Abstract
Invariant CD1d-restricted natural killer T (NKT) cells play important roles in regulating both innate and adaptive immunity. They are targeted by HIV-1 infection and severely reduced in number or even lost in many infected subjects. Here, we have investigated the characteristics of NKT cells retained by some patients despite chronic HIV-1 infection. NKT cells preserved under these circumstances displayed an impaired ability to proliferate and produce IFNγ in response to CD1d-restricted lipid antigen as compared to cells from uninfected control subjects. HIV-1 infection was associated with an elevated expression of the inhibitory programmed death-1 (PD-1) receptor (CD279) on the CD4- subset of NKT cells. However, blocking experiments indicated that the functional defects in NKT cells were largely PD-1 independent. Furthermore, the elevated PD-1 expression and the functional defects were not restored by antiretroviral treatment (ART), and the NKT cell numbers in blood did not recover significantly in response to treatment. The functional phenotype of NKT cells in these patients suggests an irreversible immune exhaustion due to chronic activation in vivo. The data demonstrate a severe functional impairment in the remaining NKT cell compartment in HIV-1 infected patients which limits the prospects to mobilize these cells in immunotherapy approaches in patients.
Keywords: Human, NKT cells, HIV, CD1d, PD-1
Introduction
Natural killer T (NKT) cells are unconventional T lymphocytes that operate on the border between the innate and the adaptive immune systems, and have some characteristics of both systems [1-3]. Their invariant TCR recognize lipid and glycolipid antigens in complex with CD1d molecules expressed primarily on dendritic cells (DCs), monocytes and B lymphocytes [4-6]. In line with their capacity to influence both innate and adaptive immunity, they are believed to be important regulatory cells in diverse settings of autoimmunity [7], cancer [8], allergy [9], and infectious diseases [10]. The observation that NKT cells are efficiently targeted and lost in HIV-1 infection was therefore of significant interest [11-13], as their loss may play a role in the severe immune dysregulation and chronic immune activation that is characteristic of this infection [14-18].
Human NKT cells have a constitutive memory T-cell like phenotype which includes expression of HIV-1 co-receptors CCR5 and CXCR4 [11, 13, 19], and direct infection may be a primary cause of their loss in infected subjects. However, there are several subsets of NKT cells which differ in their expression of surface receptors. CD4 functions as a co-receptor in NKT cells [20, 21], and also defines a functionally distinct subset that usually represents roughly half of cells [22-24]. Although the CD4+ subset is more susceptible to HIV-1 infection in vitro and probably also in vivo [11, 13], loss of NKT cells in patients may not be restricted to the CD4 expressing cells [11, 12, 25]. The recovery of NKT cells in patients starting anti-retroviral treatment (ART) is probably slow at best [25], and in some cohorts insignificant during the first year of treatment [26, 27]. However, one study reported rapid recovery of the CD4- NKT cell subset already three months after the onset of ART [28]. This issue thus warrants further study. The balance between CD4+ and CD4- subsets of NKT cells and the alterations in this balance caused by HIV-1 infection may have significant clinical importance given the distinct functional profiles exhibited by these two subsets [1, 22, 23]. Notably, however, there is significant individual variation with regard to the extent of NKT cell loss, with some patients retaining almost normal NKT cell counts whereas other have counts below detection.
In this study, we investigated the phenotypic and functional characteristics of NKT cells retained by some patients during chronic untreated HIV-1 infection, and the ability of ART to restore these cells. The data show that the NKT cells present in these patients display poor capacity to expand in response to stimulation with α-galactosyl ceramide (αGalCer), low IFNγ production at the single cell level and elevated expression of the inhibitory receptor programmed death 1 (PD-1) [29]. This functional phenotype is indicative of immune exhaustion suggesting chronic ongoing activation of these cells in vivo. This state of functional exhaustion was not restored by ART and the NKT cell numbers did not recover in response to this treatment. Together the data indicate that patients who retain relatively healthy numbers of NKT cells in chronic HIV-1 infection nevertheless have a severe functional impairment in the NKT cell compartment.
