SUMMARY
Iron acquisition, mediated by specific outer membrane receptors, is critical for colonization of the urinary tract by uropathogenic Escherichia coli (UPEC). The role of specific iron sources in vivo, however, remains largely unknown. In this study, we identified a 79 kDa heme receptor, heme acquisition protein Hma, and established that it functions independently of ChuA to mediate hemin uptake by UPEC strain CFT073. We demonstrated that expression of hma promotes TonB-dependent hemin utilization and the Hma protein binds hemin with high affinity (Kd=8 μM). Hma, however, lacks conserved His residues shown to mediate heme uptake by other bacterial receptors. In contrast, we identified Tyr126 as a residue necessary for Hmamediated hemin utilization. In a murine co-infection model of UTI, an isogenic hma mutant was outcompeted by wildtype CFT073 in the kidneys (P<0.001) and spleens (P<0.0001) of infected mice, indicating its expression provided a competitive advantage in these organs. Furthermore, a hma chuA double mutant, which is unable to utilize hemin, was unable to colonize the kidneys to wildtype levels during independent infection (P=0.02). Thus, we demonstrate that UPEC requires heme for kidney colonization and that uptake of this iron source is mediated, in part, by the novel receptor, Hma.
Keywords: c2482, iron, outer membrane, UPEC, UTI
INTRODUCTION
Bacteria have evolved highly specialized systems to acquire iron, an essential nutrient, from their environment. For example, iron-chelating siderophores, secreted by many bacterial species, function to scavenge iron from host proteins or the environment. The near absence of free iron within mammalian hosts makes these uptake systems essential for bacterial pathogens during infection.
In Gram negative bacteria, uptake of ferrisiderophores and other iron complexes is facilitated by specific outer membrane receptors. These 70–80 kDa proteins are structurally conserved, forming transmembrane beta-barrels with an N-terminal plug domain obstructing the pore of the protein (Buchanan et al., 1999; Ferguson et al., 1998). To function, these receptors require the energy-transducing activity of an inner membrane-periplasmic protein complex composed of ExbB, ExbD, and TonB (Fischer et al., 1989; Skare et al., 1993).
In addition to siderophore-mediated iron acquisition, many bacterial species can scavenge heme-bound iron. Specific outer membrane receptors bind host hemoproteins and transfer the coordinated heme molecule into the periplasm where an ABC transport system delivers it to the cytoplasm. Alternatively, hemophores scavenge heme and subsequently transfer it to specific outer membrane receptors in a process analogous to siderophore-mediated iron uptake (Wandersman and Stojiljkovic, 2000).
The majority of high affinity heme or hemoglobin receptors share four conserved histidine residues and two motifs, the FRAP and NPNL domains (Bracken et al., 1999). Two of these conserved histidines are required for HemR-, HmuR-, or ShuA-mediated heme utilization in Yersinia enterocolitica, Porphyromonas gingivalis, or Shigella dysenteriae, respectively (corresponding to His128 and His461 in HemR) (Bracken et al., 1999; Burkhard and Wilks, 2007; Liu et al., 2006). Structural modeling has predicted these residues to reside extracellularly and recent evidence indicates they function to ligate heme (Burkhard and Wilks, 2007).
In pathogenic E. coli, heme uptake is facilitated by the ChuA receptor, which shares >99% amino acid sequence identity with ShuA, the S. dysenteriae heme-hemoglobin receptor (Torres and Payne, 1997). A study examining the distribution of shuA homologs in pathogenic E. coli by Southern hybridization found that, indeed, most heme-utilizing E. coli contain the shu locus (Wyckoff et al., 1998). However, several heme-utilizing strains were shuA-negative, even under reduced stringency conditions. Thus, the authors predicted the presence of an additional heme uptake gene in these strains whose sequence differs significantly from that of shuA (Wyckoff et al., 1998).
Like other bacterial pathogens, uropathogenic E. coli (UPEC), the primary cause of uncomplicated urinary tract infections, requires TonB-dependent outer membrane iron receptors for host colonization (Torres et al., 2001). Reflecting the importance of iron acquisition for UPEC pathogenesis, the genome of the representative pyelonephritis strain CFT073 encodes at least 14 different outer membrane iron receptors (Welch et al., 2002). While several of these have been shown to contribute to the fitness of UPEC in vivo (Johnson et al., 2005; Russo et al., 2001; Russo et al., 2002; Torres et al., 2001), the importance of specific sources of host iron remains unknown.
Putative iron receptor c2482 was identified by our laboratory as an antigenic outer membrane protein expressed under iron limitation and induced during growth in human urine (Alteri and Mobley, 2007; Hagan and Mobley, 2007). Like other outer membrane iron receptors, the 2148 bp c2482 gene encodes a 79,100 Da protein that is predicted to adopt a beta-barrel structure. No iron transport or processing genes are found in the sequences flanking c2482. However, the promoter region of c2482 contains a putative Fur box and, indeed, work from our laboratory has shown that transcription of this gene is iron-responsive (Alteri and Mobley, 2007). Furthermore, the N-terminal region of c2482 contains a putative TonB box, suggesting that, like other iron receptors, the protein interacts with the inner membrane protein, TonB. Thus, initial evidence suggests that c2482 may function as a receptor for an iron compound.
The c2482 gene appears to be conserved among pathogenic strains of E. coli. DNA dot blot analysis of a panel of E. coli strains showed that c2482 or a close homolog was present in 69% and 50% of uropathogenic and intestinal pathogenic isolates tested, respectively. This differed significantly from the 17% of fecal-commensal E. coli strains that possessed c2482 (Hagan and Mobley, 2007). In addition, c2482 was among 131 genes present in all of 11 UPEC strains, but none of the 6 fecal-commensal strains examined by a recent comparative genomic hybridization study (Lloyd et al., 2007). These findings indicate that c2482 is present more frequently among pathogenic E. coli and suggest that this gene may contribute to the virulence of these pathogens.
Here we show that c2482 functions as a high affinity receptor for heme and demonstrate that heme uptake is required by UPEC for kidney colonization. Thus, we will refer to c2482 as Hma, heme acquisition protein. Furthermore, we identify residues required for Hma-mediated hemin utilization and propose that this protein represents a novel class of heme receptors that are conserved among pathogenic E. coli.
RESULTS
hma contributes to the fitness of CFT073 in vivo
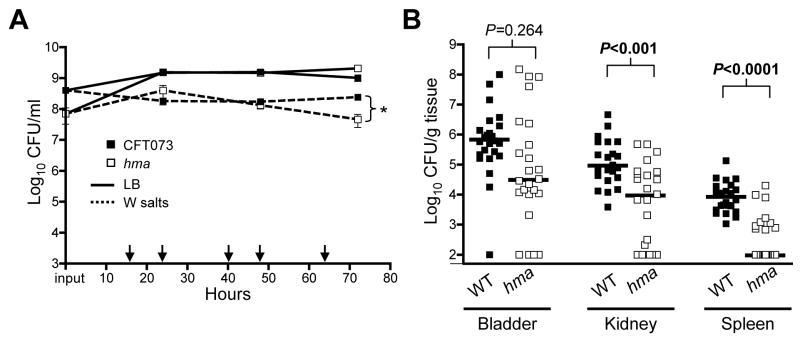
To examine the role of hma in iron acquisition, we constructed a deletion mutant in UPEC strain CFT073. In independent culture in LB medium, the hma mutant had a growth rate similar to wildtype, even in the presence of high concentrations of iron chelator (600 μM 2’2-dipyridyl [DIP]) (data not shown). Since subtle growth defects may not be detectable during independent culture, co-cultures were conducted to compare the ability of the hma mutant to directly compete with wildtype for limited nutrients. Wildtype and mutant were inoculated approximately 1:1 into the same medium and continually re-passaged into fresh medium for 72 hours. In rich medium, the hma mutant reached densities similar to those of wildtype throughout the duration of the experiment, despite an approximately half log lower inoculum (Fig. 1A), demonstrating that no growth defect exists in the mutant strain under these conditions. However in minimal medium (containing no supplemented iron), the hma mutant maintained similar cell densities initially, but was outcompeted by wildtype by 72 hours (P=0.03). Together, these data indicate that hma is not required for growth in vitro in rich medium, but may play a role during nutrient-depleted conditions.
