Abstract
Background
Preoperative brain injury is an increasingly recognized phenomenon in neonates with complex congenital heart disease. Recently, reports have been published that associate preoperative brain injury in neonates with transposition of the great arteries with the performance of balloon atrial septostomy (BAS), a procedure that improves systemic oxygenation preoperatively. It is unclear whether BAS is the cause of brain injury or is a confounder, because neonates who require BAS are typically more hypoxemic. We sought to determine the relationship between preoperative brain injury in neonates with transposition of the great arteries and the performance of BAS. We hypothesized that brain injury results from hypoxic injury, not from the BAS itself.
Methods and Results
Infants with transposition of the great arteries (n=26) were retrospectively included from a larger cohort of infants with congenital heart disease who underwent preoperative brain MRI as part of 2 separate prospective studies. Data collected included all preoperative pulse oximetry recordings, all values from preoperative arterial blood gas measurements, and BAS procedure data. MRI scans were performed on the day of surgery, before the surgical repair. Of the 26 neonates, 14 underwent BAS. No stroke was seen in the entire cohort, whereas 10 (38%) of 26 patients were found to have hypoxic brain injury in the form of periventricular leukomalacia. Periventricular leukomalacia was not associated with BAS; however, neonates with periventricular leukomalacia had lower preoperative oxygenation (P=0.026) and a longer time to surgery (P=0.028) than those without periventricular leukomalacia.
Conclusions
Preoperative brain injury in neonates with transposition of the great arteries is associated with hypoxemia and longer time to surgery. We found no association between BAS and brain injury.
Keywords: heart defects, congenital; oxygen; brain; catheterization; magnetic resonance imaging
Widespread recognition exists that for patients with complex congenital heart disease (CHD), brain development is adversely affected not only by operative strategies but also by perioperative and fetal environmental factors. Increasingly, studies demonstrate that neonates with complex CHD have abnormal brain development, delayed brain maturation, and brain injury before any surgical intervention.1-5 For example, neonates with hypoplastic left heart syndrome and transposition of the great arteries (TGA) have smaller head circumferences and brain volumes than normal neonates.6-8 Because fetal cerebral blood flow patterns in these neonates are altered, it has been proposed that hypoxemia and abnormal cerebrovascular reactivity may contribute to neurological maldevelopment and brain injury.2,9-11
Neonates with TGA provide a unique opportunity to study the impact of preoperative management on the brain, because the majority of these infants are free of identifiable genetic abnormalities. Infants with unrepaired TGA have been noted to have preoperative evidence of hypoxic brain injury by ultrasound and magnetic resonance imaging (MRI).3,12-14 At 8 years of age, children who underwent surgical repair by arterial switch operation who participated in the Boston Circulatory Arrest Study had an increased risk of behavioral, motor, and cognitive impairment.15,16 Recently, a link has been reported between neonatal brain injury and balloon atrial septostomy (BAS), an intervention aimed at relieving severe cyanosis in neonates with TGA who have limited mixing of oxygenated and deoxygenated blood.17,18 In the original report, 12 of 19 neonates with TGA undergoing BAS were found to have preoperative brain injury compared with 0 of 10 patients who did not undergo BAS. Although the authors accounted for confounding factors in the statistical analysis, the relationship between BAS and brain injury in this setting is unclear, because infants who underwent BAS were more significantly hypoxemic than those without BAS, and infants found to have brain injury had lower Apgar scores. Brain injury could have resulted from both cerebral hypoxemia and hypoperfusion in these cases. Although infants undergoing BAS, which is indicated for severe cyanosis, might be expected to experience hypoxic brain injury, these reports linked BAS predominantly to stroke, a thromboembolic or hemorrhagic phenomenon. Because BAS is critical in the management of infants with TGA and poor pulmonary-systemic mixing, we sought to elucidate the relationship between brain injury and BAS in our experience. Our hypothesis is that preoperative brain injury in the setting of TGA is related to exposure of the neonatal brain to hypoxemia, not to BAS.
Methods
Patient Population
Patients born with TGA between January 1, 2003, and April 1, 2007, were considered for inclusion in the present study. Patients were included from 2 prospective research protocols that used brain MRI to study the incidence of preoperative brain injury in neonates with all forms of CHD.10,11,19 For these studies, inclusion criteria included full-term gestational age (40±4 weeks), an intention to undergo surgical intervention with cardiopulmonary bypass with or without deep hypothermic circulatory arrest, and medical stability for 24 hours before surgery. All patients were considered for inclusion who weighed ≥2 kg and had a 5-minute Apgar score >5 or a cord pH of ≥7.0, a serum creatinine <2.0 mg/dL, and liver function tests <2 times normal. Reasons for failure to enroll included unavailability of parents for the consent process or parental refusal. Patients with TGA (n=26) from the larger prospective study were included in the present retrospective study. The Investigational Review Board of The Children’s Hospital of Philadelphia approved the study protocol.
