Anyone who has worked in an intensive care unit is aware of the phenomenon commonly referred to as the “nonthyroidal illness syndrome” or “euthyroid sick syndrome”, observed in approximately 44% of these patients (1). Low circulating levels of thyroid hormone but seemingly inappropriately low or normal TSH and diminished TSH pulsatility, suggesting the presence of central hypothyroidism, characterizes this syndrome. The low thyroid hormone levels are predictive of outcomes in the intensive care unit setting, and perhaps even a better indicator of the severity of illness than the APACHE II score (2). When thyroxine (T4) falls to <4 µg/dl, the risk of death rises to ~50%, and when T4 falls to <2 µg/dl, mortality increases even more to ~80% (3, 4). One of the dilemmas that physicians face when confronted with the nonthyroidal illness syndrome, therefore, is whether the fall in thyroid hormone levels is adaptive and simply a normal, physiologic response to conserve energy and should not be treated other than with nutritional support, or whether it is maladaptive and should be vigorously treated to restore circulating thyroid hormone levels to normal. Unraveling the physiological and/or pathophysiological mechanisms involved in precipitation of the nonthyroidal illness syndrome is one approach that has been taken to resolve this dilemma, and significant progress has been made.
Maintenance of normal thyroid function (euthyroidism) is dependent upon a complex interplay between the hypothalamus, anterior pituitary, and thyroid gland as well as a number other factors illustrated in Fig. 1. In addition to supporting peripheral tissues through effects on protein synthesis and brain development during fetal growth and early infancy, thyroid hormone has an important role in the regulation of energy expenditure by affecting obligatory thermogenesis (energy expenditure necessary to sustain basal homeostatic functions) and adaptive thermogenesis (additional heat produced in response to triggering signals to sustain core temperature). Indeed, basal metabolic rate can be reduced by as much as 30% in the absence of thyroid hormone, and adaptive thermogenesis in cold exposed animals is markedly impaired (5). In addition, thyroid hormone has effects on lipogenesis and appetite regulation, affecting genes coding for lipogenic enzymes (6, 7) and exerting direct effects on hypothalamic feeding centers (8).
Figure 1.
Neuroregulatory control systems involved in the secretion of thyroid hormone. Bold lines denote the negative feedback loop of thyroid hormone on thyrotropin-releasing hormone (TRH) secretion from the hypothalamus and TSH secretion from the anterior pituitary. Both the hypothalamic TRH neurons and anterior pituitary thyrotropes are impinged upon by numerous other potential regulatory influences that are activated under specific physiological or pathological conditions. PVN = paraventricular nucleus; ARC = arcuate nucleus, ME = median eminence; III = third ventricle (Courtesy, Dr. Praful Singru, Tufts Medical Center)
So-called “hypophysiotropic” thyrotropin-releasing hormone (TRH) neurons that regulate anterior pituitary TSH secretion are located in the hypothalamic paraventricular nucleus (PVN), a triangular shaped nucleus at the dorsal limits of the third ventricle (Fig. 1). Axons of these neurons project to the external zone of the median eminence where TRH is released into the pituitary portal system. Thyroid hormone selectively inhibits both the gene expression and the posttranslational processing of TRH in hypophysiotropic neurons, but has no effect on other TRH-synthesizing neuronal groups in the forebrain (9, 10). When circulating levels of thyroid hormones fall below normal values (hypothyroidism), the content of proTRH and TRH mRNA increases in the PVN (11) accompanied by a decline in the content of TRH in the median eminence due increased secretion of TRH into the portal blood for conveyance to the anterior pituitary (12–14). Conversely, increased circulating levels of T4 cause marked suppression of TRH gene expression in the PVN and a reduction in the secretion of TRH into the portal plexus (9, 10, 15), establishing an inverse relationship between thyroid hormone and the biosynthesis and secretion of hypophysiotropic TRH. The amount of hypophysiotropic TRH secreted into the portal system is important to establish the set point for feedback regulation of anterior pituitary TSH secretion by thyroid hormone. Thus, when portal blood TRH concentrations are low, TSH can be suppressed by less T4 circulating in the blood-stream (reduced set point), while high portal blood TRH concentrations raises the set point for feedback regulation by thyroid hormone (16, 17).
