Abstract
Chronic opiate exposure produces tolerance and hypersensitivity to mechanical and thermal stimulation that involves increased pain facilitation from the rostral ventromedial medulla (RVM). The aim of the present study was to determine the effect of sustained systemic morphine exposure on RVM neurons. Three cell types in the RVM have been described: on-cells, off-cells and neutral cells. The activity of on-cells increases in response to noxious stimulation, whereas the activity of off-cells decreases following noxious stimulation. Neutral cells remain relatively unaffected. In lightly anesthetized rats, systematic exploration throughout the RVM using single-unit extracellular recordings was used to examine both the relative proportion and the neuronal properties of the different cell classes in chronic morphine and placebo treated animals. Seven days after implanting either morphine (150 mg, s.c.) or placebo pellets a total of four electrode penetrations through the RVM were made in each animal at identical coordinates along midline. Neuronal responses related to radiant heat-evoked paw withdrawals were recorded. When compared to placebo treated rats, chronic morphine increased the number of on-cells and decreased the number of neutral cells, while the number of off-cells remained unchanged. Chronic morphine exposure had no effect on the spontaneous or heat evoked discharges in on-, off-, or neutral cells. These results indicate that chronic morphine may sensitize a subpopulation of RVM neurons to noxious stimulation, which would be expected to increase descending facilitation and promote tolerance and chronic morphine-induced paradoxical pain.
Keywords: nucleus raphe magnus, morphine, pain
Introduction
Prolonged exposure to opiates can produce tolerance to its analgesic effects and, in some cases, induce paradoxical pain (Compton et al. 2001; Doverty et al. 2001; King et al. 2005b; Sjogren et al. 1994). In animal models, chronic administration of either systemic or spinal morphine produces tolerance and hypersensitivity to both mechanical and thermal stimulation (Gardell et al. 2002; Laulin et al. 1999; Mao et al. 1994; Vanderah et al. 2001a; Vanderah et al. 2001b). Several neuroplastic changes involving the up-regulation of pronociceptive systems have been described that could contribute to chronic morphine induced hypersensitivity. Sustained morphine exposure increases calcitonin gene-related peptide (CGRP) content in dorsal root ganglion neurons both in vivo and in cell cultures (Belanger et al. 2002; Gardell et al. 2002; Menard et al. 1996) and potentiates capsaicin-evoked release of CGRP and substance P from spinal cord tissue (Gardell et al. 2002; King et al. 2005a). Additionally, sustained morphine increases expression of spinal dynorphin, a peptide with pronociceptive effects possibly mediated through NMDA receptors, and downregulates glutamate transporters in spinal cord tissue (Gardell et al. 2002; Mao et al. 2002; Vanderah et al. 2000; Vanderah et al. 2001a).
Many of these chronic morphine-induced events require input from the rostral ventromedial medulla (RVM). The RVM, which includes neurons in the midline nucleus raphe magnus and the adjacent reticular formation, modulates nociception through both indirect and, via the dorsolateral funiculus (DLF), direct projections to the spinal and medullary dorsal horn (Fields et al. 1991). Lidocaine microinjections into the RVM reverses mechanical and thermal hypersensitivity and attenuates tolerance produced by prolonged administration of morphine (Vanderah et al. 2001b). Furthermore, lesions of the DLF prevent sustained morphine-induced hypersensitivity as well as the increases in evoked CGRP release and dynorphin content from spinal cord tissue (Gardell et al. 2002). These data suggest that sustained morphine exposure increases the activity of RVM neurons involved in facilitating nociceptive signals at the level of the dorsal horn.
Noxious stimulation produces three distinct patterns of activity in RVM neurons that are correlated with withdrawal reflexes (Fields et al. 1983a; Fields et al. 1991). On-cell activity increases with the occurrence of a nociceptive reflex, off-cell activity decreases with the nociceptive reflex and neutral cells show little or no consistent change in firing rate related to withdrawal responses. Strong evidence implicates on-cell activity in facilitating and off-cell activity in inhibiting nociceptive transmission (Bederson et al. 1990; Fields et al. 1983b; Foo and Mason 2003; Heinricher et al. 2004a; Heinricher et al. 1994; Heinricher and Neubert 2004; Kaplan and Fields 1991; Kincaid et al. 2006; Meng et al. 1998; Mitchell et al. 1998; Neubert et al. 2004). The present study examined the effect of sustained systemic administration of morphine on the physiological characteristics and relative proportions of each RVM neuronal cell type.
