Abstract
Objectives
To evaluate both shared and unique risk factors for maintaining physical and cognitive function into the 9th decade and beyond.
Design
Longitudinal cohort study.
Setting
Four US communities.
Participants
1677 participants in the Cardiovascular Health Study All Stars Study, assessed in 2005/06. Median age: 85 (range 77-102), 66.5% women, 16.6% black.
Measurements
Intact function was defined as no difficulty with any activities of daily living and a score >=80 on the Modified Mini-Mental Exam. Baseline characteristics assessed in 1992/93 included demographics, behavioral health factors, chronic disease history, subclinical disease markers, cardiovascular risk factors and inflammatory markers. Multinomial logistic regression was used to compare risk for physical disability, cognitive impairment, combined impairments vs. no functional impairment.
Results
Of the 1677 participants evaluated in both domains, 891 (53%) were functionally intact. Continuous measures of function, including the Digit Symbol Substitution Test and gait speed showed that all groups, including the most functional, had declined over time. The functional group had less decline, but also tended to have higher starting values. Functional individuals had a very high baseline health profile compared to those with either physical or cognitive impairment or both impairments combined. Women and individuals with higher weight had higher rates of physical impairment but not cognitive impairment. Risk factors common to both types of impairment included cardiovascular disease and hypertension.
Conclusion
Intact function was found in only about half of these older adults in the 9th decade and beyond. High baseline function and low vascular disease risk characterized functional aging.
Keywords: (3-5) epidemiology, physical function, cognitive function, aging, extreme old age
Introduction
Optimal function in old age requires the absence of both physical disability and cognitive impairment. With very advanced old age, individuals with intact function in both domains become increasingly exceptional. To improve the potential for maintaining function very late in life, more work is needed to identify the long term unique and shared risk factors for these two aspects of functional decline. These adverse outcomes have some shared risk factors, including cardiovascular disease1,2,3 and cardiovascular risk factors.4,5,6,7 Few studies have examined shared risk factors within the same cohort and over long periods of time to very late in life.
As a follow-up examination of the Cardiovascular Health Study (CHS) cohort, we had a unique opportunity to reassess function in individuals who had been followed for many years and to reassess functional status. Using the high quality, longitudinal data previously collected in CHS, we sought to identify factors from a comprehensive examination 13 years prior that characterized the most functional individuals. In this report, we describe the age, race, and sex distribution of functional survival and the magnitude of their functional decline. We also examined the unique and shared factors contributing to physical and cognitive status in late life, hypothesizing the healthy lifestyle behaviors would characterize those most likely to remain functional. We also hypothesized that prior low levels of cardiovascular risk factors would be strong and independent, long-term predictors of functional aging.
Methods
Population
From April 2005 - May 2006, the CHS cohort was re-recruited to reevaluate physical and cognitive functioning for the CHS All Stars Study. The CHS originally enrolled 5888 men and women in an ongoing study of the risk factors for cardiovascular disease in men and women aged 65 and older. Initial enrollment included 5201 men and women at 4 US field centers from June 1989 - July 1990 (Forsyth County, North Carolina, Sacramento County, California, Washington County, Maryland and Allegheny County, Pennsylvania) and an additional 687 African-American men and women in 1992/93. For optimal comparability of assessments in the original and minority cohort, we chose 1992/93 as the baseline year for this follow-up study. Of the 5553 individuals alive in 1992/93, 1677 (30.2 %) participated in the follow-up CHS All Stars examination. A large proportion (3272, 58.9 %) of the 5553 had died, 339 (6.1 %) were alive but did not give consent for the CHS All Stars study examination and 265 (4.8%) participated in the CHS phone follow-up but not the new functional assessment. For the 1677 participants, examinations were conducted either at home only (429, 25.6%), in the clinic only (648, 38.6%), at both home and clinic (66, 3.9%) or by telephone (534, 31.8% including 144 telephone proxy interviews).
Compared to those who had died, participants in the follow-up exam were somewhat younger at baseline, more likely to be women, married, and of higher income and education. They also had better reported and measured health for all variables except arthritis. Among those who were alive, the differences between those who did or did not participate were much smaller when compared to the differences between decedents and survivors. Lower participation was related to demographics and function with significantly lower rates noted for African-Americans, those with lower education, poorer self-reported health, more difficulty with ADLs, higher rates of hypertension and cardiovascular disease and lower physical activity. Thus, the participants in the CHS All-Stars exam had a somewhat better baseline health status than those who were alive but did not participate, but were generally representative of the surviving cohort.
CHS All Stars examination
The 2005/06 CHS All Stars examination included updated medical history and medication inventory, social history (education, income, marital status, living situation), self-reported health and physical function, physical activity8, a modified Center for the Study of Depression (CES-D) scale,9 modified mini-mental status examination (3MSE),10 Digit Symbol Substitution Test (DSST),11 isometric grip and lower extremity strength, gait speed, chair stand time, and fasting blood sample.12
Functional outcomes
Functional survival was defined at the time of the follow-up examination as being free of physical disability and free of cognitive impairment. Physical disability was defined as reporting any difficulty with at least 1 of 5 activities of daily living (ADLs) including transferring, bathing, dressing, eating, and toileting. Cognitive impairment was defined as a score of less than 80/100 on the 3MSE. For individuals without an in-person examination, a Telephone Interview for Cognitive Status (TICS)13 was obtained and for those with a proxy interview, the Informant Questionnaire for Cognitive Decline in the Elderly (IQCODE)14 was administered. Based on a prior validation study, estimated 3MSE scores were calculated based on these tests to classify current level of cognitive function. All 1677 participants had data on either physical or cognitive function, while 1649 could be classified in both domains. For those with an in-person examination (n=1143), functional change was also characterized for gait speed, grip strength, 3MSE and DSST by subtracting the current (2005/06) from baseline (1992/93) scores.