Preoperative Data
Patient data reviewed included demographics, birth data, and timing of diagnosis. All preoperative arterial blood gas (ABG) values (including pH, Po2, Pco2, serum bicarbonate level, base deficit, lactate, and hemoglobin concentrations) were collected, and a daily mean value was calculated for each patient for each aspect of the ABG. For patients who underwent BAS (BAS group), these ABG values were separated as pre- and post-BAS. Preductal transcutaneous pulse oximetry recordings as they appeared on the patients’ bedside charts were also collected. Preductal and postductal saturation data are normally recorded on the bedside charts at least once per hour. For patients who underwent BAS, the method, location of procedure (referral hospital or our institution, and bedside or in the catheterization laboratory), and complications due to BAS were recorded. Use, timing, and duration of prostaglandin (PGE1) infusion were documented. Anatomic diagnosis was confirmed by transthoracic echocardiography.
Balloon Atrial Septostomy
BAS was performed in 14 of the 26 patients. Eleven of the 14 procedures were performed at The Children’s Hospital of Philadelphia; 3 were performed at referring hospitals before transport. BAS was performed at the discretion of the treating clinician but was indicated by cyanosis refractory to PGE1 infusion. BAS procedures in the catheterization laboratory (n=9) were performed under fluoroscopic guidance, whereas those performed at the bedside (n=5) used echocardiographic guidance. Either the umbilical vein (n=10) or a femoral vein (n=4) was used for vascular access. Heparin was not administered during any of the cases. A Rashkind (Medtronic Corp, Minneapolis, Minn) or Miller (Edwards Lifesciences, Irvine, Calif) septostomy balloon catheter was passed through a 6F or 7F venous sheath and passed across the atrial septum. The balloon was inflated with normal saline or dilute contrast and then rapidly pulled across the atrial septum. The technique was repeated until an adequate atrial communication was created and systemic oxygen saturation improved. The procedure was successful in achieving immediate improvement in arterial oxygenation in all cases, and no short-term complications were reported.
Brain MRI
Preoperative brain MRI scans performed before January 1, 2005 (n=16) were completed on a Siemens 1.5T Sonata (Siemens Corp, New York, NY), and those performed after February 1, 2006 (n=10) were completed on a Siemens 3T Trio scanner. All neonates underwent preoperative brain MRI scanning on the day of surgery. Study patients were transported by the cardiac anesthesia team from the cardiac intensive care unit to the operating room for induction of general anesthesia and then were transported to the MRI suite, where brain MRI was performed. On completion of the scan, patients were then transported back to the operating room for surgery.
Routine structural scans performed on the 1.5T scanner included axial, sagittal, and coronal T1, axial and coronal T2, axial fluid attenuation inversion recovery, and diffusion-weighted imaging, as well as echo gradient sequences for blood product imaging. The slice thickness was 3 mm, with a 3-mm slice gap and a repetition time of 1500 ms (T1) and 6000 ms (T2). Routine sequences performed on the 3T scanner included volumetric 3-dimensional multiplanar reconstruction T1 and T2 sampling perfection with application-optimized contrasts (SPACE) sequences that were acquired in the axial plane, with a slice thickness of 0.9 mm, no interslice gap, and a repetition time of 2050 ms (T1) and 3200 ms (T2). The axial images were reconstructed in the sagittal and coronal planes. Other sequences at 3T included fluid attenuation inversion recovery, susceptibility-weighted imaging, gradient echo, and diffusion-weighted imaging.
A neuroradiologist (R.A.Z.), blinded to the patients’ clinical history and gestational age, reviewed all brain MRI images for congenital and acquired abnormalities. For the purposes of the present study, all scans were re-reviewed carefully (R.A.Z.), with particular attention paid to evidence of stroke and periventricular leukomalacia (PVL). Focal acquired abnormalities included subdural hemorrhage, choroid plexus hemorrhage, stroke (infarction), and PVL. Stroke was defined as a focal area of diffusion restriction in an arterial territory. PVL was defined as a punctate periventricular white matter lesion associated with T1 hyperintensity with or without restriction of water diffusion on diffusion-weighted imaging. PVL was graded with a published 4-point scale ranging from none to severe.3 The status of the operculum (open or closed) was also noted.