Fig. 1. Fitness of hma mutant in vitro and in vivo.
(A) in vitro culture competition assay of wildtype CFT073 (filled symbols) and hma mutant (open symbols) cultured in Luria broth (solid line) or W salts minimal medium (dashed line). After inoculation (input CFU/ml plotted on y-axis), cultures were passaged into fresh medium every 8 (1:50 dilution) or 16 (1:500 dilution) hours. Arrows indicate culture passages. Means of triplicate cultures are plotted. *P=0.03.
(B) 72 hour CBA/J mouse co-infection with 108 CFU mixture of wildtype (WT) and hma mutant. Data points represent CFU/g of individual animals in the organs indicated; bars show median values (n=24).
Because iron acquisition is required for UPEC pathogenesis, we used a murine model of ascending UTI to investigate the contribution of hma to virulence during experimental infection. Given the redundancy of iron uptake systems in UPEC, we used a co-infection model, transurethrally inoculating mice with a 1:1 ratio of wildtype and mutant in an effort to detect subtle differences in fitness. Total inoculum equaled ~1 × 108 CFU per mouse. At 72 hours post-inoculation, the hma mutant was significantly outcompeted by wildtype in the kidneys (8-fold reduction; P<0.001) and spleens (80-fold reduction; P<0.0001) of infected mice (Fig. 1B). Moreover, the hma mutant was undetectable in the kidneys and spleens of infected mice significantly more frequently than wildtype (P=0.021, P<0.0001, respectively). Thus, hma contributes to the ability of CFT073 to colonize the kidneys and disseminate into the bloodstream. Interestingly, the hma mutant was not significantly outcompeted in the bladders of infected mice, suggesting either localized expression of this gene or localization of the receptor’s iron substrate to the kidneys and bloodstream.
Expression of hma promotes hemin utilization
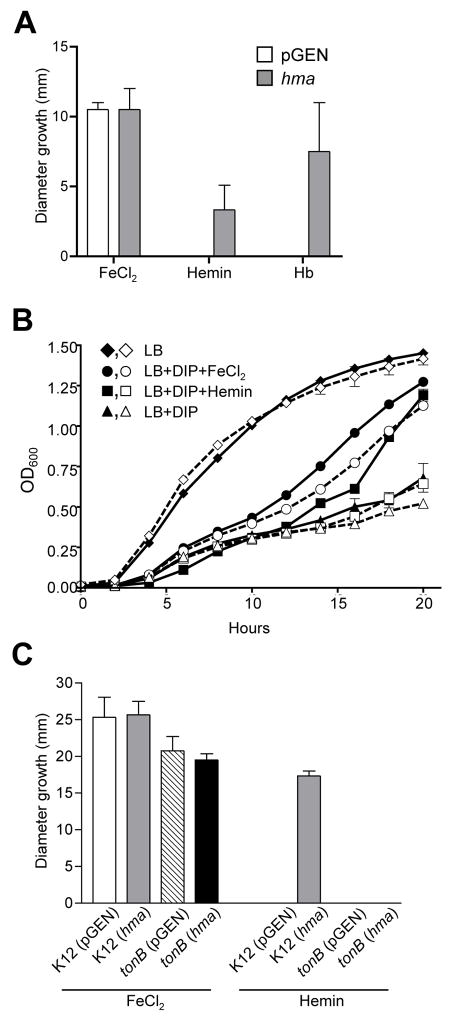
To identify the iron substrate recognized by Hma, a gain-of-function approach was taken. hma was expressed from its native promoter (pnativehma) in E. coli HB101 ent, a laboratory strain deficient in the production of enterobactin, the major siderophore, making it highly susceptible to iron limitation (Torres and Payne, 1997). To screen iron compounds for putative substrates of Hma, iron sources (10 μl) were spotted onto iron-depleted agar overlaid with 105 CFU E. coli HB101 ent. While FeCl2 (1 mM) supported the growth of strains carrying either empty vector or pnativehma, hemin (10 μM) and hemoglobin (1 mg/ml) only promoted the growth of the strain expressing hma (Fig 2A). Lactoferrin, transferrin, and albumin did not promote the growth of either strain (data not shown). A similar result was observed for growth of these strains in broth culture. HB101 ent containing vector control or pnativehma grew similarly in LB, chelated LB, and chelated LB supplemented with 20 μM FeCl2 (Fig. 2B). However, only growth of the strain expressing hma was enhanced by the addition of 10 μM hemin. Together, these data indicate that expression of hma promotes the utilization of hemin and suggests that it likely functions as a receptor for this iron compound.
Fig. 2. Hemin utilization by E. coli strains expressing hma.
(A) Growth of E. coli K12 carrying pGEN (open bars) or pnativehma (gray bars) on iron-depleted agar spotted with 1 mM FeCl2, 10 μM hemin, or 1 mg/ml hemoglobin (Hb). Bars represent mean diameter (mm) growth surrounding indicated iron source (n=3).
(B) Growth of E. coli HB101 ent carrying pGEN vector control (open symbols, dashed lines) or pnativehma (filled symbols, solid lines) in LB (diamonds) or LB + 300 μM DIP supplemented with 20 μM FeCl2 (circles), 10 μM hemin (squares), or no additional iron source (triangles). Cultures were iron-limited overnight prior to inoculation into the media indicated. The mean OD600 of triplicate cultures is plotted.
(C) Growth of E. coli K12 wildtype and tonB mutant on iron-depleted agar spotted with 10 mM FeCl2 or 10 mM hemin. Bars represent mean diameter (mm) growth of E. coli K12 pGEN (open bars), K12 pnativehma (gray bars), tonB pGEN (hatched bars), and tonB pnativehma (black bars) surrounding the indicated iron source (n≥3).
Hma function is TonB-dependent
Other outer membrane iron transporters characterized to date are dependent on the energy-transducing function of the inner membrane protein TonB. Indeed, the N-terminal region of Hma contains a putative TonB interaction site (ETLVV, residues 39–43). To determine if Hma activity requires TonB, hma was expressed from pnativehma in an E. coli K12 tonB mutant. While FeCl2 supported growth of both the parent and mutant strains on iron-depleted medium, expression of hma only promoted hemin utilization by wildtype K12, not the tonB mutant (Fig. 2C). Thus, Hma was unable to function in the absence of TonB, indicating that it is indeed a TonB-dependent receptor.