Risk factors
Similar to the current examination, numerous factors had been assessed in 1992/93.12 This analysis is focused on the risk factors assessed at the 1992/93 examination. Hypertension was defined by report of a physician diagnosis confirmed by use of an antihypertensive or by measured blood pressure >140/90 mmHg. Diabetes was defined as self-report of a physician diagnosis and hypoglycemic medication use or fasting blood glucose ≥ 126 mg/dl; impaired fasting glucose was defined as fasting glucose ≥ 110 mg/dl and < 126 mg/dl.15 Physical activity was assessed using a modified scale which was converted to kilograms of energy expenditure based on metabolic equivalents.8 Prevalent cardiovascular disease, including myocardial infarction, angina, stroke, transient ischemic attack (TIA) or peripheral arterial disease (PAD) was defined in 1989/90 by self-report confirmed by medication use or electrocardiogram (ECG) and this was updated using incident cases between 1989/90 and 1992/93 that had been reviewed and confirmed by a committee.16 Other measures assessed in 1992/93 included medical history and medication inventory, social history (education, income, marital status, living situation), self-reported health and physical function, CES-D,9 3MSE,10 DSST.11 Chronic pulmonary disease was defined as self report of chronic bronchitis, emphysema or asthma. Activities of daily living, instrumental activities of daily living and mobility activities were assessed.17 Non-invasive tests for measuring the extent of vascular disease included carotid ultrasound,18 ankle- arm index,19 ECG20 and a magnetic resonance image (MRI) of the brain in a substantial subset. White matter grade was defined on a scale of 0-9.21 Laboratory assays from 1992/93 included fasting glucose, total, high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol, triglycerides, and C-reactive protein.22
Analysis
Means and proportions of risk factors for those evaluated in 2005/06 were compared against those who died before 2005/06 and those who were alive, but who were not assessed for functional status, using analysis of variance and chi-squared tests, respectively. Skewed variables were reported as geometric means. Functional impairment was defined by combining physical and cognitive impairment into any impairment, and by examining separately groups defined by physical impairment only, cognitive impairment only, and both physical and cognitive impairments combined. Binomial and multinomial logistic regression models were used to explore the associations of risk factors with incident impairment among survivors, among 1459 individuals with intact physical and cognitive function at the baseline examination. For the multinomial model, the functional group was the reference group, and relative risk regression estimation was used, so the coefficients represent the risk of a particular impairment compared to the functional group, for a 1 unit change in the risk factor. Significance tests were also done to compare risk factors between groups. The risk factors were tested for significance and entered into each of the models in 4 stages: Stage 1 (demographics): age, sex, race, education, income, married (yes/no); Stage 2 (medical history): any cardiovascular disease, hypertension, diabetes; Stage 3 (cardiovascular disease risk factors): waist circumference, body mass index (BMI), weight, kilocalories of physical activity, systolic blood pressure, diastolic blood pressure, HDL, LDL, triglycerides, ln(C-reactive protein), ln(fasting glucose); Stage 4 (subclinical cardiovascular disease): maximum % stenosis of the internal carotid artery, internal and common carotid wall thicknesses, ankle arm index < 0.9. Coefficients that were significantly different from 0 for one or more of the outcome groups at each stage were kept in the model. Significance was tested using Wald tests with level 5%.
For participants who had gait speed, grip strength and DSST score measured in 2005/06, we summarized changes in these measures from baseline by sex and age groups and by sex and functional status at the All Stars exam. Differences in amount of change across age groups or functional status groups were assessed by linear regression.
Results
Median age of the CHS All Stars participants was 85 (range 77-102); 66.5% were women and 16.6% were black. There had been a large attrition due to death (3272, 58.9%) since the baseline. The cohort age in 2005-06 was quite advanced. In 1992-93, 379 (7%) were aged 85 or older, while among the CHS All Stars participants in 2005-06, 899, or 53.6% were 85 or older. Of the 1677 participants in 2005/06, 891 (53%) were both physically and cognitively intact while 758 (45%) were impaired; either physically impaired (n=430, 26%) or cognitively impaired (n=167, 10%) or impaired in both domains (n=161, 10%).
