Abstract
Objectives
Nano-particles of dicalcium phosphate anhydrous (DCPA) were synthesized in our laboratory for the first time and incorporated into a dental resin. Our goal was to develop a mechanically strong dental composite that has Ca and PO4 ion release to combat tooth caries, and to investigate the effects of whisker reinforcement, DCPA particle size and silanization.
Methods
DCPA nano-particles and two larger DCPA particles were used with nano-silica-fused whiskers as fillers in a resin matrix. Composite mechanical properties were measured via three-point flexure, and the release of Ca and PO4 ions were measured vs. time.
Results
Using DCPA nano-particles with a diameter of 112 nm, the composite at a DCPA:whisker mass ratio of 1:1 had a flexural strength (mean ± sd; n = 5) of (112 ± 17) MPa, not significantly different from (112 ± 14) MPa of a commercial non-releasing composite; both were higher than (29 ± 7) MPa for the composite at DCPA:whisker of 1:0 (p < 0.05). The composite with DCPA particle size of 112 nm released Ca to a concentration of 0.85 mmol/L and PO4 of 3.48 mmol/L, higher than Ca of 0.67 mmol/L and PO4 of 1.11 mmol/L using DCPA with 12 μm particle size (p < 0.05). Silanization of DCPA increased the composite strength at DCPA:whisker of 1:0 compared to that without silanization, but decreased the Ca and PO4 release (p < 0.05). Increasing the DCPA particle surface area increased the Ca and PO4 release.
Significance
Decreasing the DCPA particle size increased the Ca and PO4 release; whisker reinforcement increased the composite strength by 2 to 3 fold. The nano DCPA-whisker composites, with high strength and Ca and PO4 release, may provide the needed, unique combination of stress-bearing and caries-inhibiting capabilities.
Keywords: dental composite, nanoparticles, whisker reinforcement, particle size, flexural strength, elastic modulus, tooth caries inhibition, Ca and PO4 ion release
1. Introduction
Dental resin composites are composed of reinforcing fillers in an acrylic monomer matrix that is polymerized to form a solid restoration [1–6]. Resin compositions, fillers and cure conditions have been significantly improved [7–11]. However, recent reports on composite restorations still indicate that “The two main challenges are secondary caries and bulk fracture” [12,13]. Secondary caries at the tooth restoration margins is the most frequent reason for replacement of existing restorations [14]. Replacement of existing restorations accounts for about 70 % of all operative work [15]. Replacement dentistry costs about $5 billion/year in the U. S. alone [16]. Therefore, there is a major need to develop advanced tooth restorative materials that can not only resist fracture, but also inhibit secondary caries.
Several calcium-phosphate phases are regarded as biological precursors that form initially and then transform to apatites [17]. Hydroxyapatite [Ca10(PO4)6(OH)2], the structural prototype of the major mineral component of teeth and bones, is the final stable product in the precipitation of calcium and phosphate ions from neutral or basic solutions (pH of 7 to 9 at 37 °C) [17]. Hence there is a wide range of solution conditions when the precipitation of hydroxyapatite occurs spontaneously. Indeed, several bioactive composites have been developed that release calcium and phosphate ions in the presence of an aqueous solution, which then precipitate to form hydroxyapatite [18–21]. The calcium ions are conveniently referred to as Ca, and the phosphate ions as PO4, following previous studies [20,21]. These therapeutic composites have been shown to effectively remineralize enamel and dentin lesions in vitro. However, the calcium phosphate fillers do not reinforce the resin like the glass fillers. For example, a calcium phosphate composite had a flexural strength of about half of the strength of the unfilled resin [19]. Such low strengths were recognized as being “inadequate to make these composites acceptable as bulk restoratives” [20].
Recent studies have demonstrated the reinforcement efficacy of nano-silica-fused, silicon nitride and silicon carbide whiskers, yielding dental composites with strength and fracture toughness nearly 2-fold higher than those of commercial stress-bearing composites [22]. The whisker composites have shown superior performance in thermal cycling [23], water-aging [24], and three-body wear [25]. The whisker composites have been shown to be non-cytotoxic and supported cell attachment and proliferation in vitro [26].
Nano-particles of hydroxyapatite, α-tricalcium phosphate (α-TCP) and β-tricalcium phosphate (β-TCP) have been synthesized previously [27–29]. Nano-particles of DCPA were never reported until recently, when they were synthesized via a spray-drying technique in our laboratory for the first time [30–32]. These nano-particles were incorporated into dental resin composites which showed promise for both caries-inhibiting and stress-bearing capabilities [32]. However, the effects of DCPA particle size and silanization have not been investigated.
Previous studies on calcium phosphate composites used single particle sizes (e.g., a particle size of 1.1 μm for dicalcium phosphate anhydrous [CaHPO4], or DCPA) [21], without varying the particle size and examining this effect. Previous studies used unsilanized calcium phosphate fillers, without investigating whether the silane would improve the composite mechanical properties or retard the release of Ca and PO4 ions. The silane coupling agent was a bifunctional coating on the filler that would enhance the bonding between the filler and the resin matrix. Hence another issue to be investigated would be the effect of silanization of the releasing fillers on the Ca and PO4 release and the composite mechanical properties.
Accordingly, the objectives of the present study were to investigate the effects of reinforcement, particle size and silanization in the process of developing dental nano-composites with high strength and Ca and PO4 release capabilities. It was hypothesized that when DCPA particles and nano-silica-fused whiskers were both used as fillers in the resin, the former would release Ca and PO4 ions, while the later would provide the needed strength. It was further hypothesized that the DCPA particle size and the silane treatment would significantly affect the composite mechanical properties and the Ca and PO4 release.
2. Materials and methods
2.1 Fillers
Two types of fillers were used: DCPA particles, and nano-silica-fused whiskers.