Materials and Methods
Studies were conducted using male Sprague-Dawley rats weighing 200–250 g at the time of recording. Animals were housed two per cage in a room maintained with an alternating 12 h light-dark cycle. The present study was approved by the Committee on Animal Research at University of California San Francisco and the University of New England, and animals were treated according to the policies and recommendations of the NIH guidelines for the handling and use of laboratory animals.
Surgery
Seven days prior to recording, rats were implanted subcutaneously with two pellets containing either morphine sulfate (75 mg pellets) or placebo under isoflurane anesthesia. On the day of recording, rats were injected with sodium pentobarbital (60 – 70 mg/kg, i.p.) and a catheter inserted into the external jugular vein for administration of anesthetics. The femoral artery was cannulated for measuring blood pressure. Mean arterial pressure (MAP), heart rate and respiratory rate were recorded prior to each electrode penetration during the experiment. Body temperature was maintained at 37°C with a hot water circulating heating pad. After placing the rat into a stereotaxic holder, a hole was drilled in the interparietal bone for insertion of an electrode into the medulla. Anesthesia was maintained with a constant infusion of sodium pentobarbital (1–10 mg/kg/hr) at a level to prevent signs of discomfort and allow stable paw withdrawals with an average latency between 3.0 and 4.0 s using a feedback controlled projector lamp. Movement of the hind paw was recorded using a mechanical transducer, and the rate of rise in temperature and peak holding temperature were identical in all experiments. Cell recordings were initiated at least 45 min after starting the infusion of sodium pentobarbital.
Electrophysiological recordings
A total of four electrode penetrations at 0.4 mm intervals were made in each animal in similar coordinates along midline, from 1.2–2.4 mm caudal to interaural zero. Extracellular single unit activity was recorded from 7.5 –9.0 mm below the surface of the cerebellum using tungsten electrodes (3 Mohm, FHC, Bowdoinham, ME) using methods as previously described (Harasawa et al. 2000; Meng and Johansen 2004; Meng et al. 2005). Briefly, the amplified, filtered signal was passed through a window discriminator and monitored with digital and storage oscilloscopes. Discriminated units triggered a digital oscilloscope to confirm constant spike shape and amplitude. Data was acquired and analyzed off-line using LabView (National Instruments, Austin, TX).
Neurons from the RVM were systematically sampled to allow for comparisons between treatment groups. Periodic light brushing and pressure of the hind paw as well as heat evoked paw withdrawals were used to search for neurons. Ample time was given between stimuli in order to allow ongoing activity of cells to return. In order to minimize the possibility of sample bias, every neuron that could be isolated with a signal to noise ratio of at least 3:1 was characterized with noxious thermal stimulation of the hindpaw. At least two paw withdrawal trials were performed at 3–5 min intervals, and baseline and heat evoked changes in activity were analyzed.
On-, off-, and neutral cells were categorized according to their pattern of neuronal activity as it related to the paw withdrawal reflex (Heinricher et al. 2004a; Neubert et al. 2004). On-cells were identified by a sudden burst of activity while off-cells demonstrated a pause in activity that occurred at the time of withdrawal. Neutral cells demonstrated continuous firing that was not affected by the noxious thermal stimulus. The lack of response to thermal stimulation was also confirmed with noxious mechanical stimulation applied to the tail and hindpaw. The paw withdrawal reflex was used instead of the tail flick because previous studies have demonstrated behavioral hypersensitivity using paw stimulation (Gardell et al. 2002; King et al. 2005a; Vanderah et al. 2001b; Xie et al. 2005).
In separate experiments, the presence of tolerance and chronic morphine induced hypersensitivity was examined following morphine pellet implantation. Pa w withdrawal latencies were measured using radiant heat delivered from a Hargreaves apparatus immediately prior to and 7 days following pellet implantation.
