance, respectively. Although the focus of this analysis was on genotype × age differences in the magnitude of rapid and chronic tolerance, the interested reader is referred to Table 3 for raw sleep time and BEC at regain data.
Figure 2.
Development of rapid and chronic tolerance to ethanol-induced LORR (4.0 g/kg) in adolescent and adult C57BL/6J and DBA2/J mice. A. Change in Blood ethanol concentrations after regaining the righting reflex on day 2 compared to day 1. B. Change in the duration of LORR in adolescent and adult mice on day 2 compared to day 1. C. Change in Blood ethanol concentrations after regaining the righting reflex on day 5 compared to day 1. D. Change in the duration of LORR in adolescent and adult mice on day 5 compared to day 1. All change score were calculated by subtracting the second or fifth days values from those of day 1. Positive BEC change scores represent increases in BEC. Negative LORR change scores represent decreases in LORR duration.
Table 3.
LORR Duration and BEC Over Days
| 4.0 Tol - Raw | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | Age | N (Rapid) |
Day 2 LORR (min) |
Day 2 BEC (mg/dL) |
N (Chronic) |
Day 5 LORR (min) |
Day 5 BEC (mg/dL) |
||
| C57BL/6J | Adol | 20 | 35.7 ± 3.1 | 318.1 ± 11.3 | 29 | 40.2 ± 3.5 | 363.5 ± 10.0 | ||
| Adult | 19 | 50.1 ± 3.8 | 357.7 ± 10.2 *** | 21 | 53.7 ± 4.5 *** | 360.3 ± 12.9 | |||
| DBA/2J | Adol | 17 | 25.1 ± 5.4 | 344.3 ± 8.5 | 20 | 47.5 ± 4.9 | 363.2 ± 13.8 | ||
| Adult | 19 | 73.6 ± 7.2 * | 338.5 ± 12.9 * | 19 | 62.0 ± 6.3 *** | 331.6 ± 14.8 | |||
Significant differences were calculated by comparing day 1 values with values on days 2 and 5 using T-tests
(p<.05)
(p<.01)
(p<.001)
Day 2 BEC at regain and duration of LORR following administration of the 4.0 g/kg ethanol dose are shown in Figures 2A and 2B, respectively. Analysis of rapid tolerance indicated a significant main effect of age [F(1, 71)=4.6 P<.05] for BEC at regain with no significant difference in LORR duration, which may suggest the development of greater rapid tolerance among the adult members of both genotypes. Day 5 BEC at regain and duration of LORR following administration of the 4.0 g/kg ethanol dose are shown in Figures 2C and 2D, respectively. Analysis of chronic tolerance indicated a significant main effect of age [F(1, 85)=14.38 P<.001] for duration of LORR with no significant differences in BEC at regain. These results suggest possible pharmacokinetic differences between the age groups on day 5; adult members of both strains may absorb and/or metabolize ethanol faster than adolescents following five days of ethanol treatment at this dose.
Experiment 2 – Pharmacokinetic Sensitivity
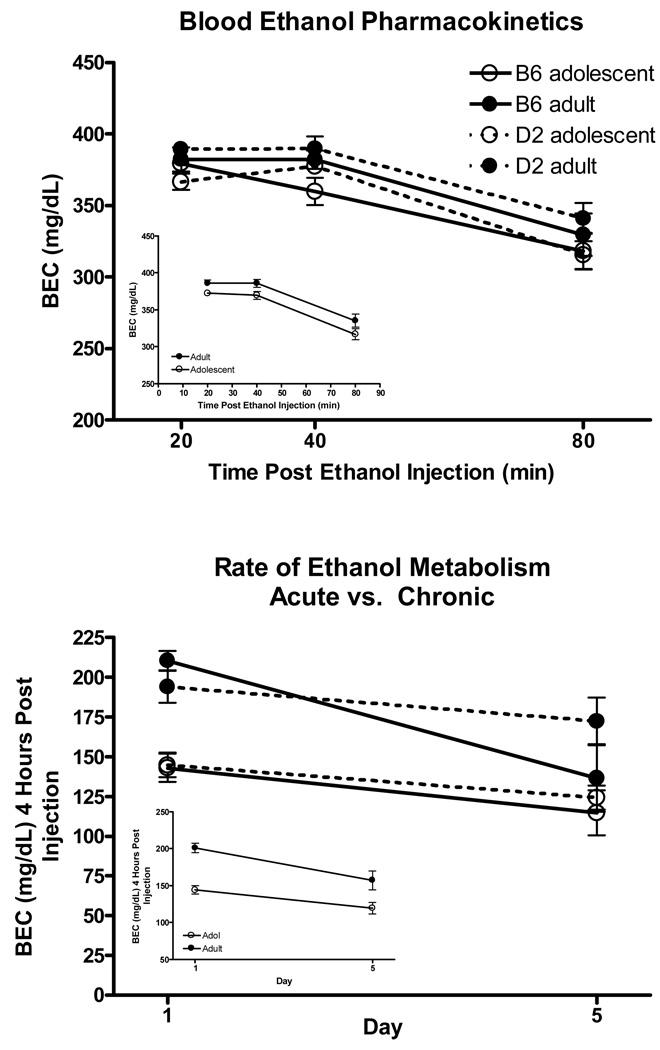
Overall differences in BEC at the different time intervals following acute administration of 4.0 g/kg ethanol can be seen in Figure 3C. Four-way analysis of BEC at the 20, 40 and 80 minute time intervals indicated significant main effects of age [F(1, 30)=8.02 P<.01], with adolescent animals having lower overall BECs than adults, and bin [F(2, 60)=50.95 P<.001] with BEC’s decreasing over time. The lack of any significant time interactions (P>.05) indicated that there were no differences in the slopes of the lines between any of the 3 times points.
