Abstract
The CDC estimates that by 2015, half of all persons living with HIV/AIDS in the USA will be over the age of 50. Despite increasing HIV seroprevalence rates in older adults, most research examining adherence to antiretroviral therapy (ART) has focused on young HIV-infected persons and, in general, has been atheoretical in nature. This study examined two ART adherence conceptual frameworks to determine whether these models generalize to HIV-seropositive older adults. Two hundred and forty-four HIV-positive adults 50-plus years of age were recruited through AIDS service organizations in Ohio and New York. Participants completed a neuropsychological battery and an audio computer-assisted self-interview. FIML SEM analyses revealed that neuropsychological functioning was not associated with adherence. Fit indices supported a stress and coping model, with negative affect mediating the effects of social support and maladaptive coping on ART adherence. Results were consistent with stress and coping models and suggest that interventions intending to increase adherence to ART in HIV-infected older adults may be more effective if they address negative affect and enhance adaptive coping and social support.
Keywords: HIV, antiretroviral adherence, older adults, negative affect, structural equation modeling
Consistent adherence to antiretroviral therapy (ART) is the cornerstone of effective HIV treatment. When used correctly, antiretroviral (ARV) medications decrease viral load and improve immune system functioning (Bangsberg et al., 2000; Paterson et al., 2000). However, the potential for viral mutations and the chronic nature of HIV infection necessitates near perfect adherence (≥95%) over sustained periods (Conway, 2007; Hogg et al., 2002). Unfortunately, 25–46% of HIV-positive persons on ART are nonadherent (<95% adherence; Gwadz et al., 1999; Paterson et al. 2000). Adherence <95% permits HIV to resume rapid replication producing drug-resistant strains that worsen patient health and complicate treatment (Bangsberg et al., 2000; Bartlett, 2002).
ARVs have reduced HIV/AIDS-related morbidity and mortality and have increased survival, contributing to a changing demography of the US HIV/AIDS epidemic (Centers for Disease Control and Prevention [CDC], 2007; Goodkin et al., 2001; Porter et al., 2003). While adults 50+ years old represented 12% of the cumulative number of AIDS cases through 2005, this group represented 19% of estimated AIDS cases in 2005 alone (CDC) and is projected to comprise 50% of all HIV-seropositive persons in the US by 2015.
Despite the changing demography, little research has examined adherence to ART in HIV-infected older adults. Of the relevant studies, many report high rates of adherence (Barclay et al., 2007; Goodkin et al., 2001; Hinkin et al., 2002; 2004). Hinkin et al. (2004) reported a mean adherence rate of 87% in persons 50+ years of age and noted that older persons were more likely to adhere than persons < 50 years. However, others have reported lower rates of adherence in older adults (Catz, Heckman, Kochman, & DiMarco, 2001), underscoring the need to better understand and develop effective interventions for this group.
The influence of neuropsychological functioning (NPF) on ART adherence has received considerable attention (Albert et al., 1999; Chesney, Morin, & Sherr, 2000; Hinkin et al., 2002, 2004). Recently, Barclay et al. (2007) found that adherence in older adults (mean age = 56.3 years, SD = 4.8) was predicted solely by NPF; no psychosocial or demographic factors predicted adherence in their sample. Other researchers assert that NPF be considered when investigating ART adherence (Rausch & Stover, 2001; Reger, Welsh, Razani, Martin, & Boone, 2002).
With some notable exceptions (Gonzalez et al., 2004; Simoni, Frick, & Huang, 2006a; Weaver et al., 2005), contemporary research on ART adherence has been atheoretical in nature, characterizing relationships among adherence and demographic, psychosocial, and health variables. Among the theory-driven and empirically validated models, Barclay et al. (2007) examined ART adherence within the context of the Health Beliefs Model (HBM) and found that the HBM had some utility in explaining adherence in younger persons but not in older adults. In their theory-driven research, Gonzalez et al. highlighted the roles of depression and positive states of mind as mediators of the association between social support and adherence. Weaver et al. examined a stress and coping model of adherence and emphasized the importance of social support, negative affect, and avoidant coping. Simoni et al. reported that negative affect and spirituality mediated social support's impact on self-efficacy which, in turn, influenced adherence. While these theory-driven studies converge on the importance of constructs such as social support, negative affect, and coping in modeling adherence, it is unclear if these models (evaluated using samples comprised largely of younger HIV-positive persons) generalize to HIV-infected older adults.