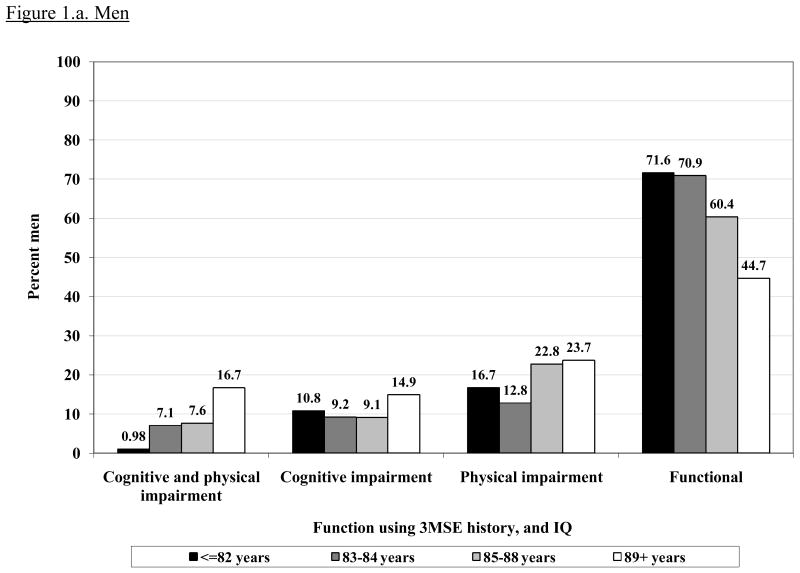
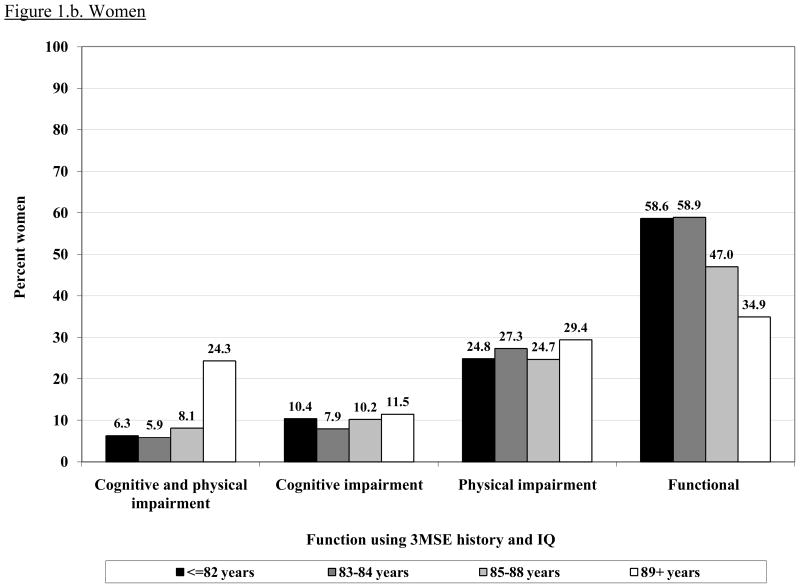
Figure 1 shows the prevalence of intact or impaired function according to age group and gender. The prevalence of intact physical and cognitive function decreased sharply after age 85 in both men and women. The largest age gradient was seen for having both impairments, which was very uncommon in the younger old and increased markedly in the oldest age group.
Figure 1.
Prevalence of each function group (physical and cognitive impairment, cognitive impairment alone, physical impairment alone and intact function) by age in men and women. CHS All Stars cohort (n= 1677).
Although a large proportion of these survivors in the CHS study remained intact, there was a substantial decline in other, continuous measures of functional performance from 13 years prior. A subset of 1143 with measures of gait speed, grip strength, DSST and 3MSE at the CHS All Stars examination showed marked declines in most measures of function. For 3MSE and the DSST, this decline was significantly, linearly related to age, with or without adjustment for the baseline value. Grip strength was significantly but less strongly related to age, while decline in gait speed was not associated with age (Figure 2). The changes were similar in men and women, except for grip strength which declined much more in the men, and which showed a significant non-linear trend in women. However, men started with significantly higher grip strength than women and remained higher in spite of the greater decline.
Figure 2.
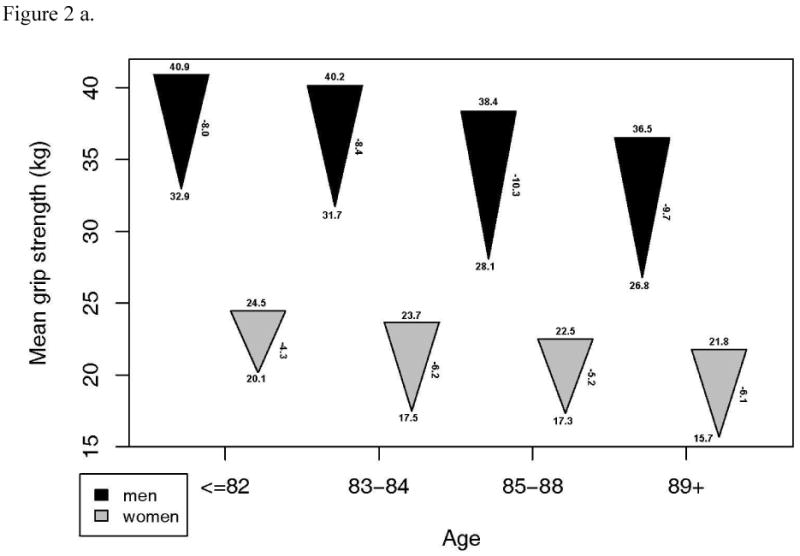