Hma is a hemin-binding protein
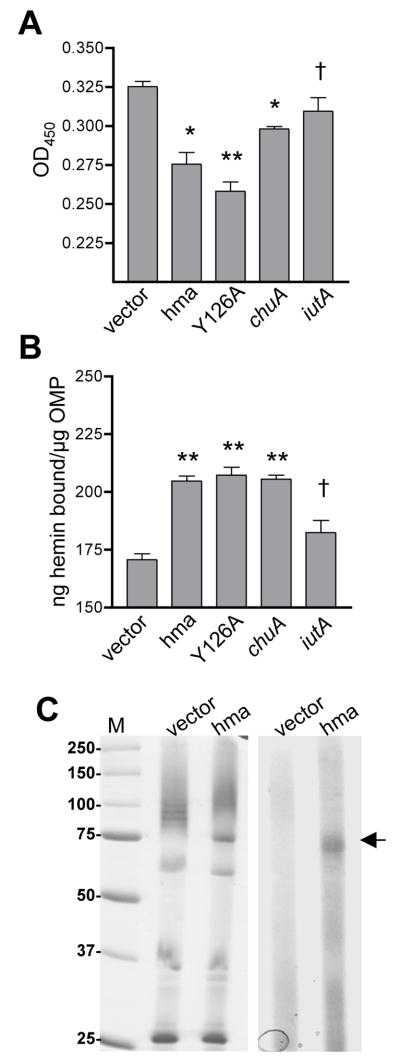
To further test the hypothesis that Hma is a heme receptor, the ability of Hma to directly bind hemin was examined. E. coli K12 whole cells expressing hma, heme receptor chuA, or siderophore receptor iutA, or carrying empty vector were incubated with hemin and pelleted. Hemin bound by the pelleted cells was removed from solution, resulting in a measurable decrease in the heme concentration of the supernatant. Using the intrinsic peroxidase activity of hemin as an indirect measure of heme quantity, we found that cells expressing hma or chuA bound and removed significantly more hemin from the solution than did cells containing a vector control (P=0.001, P=0.0004, respectively) (Fig. 3A). While bacteria expressing iutA bound slightly more hemin than the vector control, this difference was not significant (P=0.145). Similarly, outer membranes isolated from E. coli expressing hma bound an average of 205 ng hemin/μg protein, as compared to 171 ng hemin/μg bound by outer membranes from E. coli carrying empty vector (P<0.0001) (Fig. 3B). These data indicate that hemin binds to cells containing Hma and that at least part of this heme-binding activity is due to a component of the outer membrane.
Fig. 3. Hemin binding activity of Hma.
(A) Hemin binding to E. coli K12 carrying phma, pY126A, pchuA, piutA, or vector control. Induced cells were incubated with 50 μM hemin, pelleted, and hemin remaining in the supernatant detected with a peroxidase substrate. **P<0.0001, *P≤0.001, † not significant (as compared to vector control).
(B) Hemin binding to outer membrane proteins isolated from the strains in (A), as measured by microtiter plate assay. Wells were coated with 0.5 μg protein and incubated with 50 μM hemin. Unbound hemin was removed by washing and, after the addition of a peroxidase substrate, hemin binding was calculated from a standard curve using the OD450. Bars represent the mean (n≥5) and symbols are as in (A).
(C) Hemin binding to Hma protein. OMPs isolated from the strains in (A) were incubated with 85 μM hemin and separated on a non-reducing SDS-PAGE gel. Left panel is Coomassie stained gel and right panel is TMBZ stain of heme-associated peroxidase activity. Arrow indicates Hma band. M, molecular weight standards in kDa.
To detect direct heme-Hma interaction, we incubated outer membrane proteins from E. coli K12 either expressing or not expressing hma with hemin and separated the hemin-protein mixtures on a non-reducing SDS-PAGE gel. The gel was stained with 3,3′,5,5′-tetramethylbenzidine (TMBZ), a chromogenic compound that changes color in the presence of heme-associated peroxidase activity. This activity was localized to an ~80 kDa band, consistent with the size of Hma, that was absent from the vector control lane (Fig. 3C). Together with our previous findings, these data demonstrate that Hma can function as a heme receptor.
Hma binds hemin with high affinity
To define the affinity for which Hma binds heme, we measured the amount of hemin bound by purified Hma-His6 over a range of substrate concentrations. Hemin binding to Hma was saturable and each μg Hma protein bound a maximum of approximately 340 ng hemin (Fig. 4). Using nonlinear regression analysis (R2≥0.820) we estimated the dissociation constant (Kd) for Hma-hemin binding to be 8 μM. Although ChuA-His6 maximally bound less hemin than Hma-His6, it had an identical affinity constant in this assay. Because we were concerned about heme binding by the His6 tag, we also tested purified IutA-His6 and this protein bound hemin with approximately 10-fold lower affinity (Kd=90 μM) than Hma- or ChuA-His6. Thus, heme binding to Hma is specific and occurs with high affinity.
Fig. 4. Heme binding curve.
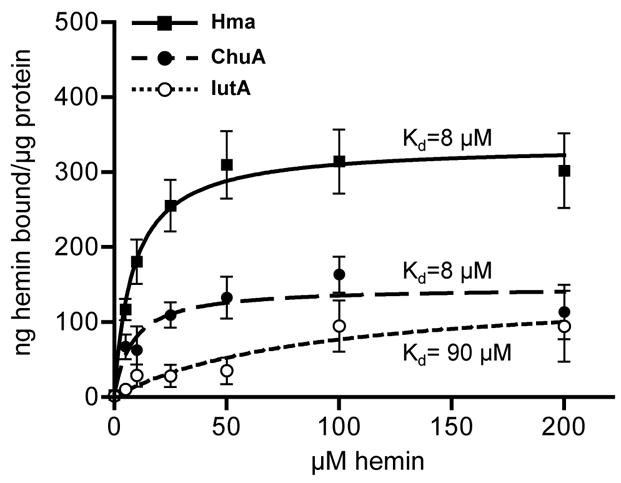
Hemin bound by purified Hma-His6 (solid squares), ChuA-His6 (solid circles), or IutA-His6 (open circles) as a function of substrate concentration. Protein (0.2 μg) was coated onto microtiter plate wells, incubated with hemin (0–200 μM), and bound hemin detected by addition of a peroxidase substrate. Hemin standards were used to calculate ng hemin bound per μg purified protein. Mean values of triplicate samples are plotted. Saturation curves for Hma (solid line, R2=0.820), ChuA (dashed line, R2=0.594), and IutA (dotted line, R2=0.455), determined by nonlinear regression analysis, are also plotted. Dissociation constant (Kd) values for each curve are indicated.
Tyr126 is required for Hma function
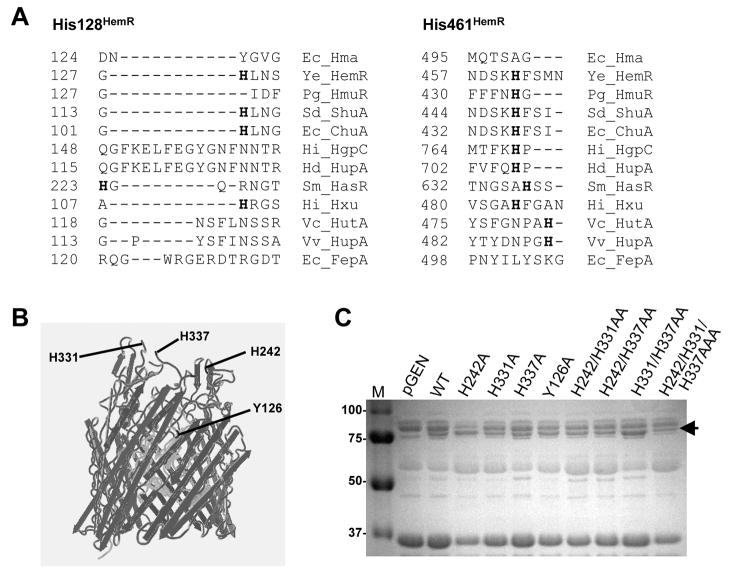
Previous studies have identified four histidine residues conserved among bacterial heme receptors, two of which are required for receptor function (Bracken et al., 1999; Burkhard and Wilks, 2007; Liu et al., 2006). However, while Hma contains a moderately-conserved FRAP-NPNL domain characteristic of other heme receptors, it lacks these conserved His residues (Fig. 5A). To identify other residues that may be important for Hma function, we employed site-directed mutagenesis. By aligning the Hma amino acid sequence with the crystal structure of FepA (Buchanan et al., 1999), a prototypic E. coli outer membrane iron receptor, we predicted extracellular residues that may function in heme binding or transport. His242, His331, and His337 are located in putative extracellular loops of Hma, while Tyr126 is predicted to be on the extracellular face of the N-terminal plug domain of the molecule (Fig. 5B). Furthermore, amino acid alignment of Hma with other heme receptors indicates that Tyr126 aligns with His128 of Y. enterocolitica HemR (Fig. 5A), a residue necessary for receptor function (Bracken et al., 1999).