Figure 3.
Development of rapid and chronic tolerance to ethanol-induced LORR (3.5 g/kg) in adolescent and adult C57BL/6J and DBA2/J mice. A. Change in Blood ethanol concentrations after regaining the righting reflex (4.0 g/kg) on day 5 compared to day 1. B. Change in the duration of LORR in adolescent and adult mice (4.0 g/kg) on day 5 compared to day 1. C. Change in Blood ethanol concentrations after regaining the righting reflex (3.5 g/kg) on day 5 compared to day 1. D. Change in the duration of LORR in adolescent and adult mice (3.5 g/kg) on day 5 compared to day 1. All change score were calculated by subtracting the second or fifth day values from those of day 1. Positive BEC change scores represent increases in BEC. Negative LORR change scores represent decreases in LORR duration.
Experiment 2 – Pharmacokinetic Tolerance
Changes in ethanol metabolism following chronic administration of the 4.0 g/kg dose can be seen in Figure 3D. Three-way analysis of BEC at the 4 hour time point on days 1 and 5 revealed significant main effects of age [F(1, 33)=33.8 P<.001], day [F(1, 33)=19.3 P<.001], and sex [F(1,33)=5.1 P<.05]. Overall, adolescents had lower BEC’s than adults, BEC’s were significantly decreased on day 5 compared to day 1, and females had lower BEC’s than males.
Experiments 3 and 4 – Sensitivity
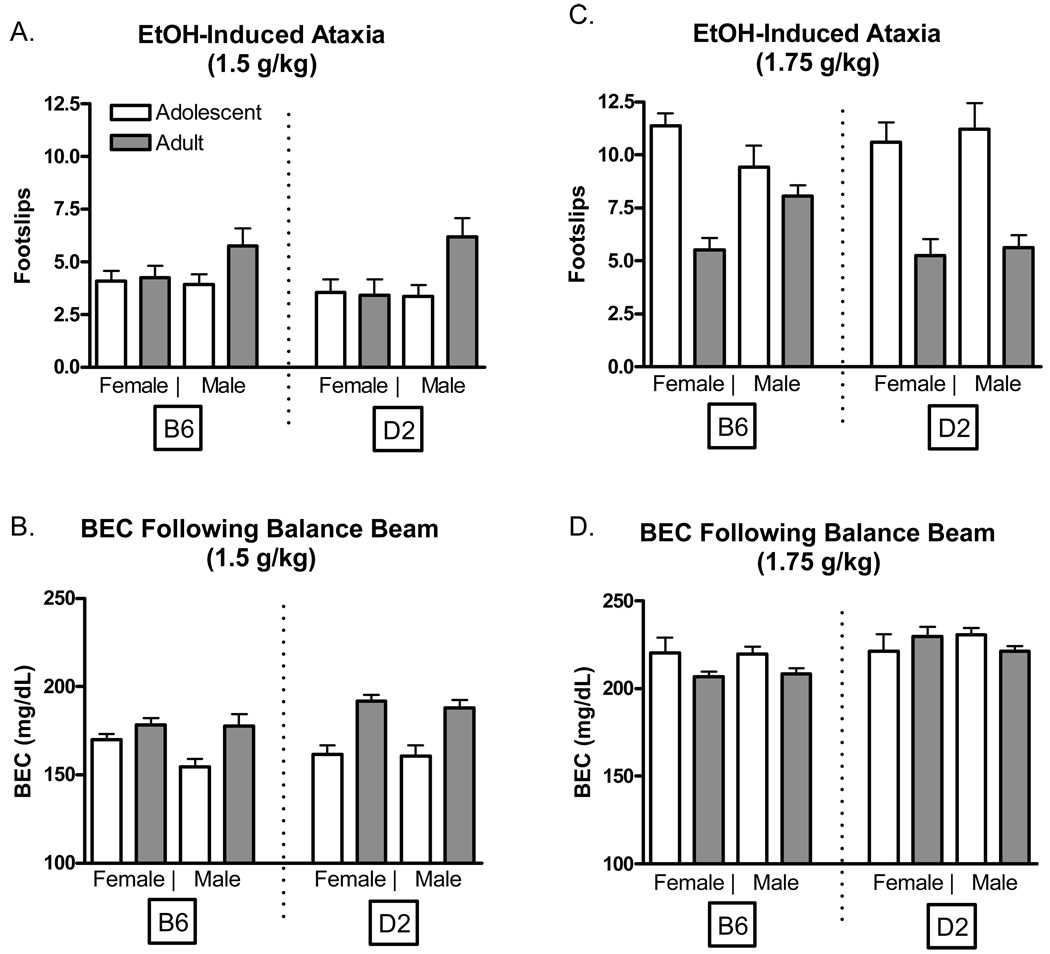
Sensitivity to ethanol’s ataxic effects at the 1.5 g/kg ethanol dose are shown in Figures 4A and 4B. The four-way analysis of hind footslips indicated significant main effects of sex [F(1, 89)=4.46 P<.05] and age [F(1, 89)=6.3 P<.05], as well as a significant sex*age interaction [F(1, 89)=6.1 P<.05]. Although adult males of both genotypes appeared to display a greater number of footslips at testing, post-hoc analysis did not detect any significant group effects. Analysis of BECs at the 1.5 g/kg dose indicated a significant main effect of age [F(1, 89)=41.54 P<.001] suggesting differences in the rate of ethanol absorption. Adult mice exhibited higher BECs immediately following testing, perhaps indicating that at least at this ethanol dose they may absorb ethanol at a faster rate than do adolescents.