Statistical Analysis
Continuous variables are expressed as mean±SD or median (range) where appropriate. Nominal variables are expressed as frequency and percentage. Patient characteristics were compared with χ2, Fisher exact, and Student t tests. For each patient, we computed daily and overall preoperative summaries of all ABG values, with means and SDs. For those patients who underwent BAS, the same summaries were also calculated for pre- and post-BAS time periods. Daily mean ABG values and daily mean oxygen saturations in the group with brain injury were compared with the group without injury by use of repeated-measures ANOVA. Logistic regression was used to model the dichotomous variable PVL as a function of independent factors. The list of candidate predictors entered into the analysis included performance of BAS (yes/no), time to surgery (days), weight (kilograms), gestational age (weeks), and lowest/mean/daily mean oxygen saturation, as well as each of the ABG summaries that were significant (P<0.05) in the ANOVA analyses. Finally, a backward stepwise model selection process, which removed the least significant variables until all remaining predictors had probability values <0.15, was performed to simplify the model while preserving good prediction properties. The final model is described in the Results. Statistical analyses were performed with Prism 5.0 (Graph-Pad Software, Inc, San Diego, Calif) and R (R Foundation for Statistical Computing, Vienna, Austria).
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Mean gestational age was 39.0±1.2 weeks, and mean birth weight was 3.46±0.61 kg (Table 1). Six patients had a ventricular septal defect associated with TGA, whereas 20 had TGA with intact ventricular septum. The only demographic difference between the BAS group (n=14) and those infants who did not undergo BAS (non-BAS group, n=12) was gender, because the BAS group was predominantly male (P=0.04). The lowest recorded oxygen saturation in the BAS group on the first day of life was 53.9%, whereas it was 77.7% in the non-BAS group (P<0.01). Similarly, the lowest recorded Po2 on the first day of life was also significantly lower in the BAS group (22.2 versus 36.4 mm Hg, P<0.01). No differences were found between the 2 groups with regard to mean arterial pH, base deficit, or other ABG values.
Table 1. Characteristics of Patients Who Underwent BAS Compared With Those Who Did Not Undergo the Procedure (No BAS).
| BAS (n=14) | No BAS (n=12) | P | |
|---|---|---|---|
| Gestational age, wk | 38.6±1.2 | 39.5±1.0 | 0.08 |
| Birth weight, kg | 3.30±0.60 | 3.67±0.59 | 0.14 |
| Head circumference, cm | 33.3±1.9 | 34.0±1.2 | NS |
| Female, n (%) | 3 (21) | 8 (67) | 0.04 |
| Prenatal diagnosis, n (%) | 4 (29) | 2 (17) | NS |
| Anatomic diagnosis, n | |||
| dTGA-IVS | 12 | 8 | NS |
| dTGA-VSD | 2 | 4 | |
| Lowest O2 saturation day 1, % | 53.9±14.4 | 77.7±15.1 | <0.01 |
| Mean O2 saturation day 1, % | 78.8±9.3 | 86.4±6.5 | 0.04 |
| Lowest Po2 day 1, mm Hg | 31.9±5.7 | 37.7±9.1 | <0.01 |
| Mean Po2 day 1, mm Hg | 27.0±5.9 | 40.8±10.8 | <0.01 |
| Mean arterial pH day 1 | 7.37±0.09 | 7.40±0.10 | NS |
| ABGs per day, n | 7.7±1.8 | 6.0±2.3 | 0.08 |
| Time to surgery, d | 4.1±3.4 | 5.1±1.3 | NS |
Values are expressed as mean±SD where appropriate. TGA-IVS indicates dextro TGA with intact ventricular septum; dTGA-VSD, dextro TGA with ventricular septal defect; day 1, first day of life; and NS, not significant.
Student t testing was performed for continuous variables, and Fisher exact testing was performed on categorical variables to determine significance. The groups differed only in gender and arterial oxygenation.
For the BAS group, mean age at BAS was 17.1±19.1 hours. After BAS, arterial Po2 improved from a mean of 27.9±6.1 mm Hg before BAS to 38.7±4.0 mm Hg (P<0.01; Figure 1). In 5 of the 14 patients who underwent BAS, PGE1 was continued or restarted after BAS to maintain adequate arterial oxygenation. Patients undergoing BAS underwent arterial switch operation at a mean age of 4.1 days of life, compared with 5.1 days of life for the non-BAS patients (P=0.45).
Figure 1.