Data Analysis
Analysis was performed on the ongoing (spontaneous) activity and heat-evoked changes in discharge of all cell types. Ongoing activity was calculated as the average frequency over a 60 s epoch immediately prior to the heat onset. Paw withdrawal related activity was calculated as the average frequency of activity over a 10 s period following the heat onset. In addition, the average frequency of activity occurring during the 10 s immediately after the withdrawal reflex was determined. Results were averaged across trials for each cell. Treatment comparisons for MAP, heart rate, respiratory rate and paw withdrawal latencies were performed using the Student’s paired t-test. For each cell type, group comparisons between morphine and placebo pelleted animals was performed using a 2-way ANOVA with Tukey-Kramer post hoc for comparisons. The total numbers of on-, off- and neutral cells encountered were also compared between groups. A Chi-square analysis was performed to compare the frequency distribution of each cell type between treatment groups and multiple t-tests with the Bonferroni correction were performed after calculating the numbers of on, off- and neutral cells as percent of total number of cells. In all tests, group differences with a p < 0.05 is considered statistically significant.
Histological Verification
At the end of the final track in each experiment electrolytic lesions (15 μA, 20 s) were made to mark the recording electrode site. Animals were perfused with 10% formalin, the brain was removed, incubated in 30% sucrose and cut on a freezing microtome. The location of recording site was verified in Cresyl Violet (0.1%) stained coronal sections according to the atlas of Paxinos and Watson (1986).
Results
A total of 168 and 182 neurons were sampled from 10 placebo and 10 morphine pelleted rats, respectively. The average number of cells isolated and analyzed in each animal was not statistically different between placebo and morphine treated animals (16.8±1.6 cells per animal in placebo versus 18.2±2.1 cells per animal in morphine treated animals, p = 0.57). Electrolytic lesions of the end of the final recording track indicated that all electrode penetrations in both placebo and morphine treated animals traversed through the RVM. In order to assure that anesthetic level was equivalent between treatment groups, the infusion rate of pentobarbital administration was adjusted based on paw withdrawal latencies. This approach is reflected in the comparable withdrawal latencies recorded in placebo (3.5±0.1 s) and chronic morphine (3.9 ± 0.2 s) treated animals. Under these conditions, the mean heart rate in placebo animals (392±2.8 beats/min) was similar to that in morphine treated animals (385 ± 5.2 beats/min). Likewise, the MAP in placebo and morphine treated animals did not differ (120.7 ± 5.1 mmHg in placebo animals versus 118.3 ± 5.1 mmHg in morphine treated animals). However, chronic morphine exposure produced a significant decrease in respiratory rate when compared to placebo animals (60.7 ± 3.7 versus 84.3 ± 2.2 breaths/min, p < 0.001). In separate experiments in which paw withdrawal latencies were examined in unanesthetized animals, morphine pellets produced a significant decrease in withdrawal latencies 7 days after morphine pellet implants (from 14.7±1.2 s to 10.8±1.0 s, p<0.05), confirming previously published results using an identical method of sustained morphine exposure (Gardell et al. 2002; Vanderah et al. 2001b).
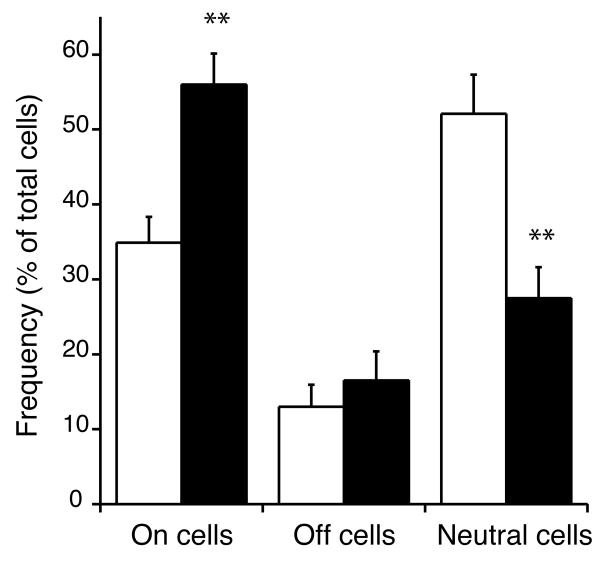
The frequency distribution of on-, off-, and neutral cells differed between treatment groups (Table 1, Chi-square, p < 0.001). More specifically, morphine treated animals had a greater proportion of on-cells and a lower proportion of neutral cells when compared to placebo controls (Figure 1). When expressed as a percentage of the total number of cells, 35 % of neurons were characterized as on-cells in placebo treated animals, compared to 56% in chronic morphine treated animals. In contrast, 52 % of the neurons were characterized as neutral cells in placebo animals compared to only 27% in chronic morphine animals. The percentage of off-cells was similar among placebo (13%) and chronic morphine (17%) treated animals.