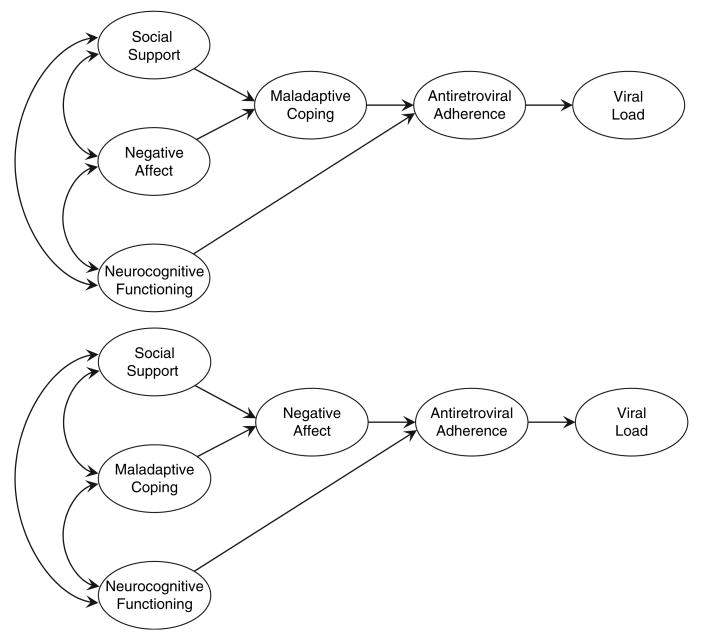
This study examined contemporary theoretical frameworks of ART adherence suggested by prior research (Gonzalez et al., 2004; Simoni et al., 2006a; Weaver et al., 2005) to determine if these models are applicable to HIV-infected older adults. Moreover, this research investigated NPF and adherence; namely, whether the inclusion of neuropsychological variables improves predictive validity. We compared two models of adherence, shown in Figure 1: the maladaptive coping mediational model (adapted from Weaver et al.), and the mood mediational model (based on Simoni et al. and Weaver et al.).
Figure 1.
Hypothesized models of antiretroviral medication adherence in HIV-seropositive older adults. The top panel of the figure shows the hypothesized maladaptive coping mediational model of antiretroviral medication adherence based on Barclay et al. (2007) and Weaver et al. (2005). The bottom panel of the figure shows the hypothesized negative affect mediational model of antiretroviral medication adherence based on Barclay et al. (2007), Simoni et al. (2006a), and Weaver et al. (2005).
Method
Data were collected as part of a randomized clinical trial (RCT) of a coping improvement intervention for HIV-positive older adults. Recruitment procedures and eligibility criteria are described in Lovejoy et al. (in press).
Participants
Of 349 eligible participants, 310 completed a pre-intervention assessment. The present study focused on modeling ART adherence; data from participants not taking ARVs (n = 66) were excluded, yielding a sample of 244. Demographic data are presented in Table 1.
Table 1.
Demographic data for HIV-positive older adults (N = 244).
| Variable | n (%) | M (SD) | Median | Range |
|---|---|---|---|---|
| Sex | ||||
| Female | 71 (29.1) | |||
| Male | 173 (70.9) | |||
| Ethnicity | ||||
| African American, non-Hispanic | 120 (49.2) | |||
| White, non-Hispanic | 74 (30.3) | |||
| African American, Hispanic | 19 (7.8) | |||
| Hispanic/Latino | 16 (6.6) | |||
| Native American | 6 (2.5) | |||
| Other | 9 (3.7) | |||
| Sexual orientation | ||||
| Gay/Lesbian | 117 (48.0) | |||
| Heterosexual | 105 (43.0) | |||
| Bisexual | 22 (9.0) | |||
| Relationship status | ||||
| Unpartnered | 179 (73.4) | |||
| Partnered | 65 (26.6) | |||
| Annual income less than $20,000 | 217 (88.9) | |||
| Taking 3 or more ARV medications | 110 (45.1) | |||
| Study site | ||||
| New York, NY | 190 (77.9) | |||
| Columbus, OH | 25 (10.2) | |||
| Cincinnati, OH | 29 (11.9) | |||
| Adherence | ||||
| Taking all HIV medications ≥95% | 195 (79.9) | |||
| Following specific instructions ≥95% | 202 (82.8) | |||
| Age (years) | 55.5 (4.8) | 54 | 50–73 | |
| Education | 13.1 (2.5) | 13 | 6–17 | |
| Years since HIV diagnosis | 12.8 (5.2) | 13 | 0–25 |
Note: HIV, Human Immunodeficiency Virus; ARV, antiretroviral.
Procedure
Participants completed an assessment battery via audio computer-assisted self-interview (ACASI) and received a $30 incentive. Use of ACASI increases privacy, improves understanding of questions, and encourages honesty when answering sensitive items (Schroder, Carey, & Vanable, 2003).
Measures
Structural equation modeling (SEM) was used to examine the relationships between seven latent constructs, each comprised of indicator variables from the measures below. Each coefficient alpha reported is based on data from the present study.