Figure 4.
Sensitivity to ethanol-induced ataxia (1.5 and 1.75 k/kg) in adolescent and adult C57BL/6J and DBA2/J mice. A. Number of footslips in adolescent and adult mice (1.5 g/kg). B. Blood ethanol concentrations after performance on the balance beam (1.5 g/kg). C. Number of footslips in adolescent and adult mice (1.75 g/kg). D. Blood ethanol concentrations after performance on the balance beam (1.75 g/kg).
Sensitivity to ethanol’s ataxic effects at the 1.75 g/kg ethanol dose are shown in Figures 4C and 4D. Analysis of hind footslips at the 1.75 g/kg dose indicated significant main effects of age [F(1, 104)=59.62 P<.001] and a significant age*sex*genotype interaction [F(1, 104)=4.03 P<.05]. Post-hoc tests revealed that the three-way interaction was driven largely by the male B6 adult animals which had significantly more hind footslips than adult D2 males and females, and significantly fewer footslips than adolescent D2 males (P’s<.05). Although adult B6 male and female mice appeared to exhibit slower absorption compared to all other groups following administration of the 1.75 g/kg dose, BEC analysis indicated only a significant main effect of genotype [F(1, 104)=7.27 P<.01].
Experiments 3 and 4 – Tolerance
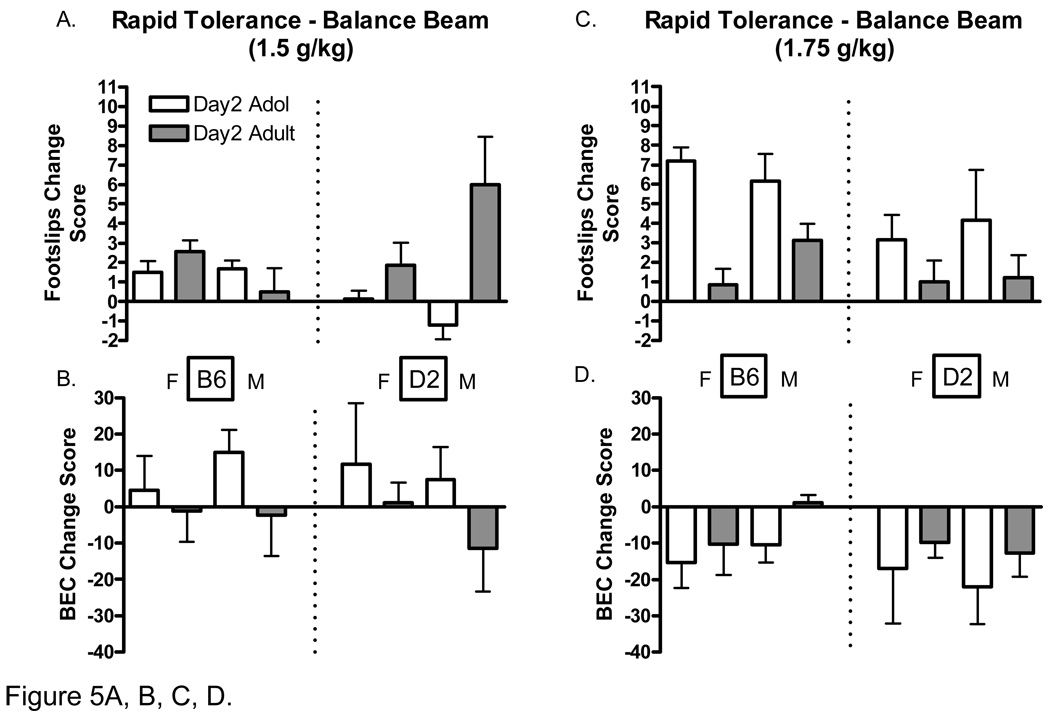
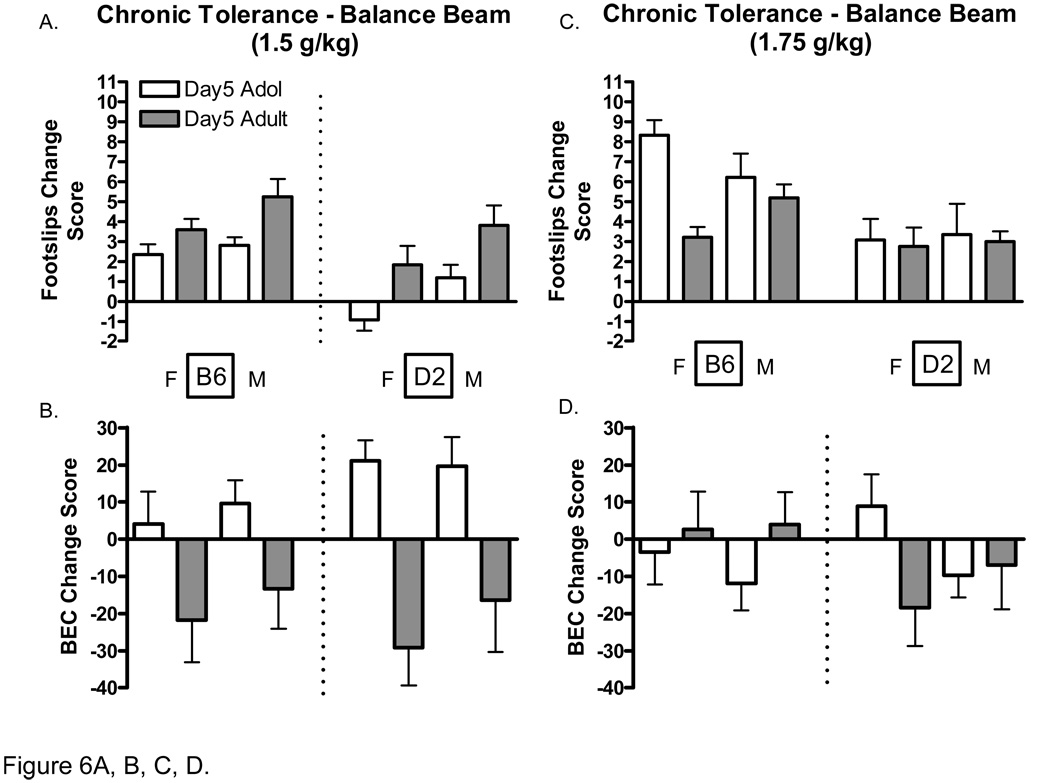
Differences in the development of rapid and chronic tolerance to the ataxic effects of ethanol can be seen in figure 5 and figure 6, respectively. Change scores were calculated by subtracting day 2 values from those on day 1 for footslips (day1 – day2 = footslips change score), and by subtracting day 1 values from those on day 2 for BECs (day2 – day1 = BEC change score). Whereas positive BEC change scores indicate increased rate of absorption compared to day one, negative values indicate decreased. Positive footslip change scores indicate a decreased number of footslips (or tolerance), whereas negative values indicate increased footslips compared to day one. Although the focus of this analysis was on differences between age groups and genotypes the interested reader is referred to Table 4 for the raw balance beam tolerance data.