Table 1.
Total numbers of On, Off, and Neutral cells recorded in the RVM of chronic morphine and placebo treated rats.
| On cells | Off cells | Neutral cells | Total cells | |
|---|---|---|---|---|
| Placebo | 55 | 21 | 92 | 168 |
| Morphine | 97 | 29 | 56 | 182 |
Chi-square analysis of the distribution revealed a statistically significant difference between the placebo and morphine treated animals (p < 0.001). N = 10 rats per treatment group.
Figure 1.
Sustained morphine exposure for 7 days increased the proportion of on-cells and decreased the proportion of neutral cells when compared to placebo controls. Data is expressed as a percentage of the total number of cells recorded in each experiment. Open bars, placebo treatment; solid bars, morphine treatment. ** p < 0.01 compared to placebo controls.
Comparison of the response properties of on-, off- and neutral cells revealed no statistically significant differences between placebo and morphine treated animals (Table 2). For all cell types, average baseline activity in placebo and morphine rats was similar. Furthermore, placebo and morphine animals demonstrated comparable increases in on-cell activity and decreases in off-cell activity evoked by thermal stimulation of the hind paw. On-cell paw withdrawal related activity increased to an average of 248 % of control in placebo and 224 % of control in morphine treated animals. These results were comparable to those calculated using activity evoked for the 10 s after paw withdrawal (data not shown). Off-cell activity during paw heat decreased to an average of 61 % of control and 54 % of control in placebo and morphine treated animals, respectively. The activity of neutral cells was not affected by thermal stimulation in either treatment group. In addition to finding no differences in the mean neuronal activity, an examination of the frequency distribution for the activity of individual neurons in each cell category also did not reveal any significant differences among treatment groups.
Table 2.
Baseline and heat evoked changes in On, Off and Neutral cell activity in morphine and placebo treated rats.
| On cells | Off cells | Neutral cells | ||||
|---|---|---|---|---|---|---|
| Baseline | Heat | Baseline | Heat | Baseline | Heat | |
| Placebo | 2.9 ± 0.7 | 7.1 ± 1.0** | 17.1 ± 3.4 | 10.5 ± 2.3** | 9.8 ± 0.8 | 9.3 ± 0.7 |
| Morphine | 2.8 ± 0.4 | 6.3 ± 0.5** | 13.1 ± 1.6 | 7.1 ± 0.9** | 8.6 ± 0.9 | 7.5 ± 0.9 |
Baseline activity was measure during the 10 s prior to the onset of paw heat stimulation. Heat evoked activity was measured for 10 s beginning at the onset of the thermal stimulus.
p < 0.01 versus baseline activity from the same cell class.
Discussion
Previous studies have demonstrated the involvement of the RVM in sustained morphine-induced behavioral tolerance, hypersensitivity and increased capsaicin-evoked CGRP release from dorsal horn tissue (Gardell et al. 2002; Vanderah et al. 2001a). The current study found that sustained exposure to morphine produced an increase in the proportion of on-cells and a decrease in the proportion of neutral cells recorded from the RVM in rats. Under our experimental conditions, however, the response characteristics of RVM neurons were not different between treatment groups. The increase in on-cell proportion was not due to morphine withdrawal, which increases on-cell activity (Bederson et al. 1990), because morphine exposure was sustained throughout the recording period. Furthermore, plasma morphine levels measured in rats implanted with two 75 mg morphine pellets remain constant around 450 ng/ml for 7 days, with the only decrease (below 300 ng/ml) occurring on day 3 after the pellet implant (personal communication, Dr. Michael Ossipov, University of Arizona). In the present experiment, we used an identical method of sustained morphine administration and confirmed the presence of hypersensitivity to thermal stimulation after 7 days.