Antiretroviral (ARV) adherence
Adherence was assessed via items adapted from the ACTG adherence questionnaire (Chesney et al., 2000). Participants were asked: (a) “Using a scale of 0 to 100, where 0 is ‘not at all’ and 100 is ‘all the time,’ over the past week, how much of the time have you taken all of your HIV/AIDS medicines?” (All HIV Meds); and (b) “Using a scale of 0 to 100, where 0 is ‘not at all’ and 100 is ‘all the time,’ over the past week, how much of the time have you followed the specific instructions from your doctor or pharmacist for taking your HIV/AIDS medicines?” (Instructions).
Viral load
Participants were asked, “What was the result of your most recent viral load? If it was undetectable, please answer with zero.” (Viral Load).
Social support
Social support was assessed using the Functional Assessment of Chronic Illness Therapy (FACIT; Webster, Odom, Peterman, Lent, & Cella, 1999) and the Provision of Social Relations Scale (PSR; Turner, Frankel, & Levin, 1983). Participants reported perceived social support via the eight-item Social Well-Being (SWB) subscale of the FACIT (α = 0.88). The PSR includes two subscales: support from Friends (α =0.88, nine items) and Family (α =0.84, six items).
Maladaptive coping
Coping strategies used by respondents to resolve HIV-related stressors were examined via the Ways of Coping Questionnaire (WOC; Folkman & Lazarus, 1988), the Coping With Illness scale (CWI; Murphy, Rotheram-Borus, & Marelich, 2003), and items created for the RCT (available from N.B.H.). Participants rated how often they engaged in each coping behavior. Guided by prior research examining coping strategies in HIV-positive individuals (Hansen et al., 2006; Tarakeshwar, Hansen, Kochman, & Sikkema, 2005; Tate, van den Berg, Hansen, Kochman, & Sikkema, 2006), 20 items were selected that were theoretically related to maladaptive coping strategies, including seven used to form an escape-avoidance (Escape) indicator (α = 0.75); four to form an avoidant (Avoid) indicator (α = 0.56); and nine to form a self-destructive (Destruct) indicator (α = 0.68).
Affect
Measures of depressive symptomatology, anxiety, and general psychological well-being assessed affect. Cognitive and affective symptoms of depression were measured using the 30-item (α = 0.78) Geriatric Depression Scale (GDS; Yesavage et al., 1983). Severity of anxiety symptomatology was assessed with the 21-item (α =0.92) Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988). The 22-item Psychological General Well-Being Schedule (PGWB; Dupuy, 1984; α =0.96) measured overall levels of psychological health.
Neuropsychological functioning (NPF)
Participants completed a neuropsychological battery that assessed executive functioning (Trail Making Test, Part B; Trails B), verbal fluency (Controlled Oral Word Association Test, Letters FAS; COWA), and global functioning (Modified Mini-Mental State Examination (3MS), Teng & Chui, 1987). Neuropsychological test scores were adjusted for demographic characteristics (age, education) using published norms (3MS adjusted according to Jones et al., 2002; Trails B norms from Tombaugh, 2004; COWA norms from Tombaugh, Kozak, & Rees, 1999). Scores on the 3MS potentially range from 0 to 100. Lower scores indicate greater impairment. The mean was 93.2 (SD = 5.4), indicating relatively high functioning in the current sample.
Demographics
Standard demographic items were administered. HIV-specific information was collected: number of years since HIV diagnosis (Time since Dx) and AIDS status.
Data analysis
Preliminary analyses examined bivariate associations among adherence variables and potentially confounding demographic variables (age, gender, ethnicity, education, income, sexual orientation, relationship status, and study site) and medically relevant variables (time since HIV diagnosis, number of ARVs, and substance use).
SEM examined models of adherence using Full Information Maximum Likelihood (FIML) with LISREL v8.80 (Jöreskog, & Sörbom, 2006). A model was judged to be supported by the data when the absolute fit index root mean square error of approximation (RMSEA) was less than 0.06 (Hu & Bentler, 1999; Marsh, Hau, & Wen, 2004) and the 90% confidence interval included 0.05 (Bollen & Long, 1993; Browne & Cudeck, 1993).
Anderson and Gerbing's (1988) two-step approach was used to examine factor loadings of the measurement models (MMT) and investigate relations among structural components. Modification indices (MIs) were inspected to isolate sources of ill fit. Model parameters under alternative specifications were examined to identify whether they changed substantially as various models were tested.
Results
Prior to analysis, distributions of variables were examined. To adjust for skewness, an arcsine transformation for proportions (i.e. variables bounded by zero and unity; Cohen, Cohen, West, & Aiken, 2003, pp. 240–241) was applied to the adherence and NPF variables; viral load was natural log transformed. Variances, covariances, means, and standard deviations are shown in Table 2. Means shown in Table 2 represent the average response across scale items, with the exception of NPF, adherence, and viral load which were transformed. With respect to ART adherence, 80% of participants reported being adherent ≥95% (All HIV Meds) and 83% indicated following specific instructions ≥95% (Instructions). Time since Dx was associated with both adherence variables (r = 0.17, p <0.01, All HIV Meds; r = 0.19, p <0.01, Instructions) and was included as a control variable in SEM analyses. Adherence was not significantly associated with other demographic or medically related variables (age, gender, ethnicity, education, income, sexual orientation, relationship status, substance use, study site).