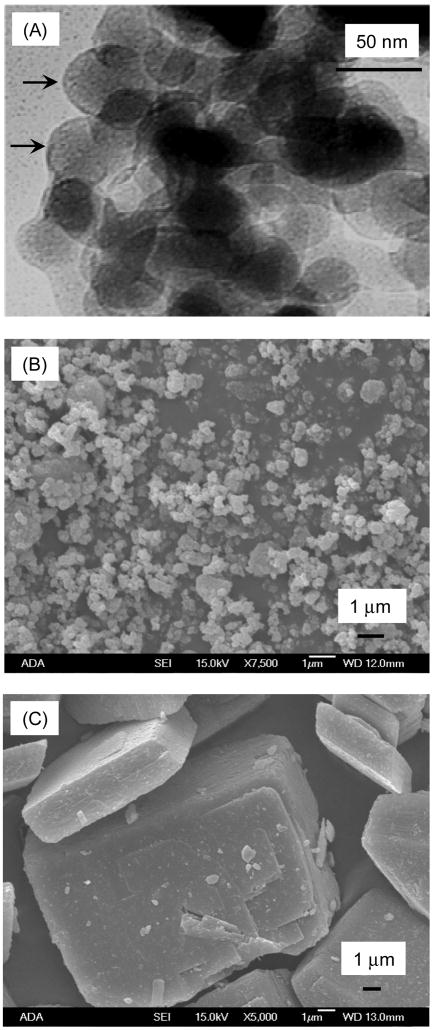
The DCPA nano-particles were synthesized via a spray-drying technique in our laboratory [30–32]. X-ray diffraction indicated that the powder was DCPA [32]. This nano DCPA powder was used in the present study along with two other DCPA powders, as listed in Table 1. A commercial DCPA powder was also used as fillers for the resin (Baker Analyzed Reagents, J. T. Baker Chemical, Phillipsburg, NJ). To obtain a finer powder, the as-received commercial DCPA was ball-milled (Retsch PM4, Brinkman, NY) in ethanol for 24 h to obtain a fine DCPA powder. For the as-received DCPA and the ground DCPA in Table 1, the particle size distribution was measured by a sedimentation method with the use of a centrifugal particle analyzer (SA-CP3, Shimazu, Kyoto, Japan). The nano DCPA could not be analyzed using the particle analyzer, hence the specific surface area of the nano powder was measured using multipoint-BET (AUTOSORB-1, Quantachrome Instruments, Boynton Beach, FL).
Table 1.
The three DCPA powders used as fillers in dental composites*
| Manufacturer | Median Particle Size | Particle Size Range | Appearance | |
|---|---|---|---|---|
| Nano DCPA | Synthesized in our lab | 112 nm (0.112 μm) | Approximately 50 nm to 200 nm | Fig. 1A |
| Ground DCPA | Grinding the Baker powder for 24 h | 0.88 μm | 0.1 μm to 2.0 μm | Fig. 1B |
| As-received DCPA | Baker Analyzed Reagents, J. T. Baker Chemical, Phillipsburg, NJ | 12.0 μm | 1 μm to 60 μm | Fig. 1C |
The particle size of nano DCPA was calculated from the BET-surface area measurement (AUTOSORB-1, Quantachrome Instruments, Boynton Beach, FL). The other DCPA particle sizes were measured by a sedimentation method with the use of a centrifugal particle analyzer (SA-CP3, Shimazu, Kyoto, Japan).
Silicon nitride whiskers were obtained from a commercial source (α-Si3N4, Nanostructured and Amorphous Materials Inc., Los Alamos, NM). Because the particle size analyzer yielded only an equivalent spherical particle diameter, the whiskers were examined using a scanning electron microscope (SEM, 5300, JEOL, Peabody, MA). The average whisker length of 100 randomly-selected whiskers was measured to be 14 μm, with a range from approximately 3 μm to 55 μm. The average whisker diameter was 0.5 μm, with a range from about 0.1 μm to 1.0 μm. The whiskers were mixed with silica (Aerosil-OX50, Degussa, Ridgefield, NJ) having a diameter of about 40 nm. The silicon nitride:silica mass ratio was 5:1. The mixture was heated in a furnace at 800 °C for 30 min to fuse the silica onto the whiskers. This method did not produce a monolayer of silica on the whiskers as some silica agglomeration occurred [Fig. 1A, Ref. 25]; however, it did yield a resin composite with high strength, fracture toughness and wear resistance [25]. The powder was then silanized with 4 % 3-methacryloxypropyltrimethoxysilane and 2 % n-propylamine (mass fractions) [25].
Figure 1.
Microscopy of the three DCPA powders. (A) TEM microscopy of nano-particles of DCPA with arrows indicating particles of about 50 nm in size. (B) SEM of a commercial DCPA powder ground for 24 h, yielding a median particle diameter of 0.88 μm. (C) SEM of the as-received commercial DCPA powder with a median diameter of 12.0 μm.
The nano-silica-fused whiskers in the present study were always silanized. The DCPA powders were either silanized or unsilanized, as specified below.
2.2 Resin composite fabrication
A monomer consisting of 48.975 % Bis-GMA (bisphenol glycidyl dimethacrylate), 48.975% TEGDMA (triethylene glycol dimethacrylate), 0.050 % 2,6-di-tert-butyl-4-methylphenol, and 2.000 % benzoyl peroxide formed part I, the initiator, of a two-part chemically-activated resin [32]. Part II, the accelerator resin, consisted of 49.5 % Bis-GMA, 49.5 % TEGDMA, and 1.0 % N,N-dihydroxyethyl-p-toluidine.
The fillers consisted of nano-silica-fused whiskers and DCPA particles at a mass ratio of DCPA:whisker of 1:1. This ratio was selected following a previous study [32]. The filler mass fraction ([DCPA + whiskers]/[DCPA + whiskers + resin]) was 65 %, selected to achieve a relatively flowable paste. Equal masses of the two pastes, part I and part II, were mixed and filled into a rectangular stainless steel mold with (2 × 2 × 25) mm3 dimensions. The specimens were incubated at 37 °C with a relative humidity of approximately 50 % for 24 h prior to testing.
Two groups of specimens were fabricated. The first group was made to study the effects of DCPA particle size and whisker reinforcement. A 3 × 2 full factorial design was used with two levels of whisker reinforcement (DCPA:whisker mass ratio = 1:0 or no whiskers; DCPA:whisker = 1:1), and three levels of DCPA particle size (112 nm, 0.88 μm, 12.0 μm). The DCPA powders were not silanized so that the Ca and PO4 release would not be inhibited, following previous studies on calcium phosphate composites [19,21,32]. The nano-silica-fused whiskers were silanized to provide maximum reinforcement.