Figure 5.
Development of rapid tolerance to ethanol-induced ataxia (1.5 and 1.75 k/kg) in adolescent and adult C57BL/6J and DBA2/J mice. A. Change in the number of footlslips in adolescent and adult mice (1.5) on day 2 compared to day 1. B. Change in Blood ethanol concentrations after performance on the balance beam (1.5 g/kg) on day 2 compared to day 1. C. Change in the number of footlslips in adolescent and adult mice (1.75) on day 2 compared to day 1. D. Change in Blood ethanol concentrations after performance on the balance beam (1.75 g/kg) on day 2 compared to day 1. Footslip change scores were calculated by subtracting the 1st day values from those of day 2. BEC change scores were calculated by subtracting the 2nd day values from those of day 1. Positive footslip change scores represent decreases in footslips. Positive BEC change scores represent increases in rates of ethanol absorption (BEC).
Figure 6.
Development of chronic tolerance to ethanol-induced ataxia (1.5 and 1.75 k/kg) in adolescent and adult C57BL/6J and DBA2/J mice. A. Change in the number of footlslips in adolescent and adult mice (1.5) on day 5 compared to day 1. B. Change in Blood ethanol concentrations after performance on the balance beam (1.5 g/kg) on day 5 compared to day 1. C. Change in the number of footlslips in adolescent and adult mice (1.75) on day 5 compared to day 1. D. Change in Blood ethanol concentrations after performance on the balance beam (1.75 g/kg) on day 5 compared to day 1. Footslip change scores were calculated by subtracting the 1st day values from those of day 5. BEC change scores were calculated by subtracting the 5th day values from those of day 1. Positive footslip change scores represent decreases in footslips. Positive BEC change scores represent increases in rates of ethanol absorption (BEC).
Table 4.