On the basis of the normal physiology described above, it might seem paradoxical that when circulating thyroid hormone levels fall in association with the nonthyroidal illness syndrome, a compensatory rise in TSH is not observed. The explanation for this observation, however, has become clearer in recent years as a result of extensive studies in experimental animals and man using fasting or hypocaloric diets as models for nonthyroidal illness. While decreased type 1 iodothyronine deiodinase (D1) and increased type 3 iodothyronine deiodinase (D3) activity in liver and/or muscle contribute to rapid reduction in thyroid hormone levels (18–20), reduced thyroid hormone output from the thyroid gland as a result of central hypothyroidism is now well established (21, 22). The latter response is orchestrated by leptin, an adipose-derived hormone, which declines in the circulation with fasting and restored to normal levels by refeeding. If leptin is administered systemically or intracerebroven-tricularly to fasting animals, the reduction in circulating levels of thyroid hormone and TSH are prevented (23, 24). Similarly, the administration of leptin to fasting or caloric-deprived healthy human subjects restores thyroid hormone levels to or toward normal and restores TSH pulsatility (25–27).
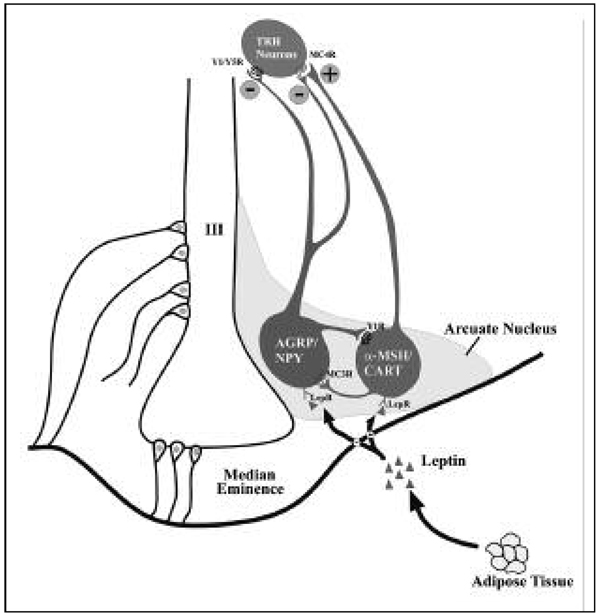
The primary action of leptin on the hypothalamic-pituitary-thyroid axis appears to be the hypothalamus by changing the set point for feedback sensitivity of hypophysiotropic TRH-producing neurons in the PVN to thyroid hormone, lowering the set point when leptin levels are suppressed during fasting (28). As illustrated in Fig. 2, at least two anatomically distinct and functionally antagonistic populations of neurons present in the hypothalamic arcuate nucleus, α-melanocortin-stimulating hormone (α-MSH)-producing neurons that co-express cocaine and amphetamine-regulated transcript (CART), and neuropeptide Y (NPY)-producing neurons that co-express agouti-related peptide (AGRP), are responsible for the actions of leptin on hypophysiotropic TRH. Alpha-MSH has profound activating effects on hypophysiotropic TRH neurons and when administered intracerebroventricularly, restores fasting-induced suppression of TRH mRNA in hypophysiotropic neurons to levels in ad lib fed animals by phosphorylating the nuclear transcription factor, CREB (29, 30). Conversely, both NPY and AGRP have inhibitory effects on TRH gene expression in hypophysiotropic neurons when administered intracerebroventricularly, and replicate many of the changes in the hypothalamic-pituitary-thyroid axis observed during fasting despite continued feeding (31, 32). It is presumed that the inhibitory effect of AGRP on TRH gene expression is the result of antagonizing the activating effects of α-MSH at the melanocortin 4 receptor on the surface of hypophysiotropic TRH neurons, whereas the inhibitory effect of NPY occurs by reducing cAMP (33). Thus, during fasting when circulating leptin levels decline, the simultaneous inhibition of α-MSH production and increase in AGRP and NPY production in arcuate nucleus neurons reduce CREB phosphorylation in TRH neurons, essentially reducing the set point for feed-back inhibition of the TRH gene by thyroid hormone. A direct action of leptin on hypophysiotropic TRH neurons has also been proposed (34).
Figure 2.