Fig. 5. Residues required for Hma-mediated heme utilization.
(A) Partial amino acid alignment of Hma with bacterial heme receptors, indicating conserved His residues (bolded) critical for function of HemR (His 128HemR and His 461HemR). Ec, E. coli CFT073; Ye, Y. enterocolitica; Pg, P. gingivalis; Sd, S. dysenteriae; Hi, Haemophilus influenzae; Hd, H. ducreyi; Sm, Serratia marcescens; Vc, Vibrio cholerae; Vv, V. vulnificus.
(B) Structure alignment of Hma with FepA, showing the predicted locations of H242, H331, H337, and Y126 (black). Beta barrel domain (dark gray) and N-terminal plug domain (light gray) are also shown.
(C) SDS-PAGE gel of outer membrane fractions (10 μg) isolated from E. coli K12 containing empty vector, pnativehma, or pnativehma with H242A, H331A, H337A, Y126A, H242A H331A, H242A H337A, H331 337A, or H242A H331A H337A mutations. Strains were iron-limited ~7 h in LB with 200 μM DIP prior to outer membrane fractionation. Arrow indicates ~80 kDa Hma band.
These four residues (His242, His331, His337, and Tyr126) were mutated to Ala in pnativehma and the resulting proteins were expressed in the E. coli K12 outer membrane at wildtype levels (Fig. 5C). We used this evidence of appropriate expression and membrane localization as an indirect indicator of correct protein folding, although it is possible that the mutation(s) disrupted Hma structure. The ability of the mutated Hma proteins to promote heme utilization was assessed by plating these strains on iron-depleted agar containing either FeCl2 or hemin at various concentrations. The lowest concentration of iron compound capable of supporting growth was identified as the minimal supplementary concentration for each strain. E. coli K12 expressing the H242A, H331A, or H337A mutants grew on hemin to the same extent as strains expressing wildtype Hma, indicating that these residues alone are not required for heme utilization (Table 1). To examine the possibility of functional redundancy with respect to the extracellular loop His residues (H242, H331, and H337), double and triple mutants of these residues were tested. Again, all of these mutant Hma proteins were able to facilitate heme utilization to the same extent as wildtype (Table 1). However, function of the Y126A protein was abolished, as the strain expressing this protein could not use even high concentrations of hemin (100 μM). The Y126A mutant Hma retained its hemin binding activity (Fig. 3A, 3B), though, suggesting the importance of this residue in the transport, rather than binding, of heme. Therefore, these data indicate that Tyr126, but none of the putative extracellular loop His residues, is required for the heme-uptake activity of Hma.
Table 1.
Ability of Hma site-directed mutants to mediate hemin utilization.
| Straina | Minimum conc. (μM)required to support growthb |
|
|---|---|---|
| FeCl2 | Hemin | |
| pGEN | 10 | >100 |
| wildtype Hma | 10 | 25 |
| H242A | 10 | 25 |
| H331A | 10 | 25 |
| H337A | 10 | 25 |
| H242A H331A | 10 | 25 |
| H242A H337A | 10 | 25 |
| H331A H337A | 10 | 25 |
| H242A H331A H337A | 10 | 50 |
| Y126A | 10 | >100 |
E. coli K12 containing pGEN vector alone or pnativehma with indicated mutation
growth on sorbitol-MacConkey agar supplemented with 350 μM DIP
Both chuA and hma contribute to CFT073 heme utilization
In addition to Hma, E. coli CFT073 contains another heme or hemoglobin receptor, ChuA. To examine the contribution of each of these proteins to heme utilization by CFT073, a hma chuA isogenic mutant was constructed. Together with the single mutants, the ability of hma chuA to utilize heme as a sole iron source was assessed. Wildtype, the single mutants, and the hma chuA double mutant all required the same concentration of FeCl2 for growth (Table 2). However, the chuA mutant required a higher concentration of hemin as compared to either wildtype or the hma mutant (25 μM as compared to 1 μM) and the double mutant was unable to grow even with the highest concentration of hemin (100 μM). This defect could be complemented by expression of hma from pnativehma, but not with the pGEN empty vector. Therefore, although it appears that chuA contributes more to heme uptake, either chuA or hma is sufficient for hemin utilization by CFT073 in vitro.
Table 2.
Ability of CFT073 heme uptake mutants to utilize hemin as a sole iron source.
| Strain | Minimum conc. (μM)required to support growtha |
|
|---|---|---|
| FeCl2 | Hemin | |
| CFT073 | 10 | 1 |
| hma | 10 | 1 |
| chuA | 10 | 25 |
| hma chuA | 10 | >100 |
| hma chuA (pGEN) | 5 | >100 |
| hma chuA (pnativehma) | 25 | 25 |
growth on sorbitol-MacConkey agar supplemented with 350 μM DIP
Heme uptake is required for maximum kidney colonization
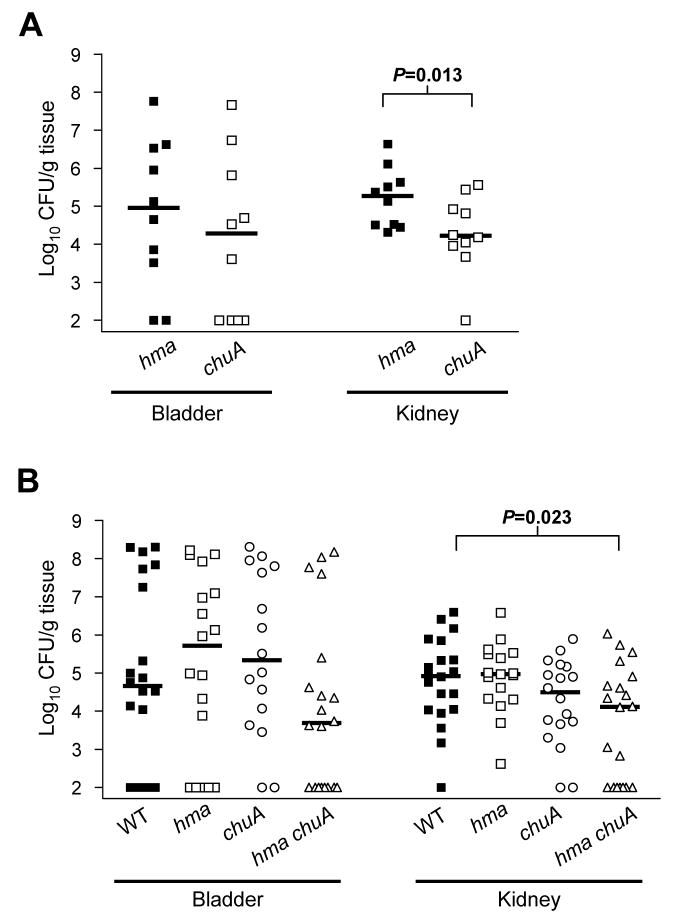
To identify the role of heme uptake for urinary tract colonization by CFT073, as well as define the relative contributions of hma and chuA to this process in vivo, the heme receptor mutants were tested in the mouse model of UTI. After a 72 hour co-infection with a 1:1 mixture of 108 CFU of the chuA and hma mutants, the chuA mutant was found at significantly lower levels in the kidneys of infected animals (P<0.05) (Fig. 6A). This demonstrates that, in the kidney, the strain lacking hma was better able to compete for heme than was the chuA mutant, indicating that the ChuA receptor contributes more to heme uptake in vivo than does Hma. When the chuA and hma mutants were independently inoculated into separate mice, these strains colonized the bladder and kidneys to the same extent as wildtype (Fig. 6B). However, the hma chuA double mutant was found at significantly lower levels in the kidneys of infected mice during independent infection (P=0.023), suggesting the importance of an intact heme uptake system for kidney colonization. While there was only an approximately one log difference between the median CFU/g kidney tissue of hma chuA and wildtype, a significant number of mice inoculated with hma chuA failed to produce a kidney infection (7/20 hma chuA-inoculated mice were uninfected as compared to only 1/20 mice uninfected that were wildtype-inoculated, P=0.044). Together, these data demonstrate the requirement of a heme receptor (either hma or chuA) for efficient kidney colonization by CFT073, as well as provide evidence that heme is an essential source of iron for this pathogen during kidney infection.
