Abstract
BACKGROUND
Pediatric mastocytosis consists of a spectrum of clinical variants characterized by increased numbers of resident mast cells in various organ systems. Mast cells are instrumental in mediating anaphylaxis and patients with mastocytosis are at risk to develop provoked and unprovoked episodes of anaphylaxis.
METHODS
The authors examined peri-anesthetic records of patients with pediatric mastocytosis who were anesthetized for diagnostic and surgical procedures from 1993 to 2006. In addition, the authors conducted a literature review of the experience of the use anesthetics in pediatric mastocytosis.
RESULTS
Twenty-two patients with pediatric mastocytosis, with a median age of 3.2 years (range 6 months to 20 years) at the time of the procedure, were anesthetized for 29 diagnostic and surgical procedures. All variants of the disease are represented in this series. Most patients had a history of flushing, pruritus, GERD and abdominal pain; one patient had history of spontaneous anaphylaxis. Routine anesthetic techniques were used and despite the complexity of the disease, the peri-operative courses were uncomplicated and without serious adverse events.
CONCLUSIONS
We review the main features of pediatric mastocytosis, its anesthetic and perioperative implications, and describe a practical approach to the anesthetic management of pediatric patients with the disease. While many drugs used routinely in anesthesia reportedly cause mast cell degranulation, deviations from routine anesthesia techniques are not necessarily warranted. However, an understanding of the anesthetic implications of the disease and meticulous preparation to treat possible adverse events are advised.
INTRODUCTION
Mastocytosis represents a spectrum of disease variants characterized by a pathologic increase of mast cells in cutaneous and extracutaneous sites including the gastrointestinal tract, liver, spleen, lymphoid tissues, and bone marrow. The prevalence of the disease is approximately one in 25-30,000 and its clinical manifestations vary with age of onset (pediatric vs. adult), disease variant (systemic vs. cutaneous), severity (indolent vs. aggressive) and associated hematologic disorders. The symptoms of mastocytosis are related to an increased mast cell burden in specific organ systems, spontaneous or induced (immunological and non-immunological) release of mediators from mast cells, and the associated hematologic consequences. Patients with all variants of disease may exhibit symptoms of gastro-esophageal reflux disease (GERD), flushing, pruritus, urticaria, and hypotension. More importantly, all patients with mastocytosis are at risk for unprovoked anaphylaxis that can occur during anesthesia.1, 2
Patients with pediatric mastocytosis often need diagnostic and therapeutic procedures that require sedation or anesthesia. Because mast cells are implicated in the pathophysiology of anaphylaxis and patients with mastocytosis have an increased mast cell burden, drugs used in anesthesia which degranulate mast cells raise justified concerns about potential adverse reactions. These concerns are re-enforced by existing literature implicating opioids, muscle relaxants, analgesics, and volatile anesthetics as drugs that may directly or indirectly activate mast cells.3, 4 However, while heightened awareness of potential consequences of mast-cell mediator release is warranted, the fear of anaphylaxis should not prevent the use of opioids or muscle relaxant deemed beneficial during the peri-operative period.
The dearth of information available on anesthetic management of children and adolescents with mastocytosis creates a challenge. The prevailing information consists mainly of single case reports,5-8 and one series from 1987 with data from patients with two of the four sub-variants of pediatric-onset cutaneous mastocytosis who received drugs currently seldom used in pediatric anesthesia.9 In three of the single case reports, all patients had uncomplicated anesthetics after pre-medication with H1 antihistamines.5-7. In the fourth case study, the patient had cardiac arrest.8 The remaining study consists of a retrospective analysis of 15 children with cutaneous mastocytosis, urticaria pigmentosa (N=12) and solitary mastocytoma (N=3), who received general anesthesia for 29 procedures.9 Two patients were pre-medicated with H1 antihistamines and most anesthetics were uncomplicated, although two children had cutaneous eruptions after administration of codeine. In this report, we contribute to this experience by reviewing the anesthetic management of 22 patients encompassing multiple variants of pediatric mastocytosis.