Although no studies have recorded from the RVM after 7 days of sustained morphine administration, other studies have examined the properties of on- and off-cells after tolerance to morphine administered directly into the periaqueductal gray (PAG). In these studies, no difference in the properties of on- or off-cells was found after the development of morphine tolerance, except under conditions of a naloxone challenge in which there was an increase in on-cell activity (Lane et al. 2004; Tortorici et al. 2001). Sustained morphine administered to the PAG, however, has not been demonstrated to induce behavioral hypersensitivity. Furthermore, these experiments were conducted after only three days of morphine exposure, while hypersensitivity to sustained systemic morphine administration occurs only after the fourth day (Gardell et al. 2002; Vanderah et al. 2001b).
The finding that on-cell activity remained unchanged after sustained morphine exposure was initially surprising. The recordings occurred at a time during which significant hyperalgesia has been demonstrated, and several studies have provided evidence that increases in the ongoing activity of on-cells produces hyperalgesia. For example, both CCK and low doses of neurotensin microinjected into the RVM excites on-cells and decreases paw withdrawal latencies (Heinricher and Neubert 2004; Neubert et al. 2004). Under the experimental conditions of the present study, however, anesthesia was titrated to give comparable paw withdrawal latencies. With the absence of hyperalgesia in chronic morphine treated animals under these conditions, and the observation that increased depth of anesthesia decreases on-cell activity (Leung and Mason 1995), the absence of any difference on on-cell ongoing or evoked activity should not be unexpected. Alternatively, it is possible that neurons were not evaluated for long enough periods of time to observe changes in the amount of time on-cells spend in their active phase (Carlson et al. 2005; Heinricher 2005; Kincaid et al. 2006).
While recording from the RVM in lightly anesthetized animals allows for the correlation of neuronal activity with a nociceptive reflex, one limitation with this method is the inability to perform full stimulus-response functions. This inability may account for the absence of differences found in the activity of RVM neurons. Future studies will record from deeply anesthetized animals in order to assess the activity evoked by a full range of stimulus intensities.
The increase in number of physiologically characterized on-cells supports the notion that chronic morphine administration increases descending facilitation from the RVM, contributing to both tolerance and morphine-induced paradoxical pain. While the activation of off-cells is important for the production of systemic or brain stem mediated morphine analgesia (Fields et al. 1983b; Heinricher et al. 1999; Heinricher et al. 1994; Heinricher et al. 2001; Mitchell et al. 1998), more recent evidence strongly implicates on-cell activity in the facilitation of nociceptive reflexes. In early studies, acute naloxone precipitated withdrawal was shown to activate on-cells and produce hyperalgesia (Bederson et al. 1990; Kaplan and Fields 1991; Kim et al. 1990). This hyperalgesia was attenuated by RVM microinjection of lidocaine, supporting the importance of on-cell activity to descending facilitation (Kaplan and Fields 1991). Other studies have demonstrated that RVM on-cell activation contributes to secondary hyperalgesia and sensitization produced by application of mustard oil to the hindlimb (Kincaid et al. 2006; Urban and Gebhart 1999a; Urban and Gebhart 1999b; Urban et al. 1996).
One possible mechanism for the increase in number of on-cells is the sensitization of neurons to nociceptive input, so that neurons initially unresponsive to noxious stimulation become responsive following chronic morphine exposure. In fact, such a phenomenon has been demonstrated in a model of secondary hyperalgesia. Application of mustard oil to the knee joint, which causes an RVM dependent decrease in paw withdrawal latencies, resulted in the sudden recruitment of a previously silent on cell (Kincaid et al. 2006, see Fig 3). Results from in vitro RVM slice recordings also appear to support such a proposed mechanism. Chronic morphine treatment increases glutamatergic input onto secondary cells, a specific class of neurons recorded in RVM slices believed to represent on-cells (Bie and Pan 2005). The chronic morphine induced increase in excitatory transmission, which was restricted to secondary cells, could lead to the activation of these neurons under normally sub-threshold conditions.