Fig. 6. Heme uptake mutants in a mouse model of UTI.
(A) 72 hour CBA/J mouse co-infection with 108 CFU mixture of hma (solid symbols) and chuA (open symbols) mutants. Symbols represent CFU/g tissue in individual animals and bars indicate the median (n=10).
(B) 72 hour independent infections with 108 CFU of wildtype CFT073 (filled squares), hma (open squares), chuA (open circles), or hma chuA (open triangles) mutants (n=20).
chuA and hma are differentially expressed in vivo
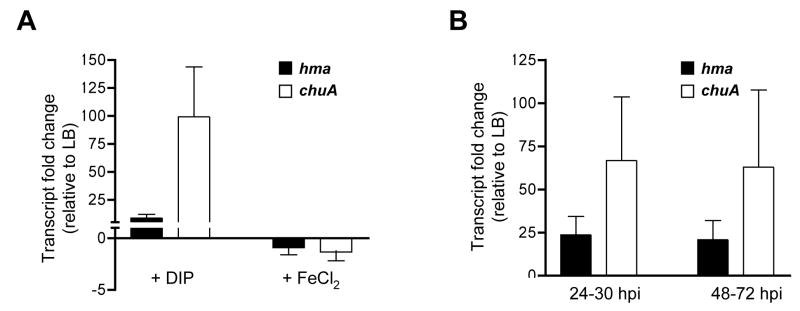
As chuA and hma each encodes a heme receptor, we were surprised to note the phenotypic differences of these two mutants, both in vitro (Table 2) and in vivo (Fig. 6A). While chuA appears to contribute more to heme utilization, both receptors have similar affinities for hemin (Fig 4). To examine potential differences in expression, we compared transcript levels using real-time qPCR of chuA and hma from bacteria cultured in vitro or isolated in vivo. As compared to LB-cultured CFT073, chuA transcript increased an average of 99-fold when bacteria were cultured under iron-limitation, while hma was just 8.8-fold upregulated (Fig. 7A). In the presence of excess FeCl2, transcripts for both genes were slightly decreased (−1.3-fold as compared to LB). Similarly, bacterial transcripts isolated from the urine of CFT073-infected mice showed that chuA was upregulated an average of 67-fold in vivo, while hma increased 24-fold as compared to LB-cultured bacteria (Fig. 7B). Urine samples from PBS-infected control mice did not show significant amplification (data not shown). Together these data indicate that, under the iron-limiting conditions found in vivo, chuA is expressed more highly than hma. This difference in expression level is likely an important factor in the relative contributions of these two receptors to heme utilization.
Fig. 7. in vivo expression of chuA and hma by real-time qPCR.
(A) Fold change of hma (solid bars) and chuA (open bars) transcript levels in E. coli CFT073 cultured in LB supplemented with 200 μM DIP (left) or 10 μM FeCl2 (right), relative to expression in LB alone. Bars represent the mean of four independent experiments.
(B) Fold change of hma (solid bars) and chuA (open bars) transcript levels in the urine of CBA/J mice transurethrally inoculated with 108 CFU of E. coli CFT073, relative to expression in LB. Bars represent the mean of triplicate samples, each sample containing urine collected from 5 animals (n=15) during the timepoints indicated (24–30 hpi, right; 48–72 hpi, left).
DISCUSSION
Hma functions as a heme receptor in uropathogenic E. coli and heme acquisition is necessary for upper urinary tract colonization by this pathogen. Expression of hma promotes TonB-dependent hemin utilization by a laboratory strain of E. coli and confers an ability to bind hemin. Furthermore, purified Hma binds hemin with high affinity (Kd=8 μM). In UPEC, Hma functions independently of ChuA to mediate heme uptake and a strain lacking both of these receptors is deficient for kidney colonization in a mouse model of UTI. Additionally, we demonstrate that, unlike the bacterial heme receptors characterized to date, Tyr126 is required for Hma-dependent hemin utilization. Therefore, we suggest that Hma represents a novel class of heme receptors that is distinct from the HemR family of bacterial heme receptors.
Hma has only limited homology to other characterized bacterial heme receptors. While ChuA shares 70% amino acid sequence identity with HemR, Hma is only 18% identical. By BLAST analysis, Hma is most closely related to TonB-dependent receptors of Dinoroseobacter and Desulfuromonas, marine photosynthetic and sulfur-metabolizing bacteria. In addition to CFT073, copies of hma are present in all sequenced UPEC (F11, UTI89, 538) and enterohemorrhagic E. coli strains (EDL933, Sakai, EC508, EC4042), and a close homolog (73% identical) is found in the infrequent uropathogen Citrobacter koseri. Furthermore, the G+C content of the hma ORF is considerably less than that of the CFT073 genome (45.3% as compared to 50.5%), implying that it may have been acquired horizontally. These findings suggest that hma likely evolved separately from chuA and hemR and may have been conserved among pathogens due to the selective advantage it conferred in vivo.
Previous structure-function studies identified two His residues conserved among heme receptors of Gram negative bacteria that are required for heme uptake (Bracken et al., 1999; Burkhard and Wilks, 2007; Liu et al., 2006). Corresponding to His128 and His461 in Y. enterocolitica HemR, these residues are absent from Hma (Fig. 5A). As His128 and His461 are predicted to be located on the extracellular face of the N-terminal plug domain and on an extracellular loop, respectively (Burkhard and Wilks, 2007), we predicted the structure of Hma to identify putative heme-binding residues in these locations. Tyr126 was predicted to reside extracellularly on the N-terminal plug domain (Fig. 5B) and in amino acid sequence alignments, Hma Tyr126 aligned with HemR His128 (Fig. 5A). Here we show that Tyr126 is required for Hma-mediated hemin utilization (Table 1). While tyrosine is known to occasionally coordinate heme ligands (Arnoux et al., 1999), the requirement of a Tyr residue at this location represents a significant difference between Hma and the previously-studied heme receptors and provides further evidence that hma may have evolved independently of ChuA and HemR.
His242, His331, and His337 are all located on putative extracellular loops of Hma (Fig. 5B); however they are not required for receptor function, either alone or in combination (Table 1). As the Y126A mutant retained its hemin-binding activity, it is likely that additional residue(s) function in binding/transport and compensated for the loss of Tyr126 in this mutant. A number of Tyr residues reside on the putative extracellular loops and additional work is needed to determine if they, or an alternative residue, participate with Tyr126 in Hma-mediated heme uptake.
We estimated the affinity of Hma for hemin to be in the micromolar range (Kd=8 μM). Observed hemin binding to IutA (Kd=90 μM) likely represented binding to the His6 tag present on the purified proteins or other nonspecific interactions. While the affinities of most heme receptors, including HemR, are unknown, our result is similar to the Kd=5 μM and Kd=24 μM measured for S. marsescens HasR and P. gingivalis HmuR receptors, respectively (Izadi-Pruneyre et al., 2006; Olczak et al., 2001).