MATERIALS AND METHODS
Patients with pediatric mastocytosis who required anesthesia for invasive procedures were included in this study. Patients were evaluated at the NIH from 1993 to 2006 as participants in an IRB-approved research protocol designed to study the pathogenesis, natural history, and the management of pediatric mastocytosis. Consent was obtained from parents or guardians for participation in the study and for review of operative records. In addition, assent was obtained from children older than six years. Although all participants were enrolled in a research protocol at the NIH Clinical Center, some procedures were performed at other institutions. Of the 22 patients, 17 children had 23 procedures performed at the NIH and five had six procedures performed outside the NIH. The anesthetic technique used for the procedures was chosen at the discretion of each anesthesiologist. In some cases, an intensivist administered sedation. A multidisciplinary team was involved in the care of these patients and included an allergist at NIH. Unless there was a clear history of adverse reactions to a given drug, no anesthetics or analgesics were specifically avoided.
The authors collected data on patient demographics, primary diagnosis, disease variant following the WHO classification of mastocytosis (Table 1),10 physical findings, genetic analysis,11 diagnostic and therapeutic procedures, anesthetic techniques, drugs used peri-operatively, length of anesthetics, and peri-anesthetic events. We evaluated anesthetic and procedural complications and noted hemodynamic lability, temperature changes, airway complications, changes in oxygen saturation, and cutaneous eruptions up to 48 hours after the procedures.
Table 1.
World Health Organization Consensus Classification on Mastocytosis 1
| Variant Term | Abbreviation | Sub variants |
|---|---|---|
| Cutaneous Mastocytosis | CM | Urticaria Pigmentosa (UP) |
| Maculopapular CM (MPCM) | ||
| Diffuse CM (DCM) | ||
| Mastocytoma of Skin (MAST) | ||
| Indolent Systemic Mastocytosis | ISM | Smoldering SM |
| Isolated bone marrow mastocytosis | ||
| Systemic Mastocytosis with an | SM-AHNMD | SM-AML |
| Associated Clonal Hematologic | SM-MDS | |
| Non-Mast Cell Lineage Disease | SM-MPD | |
| SM-CMML | ||
| SM-NHL | ||
| Aggressive Systemic Mastocytosis | ASM | |
| Mast Cell Leukemia | MCL | Aleukemic MCL |
| Mast Cell Sarcoma | ||
| Extracutaneous Mastocytoma |
adapted from reference 10
RESULTS
Patient Demographics
Twenty-two patients with pediatric mastocytosis were anesthetized for 29 diagnostic and surgical procedures (median age at time of first anesthetic = 3.2 years [range 6mos - 20 yrs], Table 2). Among the cohort of patients (15 males and 7 females), 14 had cutaneous mastocytosis (CM) without systemic involvement: six had urticaria pigmentosa (UP), two had maculopapular CM (MPCM), five had diffuse cutaneous mastocytosis (DCM), and one had a mastocytoma (MAST). Eight patients had indolent systemic mastocytosis (ISM), of which all had UP (Table 2). The onset of disease ranged from birth to 12 months.
Table 2.