Table 2.
Variances and covariances of observed variables in structural equation models examining antiretroviral medication adherence in 244 HIV-positive older adults.
| Construct | Indicator | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Social support | 1. Social well-being | 1.03 | 0.40 | 0.42 | −0.21 | −0.06 | −0.24 | −0.06 | −0.13 | 0.47 | 0.03 | 0.07 | 0.07 | 0.13 | 0.14 | 0.01 | 0.29 |
| 2. Friends | 0.97 | 0.61 | −0.14 | −0.05 | −0.17 | −0.03 | −0.16 | 0.37 | −0.01 | 0.25 | 0.12 | 0.09 | 0.08 | 0.28 | 0.21 | ||
| 3. Family | 1.11 | −0.12 | −0.01 | −0.10 | −0.03 | −0.13 | 0.32 | 0.01 | 0.13 | 0.05 | 0.08 | 0.07 | −0.13 | −0.47 | |||
| Maladaptive coping | 4. Escape | 0.37 | 0.06 | 0.21 | 0.03 | 0.16 | −0.24 | −0.01 | −0.01 | −0.01 | −0.04 | −0.05 | 0.19 | 0.01 | |||
| 5. Avoid | 0.08 | 0.07 | 0.01 | 0.04 | −0.07 | −0.01 | −0.01 | −0.01 | −0.03 | −0.04 | 0.15 | −0.21 | |||||
| 6. Destruct | 0.35 | 0.05 | 0.14 | −0.26 | −0.01 | −0.03 | −0.06 | −0.06 | −0.04 | 0.07 | −0.07 | ||||||
| Negative affect | 7. Depression | 0.03 | 0.04 | −0.08 | 0.00 | −0.01 | −0.01 | −0.02 | −0.03 | 0.06 | −0.04 | ||||||
| 8. Anxiety | 0.29 | −0.28 | −0.01 | −0.05 | 0.00 | −0.06 | −0.08 | 0.17 | −0.22 | ||||||||
| 9. Psychological general well-being | 0.84 | 0.01 | 0.13 | 0.06 | 0.13 | 0.18 | 0.00 | 0.11 | |||||||||
| Neuropsychological functioning | 10. 3MS | 0.05 | 0.01 | 0.06 | 0.00 | 0.01 | 0.03 | 0.07 | |||||||||
| 11. Trails B | 0.81 | 0.23 | 0.02 | −0.02 | −0.01 | 0.28 | |||||||||||
| 12. COWA | 0.64 | 0.01 | −0.02 | 0.24 | 0.39 | ||||||||||||
| ART adherence | 13. All HIV medications | 0.38 | 0.32 | −0.77 | 0.56 | ||||||||||||
| 14. Instructions | 0.56 | −0.76 | 0.81 | ||||||||||||||
| Viral load | 15. Ln viral load | 13.77 | −1.19 | ||||||||||||||
| Time since HIV diagnosis | 16. Years since HIV-positive diagnosis | 27.11 | |||||||||||||||
| M | 2.28 | 3.62 | 3.48 | 1.82 | 2.03 | 1.20 | 0.50 | 0.87 | 3.04 | 2.70 | 1.08 | 1.16 | 2.82 | 2.82 | 2.26 | 12.90 | |
| SD | 1.01 | 0.98 | 1.05 | 0.61 | 0.28 | 0.59 | 0.17 | 0.54 | 0.92 | 0.22 | 0.90 | 0.80 | 0.62 | 0.75 | 3.71 | 5.21 |
Note: Variances are shown in the diagonal of the matrix. ART, Antiretroviral therapy; 3MS, Modified Mini-Mental State Examination; COWA, Controlled Oral Word Association; Ln, Natural log.
Measurement models (MMTs)
Modeling began by testing a MMT with seven latent variables formed using the indicators described above. We included a “dummy” latent variable with a single Viral Load indicator. In the instance of a single indicator, using prior research to inform model specification and improve parameter estimation is recommended (Kline, 2005, pp. 229–231). Thus, we fixed the error variance of the viral load indicator to 27%, a value derived from the average of the standardized error variance terms reported by Weaver et al. (2005).