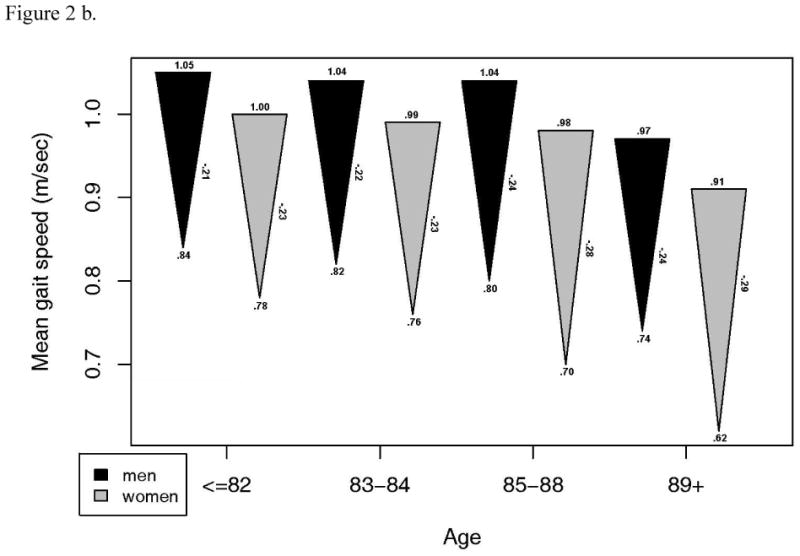
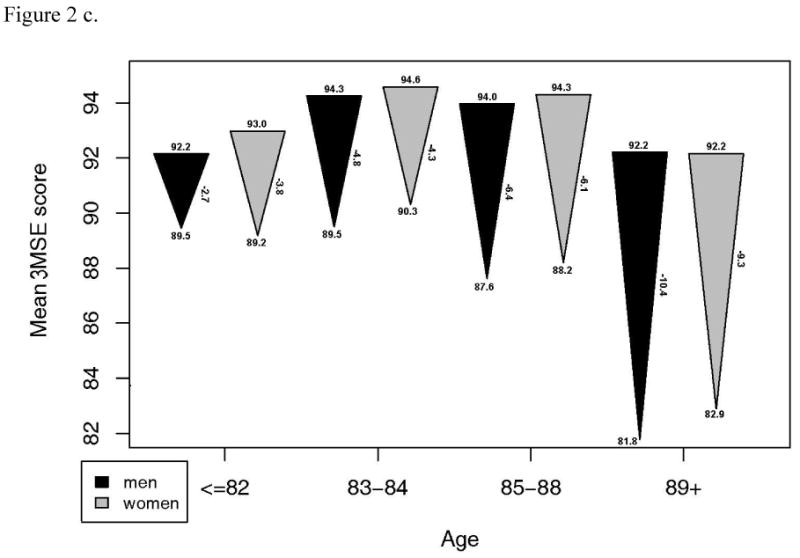
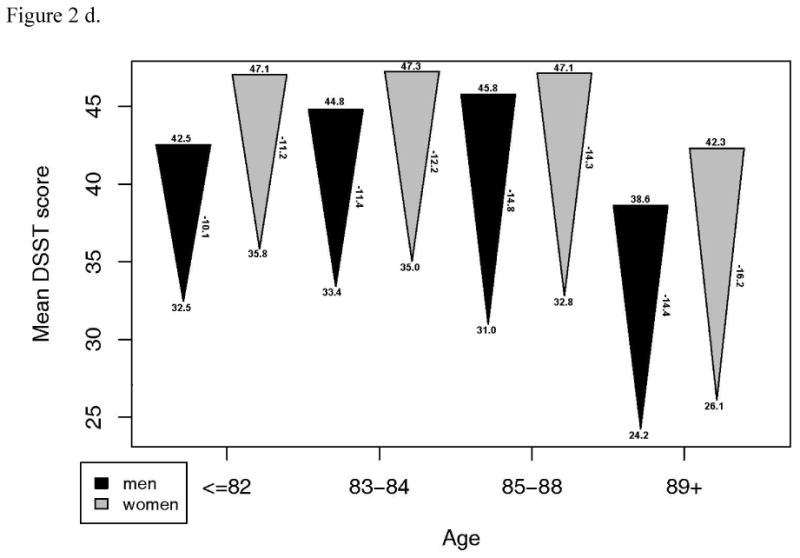
- Grip strength: a. Starting value in men p=.0001, women, p<.001. b. End value in men, p<.001, women, p <.001. c. Change in men, p=.045, women, p=.003.
- Gait speed: a. Starting value in men p=.158, women, p=.002. b. End value in men, p=.050, women, p <.001. c. Change in men, p=.842, women, p=.057.
- Modified mini-mental status examination: a. Starting value in men p=.013, women, p<.001. b. End value in men, p<.001, women, p <.001. c. Change in men, p<.001, women, p<.001.
- Digit symbol substitution test: a. Starting value in men p<.001, women, p=.001. b. End value in men, p<.001, women, p <.001. c. Change in men, p=.002, women, p=.001.
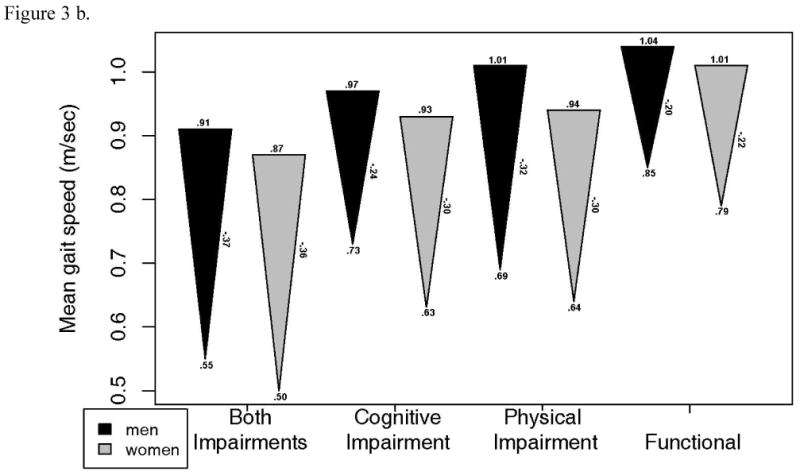
Changes in grip strength, gait speed and DSST were also examined in each of the 4 functional status groups (Figure 3). 3MSE change was not examined as it was used to define intact cognitive function. These plots illustrate substantial declines in all groups, including the functional group. After adjustment for age, grip strength decline was greater in those who had combined physical and cognitive impairment compared to the functional group. DSST decline was greater in all impairment groups compared to the functional group. Gait speed decline was significantly less in the functional group than in all other groups, except the cognitively impaired only group in men. Of note, most of the baseline values in the functionally intact group were quite high, suggesting that they had substantial functional reserve.