The second group was to investigate the effect of DCPA silanization. A 2 × 2 full factorial design was used with two levels of silanization for DCPA (silane treatment; no silane), and two levels of whiskers (DCPA:whisker mass ratio = 1:0; DCPA:whisker mass ratio = 1:1). The DCPA with a particle size of 0.112 μm was used for this experiment because preliminary studies showed that the composite filled with this DCPA released more Ca and PO4 than the other two DCPA powders.
A hybrid composite (TPH, Caulk/Dentsply, Milford, DE) was used as a non-releasing control. It consisted of a (hybrid) combination of barium silicate glass particles and fumed silica particles, with a mean particle size of about 0.8 μm, at 78 % filler level by mass in a urethane-modified Bis-GMA-TEGDMA resin. The specimens were photo-cured (Triad-2000, Dentsply, York, PA) for 1 min on each open side of the specimen using the same stainless steel molds.
2.3 Flexural testing
The flexural strength and elastic modulus of the composites were measured using a three-point flexural test with a 20 mm span at a crosshead-speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC). The flexural strength was calculated by S = 3 Pmax L/(2 b h2), where Pmax is the maximum load on the load-displacement curve, L is flexure span, b is specimen width, and h is specimen thickness [33]. The elastic modulus was calculated by E = (P/d) (L3/[4 b h3]), where the load P divided by the corresponding displacement d is the slope of the load-displacement curve in the linear elastic region [34]. The standard uncertainty of the flexural measurement was estimated to be 1 % due to specimen dimension measurement [35].
2.4 Ca and PO4 release
A NaCl solution (133 mmol/L) buffered with 50 mmol/L HEPES (pH = 7.4; 37 °C) was used. Following a previous study [32], three specimens of approximately 2 mm × 2 mm × 12 mm were immersed in 50 mL solution, yielding a specimen volume/solution of 2.9 mm3/mL. This compared to a specimen volume per solution of approximately 3.0 mm3/mL in a previous study [19]. The concentrations of Ca and PO4 released from the specimens were measured vs. immersion time: 1 day (d), 2 d, 4 d, 7 d, 14 d, 21 d, 28 d, 35 d, 42 d, 49 d, and 56 d. At each time period, aliquots of 0.5 mL were removed and replaced by fresh solution. The aliquots were analyzed for Ca and PO4 concentrations via spectrophotometric methods (DMS-80 UV-visible, Varian, Palo Alto, CA) following previous studies [19,21]. The standard uncertainty for the Ca and PO4 release measurements was estimated to be 3 % [36].
Transmission electron microscopy (TEM, 3010-HREM, JEOL, Peabody, MA) was used to examine the nano DCPA powder. To minimize agglomeration, an acetone suspension of the nano-particles was ultrasonicated and drops of the suspension were deposited onto the TEM grids. The other DCPA powders were sputter coated with gold and examined with the SEM. One-way and two-way ANOVA were performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used to compare the measured data at a p value of 0.05.
3. Results
The DCPA nano-particles are shown in the TEM micrograph in Fig. 1A. The arrows indicate particles of about 50 nm in size. Some agglomerates were up to 200 nm in size. Due to agglomeration, the BET surface-area measurement yielded a particle diameter of 112 nm. Grinding the as-received DCPA yielded a fine powder as shown in the SEM micrograph in (B); it had a median particle diameter (50th percentile by mass) of 0.88 μm, and a range from 0.1 μm to 2.0 μm. The as-received DCPA powder is shown in (C); it had a median particle diameter of 12.0 μm, with a range from 1 μm to 60 μm.
Effect of DCPA particle size and DCPA:whisker ratio on mechanical properties
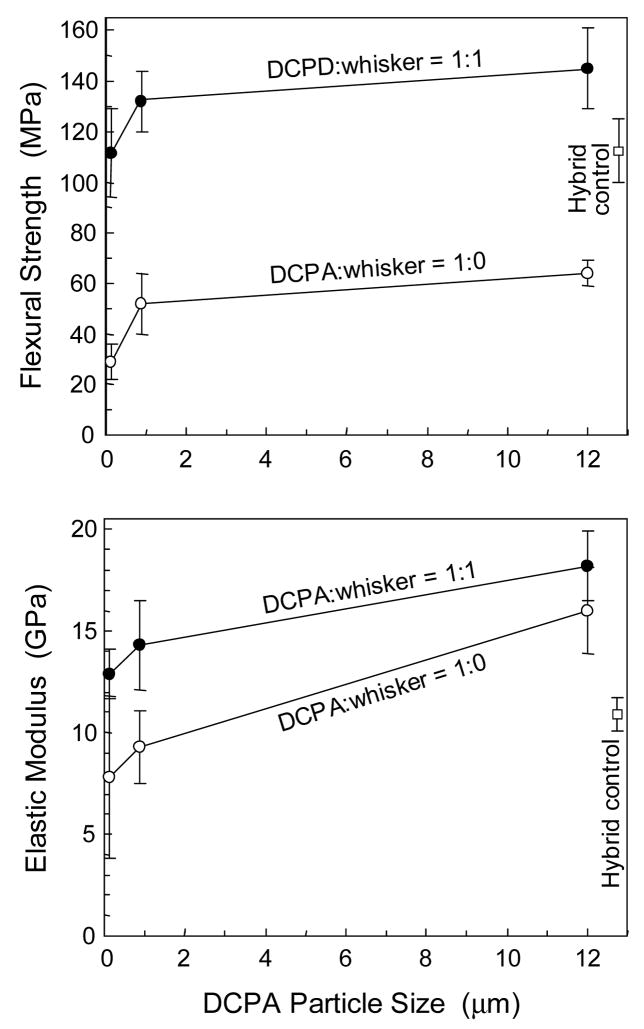
The flexural testing results are plotted in Fig. 2. Two-way ANOVA showed significant effects of particle size and whisker reinforcement (p < 0.05), with no significant interaction between the two variables (p > 0.1). Increasing the DCPA particle size significantly increased the composite strength and elastic modulus (p < 0.05). At each particle size, the composite at a DCPA:whisker ratio of 1:1 had significantly higher strength and elastic modulus than the composite at a DCPA:whisker ratio of 1:0 (p < 0.05), except for the modulus of composites containing 12-μm DCPA (p > 0.10). The hybrid control composite had a flexural strength (mean ± sd; n = 5) of (112 ± 14) MPa, significantly higher then those of the composite at a DCPA:whisker ratio of 1:0 (p < 0.05). However, the composite at a DCPA:whisker ratio of 1:1 using the 12-μm DCPA particles had a strength of (147 ± 16) MPa, significantly higher than that of the hybrid control (p < 0.05). The composite at a DCPA:whisker ratio of 1:1 using DCPA particles of 112 nm had a strength of (112 ± 17) MPa, not significantly different from that of the hybrid control (p > 0.1).