An initial test of the MMT suggested satisfactory data-model fit, FIML χ2 (85, N = 244) = 160.67, p <0.001, RMSEA = 0.06 (CI90% = 0.05, 0.07). Indicators loaded significantly on their respective factors, parameter estimates were reasonable, and factors were measured adequately. Intercorrelations among the constructs are shown in Table 3. The correlation between the adherence and NPF latent constructs was nonsignificant. NPF was excluded from the model and data-model fit reexamined. Fit indices for the second MMT suggested a marginal fit, FIML χ2(52, N = 244) = 112.81, p <0.001, RMSEA = 0.06 (CI90% = 0.05, 0.08). MIs were inspected to isolate sources of ill fit. The highest MI represented an error covariance between the Friends and Family subscales of the PSR, which when allowed to correlate resulted in a statistically significant improvement (FIML χ2(51, N = 244) = 89.39, p <0.001, RMSEA = 0.05; CI90% = 0.03, 0.07) and was considered a reasonable modification as this likely represented non-random error (i.e. subscales of the same instrument; Gerbing & Anderson, 1984). All latent constructs were significantly correlated with adherence in the final MMT.
Table 3.
Intercorrelations among the latent constructs included in the initial measurement model in 244 HIV-positive older adults.
| Indicator | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Social support | – | −0.44*** | −0.62*** | 0.18* | 0.24** | 0.04 | 0.00 |
| 2. Maladaptive coping | – | 0.76*** | −0.14 | −0.22** | 0.11 | −0.04 | |
| 3. Negative affect | – | −0.09 | −0.36*** | 0.07 | −0.06 | ||
| 4. Neuropsychological functioning | – | 0.00 | 0.08 | 0.11 | |||
| 5. ART adherence | – | −0.47*** | 0.23** | ||||
| 6. Viral load | – | −0.08 | |||||
| 7. Years since HIV diagnosis | – |
Note: ART, Antiretroviral therapy.
p <0.05;
p <0.01;
p <0.001.
Psychosocial models
Based on previous research (Gonzalez, et al., 2004; Simoni et al., 2006a; Weaver et al., 2005) and guided by Lazarus and Folkman's (1984) Transactional Model of Stress and Coping, two competing models examining stress and coping constructs as determinants of adherence and viral load were considered (Figure 1). We examined fit indices for the non-nested models independently and applied the criteria described above when judging data-model adequacy.
Maladaptive coping mediational model
To determine whether maladaptive coping mediated the relationships between social support, affect, and adherence, data were fit to the hypothesized maladaptive coping mediational model. The path from social support to maladaptive coping was nonsignificant (standardized coefficient = −0.09, p > 0.05) and fit indices suggested that data-model fit was inadequate (FIML χ2 (56, N = 244) = 103.25, p <0.001, RMSEA =0.06 (CI90% =0.04, 0.08). The contention that maladaptive coping mediates the associations between negative affect, social support, and adherence was not supported.
Negative affect mediational model
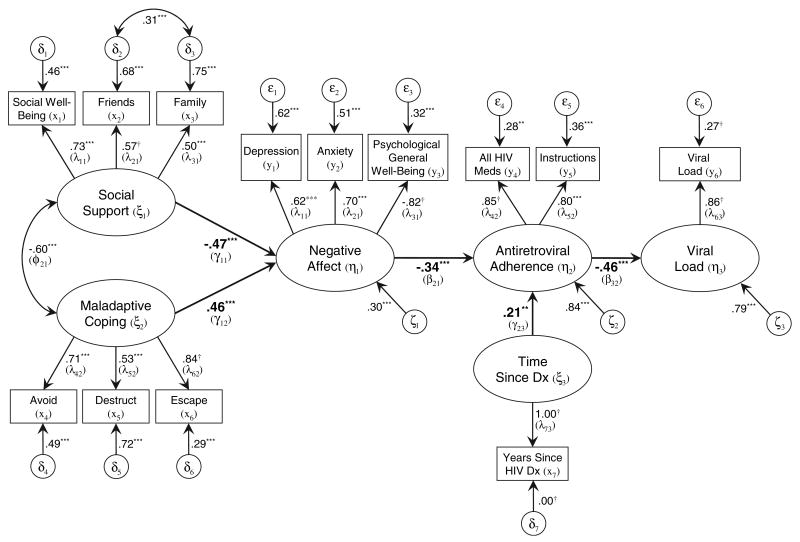
Data were fit to the second hypothesized structural model. Results supported the notion that affect mediates the relationships among social support, maladaptive coping, and adherence (FIML χ2(56, N = 244) = 94.81, p <0.001, RMSEA = 0.05 (CI90% = 0.03, 0.07). All structural paths were statistically significant (p's < 0.001). The model accounted for 70% of the variance in negative affect, 16% in adherence, and 21% in viral load. The final negative affect mediational model is shown in Figure 2.
Figure 2.
Standardized parameter estimates of negative affect mediational model of antiretroviral medication adherence in HIV-seropositive older adults. Structural paths and coefficients shown in bold. Nonsignificant correlations among exogenous variables and nonsignificant structural paths not shown. LISREL notation shown in parentheses. Meds = medications. Dx = diagnosis. †fixed parameter. *p <0.05; **p <0.01; ***p <0.001.