Demographics and Serum Tryptase Levels in Patients with Pediatric Mastocytosis
| Patient | Diagnosis | Gender | Age of onset (months) |
Age at first procedure (years) |
Serum Tryptase (normal <20 ng/ml) |
|---|---|---|---|---|---|
| 1 | MPCM | F | 2 | 2.0 | 7 |
| 2 | MPCM | M | Birth | 6.0 | 6 |
| 3 | UP | M | Birth | 1.6 | 8 |
| 4 | UP | M | 3 | 20.0 | 5 |
| 5 | UP | F | Birth | 6.1 | 5 |
| 6 | UP | M | Birth | 9.2 | 2 |
| 7 | UP | F | 3 | 1.0 | 27 |
| 8 | UP | M | 12 | 3.2 | 5 |
| 9 | DCM | M | 4 | 1.3 | 74 |
| 10 | DCM | M | 3 | 0.8 | 167 |
| 11 | DCM | M | 9 | 4.2 | 5 |
| 12 | DCM | F | 4 | 3.4 | 18 |
| 13 | DCM | M | Birth | 7.3 | 126 |
| 14 | ISM | F | 1 | 5.6 | 109 |
| 15 | ISM | M | 6 | 1.9 | 22 |
| 16 | ISM | M | 5 | 14.8 | 45 |
| 17 | ISM | M | 3 | 2.9 | 54 |
| 18 | ISM | F | Birth | 2.4 | 348 |
| 19 | ISM | F | Birth | 3.2 | 238 |
| 20 | ISM | M | Birth | 0.5 | 124 |
| 21 | ISM | M | Birth | 1.0 | 440 |
| 22 | MAST | M | 3 | 20.3 | 21 |
MPCM = Maculopapular Cutaneous Mastocytosis; UP = Urticaria Pigmentosa;
DCM = Diffuse Cutaneous Mastocytosis; ISM = Indolent Systemic Mastocytosis;
MAST = Mastocytoma.
Upon questioning, parents would often report “allergies” to opioids and other histamine releasing drugs in their children. However, further questioning revealed that in most cases, these reports reflected parents’ concerns about the administration of those drugs to their children given their diagnosis of mastocytosis rather than an actual history of adverse reaction to those drugs.
One patient (5 %) was on chronic corticosteroid therapy, nine (41%) on H1 and H2 antihistamines, and five (23%) on other drugs including inhaled beta-agonists, inhaled corticosteroids, stimulant therapy for attention deficit disorder, and proton-pump inhibitors. All drugs were administered on routine schedules throughout patients’ hospitalizations.
Pre-operative symptoms consisted of those associated with cutaneous and systemic mastocytosis including cutaneous, gastrointestinal and neurologic manifestations. The most common cutaneous manifestations were flushing (N=19) and pruritus (N=17). Gastrointestinal symptoms including nausea, vomiting, diarrhea, and/or abdominal pain, were seen in ten patients. GERD was reported in five patients whose symptoms were controlled on H2 antihistamines or proton pump inhibitors. Other symptoms included headache in five, syncope in three and anaphylaxis in one patient (Table 3). This latter patient with systemic disease had episodes of unprovoked anaphylaxis documented by hypotension, loss of consciousness, and significant increases in serum tryptase (a marker of mast cell burden) over its baseline value. Among the 22 patients, median serum tryptase was 24.5 ng/ml (range: 2 ng/ml to 440 ng/ml; normal value <20 ng/ml, Mayo Clinic Diagnostic Laboratories, (Table 2).
Table 3.
Pre-Operative Symptom Frequency and Post-Operative Adverse Reactions.
| Signs & Symptoms | Number of Patients (%) |
Intra-Op or Post-Op Adverse Reaction (%) |
|---|---|---|
| Cutaneous | ||
| Flushing | 19 (86)1 | 2 (9) |
| Pruritus | 17 (77) | 0 |
| Blistering | 4 (18) | 0 |
| Gastrointestinal | ||
| N/V/ Diarrhea | 10 (45) | 41 (18) |
| Abdominal pain | 9 (41) | 0 |
| Hepatosplenomegaly | 5 (23) | -- |
| GERD | 5 (23) | 0 |
| PUD | 2 (9) | 0 |
| Neurological | ||
| Headache | 5 (23) | 0 |
| Cardiovascular | ||
| Hypotension | 0 (0) | 0 |
| Syncope | 3 (14) | 0 |
| Anaphylaxis | 1 (5) | 0 |
Total number of patients = 22
All 4 patients had nausea and vomiting only.
