Abstract
BACKGROUND
Cancer-associated stroma contributes to the malignant behavior of adenocarcinomas of the prostate and other organs. CD90 is a marker of mesenchymal stem cells (MSCs) and its expression is higher in prostate cancer stroma compared to normal tissue. Cultured prostate cancer-associated fibroblasts (CAFs) expressing high versus low levels of CD90 were analyzed for an MSC-like or tumor-promoting phenotype.
METHODS
CD90hi and CD90lo cells were collected by fluorescence-activated cell sorting (FACS). Expression of genes associated with MSCs and/or tumor-promoting activities was measured by quantitative polymerase chain reaction (qPCR). Effects of stromal cell co-culture or conditioned media were tested on BPH-1 epithelial cells.
RESULTS
The pattern of gene expression did not support the hypothesis that CD90hi cells were MSCs. However, CD90hi cells expressed higher levels of many genes associated with tumor promotion, including cytokines, angiogenic factors, hedgehog signaling components, and transforming growth factor (TGF)-β. Co-culture or conditioned medium from CD90hi cells increased CXCR4 expression in BPH-1 cells, at least in part due to TGF-β, and protected BPH-1 cells from apoptosis.
CONCLUSIONS
Our results suggest that the elevated expression of CD90 previously observed in the cancer-associated stroma of the human prostate is biologically significant. Although our results do not support the idea that CD90hi cells cultured from the cancer stroma are MSCs, our findings suggest that the phenotype of these cells is more tumor-promoting than that of cells expressing low CD90.
Keywords: prostate cancer, stroma, CD90, mesenchymal stem cells
INTRODUCTION
The cancer-associated stroma is recognized as a significant contributor to the development and progression of adenocarcinomas. This stroma is characterized as a “reactive” stroma [1,2], with properties similar to that of a wound repair stroma [3]. Specifically, the fibroblastic component of the stroma becomes constitutively myofibroblastic. The origin of the reactive stroma in the prostate and other cancers is not clear. While a reactive stroma may develop from conversion of the resident stroma in response to paracrine signals from the malignant epithelium, the phenotypic changes appear to be permanent and are retained even in the absence of the epithelium. One possibility is that the reactive stroma arises from the malignant epithelium itself by a process of epithelial-mesenchymal transition (EMT) [4,5]. There is evidence for this in some types of cancers [6,7], but little evidence in prostate cancer. More recently, the reactive stroma has been suggested to arise from mesenchymal stem cells (MSCs) [8]. These MSCs may be intrinsic components of the local stromal tissue, or may originate in the bone marrow.
There are no definitive markers of MSCs, but these cells are typically defined as positive for CD90 [9]. CD90 (Thy-1) is a 25–37 kDa glycosylphosphatidy-linositol (GPI)-anchored glycoprotein involved in cell-cell and cell-matrix interactions [10]. Our attention was drawn to CD90-expressing cells in the prostate cancer stroma for several reasons. First, Liu et al. [11] reported that CD90 expression was elevated in the stroma of prostate cancer compared to that of normal tissue or benign prostatic hyperplasia. Secondly, we found that CD90 expression was higher in stromal cells cultured from prostate cancer compared to those from benign prostate tissues [12]. The association of CD90 with MSCs led us to question whether CD90-positive cells in the prostate cancer stroma were MSCs, and/or whether CD90-positive cells have a particular role in stromal-epithelial interactions that promote progression of prostate cancer.
In this study, we isolated cells expressing high (CD90hi) versus low (CD90lo) levels of CD90 from primary cultures of stromal cells from prostate cancers of Gleason grade 3 and/or 4. These cells were characterized for expression of genes associated with stemness and cancer-promoting properties. The effects of co-culture or conditioned media from these cells on immortal but non-malignant prostatic epithelial cells, BPH-1 [13], were also examined. These cells were chosen as the epithelial target of paracrine activity of stromal cells because prostatic cancer-associated fibroblasts (CAFs) have been shown to irreversibly convert BPH-1 cells to malignancy [14].
MATERIALS AND METHODS
Cell Culture
Primary cultures of human prostatic stromal cells were established from histologically confirmed prostate cancer according to previously described methods [15]. The cell cultures (F-CA-56, -60, -78, and -85) used in this study are listed in Table I. The presence of contaminating epithelial cells was ruled out by the absence of staining with antibodies against epithelial keratins 5 and 18 (Enzo Life Sciences, Inc., Farmingdale, NY). CAFs were serially passaged in stromal medium: MCDB 105 (Sigma-Aldrich, St. Louis, MO) supplemented with 5 µg/ml of insulin, 5 ng/ml of fibroblast growth factor (FGF)-2, 5% fetal bovine serum (FBS), and 100 µg/ml of gentamycin. BPH-1 cells (obtained from Dr. Simon Hayward, Vanderbilt University, TN) were grown in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 5% FBS and 100 µg/ml of gentamycin.
TABLE I.
Summary of Cell Cultures Used in the Study
| Name | Age (years) | Gleason grade of cancer |
|---|---|---|
| F-CA-56 | 50 | 3/4 |
| F-CA-60 | 50 | 3/4 |
| F-CA-78 | 62 | 4/4 |
| F-CA-85 | 69 | 4/4 |
Fluorescence-Activated Cell Sorting (FACS) by CD90 Expression
Five to 10 million CAFs were harvested at passages 8–13 with TrypLE Express (Gibco, Grand Island, NY) and suspended in 1 ml of phosphate buffered saline (PBS). Cells were filtered through a 70-µm cell strainer to obtain a single cell suspension. CD90 was labeled by incubating with ab11154, a mouse monoclonal antibody conjugated to biotin (Abcam, Cambridge, MA), at 1:20 dilution for 20 min on ice. Cells were then washed three times with PBS and incubated with avidin conjugated to Alexa Fluor 488 (Molecular Probes, Carlsbad, CA) at 1:200 dilution for 20 min on ice. DAPI (1 µg/ml) was used to distinguish live versus dead cells. The stained cells were sorted on a FACS Aria (BD Biosciences, San Diego, CA) using FACSDiva software (BD Biosciences). Data were additionally analyzed and presented using FlowJo software (Tree Star, Ashland, OR). A 100-µm ceramic nozzle (BD Biosciences), sheath pressure of 20–25 pounds per square inch, and an acquisition rate of 1,000–3,000 events per sec were used as conditions optimized for stromal cell sorting.