Figure 2.
Mechanical properties of the composites. Each value is the mean of five measurements with the error bar showing one standard deviation (mean ± sd; n = 5). “Whisker” refers to the nano-silica-fused whiskers that were silanized. DCPA was not silanized. The DCPA:whisker number is a mass ratio. The hybrid control is a commercial composite (TPH) with no release of Ca or PO4. Decreasing the DCPA particle size slightly decreased the composite properties; whisker reinforcement substantially improved the composite mechanical properties.
The elastic modulus of the composite using a DCPA particle size of 112 nm with DCPA:whisker of 1:1 was (12.9 ± 1.3) GPa, not significantly different from the (10.9 ± 0.8) GPa of the hybrid control (p > 0.1). Both were significantly lower than the (18.2 ± 1.7) GPa of the composite with DCPA:whisker of 1:1 and a DCPA particle size of 12 μm (p < 0.05).
Effect of DCPA silanization on mechanical properties
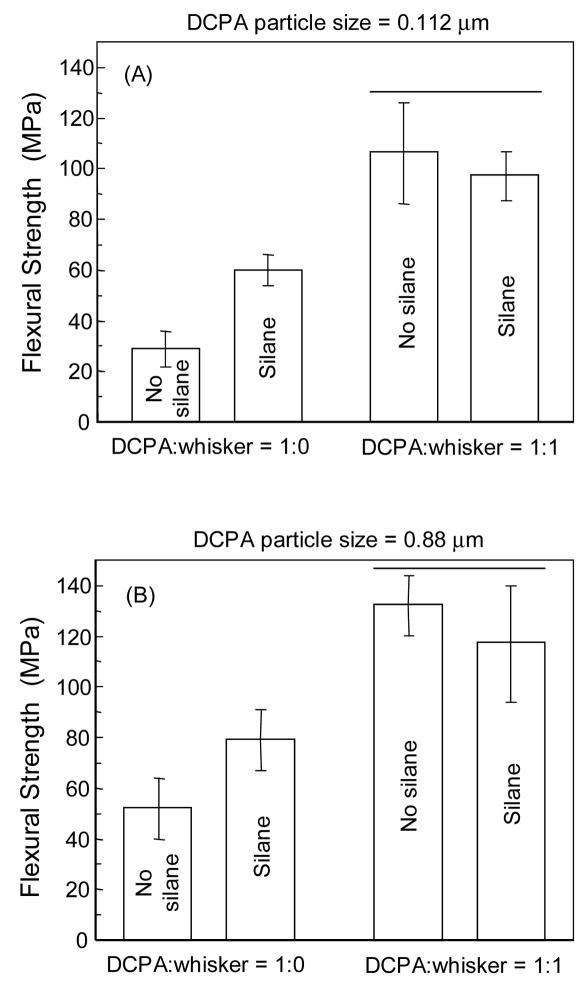
In Fig. 3A, the nano-silica-fused whiskers were silanized, and the DCPA particles (particle size of 112 nm) were either silanized or unsilanized. Two-way ANOVA showed significant effects of silanization and whisker reinforcement (p < 0.05), with a significant interaction between the two parameters (p < 0.05). The strength of composite containing silanized DCPA at DCPA:whisker of 1:0 was significantly higher than that containing unsilanized DCPA (p < 0.05). However, at DCPA:whisker of 1:1, whether the DCPA was silanized or not had no significant effect on strength (p > 0.1). In addition, the composites at DCPA:whisker of 1:1 had higher strength than those at DCPA:whisker of 1:0, whether the DCPA was silanized or not.
Figure 3.
Effect of DCPA silanization on composite strength. “Whisker” refers to the nano-silica-fused whiskers that were silanized. DCPA was either silanized or not silanized. Each value is mean ± sd; n = 5. At DCPA:whisker of 1:0, DCPA silanization increased the composite strength. At DCPA:whisker of 1:1, DCPA silanization had no significant effect. These trends were true for both 0.112-μm DCPA (A) and 0.88-μm DCPA (B). The horizontal bar indicates values that are not significantly different (p > 0.1).
To confirm the effect of silanization on the composites with DCPA particle size of 112 nm observed in Fig. 3A, the test was also performed on the composite with DCPA particles of 0.88 μm. The data are plotted in Fig. 3B, showing a similar silanization effect as in Fig. 3A.