As for most bacterial pathogens, iron acquisition within the iron-limited host is crucial to the virulence of UPEC. A tonB mutant was severely attenuated in vivo, indicating that TonB-dependent systems are required for UPEC colonization (Torres et al., 2001). While no single uptake system has been found to be necessary for colonization, disruption of chuA-mediated heme uptake (Torres et al., 2001) or enterobactin (Johnson et al., 2005), salmochelin (Russo et al., 2002), or aerobactin (Torres et al., 2001) siderophore uptake resulted in outcompetition by a wildtype strain in vivo. Thus, considerable functional redundancy exists among these systems.
Although siderophore and heme uptake systems contribute to the fitness of UPEC, the role of specific iron sources in the host remains largely unknown. Here we show that a CFT073 strain deficient for heme utilization is unable to colonize the murine kidney to wildtype levels (Fig. 6B). This represents the first evidence that heme is a required source of iron for UPEC in vivo. The importance of heme uptake in the kidney is further supported by our and others’ findings that both the hma and chuA mutants are outcompeted by wildtype CFT073 in the kidneys of infected mice during co-infection experiments (Fig. 1B) (Torres et al., 2001). It is interesting to note that although ChuA appears to contribute more to heme uptake in vivo (Fig. 6A), Hma alone is sufficient for kidney colonization, as the chuA mutant independently colonized the kidneys at levels similar to wildtype (Fig. 6B).
Both in vitro and in vivo, we observed a striking difference between the hma and chuA mutants, with chuA appearing to play a greater role in heme utilization. We propose that this difference is due, at least in part, to the relative expression levels of the two receptors. As compared to bacteria cultured in rich medium, bacteria cultured under iron limitation or isolated from the urine of infected mice upregulated chuA to a greater extent than hma (Fig. 7B). The qPCR results shown here are replicated at the protein level, as quantitative profiling of CFT073 cultured in urine measured considerably more ChuA than Hma in the outer membrane (Alteri and Mobley, 2007). Thus, a chuA mutant would likely contain significantly less heme receptor on its surface than would an hma mutant.
While heme uptake is critical for UPEC to colonize the murine kidney, it appears to play a lesser role in bladder colonization. In both co-infection (Fig. 1B) and independent infection (Fig. 6B) experiments, all heme uptake mutants infected the bladder to levels indistinguishable from wildtype. Similarly, the chuA mutant, outcompeted by the hma mutant in the kidneys of infected mice, colonized the bladders effectively in the presence of the competing strain (Fig. 6A). However, we show that both hma and chuA are highly upregulated in urine from infected mice (Fig. 7B), indicating that they are expressed in the iron-limited bladder. Instead, we hypothesize that non-heme sources of iron are more prevalent in the bladder and therefore more important during UPEC colonization of this site.
Free heme is not readily available in the host, as the majority is bound by hemoglobin and sequestered within erythrocytes or bound by other serum proteins (Wandersman and Stojiljkovic, 2000). Thus, it is unlikely that free heme is the substrate for Hma in vivo. Hemoglobin, which is utilized by both ChuA (Torres and Payne, 1997) and Hma, is a potential heme source in vivo, especially in the blood-rich kidneys. To facilitate use of this iron source, many UPEC strains secrete hemolysin, which lyses red blood cells (Gadeberg and Orskov, 1984) and releases hemoglobin. Additionally, in abscess-forming E. coli, a secreted hemoglobin protease (Hbp) degrades hemoglobin, binds the released heme, and is hypothesized to transfer this heme to the bacteria for receptor-mediated import (Otto et al., 1998). A homolog of Hbp is found in UPEC strains, as well, although its protease activity remains unclear (Heimer et al., 2004). Further work is needed to elucidate the role these secreted proteins may play in ChuA-and Hma-mediated heme acquisition.
EXPERIMENTAL PROCEDURES
Bacterial strains and culture conditions
All strains used in this study are listed in Table 3. Bacteria were routinely cultured in Luria broth (LB) at 37°C with aeration and appropriate antibiotics. W salts minimal medium (Smith et al., 1971) consisted of 60 mM K2HPO4, 30 mM KH2PO4, 0.4 mM MgSO4, 2% NaCl, 0.4% glucose, 0.005% thiamine, and 10 mM NH4Cl.
Table 3.
Bacterial strains and plasmids.
| E. coli strain | Descriptiona | Reference or source |
|---|---|---|
| CFT073 | Pyelonephritis isolate | (Mobley et al., 1990) |
| K12 | MG1655, laboratory strain | (Blattner et al., 1997) |
| HB101 ent | HB101 ent::Tn5 strain 1017; Kanr | (Torres and Payne, 1997) |
| chuA | CFT073 chuA::cat; Camr | (Torres et al., 2001) |
| hma | CFT073 Δc2482::kan; Kanr | This study |
| hma chuA | CFT073 Δc2482::kan chuA::cat; Kanr, Camr | This study |
| tonB | MG1655 ΔtonB::kan, Kanr | This study |
|
| ||
| Plamid | ||
| pKD4 | λ Red template vector; Kanr Ampr | (Datsenko and Wanner, 2000) |
| pKD46 | Red recombinase helper plasmid, temp-sensitive; Ampr | (Datsenko and Wanner, 2000) |
| pGEN | pGEN-MCS, promoter-less expression vector, p15A ori (copy number ~15), par hok sok mok parM parR; Ampr | (Galen et al., 1999; Lane et al., 2007) |
| pnativehma | hma with native promoter (900 bp upstream) in pGEN-MCS | This study |
| pBAD-myc-HisA | Expression vector, pBR322 ori (low copy), araBAD promoter (arabinose-inducible), araC; Ampr | Commercial (Invitrogen) |
| phma | hma in pBAD | This study |
| pY126A | hmaY126A in pBAD | This study |
| pchuA | chuA in pBAD | This study |
| piutA | iutA in pBAD | This study |
| phma-His | hma in pBAD with C-term. His6 tag | This study |
| pchuA-His | chuA in pBAD with C-term. His6 tag | This study |
| piutA-His | iutA in pBAD with C-term. His6 tag | This study |
Kan, kanamycin; Cam, cholamphenicol; Amp, ampicillin
Mutant construction
Deletion of hma (in both wildtype CFT073 and chuA::cat backgrounds) and tonB (in MG1655) was achieved using the λ Red recombinase system (Datsenko and Wanner, 2000). Using primers containing sequences in the 5’ and 3’ ends of hma or tonB, a kanamycin resistance gene was PCR amplified from the template plasmid pKD4 (Table 3). The resulting product was used to replace >80% of the hma or tonB gene by Red recombinase-mediated homologous recombination (recombinase expressed from pKD46). Mutants were verified by PCR and differential EagI digestion.
Expression and purification of recombinant proteins
An approximately 3 kb fragment containing the hma ORF plus 900 bp upstream was PCR amplified from CFT073 chromosomal DNA and cloned into the NdeI - EagI restriction sites of pGEN-MCS (pnativehma) (Table 3). The hma ORF (minus upstream region) was also PCR amplified and cloned into the NcoI - BglII restriction sites of pBAD-myc-HisA (Table 3), both in- and out-of-frame with the vector’s C-terminal 6x His tag (phma-His and phma, respectively). The chuA and iutA ORFs were PCR amplified and similarly cloned into the NcoI - XhoI sites of pBAD, both in- and out-of-frame with the C-terminal 6x His tag (pchuA-His, piutA-His and pchuA, piutA, respectively). Expression of hma, chuA, and iutA from PBAD was induced by addition of L-arabinose to 100 μM. Using a nickel-nitriloacetic-agarose column (Qiagen), His6 fusions were purified from E. coli TOP10® (Invitrogen) outer membrane fractions (see below) in the presence of 8 M urea. Buffer exchange at 4°C was used to solubilize the purified protein in PBS with 0.05% Zwittergent® (Calbiochem).