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA from sorted CD90hi and CD90lo cells was isolated using Trizol (Invitrogen) and reverse transcribed using SuperScript III Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. cDNA product was then mixed with SYBR® GreenER™ qPCR super mix (Invitrogen) and primers of interest in the subsequent PCR using a M×3005Ps qPCR System (Stratagene, La Jolla, CA). Transcript level in each sample was determined in triplicate to minimize the experimental variation (standard deviation was calculated for each reaction). Transcript level of TATA box binding protein (TBP) was assayed simultaneously as an internal control to normalize transcript levels of genes of interest. The primer sequences used are listed in Table II.
TABLE II.
Primer Sequences Used in qPCR
| Name | Sequence | Name | Sequence |
|---|---|---|---|
| AR-forward | agtcccacttgtgtcaaaagc | IGF1-forward | tggatgctcttcagttcgtg |
| AR-reverse | acttctgtttcccttcagcg | IGF1-reverse | ttgtttcctgcactccctct |
| CCL5-forward | gcacttgccactggtgtaga | IL6-forward | gaagattccaaagatgtagcccg |
| CCL5-reverse | cgctgtcatcctcattgcta | IL6-reverse | tgttttctgccagtgcctc |
| CD13-forward | caggtcacacccctcttca | OCT4-forward | agtgagaggcaacctggaga |
| CD13-reverse | ggtgctgatggcattaacct | OCT4-reverse | acactcggaccacatccttc |
| CD105-forward | tccctgtgtccacttctcct | OGN-forward | tggaatccgtgcctcttaat |
| CD105-reverse | ggccttatcctgtgtccagt | OGN-reverse | tggtgtcattagccttgcag |
| CD166-forward | cttgcacagcagaaaaccaa | PTCH1-forward | cgatggagtccttgcctacaa |
| CD166-reverse | ccagtagacgacaccagcaa | PTCH1-reverse | ccaccagacgctgtttagtca |
| CD29-forward | cgaggtcatggttcatgttg | S100A4-forward | gatgagcaacttggacagca |
| CD29-reverse | cccatttggcattcattttc | S100A4-reverse | cttcctgggctgcttatctg |
| CD44-forward | aaggtggagcaaacacaacc | SEPT6-forward | tctgcttcaacatcctgtgc |
| CD44-reverse | actgcaatgcaaactgtcag | SEPT6-reverse | gctttagcctcacgttgctc |
| CTGF-forward | ttagcgtgctcactgacctg | SFRP1-forward | cgagtttgcactgaggatga |
| CTGF-reverse | ttcacttgccacaagctgtc | SFRP1-reverse | gaagtggtggctgaggttgt |
| CXCL12-forward | agagccaacgtcaagcatct | SMO-forward | ttaccttcagctgccacttctacg |
| CXCL12-reverse | ctttagcttcgggtcaatgc | SMO-reverse | gccttggcaatcatcttgctcttc |
| ENPP2-forward | acaacgaggagagctgcaat | TBP-forward | tgctgagaagagtgtgctggag |
| ENPP2-reverse | agaagtccaggctggtgaga | TBP-reverse | tctgaataggctgtggggtc |
| FGF2-forward | agagcgaccctcacatcaag | TGF-β1-forward | gtggaaacccacaacgaaat |
| FGF2-reverse | actgcccagttcgtttcaagt | TGF-β1-reverse | cacgtgctgctccactttta |
| FGFR1-forward | cgatgtgcagagcatcaact | TGF-β2-forward | cgccaaggaggtttacaaaa |
| FGFR1-reverse | tgcggttacgcaagcatag | TGF-β2-reverse | ctccattgctgagacgtcaa |
| GLI1-forward | tactcacgcctcgaaaacct | TGF-β3-forward | ggttttccgcttcaatgtgt |
| GLI1-reverse | gtctgctttcctccctgatg | TGF-β3-reverse | gctcgatcctctgctcattc |
| HGF-forward | ctggttccccttcaatagca | VEGFA-forward | cccactgaggagtccaacat |
| HGF-reverse | ctccagggctgacatttgat | VEGFA-reverse | aaatgctttctccgctctga |
| ICAM4-forward | atggggtctctgttccctct | WDR7-forward | tacatggatgtgtccgctgt |
| ICAM4-reverse | tgcaattgagctgcactgac | WDR7-reverse | ttggctatggtggtgatgaa |
Co-Culture of BPH-1 and Sorted CAFs and Immunofluorescence Staining of CXCR4
Freshly sorted CD90hi and CD90lo cells were seeded in stromal medium (described above) at a density of 250,000 per 60-mm dish and allowed to attach overnight. After removal of stromal medium, 100,000 BPH-1 cells were seeded in serum-free RPMI 1640 in each dish containing sorted CAFs. BPH-1 cells cultured in serum-free RPMI 1640 (Invitrogen) with or without transforming growth factor (TGF)-βl (5 ng/ml) (PeproTech, Inc., Rocky Hill, NJ) were used as positive or negative control. Cells were stained for CXCR4 expression after 24 hr incubation at 37°C. Cells were fixed in 2% paraformaldehyde. Horse serum (10% in PBS) was used to block non-specific binding of antibodies. The cells were then incubated at room temperature (RT) for 30 min with a mouse monoclonal antibody against human CXCR4 (R&D Systems, Minneapolis, MN) at 25 µg/ml. After three washes with PBS, cells were incubated with an Alexa Fluro 488-conjugated goat anti-mouse secondary antibody (Invitrogen) at a 1:200 dilution at RT for 30 min. Fluorescence signal was visualized using a Nikon Eclipse E800 microscope (Nikon Instruments, Inc., Melville, NY).
Conditioned Medium and Flow Cytometry Analysis of CXCR4 Expression
Freshly sorted CD90hi and CD90lo cells were seeded in stromal medium (described above) at a density of 500,000 per 60-mm dish and allowed to attach overnight. Medium was then replaced with serum-free RPMI 1640 (Invitrogen). The cells were incubated at 37°C for a further 24 hr. The medium was collected, centrifuged, and stored at −80°C for later use.