Results
Some patients with chronic HIV-1 infection retain CD1d-restricted NKT cells in circulation irrespective of treatment
CD1d-restricted invariant NKT cells have a constitutive memory T cell-like phenotype with a high level of CCR5 expression making them good targets for HIV-1 infection [11-13, 24, 30]. Nevertheless, some HIV-positive subjects retain almost healthy numbers of these cells despite ongoing viral replication. The functional properties of these NKT cells have not been studied in detail. As this may be of importance for the participation of NKT cells in regulation and activation of immune responses in HIV+ subjects, we here studied prospectively four groups of patients with chronic HIV-1 infection for a period of up to 40 months: 1) untreated patients with ongoing viral replication; 2) patients on ART with no detectable virus; 3) patients on ART with unstable control of virus; and 4) patients starting ART after the first blood sample was drawn (Table 1). Fresh PBMC from patients and healthy control donors were stained for Vα24/Vβ11 NKT cells and analyzed by flow cytometry (Fig. 1A). The Vα24/Vβ11 mAb combination consistently, in both infected subjects and uninfected controls, identified cells binding the αGalCer-loaded CD1d DimerX reagent. In a cross-sectional analysis at baseline 11 out of a total of 36 patients (31%) had measurable numbers of NKT cells (Fig. 1B). There was no apparent difference between subjects on ART where five out of 19 (26%) displayed measurable NKT cells, and untreated patients where six out of 16 (38%) had detectable NKT cells. Although numbers fluctuated somewhat over time, NKT cell counts were generally stable in longitudinal analysis and no patients who were initially negative gained detectable NKT cells over time. Notably, this was also true for patients in group 4 who initiated ART. Whereas these patients showed a significant recovery of their CD4 T cell counts (P = 0.022), no such effect of ART could be seen for NKT cells (Fig. 1B, Group 4). Thus, a minority of patients in this study had detectable NKT cell counts in chronic stages of HIV-1 infection. Patients without NKT cells at baseline did not recover detectable counts over time irrespective of ART, and ART was unable to support an increase in NKT cells in patients starting treatment.
Table 1.
Patient group characteristics at baseline
| Characteristic | Group 1 n=9 | Group 2 n=9 | Group 3 n=10 | Group 4 n=7 |
|---|---|---|---|---|
| Initial CD4 T cell count, median cells/μL (range) | 535 (691-1082) | 572 (349-1085) | 364 (35-1261) | 224 (3-343) |
| Initial HIV-1 RNA, median log10 copies/mL (range) | 4 (2.5-4.7) | <1.7 | 2.2 (<1.7-5.1) | 4.8 (4.2-6.2) |
| Gender, male (%) | 44 | 78 | 80 | 71 |
| Age, median years (range) | 42 (26-63) | 40 (31-52) | 41 (30-55) | 36 (19-47) |
| Ethnicity, caucasian (%) | 67 | 67 | 70 | 71 |
| Transmission, sexual (%) | 56 | 89 | 80 | 86 |
| Month since positive test, median (range) | 54 (7-218) | 97 (20-157) | 175 (33-225) | 98 (35-241) |
| Months on ART, median (range) | N/A | 69 (7-83) | 83 (14-101) | N/A |
| CD4 T cell count before initiation of ART, median cells/μL (range) | N/A | 202 (50-440) | 240 (20-370) | 244 (3-343) |
Figure 1.
Some HIV-1 infected patients have NKT cells that persist over time irrespective of ART. (A) Identification of CD1d-restricted NKT cells in peripheral blood. CD1d DimerX-αGalCer binding NKT cells were identified among CD3+ cells by co-expression of the T cell receptor chains Vα24 and Vβ11 as determined by flow cytometry. (B) Longitudinal measurement of NKT cells counts (cells/μl), CD4+ T cell counts (cells/μl) and viral load (copies/ml, horizontal line indicates the limit of detection) in (Group 1, n=9) untreated patients with ongoing viral replication; (Group 2, n=9) patients on ART with no detectable virus; (Group 3, n=10) patients on ART with unstable control of virus; and (Group 4, n=7) patients starting ART after the first blood sample was drawn. Patients with NKT cell counts above the limit of detection are indicated in red. The vertical line in panel (Group 4) indicates the start of treatment. *** indicates P < 0.001, and n.s. indicates non-significant as determined by the paired T-test.