Regulation of hypophysiotropic TRH neurons by leptin-sensitive hypothalamic arcuate nucleus neurons. Two, major sets of neurons are noted including those that produce AGRP/NPY, that inhibit TRH neurons through Y1 and Y5 receptors (Y1/Y5R), and α-MSH, that stimulates TRH neurons through the MC4 receptor (MC4R). CART also activates TRH neurons but by an unknown mechanism(s). Reciprocal, inhibitory interactions between NPY/AGRP and α-MSH neurons also occur. The location of tanycyte cell bodies in the floor and infralateral walls of the third ventricle and their cytoplasmic projections are depicted in the left half of the diagram (Courtesy, Dr. Praful Singru, Tufts Medical Center)
Given current understanding of the elaborate and highly regulated physiology of the hypothalamic-pituitary-thyroid axis by fasting described above, it would be difficult to argue that the associated fall in circulating thyroid hormone levels is maladaptive. Rather, this mechanism is likely an important homeostatic response to conserve energy, a concept in keeping with observations by Gardner et al (35) and Burman et al (36) that T3 administration to fasting human subjects leads to increased urinary nitrogen excretion. The appropriate treatment for fasting-induced reduction in thyroid hormone levels, therefore, would be the replacement of calories, and not the administration of thyroid hormone.
In addition to fasting, the nonthyroidal illness syndrome can be induced by a number of different disorders including sepsis, trauma, burns, surgery, and cardiovascular, renal, and liver disease (3, 4), and often several of these disorders occurring simultaneously. The mechanisms responsible for the fall in circulating thyroid hormone levels, however, may not be the same as that responsible for the fall in circulating thyroid hormone levels associated with fasting. This is suggested by the observation that endotoxin administration, which simulates infection, increases rather than decreases α-MSH gene expression and does not alter the expression of NPY in arcuate nucleus neurons (37), responses that would ordinarily predict increased TRH gene expression. Nevertheless, there is strong experimental support to indicate that like fasting, central hypothyroidism contributes to the fall in thyroid hormone levels observed in other disorders that give rise to the nonthyroidal illness syndrome. Namely, TRH mRNA is reduced in the PVN of patients dying of chronic, severe illness (38), continuous, exogenous administration of TRH (together with a growth hormone secretagogue) is effective in restoring TSH and circulating thyroid hormone levels to normal (39), and a rise in TSH usually heralds return of the thyroid axis to normal following recovery from severe illness (40).
Recent studies in rats, mice and rabbits have raised the possibility that endotoxin-induced upregulation of type 2 iodothyronine deiodinase (D2) in tanycytes, specialized ependymal cells lining the floor and infralateral borders of the third ventricle in the mediobasal hypothalamus (Fig. 2), may explain central hypothyroidism associated with infection (41, 42). Endotoxin induces a 4-fold increase in D2 mRNA and activity that is independent of the associated fall in circulating thyroid hormone levels (42). As D2 is the major enzyme in the brain responsible for converting T4 to its more potent, biologically active metabolite, T3 (43), it is hypothesized that the increase in tanycyte D2 activity may cause tissue-specific thyrotoxicosis in the mediobasal hypothalamus by increasing the conversion of T4 to T3, ultimately leading to direct suppression of hypophysiotropic TRH neurons in the PVN (44). T3 released into the portal capillary system may also inhibit the secretion of TSH from anterior pituitary thyrotrops.
Under normal circumstances, tanycytes may participate in feedback regulation of hypophysiotropic TRH neurons by thyroid hormone. While both T4 and T3 are present in the circulating blood, trafficking of the less active T4 into the brain and then conversion to T3 is a necessary step in the thyroid hormone feedback mechanism (45). As demonstrated by Kakucska et al. (45), restoration of normal peripheral T3 levels in hypothyroid rats by the systemic administration of T3, alone, is not sufficient to inhibit increased TRH gene expression in hypothyroid rats. Furthermore, ~80% of T3 in the brain originates from local T4 to T3 conversion (46), primarily by D2 (43). As the PVN contains little, if any D2 activity or D2 mRNA (47, 48), T3 must derive from another locus within the brain where D2 is synthesized, and then be transported to the PVN.
D2 is expressed in tanycytes in all animal species studied thus far including man (44, 49), suggesting an important homeostatic function. Being at the interface of the cerebrospinal fluid (CSF) by nature of its location in the third ventricle, and the vascular system, through long cytoplasmic projections that contact portal vessels and envelop blood vessels in the hypothalamus (50), tanycytes are in strategic position to extract T4 from the bloodstream or the CSF, convert T4 to T3, and then release T3 into the hypothalamus. T3 released into the CSF could reach hypophysiotropic TRH neurons by volume transmission, moving between ependymal cells lining the third ventricle, and/or taken up by TRH axon terminals in the mediobasal hypothalamus and transported retrogradely to the PVN. Tanycyte D2 may also be involved in regulating hypothalamic levels of T3, as suggested by the large number of hypothalamic neurons that contain thyroid hormone receptors (51) and evidence for tanycyte-neuronal interactions in the arcuate nucleus that may be involved in regulating UCP 2-dependent mitochondrial uncoupling in NPY/AGRP neurons (52). It remains unclear, however, whether endotoxin-induced upregulation of tanycyte D2 is simply another physiological, regulatory response that promotes energy conservation under these adverse conditions, or an epiphenomenon that leads to inappropriate suppression of hypophysiotropic TRH neurons.