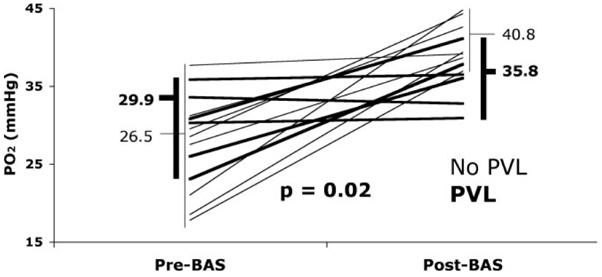
Arterial oxygenation before and after BAS. Six neonates were found to have PVL on MRI imaging (heavy lines). Those 6 patients with PVL had a mean improvement in arterial Po2 of 5.9 mm Hg compared with an improvement of 14.3 mm Hg in the no-PVL group (thin lines). Student t testing demonstrated a significant difference between the mean improvement in BAS patients with PVL compared with those who did not have PVL (P=0.02).
Stroke was seen in none of the patients on preoperative brain MRI; however, a total of 10 patients (38%) had PVL (Table 2). Of these 10 patients, the PVL was graded as mild in 3 patients and moderate in 7 (Figure 2). Clinically insignificant lesions found on brain MRI scan included mild choroid plexus hemorrhage (n=2), grade 1 intraventricular hemorrhage (n=6), and open operculum (n=8).
Table 2. Characteristics of Patients Found to Have Preoperative White Matter Brain Injury (PVL Group) Compared With Those Without Brain Injury (No PVL).
| PVL (n=10) | No PVL (n=16) | P | |
|---|---|---|---|
| BAS, n (%) | 6 (60) | 8 (50) | NS |
| Gestational age, wk | 39.0±1.2 | 38.9±1.1 | NS |
| Birth weight, kg | 3.41±0.59 | 3.50±0.64 | NS |
| Head circumference, cm | 33.1±1.6 | 33.8±1.6 | NS |
| TGA with VSD, n (%) | 3 (30) | 3 (19) | NS |
| Female, n (%) | 5 (50) | 6 (37) | NS |
| Preoperative measures | |||
| pH | 7.44±0.06 | 7.43±0.06 | NS |
| Pco2, mm Hg | 38.8±2.9 | 39.6±3.8 | NS |
| Po2, mm Hg | 36.9±1.5 | 41.9±5.0 | 0.026 |
| Hemoglobin, g/dL | 13.8±0.9 | 14.8±2.1 | NS |
| Base excess, mEq/L | 2.43±3.5 | 1.63±2.9 | NS |
| Lactate | 2.9±0.8 | 3.9±1.2 | NS |
| ABGs per day, n | 6.8±2.3 | 7.1±2.1 | NS |
| Lowest O2 saturation, % | 76.1±9.0 | 75.3±16.4 | NS |
| Time to surgery, d | 5.6±2.9 | 3.9±2.2 | 0.028 |
Values are expressed as mean±SD where appropriate. NS indicates P>0.15.
Student t testing was performed for continuous variables, and Fisher exact testing was performed on categorical variables to determine significance. Means are calculated based on values available from time of birth until time of surgical repair.
Figure 2.
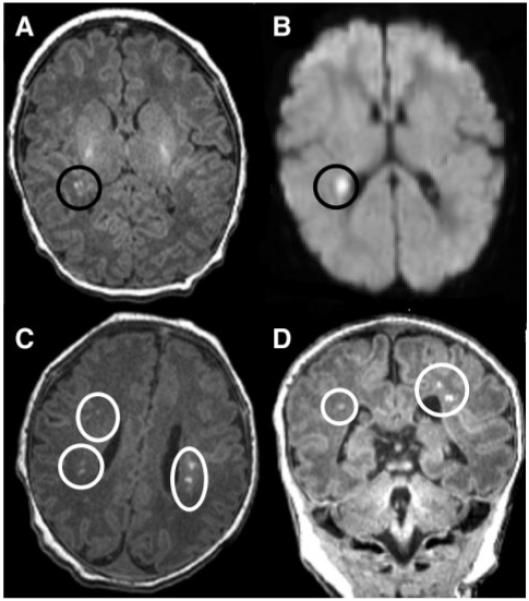
Brain MRI of preoperative infants with TGA: T1 imaging (A) and diffusion-weighted imaging (B) in a patient with mild PVL, which is a unifocal, small (<3 mm) white matter lesion. The lower MRI images demonstrate axial (C) and coronal (D) T1 imaging in a patient with bilateral, multifocal (moderate PVL) white matter disease.