GERD = Gastroesophageal Reflux Disease
PUD = Peptic Ulcer Disease
PROCEDURES AND ANESTHETIC MANAGEMENT
The anesthetic management of 29 procedures (23 performed at the NIH and six at other institutions) is shown in Table 4. Preoperative allergy skin testing to drugs used during the anesthetics was not performed. Routine prophylactic H1 and H2 blockers and steroids were not administered prior to anesthetics; however, if patients were on chronic therapy (N=13), their medications were continued as scheduled. Fifteen patients (68%) were pre-medicated with midazolam (0.1 to 0.5 mg/kg) and one (5%) with fentanyl (1.0 mcg/kg).
Table 4.
Anesthetic management of patients with pediatric-onset mastocytosis*
| Patient | Age | Surgical time |
Anesthetic time |
Antihistamine | Procedure | Drugs used perioperatively | Adverse events | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Intra | Post | |||||||
| 1 | 2 | 15 | 30 | skin biopsy and bone marrow biopsy and aspirate |
midazolam | sodium thiopental, lidocaine, ketamine |
none | ||
| 2a | 12 | 90 | 120 | diphenhydramine ranitidine |
EGD, colonoscopy | midazolam | propofol, isoflurane | none | |
| 2b | 13 | 25 | 50 | bone marrow biopsy | midazolam, ketamine | acetaminophen | none | ||
| 3 | 13 | 30 | 45 | bone marrow biopsy and aspirate |
lidocaine, bupivacaine, isoflurane, sevoflurane, nitrous oxide |
fentanyl | vomiting | ||
| 4 | 4 | 30 | 48 | bone marrow biopsy and aspirate |
lidocaine, sevoflurane, nitrous oxide, fentanyl |
ondansetron | flushing | ||
| 5a | 0.9 | 15 | 48 | bone marrow biopsy and aspirate |
fentanyl, midazolam |
fentanyl | none | ||
| 5b | 3 | 70 | 85 | EGD | midazolam | sevoflurane | none | ||
| 6a | 1 | 15 | 30 | bilateral myringotomy tubes |
sevoflurane, nitrous oxide |
acetaminophen | none | ||
| 6b | 5 | 54 | 63 | diphenhydramine ranitidine |
cardiac catheterization | lidocaine, midazolam heparin |
acetaminophen | none | |
| 7a | 0.6 | 30 | 45 | bone marrow biopsy and aspirate |
lidocaine, chloral hydrate |
none | |||
| 7b | 19 | 60 | 75 | bone marrow biopsy and aspirate |
midazolam | lidocaine, propofol, fentanyl, ephedrine |
fentanyl, acetaminophen |
none | |
| 8 | 20 | 105 | 125 | laproscopic cholecystectomy |
midazolam | propofol, rocuronium, fentanyl, ondansetron, neostigmine, glycopyrrolate |
acetaminophen, hydrocodone |
vomiting | |
| 9 | 1 | 35 | 65 | bone marrow biopsy and aspirate |
lidocaine, sevoflurane nitrous oxide, propofol |
none | |||
| 10 | 6 | 18 | 27 | bone marrow biopsy and aspirate |
midazolam | prilocaine, ketamine | none | ||
| 11 | 9 | 75 | 90 | CT guided spleen biopsy |
midazolam | lidocaine, propofol, isoflurane, vercuronium, fentanyl, neostigmine, glycopyrrolate |
none | ||
| 12 | 0.8 | 20 | 40 | hydroxyzine ranitidine |
bone marrow biopsy and aspirate |
lidocaine, sevoflurane, nitrous oxide |
acetaminophen, fentanyl |
none | |
| 13 | 20 | 15 | 20 | bone marrow biopsy and aspirate |
lidocaine, midazolam, fentanyl |
none | |||
| 14 | 4 | 30 | 40 | ranitidine | bilateral myringotomy tubes and adenoidectomy |
midazolam methylpred -nisone |
sevoflurane, propofol | ondansetron, acetaminophen, fentanyl |
vomiting |
| 15 | 3 | 40 | 55 | hydroxyzine diphenhydramine ranitidine |
bone marrow and skin biopsy |
atropine, sevoflurane, nitrous oxide |
none | ||
| 16 | 1 | 30 | 55 | diphenhydramine ranitidine |
bone marrow biopsy and aspirate |
midazolam | lidocaine, propofol, ketamine |
acetaminophen, ibuprofen |
vomiting |
| 17 | 13 | 30 | 45 | cetirizine ranitidine |
bone marrow biopsy and aspirate |
midazolam | lidocaine, propofol. sevoflurane, isoflurane, nitrous oxide |