BPH-1 cells were seeded into a 24-well plate with 100,000 cells/well in RPMI 1640 supplemented with 5% FBS and 100 µg/ml of gentamycin, and incubated at 37°C overnight. The medium was then replaced with either serum-free RPMI 1640, serum-free RPMI 1640 conditioned by CD90hi/CD90lo cells with or without neutralizing antibody against TGF-β (R&D Systems) at 10 µg/ml. After 24 hr, cells were harvested with TrypLE Express and suspended in 100 µl of PBS. CXCR4 was labeled as described above. The stained cells were analyzed on FACSAria (BD Biosciences) using FACSDiva software (BD Biosciences). Data were additionally analyzed and presented using FlowJo software (Tree Star).
Cell Death Assay
BPH-1 cells were seeded into 48-well plates at a density of 10,000 cells/well in RPMI 1640 supplemented with 5% FBS and 100 µg/ml of gentamycin, and incubated at 37°C overnight. Medium was removed and replaced with either serum-free RPMI 1640, serum-free RPMI 1640 with 100 µM hydrogen peroxide (H2O2) (Fisher Science Education, Rochester, NY), or serum-free RPMI 1640 conditioned by CD90hi/CD90lo cells with 100 µM H2O2. After 24 hr, media were collected from each well and centrifuged to gather floating cells. These were combined with cells detached from each well by TripLE Express. Cells were stained with 0.04% trypan blue (Sigma-Aldrich) for 4 min. The percentage of cells with blue nuclei (dead cells) was determined by counting under a Nikon Diaphot microscope (Nikon Instruments, Inc.).
RESULTS
Stratification of Cancer-Associated Fibroblasts Into Populations of CD90hi Versus CD90lo Cells
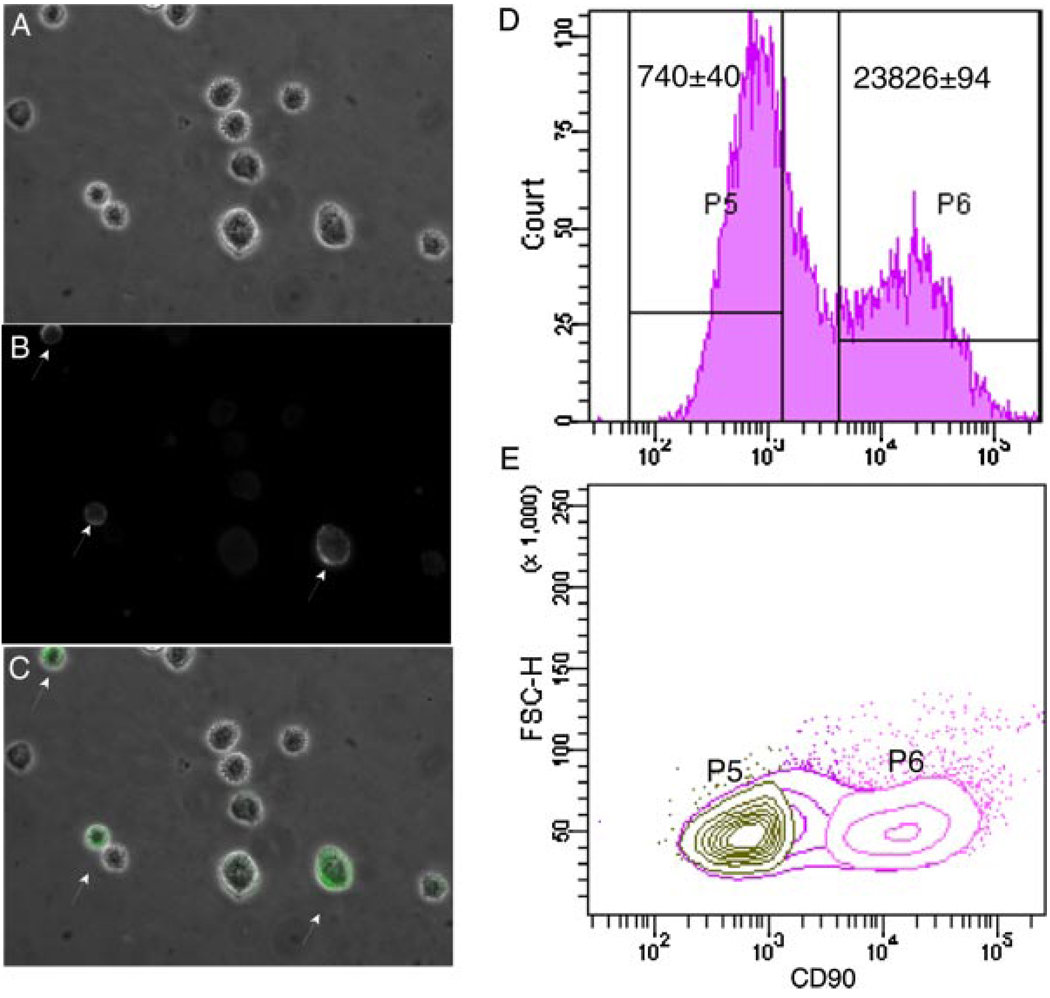
We determined whether CAFs differentially expressed CD90 by immunofluorescent staining. We surveyed four primary cultures of fibroblasts grown from human prostatic adenocarcinomas (Table I). After staining with antibody against CD90, three of four cultures showed heterogeneity in CD90 expression by flow cytometry analysis, although subpopulations expressing high (CD90hi) or low (CD90lo) levels of CD90 were more distinct in certain cultures (data not shown). We chose to use F-CA-56 cells for the majority of experiments in our study because the clear separation of CD90hi and CD90lo cells gave the highest yield of cells during sorting by FACS (Fig. 1). A subset of F-CA-56 cells showed significantly higher expression of CD90 with bright fluorescent signal concentrated on the cell membrane (arrows in Fig. 1B). This was not an artifact of the imaging, because cells with bright or dim CD90 signals were on the same focal plane in the merged image (Fig. 1C) of fluorescence staining and phase contrast microscopy (Fig. 1A). Cells were collected based on their CD90 expression. As shown in the histogram of CD90 expression in Figure 1D, cells that expressed CD90 in the lower 30% range (CD90lo) were separated from those in the higher 30% range (CD90hi). The average level of CD90 expression in the CD90hi population was 20- to 30-fold higher than that of the CD90lo population. F-CA-85 cells were similarly sorted into CD90hi and CD90lo populations and used for a subset of the experiments.
Fig. 1.