Direct effects
Direct effects of predictor variables on adherence are shown in Figure 2; non-significant effects have been omitted for simplicity. The model indicated that social support (γ = −0.47, p <0.001) and maladaptive coping (γ = 0.46, p <0.001) predict negative affect which, in turn, predicts adherence (β = −0.34, p <0.001). Time since Dx was significantly associated with adherence (γ = 0.21, p <0.01). The direct effect of adherence on viral load (β = −0.46, p <0.001) evidenced that participants who were more adherent were significantly more likely to have a reduced viral load.
Total and indirect effects
Table 4 shows the standardized total and indirect effects (indirect effects include the respective overall indirect paths). Time since Dx had an indirect effect on viral load (standardized coefficient = −0.10, p <0.01). The results indicated that 100% of the total effect of both social support (standardized coefficient = 0.16, p <0.01) and maladaptive coping (standardized coefficient = −0.15, p <0.01) on adherence was delivered indirectly through negative affect. Negative affect fully mediates these relationships as: (a) bivariate relationships between adherence and social support and coping are significant; (b) there is an independent relationship between negative affect and adherence; (c) there are significant indirect paths between adherence and social support (Sobel statistic z = 2.81, p <0.01) and coping (Sobel statistic z = −2.91, p <0.01); and (d) significant direct relationships between exogenous constructs and adherence were absent (Preacher & Hayes, 2004). Additionally, 100% of the total effects of the psychosocial constructs on viral load was delivered indirectly through adherence (standardized coefficient = −0.07, p <0.01 social support; standardized coefficient = 0.07, p <0.01 maladaptive coping; standardized coefficient = 0.15, p <0.01 negative affect).
Table 4.
Standardized total and indirect effects for structural model of antiretroviral medication adherence in 244 HIV-positive older adults.
| Dependent variable | |||
|---|---|---|---|
| Independent variable | Negative affect | ART adherence | Viral load |
| Total effects | |||
| Social support | −0.47*** | 0.16** | −0.07** |
| Maladaptive coping | 0.46*** | −0.15** | 0.07** |
| Years since HIV diagnosis | −0.01 | 0.21** | −0.08 |
| Negative affect | −0.34*** | 0.15*** | |
| ART adherence | −0.46*** | ||
| Indirect effects | |||
| Social support | 0.16** | −0.07** | |
| Maladaptive coping | −0.15** | 0.07** | |
| Years since HIV diagnosis | 0.00 | −0.10** | |
| Negative affect | 0.15*** | ||
Note: ART, Antiretroviral therapy.
p <0.01;
p <0.001.
Discussion
Although previous studies have investigated correlates of ART adherence, HIV/AIDS researchers, healthcare providers, and HIV-seropositive individuals would benefit from empirically validated models that refine current theory and inform adherence interventions (Simoni et al., 2006a). The present study used SEM to evaluate psychosocial models of ART adherence in 244 HIV-positive older adults, a group that is growing rapidly in size and for whom little is known about ART adherence. SEM analyses examined social support, maladaptive coping, negative affect and NPF as potential determinants of adherence and viral load. Results supported a stress and coping model concinnous with an accepted and widely used framework (i.e. Lazarus & Folkman's Transactional Model of Stress and Coping); negative affect fully mediated the associations of social support and maladaptive coping with adherence. The present results are in accord with studies (e.g. Gonzalez et al., 2004; Paterson et al., 2000) that have emphasized relationships among social support, coping, affect, and adherence.
The current study added to contemporary models of adherence by including NPF, testing alternative models and, in part, replicating prior research using different indicators of each latent construct. Contrary to Barclay et al. (2007), this study did not find an association between NPF and adherence in HIV-infected older adults. The relatively high level of NPF observed in the present sample may account for the unsuccessful replication of Barclay et al.'s results.
Self-reported adherence levels in the present study (80% All HIV Meds, 83% Instructions) were similar to Barclay et al.'s (2007) and Hinkin et al.'s (2004) observations obtained using MEMS (85% and 87% adherence, respectively). This finding appears to support the criterion-related validity of the present study and is consistent with literature supporting the validity of self-reported adherence data (Nieuwkerk & Oort, 2005; Simoni et al., 2006b).
Study results have implications for experimental research and clinical practice. Specifically, interventions aimed at improving adherence in HIV-infected older adults are more likely to be successful if social support, adaptive coping strategies, and affect are enhanced. This study underscores the importance of social support in influencing health behaviors and health outcomes, and supports the use of group interventions focused on coping enhancement and alleviating psychological distress (e.g. Sikkema et al., 2006; Sikkema et al., 2007). It is likely that enhancing coping, social support, and affect will positively impact adherence in older adults. Additionally, study results suggest that healthcare providers should regularly assess affect and encourage patients to seek social support. For example, online or telephone-based support groups may provide isolated or stigmatized older adults with opportunities to interact with HIV-seropositive peers who face the unique challenges of aging with HIV (Heckman et al., 2006).