NKT cells in HIV-infected subjects display poor proliferative capacity
With the data at hand showing that some patients in the cohort retain CD1d-restricted NKT cells during chronic untreated or treated HIV-1 infection, we next studied the functionality of these cells. The ability of NKT cells in peripheral blood samples from patients and healthy control subjects to expand in response to ex vivo stimulation with αGalCer + IL-2 was assessed over 13 days in the presence of the anti-retroviral drug AZT. NKT cell numbers in PBMC from healthy donors expanded more than 250-fold over the course of the assay (Fig. 2A). Interestingly, NKT cells in samples from the chronically HIV-1 infected subjects displayed a severely impaired ability to proliferate in this assay, and this was true for both CD4+ and CD4- NKT cells (P < 0.001) (Fig. 2A). Moreover, this defect in proliferation was not restored by ART (Fig. 2B). The trend observed in healthy donors that CD4+ NKT cells had greater proliferative capacity than their CD4- counterparts was maintained and exaggerated in the HIV-1 infected patients indicating that the proliferative impairment was more severe in the CD4- NKT cells (Fig. 2C). CD4+ NKT cells expanded as a percentage of the total NKT cell population in samples from both untreated and treated patients (P < 0.013 and P < 0.001, respectively). Taken together, these data indicate that the invariant NKT cells which remain in some patients with chronic HIV-1 infection are severely defective in their proliferative capacity.
Figure 2.

Poor proliferative capacity in NKT cells from HIV-1 infected subjects. The proliferative capacity of NKT cells was assessed by culturing PBMC in triplicates in the presence of antigen (αGalCer, 100 ng/ml), IL-2 (10 ng/ml) and the anti-retroviral drug AZT (10 μM). NKT cell frequencies were determined by FACS analysis at day 0 and 13, and the average fold expansion was calculated for each donor. (A-B) Comparison of the proliferative capacity of NKT cells and CD4- and CD4+ NKT cell subsets from healthy donors (HIV-, n=13) and HIV-1 infected subjects (HIV+, n=21), as well as untreated (n=9) and treated patients (n=17), respectively. NKT cell numbers were assessed before (d0) and after culture for 13 days (d13), and the fold expansion of NKT cells was calculated. (C) Proportion of CD4+ NKT cells before (d0) and after culture for 13 days (d13). Data are shown as mean ± SD. * indicates P < 0.05, *** indicates P < 0.001, and n.s. indicates non-significant as determined by the Mann-Whitney Rank Sum Test (A) and paired T-test (C).
Impaired IFNγ production in NKT cells in HIV-infected subjects
Rapid production of large amounts of IFNγ upon activation is an important part of the innate-like NKT cell response, which is critical to the ability of these cells to activate and regulate other immune cells. Therefore, the capacity of NKT cells in blood from HIV-1 infected patients to produce IFNγ in response to αGalCer ex vivo was assessed at the single cell level by intracellular cytokine flow cytometry (Fig. 3A). Percent IFNγ-positive NKT cells was significantly reduced in infected subjects as compared to healthy donors (P = 0.007) (Fig. 3B). The IFNγ response in NKT cells was not significantly stronger in the patients on ART, suggesting that the partial loss of IFNγ production could not be reversed by treatment in this cohort (Fig. 3C). Thus, the CD1d-restricted NKT cells retained in circulation in some chronically HIV-infected subjects display an impaired ability to produce IFNγ.
Figure 3.
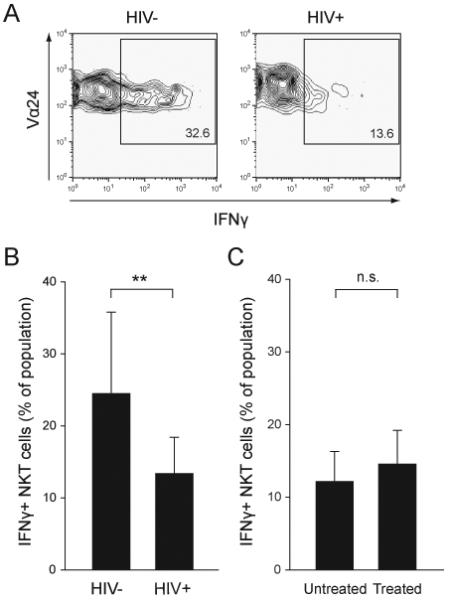
Impaired IFNγ production in residual NKT cells in HIV-1 infected patients. The production of IFNγ in NKT cells was measured at the single cell level by intracellular staining and FACS analysis after stimulation with αGalCer. (A) Representative contour plots showing the expression of IFNγ in NKT cells from a healthy donor (HIV-) and a HIV-1 infected patient (HIV+). (B-C) graphs showing mean percentages of IFNγ-positive NKT cells ± SD from HIV- (n=12) and HIV+ (n=11) subjects, and untreated (n=5) and treated patients (n=9), respectively. Data shown are mean ± SD. ** indicates P < 0.01, and n.s. indicates non-significant as determined by the T-test.