Quantitation and collection of CD90hi versus CD90lo cells from F-CA-56 cells by FACS. A: F-CA-56 cells in suspension under phase contrast microscope. B: A subset of F-CA-56 cells in suspension stained with anti-CD90 showed intense fluorescent signal on cell membrane (arrows). C: Merged image of A and B. D: A histogram of CD90 expression in F-CA-56 cells by flow cytometry. Average intensity among cells defined by gates P5 and P6 is indicated. E: Acontour plot of cells defined by gates P5 and P6 showed two well-separated populations. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
Expression of Genes Associated With MSCs
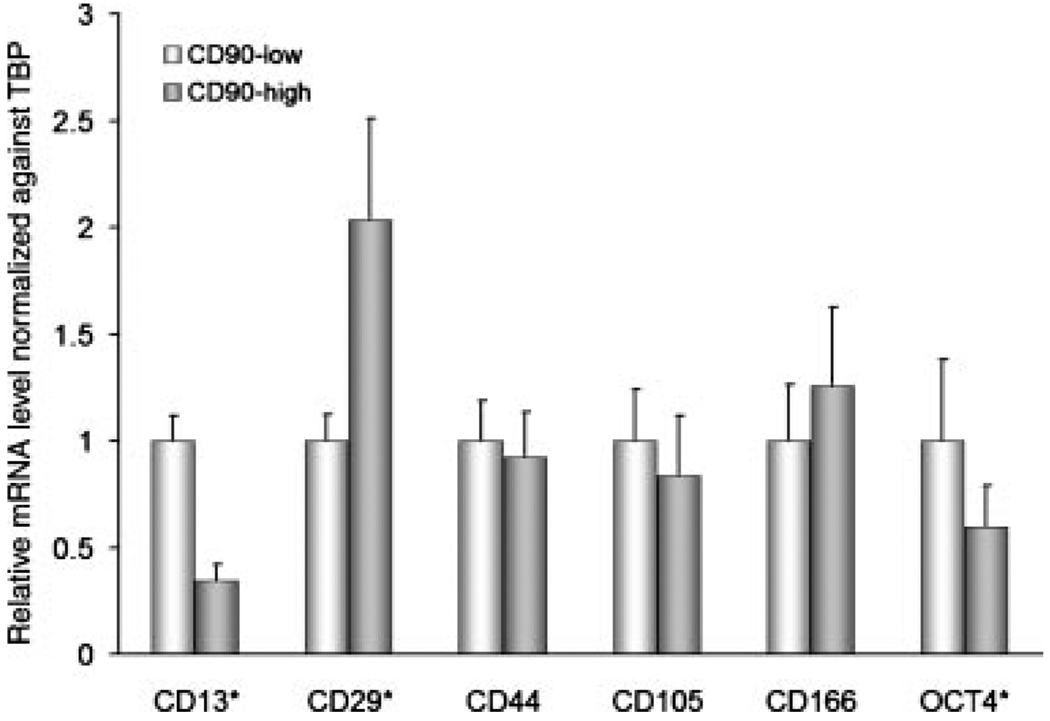
In addition to CD90, other genes typically expressed by MSCs include CD13, CD29, CD44, CD105, CD166, and OCT4 [16,17]. Levels of mRNA transcripts of each of these genes in CD90hi versus CD90lo F-CA-56 cells were compared by qPCR. Figure 2 shows that only CD29 was expressed at a significantly higher level in CD90hi compared to CD90lo cells. The other CD antigens were either expressed at similar levels in the two cell populations, or, as in the case of CD13 and OCT4, were significantly lower in CD90hi cells. These results do not support the hypothesis that CD90hi cells are enriched with MSCs.
Fig. 2.
Relative gene expression of MSC-associated stem cell markers in CD90hi versus CD90lo cells from F-CA-56 determined by qPCR. Error bars represent standard deviation. Asterisks indicate statistical significances by t-test (P < 0.05).
Expression of Cancer-Promoting Genes
The secretion of certain growth factors and cytokines has been ascribed to tumor-associated stroma [18]. The activities of these factors are considered to be important in the promotion of the development and progression of the malignant epithelium by the stroma. Therefore, we examined the mRNA expression of a number of these cytokines and growth factors in CD90hi versus CD90lo cells sorted from F-CA-56 and F-CA-85 by qPCR. Factors particularly implicated in prostate cancer-promoting activity were chosen for analysis. The selected genes included IL6, CXCL12, VEGFA, FGF2, SFRP1, CCL5, HGF, IGF1, and S100A4. In F-CA-56 cells, Figure 3A shows that a number of these growth factors and cytokines—IL6, VEGFA, FGF2, and CXCL12—were expressed at significantly higher levels (2- to 7-fold) in CD90hi versus CD90lo populations. In F-CA-85 cells, IL6, VEGFA, and FGF2 were also significantly higher in CD90hi versus CD90lo populations as in F-CA-56 cells. CXCL12 was slightly higher (1.3-fold) in CD90hi versus CD90lo populations, barely missing the statistical significance threshold (P = 0.07) (Fig. 3B). CCL5 and HGF were also significantly higher in CD90hi versus CD90lo populations from F-CA-85, but not F-CA-56 cells. These results suggest that CD90hi cells could have more tumor-promoting activity than CD90lo cells. AR was also measured and was significantly lower in CD90hi compared to CD90lo cells from F-CA-56, but not in F-CA-85 cells (Fig. 3). Similar results were seen with S100A4 (Fig. 3).
Fig. 3.
Relative gene expression of cancer-promoting factors in CD90hi versus CD90lo cells determined by qPCR. Two CAFs were used in the analysis shown in (A) (F-CA-56) and (B) (F-CA-85). For both A and B, error bars represent standard deviation. Asterisks indicate statistical significances by t-test (P < 0.05).
Hedgehog Signaling Pathway
Figure 3 also shows the relative mRNA expression of key genes in the hedgehog signaling pathway—PTCH1, SMO, and GLI1—in CD90hi versus CD90lo cells as measured by qPCR. In both F-CA-56 and F-CA-85, all three genes were expressed at significantly higher levels in CD90hi compared to CD90lo cells. Specifically, PTCH1 and SMO were expressed 5- and 2-fold higher, respectively, in CD90hi cells from F-CA-56. GLI1, the mediator of hedgehog signaling, was∼11-fold higher in CD90hi cells from F-CA-56. For F-CA-85, PTCH1 and SMO expression were higher in CD90hi cells by 19- and 28-fold, respectively, while GLI1 expression was elevated by threefold.