The present study had several advantages and limitations. Data were collected via ACASI interviews to maximize integrity and confidentiality. While some research suggests that HIV-infected persons may overestimate ART adherence (Garber, Nau, Erickson, Aikens, & Lawrence, 2004), recent reviews indicate that self-report methods are viable assessment strategies (Nieuwkerk and Oort, 2005). Simoni et al.'s (2006b) review maintains that, “… even brief self-report measures of ARV adherence can be robust…” (p. 227). Nevertheless, the present study cannot rule out possible reporting bias. Some indicators of maladaptive coping included unpublished items. The viral load variable was self-reported. To address limitations of the single viral load indicator, we fixed the error variance of the viral load indicator based on prior research that included objective measures (assays of venous blood samples; Weaver et al., 2005). Participants were from large urban cities in the USA; findings may not generalize to other geographic regions or non-metropolitan areas. The sample included participants who reported at least some depressive symptoms, had generally high NPF, and were affiliated with an AIDS service organization (ASO) or other community-based organization. Future studies could address these limitations by sampling a more expansive spectrum with regard to affect, neurocognitive variables, geography, and formal support networks.
Future research should continue to refine models of ART adherence in HIV-positive older adults and include additional theoretically grounded constructs such as self-efficacy, locus of control, motivation to adhere, and patient–provider relationships. We emphasize that additional research is needed to clarify the role of NPF in HIV-infected older adults with respect to ART adherence. Taken as a whole, the current study suggests that interventions that increase social support and reduce maladaptive coping may be effective at improving ART adherence in the growing population of HIV-seropositive older adults.
Acknowledgments
This research was supported by Grant R01-MH067568 from the National Institute of Mental Health and the National Institute on Nursing Research to Timothy G. Heckman, Ph.D. We are grateful to all study participants, the AIDS service organizations that made this research possible (The Columbus AIDS Task Force, AIDS Volunteers of Cincinnati, and Callen-Lorde in NYC), and Sharon Neufeld and Allyson DeLorenzo.
References
- Albert SM, Weber CM, Todak G, Polanco C, Clouse R, McElhiney M. An observed performance test of medication management ability in HIV: Relation to neuropsychological status and medication adherence outcomes. AIDS and Behavior. 1999;3:121–128. [Google Scholar]
- Anderson JC, Gerbing DW. Structural equation modeling in practice: A review and recommended two-step approach. Psychological Bulletin. 1988;103:411–423. [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Zolop AR, Holodniy M, Shiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, et al. Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JA. Addressing the challenges of adherence. Journal of Acquired Immune Deficiency Syndromes. 2002;29(1):S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Catz SL, Heckman TG, Kochman A, DiMarco M. Rates and correlates of HIV treatment adherence among late middle-aged and older adults living with HIV disease. Psychology, Health & Medicine. 2001;6:47–58. [Google Scholar]
- Centers for Disease Control and Prevention. HIV/ AIDS surveillance report: HIV infection and AIDS in the United States and dependent areas, 2005. 2007 Retrieved September 2, 2007, from http://www.cdc.gov/hiv/topics/surveillance/basic.htm.