| 1.5 Tolerance | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | Age | Sex |
N (Day 2) |
Footslips (Day 2) |
BEC (Day 2) |
N (Day 5) |
Footslips (Day 5) |
BEC (Day 5) |
|
| C57BL/6J | Adol | Male | 6 | 2.0 ±0.36 | 170.5 ± 7.33 | 15 | 1.13 ± 0.21 | 164.3 ± 6.11 | |
| Female | 6 | 3.0 ±0.44 | 172.16 ± 7.64 | 11 | 1.72 ± 0.33 | 174.1 ± 7.79 | |||
| Adult | Male | 4 | 4.25 ± 0.85 | 180.47 ± 8.77 | 12 | 0.5 ± 0.19 | 164.25 ± 7.35 | ||
| Female | 8 | 3.12 ± 0.61 | 170.01 ± 13.06 | 13 | 4.46 ± 0.44 | 182.77 ± 2.92 | |||
| DBA/2J | Adol | Male | 5 | 3.6 ± 0.6 | 172.4 ± 10.4 | 11 | 2.18 ± 0.26 | 180.27 ± 2.83 | |
| Female | 7 | 2.14 ± 0.34 | 173.67 ± 7.17 | 13 | 0.66 ± 0.14 | 156.45 ± 0.14 | |||
| Adult | Male | 4 | 1.25 ± 0.25 | 184.7 ± 11.03 | 11 | 2.36 ± 0.7 | 171.63 ± 12.17 | ||
| Female | 7 | 1.85 ± 0.34 | 190.64 ± 3.43 | 12 | 1.58 ± 0.35 | 162.5 ± 9.22 | |||
| 1.75 Tolerance | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | Age | Sex |
N (Day 2) |
Footslips (Day 2) |
BEC (Day 2) |
N (Day 5) |
Footslips (Day 5) |
BEC (Day 5) |
|
| C57BL/6J | Adol | Male | 6 | 4.5 ± 0.76 | 208.2 ± 7.92 | 14 | 3.21 ± 0.43 | 207.88 ± 8 | |
| Female | 10 | 4.4 ± 0.22 | 201.02 ± 10.97 | 18 | 3.05 ± 0.33 | 216.99 ± 5.49 | |||
| Adult | Male | 8 | 5.12 ± 0.76 | 207.13 ± 2.47 | 15 | 2.86 ± 0.46 | 212.3 ± 9.05 | ||
| Female | 6 | 5 ± 0.57 | 202.83 ± 4.79 | 13 | 2.3 ± 0.48 | 209.34 ± 9.46 | |||
| DBA/2J | Adol | Male | 6 | 6.83 ± 0.47 | 204.86 ± 18.07 | 14 | 7.85 ± 0.98 | 220.9 ± 3.83 | |
| Female | 8 | 8.5 ± 0.8 | 211.48 ± 12.72 | 15 | 7.53 ± 0.49 | 230.34 ± 2.46 | |||
| Adult | Male | 5 | 4.2 ± 0.8 | 208.22 ± 6.85 | 11 | 2.63 ± 0.49 | 214.33 ± 11.44 | ||
| Female | 6 | 5.0 ± 1 | 220.6 ± 8.78 | 12 | 2.5 ± 0.45 | 211.45 ± 9.1 | |||
To examine the possibility that experience with the balance beam on day 2 might influence subsequent performance on day 5, only half the animals tested for sensitivity on day 1 were tested for rapid tolerance on day 2 (all mice were tested for chronic tolerance on day 5). Therefore, although all mice received ethanol injections on day 2, only a subset of them were actually tested for balance beam performance on that day. However, there were no significant main effects or interactions in day 5 performance between mice that had been tested on day 2 and mice that had not, so footslip change scores for all mice were collapsed for subsequent analysis of chronic tolerance on day 5. It is nevertheless important to realize that our efforts to control for possible practice effects significantly reduced our N on day 2. For this reason our power to detect significant effects was somewhat limited on that day.
Day 2 balance beam performance and corresponding BECs following administration of the 1.5 g/kg and 1.75 g/k ethanol dose are shown in Figure 5. Analysis of hind footslip data following administration of the 1.5 g/kg dose indicated a significant main effect of age [F(1, 39 =10.75 P<.01]. There were also significant age*genotype [F(1, 39 =11.22 P<.01] and age*genotype*sex [F(1, 39 =8.17 P<.01] interactions. Post-hoc tests confirmed that whereas the age*genotype*sex interaction was largely due to the apparent greater rapid tolerance developed by D2 adult males compared to all other groups (P<.05), the age*genotype interaction was largely driven by the relatively poorer performance of the D2 adolescents compared to all other groups (P’s≤.05). These results are shown in Figure 5A. Analysis of hind footslip data following administration of the 1.75 g/kg dose indicated significant main effects of age [F(1, 46 =15.81 P<.001] and genotype [F(1, 46 =4.58 P<.05]; adolescents generally developed greater rapid tolerance than adults, and B6 mice generally developed greater rapid tolerance than D2 mice, at the 1.75 g/kg dose (Figure 5C). There were no significant differences between groups in the rate of absorption (BECs) at either dose (Figures 5B and 5D).
Day 5 balance beam performance and corresponding BECs following administration of the 1.5 g/kg and 1.75 g/k ethanol dose are shown in Figure 6. Analysis of hind footslip data following administration of the 1.5 g/kg ethanol dose indicated significant main effects of age [F(1, 89 =20.77 P<.001], genotype [F(1, 89 =16.54 P<.001], and sex [F(1, 89 =9.7 P<.01]. However, there was also a significant main effect of age for BECs with adolescent mice exhibiting overall higher BECs than adult mice, suggesting that differences in the rate of ethanol absorption may have accounted for at least some of the observable behavioral differences in chronic tolerance developed at this dose. Analysis of hind footslip data following administration of the 1.75 g/kg dose indicated a significant main effects of age [F(1, 104 =5.96 P<.05] and genotype [F(1, 104 =15.09 P<.001] with adolescents generally exhibiting a greater number of footslips compared to adults, and B6 mice generally exhibiting a greater number of footslips than D2 mice. There were no significant differences in the rate of absorption (BECs) following administration of the 1.75 g/kg ethanol dose.