Neonates found to have PVL were similar to the no-PVL group with regard to gestational age, birth weight, gender, head circumference, timing of diagnosis, and diagnosis (TGA with ventricular septal defect versus intact ventricular septum; Table 2). Furthermore, no significant differences were found between the PVL and no-PVL group with regard to mean arterial pH, lactate, Pco2, base excess, or continued use of PGE1. BAS was not associated with PVL; PVL was seen in 6 (43%) of the 14 patients who had undergone BAS, whereas PVL was seen in 4 (33%) of 12 patients without BAS (P=0.68). The 6 patients who underwent BAS and were found to have PVL had a smaller improvement in arterial Po2 after BAS (mean increase 5.9±6.6 mm Hg) than those who did not have PVL (mean increase 14.3±7.1 mm Hg, P=0.02; Figure 1).
Although performance of BAS was not associated with PVL, lower arterial oxygenation correlated strongly with the presence of PVL on preoperative brain MRI. Daily mean Po2 preoperatively was significantly lower for the PVL group than for the no-PVL group (P=0.02; Figure 3). The PVL group’s mean preoperative arterial Po2 remained below 40 mm Hg for the entire preoperative course. A multivariate analysis revealed that lower mean preoperative Po2 and longer time to surgery were associated with PVL and were additive risk factors for PVL (Figure 4). The PVL group had a mean preoperative Po2 of 36.9±1.5 mm Hg compared with 41.9±5.0 mm Hg for the no-PVL group (P=0.026). Furthermore, the PVL group underwent surgery at a mean age of 5.6±2.9 days, compared with 3.9±2.2 days for the no-PVL group (P=0.028). No clinical indications were identified that resulted in delay of surgery in the PVL group. Logistic regression analysis revealed that the probability of PVL was negatively associated with arterial Po2 and positively associated with time to surgery as described by the formula:
Figure 3.
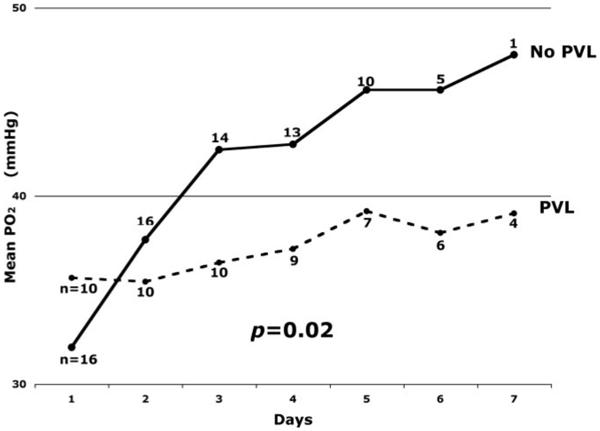
A daily mean Po2 was calculated for the PVL and no-PVL groups. Repeated-measures ANOVA demonstrated a significant difference in mean daily Po2 between the PVL group (dashed line) and the no-PVL group (solid line; P=0.02). The PVL group never achieved a mean daily Po2 >40 mm Hg.
Figure 4.
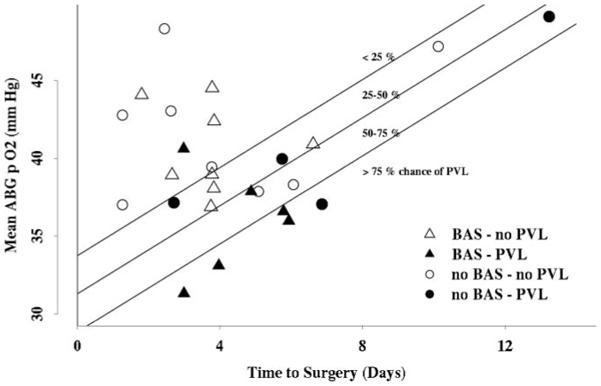
Risk model of PVL: Mean preoperative arterial Po2 is plotted against time to surgery for each patient in the entire cohort. Quartiles of risk were derived by logistic regression analysis. Patients who underwent BAS are represented by triangles, whereas those who did not undergo BAS are represented by circles. The patients found to have PVL (solid black characters) had lower preoperative Po2 and longer time to surgery.
Thus, lower mean Po2 and longer time to surgery were both independent risk factors for development of PVL. Using the nonlinear logistic link function (Figure 5), we can estimate probability of PVL based on values of the independent variables. For example, a neonate with a mean preoperative Po2 of 40 mm Hg who underwent surgery at day of life 4 would have an estimated log odds of PVL of -1.48, which translates to a probability of 20.3%, placing the patient in the 0% to 25% risk quartile. For that same case, a lower mean preoperative arterial Po2 of 1 mm Hg increases the probability by 8.2 percentage points to 28.5%. On the other hand, surgery 1 day later increases the probability by 12.1 percentage points to 32.4%.
Figure 5.