fentanyl | none |
| 18 | 2 | 20 | 30 | bone marrow biopsy and aspirate |
midazolam | isoflurane, sevoflurane, nitrous oxide, fentanyl |
none | ||
| 19a | 2 | 23 | 38 | bone marrow biopsy and aspirate |
midazolam | atropine, ketamine, midazolam |
none | ||
| 19b | 9 | 40 | 55 | bone marrow biopsy and aspirate |
lidocaine, nitrous oxide,fentanyl sevoflurane, isoflurane, |
flushing | |||
| 20 | 3 | 30 | 45 | bone marrow biopsy and EGD |
lidocaine, sevoflurane, fentanyl isoflurane, nitrous oxide |
none | |||
| 21 | 3 | 20 | 30 | hydroxyzine cetirizine ranitidine |
bone marrow biopsy and aspirate |
lidocaine, chloral hydrate |
none | ||
| 22a | 7 | 120 | 135 | dental rehabilitation | midazolam | lidocaine, propofol, vecuronium, sodium thiopental |
fentanyl | none | |
| 22b | 14 | 45 | 50 | EGD | midazolam | propofol, isoflurane, nitrous oxide, fentanyl succinylcholine, cisatracurium |
none | ||
| 22c | 16 | 340 | 360 | cetirizine | otoplasty and bone marrow biopsy and aspitrate |
ceftriaxone | lidocaine, propofol, isoflurane, nitrous oxide, cisatracurium, fetanyl ondansetron |
diphenhydramine | none |
Age, in years and anesthetic and surgicaltimes in minutes. EGD= esophagogastroduodenoscopy.
General anesthesia was administered for 24 procedures and sedation and local anesthesia for five (Table 4). General anesthesia was induced with volatile anesthetics in 11 procedures, propofol in eight, sodium thiopental in two, and ketamine in three procedures. After induction of general anesthesia, the trachea was intubated in 7 patients (5 with and 2 without muscle relaxant), a laryngeal mask airway was inserted in one, and mask ventilation was continued in five patients. For the other 11 general anesthetics, the patients were allowed to spontaneously ventilate and oxygen was delivered by nasal canula. Over all 29 procedures, the average duration of anesthetics was 67 minutes (range 20-360 minutes).
All peri-operative courses were uncomplicated and no patient exhibited hemodynamic instability, signs of hypermetabolism, or significant changes in temperature. One patient with DCM (patient 13) developed induration on his left heel after a 6-hour procedure, despite careful attention to appropriate positioning and padding of pressure points. The area was treated with heat and foot elevation and the injury resolved completely within 18 hours. Two patients developed flushing without hemodynamic lability during or after the procedures. Four patients experienced nausea and vomiting shortly after the procedure, which was treated with ondansetron. Hypotension or bronchospasm associated with mast cell mediator release were not observed during any anesthetic. Intravenous opioids (fentanyl, morphine, or meperidine) were used during and after the procedures followed by oral acetaminophen or ibuprofen as needed for pain (Table 4).
DISCUSSION
We report a series of 29 anesthetics in 22 patients with pediatric mastocytosis where commonly used anesthetic regimens were used. Preoperative drug skin testing was not performed, prophylactic antihistamines or corticosteroids were not administered, and scheduled maintenance medications were continued. We adopt an approach that advocate the administration of incremental, rather than single boluses of needed drugs (opioids, muscle relaxants) known to activate mast cells in those patients without a previous history of adverse events. In addition, we recommend a thorough understanding of mastocytosis and its manifestations and meticulous preparation to treat, albeit rare, possible adverse events during anesthetics.