Discussion
The available literature suggests that adolescents exhibit altered behavioral sensitivity and tolerance to ethanol (see Spear, 2000 for review). The present data add to this literature by suggesting that adolescent and adult B6 and D2 inbred mice exhibit altered sensitivity and tolerance to ethanol’s hypnotic and motor impairing actions. In experiment 1, we demonstrated that adolescent D2 mice are less sensitive to the hypnotic actions of a 4 g/kg ethanol dose than adults of the same genotype (and all other groups), and that there are fundamental differences in the development of both rapid and chronic tolerance between age groups, likely due to shifts in both pharmacokinetics and brain sensitivity. In experiment 2, we extended these findings to examine the magnitude of pharmacokinetic differences inferred by the experiment 1 behavioral data. These results demonstrated that adolescent mice had overall lower BEC’s following both acute and chronic administration 4.0 g/kg ethanol administration. Therefore, although all groups developed various degrees of behavioral and/or metabolic tolerance, the apparent age differences may have been due in part to differences in the relative amount of ethanol exposure both acutely and as a function of time. Together these results support recent mouse literature indicating differences in sensitivity to LORR during adolescence in mice (Hefner and Holmes, 2007) while providing additional evidence in support of significant genetic and pharmacokinetic contributions to both sensitivity and tolerance.
In experiments 3 and 4, we assessed sensitivity and tolerance to ethanol’s motor impairing actions using the balance beam apparatus. Our first experiment at the 1.5 g/kg dose indicated that adolescents had faster rates of ethanol absorption which most likely contributed at least in part to the observed behavioral differences. Taking these differences into account, female adolescents were similarly (B6) if not more (D2) impaired than female adults of the same genotype at this dose. At the slightly higher 1.75 g/kg dose, adolescents of both genotypes were found to be more sensitive to ethanol’s ataxic effects with the exception of the adolescent B6 males which were not statistically different from adult males of the same genotype. There were also genotype differences in ethanol absorption with D2 animals exhibiting generally faster rates than B6 animals. Furthermore, there were age and genotype differences in the development of rapid and chronic tolerance although the differences in chronic tolerance may have been in part due to differences in the rates of ethanol absorption. The current results add to the growing literature suggesting altered adolescent sensitivity to ethanol’s motor incoordinating actions. Whereas Hollstedt et al. (1980) and White (2002) demonstrated decreased sensitivity to the ataxic effects of ethanol in adolescent rats using the tilting-plane apparatus, recent work in mice (Hefner and Holmes, 2007) using the rotorod reported increased sensitivity to these effects. Our data support the later findings indicating the existence of species and/or task related differences with respect to ethanol-induced ataxia in adolescent and adult mice, and suggest that genotype is a contributing factor.
Sensitivity to ethanol’s hypnotic actions
Data showing decreased sensitivity to the hypnotic actions of ethanol in adolescent animals compared to adults is consistent with the current rat (Silveri and Spear, 1998) and mouse (Hefner and Holmes, 2007) literature. However, whereas Hefner and Holmes (2007) found no differences in BECs at recovery from LORR between age groups, our results indicate that these differences may in fact exist in mice but that they tend to emerge as a function of genotype. Our findings in B6 mice at the 4.0 g/kg dose are consistent with Hefner and Holmes (2007) data showing shorter sleep times in adolescents with no age-related differences in BEC at regain. These results together with our metabolism data suggest that sensitivity to ethanol’s hypnotic effects between age groups in B6 mice is not qualitatively different at this dose. These results are more interesting in light of data indicating that compared to their adult counterparts, D2 adolescents are less sensitive to ethanol’s hypnotic actions at the same dose despite evident differences in ethanol pharmacokinetics.
Tolerance to ethanol’s hypnotic actions
Animals that develop tolerance to LORR are expected to recover the righting response at higher BECs than they had on the previous day or days. If one assumes a constant rate of blood ethanol clearance, tolerant animals should regain their righting reflex earlier on the descending limb of the blood ethanol concentration curve (Boehm et al. 2003, 2004). In other words, tolerance can be defined as the ability of an animal to show a particular behavior (in this case, regain of the righting response) at a higher BEC than it had previously. Although the observed changes in BEC’s were generally in the expected direction (higher on days 2 and 5), significantly shorter LORR durations were not always paralleled by significantly higher BEC’s. Whereas these results may be suggestive of pharmacokinetic influences such as changes in the rate of blood ethanol absorption/metabolism with repeated exposures (metabolic tolerance), the development of within session tolerance (acute functional tolerance) or even changes in the magnitude and/or rate of acute functional tolerance over repeated ethanol exposures cannot be ruled out.
Despite the possibility that pharmacokinetic and/or acute functional tolerance may have contributed to the current findings, it is likely that both adolescent and adult animals developed at least some rapid and chronic tolerance to ethanol-induced LORR. Indeed, both age groups exhibited higher BECs upon recovery from LORR on days 2 and 5 compared to day 1, although these effects were only ever statistically significant for adults on day 2 (table 3). Thus, given the fact that we tested mice during early adolescence, the present results appear to be consistent with the rat literature suggesting that the ability to develop rapid tolerance emerges at some point during adolescence (Silveri and Spear 1999), and that younger animals show a decreased ability to develop chronic tolerance than their adult counterparts (Lagerspetz, 1972). Our primary interest was to evaluate the degree of rapid and chronic tolerance developed in adolescent and adult mice. To that end, our data suggest that age- and genotype-dependent differences in the degree of rapid and chronic tolerance, not independent of pharmacokinetic alterations, do in fact exist.