Logistic link function estimating risk of PVL by preoperative Po2 and time to surgery. The probability of PVL (y-axis) rises with the log odds (x-axis), which is derived with the equation: Log odds=14.04-0.45 (Po2)+0.63 (days to surgery). The effects of oxygenation and time to surgery are linear in the log-odds scale (x-axis) but are nonlinear in the probability scale (y-axis).
Discussion
BAS was introduced in 1966 by William Rashkind as a palliative technique to improve systemic and pulmonary arterial mixing in neonates with TGA.20,21 In the infant with unrepaired TGA, delivery of oxygen to tissues, including the brain, is dependent on mixing between the systemic and pulmonary circulations. In the presence of a restrictive or intact atrial septum, infants with TGA may develop profound hypoxemia and severe lactic acidosis. In these infants, ductal patency, maintained with PGE1, may not provide adequate mixing, and BAS may be indicated. Successful BAS improves systemic oxygenation by allowing for atrial-level mixing of the parallel systemic and pulmonary circulations until surgical correction can be performed. In many cases, PGE1 can be discontinued after successful BAS.
The recent published findings of McQuillen et al17,18 report a significant risk of stroke associated with performance of BAS. Although we did not detect this form of brain injury in any of the patients in the present study, these reports highlight the increased risk in these patients of cerebral embolus from any source, including intravenous access and interventional catheterization. In comparing the 2 studies, it should be noted that the MRI scans in the present study were done immediately before surgery, at a mean age of 4.6±2.6 days, whereas in the aforementioned study, preoperative MRI scans were performed “as soon as the baby was stable enough.”17 Thus, lack of stroke in the present study is not due to “missing” strokes that might have occurred, or been more apparent, later in the preoperative course. In fact, some cases of PVL may have gone undiscovered in the previous report because the MRI scans were performed earlier in the preoperative course. Despite the potential risks associated with BAS, it is often a necessary palliation in the infant with profound hypoxemia and acidosis. In the neonate with hypoxemia but without acidosis, the potential risks of BAS must be weighed against the potential benefits. The present study suggests that infants in this condition are at ongoing risk of brain injury. Importantly, the brain injury incurred as a result of hypoxemia (PVL) has been demonstrated to increase the risk of adverse neurocognitive outcomes in preterm infants.22
A surprising lack of consensus exists on the classification of lesions found on brain MRI in neonates. Stroke and PVL are forms of brain injury with different causes and anatomic distributions. Stroke, or focal infarct, is seen in discrete arterial territories. White matter injury (PVL), on the other hand, is thought to represent watershed infarct that occurs in the setting of hypoxemia and/or cerebral hypoperfusion.2,23 Furthermore, because PVL can be a watershed event, the full extent of the white matter injury may be underappreciated on routine MRI sequences.24,25 In the present study, PVL was defined as white matter injury in the periventricular area that forms the watershed between cortical perforating arteries and the ventriculofugal arteries that penetrate the white matter at right angles from the ventricle surface.26,27 Stroke was defined as any cortical or subcortical lesion in an arterial or endarterial distribution. Stroke was not seen among the cohort of patients with TGA but was seen in the larger prospective cohort, which included neonates with hypoplastic left heart syndrome. Among the 10 patients in the present study who were found to have white matter disease, 7 had moderate (bilateral and multifocal) PVL. The present report identifies a significantly higher incidence of white matter injury than all prior published reports. The reasons for this are unclear but may relate to institutional differences in the classification of brain injury.
The issue of neurological injury in neonates with TGA is significant given the literature highlighting cognitive, motor, and behavioral impairments in these children when they reach school age.15,28 Despite the lack of consensus on classification of the lesions found on brain MRI, a number of studies consistently demonstrate that neonates with CHD are at risk for preoperative brain injury.3,19,29,30 The McQuillen study17 and the present study found a similar rate of preoperative brain injury if all types of injury were included. The initial study by McQuillen et al reported an association between BAS and all forms of brain injury, focusing on embolic mechanisms. A subsequent report with an expanded cohort found that BAS was only associated with stroke, not PVL.18 These data might suggest that BAS should be avoided in neonates with unrepaired TGA. An alternative interpretation might be that the most profoundly hypoxic infants who are selected to receive the intervention are at the highest risk for hypoxic injury to white matter. Consistent with this, the present data indicate that preoperative brain injury is related to severity and duration of exposure to hypoxemia. This alternate interpretation may better explain the fact that 5 (42%) of 12 patients in the McQuillen study with brain injury had white matter injury. The present findings are significant because these factors (oxygenation and time to surgery) are potentially modifiable risk factors. Lower oxygenation can be treated with BAS, continued use of PGE1 infusion, or both, and time to surgery can be shortened in specific cases.