Effect of DCPA particle size and DCPA:whisker ratio on Ca and PO4 release
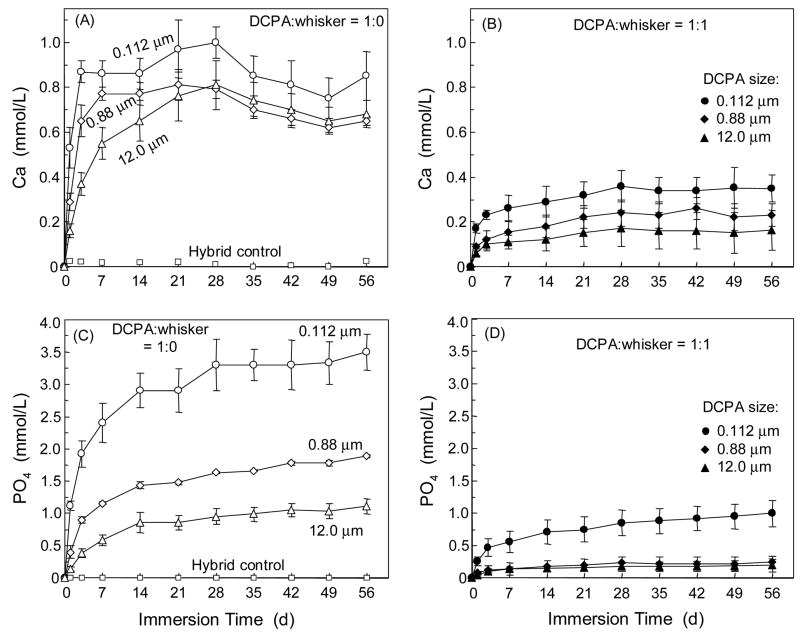
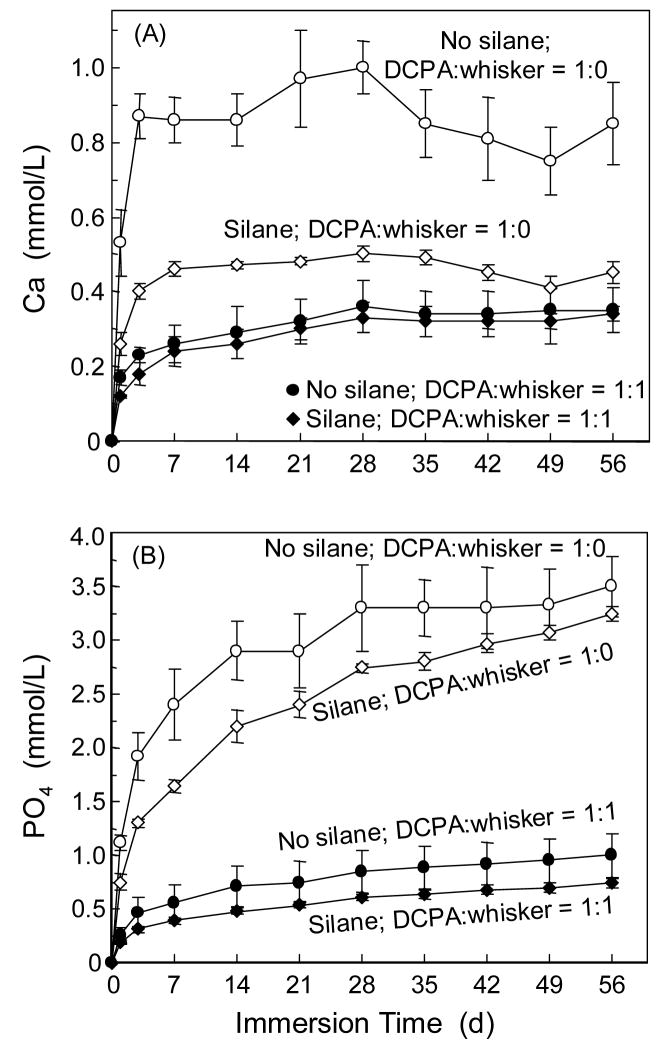
The effects of DCPA size and DCPA:whisker ratio on the amount of Ca and PO4 release are shown in Fig. 4. Two-way ANOVA showed significant effects of DCPA particle size and whisker reinforcement (p < 0.05); there was a significant interaction between the two variables (p < 0.05). The nano-composite with a DCPA particle size of 112 nm released significantly more Ca and PO4 than the other two composites with DCPA sizes of 0.88 μm and 12 μm. The composites with no whiskers released more Ca and PO4 than those with whiskers at the same DCPA particle size. The amount of Ca and PO4 release was increased with time in the first month. Then the PO4 concentration started to plateau, while the Ca concentration decreased in Fig. 4A. At 56 d, the Ca concentration (mean ± sd; n = 3) for the composite with DCPA size of 112 nm was (0.85 ± 0.11) mmmol/L at DCPA:whisker of 1:0, significantly higher than (0.35 ± 0.06) mmol/L at DCPA:whisker of 1:1 (p < 0.05). For the composite with DCPA size of 112 nm, the PO4 concentration was (3.48 ± 0.28) mmol/L at DCPA:whisker of 1:0, significantly higher than (0.99 ± 35) mmol/L at DCPA:whisker of 1:1 (p < 0.05). The commercial hybrid control had no detectable Ca or PO4 release.
Figure 4.
Ca and PO4 release: effect of DCPA particle size. “Whisker” refers to the silanized nano-silica-fused whiskers. DCPA was not silanized. The numbers (e.g., 12 μm, refer to the DCPA particle size). Each value is mean ± sd; n = 3. The nano-composite with DCPA particle size of 0.112 μm had significantly higher Ca and PO4 release than the other two composites with DCPA sizes of 0.88 μm and 12 μm. The hybrid control composite had no detectable Ca or PO4 release.
Effect of DCPA silanization on Ca and PO4 release
Fig. 5 shows the effect of silanization on the amount of Ca and PO4 released from the composites with DCPA:whisker mass ratios of 1:0 and 1:1, respectively, both using the DCPA with a particle size of 112 nm. Two-way ANOVA showed significant effects of silanization and whisker reinforcement (p < 0.05), with a significant interaction between the two variables (p < 0.05). In Fig. 5A, at DCPA:whisker of 1:0, the composite containing unsilanized DCPA released significantly more Ca ions than the composite containing silanized DCPA (p < 0.05). At DCPA:whisker of 1:1, whether the DCPA was silanized or not had little effect on the amount of Ca released. The same was true for the PO4 release in Fig. 5B.
Figure 5.
Ca and PO4 release: effect of DCPA silanization. “Whisker” refers to the nano-silica-fused whiskers that were silanized. The composite contained DCPA powder (particle size of 0.112 μm) that was not silanized. Each value is mean ± sd; n = 3.
4. Discussion
Effect of DCPA particle size
Previous studies on remineralizing calcium phosphate composites did not examine the effect of filler particle size. For example, a previous study developed a pulp capping/base composite containing DCPA particles with a single median size of 1.1 μm [21]. In the present study, the DCPA particle size was varied from about 112 nm to 12.0 μm, and the effects on mechanical properties and Ca and PO4 released were investigated for the first time. Decreasing the DCPA particle size decreased the composite mechanical properties (Fig. 2). This was likely because, at the same filler level, decreasing the particle size increased the filler particle surface area in the matrix resin. This would in turn increase the concentration of entrapped air in the composite, thus moderately decreasing the resin’s polymerization conversion. The degree of polymerization conversion (DC) of the six composites in Fig. 2 was measured using a Fourier Transform Infrared Spectrometer as described previously [35]. The results are listed in Table 2. Indeed, the DC was 73.1 % for the composite with the 12.0 μm DCPA (no whiskers), significantly higher than 59.8 % at a DCPA particle size of 112 nm (p < 0.05). At DCPA:whisker of 1:1, the DC was 78.3 % at 12.0 μm DCPA, significantly higher than 69.6 % at a DCPA particle size of 112 nm (p < 0.05). Hence the DC was proportional to the DCPA particle size (or inversely proportional to DCPA filler surface area). The benefit with whisker reinforcement was that, even when using the smallest DCPA particles (112 nm) with the highest surface area, the composite strength still matched that of a commercial stress-bearing, non-releasing composite.