Expression of Genes Differentially Expressed by Stromal Cells From Cancer and Normal Tissues
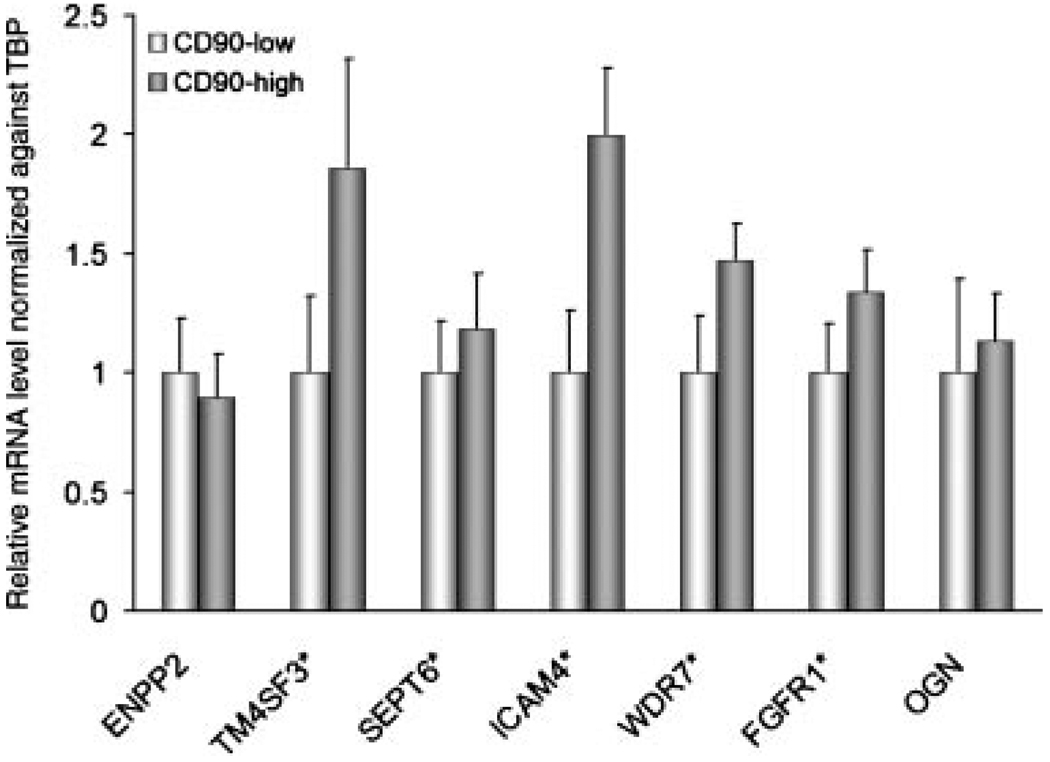
Previously, we cultured stromal cells from normal prostatic tissues, benign prostatic hyperplasia, and prostate cancer [12]. Gene expression profiling by cDNA microarray analysis yielded a list of genes differentially overexpressed in CAFs compared to stromal cells from benign tissues. CD90, in fact, was one of these genes. The expression of the top seven genes in the significance analysis of microarray (SAM) list that were also validated by qPCR was measured in CD90hi and CD90lo F-CA-56 cells by qPCR. Five of these genes (TM4SF3, SEPT6, ICAM4, WDR7, and FGFR1) were significantly higher in CD90hi cells (Fig. 4). This suggests that CD90hi cells in CAFs may be key contributors to differential gene expression observed between stromal cells cultured from benign versus malignant tissues.
Fig. 4.
Relative expression of genes differentially expressed in prostate cancer-associated stromal cells in CD90hi versus CD90lo cells from F-CA-56 determined by qPCR. Error bars represent standard deviation. Asterisks indicate statistical significances by t-test (P < 0.05).
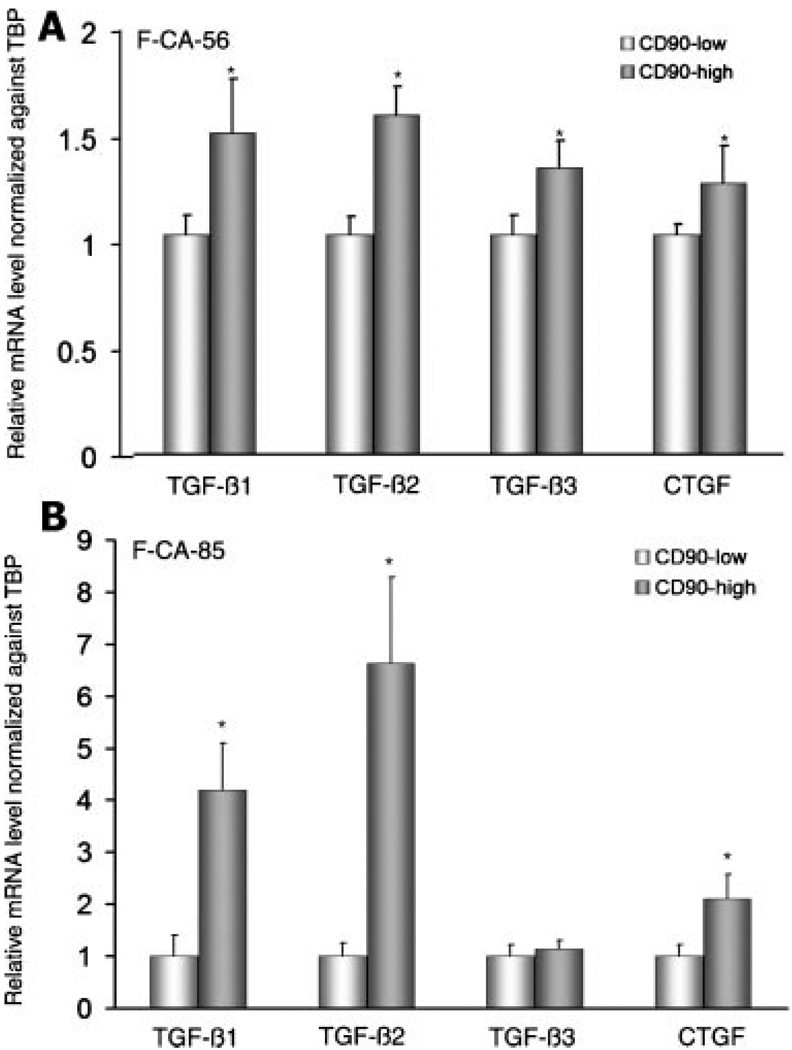
TGF-β Expression Is Higher in CD90hi Cells
The mRNA expression of three isoforms of TGF-β—1, -2 and -3—as well as a downstream target of TGF-β, CTGF, was measured in CD90hi and CD90lo cells by qPCR in F-CA-56. All of these genes were expressed at significantly higher levels in CD90hi cells (Fig. 5A). Similar results were observed in F-CA-85 cells except for TGF-β-3, which was 1.3-fold higher in CD90hi cells but not statistically significant (Fig. 5B).