Elevated PD-1 expression in NKT cells in HIV-infected subjects
PD-1 delivers negative signals that regulate the activation of conventional T cells [29]. The expression and function of PD-1 in human CD1d-restricted NKT cells has not, however, been investigated. We therefore assessed the expression of this receptor in NKT cells in blood (Fig. 4A). Interestingly, HIV-infected patients had an elevated PD-1 expression on NKT cells as compared to healthy control subjects (P = 0.021) (Fig. 4B). The expression of this inhibitory receptor on NKT cells in the infected subjects was close to double that seen on conventional T cells (P < 0.001) (Fig. 4C). Furthermore, the increase in PD-1 expression was mostly confined to the CD4- NKT cell subset (Fig. 4D).
Figure 4.
Elevated PD-1 expression in CD4- NKT cells in HIV-1 infected subjects. (A) PD-1 expression was determined in NKT cells and T cells from HIV- and HIV+ subjects by flow cytometry. Representative contour plots including an isotype control are shown. (B) Percentage of PD-1 expressing NKT cells in healthy controls (HIV-, n=12) and HIV-1 infected patients (HIV+, n=11). (C) PD-1+ NKT cells and T cells in HIV+ subjects. (D) Graphs comparing the percentage of PD-1 positive cells in HIV- and HIV+ subjects in CD4- and CD4+ NKT cell subpopulations. (E) Graphs comparing the proliferative capacity of NKT cells and CD4- and CD4+ NKT cell subsets from HIV-1 infected subjects (n=11) in the presence of anti-PD-L1 plus anti-PD-L2 antibodies or isotype control. (F) Expression of IFNγ in NKT cells from HIV-1 infected patients (n=6) in the presence and absence of anti-PD-L1 plus anti-PD-L2 antibodies. Data are shown as mean ± SD. * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001, and n.s. indicates non-significant as determined by T-test, or alternatively the Mann-Whitney Rank Sum Test in case data failed the normality test.
Whereas the functional capacity of NKT cells was impaired, and PD-1 expression on these cells was elevated in HIV-infected subjects, there was still considerable heterogeneity among the patients with regard to these parameters. We therefore investigated if the PD-1 expression level might correlate with the proliferative capacity or IFNγ production in NKT cells. In healthy control subjects there was no trend towards a correlation between PD-1 expression and proliferative capacity in NKT cells, irrespective of whether the entire population was included or if the CD4+ or CD4- NKT cells were separately analyzed (data not shown). Similarly, IFNγ production in response to αGalCer showed no relationship with PD-1 surface expression in neither healthy subjects nor HIV-1 infected patients (data not shown). In contrast, the proliferative ability of NKT cells in infected subjects tended to decrease with higher PD-1 expression. This trend towards significance was most marked in the CD4- subset of NKT cells (P = 0.097) (data not shown). However, when anti-PD-L1 and anti-PD-L2 antibodies were used to block their interaction with PD-1 there was no significant effect on either proliferative capacity (Fig. 4E), or IFNγ production in response to αGalCer (Fig. 4F).
Taken together, these findings demonstrate that HIV-1 infection is associated with an elevated PD-1 expression in CD4- NKT cells that persist in infected subjects. However, the functional impairment of these cells appears to be largely PD-1 independent.
Discussion
It has been known for some time that CD1d-restricted NKT cells are highly susceptible to HIV-1 infection and lost in many patients. However, less attention has been directed to studies of the functional properties of NKT cells that persist in some patients despite chronic untreated HIV-1 infection. This is likely to have clinical importance given the diverse roles of NKT cells in the regulation and activation of both adaptive and innate immune responses. Here, we have found that the NKT cells retained in some patients proliferate poorly in response to stimulation with αGalCer, have an impaired IFNγ production and high expression of PD-1. These data indicate that the impairment of the NKT cell compartment in HIV-1 infection is multi-faceted, with direct infection and loss in many patients, and a functional phenotype indicative of immune exhaustion in NKT cells that are retained in chronically infected patients.