Outer membrane isolation
Bacteria were harvested by centrifugation (10 min, 8000×g, 4°C), resuspended in 10 mM HEPES pH 7.0, and lysed by two passages through a French pressure cell (20,000 psi). After the lysate was cleared by centrifugation (10 min, 8000×g, 4°C), membranes were isolated from the cleared lysate by ultracentrifugation (30 min, 100,000×g, 4°C). The membrane pellet was resuspended in 2% sarcosine, incubated 30 min at room temp, and ultracentrifuged (30 min, 100,000×g, 4°C) to isolate the sarcosine-insoluble outer membranes. Outer membranes were resuspended in 10 mM HEPES pH 7.0 or solubilized in 0.2% Zwittergent® (Calbiochem).
in vitro competition assay
in vitro co-cultures were performed as previously described (Lane et al., 2005). Briefly, wildtype CFT073 and the hma mutant were grown to late exponential phase and the OD600 of each culture was standardized to 0.8. Standardized cultures were mixed 1:1, diluted 1:500 into LB or W salts minimal medium and incubated at 37°C with aeration. Every 8 or 16 hours, cultures were passaged into fresh medium at 1:500 or 1:50, respectively. At 24, 48, and 72 hours post-inoculation, cultures were plated on LB agar and LB containing 25 μg/ml kanamycin to determine wildtype and mutant CFU/ml. All cultures were plated using an Autoplate 4000® (Spiral Biotech) spiral plater and enumerated with a Q-Count automatic colony counting system (Spiral Biotech).
Iron source growth assays
Growth promotion assays were performed as described (Torres and Payne, 1997), with modification. Prior to inoculation, bacteria were cultured in LB containing 200 μM DIP for at least 6 hours and washed in PBS. Approximately 105 CFU were plated onto LB supplemented with 375 μM DIP (Sigma). Iron sources (10 μl) were spotted directly onto the plate (1 mM FeCl2, 10 μM hemin, 1 mg/ml hemoglobin, 10 mg/ml lactoferrin, 10 mg/ml holo-transferrin, and 10 mg/ml bovine serum albumin [Sigma]) and incubated 48–72 hours at 37°C.
All other plate assays utilized sorbitol-MacConkey agar (Difco) supplemented with 350 μM DIP and an iron source at the indicated concentration. For heme titration experiments, 1–50 μM FeCl2 and 10 nM-100 μM hemin were used. Prior to inoculation, bacteria were cultured in LB containing 200–400 μM DIP for at least 6 hours and washed in PBS. The OD600 was standardized to ~1.0 and approximately 200 CFU were spread per plate.
For growth curves, strains were iron-limited overnight by culturing in LB containing 200 μM DIP. Prior to inoculation, strains were washed in PBS and ~105 CFU inoculated into LB containing 300 μM DIP and 20 μM FeCl2, 10 μM hemin, or no additional iron source. Growth curves were performed in a Bioscreen C Growth Curve Analyzer (Growth Curves, USA) at 37°C with aeration.
Hemin-binding assays
Hemin binding to whole cells was determined as previously described (Olczak et al., 2001). MG1655 containing phma, pY126A, pchuA, piutA or empty vector were induced for 3 h with 100 μM arabinose, washed and resuspended in PBS. The OD600 was standardized to 1.0 and 800 μl samples of this cell suspension were mixed with 200 μl 50 μM hemin. After 1 h incubation at 37°C, bacteria were pelleted at 16,000×g for 3 min and 20 μl supernatant was incubated with 80 μl 1-Step™ Turbo TMB-ELISA (Sigma) for 20 min at room temp. Reactions were stopped by the addition of 100 μl 1.0 N H2SO4 and the OD450 measured.
Hemin binding to outer membranes or purified protein was determined as previously described (Asuthkar et al., 2007), with modification. Purified proteins or outer membranes from MG1655 containing phma, pY126A, pchuA, or piutA were prepared as described. Protein was diluted in coating buffer (50 mM Na2CO3, 50 mM NaHCO3, pH 9.6) and coated onto a microtiter plate (0.1–0.5 μg per well) at 37°C overnight. Wells were blocked for 1 h with 2% BSA in PBS, washed with PBS and incubated at 37°C for 1 h with 100 μl hemin solution (for outer membranes 50 μM hemin, for purified protein 0–200 μM). Wells were washed 4x with PBS and 100 μl 1-Step Turbo-TMB peroxidase substrate was added. After 20 min at room temp, the OD450 was measured. Amount of hemin bound by each sample was calculated from a standard curve.
A modified in-gel TMBZ staining method was used to detect Hma-associated heme (Stugard et al., 1989). Outer membranes from MG1655 carrying phma (10 μg) were incubated with 85 μM hemin for 1 h at 37°C. SDS-PAGE loading buffer lacking dithiothreitol was added to samples, which were elecrophoresed in the presence of 0.1% SDS on a 10% acrylamide gel (3.75% stacking gel) at 200V for 1 h at 4°C in the dark. Gels were fixed for 1 h in a pre-chilled solution of 0.25 M sodium acetate pH 5-methanol-H2O (6:3:1). To detect heme-associated peroxidase activity, gels were stained as described in 2 parts freshly-prepared 6.3 mM TMBZ (Sigma) in methanol, 7 parts 0.25 M sodium acetate pH 5, and 1 part H2O for 35 min (Thomas et al., 1976). Color development was achieved by adding H2O2 to a final concentration of 0.1% and incubating 30 min. Gels were washed in acetate-buffered 30% isopropanol and imaged immediately. All fixing/staining steps were performed at 4°C in the dark.
Site-directed mutagenesis
The amino acid sequence of Hma was aligned with the structure of E. coli FepA using Cn3D version 4.1 (NCBI). Residue changes in Hma were made using the QuikChange® II Site-Directed Mutagenesis protocol (Stratagene), with pnativehma as the template. Mutagenic primers are listed in Table 4 and all reactions were carried out according to the manufacturer’s instructions. All mutations were confirmed by sequencing (University of Michigan DNA Core Facility).
Table 4.
Site-directed mutagenesis primer sequencesa.
| Mutation | Forward | Reverse |
|---|---|---|
| H242A | GGTTATAACTCCGGAAACGCTCGTTTTGGCCTCTCGC | GCGAGAGGCCAAAACGAGCGTTTCCGGAGTTATAACC |
| H331A | CAGGCTCTGACCGTTGCTAACAAGACTGACACCCATG | CATGGGTGTCAGTCTTGTTAGCAACGGTCAGAGCCTG |
| H337A | CATAACAAGACTGACACCGCTGATAAGCAATACACTC | GAGTGTATTGCTTATCAGCGGTGTCAGTCTTGTTATG |
| Y126A | GCGCGCCGGAGATAATGCTGGTGTGGGACTGTTG | CAACAGTCCCACACCAGCATTATCTCCGGCGCGC |
all sequences listed 5’→ 3’
CBA mouse model of ascending UTI
Female 6- to 8-week-old CBA/J mice were transurethrally inoculated as previously described (Hagberg et al., 1983). A sterile 0.28 mm diameter polyethylene catheter attached to an infusion pump (Harvard Apparatus) was used to deliver 50 μl of bacterial suspension containing 108 CFU per mouse. Cultures were grown overnight with aeration and resuspended in PBS prior to inoculation. For co-infection experiments, resuspended strains were mixed at a 1:1 ratio and inoculated into the same mouse. 72 hours post-inoculation, mice were euthanized and bladder, kidneys, and spleens removed and homogenized in 3 ml PBS using an Omni TH homogenizer (Omni International). Dilutions of this homogenate were plated on LB to determine CFU/g tissue. For co-infection experiments, homogenate was also plated on appropriate antibiotics to differentiate wildtype and mutant strains.