Review of the literature from 1968 to August 2006 using MeSH headings mastocytosis, anesthesia and analgesia, and anaphylaxis reveals reports of serious adverse reactions in adults with mastocytosis. 1-3, 12-15 In contrast to adults,2 we found no reports of anesthesia-related deaths,5-9 and few reports of serious anesthesia-related complications in children with mastocytosis.8,9 Our experience in pediatric mastocytosis supplement the scarce literature by describing anesthetics in children including those with systemic disease, a variant not included in earlier reports.
Several drugs used in this series (NSAIDS, opioids, sedative hypnotics, and volatile anesthetics) are reported to cause mast cell mediator release. However, previous studies of drug-induced mast cell activation were conducted in vitro or in animals and may not reflect the human response.16 In limited human studies, d-tubocurarine, tubocurarine, pancurionum and gallamine triethiodide are associated with histamine release; however, these agents are seldom used in current anesthesia practice and alternatives were used in our patients.17-19 Meperidine and morphine cause increases in histamine levels in humans more frequently than fentanyl and sulfentanil.20 We used fentanyl, morphine and meperidine and observed no evidence of hemodynamic lability. With regard to NSAIDs, as sensitivity occurs within the general population and it is expected some mastocytosis patients would have adverse reactions to NSAIDs. In fact, a lethal idiosyncratic reaction to ketorolac was observed in one adult with mastocytosis at this institution (unpublished data). Therefore, we administer NSAIDs with caution in mastocytosis patients and only in the absence of a clinical history of sensitivity. We perform graded administration of an NSAID on any patient who has no history of their use to establish safety.
One patient with DCM (# 14) developed an area of induration on the heel after a six hour procedure. The basis of such an event can be appreciated when one considers that patients with DCM (Fig 1) have marked skin infiltration with mast cells, as compared to the UP seen in cutaneous disease (Fig 2). In mastocytosis patients, mechanical pressure can sometimes lead to blister formation.21 Therefore, special attention to position and to protection of pressure points has to be given to patients with pediatric mastocytosis during anesthesia.
Figure 1. Diffuse Cutaneous Mastocytosis.
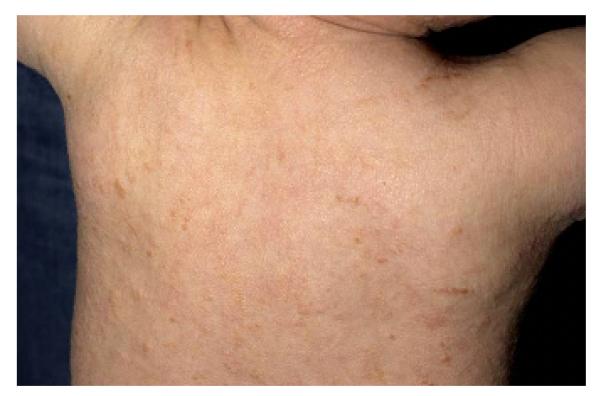
An infant with Diffuse Cutaneous Mastocytosis. The skin is diffusely infiltrated with mast cells demonstrating a peau d’ orange appearance without distinct uritcaria pigmentosa lesions.
Figure 2. Urticaria Pigmentosa.