Table 2.
Degree of polymerization conversion for DCPA-whisker composites in Fig. 2
| DCPA:Whisker Mass Ratio | DCPA Particle Size | Degree of Conversion (%) (mean ± sd; n = 5) |
|---|---|---|
| 1:0 | 112 nm | 59.8 ± 2.4 |
| 1:0 | 0.88 μm | 62.2 ± 0.4 |
| 1:0 | 12.0 μm | 73.1 ± 0.9 |
| 1:1 | 112 nm | 69.6 ± 0.3 |
| 1:1 | 0.88 μm | 75.4 ± 3.1 |
| 1:1 | 12.0 μm | 78.3 ± 1.3 |
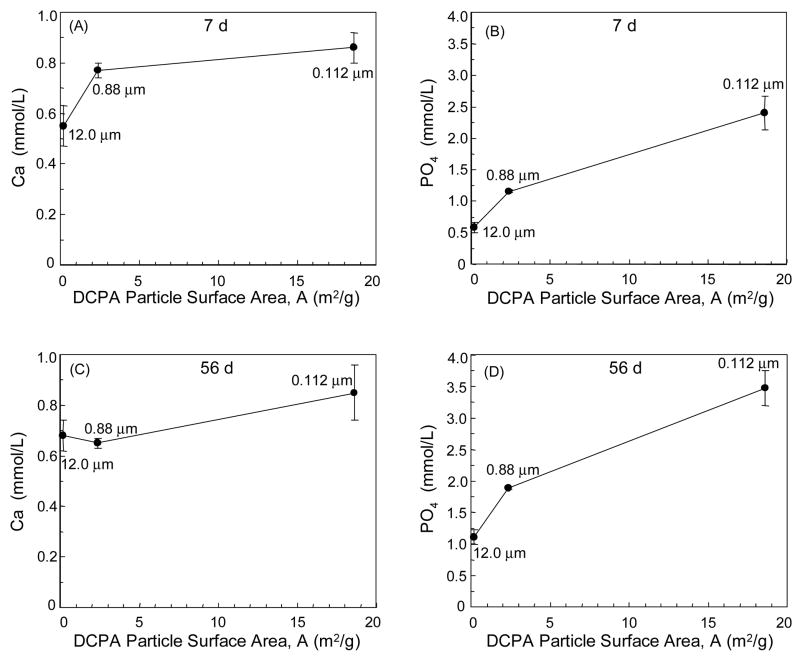
An advantage of using smaller DCPA particles was their higher surface area, which appeared to facilitate the release of Ca and PO4 ions. Particle specific surface area A = 6/(d ρ), where d is the equivalent spherical diameter of the particles, and ρ is density (ρ = 2.89 g/cm3 for DCPA) [37]. Smaller particles with a larger surface area may have faster release, resulting in higher ionic concentrations. The A values were calculated for the three DCPA powders in the present study, and plotted in Fig. 6 vs. the experimental Ca and PO4 concentrations. Figs. 6A–B show the initial Ca and PO4 release (7 d), while Figs. 6C–D show the 56 d release, for the nano DCPA composite without whiskers. They showed an increasing ion release with increasing the DCPA particle surface area. This study was limited to three different DCPA particles sizes; it would require more particle sizes to establish an empirical equation (such as a power-law relationship) between the amount of ion release and filler particle surface area. Nonetheless, the results in Fig. 6 demonstrated the importance of DCPA particle surface area on the release of Ca and PO4 for the first time. This could provide guidance on the development of other biomedical releasing materials.
Figure 6.
Effect of DCPA particle surface area on Ca and PO4 release for composite containing unsilanized nano DCPA at DCPA:whisker of 1:0. The particle specific surface area was calculated by A = 6/(d ρ), where d is diameter and ρ is density (2.89 g/cm3 for DCPA). (A–B) Initial Ca and PO4 release from Fig. 4A and 4C (7-day data). (C–D) 56-d release data from Fig. 4A and 4C. Each Ca and PO4 concentration value is mean ± sd; n = 3. Smaller particles with a larger surface area had more ion release.
Effect of DCPA particle silanization
Previous studies used unsilanized calcium phosphate fillers in dental resins [19,21,32]. The present study examined the effects of silanization of the DCPA fillers on the amounts of Ca and PO4 released and the composite strength. For composites containing DCPA without whiskers, silanization of the DCPA significantly increased the composite strength (Fig. 3). The trade-off was a decrease in the amount of Ca and PO4 released (Fig. 5), likely due to the hydrophobic silane coating on the DCPA particles retarding the access of water to the particles slowing their dissolution. At a DCPA:whisker ratio of 1:1, the silanization of DCPA did not significantly change the composite strength (Fig. 3), probably because the whisker reinforcement exerted a dominant effect, and the DCPA silanization effect was negligible in comparison. Therefore, for stress-bearing restorations, it would be desirable to use a composite with a DCPA:whisker ratio near 1:1 using unsilanized DCPA nano-particles, which produced a Ca concentration of 0.35 mmol/L and PO4 of 0.99 mmol/L (at 56 d). These concentrations matched or exceeded the reported Ca and PO4 concentrations measured using a similar method for previous calcium phosphate composites known to remineralize enamel and dentin lesions in vitro [18–21]. For example, amorphous calcium phosphate (ACP) composites yielded a Ca concentration of 0.3 mmol/L to 1.0 mmol/L, and a PO4 concentration of 0.2 mmol/L to 0.7 mmol/L (Figs. 2–3 in Ref. 19). Another study on remineralizing Ca-PO4 composites reported a Ca concentration of 0.5 mmol/L and a PO4 concentration of 0.1 mmol/L in buffered saline [21].