The functionally exhausted state of the NKT cell compartment observed here may have clinical importance on several levels. Previous observations have indicated that the CD4+ subset is more sensitive to HIV-1 infection, suggesting that this infection leaves a biased NKT cell compartment with the functions mediated by CD4- NKT cells less affected. Because of the different functional profiles of CD4+ and CD4- NKT cells this could in turn mean that the residual NKT cell compartment in patients would be biased towards a Th1 inflammatory and NK cell-activating profile. Our data presented here suggest that also these functions may be suppressed in vivo, as the functional impairment of NKT cells was broad-based and the elevated PD-1 expression was mostly confined to the CD4- subset. It is thus likely that the effect of HIV-1 infection on the NKT cell compartment, at least in chronic stages of infection, is a broad-based impairment rather than a functional biasing.
The functional characteristics of NKT cells in different diseases may be important not only to understand the role of these cells in pathogenesis, but also in the context of therapeutic approaches. The poor function of NKT cells in chronic HIV-1 infection suggests that treatment or vaccine regimens including NKT cell activation may not be suitable in this disease. Whereas ART alone is largely ineffective in restoring the numbers and function of these cells, it remains possible that combination treatment with cytokines such as IL-2 may be more effective. This idea is supported by the previous observation that ART+IL-2 combination treatment can expand NKT cells in vivo in patients with primary HIV-1 infection [26]. In contrast, ART and interferon-α+ribavirin combination treatment in patient with chronic hepatitis C virus and HIV-1 co-infection was unable to support reconstitution of a depleted NKT cell compartment [31].
The high PD-1 expression was mostly confined to the CD4- subset of NKT cells. PD-1 negatively regulates immune responses, and the expression of this receptor is taken to indicate activation-induced exhaustion in conventional MHC-restricted CD8 and CD4 T cells in HIV-infected patients [32-35]. Together, these observations suggest that the CD4- NKT cells are actively involved in attempts by the immune system to control viral replication. This idea is also supported by the recent observation of an elevated expression of activation markers by NKT cells in HIV infection [36]. The nature of such potential NKT cell-mediated anti-viral responses is at present unclear, but the observation that HIV-1 actively down-regulates CD1d expression in antigen-presenting cells in an apparent mode of immune escape supports this notion [37, 38].
Several reports have convincingly shown that PD-1 is highly expressed in conventional MHC-restricted T cells in HIV-1 infection [32-35]. Three reports found PD-1 expression to be associated with exhaustion of HIV-specific CD8 T cell responses and with predictors of disease progression [32, 33, 35], whereas this was less clear in a fourth cohort [34]. Furthermore, PD-1 expression was lower in patients with long-term non-progressive disease [35]. However, the increase in PD-1 was not restricted to HIV-specific CD8 T cells as EBV-specific cells in the same patients were also high in their expression of this receptor [32-34]. On a functional level, PD-1 was linked to an inability of CD8 T cells to proliferate, similar to our observations here with NKT cells. Therapeutic blockade of PD-1 signaling is being considered as a potential way to enhance immunity in chronic infections [39]. However, our results suggest that the poor functionality of NKT cells in this disease is largely independent of PD-1, indicating that such approaches may not benefit the NKT cell compartment.
We report here the functional impairment of residual NKT cells in chronic HIV-1 infection. The elevated PD-1 expression and poor proliferative capacity of NKT cells in this disease has to our knowledge not been reported before. A recent study reported suppressed IFNγ production in NKT cell-enriched PBMC samples from patients with primary infection [27], and this defect was, in contrast to our findings, restored by ART. Direct comparison with the present study is hampered by the fact that whereas we assessed IFNγ directly on the single cell level by intracellular staining, Vasan et al. used indirect measurement in supernatant of an NKT cell-enriched PBMC sample. Another difference is that the present study investigated chronic infection, whereas Vasan et al. studied primary infection. The basis for the diverging results with regard to the ability of ART to restore IFNγ production is at present unclear. However, one may speculate that NKT cells may in an early stage of the disease recover their functional capacity more efficiently in response to ART-mediated suppression of viral replication.
In this study we have measured NKT cell responses to αGalCer added directly to PBMC cultures. A possible concern with this experimental approach is that it depends on the CD1d expression of endogenous antigen presenting cells, and HIV-1 has been reported to down-regulate CD1d [37, 38]. However, we have measured CD1d expression on myeloid DCs in PBMC from the patients included in this study and it does not deviate from that observed in the healthy control subjects (data not shown). This result is not unexpected as productive HIV-1 infection of antigen presenting cells (APCs) in blood is probably very rare. In a second attempt to address this concern, we added αGalCer-loaded CD1d DimerX to PBMC cultures as a direct APC-independent stimulus. This approach generated results very similar to those seen with addition of αGalCer alone to the cultures, i.e. responses were suppressed in the HIV-1 infected patients (data not shown). Thus, the overall picture emerging from our investigations is that the defective IFNγ production and proliferation by NKT cells in HIV+ subjects is most probably NKT cell intrinsic.