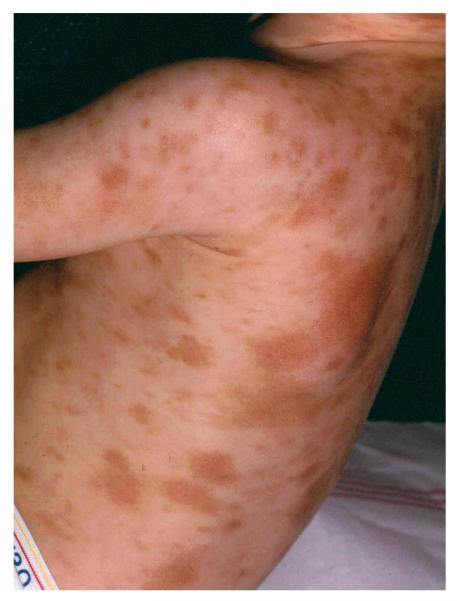
A child with Urtcaria Pigmentosa demonstrating typical lesions seen in patients with cutaneous mastocytosis. The lesions are varied in size and reddish-brown in color on a background of normal appearing skin.
Serum tryptase (constitutively expressed in patients with mastocytosis) levels are a reflection of mast cell burden. In patients with mastocytosis, further elevations of serum tryptase levels from baseline strongly suggest the diagnosis of anaphylaxis and mast cell degranulation. We thus routinely obtain baseline serum tryptase level to serve as a reference point that can be valuable in the diagnosis of possible anesthesia-associated adverse events.22
In our series, routine skin testing to anesthetic drugs, muscle relaxants or opioids was not performed prior to anesthetics. Skin tests, in general, are not reliable predictors of adverse reactions to drugs because only the intact drug, not their metabolite (which in some are responsible for the allergic reaction) are examined. Some drugs directly degranulate mast cells in the skin (codeine),23 but may be used in most individuals without a problem. Therefore, we advocate conducting a detailed review of prior clinical reactions to any agent and such agents be avoided.
Our experience with the anesthetic management of children with mastocytosis is in general agreement with reports in the literature and suggests that when needed, agents such as opioids and muscle relaxants can be used. However, one should consider the details of the patient’s history, be cognizant that potential serious anesthesia-related adverse events may occur, and treatment for those events should be readily available. Further, we suggest that routine preoperative drug testing is unnecessary and baseline serum tryptase levels are valuable for the diagnosis of intraoperative events.
Acknowledgments
Financial Support: Division of Intramural Research, NIAID & NIH Clinical Center
Footnotes
Implication statement
Pediatric mastocytosis is characterized by a spectrum of clinical variants which have in common, an increase in mast cells in various organ systems; and that can be associated with unprovoked anaphylaxis. Given the complex nature of the disease, understanding the pathophysiology is important for the management of patients with pediatric mastocytosis. This article suggests that commonly administered anesthetics may be used for patients with pediatric mastocytosis.
Conflict of interest: None
REFERENCES
- 1.Desborough JP, Taylor I, Hattersley A, Garden A, Wolff A, Bloom SR, Morgan M. Massive histamine release in a patient with systemic mastocytosis. Br J Anaesth. 1990;65:833–6. doi: 10.1093/bja/65.6.833. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan ST, Jones GN. Systemic mastocytosis presenting as profound cardiovascular collapse during anaesthesia. Anaesthesia. 1998;53:804–7. doi: 10.1046/j.1365-2044.1998.00536.x. [DOI] [PubMed] [Google Scholar]
- 3.Greenblatt EP, Chen L. Urticaria pigmentosa: an anesthetic challenge. J Clin Anesth. 1990;2:108–15. doi: 10.1016/0952-8180(90)90063-9. [DOI] [PubMed] [Google Scholar]
- 4.Stellato C, de Paulis A, Cirillo R, Mastronardi P, Mazzarella B, Marone G. Heterogeneity of human mast cells and basophils in response to muscle relaxants. Anesthesiology. 1991;74:1078–86. doi: 10.1097/00000542-199106000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Coleman MA, Liberthson RR, Crone RK, Levine FH. General anesthesia in a child with urticaria pigmentosa. Anesth Analg. 1980;59:704–6. [PubMed] [Google Scholar]