Another explanation for the increase in on-cells and concurrent decrease in neutral cells is a phenotypic shift from neutral cells to on-cells. Such a change in the response properties of RVM neurons has been suggested to occur following inflammation, although in these studies neutral cells also began to behave as off-cells (Miki et al. 2002). Unfortunately, given the relatively long time-course of chronic morphine-induced changes in RVM neurons, we were unable to observe neurons undergoing such changes. Identification of these neurons in future studies may be possible by examining the properties of RVM neurons prior to the development of maximal hypersensitivity after sustained morphine exposure.
The general proportions of on-cells in placebo treated rats appear to be consistent with a previous report on the properties of systematically surveyed RVM neurons (Leung and Mason 1998). A thorough sampling of RVM neurons found that 36 % of the isolated cells could be classified as on-cells and 23 % were identified as off-cells. This compares with the present study, in which 35 % of the neurons were on-cells and 13 % off-cells in placebo treated animals. The consistency with our results is noteworthy, especially given the use of different anesthetics (isoflurane versus pentobarbital) and withdrawal reflexes (tail versus paw). The lower percentage of off-cells we found may have resulted from experimenter differences in the use of tactile stimulation as a search stimulus. A greater frequency of searching using periodic hind paw mechanical pressure could have the effect of inhibiting off-cells so that fewer would be identified. The similar frequency of off-cells in placebo versus morphine treated animals, however, indicates that comparable search strategies were employed between treatment groups.
The increase in RVM on-cells and subsequent downstream effects could have important consequences, possibly leading to the observation of tolerance, morphine induced paradoxical pain, and medication overuse headache in migraine sufferers (Compton et al. 2001; Doverty et al. 2001; King et al. 2005b; Sjogren et al. 1994; Limmroth and Katsarava 2004; Meng and Porreca 2004; Smith and Stoneman 2004).
Conclusion
In summary, the sustained administration of morphine for one week increased the proportion of on-cells and decreased the proportion of neutral cells recorded in the RVM of rats. These changes may be responsible for the chronic morphine-induced behavioral hypersensitivity and increased excitability in the dorsal horn. Further investigations are necessary to determine the mechanisms by which chronic morphine increases the proportion of on-cells as well as the mechanisms for the subsequent on-cell mediated increase in nociceptive signaling at the level of the dorsal horn.
Acknowledgments
This work was supported by grant K02DA018408 to I.D.M. from the National Institutes of Health, National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- Belanger S, Ma W, Chabot JG, Quirion R. Expression of calcitonin gene-related peptide, substance P and protein kinase C in cultured dorsal root ganglion neurons following chronic exposure to mu, delta and kappa opiates. Neuroscience. 2002;115:441–53. doi: 10.1016/s0306-4522(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Bie B, Pan ZZ. Increased glutamate synaptic transmission in the nucleus raphe magnus neurons from morphine-tolerant rats. Mol Pain. 2005;1:7. doi: 10.1186/1744-8069-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JD, Selden NR, Heinricher MM. Nocifensive reflex-related on- and off-cells in the pedunculopontine tegmental nucleus, cuneiform nucleus, and lateral dorsal tegmental nucleus. Brain Res. 2005;1063:187–94. doi: 10.1016/j.brainres.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–46. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–6. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. Journal of Neuroscience. 1983a;3:545–552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annual Review of Neuroscience. 1991;14:219–45. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983b;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Discharge of raphe magnus ON and OFF cells is predictive of the motor facilitation evoked by repeated laser stimulation. J Neurosci. 2003;23:1933–40. doi: 10.1523/JNEUROSCI.23-05-01933.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–55. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasawa I, Fields HL, Meng ID. Delta opioid receptor mediated actions in the rostral ventromedial medulla on tail flick latency and nociceptive modulatory neurons. Pain. 2000;85:255–62. doi: 10.1016/s0304-3959(99)00280-8. [DOI] [PubMed] [Google Scholar]