Mechanical properties
Besides matching/exceeding the Ca and PO4 release of previous calcium phosphate composites, the composite with DCPA:whisker of 1:1 using unsilanized nano DCPA had a flexural strength of 112 MPa, more than 2-fold higher than the strengths of previous calcium phosphate composites. Using specimens without immersion, the ACP composite had a flexural strength of about 50 MPa [19,38]. This was consistent with the observation that “all the amorphous calcium phosphate fillers yielded polymerized materials weaker than unfilled polymers” [19]. Another composite containing DCPA particles also had a flexural strength of around 40 MPa to 50 MPa for specimens without immersion [21,39]. The flexural strength of the composite of the present study (with DCPA:whisker of 1:1 using unsilanized nano DCPA) matched that of a commercial stress-bearing, non-releasing composite. For clarity, these properties of several materials are listed in Table 3.
Table 3.
Mechanical properties and Ca and PO4 release of dental composites*
| Flexural Strength (MPa) |
Elastic Modulus (GPa) |
Ca Release (mmol/L) |
PO4 Release (mmol/L) |
|
|---|---|---|---|---|
| Nano DCPA:whisker composite (this study) | 112 ± 17 | 12.9 ± 1.3 | 0.35 | 0.99 |
| Hybrid composite control (this study) | 112 ± 14 | 10.9 ± 0.8 | 0 | 0 |
| ACP composite [19,38] | 50 – 60 | 0.3 – 1.0 | 0.3 – 0.7 | |
| Ca-PO4 composite [21,39] | 40 – 60 | 0.5 | 0.1 |
The nano DCPA:whisker composite had a DCPA:whisker of 1:1 mass ratio, and the nano DCPA was not silanized. The hybrid control was TPH (Caulk/Dentsply).
The DCPA-whisker composites had elastic modulus ranging from 12.9 GPa to 18.2 GPa. They either were lower than or matched the 18 GPa of dentin [40]. But they were all higher than the 10.9 GPa of the commercial stress-bearing, non-releasing hybrid control composite.
In a previous study on different resins and curing conditions [32], the flexural strengths of nano DCPA-whisker composites were measured after water immersion. After 56 days of immersion, the flexural strength was about 100 MPa for two-part, self-cured composites at nano DCPA:whisker of 1:1 [32]. Hence the strength after 56 days was still much higher than those of previous calcium phosphate composites using dry specimens without any immersion (Table 3).
Effect of synergistic use of nano-releasing fillers/reinforcing fillers in resin
In a previous study [21], the DCPA particle size, d, was 1.1 μm and the TTCP (tetracalcium phosphate) particle size was 16 μm. The density, ρ, is 2.89 g/cm3 for DCPA and 3.07 g/cm3 for TTCP. Hence particle specific surface area A = 1.9 m2/g for DCPA and 0.12 m2/g for TTCP were much less than the 18.6 m2/g for the nano DCPA of the present study. A major advantage of the using nano-sized DCPA particles was that one could use less fillers in the composite while still matching the Ca and PO4 release of previous remineralizing composites, thereby making room in the resin for reinforcing (but non-releasing) fillers. In the present study, this synergistic releasing nanofiller/reinforcing filler method yielded a mechanically-strong dental composite with the potential of both stress-bearing and Ca and PO4 releasing capabilities. Such a combination is not yet available in current dental materials.
The nano DCPA-whisker composite paste at a filler level of 65 % by mass was quite flowable with room for more fillers. But the nano DCPA paste without whiskers (nano DCPA:whisker = 1:0) could be mixed only up to 65 %. Therefore, to enable a direct comparison of these composites, the filler level was fixed to be the same at 65 %. The composite with nano DCPA:whisker of 1:1 could be further improved via higher filler levels for increased mechanical properties and more Ca and PO4 release. Meanwhile, the nano DCPA-whisker composite at 65% may be useful as a flowable composite to repair defective margins, with the potential benefit of remineralizing caries margins and avoiding the need to replace the entire restoration.
Previous studies [18–21] showed that when the Ca and PO4 ions were released from the composite restoration, they reprecipitated to form hydroxyapatite outside the composite and inside the tooth lesions, significantly increasing the mineral content of the lesion. The fact that the Ca and PO4 concentrations from the DCPA-whisker composite matched or exceeded those of the previous calcium phosphate composites suggests that the DCPA-whisker composite may also be an effective remineralizer. The nano DCPA-whisker composites with high strength and Ca and PO4 release were whitish in color but relatively opaque. They may be useful in stress-bearing posterior restorations, for which previous calcium phosphate composites were too weak to survive. Further studies are needed to match the refractive index of the fillers to that of the resin to improve the esthetics for anterior applications.
5. Summary
-
[1]
Nano DCPA particles were synthesized in our laboratory and incorporated into a dental resin. Two other DCPA sizes were also used to examine the effect of particle size on mechanical properties and Ca and PO4 release. Decreasing the DCPA particle size decreased the composite strength, while whisker reinforcement more than doubled the composite strength and significantly increased the elastic modulus.
-
[2]
Silanization of the DCPA particles increased the composite strength but decreased the Ca and PO4 release. The use of unsilanized nano DCPA together with whisker reinforcement appeared to be the best microstructural tailoring route to producing a composite with high strength and Ca and PO4 release.
-
[3]
The synergistic use of nano DCPA and whiskers resulted in a strong composite with flexural strength matching that of a commercial stress-bearing, non-releasing composite. The strength of the nano DCPA-whisker composite was 2-fold greater than those of previous calcium phosphate composites. The Ca and PO4 release from the nano DCPA-whisker composite matched/exceeded those of previous composites known to remineralize in vitro tooth lesions.
-
[4]
Increasing the DCPA particle surface area significantly increased the Ca and PO4 release, with the nano DCPA exhibiting the highest release. The relationship between particle surface area and release may have implications on further development of therapeutic releasing and drug-delivering materials for dental and biomedical applications.
Acknowledgments
We gratefully thank A. M. Fraser for experimental assistance, Dr. J. M. Antonucci for providing the resin monomers, and Drs. L. C. Chow and S. Takagi for discussions. This study was supported by NIDCR grant R01 DE14190, NIST, and the ADAF.