The longitudinal measurement of NKT cell numbers was performed with the combination of mAbs against TCR Vα24 and Vβ11 as these mAbs together identify the vast majority of CD1d-restricted NKT cells in humans. However, one should note that whereas the Vα24 α-chain segment is consistently used in the invariant NKT cell TCR, the β-chain can sometimes carry other Vβ segments than the Vβ11. It therefore remains possible that we have slightly underestimated the numbers of NKT cells in both patients and healthy subjects.
In summary, the results of this study indicate a severe functional impairment in the CD1d-restricted NKT cell compartment in HIV-infected patients who retain relatively healthy numbers of these cells in blood. NKT cells in these patients are drastically suppressed in their ability to proliferate and to produce IFNγ in response to glycolipid antigen. Furthermore, NKT cells have an increased expression of the inhibitory receptor PD-1, suggesting activation of these cells in vivo. These results help us understand the full impact of HIV-1 on this arm of cellular immunity and indirectly suggest that these cells may be actively involved in attempts by the immune system to control HIV-1.
Materials and methods
Patient samples and viral load measurements
Patients were followed and treated at the Infectious Diseases clinic at Karolinska University Hospital in Huddinge, Stockholm, Sweden. Patient group characteristics are listed in Table 1. Some patients were on effective ART and some patients initiated ART during the study as indicated in Table 1. For all patients, ART was a triple drug regimen including protease inhibitor. The uninfected healthy control subjects were healthy volunteers donating blood at the Stockholm Blood bank, and matched for age and sex with the average of the patient groups. The study protocols were approved by the local institutional review board. Heparinized whole blood samples were obtained after informed consent. Peripheral blood mononuclear cells (PBMC) were isolated by Lymphoprep gradient centrifugation (Axis-Shield, Oslo, Norway), and washed twice before analysis by flow cytometry. Plasma HIV-1 RNA was measured with the Amplicor HIV-1 Monitor with a lower limit of quantification at 50 copies of RNA/ml (Roche Diagnostics, Branchburg, NJ, USA).
Flow cytometry and mAbs
The following mAbs were used: anti-CD3 PerCp, anti-CD4 APC and Pacific Blue, anti-PD-1 (CD279) FITC, anti-IFNγ PE, recombinant dimeric human CD1d:Ig (DimerX) were all from BD Biosciences (San Diego, CA, USA). Anti-Vβ11 FITC and anti-Vα24 PE and APC were from Immunotech (Marseilles, France). For dead cell exclusion, the LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Invitrogen, Eugene, OR, USA) was used according to the instructions of the manufacturer. PBMCs were stained in a 96-well v-bottomed plate for 30 min at 4°C, and all washes were done in PBS with 5% FCS. Multicolor flow cytometry data were acquired on CyAn ADP (Dako, Copenhagen, Denmark) and FACSCalibur (BD Biosciences) instruments, and analyzed using FlowJo software (Tree Star, OR, USA) [40].
Functional NKT cell assay
Cytokine expression in NKT cells was detected after ex vivo stimulation followed by intracellular cytokine flow cytometry as previously described [41]. Briefly, after thawing and resting over night, 1 × 106 PBMC were incubated in 500 μl RPMI 1640 medium supplemented with 10% fetal calf serum in the presence of αGalCer (200 ng/ml). After 2 h, additional 500 μl medium containing brefeldin A (GolgiPLUG, 2 mg/ml, BD Biosciences) were added, and final concentrations of αGalCer and brefeldin A were 100 ng/ml and 1 μg/ml, respectively. In some experiments, mAbs against human PD-L1 (final concentration 5 μg/ml; eBiosciences) and PD-L2 (final concentration 5 μg/ml; eBiosciences) were added to the cultures to block the interaction between PD-1 and its ligands. Purified mouse IgG1 (final concentration 10 μg/ml; BioLegend) was used as an isotype control. After a total incubation time of 8 h, cells were stained for surface markers to identify NKT cells and subsequently subjected to intracellular cytokine staining. After permeabilization with FACS permeabilizing solution 2 (BD Biosciences) at room temperature for 10 min, cells were incubated with mAb against intracellular IFNγ for 30 min at 4° and analyzed by flow cytometry. Samples were run and analyzed in duplicates.