- Heinricher MM. Nociceptin/orphanin FQ: pain, stress and neural circuits. Life Sci. 2005;77:3127–32. doi: 10.1016/j.lfs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain. 2004a;110:419–26. doi: 10.1016/j.pain.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004 doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ, Martenson ME, Goncalves L. Prostaglandin E2 in the medial preoptic area produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Neuroscience. 2004b;128:389–98. doi: 10.1016/j.neuroscience.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001;92:129–38. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991;11:1433–9. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Fields HL, Barbaro NM. Morphine analgesia and acute physical dependence: rapid onset of two opposing, dose-related processes. Brain Res. 1990;516:37–40. doi: 10.1016/0006-8993(90)90894-h. [DOI] [PubMed] [Google Scholar]
- Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33–41. doi: 10.1152/jn.00449.2005. [DOI] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005a;116:276–88. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005b;14:194–205. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- Lane DA, Tortorici V, Morgan MM. Behavioral and electrophysiological evidence for tolerance to continuous morphine administration into the ventrolateral periaqueductal gray. Neuroscience. 2004;125:63–9. doi: 10.1016/j.neuroscience.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Celerier E, Larcher A, Le Moal M, Simonnet G. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–6. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Effects of isoflurane concentration on the activity of pontomedullary raphe and medial reticular neurons in the rat. Brain Res. 1995;699:71–82. doi: 10.1016/0006-8993(95)00858-n. [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Physiological survey of medullary raphe and magnocellular reticular neurons in the anesthetized rat. J Neurophysiol. 1998;80:1630–46. doi: 10.1152/jn.1998.80.4.1630. [DOI] [PubMed] [Google Scholar]
- Limmroth V, Katsarava Z. Medication overuse headache. Curr Opin Neurol. 2004;17:301–6. doi: 10.1097/00019052-200406000-00011. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–12. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–23. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard DP, van Rossum D, Kar S, St Pierre S, Sutak M, Jhamandas K, Quirion R. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16:2342–51. doi: 10.1523/JNEUROSCI.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience. 2004;124:685–93. doi: 10.1016/j.neuroscience.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP, Harasawa I, Fields HL. Kappa opioids inhibit physiologically identified medullary pain modulating neurons and reduce morphine antinociception. J Neurophysiol. 2005;93:1138–44. doi: 10.1152/jn.00320.2004. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Meng ID, Porreca F. Mechanisms of medication overuse headache. Headache Currents. 2004;1:47–54. [Google Scholar]
- Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–60. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Lowe D, Fields HL. The contribution of the rostral ventromedial medulla to the antinociceptive effects of systemic morphine in restrained and unrestrained rats. Neuroscience. 1998;87:123–33. doi: 10.1016/s0306-4522(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–65. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Sjogren P, Jensen NH, Jensen TS. Disappearance of morphine-induced hyperalgesia after discontinuing or substituting morphine with other opioid agonists. Pain. 1994;59:313–6. doi: 10.1016/0304-3959(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Smith TR, Stoneman J. Medication overuse headache from antimigraine therapy: clinical features, pathogenesis and management. Drugs. 2004;64:2503–14. doi: 10.2165/00003495-200464220-00002. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Morgan MM, Vanegas H. Tolerance to repeated microinjection of morphine into the periaqueductal gray is associated with changes in the behavior of off- and on-cells in the rostral ventromedial medulla of rats. Pain. 2001;89:237–44. doi: 10.1016/s0304-3959(00)00367-5. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Central mechanisms in pain. Med Clin North Am. 1999a;83:585–96. doi: 10.1016/s0025-7125(05)70125-5. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999b;96:7687–92. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Jiang MC, Gebhart GF. Participation of central descending nociceptive facilitatory systems in secondary hyperalgesia produced by mustard oil. Brain Res. 1996;737:83–91. doi: 10.1016/0006-8993(96)00631-2. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–9. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001a;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001b;21:279–86. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–16. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]