Fig. 5.
Relative gene expression of TGF-β isoforms in CD90hi versus CD90lo cells determined by qPCR. Two CAFs were used in the analysis shown in (A) (F-CA-56) and (B) (F-CA-85). For both A and B, error bars represent standard deviation. Asterisks indicate statistical significances by t-test (P < 0.05).
Impact of Overexpression of TGF-β by CD90hi Cells on Stromal-Epithelial Interactions
It has been reported that TGF-β upregulates the expression of CXCR4, the receptor for CXCL12, in epithelial cells [19]. Since we observed that gene expression of TGF-β was elevated in CD90hi compared to CD90lo cells, we performed a functional assay to test the potential impact on stromal-epithelial interactions. After collection by FACS, CD90hi and CD90lo F-CA-56 cells were placed into short-term culture and conditioned medium was collected after 24 hr. BPH-1 epithelial cells were maintained in control medium or conditioned medium for 24 hr, then cell-surface expression of CXCR4 was measured by FACS. The percentage of BPH-1 cells expressing high levels of CXCR4 was increased after exposure to conditioned medium from CD90hi cells compared to conditioned medium from CD90lo cells or control medium (Fig. 6). In the fraction of cells expressing high levels of CXCR4, the intensity of expression was increased ∼30-fold. This elevated expression did not occur if BPH-1 cells were maintained in conditioned medium from CD90hi cells with added neutralizing antibody against TGF-β, suggesting that the paracrine activity of TGF-β from CD90hi cells induced CXCR4. Co-culture of CD90hi cells with BPH-1 cells similarly increased CXCR4 expression (Fig. 6).
Fig. 6.
CXCR4 expression in BPH-1 epithelial cells in response to CD90hi versus CD90lo stromal cells. A: Histograms of CXCR4 expression in BPH-1 cells determined by flow cytometry. BPH-1 cells were cultured either in serum-free RPMI 1640, or serum-free RPMI 1640 conditioned by CD90hi/CD90lo cells from F-CA-56 with or without neutralizing antibody against TGF-β at 10 µg/ml. After 24 hr, cells were harvested and CXCR.4 expression was measured by FACS. B–E: Immunofluorescence staining of CXCR4 in BPH-1 cells. BPH-1 cells were cultured either in serum-free RPMI 1640 without TGF-β (B), with TGF-β (D), with CD90lo CAFs (C), or with CD90hi CAFs (E).CXCR4-expressing BPH-1 cells are indicated by arrows. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
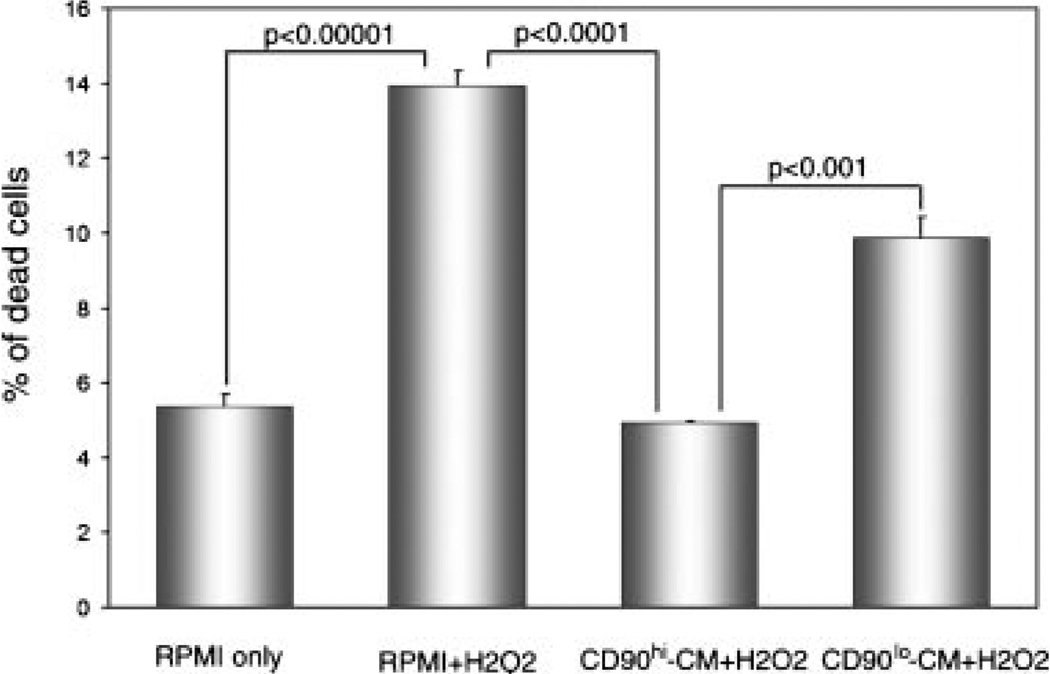
CD90hi Conditioned Medium Protects BPH-1 Cells From H2O2-Induced Cell Death
Co-culture with reactive stromal cells has been reported to protect prostate cancer cells from apoptosis [20]. BPH-1 cells were induced to undergo cell death by H2O2, and the ability of medium conditioned by CD90hi or CD90lo cells to protect BPH-1 cells from H2O2-induced death was examined (Fig. 7). H2O2 approximately doubled the basal rate of death in BPH-1 cells. Conditioned medium from CD90hi cells protected BPH-1 cells from H2O2-induced death, and conditioned medium from CD90lo cells only partially protected cells from H2O2-induced death. Overall, these results suggest that factors secreted by CD90hi stromal cells increased the survival of BPH-1 epithelial cells in the presence of an apoptosis-inducing agent to a greater degree than CD90lo stromal cells.