NKT cell expansion assay
The proliferative capacity of NKT cells was assessed after ex vivo stimulation. After thawing and resting over night, 1 × 105 PBMC were incubated in triplicates in 96-well plates in RPMI 1640 medium supplemented with 10% fetal calf serum, αGalCer (100 ng/ml) and recombinant human IL-2 (10 ng/ml, PeproTech EC, UK). In some experiments, anti-PD-L1 and anti-PD-L2 (5 μg/ml each) or purified mouse IgG1 (10 μg/ml) were added to the cultures to block interaction with PD-1. In order to prevent virus outgrowth, the anti-retroviral drug 3′-azido-3′deoxythymidine (AZT, 10 μM, Sigma-Aldrich, St. Louis, MO, USA) was added to all cultures. AZT was also added in equivalent amounts to all cultures with PBMC from healthy donors. Medium was replenished at day 7 and cultures were analyzed for NKT cell frequencies at day 0 and 13 using flow cytometry.
Statistical analysis
The flow cytometry data and clinical data obtained were analyzed by descriptive statistics, linear regression, T-test, and the Mann-Whitney Rank Sum test, paired T-test and Wilcoxon signed rank test, as appropriate using Sigma Stat software (SPSS, Chicago, IL).
Acknowledgements
This work was supported by grants from the Swedish Research Council (M.M. and J.K.S), the Swedish International Development Agency (J.K.S.), the Swedish Foundation for Strategic Research (M.M. and J.K.S.), the Swedish Physicians Against AIDS Research Foundation (M.M.), the Åke Wiberg Foundation (M.M.), the Jeansson Foundation (M.M.), the Clas Groshinsky Foundation (M.M.), the Swedish National Board of Health and Welfare (J.K.S.), and the US National Institutes of Health grant AI52731 (J.K.S.). We thank Dr. Hans-Gustaf Ljunggren for critical reading of the manuscript, and the nurses Margit Halvarsson and Marja Ahlqvist for help with patient samples.
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandberg JK, Ljunggren HG. Development and function of CD1d-restricted NKT cells: influence of sphingolipids, SAP and sex. Trends Immunol. 2005;26:347–349. doi: 10.1016/j.it.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 4.Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. Curr. Top. Microbiol. Immunol. 2007;314:113–141. doi: 10.1007/978-3-540-69511-0_5. [DOI] [PubMed] [Google Scholar]
- 5.Stronge VS, Salio M, Jones EY, Cerundolo V. A closer look at CD1d molecules: new horizons in studying NKT cells. Trends Immunol. 2007;28:455–462. doi: 10.1016/j.it.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Smed-Sorensen A, Moll M, Cheng TY, Lore K, Norlin AC, Perbeck L, Moody DB, et al. IgG regulates the CD1 expression profile and lipid antigen-presenting function in human dendritic cells via FcγRIIa. Blood. 2008;111:5037–5046. doi: 10.1182/blood-2007-07-099549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamura T, Sakuishi K, Illes Z, Miyake S. Understanding the behavior of invariant NKT cells in autoimmune diseases. J. Neuroimmunol. 2007;191:8–15. doi: 10.1016/j.jneuroim.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Swann JB, Coquet JM, Smyth MJ, Godfrey DI. CD1-restricted T cells and tumor immunity. Curr. Top. Microbiol. Immunol. 2007;314:293–323. doi: 10.1007/978-3-540-69511-0_12. [DOI] [PubMed] [Google Scholar]
- 9.Meyer EH, DeKruyff RH, Umetsu DT. iNKT cells in allergic disease. Curr. Top. Microbiol. Immunol. 2007;314:269–291. doi: 10.1007/978-3-540-69511-0_11. [DOI] [PubMed] [Google Scholar]
- 10.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 11.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J. Exp. Med. 2002;195:869–879. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Vliet HJ, von Blomberg BM, Hazenberg MD, Nishi N, Otto SA, van Benthem BH, Prins M, et al. Selective decrease in circulating Vα24+ Vβ11+ NKT cells during HIV type 1 infection. J. Immunol. 2002;168:1490–1495. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 13.Sandberg JK, Fast NM, Palacios EH, Fennelly G, Dobroszycki J, Palumbo P, Wiznia A, et al. Selective loss of innate CD4+ Vα24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 2002;76:7528–7534. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 15.Hazenberg MD, Stuart JW, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 16.Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 17.