A number of small clinical trials have attempted to determine whether thyroid hormone replacement in intensive care unit patients has any beneficial or detrimental effects on overall outcomes (3, 4, 53). Most have shown T4 or T3 to be safe and well tolerated (54, 55). It could be argued, however, that because of the associated rise in D3 in these patients, neither T4 nor T3 are appropriate therapy due to the effects of this enzyme to increase the conversion of T4 to reverse T3 (rather than T3) or degrade T3, respectively (3). Nevertheless, improvement in cardiac hemodynamic parameters including cardiac output, end diastolic volume and stroke volume, and a reduction in peripheral arterial resistance have been observed in patients receiving T3 following coronary artery bypass surgery and/or in patients with dilated cardiomyopathy (53, 56–58), but at the expense of further suppression in circulating levels of TSH. A novel approach to the treatment of the nonthyroidal illness syndrome in critically ill patients has recently been suggested Van den Berghe et al (39, 59). Using a continuous infusion of TRH together with a growth hormone secretagogue, not only were thyroid hormone levels and TSH pulsatility restored in these patients, but catabolic parameters were also improved.
The dilemma of whether or not to treat patients with the nonthyroidal illness syndrome with thyroid hormone remains unresolved, but progress is being made. With better understanding of the pathophysiology underlying each of the various disorders giving rise to the fall in thyroid hormone levels in critically ill patients, the answer to this question will ultimately be found and lead to the appropriate approach to therapy.
References
- 1.Plikat K, Langgartner J, Buettner R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56:239–244. doi: 10.1016/j.metabol.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Lawler PG. Prediction of outcome in intensive care patients using endocrine parameters. Crit Care Med. 1995;23:78–83. doi: 10.1097/00003246-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 3.De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin. 2006;22:57–86. doi: 10.1016/j.ccc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am. 2007;36:657–672. doi: 10.1016/j.ecl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med. 2003;139:205–213. [PubMed] [Google Scholar]
- 6.Bianco AC, Carvalho SD, Carvalho CR, Rabelo R, Moriscot AS. Thyroxine 5’-deiodination mediates norepinephrine-induced lipogenesis in dispersed brown adipocytes. Endocrinology. 1998;139:571–578. doi: 10.1210/endo.139.2.5737. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer JH, Schwartz HL, Lane JT, Thompson MP. Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Invest. 1991;87:125–132. doi: 10.1172/JCI114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong WM, Martin NM, Smith KL, et al. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology. 2004;145:5252–5258. doi: 10.1210/en.2004-0545. [DOI] [PubMed] [Google Scholar]
- 9.Lechan RM, Kakucska I. Feedback regulation of thyrotropin-releasing hormone gene expression by thyroid hormone in the hypothalamic paraventricular nucleus. Ciba Found Symp. 1992;168:144–158. doi: 10.1002/9780470514283.ch10. [DOI] [PubMed] [Google Scholar]
- 10.Perello M, Friedman T, Paez-Espinosa V, Shen X, Stuart RC, Nillni EA. Thyroid hormones selectively regulate the posttranslational processing of prothyrotropin-releasing hormone in the paraventricular nucleus of the hypothalamus. Endocrinology. 2006;147:2705–2716. doi: 10.1210/en.2005-1609. [DOI] [PubMed] [Google Scholar]
- 11.Lechan RM, Kakucska I. Functional Anatomy of the Neuroendocrine Hypothalamus, Ciba foundation Symposium 168. Chichester: John Wiley & Sons Ltd; 1992. Feedback regulation of thyrotropin-releasing hormone gene expression by thyroid hormone in the hypothalamic paraventricular nucleus; pp. 144–164. [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Yamada M. Thyroid hormones regulate the amount of thyrotrophin-releasing hormone in the hypothalamic median eminence of the rat. J Endocrinol. 1987;114:443–448. doi: 10.1677/joe.0.1140443. [DOI] [PubMed] [Google Scholar]