Fig. 7.
Protection of BPH-1 epithelial cells from H2O2-induced cell death. BPH-1 cells were either cultured in serum-free RPMI 1640 without H2O2, with H2O2 (100 µM), with conditioned medium (CM) from CD90hi F-CA-56 cells and H2O2, or CM from CD90lo F-CA-56 cells and H2O2. Student’s t-test was used to determine statistical significance.
DISCUSSION
We compared expression of stem cell markers and cancer-promoting genes in CD90hi versus CD90lo prostatic CAFs at the transcript level. Our results demonstrated that a host of cancer-promoting genes but not stem cell markers were expressed at a higher level in CD90hi compared to CD90lo cells. In addition, CD90hi cells expressed higher levels of TGF-β, which acted as a paracrine signal to induce CXCR12/CXCR4 pathway activity, critical in malignant transformation of benign prostatic epithelial cells, BPH-1. Moreover, CD90hi cell conditioned-medium protected BPH-1 cells from H2O2-induced cell death to a greater degree than medium conditioned by CD90lo cells. Although our study did not address the biological function of CD90 itself in prostatic CAFs, partly due to the poor viability of cells after collection by FACS, our results suggest that CD90hi cells may have more cancer-promoting potential than CD90lo cells without exhibiting an MSC-like phenotype.
Our data do not support the hypothesis that high expression of CD90 in the prostate cancer-associated stroma is reflective of infiltration by CD90-positive MSCs. The majority of genes commonly considered to be stem cell-associated markers of MSCs were not more highly expressed in CD90hi than in CD90lo cells. In fact, the only MSC-associated gene significantly elevated in CD90hi cells was CD29, also known as β1-integrin, which has a major role in cell-matrix interactions and stem cell differentiation [21]. In future studies, it may be of interest to investigate the biological role of CD29 in CD90hi cells and in stromal-epithelial interactions in prostate cancer. On the other hand, MSCs share molecular signatures with tumor mesenchymal cells [22] and express pro-angiogenic genes, growth factors, and cytokines (reviewed in Ref. [23]). We found many of these genes, including IL6, VEGFA, FGF-2, and CXCL12, expressed at higher levels in CD90hi cells. The similarity of the hematopoietic stem cell niche created by bone marrow MSCs to the tumor mesenchyme has led some to suggest that the cancer stroma provides a similar “niche” for cancer stem cells [8,24]. Our data suggest that if such a “niche” exists in prostate cancer, it is likely to be composed of CD90hi cells.
Many of the genes that we found over-expressed in CD90hi cells have been implicated in prostate cancer promotion by the stroma. For instance, it is believed that one important property of reactive stroma is its ability to promote angiogenesis [25]. VEGFA, FGF-2, and CTGF, all overexpressed in CD90hi compared to CD90lo cells, are key angiogenic molecules. FGF-2 also has growth-stimulatory activity in malignant epithelial cells as well as in stromal cells themselves [26]. Its receptor, FGFR1, was also overexpressed by CD90hi cells, suggesting activity of an autocrine growth-promoting loop. In addition, we found elevated expression of major hedgehog (Hh) signaling pathway components in CD90hi cells including SMO, PTCH1, and GLI1. It has been reported that overexpression of Hh pathway ligands in LNCaP prostate cancer cells dramatically accelerates tumor growth in a xenograft model by activating GLI1 expression in the tumor stroma [27]. A similar phenomenon has been observed in colon cancer [28], suggesting a common promoting role of a paracrine-autocrine loop of Hh signaling between stromal and epithelial cells in cancer growth. Finally, we found that AR mRNA expression was lower in CD90hi compared to CD90lo cells in F-CA-56. Ample evidence suggests that decreased AR signaling in stromal cells may function as a paracrine factor to stimulate proliferation and invasion of prostate cancer cells in vitro and in vivo [29–31]. However, AR expression was not significantly lower in CD90hi compared to CD90lo cells in F-CA-85. This may be related to the different histopathologies of the cancers from which these cultures were derived. Indeed, two of the cancer-promoting genes, CCL5 and HGF, showed significantly higher expression in CD90hi cells from F-CA-85, but not F-CA-56 cells. Nonetheless, overall our data indicate that CD90hi CAFs may play a leading role in promoting cancer progression by increased expression of angiogenic factors, activated Hh signaling, and reduced AR signaling.
Perhaps the most intriguing finding from our study is that CD90hi cells showed higher expression of TGF-β and its target genes. TGF-β is known to play an important role in the tumor microenvironment (reviewed in Ref. [32]) and many studies have found TGF-β overexpression in prostate CAFs [12,19,33]. As a multipotent molecule, TGF-β increases stromal cell expression of angiogenic-relevant molecules such as VEGF, CTGF, and FGF-2 [34,35]. Inhibition of TGF-β in prostate xenograft tumors resulted in tumors that were poorly vascularized [36], while overexpression of CTGF by engineered prostate stromal cells significantly elevated LNCaP tumorigenesis and angiogenesis in vivo [37]. Furthermore, TGF-β induces CXCR4 expression in immortal but non-malignant prostatic epithelial cells, BPH-1. This increased CXCR4 signaling was shown to be required for induction of tumorigenesis of BPH-1 by CAFs, as neutralizing antibody against TGF-β blocked the ability of CAFs to irreversibly convert BPH-1 into tumor cells [19]. Altogether, these studies point to the critical tumor-promoting roles of stromal TGF-β in prostate cancer.
Our results suggest that CD90hi cells in particular may be responsible for these TGF-β-mediated cancer-promoting activities of CAFs. First, all three forms of TGF-β (1, 2, and 3) as well as CTGF, a classic downstream target of TGF-β, were elevated in CD90hi cells. Second, either co-culture with CD90hi cells or culture in medium conditioned by CD90hi cells increased expression of CXCR4 in BPH-1 cells, which could be reversed by TGF-β inhibition. Finally, TGF-β production by stromal cells has been shown to promote tumor progression by increasing the survival of malignant epithelial cells in a xenograft model [20]. Similarly, we found that culture of BPH-1 cells in medium conditioned by CD90hi cells protected the epithelial cells from H2O2-induced apoptosis to a greater degree than CD90lo cells.