There are several caveats to the above discussion. First, a short sleep time upon first ethanol exposure provides little room (floor effect) for behavioral tolerance to develop. This may explain why significant tolerance to the duration of sleep time was only observed in adult animals. Second, there were a surprising number of adolescent animals that did not loose the righting reflex on day 2 or day 5, either because they had become completely tolerant to the hypnotic effects of ethanol, or because their relative smaller size made successful intraperitoneal injection more difficult. A similar problem was reported by Hefner and Holmes (2007) following administration of the 3.5 g/kg dose. These animals had to be eliminated from the study (see Table 1 for a detailed list of animals that failed to loose the righting response). Nevertheless, if some of these adolescents had actually become completely insensitive to the repeated ethanol administrations, our adolescent group means may not reflect the true level of tolerance achieved by these animals. One way to address this problem may be to simply repeat the test using increasingly larger ethanol doses. However, adult animals would likely begin to experience harmful metabolic and hypothermic effects that could complicate and confound our behavioral measure. Indeed, ethanol induced hypothermia has been shown increases with dose and to influence the rates of metabolism in adult mice (Romm &Collins, 1987). As hypothermic influences may have already been in play within the current data set, future studies examining age differences in sensitivity and tolerance to the hypothermic effects of ethanol are currently under way.
Finally, if groups of animals differ in the rate of ethanol absorption and/or metabolism, it may be that any differences in the development of tolerance are due to the relative degree of exposure in this group per unit time. For example, if the duration of ethanol exposure were the only variable driving the development of tolerance, adult animals would consistently develop greater degrees of tolerance. However, while the duration of ethanol in blood and brain likely play a major role in the development of tolerance, these influences are but one possible contribution to the observed group differences (or lack thereof) which future studies looking into acute functional tolerance will surely begin to elucidate.
Sensitivity to ethanol’s ataxic effects
Adolescent mice were generally found to be significantly more sensitive than adults to the ataxic effects of ethanol. However, it is possible that the influence of ethanol coupled with the smaller scaled beam combined synergistically to create a comparatively more difficult task. Our decision to scale the beam was based on considerable literature examining size and gate differences in adolescent and adult rats (Doremus et al., 2003, 2004, 2006; Varlinskaya and Spear, 2002, 2006, 2007). To our knowledge no work characterizing mouse size in relationship to task performance on the balance beam apparatus have been published. Further evaluation of the relative size difference between adolescent and adult mice and the degree to which these differences may lead to different degrees of behavioral performance irrespective of ethanol challenge is warranted.
Assuming our scaling of the beam was appropriate, an alternative explanation for the observed age-dependent differences in behavioral sensitivity on first exposure could have been that the age groups differed in BEC at the time of testing. That is, differences in absorption/metabolism of ethanol rather than differences in performance under similar levels of “intoxication” could have lead to the observed age differences in the number of ethanol-induced hind footslips. However, in experiment 5, an increased sensitivity in adolescents was observed following administration of the higher ethanol dose (1.75 g/kg) despite detecting no significant differences in BEC immediately following testing. Thus, the present data would appear to more appropriately reflect differences in behavioral impairment under similar levels of intoxication.
Tolerance to ethanol’s ataxic effects
To our knowledge this report is the first to examine tolerance to the motor incoordinating actions of ethanol in adolescent mice. The results of experiment 3 and 4 indicate that there were differences in the development of rapid and chronic tolerance to the ataxic effects of ethanol between all groups, but that the significance of these effects depended greatly on dose. For example, although there were no significant differences in ethanol pharmacokinetics on the second day of testing (rapid tolerance) at either dose, age-dependent behavioral differences were in opposite directions with larger improvements in adults at the 1.5 g/kg dose and larger improvements in adolescents at the 1.75 g/kg dose. Furthermore, age-dependent differences in BEC’s on the 5th day of testing (chronic tolerance) following administration of the 1.5 g/kg dose strongly suggest pharmacokinetic influences, whereas the lack of such effects following administration of the 1.75 g/kg dose suggests that these effects are independent of such influence. These results may argue in favor of age-related bidirectional dose-dependent changes in the development of tolerance to ethanol’s motor incoordinating actions, with younger animals developing tolerance at higher doses and adult animals developing tolerance at lower doses. Importantly, the previously mentioned differences in sensitivity to ethanol-induced ataxia on this apparatus may contribute to the relative differences in tolerance. Additional work will be necessary to more adequately describe this complicated picture.
Conclusion
The present results add to the growing literature suggesting that adolescents and adults differ in sensitivity and tolerance to ethanol’s hypnotic and ataxic effects. Our goal was to compare sensitivity to these ethanol behavioral actions in B6 and D2 inbred mouse strains known to differ widely in sensitivity to many ethanol related behavioral phenotypes. We predicted that adolescent mice would be less sensitive and develop greater tolerance to these ethanol behavioral measures, and that the two genotypes would differ in sensitivity to these ethanol behavioral responses at both ages.
Our results were mixed. Surprisingly, only adolescent D2 animals were found to be less sensitive than adults of the same genotype to ethanol-induced hypnosis. Age differences in the degree of tolerance to this effect varied by number of ethanol exposures. Whereas adult animals showed decreased sensitivity on their second exposure compared to adolescents (rapid tolerance), after repeated exposures greater metabolic contributions emerged (chronic metabolic tolerance). Ethanol-induced ataxia varied considerably by dose. Overall, adolescents were equally or more sensitive than adults to ethanol’s motor incoordinating actions. Interestingly, across all experiments, groups that were the most sensitive also showed the largest degrees of tolerance within a particular dose. Although additional ethanol doses would undoubtedly provide a more complete picture, it is tempting to speculate that sensitivity to ataxia is predictive of tolerance to the same behavior. If reduced sensitivity and enhanced tolerance development to ethanol’s actions does in part predispose adolescents to consume larger amounts of ethanol compared to their adult counterparts (Spear, 2000), than perhaps the observed differences in adolescent sensitivity to ethanol-induced ataxia (particularly in D2 mice) are less influential then are the age differences in sensitivity to ethanol’s hypnotic actions. Future studies should examine sensitivity and tolerance to these and other ethanol related behavioral measures using multiple age groups and a panel of inbred mouse strains to more fully elucidate possible genetic contributions influencing ontogenetic differences in behavioral sensitivity to ethanol.