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 18.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 19.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+ Vβ11+ NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–16. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 20.Thedrez A, de Lalla C, Allain S, Zaccagnino L, Sidobre S, Garavaglia C, Borsellino G, et al. CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood. 2007;110:251–258. doi: 10.1182/blood-2007-01-066217. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Wang X, Besra GS, Gumperz JE. Modulation of CD1d-restricted NKT cell responses by CD4. J. Leukoc. Biol. 2007;82:1455–1465. doi: 10.1189/jlb.0307163. [DOI] [PubMed] [Google Scholar]
- 22.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandberg JK, Stoddart CA, Brilot F, Jordan KA, Nixon DF. Development of innate CD4+ alpha-chain variable gene segment 24 (Vα24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7058–7063. doi: 10.1073/pnas.0305986101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang OO, Wilson SB, Hultin LE, Detels R, Hultin PM, Ibarrondo FJ, Jamieson BD. Delayed reconstitution of CD4+ iNKT cells after effective HIV type 1 therapy. AIDS Res. Hum. Retroviruses. 2007;23:913–922. doi: 10.1089/aid.2006.0253. [DOI] [PubMed] [Google Scholar]
- 26.Moll M, Snyder-Cappione J, Spotts G, Hecht FM, Sandberg JK, Nixon DF. Expansion of CD1d-restricted NKT cells in patients with primary HIV-1 infection treated with interleukin-2. Blood. 2006;107:3081–3083. doi: 10.1182/blood-2005-09-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasan S, Poles MA, Horowitz A, Siladji EE, Markowitz M, Tsuji M. Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int. Immunol. 2007;19:943–951. doi: 10.1093/intimm/dxm055. [DOI] [PubMed] [Google Scholar]
- 28.van der Vliet HJ, van Vonderen MG, Molling JW, Bontkes HJ, Reijm M, Reiss P, van Agtmael MA, et al. Cutting edge: Rapid recovery of NKT cells upon institution of highly active antiretroviral therapy for HIV-1 infection. J. Immunol. 2006;177:5775–5778. doi: 10.4049/jimmunol.177.9.5775. [DOI] [PubMed] [Google Scholar]
- 29.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Der Vliet HJ, Nishi N, de Gruijl TD, von Blomberg BM, van den Eertwegh AJ, Pinedo HM, Giaccone G, Scheper RJ. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95:2440–2442. [PubMed] [Google Scholar]
- 31.Gonzalez VD, Falconer K, Michaelsson J, Moll M, Reichard O, Alaeus A, Sandberg JK. Expansion of CD56- NK cells in chronic HCV/HIV-1 co-infection: reversion by antiviral treatment with pegylated IFNα and ribavirin. Clin. Immunol. 2008;128:46–56. doi: 10.1016/j.clim.2008.03.521. [DOI] [PubMed] [Google Scholar]
- 32.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 33.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 34.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 36.Montoya CJ, Catano JC, Ramirez Z, Rugeles MT, Wilson SB, Landay AL. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin. Immunol. 2008;127:1–6. doi: 10.1016/j.clim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Cho S, Knox KS, Kohli LM, He JJ, Exley MA, Wilson SB, Brutkiewicz RR. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology. 2005;337:242–252. doi: 10.1016/j.virol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, McMichael AJ, Screaton GR, Xu XN. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 2006;36:278–286. doi: 10.1002/eji.200535487. [DOI] [PubMed] [Google Scholar]
- 39.Riley JL, June CH. The road to recovery: translating PD-1 biology into clinical benefit. Trends Immunol. 2007;28:48–50. doi: 10.1016/j.it.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez VD, Bjorkstrom NK, Malmberg KJ, Moll M, Kuylenstierna C, Michaelsson J, Ljunggren HG, Sandberg JK. Application of nine-color flow cytometry for detailed studies of the phenotypic complexity and functional heterogeneity of human lymphocyte subsets. J. Immunol. Methods. 2008;330:64–74. doi: 10.1016/j.jim.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandberg JK, Bhardwaj N, Nixon DF. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Vα24 NKT cell compartment. Eur. J. Immunol. 2003;33:588–596. doi: 10.1002/eji.200323707. [DOI] [PubMed] [Google Scholar]