Figure 3.
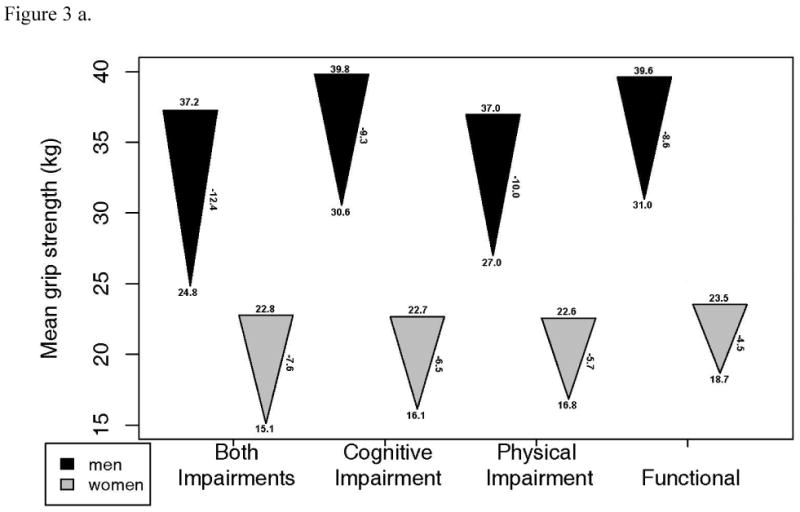
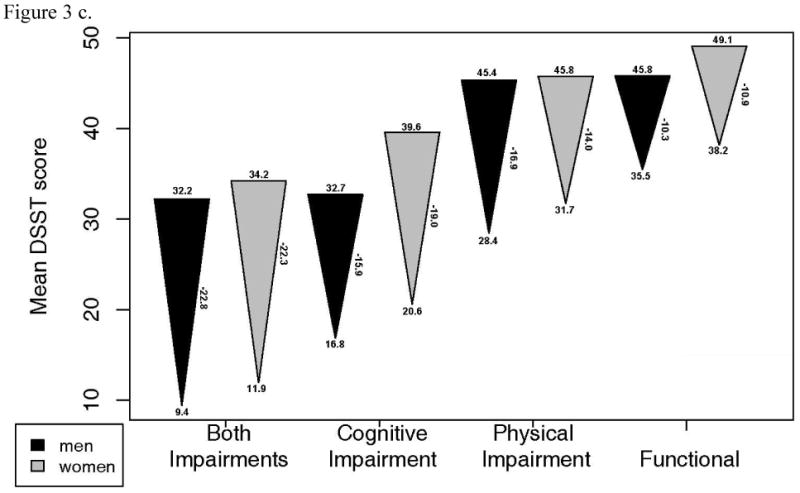
- Grip strength: a. Starting value in men p=.039, women, p=.185. b. End value in men, p<.001, women, p <.001. c. Change in men, p=.043, women, p=.001.
- Gait speed: a. Starting value in men p=.067, women, p<.001. b. End value in men, p<.001, women, p <.001. c. Change in men, p=.002, women, p<.001.
- Digit symbol substitution test a. Starting value in men p<.001, women, p<.001. b. End value in men, p<.001, women, p <.001. c. Change in men, p<.001, women, p<.001.
The bivariate relationships between 1992/93 risk factors and 2005/06 function are shown in Table 2. This analysis excluded individuals with impairment in 1992/93. Again, many of the risk factors examined 13 years prior were at relatively favorable levels for all groups, though best for the group that remained functional. Those with intact function were more likely to be younger, men, married; a higher proportion were educated to high school graduation or beyond, and reported a higher income. This group had very low rates of prior diabetes, hypertension and cardiovascular disease and higher physical activity. The group with both physical and cognitive impairment had the poorest risk factor profile compared to the other groups. Measures of subclinical vascular disease were quite low in all groups and lowest in the most functional group. For example, in the functional group only 34% had >25% carotid stenosis, and only 4% had an abnormal ankle-arm index (<0.9).
Table 2.