- 13.Dahl GE, Evans NP, Thrun LA, Karsch FJ. A central negative feedback action of thyroid hormones on thyrotropin-releasing hormone secretion. Endocrinology. 1994;135:2392–2397. doi: 10.1210/endo.135.6.7988422. [DOI] [PubMed] [Google Scholar]
- 14.Rondeel JM, de Greef WJ, Klootwijk W, Visser TJ. Effects of hypothyroidism on hypothalamic release of thyrotropin-releasing hormone in rats. Endocrinology. 1992;130:651–656. doi: 10.1210/endo.130.2.1733713. [DOI] [PubMed] [Google Scholar]
- 15.Koller KJ, Wolff RS, Warden MK, Zoeller RT. Thyroid hormones regulate levels of thyrotropin-releasing-hormone mRNA in the paraventricular nucleus. Proc Natl Acad Sci USA. 1987;84:7329–7333. doi: 10.1073/pnas.84.20.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JB, Boshans R, Reichlin S. Feedback regulation of TSH secretion in rats with hypothalamic lesions. Endocrinology. 1970;87:1032–1040. doi: 10.1210/endo-87-5-1032. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan MM, Taft JA, Reichlin S, Munsat TL. Sustained rises in serum thyrotropin, thyroxine, and triiodothyronine during long term, continuous thyrotropin-releasing hormone treatment in patients with amyotrophic lateral sclerosis. J Clin Endocrinol Metab. 1986;63:808–814. doi: 10.1210/jcem-63-4-808. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Perez A, Palos-Paz F, Kaptein E, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf ) 2008;68:821–827. doi: 10.1111/j.1365-2265.2007.03102.x. [DOI] [PubMed] [Google Scholar]
- 19.Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88:3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 20.Debaveye Y, Ellger B, Mebis L, et al. Tissue deiodinase activity during prolonged critical illness: effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone-releasing peptide-2. Endocrinology. 2005;146:5604–5611. doi: 10.1210/en.2005-0963. [DOI] [PubMed] [Google Scholar]
- 21.Fekete C, Lechan RM. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front Neuroendocrinol. 2007;28:97–114. doi: 10.1016/j.yfrne.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 23.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 24.Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-nduced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 25.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JL, Matarese G, Shetty GK, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA. 2006;103:8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 28.Lechan RM, Fekete C. Feedback regulation of thyrotropin-releasing hormone (TRH): mechanisms for the non-thyroidal illness syndrome. J Endocrinol Invest. 2004;27:105–119. [PubMed] [Google Scholar]
- 29.Fekete C, Legradi G, Mihaly E, et al. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar S, Légrádi G, Lechan RM. Intracerebroventricular administration of alpha-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Res. 2002;945:50–59. doi: 10.1016/s0006-8993(02)02619-7. [DOI] [PubMed] [Google Scholar]
- 31.Fekete C, Kelly J, Mihaly E, et al. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology. 2001;142:2606–2613. doi: 10.1210/endo.142.6.8207. [DOI] [PubMed] [Google Scholar]
- 32.Fekete C, Marks DL, Sarkar S, et al. Effect of Agouti-Related Protein (Agrp) in Regulation of the Hypothalamic-Pituitary-Thyroid (Hpt) Axis in the Mc4-R Ko Mouse. Endocrinology. 2004 [Google Scholar]
- 33.Lechan RM, Fekete C. Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis. Peptides. 2006;27:310–325. doi: 10.1016/j.peptides.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Harris M, Aschkenasi C, Elias CF, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner DF, Kaplan MM, Stanley CA, Utiger RD. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med. 1979;300:579–584. doi: 10.1056/NEJM197903153001102. [DOI] [PubMed] [Google Scholar]
- 36.Burman KD, Wartofsky L, Dinterman RE, Kesler P, Wannemacher RW., Jr The effect of T3 and reverse T3 administration on muscle protein catabolism during fasting as measured by 3-methylhistidine excretion. Metabolism. 1979;28:805–813. doi: 10.1016/0026-0495(79)90206-3. [DOI] [PubMed] [Google Scholar]
- 37.Sergeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Res Mol Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 38.Fliers E, Guldenaar SEF, Wiersinga WM, Swaab DF. Decreased Hypothalamic Thyrotropin-Releasing Hormone Gene Expression in Patients with Nonthyroidal Illness. J Clin Endocrinol Metab. 1997;82:4032–4036. doi: 10.1210/jcem.82.12.4404. [DOI] [PubMed] [Google Scholar]