Taken together, our data underscore the importance of stromal-epithelial interaction in prostate cancer development and progression. More importantly, our results pinpoint a subpopulation of CAFs which may be responsible for the tumor-promoting effects of the cancer-associated stroma in prostatic adenocarcinomas. This population of cells expresses a higher level of CD90 compared to other CAFs, which may be helpful in designing therapeutic strategies to eliminate such cancer-promoting effects from CAFs.
ACKNOWLEDGMENTS
This study was performed with support from NIH CA123532 and DAMD W81XWH-06-1-0101 grants.
Grant sponsor: NIH; Grant number: CA123532; Grant sponsor: DAMD; Grant number: W81XWH-06-1-0101.
REFERENCES
- 1.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166(6):2472–2483. [PubMed] [Google Scholar]
- 2.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107(1):1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 3.Berry PA, Maitland NJ, Collins AT. Androgen receptor signalling in prostate: Effects of stromal factors on normal and cancer stem cells. Mol Cell Endocrinol. 2008;288(l–2):30–37. doi: 10.1016/j.mce.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 5.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 6.Bates RC, Pursell BM, Mercurio AM. Epithelial-mesenchymal transition and colorectal cancer: Gaining insights into tumor progression using LIM 1863 cells. Cells Tissues Organs. 2007;185(1–3):29–39. doi: 10.1159/000101300. [DOI] [PubMed] [Google Scholar]
- 7.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25(6):629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 8.Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: Tumor-associated niche cells. Genes Dev. 2008;22(5):559–574. doi: 10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis JE, Esterly K, Awadallah A, Parrish CR, Poynter GM, Goltry KL. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells. 2007;25(10):2575–2582. doi: 10.1634/stemcells.2007-0204. [DOI] [PubMed] [Google Scholar]
- 10.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20(8):1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 11.Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. Am J Pathol. 2004;165(5):1543–1556. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Ramos CF, Brooks JD, Peehl DM. Distinctive gene expression of prostatic stromal cells cultured from diseased versus normal tissues. J Cell Physiol. 2007;210(1):111–121. doi: 10.1002/jcp.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31(l):14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 14.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peehl DM, Sellers RG. Cultured stromal cells: An in vitro model of prostatic mesenchymal biology. Prostate. 2000;45(2):115–123. doi: 10.1002/1097-0045(20001001)45:2<115::aid-pros5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67–81. doi: 10.1007/978-1-59745-443-8_4. [DOI] [PubMed] [Google Scholar]
- 17.Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, Antsaklis A, Anagnou NP. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16(6):931–952. doi: 10.1089/scd.2007.0036. [DOI] [PubMed] [Google Scholar]
- 18.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 19.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 20.Singh H, Dang TD, Ayala GE, Rowley DR. Transforming growth factor-beta1 induced myofibroblasts regulate LNCaP cell death. J Urol. 2004;172(6 Pt l):2421–2425. doi: 10.1097/01.ju.0000138082.68045.48. [DOI] [PubMed] [Google Scholar]
- 21.Ridger VC, Wagner BE, Wallace WA, Hellewell PG. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol. 2001;166(5):3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- 22.Galie M, Konstantinidou G, Peroni D, Scambi I, Marchini C, Lisi V, Krampera M, Magnani P, Merigo F, Montani M, Boschi F, Marzola P, Orru R, Farace P, Sbarbati A, Amici A. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2008;27(18):2542–2551. doi: 10.1038/sj.onc.1210920. [DOI] [PubMed] [Google Scholar]
- 23.Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008;217(2):296–300. doi: 10.1002/jcp.21521. [DOI] [PubMed] [Google Scholar]
- 24.Risbridger GP, Taylor RA. Minireview: Regulation of prostatic stem cells by stromal niche in health and disease. Endocrinology. 2008;149(9):4303–4306. doi: 10.1210/en.2008-0465. [DOI] [PubMed] [Google Scholar]
- 25.Condon MS. The role of the stromal microenvironment in prostate cancer. Semin Cancer Biol. 2005;15(2):132–137. doi: 10.1016/j.semcancer.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Shain SA. Exogenous fibroblast growth factors maintain viability, promote proliferation, and suppress GADD45 alpha and GAS6 transcript content of prostate cancer cells genetically modified to lack endogenous FGF-2. Mol Cancer Res. 2004;2(11):653–661. [PubMed] [Google Scholar]
- 27.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145(8):3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 28.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Li CX, Ye H, Chen F, Melamed J, Yi P, Liu J, Wang Z, Tsou HC, Wei J, Waiden P, Garabedian MJ, Lee P. Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med. 2008;12(6b):2790–2798. doi: 10.1111/j.1582-4934.2008.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cano P, Godoy A, Escamilla R, Dhir R, Onate SA. Stromal-epithelial cell interactions and androgen receptor-coregulator recruitment is altered in the tissue microenvironment of prostate cancer. Cancer Res. 2007;67(2):511–519. doi: 10.1158/0008-5472.CAN-06-1478. [DOI] [PubMed] [Google Scholar]
- 31.Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, Delprado W, Stricker PD, Grygiel JJ, Sutherland RL. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61(2):423–427. [PubMed] [Google Scholar]
- 32.Stover DG, Bierie B, Moses HL. A delicate balance: TGF-beta and the tumor microenvironment. J Cell Biochem. 2007;101(4):851–861. doi: 10.1002/jcb.21149. [DOI] [PubMed] [Google Scholar]
- 33.San Francisco IF, DeWolf WC, Peehl DM, Olumi AF. Expression of transforming growth factor-beta 1 and growth in soft agar differentiate prostate carcinoma-associated fibroblasts from normal prostate fibroblasts. Int J Cancer. 2004;112(2):213–218. doi: 10.1002/ijc.20388. [DOI] [PubMed] [Google Scholar]
- 34.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269(9):6271–6274. [PubMed] [Google Scholar]
- 35.Yang F, Strand DW, Rowley DR. Fibroblast growth factor-2 mediates transforming growth factor-beta action in prostate cancer reactive stroma. Oncogene. 2008;27(4):450–459. doi: 10.1038/sj.onc.1210663. [DOI] [PubMed] [Google Scholar]
- 36.Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002;62(ll):3298–3307. [PubMed] [Google Scholar]
- 37.Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65(19):8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]