Multinomial logistic regression model comparing the group with intact function in 2005/06 to groups with both impaired physical and cognitive function, impaired cognitive function alone, and impaired physical function.
| Both impairments | Cognitively impaired | Physically impaired | p-value | |
|---|---|---|---|---|
| Age, years | 1.25 (1.19, 1.32) | 1.10 (1.05, 1.16) | 1.08 (1.04, 1.12) | <0.001 |
| Male sex | 0.48 (0.29, 0.79) | 0.97 (0.62, 1.54) | 0.43 (0.31, 0.59) | <0.001 |
| Black race | 1.53 (0.86, 2.69) | 1.28 (0.75, 2.19) | 0.65 (0.43, 0.98) | .025 |
| Education | ||||
| < High School | 1.00 | 1.00 | 1.00 | <.001 |
| High School | 0.78 (0.43, 1.40) | 0.32 (0.19, 0.52) | 0.74 (0.50, 1.10) | |
| > High School | 0.57 (0.32, 1.00) | 0.25 (0.15, 0.39) | 0.79 (0.55, 1.13) | |
| Cardiovascular disease | 2.19 (1.32, 3.63) | 1.30 (0.77, 2.19) | 1.31 (0.91, 1.88) | 0.021 |
| Hypertension | 1.48 (0.97, 2.28) | 1.62 (1.10, 2.38) | 1.42 (1.10, 1.85) | 0.008 |
| Weight, per 10 lbs | 1.14 (1.06, 1.24) | 0.95 (0.88,1.03) | 1.10 (1.05, 1.16) | <0.001 |
| Physical Activity (kcal) | ||||
| ≤ 0 | 1.00 | 1.00 | 1.00 | 0.021 |
| > 0 -≤270 | 0.85 (0.32, 2.30) | 1.08 (0.36, 3.20) | 0.38 (0.20, 0.73) | |
| > 270 -≤ 810 | 0.79 (0.31, 2.06) | 1.32 (0.46, 3.73) | 0.67 (0.38, 1.19) | |
| > 810 -≤ 1890 | 0.72 (0.28, 1.84) | 0.68 (0.24, 1.97) | 0.51 (0.29, 0.84) | |
| > 1890 | 0.71 (0.27, 1.84) | 1.49 (0.53, 4.18) | 0.47 (0.27, 0.83) |
Two multivariable models were examined. First, we compared the functional group to those with any impairment. This model identified higher age, female sex, lower education and income, a history of cardiovascular disease, hypertension, higher internal carotid wall thickness, higher weight and less physical activity as risk factors for impairment 13 years later (data not shown). When each functionally impaired group was compared to the group with intact physical and cognitive function (Table 2), the overall list of risk factors was similar, but differences emerged for physical as compared to cognitive impairment. Higher age was a significant risk factor for any type of impairment, most notably for combined physical and cognitive impairment. Men were much less likely to become physically impaired or to suffer both impairments, but there was no difference between men and women in risk for cognitive impairment alone. Higher education was strongly protective against impaired cognition, and physical activity against physical impairment, as expected. Risk of impairment was uniformly increased for those with a history of hypertension or cardiovascular disease, although not significant at the p=.05 level for every group. Higher weight was associated with physical impairment or combined physical and cognitive impairment. Blacks were less likely to have physical impairment only, but more likely to have combined impairments compared to whites.
Discussion
After 13 years, 891 men and women who had been participants in the CHS study were found to be alive and functionally intact in both the physical and cognitive domains. While this group is of interest for doing so well, we observed that they had experienced substantial declines in both physical and cognitive function. Individuals with intact function had very low levels of disease and higher levels of physical performance at baseline, suggesting that a major factor in maintaining function was having very high health and function to start. This supports the notion that functional reserve is critical to future functional integrity in older adults.
Factors most strongly related to long term function included low levels of cardiovascular disease at baseline and higher income, education and physical activity. Patterns of factors associated with physical functional impairment differed somewhat from those for cognitive function impairment or combined physical and cognitive impairment. Combined impairment was strongly related to age, being a woman, history of cardiovascular disease and higher weight, while cognitive impairment alone was related primarily to age, education and hypertension. Physical impairment alone was more common in women and less common in blacks. Hypertension and higher body weight were important risk factors for physical impairment while higher physical activity was related to lower risk of physical impairment. Thus physical and cognitive impairment have education and hypertension as common risk factors while higher weight at baseline emerged as a major risk factor for lower physical function in late life.
The differing patterns of risk factor associations for physical and cognitive functioning suggest that multiple targets need to be considered to fully maintain function in both domains. As major targets, prevention of cardiovascular disease and blood pressure management appear most likely to preserve function in both domains. Previous studies in CHS have documented the important influence of clinical and subclinical cardiovascular disease on both physical1 and cognitive function.2,3 It is worth noting the very low baseline levels of vascular disease in those who participated in the CHS All Stars examination. Nevertheless, baseline cardiovascular disease and hypertension each contributed independently to the physical and cognitive impairment. The mechanisms of these associations appear to be related to the correlations between hypertension and atherosclerosis with subclinical cerebrovascular disease.1
The data shown here suggest that higher weight can have a long term adverse impact on function in very late life. While obesity is less predictive of mortality in late life,23 it appears to be a critical determinant of physical disability. In the Health, Aging and Body Composition Study, body fat has been shown to be strongly related to incident mobility impairment in men and women aged 70-79, independent of muscle mass and health status.24 Physical activity was highest in the most functional group, and was of borderline statistical significance in multivariate the models. Thus obesity, physical activity and education are important lifestyle factors associated with long term functional integrity in old age. The lack of long term independent association of smoking, diabetes and C-reactive protein with function may reflect their strong prediction of mortality. Furthermore, it is important to recognize that the levels of these risk factors were very favorable at baseline. Since disability is an important predictor of mortality,25 it is possible that elevations in these and other risk factors may be more closely linked temporally to the disability that precedes death. In other words, they may be better short term than long term predictors.