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Morin M, Sherr L. Adherence to HIV combination therapy. Social Science and Medicine. 2000;50:1599–1605. doi: 10.1016/s0277-9536(99)00468-2. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd. Mahwah, NJ: Lawrence Earlbaum Associates; 2003. [Google Scholar]
- Conway B. The role of adherence to antiretroviral therapy in the management of HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2007;45:S14–S18. doi: 10.1097/QAI.0b013e3180600766. [DOI] [PubMed] [Google Scholar]
- Dupuy HJ. The Psychological General Well-Being (PGWB) Index. In: Wenger NK, Mattson ME, Furberg CD, Elinson J, editors. Assessment of quality of life in clinical trials of cardiovascular therapies. Washington, DC: Le Jacq; 1984. pp. 170–183. [DOI] [PubMed] [Google Scholar]
- Folkman L, Lazarus RS. Manual for the ways of coping questionnaire: Research edition. Palo Alto, CA: Consulting Psychologists Press; 1988. [Google Scholar]
- Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: A summary of the literature. Medical Care. 2004;42:649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- Gerbing DW, Anderson JC. On the meaning of within-factor correlated measurement errors. Journal of Consumer Research. 1984;11:572–580. [Google Scholar]
- Gonzalez JS, Penedo FJ, Antoni MH, Durán RE, McPherson-Baker S, Ironson G, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology. 2004;23:413–418. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, et al. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. Journal of Clinical Epidemiology. 2001;54:S35–S43. doi: 10.1016/s0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- Gwadz M, De Vogli R, Rotheram-Borus MJ, Diaz MM, Cisek T, James NB, et al. Behavioral practices regarding combination therapies for HIV/ AIDS. Journal of Sex Education and Therapy. 1999;24:81–88. [Google Scholar]
- Hansen NB, Ghebremichael M, Tarakeshwar N, Kochman A, Zhang H, Sikkema KJ. Longitudinal effects of coping on outcome in a randomized controlled trial of a coping group intervention for HIV-positive adults with AIDS-related bereavement. Death Studies. 2006;30:609–636. doi: 10.1080/07481180600776002. [DOI] [PubMed] [Google Scholar]
- Heckman TG, Barcikowski R, Ogles B, Suhr J, Carlson B, Holroyd K, et al. A telephone-delivered coping improvement group intervention for middle-aged and older adults living with HIV/AIDS. Annals of Behavioral Medicine. 2006;32:27–38. doi: 10.1207/s15324796abm3201_4. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasala RS, Lam MN, et al. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18:S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R, Heath K, Bangsberg DR, Yip B, Press N, O'Shaughnessy MV, et al. Intermittent use of triple combination therapy is predictive of mortality at baseline and after one year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jones TG, Schinka JA, Vanderploeg RD, Small BJ, Graves AB, Mortimer JA. 3MS normative data for the elderly. Archives of Clinical Neuropsychology. 2002;17:171–177. [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8.80. Chicago, IL: Scientific Software International; 2006. [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2nd. New York: Guilford Press; 2005. [Google Scholar]
- Lazarus RS, Folkman S. Appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- Lovejoy TI, Heckman TG, Sikkema KJ, Hansen NB, Kochman A, Suhr J, et al. Patterns and correlates of sexual activity and condom use behavior in persons 50-plus years of age living with HIV/AIDS. AIDS and Behavior. doi: 10.1007/s10461-008-9384-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, Hau K, Wen Z. In search of golden rules: Comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler's (1999) findings. Structural Equation Modeling. 2004;11:320–341. [Google Scholar]
- Murphy DA, Rotheram-Borus MJ, Marelich WD. Factor structure of a coping scale across two samples. Journal of Applied Social Psychology. 2003;3:627–647. [Google Scholar]
- Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: A meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2005;38:445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Porter K, et al. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. The Lancet. 2003;362:1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instrument, & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Rausch DM, Stover ES. Neuroscience research in AIDS. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2001;25:231–257. doi: 10.1016/s0278-5846(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Schroder KEE, Carey MP, Vanable MP. Methodological challenges in research on sexual risk behavior: II. Accuracy of self-reports. Annals of Behavioral Medicine. 2003;26:104–123. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema KJ, Hansen NB, Ghebremichael M, Kochman A, Tarakeshwar N, Meade CS, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: Longitudinal effects on grief. Health Psychology. 2006;25:563–570. doi: 10.1037/0278-6133.25.5.563. [DOI] [PubMed] [Google Scholar]
- Sikkema KJ, Hansen NB, Kochman A, Tarakeshwar N, Neufeld S, Meade CS, et al. Outcomes from a group intervention for coping with HIV/AIDS and childhood sexual abuse: Reductions in traumatic stress. AIDS and Behavior. 2007;11:49–60. doi: 10.1007/s10461-006-9149-8. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychology. 2006a;25:74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson C, Pantalone DW, Merrill J, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and Behavior. 2006b;10:227–331. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakeshwar N, Hansen NB, Kochman A, Sikkema KJ. Gender, ethnicity, and spiritual coping among bereaved HIV-positive individuals. Mental Health, Religion, and Culture. 2005;8:109–125. [Google Scholar]
- Tate DC, van den Berg JJ, Hansen NB, Kochman A, Sikkema KJ. Race, social support, and coping strategies among HIV-positive gay and bisexual men. Culture, Health & Sexuality. 2006;8:235–249. doi: 10.1080/13691050600761268. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Tombaugh TN. Trail making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology. 1999;14:167–177. [PubMed] [Google Scholar]
- Turner RJ, Frankel BG, Levin DM. Social support: Conceptualization, measurement, and implications for mental health. Research in Community and Mental Health. 1983;3:67–111. [Google Scholar]
- Weaver KE, Llabre MM, Durán RE, Antoni MH, Ironson G, Penedo FJ, et al. A stress and coping model of medication adherence and viral load in HIV-positive men and women on highly active antiretroviral therapy (HAART) Health Psychology. 2005;24:385–392. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- Webster K, Odom L, Peterman A, Lent L, Cella D. The functional assessment of chronic illness therapy (FACIT) measurement system: Validation of version 4 of the core questionnaire. Quality of Life Research. 1999;8:604. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]