Recent reports have drawn attention to the “shared selective vulnerability” of the cerebral white matter in the preterm neonatal brain and that of the term infant with complex CHD.4,19 This vulnerability may explain in part the frequency of PVL in term neonates with prolonged hypoxemia secondary to CHD. Although some investigators have concluded that white matter injury in these neonates may occur primarily in utero because of altered circulation patterns, evidence exists that further white matter injury may occur postnatally and postoperatively in neonates with TGA.1,3 In a prior study by Galli et al,1 PVL correlated with perioperative cerebral arterial oxygen content in a heterogeneous population of neonates with CHD. In that study, more than 50% of neonates (44 of 82) undergoing surgery for complex CHD were found to have PVL. A postoperative mean arterial Po2 <40 mm Hg was associated with increased risk of PVL. In the present study, the PVL group had a preoperative mean Po2 of 36.9 mm Hg, and as a group, they never achieved a mean daily Po2 >40 mm Hg. Thus, preoperative and early postoperative hypoxemia appears to be associated with the development of PVL. Interventions to optimize cerebral oxygenation before corrective surgery may in fact prove neuroprotective.
In the present study, we report a PVL incidence of 38% in a cohort of preoperative neonates with TGA deemed hemodynamically stable and without signs of end-organ damage. Stroke was not seen in any of the 26 patients in the present cohort, although in the larger prospective cohort including infants with hypoplastic left heart syndrome, stroke was seen. Preoperative brain injury in the present cohort was associated with lower arterial oxygenation. Furthermore, neonates with prolonged exposure to cyanosis but who did not undergo BAS were also at risk of PVL. The mechanisms responsible for PVL are complex and include inflammation, infection, impaired cerebrovascular autoregulation, and ischemia. Although we have demonstrated an association between 2 clinical variables and PVL, the present data do not prove a causal relationship. Yet, our findings are significant because of the potential impact on preoperative care of the infant with TGA. These data support the practice of BAS to improve mixing and to maximize systemic oxygen delivery in select newborns with TGA. This procedure, when successful at improving arterial oxygenation, decreased patient risk of preoperative white matter injury.
Improvements in the preoperative care of neonates with TGA and stabilization of oxygenation with PGE1 have resulted in many infants undergoing arterial switch operation on a semielective basis. Although continuing the use of PGE1 after BAS may aid in optimizing oxygenation, prolonged use of PGE1 to augment systemic oxygenation can be problematic because of its effects in lowering diastolic blood pressure. After successful BAS to improve systemic oxygenation, neonates often are considered adequately stable to await surgery for a number of days or longer. The data from the present study support the practice of performing arterial switch operation as early as is feasible, with or without BAS in selected cases, in an effort to optimize long-term neurodevelopmental outcomes.
Study Limitations
This is a retrospective study with a limited sample size of 26 infants, but it is similar in size to other published reports. Although the brain MRI scans were obtained in a prospective manner and interpreted in a blinded fashion, the laboratory and bedside data were reviewed retrospectively. Comparison of ABG summaries among patients was complicated by the fact that patients had a greater number of samples during times of instability and fewer during periods of stability. Furthermore, bedside recording of the arterial pulse oximetry may not truly reflect all values observed during a particular time interval. Blood pressure data, important in postoperative PVL, were not evaluated in the present study. A major difference between the present study and prior reports is that patients presenting with signs of significant preoperative hemodynamic instability were excluded. Thus, a possibility exists that the true incidence of preoperative brain injury is underestimated in the larger population of neonates with TGA.
Conclusions
Preoperative brain injury in neonates with TGA was not adversely associated with BAS in the present study. We found no neonates with preoperative stroke, although 38% of the neonates had preoperative PVL. The risk of PVL increased with longer time to surgical repair and with lower arterial oxygenation. In contrast to previous reports, we found that BAS was protective against PVL when the procedure resulted in improved oxygenation. A multicenter prospective study or registry is recommended to further clarify risk factors for preoperative brain injury in this vulnerable patient population.
Acknowledgments
Sources of Funding
Funding for this study was provided by a K-23 grant from the National Institutes of Neurological Diseases and Stroke (NINDS, NIH), awarded to Dr Licht.
Footnotes
Disclosures
None.