The disparity in physical function between men and women was quite pronounced and is consistent with other literature.26 The reasons for this disparity are not well understood. The marked difference between men and women in maintaining physical function, with or without intact cognitive function persisted and in fact increased with adjustment for baseline chronic health conditions. This gender difference was also seen when looking at loss of physical and cognitive function as a combined outcome, although there was little gender difference in cognitive impairment. This suggests that the gender difference even in combined impairments is related to the greater physical impairment in women. This is consistent with studies of dementia incidence which show that there is less difference by sex than previously thought.27
Patterns of functional decline differed between groups defined at the CHS All Stars exam. The group with cognitive impairment had lower gait speed and substantially lower DSST scores at baseline than the physically impaired or the functional groups. Others have reported that gait speed and cognitive speed are related and predict declines in cognitive and physical function respectively.28,29 Other work in CHS suggests that these aspects of decline have a common pathogenesis in the dorsolateral prefrontal cortex.30 More work is needed to more fully evaluate these trajectories of change, their shared risk factors and underlying pathology.
There are several important limitations to this study. First, in spite of our best efforts, it was very challenging to recruit and to fully examine all survivors. Because the rates of cognitive impairment that were assessed were fairly low and cognitive impairment is known to reduce rates of participation, we suspect that the prevalence of cognitive impairment was underestimated to a greater extent than physical impairment. This would likely bias risk factor estimates for cognitive impairment towards the null. Secondly, among the CHS All Stars participants, the number with performance based assessments of function was only 1143 (68%). Nevertheless, the functional decline in all of those examined was dramatic and probably an underestimate of the average for the full surviving cohort.
In summary, only a minority of CHS participants were functionally intact after 13 years of follow-up. The CHS All Stars cohort demonstrated substantial loss in function that was mitigated somewhat by very high levels of function 13 years ago. More work is needed to evaluate the correlates of the trajectories of decline. The most functionally intact individuals were characterized by low levels of cardiovascular disease and more optimal behavioral health factors. This supports the potential to increase the number of older adults to maintain function well into the 9th decade through preventive measures.
Table 1.
Characteristics in the 4 functional status groups at the CHS All Stars examination, measured in 1992/93. Analysis excludes participants with any functional impairment at baseline (difficulty with ADL or 3MSE <80).
| Both impairments (n=161) |
Cognitively impaired only (n=167) |
Physically impaired only (n=430) |
Total with either impairment (n=605) |
Functional (n=891) |
4 group p† | 2 group p‡ | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 75.1 (4.7) | 73.3 (4.5) | 72.9 (3.6) | 73.4 (4.1) | 72.0 (3.3) | <.001 | <.001 |
| Sex (men) | 30% | 36% | 27% | 29% | 39% | .001 | <.001 |
| Race (black) | 19% | 17% | 11% | 14% | 15% | .08 | .62 |
| Education | <.001 | <.001 | |||||
| < High School | 18% | 30% | 13% | 18% | 10% | ||
| High School | 39% | 34% | 35% | 36% | 35% | ||
| > High School | 43% | 36% | 52% | 47% | 55% | ||
| Household income > $12,000 | 71% | 71% | 82% | 77% | 86% | <.001 | <.001 |
| Currently married vs. not | 57% | 70% | 70% | 68% | 76% | <.001 | <.001 |
| Cardiovascular disease | 25% | 16% | 16% | 18% | 13% | .01 | .001 |
| Hypertension | 58% | 57% | 53% | 55% | 43% | <.001 | <.001 |
| Cancer | 13% | 14% | 14% | 14% | 11% | .35 | .01 |
| Chronic pulmonary disease | 7% | 10% | 14% | 12% | 8% | .04 | .05 |
| AAI <0.9 | 6% | 5% | 4% | 5% | 4% | .78 | .57 |
| Carotid stenosis > 25% | 37% | 46% | 39% | 40% | 34% | .05 | .03 |
| Diabetes | .03 | .20 | |||||
| Normal | 74% | 81% | 80% | 79% | 83% | ||
| IFG | 7% | 11% | 11% | 10% | 8% | ||
| Diabetes | 19% | 8% | 9% | 10% | 9% | ||
| Arthritis | 50% | 45% | 54% | 51% | 42% | <.001 | <.001 |
| Smoking | .53 | .75 | |||||
| Never | 48% | 49% | 53% | 51% | 51% | ||
| Former | 44% | 38% | 40% | 40% | 41% | ||
| Current | 8% | 12% | 7% | 8% | 7% | ||
| Physical activity (kcal*) (SD) | 547.4 (8.0) | 810.8 (6.0) | 554.9 (8.5) | 601.3 (7.9) | 845.6 (5.9) | .003 | .001 |
| Body mass index (SD) | 27.7 (4.7) | 26.5 (4.2) | 27.4 (4.5) | 27.3 (4.5) | 26.6 (4.1) | .004 | .004 |
| Waist circumference (cm) (SD) | 95.4 (13.0) | 91.9 (11.7) | 93.9 (12.6) | 93.7 (12.5) | 91.9 (11.9) | .01 | .01 |
| Any IADL difficulty | 21% | 11% | 17% | 17% | 9% | <.001 | <.001 |
| Self-reported health status | <.001 | <.001 | |||||
| Excellent | 5% | 8% | 6% | 6% | 13% | ||
| Very good | 29% | 38% | 37% | 36% | 45% | ||
| Good | 52% | 42% | 45% | 46% | 37% | ||
| Fair/poor | 15% | 11% | 12% | 12% | 6% | ||
| C-reactive protein* (SD) | 2.6 (3.0) | 2.3 (2.9) | 2.8 (2.9) | 2.7 (2.1) | 2.3 (3.0) | .02 | .01 |
Geometric mean
test comparing groups with both impairments, cognitively impaired, physically impaired and functional
test comparing groups total with either impairment to functional.