Acknowledgments
This work was supported in part by grants from NIAAA (AA015434) and the Center for Development and Behavioral Neuroscience at Binghamton University.
References
- 1.Boehm SL, 2nd, Peden L, Chang R, Harris RA, Blednov YA. deletion of fyn-kinase gene alters behavioral sensitivity to ethanol. Alcohol Clin Exp Res. 2003;27(7):1033–1040. doi: 10.1097/01.ALC.0000075822.80583.71. [DOI] [PubMed] [Google Scholar]
- 2.Boehm SL, 2nd, Peden L, Jennings AW, Kojima N, Harris RA, Blednov YA. Over-expression of the fyn-kinase gene reduces hypnotic sensitivity to ethanol in mice. Neurosci Lett. 2004;372(1–2):6–11. doi: 10.1016/j.neulet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham CL, Niehus DR, Malott DH, et al. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- 4.Crabbe JC, Metten P, Yu CH, Schlumbohm JP, Cameron AJ, Wahlsten D. Genotypic differences in ethanol sensitivity in two tests of motor incoordination. J Appl Physiol. 2003;95(4):1338–1351. doi: 10.1152/japplphysiol.00132.2003. [DOI] [PubMed] [Google Scholar]
- 5.Crabbe JC, Metten P, Ponomarev I, Prescott CA, Wahlsten D. Effects of genetic and procedural variation on measurement of alcohol sensitivity in mouse inbred strains. Behav Genet. 2006;36(4):536–552. doi: 10.1007/s10519-006-9067-6. [DOI] [PubMed] [Google Scholar]
- 6.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescents and adult rats. Pharmacol Biochem Behav. 2003;75(2):411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 7.Doremus TL, Varlinskaya EI, Spear LP. Age-related differences in elevated plus maze behavior between adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:427–430. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- 8.Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83(4):570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Finn DA, Bejanian M, Jones BL, McGivern RF, Syapin PJ, Crabbe JC, Alkana RL. Body temperature differentially affects ethanol sensitivity in both inbred strains and selected line of mice. J Pharmacol Exp Ther. 1990;253(3):1229–1235. [PubMed] [Google Scholar]
- 10.Hawkins JD, Graham JW, Maguin E, et al. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. I. Coordination as measured by the tilting-plane test. Med Biol. 1980;55:164–168. [PubMed] [Google Scholar]
- 12.Hoff J. Methods of Blood Collection in the Mouse. Lab Animal. 2000;29(10):47–53. [Google Scholar]
- 13.Johnston LD, O’Malley PM, Bachman JG. Monitoring the future: national results on adolescent drug use overview of key findings. 2002 NIH Publication No. 03-53742003. [Google Scholar]
- 14.Kalant H. Problems in the search for mechanisms of tolerance. Alcohol Alcohol. 1993;2:1–8. [PubMed] [Google Scholar]
- 15.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 16.Lagerspetz KY. Postnatal development of the effects of alcohol and of the induced tolerance to alcohol in mice. Acta Pharmacol Toxicol. 1972;31(5):509–520. doi: 10.1111/j.1600-0773.1972.tb03613.x. [DOI] [PubMed] [Google Scholar]
- 17.Lopez M, Simpson D, White N, Randall C. Age- and sex-related differences in alcohol and nicotine effects in C57BL/6J mice. Addict Biol. 2003;8(4):419–427. doi: 10.1080/13556210310001648176. [DOI] [PubMed] [Google Scholar]
- 18.Markwiese BJ, Acheson SK, Levin ED, et al. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- 19.McClearn G, Rodgers D. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–695. [Google Scholar]
- 20.Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behav Pharmacol. 1994;5:533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., 2nd GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88(1):105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips TJ, Huson M, Gwiazdon C, et al. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res. 1995;10:269–278. doi: 10.1111/j.1530-0277.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 23.Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther. 2002;302:257–263. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- 24.Romm E, Collins AC. Body temperature influences on ethanol elimination rate. Alcohol. 1987;6:33–38. doi: 10.1016/0741-8329(87)90042-5. [DOI] [PubMed] [Google Scholar]
- 25.Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 26.Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- 27.Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 28.Silveri MM, Spear LP. Acute, rapid and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- 29.Spear LP. The adolescent brain and age-related behavioral manifestations [Review] Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 30.Spear LP. Adolescent brain development and animal models. Ann NY Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- 31.Spear LP, Varlinskaya EI. Adolescence: Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. Review. [PubMed] [Google Scholar]
- 32.Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology. 2007;197(3):361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart DG, Brown SA. Withdrawal and dependence symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- 34.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Ethanol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 35.Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Ethanol Clin Exp Res. 2006;30:1833–1844. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;1:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 38.Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press; 2003. [PubMed] [Google Scholar]