- 39.Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Iranmanesh A, Veldhuis JD, Bouillon R. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin Endocrinol (Oxf ) 2002;56:655–669. doi: 10.1046/j.1365-2265.2002.01255.x. [DOI] [PubMed] [Google Scholar]
- 40.Bacci V, Schussler GC, Kaplan TB. The relationship between serum triiodothyronine and thyrotropin during systemic illness. J Clin Endocrinol Metab. 1982;54:1229–1235. doi: 10.1210/jcem-54-6-1229. [DOI] [PubMed] [Google Scholar]
- 41.Fekete C, Gereben B, Doleschall M, et al. Lipopolysaccha-ride induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: implications for the nonthyroidal illness syndrome. Endocrinology. 2004;145:1649–1655. doi: 10.1210/en.2003-1439. [DOI] [PubMed] [Google Scholar]
- 42.Fekete C, Sarkar S, Christoffolete MA, Emerson CH, Bianco AC, Lechan RM. Bacterial lipopolysaccharide (LPS)-induced type 2 iodothyronine deiodinase (D2) activation in the mediobasal hypothalamus (MBH) is independent of the LPS-induced fall in serum thyroid hormone levels. Brain Res. 2005;1056:97–99. doi: 10.1016/j.brainres.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 44.Lechan RM, Fekete C. Infundibular tanycytes as modulators of neuroendocrine function: hypothetical role in the regulation of the thyroid and gonadal axis. Acta Biomed. 2007;78 Suppl 1:84–98. [PubMed] [Google Scholar]
- 45.Kakucska I, Rand W, Lechan RM. Thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is dependent upon feedback regulation by both triiodothyronine and thyroxine. Endocrinology. 1992;130:2845–2850. doi: 10.1210/endo.130.5.1572297. [DOI] [PubMed] [Google Scholar]
- 46.Crantz FR, Silva JE, Larsen PR. An analysis of the sources and quantity of 3,5,3'-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982;110:367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- 47.Riskind PN, Kolodny JM, Larsen PR. The regional hypothalamic distribution of type II 5'-monodeiodinase in euthyroid and hypothyroid rats. Brain Res. 1987;420:194–198. doi: 10.1016/0006-8993(87)90260-5. [DOI] [PubMed] [Google Scholar]
- 48.Tu HM, Kim SW, Salvatore D, et al. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- 49.Fliers E, Unmehopa UA, Alkemade A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol Cell Endocrinol. 2006;251:1–8. doi: 10.1016/j.mce.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez EM, Blazquez JL, Pastor FE, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 51.Puymirat J. Thyroid receptors in the rat brain. Prog Neurobiol. 1992;39:281–294. doi: 10.1016/0301-0082(92)90019-b. [DOI] [PubMed] [Google Scholar]
- 52.Coppola A, Liu ZW, Andrews ZB, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell metabolism. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haas NA, Camphausen CK, Kececioglu D. Clinical review: thyroid hormone replacement in children after cardiac surgery - is it worth a try? Crit Care. 2006;10:213. doi: 10.1186/cc4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brent GA, Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63:1–8. doi: 10.1210/jcem-63-1-1. [DOI] [PubMed] [Google Scholar]
- 55.Becker RA, Vaughan GM, Ziegler MG, et al. Hypermetabolic low triiodothyronine syndrome of burn injury. Crit Care Med. 1982;10:870–875. doi: 10.1097/00003246-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Klemperer JD, Klein I, Gomez M, et al. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med. 1995;333:1522–1527. doi: 10.1056/NEJM199512073332302. [DOI] [PubMed] [Google Scholar]
- 57.Spratt DI, Frohnauer M, Cyr-Alves H, et al. Physiological effects of nonthyroidal illness syndrome in patients after cardiac surgery. Am J Physiol Endocrinol Metab. 2007;293:E310–E315. doi: 10.1152/ajpendo.00687.2006. [DOI] [PubMed] [Google Scholar]
- 58.Pingitore A, Galli E, Barison A, et al. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:1351–1358. doi: 10.1210/jc.2007-2210. [DOI] [PubMed] [Google Scholar]
- 59.Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab. 1999;84:1311–1323. doi: 10.1210/jcem.84.4.5636. [DOI] [PubMed] [Google Scholar]