References
- 1.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 2.Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, Tabbutt S, Durning SM, Shera DM, Gaynor JW, Spray TL, Clancy RR, Zimmerman RA, Detre JA. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841–849. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kurth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(suppl):I-109–I-114. [PubMed] [Google Scholar]
- 4.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 5.Partridge SC, Vigneron DB, Charlton NN, Berman JI, Henry RG, Mukherjee P, McQuillen PS, Karl TR, Barkovich AJ, Miller SP. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640–651. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 6.Manzar S, Nair AK, Pai MG, Al-Khusaiby SM. Head size at birth in neonates with transposition of great arteries and hypoplastic left heart syndrome. Saudi Med J. 2005;26:453–456. [PubMed] [Google Scholar]
- 7.Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol. 1996;143:505–513. doi: 10.1093/oxfordjournals.aje.a008771. [DOI] [PubMed] [Google Scholar]
- 8.Song Z, Awate SP, Licht DJ, Gee JC. Clinical neonatal brain MRI segmentation using adaptive nonparametric data models and intensity-based Markov priors. Med Image Comput Comput Assist Interv Int Conf. 2007;10:883–890. doi: 10.1007/978-3-540-75757-3_107. [DOI] [PubMed] [Google Scholar]
- 9.Jouannic JM, Benachi A, Bonnet D, Fermont L, Le BJ, Dumez Y, Dommergues M. Middle cerebral artery Doppler in fetuses with transposition of the great arteries. Ultrasound Obstet Gynecol. 2002;20:122–124. doi: 10.1046/j.1469-0705.2002.00756.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32–36. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 11.Licht DJ, Wang J, Silvestre DW, Agner SC, Tabbutt S, Nicolson SC, Montenegro LM, Wernovsky G, Shera DM, Spray TL, Zimmerman RA, Gaynor JW, Clancy RR, Detre JA. Reduced preoperative cerebrovascular reactivity in infants with severe congenital heart defects is associated with periventricular leukomalacia. Stroke. 2006;37:641. Abstract. [Google Scholar]
- 12.Krull F, Latta K, Hoyer PF, Ziemer G, Kallfelz HC. Cerebral ultrasonography before and after cardiac surgery in infants. Pediatr Cardiol. 1994;15:159–162. doi: 10.1007/BF00800668. [DOI] [PubMed] [Google Scholar]
- 13.Miller SP, McQuillen PS, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM, Hamrick SE, Azakie A, Karl TR. Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg. 2004;77:1698–1706. doi: 10.1016/j.athoracsur.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 14.Te Pas AB, van Wezel-Meijler G, Bokenkamp-Gramann R, Walther FJ. Preoperative cranial ultrasound findings in infants with major congenital heart disease. Acta Paediatr. 2005;94:1597–1603. doi: 10.1111/j.1651-2227.2005.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 15.Bellinger DC, Wypij D, duDuplessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 16.Karl TR, Hall S, Ford G, Kelly EA, Brizard CP, Mee RB, Weintraub RG, Cochrane AD, Glidden D. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2004;127:213–222. doi: 10.1016/j.jtcvs.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 17.McQuillen PS, Hamrick SE, Perez MJ, Barkovich AJ, Glidden DV, Karl TR, Teitel D, Miller SP. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–285. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 18.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, Azakie A, Karl T, Miller SP. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 19.Licht DJ, Agner SC, Montenegro LM, Nicolson SC, Silvestre DW, Tabbutt S, Detre JA, Gaynor JW, Wernovsky G, Wang J, Clancy RR, Zimmerman RA. Pre-operative MRI abnormalities are common in full-term infants with severe congenital heart defects and resemble lesions in pre-term infants. Neuropediatrics. 2006;37:S1. Abstract. [Google Scholar]
- 20.Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy: a palliative approach to complete transposition of the great arteries. JAMA. 1966;196:991–992. [PubMed] [Google Scholar]
- 21.Rashkind WJ, Miller WW. Transposition of the great arteries: results of palliation by balloon atrioseptostomy in thirty-one infants. Circulation. 1968;38:453–462. doi: 10.1161/01.cir.38.3.453. [DOI] [PubMed] [Google Scholar]
- 22.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 23.Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, Rivkees SA. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60:696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- 24.Felderhoff-Mueser U, Rutherford MA, Squier WV, Cox P, Maalouf EF, Counsell SJ, Bydder GM, Edwards AD. Relationship between MR imaging and histopathologic findings of the brain in extremely sick preterm infants. Am J Neuroradiol. 1999;20:1349–1357. [PMC free article] [PubMed] [Google Scholar]
- 25.Prager A, Roychowdhury S. Magnetic resonance imaging of the neonatal brain. Indian J Pediatr. 2007;74:173–184. doi: 10.1007/s12098-007-0012-3. [DOI] [PubMed] [Google Scholar]
- 26.Volpe JJ. Neurology of the Newborn. 4th ed. WB Saunders; Philadelphia, Pa: 2005. [Google Scholar]
- 27.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 29.Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:991–1000. [PubMed] [Google Scholar]
- 30.Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005;110:563–578. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]