- 6.Nelson LP, Savelli-Castillo I. Dental management of a pediatric patient with mastocytosis: a case report. Pediatr Dent. 2002;24:343–6. [PubMed] [Google Scholar]
- 7.Brodier C, Guyot E, Palot M, David P, Rendoing J. [Anesthesia of a child with a cutaneous mastocytosis] Cah Anesthesiol. 1993;41:77–9. [PubMed] [Google Scholar]
- 8.Tirel O, Chaumont A, Ecoffey C. [Circulatory arrest in the course of anesthesia for a child with mastocytosis] Ann Fr Anesth Reanim. 2001;20:874–5. doi: 10.1016/s0750-7658(01)00536-6. [DOI] [PubMed] [Google Scholar]
- 9.James PD, Krafchik BR, Johnston AE. Cutaneous mastocytosis in children: anaesthetic considerations. Can J Anaesth. 1987;34:522–4. doi: 10.1007/BF03014363. [DOI] [PubMed] [Google Scholar]
- 10.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, Marone G, Nunez R, Akin C, Sotlar K, Sperr WR, Wolff K, Brunning RD, Parwaresch RM, Austen KF, Lennert K, Metcalfe DD, Vardiman JW, Bennett JM. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 11.Valent P, Sillaber C, Bettelheim P. The growth and differentiation of mast cells. Prog Growth Factor Res. 1991;3:27–41. doi: 10.1016/0955-2235(91)90011-r. [DOI] [PubMed] [Google Scholar]
- 12.Lerno G, Slaats G, Coenen E, Herregods L, Rolly G. Anaesthetic management of systemic mastocytosis. Br J Anaesth. 1990;65:254–7. doi: 10.1093/bja/65.2.254. [DOI] [PubMed] [Google Scholar]
- 13.Borgeat A, Ruetsch YA. Anesthesia in a patient with malignant systemic mastocytosis using a total intravenous anesthetic technique. Anesth Analg. 1998;86:442–4. doi: 10.1097/00000539-199802000-00044. [DOI] [PubMed] [Google Scholar]
- 14.Scott HW, Jr., Parris WC, Sandidge PC, Oates JA, Roberts LJ., 2nd Hazards in operative management of patients with systemic mastocytosis. Ann Surg. 1983;197:507–14. doi: 10.1097/00000658-198305000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosking MP, Warner MA. Sudden intraoperative hypotension in a patient with asymptomatic urticaria pigmentosa. Anesth Analg. 1987;66:344–6. [PubMed] [Google Scholar]
- 16.Lorenz W. Histamine release in man. Agents Actions. 1975;5:402–16. doi: 10.1007/BF01972656. [DOI] [PubMed] [Google Scholar]
- 17.Basta SJ, Savarese JJ, Ali HH, Moss J, Gionfriddo M. Histamine-releasing potencies of atracurium, dimethyl tubocurarine and tubocurarine. Br J Anaesth. 1983;55(Suppl 1):105S–6S. [PubMed] [Google Scholar]
- 18.Sniper W. The estimation and comparison of histamine release by muscle relaxants in man. Br J Anaesth. 1952;24:232–7. doi: 10.1093/bja/24.4.232. [DOI] [PubMed] [Google Scholar]
- 19.Booij LH, Krieg N, Crul JF. Intradermal histamine releasing effect caused by Org-NC 45. A comparison with pancuronium, metocurine and d-tubocurarine. Acta Anaesthesiol Scand. 1980;24:393–4. doi: 10.1111/j.1399-6576.1980.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 20.Flacke JW, Flacke WE, Bloor BC, Van Etten AP, Kripke BJ. Histamine release by four narcotics: a double-blind study in humans. Anesth Analg. 1987;66:723–30. [PubMed] [Google Scholar]
- 21.Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545–61. doi: 10.1016/0190-9622(95)90336-4. quiz 62-4. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–6. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 23.Fisher MM. Skin testing in the preoperative diagnosis of anaesthetic allergy. Ann Fr Anesth Reanim. 1985;4:192–4. doi: 10.1016/S0750-7658(85)80199-4. [DOI] [PubMed] [Google Scholar]