RNA isolation and qPCR
For in vitro RNA samples, CFT073 was cultured with aeration to late exponential phase (OD600=0.5–0.6) in 100 ml LB or LB containing 200 μM DIP or 20 μM FeCl2. Culture aliquots (200 μl) were mixed with 25 μl cold 5% phenol-ethanol stop solution, pelleted (1 min, 10,000×g), and stored at −80°C for RNA isolation. Thawed pellets were resuspended in 100 μl RNase-free TE containing 1 mg/ml lysozyme and RNA isolated using the RNeasy protocol (Qiagen). Samples were DNase-treated according to the Turbo™ DNA-Free procedure (Ambion) and cDNA synthesized using SuperScript™ II First-Strand Synthesis reagents (Invitrogen) according to the manufacturers’ instructions. Real-time qPCR was performed using 30 ng cDNA template and Brilliant SYBR® Green reagents (Stratagene). Data were normalized to gapA transcript and analyzed using MxPro 4.0 software (Stratagene).
For in vivo RNA samples, CBA/J mice were infected with CFT073 (or PBS control) as described above. At two-hour intervals beginning at 24 hpi, urine was collected and pooled from each cage of mice (5 animals). Immediately after collection, cold 5% phenol-ethanol stop solution was added (0.125 μl solution per μl urine) and samples were pelleted (1 min, 10,000×g) and stored at −80°C. Pellets from 5–7 timepoints were combined for RNA isolation, which was performed as described above.
Statistical analyses
Statistics were performed using GraphPad InStat® statistical software. P values for co-infections and co-cultures were calculated by the Wilcoxon matched-pairs signed-ranks test, for independent infections by the Mann-Whitney test, and all others by the Student’s t-test. GraphPad Prism® was used for nonlinear regression analysis.
Acknowledgments
We would like to thank Alfredo Torres (University of Texas Medical Branch) for generously providing the CFT073 chuA::cat and HB101 ent::kan strains used in this study and Sara N. Smith and M. Chelsea Lane for assistance with our animal studies. Funding for this work was provided by Public Health Service grant AI043360 from the National Institutes of Health.
References
- Alteri CJ, Mobley HL. Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine. Infect Immun. 2007;75:2679–2688. doi: 10.1128/IAI.00076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux P, Haser R, Izadi N, Lecroisey A, Delepierre M, Wandersman C, Czjzek M. The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nat Struct Biol. 1999;6:516–520. doi: 10.1038/9281. [DOI] [PubMed] [Google Scholar]
- Asuthkar S, Velineni S, Stadlmann J, Altmann F, Sritharan M. Expression and characterization of an iron-regulated hemin-binding protein, HbpA, from Leptospira interrogans serovar Lai. Infect Immun. 2007;75:4582–4591. doi: 10.1128/IAI.00324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- Bracken CS, Baer MT, Abdur-Rashid A, Helms W, Stojiljkovic I. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J Bacteriol. 1999;181:6063–6072. doi: 10.1128/jb.181.19.6063-6072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- Burkhard KA, Wilks A. Characterization of the outer membrane receptor ShuA from the heme uptake system of Shigella dysenteriae. Substrate specificity and identification of the heme protein ligands. J Biol Chem. 2007;282:15126–15136. doi: 10.1074/jbc.M611121200. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- Fischer E, Gunter K, Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989;171:5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeberg OV, Orskov I. In vitro cytotoxic effect of alpha-hemolytic Escherichia coli on human blood granulocytes. Infect Immun. 1984;45:255–260. doi: 10.1128/iai.45.1.255-260.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen JE, Nair J, Wang JY, Wasserman SS, Tanner MK, Sztein MB, Levine MM. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect Immun. 1999;67:6424–6433. doi: 10.1128/iai.67.12.6424-6433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan EC, Mobley HL. Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect Immun. 2007;75:3941–3949. doi: 10.1128/IAI.00337-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Eden C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer SR, Rasko DA, Lockatell CV, Johnson DE, Mobley HL. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect Immun. 2004;72:593–597. doi: 10.1128/IAI.72.1.593-597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi-Pruneyre N, Huche F, Lukat-Rodgers GS, Lecroisey A, Gilli R, Rodgers KR, Wandersman C, Delepelaire P. The heme transfer from the soluble HasA hemophore to its membrane-bound receptor HasR is driven by protein-protein interaction from a high to a lower affinity binding site. J Biol Chem. 2006;281:25541–25550. doi: 10.1074/jbc.M603698200. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Jelacic S, Schoening LM, Clabots C, Shaikh N, Mobley HL, Tarr PI. The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect Immun. 2005;73:965–971. doi: 10.1128/IAI.73.2.965-971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MC, Lockatell V, Monterosso G, Lamphier D, Weinert J, Hebel JR, Johnson DE, Mobley HL. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect Immun. 2005;73:7644–7656. doi: 10.1128/IAI.73.11.7644-7656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MC, Alteri CJ, Smith SN, Mobley HL. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A. 2007;104:16669–16674. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Olczak T, Guo HC, Dixon DW, Genco CA. Identification of amino acid residues involved in heme binding and hemoprotein utilization in the Porphyromonas gingivalis heme receptor HmuR. Infect Immun. 2006;74:1222–1232. doi: 10.1128/IAI.74.2.1222-1232.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AL, Rasko DA, Mobley HL. Defining genomic islands and uropathogenspecific genes in uropathogenic Escherichia coli. J Bacteriol. 2007;189:3532–3546. doi: 10.1128/JB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olczak T, Dixon DW, Genco CA. Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metalloporphyrins. J Bacteriol. 2001;183:5599–5608. doi: 10.1128/JB.183.19.5599-5608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto BR, van Dooren SJ, Nuijens JH, Luirink J, Oudega B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J Exp Med. 1998;188:1091–1103. doi: 10.1084/jem.188.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Carlino UB, Johnson JR. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect Immun. 2001;69:6209–6216. doi: 10.1128/IAI.69.10.6209-6216.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, McFadden CD, Carlino-MacDonald UB, Beanan JM, Barnard TJ, Johnson JR. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect Immun. 2002;70:7156–7160. doi: 10.1128/IAI.70.12.7156-7160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Ahmer BM, Seachord CL, Darveau RP, Postle K. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J Biol Chem. 1993;268:16302–16308. [PubMed] [Google Scholar]
- Smith GR, Halpern YS, Magasanik B. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. 4-imidazolone-5-propionate amidohydrolase and N-formimino-L-glutamate formiminohydrolase. J Biol Chem. 1971;246:3320–3329. [PubMed] [Google Scholar]
- Stugard CE, Daskaleros PA, Payne SM. A 101-kilodalton heme-binding protein associated with congo red binding and virulence of Shigella flexneri and enteroinvasive Escherichia coli strains. Infect Immun. 1989;57:3534–3539. doi: 10.1128/iai.57.11.3534-3539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PE, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Torres AG, Payne SM. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- Torres AG, Redford P, Welch RA, Payne SM. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun. 2001;69:6179–6185. doi: 10.1128/IAI.69.10.6179-6185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C, Stojiljkovic I. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr Opin Microbiol. 2000;3:215–220. doi: 10.1016/s1369-5274(00)00078-3. [DOI] [PubMed] [Google Scholar]
- Welch RA, Burland V, Plunkett G, 3rd, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff EE, Duncan D, Torres AG, Mills M, Maase K, Payne SM. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol Microbiol. 1998;28:1139–1152. doi: 10.1046/j.1365-2958.1998.00873.x. [DOI] [PubMed] [Google Scholar]