Footnotes
DISCLAIMER
Certain commercial materials and equipment are identified to specify the experimental procedure. This does not imply recommendation or endorsement by NIST or ADAF or that the material or equipment identified is necessarily the best available for the purpose. Unless otherwise specified in the paper, one standard deviation was used as the estimated standard uncertainty of the measurements.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Söderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res. 1984;63:1248–1254. doi: 10.1177/00220345840630101701. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AJ, Burstone CJ, Hadjinikolaou I, Jancar J. Screening of matrices and fibers for reinforced thermoplastics intended for dental applications. J Biomed Mater Res. 1994;28:167–173. doi: 10.1002/jbm.820280205. [DOI] [PubMed] [Google Scholar]
- 3.Griggs JA, Thompson JY, Anusavice KJ. Effects of flaw size and auto-glaze treatment on porcelain strength. J Dent Res. 1996;75:1414–1417. doi: 10.1177/00220345960750061301. [DOI] [PubMed] [Google Scholar]
- 4.Ferracane JL, Berge HX, Condon JR. In vitro aging of dental composites in water -- Effect of degree of conversion, filler volume, and filler/matrix coupling. J Biomed Mater Res. 1998;42:465–472. doi: 10.1002/(sici)1097-4636(19981205)42:3<465::aid-jbm17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Frankenberger R, García-Godoy F, Lohbauer U, Petschelt A, Krämer N. Evaluation of resin composite materials. Part I: In vitro investigation Am J Dent. 2005;18:23–27. [PubMed] [Google Scholar]
- 6.Krämer N, García-Godoy F, Reinelt C, Frankenberger R. Clinical performance of posterior compomer restorations over 4 years. Am J Dent. 2006;19:61–66. [PubMed] [Google Scholar]
- 7.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 8.Ruddell DE, Maloney MM, Thompson JY. Effect of novel filler particles on the mechanical and wear properties of dental composites. Dent Mater. 2002;18:72–80. doi: 10.1016/s0109-5641(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 9.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater. 2002;18:436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 10.Drummond JL, Bapna MS. Static and cyclic loading of fiber-reinforced dental resin. Dent Mater. 2003;19:226–231. doi: 10.1016/s0109-5641(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. J Dent Res. 2005;84:822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 12.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. International Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 15.CDC (Center for Disease Control) . 2005 December; www.cdc.gov/OralHealth/factsheets.
- 16.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commision Projects 2–95 International Dent J. 2001;51:117–158. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 17.LeGeros RZ. In: Calcium phosphates in oral biology and medicine. Myers HM, editor. Basel, Switzerland: S. Karger; 1991. Chapters 3–4. [PubMed] [Google Scholar]
- 18.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75:1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 19.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 20.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res part B. 2000;53:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 22.Xu HHK, Quinn JB, Smith DT, Antonucci JM, Schumacher GE, Eichmiller FC. Dental resin composites containing silica-fused whiskers – Effects of whisker-to-silica ratio on fracture toughness and indentation properties. Biomaterials. 2002;23:735–742. doi: 10.1016/s0142-9612(01)00178-8. [DOI] [PubMed] [Google Scholar]
- 23.Xu HHK, Eichmiller FC, Smith DT, Schumacher GE, Giuseppetti AA, Antonucci JM. Effect of thermal cycling on whisker-reinforced dental resin composites. J Mater Sci: Mater in Med. 2002;13:875–883. doi: 10.1023/a:1016504530133. [DOI] [PubMed] [Google Scholar]
- 24.Xu HHK. Long-term water aging of whisker-reinforced polymer-matrix composites. J Dent Res. 2003;82:48–52. doi: 10.1177/154405910308200111. [DOI] [PubMed] [Google Scholar]
- 25.Xu HHK, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. J Dent Res. 2004;83:930–935. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- 26.Xu HHK, Smith DT, Simon CG. Strong and bioactive composites containing nano-silica-fused whiskers for bone repair. Biomaterials. 2004;25:4615–4626. doi: 10.1016/j.biomaterials.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Gonsalves KE. Preparation and characterization of thermally stable nanohydroxyapatite. J Mater Sci: Mater Med. 1997;8:25–28. doi: 10.1023/a:1018586128257. [DOI] [PubMed] [Google Scholar]
- 28.Sutorik AC, Paras MS, Lawrence D, Kennedy A, Hinklin T. Synthesis, characterization, and sintering behavior of calcium hydroxyapatite powders with average particle diameters of 150 nm. Ceramic Trans. 2003;147:73–82. [Google Scholar]
- 29.Bow JS, Liou SC, Chen SY. Structural characterization of room-temperature synthesized nano-sized β-tricalcium phosphate. Biomaterials. 2004;25:3155–3161. doi: 10.1016/j.biomaterials.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 30.Chow LC, Sun L, Hockey B. Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J Res NIST. 2004;109:543–551. doi: 10.6028/jres.109.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu HHK, Sun L, Takagi S, Chow LC. Dental releasing materials. 2005 U. S. Patent Application, Serial No. 11/138,182, filed on May 26. [Google Scholar]
- 32.Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ISO/FDIS 4049: Dentistry – polymer-based fillings, restorative and luting materials. 3. Geneva, Switzerland: International Organization for Standardization; 2000. [Google Scholar]
- 34.ASTM D 790-03. Standard test methods for flexural properties of unreinforced and reinforced plastic and electrical insulating materials. West Conshohocken, PA; ASTM International: 2004. [Google Scholar]
- 35.Xu HHK. Dental composite resins containing silica-fused ceramic single-crystalline whiskers with various filler levels. J Dent Res. 1999;78:1304–1311. doi: 10.1177/00220345990780070401. [DOI] [PubMed] [Google Scholar]
- 36.Xu HHK, Sun L, Weir MD, Takagi S, Chow LC, Hockey B. Effects of nano-sized monocalcium phosphate monohydrate fillers on Ca-and-PO4-releasing dental composites. J Biomed Mater Res part B. 2006 (accepted for publication) [Google Scholar]
- 37.Chow LC, Markovic M, Takagi S. Calcium phosphate cements. In: Struble LJ, editor. Cements research progress. Westerville, OH: American Ceram Soc; 1999. pp. 215–238. [Google Scholar]
- 38.O’Donnell JNR, Antonucci JM, Skrtic D. Mechanical properties of amorphous calcium phosphate composites. J Dent Res. 2005;84 doi: 10.1177/0883911506064476. (IADR Abstract No. 586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickens SH, Flaim GM, Floyd CJE. Effect of resin composition on mechanical and physical properties of calcium phosphate filled bonding systems. Polymer Preprints. 2004;45:329–330. [Google Scholar]
- 40.Marshall GW, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]