Abbreviations: Cardiovascular Health Study, CHS; Activities of daily living, ADL; Modified mini-mental status examination, 3MSE; Standard deviation, SD; Ankle arm index, AAI; Impaired fasting glucose, IFG; Activities of daily living, ADL; Instrumental activities of daily living, IADL.
Acknowledgments
Funding: The research reported in this article was supported by the National Institute on Aging R01-AG-023629. The CHS was supported from contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01-AG-15928, R01-AG-20098, and R01-AG-027058 from the National Institute on Aging, HL-075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions:
ABN: Participated in study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of the manuscript.
AMA: Participated in study design, data analysis and interpretation and preparation of manuscript.
MCS: Participated in data analysis and interpretation and preparation of the manuscript.
DGI: Contributed to the study oversight, acquisition of data, and preparation of the manuscript.
MC: Participated in laboratory analysis of samples, and preparation of the manuscript.
ESS: Participated in Study design and interpretation of data and preparation of the manuscript.
JD: Participated in acquisition of subjects and all data.
SBK: Participated in acquisition of subjects and data.
PHMC: Participated in acquisition of subjects and data.
LPF: Participated in study concept and design, acquisition of subjects and data, and preparation of manuscript.
JR: Participated in study concept and design, acquisition of subjects and data, and preparation of manuscript.
Sponsor's Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
References
- 1.Rosano C, Kuller LH, Chung H, et al. Subclinical Brain Magnetic Resonance Imaging Abnormalities Predict Physical Functional Decline in High-Functioning Older Adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer's Disease Incidence in Relationship to Cardiovascular Disease in the Cardiovascular Health Study Cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Arnold AM, Naydeck BL, et al. “Successful Aging”: Impact of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 4.Reed DW, Foley DJ, White LR, et al. Predictors of Healthy Aging in Men with High Life Expectancies. Am J Public Health. 1998;88:1463–1468. doi: 10.2105/ajph.88.10.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lincoff AM, Kleiman NS, Kereiakes DJ, et al. REPLACE-2 Investigators. Long-term efficacy of bivalirudin and provisional glycoprotein IIb/IIIa blockade vs heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary revascularization: REPLACE-2 randomized trial. JAMA. 2004;292:696–670. doi: 10.1001/jama.292.6.696. erratum appears in JAMA 2006;296:46. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson HH, Cesari M, Kritchevsky SB, et al. Predictors of combined cognitive and physical decline. J Am Geriatr Soc. 2005;53:1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- 7.Terry DF, Pencina Mj, Vasan RS, et al. Cardiovascular Risk Factors Predictive for Survival and Morbidity-Free Survival in the Oldest-Old Framingham Heart Study Participants. J Am Geriatr Soc. 2005;53:1944–1950. doi: 10.1111/j.1532-5415.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor HL, Jacobs DR, Schuker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:745–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 9.Orme J, Reis J, Herz E. Factorial and indiscriminate validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Teng EL, Chui HC. Modified mini mental state (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 11.Salthouse PA. The role of memory in the age decline in digit-symbol substitution performance. J Gerontol. 1978;33:232–238. doi: 10.1093/geronj/33.2.232. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Gallo JJ, Breitner JCS. Alzheimer's disease in the NAS-NRC Registry of Ageing Twin Veterans, IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer's dementia. Psychol Med. 1995;25:1211–1219. doi: 10.1017/s0033291700033183. [DOI] [PubMed] [Google Scholar]
- 14.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 15.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 16.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 17.Fitti JE, Kovar MG, National Center for Health Statistics . Vital and Health Statistics. Series 1, No 21. DHHS Pub No (PHS) 87-1323. Public Health Service, Government Printing Office; Washington. U.S.: Oct, 1987. The Supplement on Aging to the 1984 National Health Interview Survey. [PubMed] [Google Scholar]
- 18.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Siscovick DS, Manolio TA, et al. The Ankle- Arm Index as Marker of Atherosclerosis in the Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 20.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic Design of a Multicenter Investiation of Free-living Elderly Subjects: The Cardiovascular Health Study. J Am Soc Echiocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 21.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults: The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 22.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 23.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention phase II. Ann intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Goodpaster BH, Kritchevsky SB, et al. Health ABC Study. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J Gerontol A Biol Sci Med Sci. 2005;60A:M324–M333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Biol Sci Med Sci. 1994;49A:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 26.Newman AB, Brach JS. Gender gap in longevity and disability in older persons. Epidemiol Rev. 2001;23:343–350. doi: 10.1093/oxfordjournals.epirev.a000810. [DOI] [PubMed] [Google Scholar]
- 27.Hebert LE, Scherr PA, McCann JJ, et al. Is the risk of developing Alzheimer's disease greater for women than for men? Am J Epidemiol. 2001;153:132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson HH, Rosano C, Simonsick EM, et al. Health ABC study. Cognitive Function, Gait Speed Decline, and Comorbidities: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2007;62:M844–M850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 29.Rosano C, Simonsick EM, Harris T, et al. Association Between Physical and Cognitive Function in Healthy Elderly: The Health, Aging, Body Composition Study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- 30.Rosano C, Aizenstein HJ, Studenski S, et al. A Regions-of-Interest Volumetric Analysis of Mobility Limitations in Community-Dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2007;62:M1048–M1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]