Abstract
Background and objectives: Racial differences in mineral metabolism exist in the chronic kidney disease population, especially as it relates to intact parathyroid hormone (iPTH) levels. Few data exist on the relationship of these markers to bone biopsy findings in African-American (AA) hemodialysis patients across the spectrum of renal osteodystrophy (ROD).
Design, setting, participants, & measurements: In prevalent AA hemodialysis subjects, we prospectively evaluated subjects by performing transiliac bone biopsy and correlating biochemical and clinical data to bone histology.
Results: Study patients (n = 43) had an average age of 53.7 (±11.6) yr, with dialysis vintage of 40.4 (±24.5) mo, 30% with diabetes, and 51% male. Bone histology revealed adynamic bone disease (ABD) (16%), mild to moderate hyperparathyroidism (HPT) (72%), severe (12%) HPT, and no osteomalacia or mixed uremic osteodystrophy. At the time of biopsy, mean corrected calcium was 9.1, 8.9, and 9.4 mg/dl (P = 0.344); calcium-phosphorus (Ca × PO4) product was 42, 55, and 62 mg2/dl2 (P = 0.002); phosphorus was 4.6, 6.2, and 6.7 mg/dl (P = 0.005); and iPTH was 225, 566, and 975 pg/ml (P = 0.006), respectively. Median values for bone-specific alkaline phosphatase (BS-AP) were 16, 34, and 64 ng/ml (P < 0.0001) among the three groups.
Conclusions: These data demonstrate that across the spectrum of ROD, iPTH levels are higher than expected in AA hemodialysis subjects. iPTH, PTH peptides, and bone-specific alkaline phosphatase correlated directly with histomorphometric measurements of bone turnover and when subjects were grouped by histologic diagnosis. Only 9.5% of subjects were simultaneously within suggested Kidney Disease Outcomes Quality Initiative (K/DOQI) ranges for Ca × PO4, phosphorus, and iPTH, of which 75% demonstrated ABD on biopsy.
Abnormalities in bone and mineral metabolism occur frequently in chronic kidney disease (CKD) patients, leading to bone lesions. This disorder has more recently been termed chronic kidney disease-related mineral and bone disorder (CKD-MBD) by the Kidney Disease Improving Global Outcomes (KDIGO) foundation (1). Bone and mineral disorders in CKD patients have been associated with significant morbidity and mortality (2–5). The bone lesions, coined renal osteodystrophy (ROD), in this population can manifest symptoms such as intense pruritus, bone pain, myopathies, muscle tendon rupture, and increased incidence of fractures. Beyond symptoms, however, ROD manifests in derangements in bone histologic findings such as abnormalities in bone turnover, mineralization, and volume (6), a classification system that has been adopted by the KDIGO committee (1). Bone histology remains the “gold standard” for definitive diagnosis of the osteodystrophic lesion. However, in routine clinical practice nephrologists rely on noninvasive methods for assessment of bone turnover, such as intact parathyroid hormone (iPTH) levels.
The Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines (CPG) for Bone Metabolism and Disease in Chronic Kidney Disease, released in 2003, provided the first integrated clinical action plan for the treatment of CKD-MBD (7). The treatment goals provided by these CPGs were developed through a systematic review of the literature to date (January 1, 2001) in addition to a broad-based review by experts, organizations, and the public. However, it is unclear if the recommended goals of therapy in the guidelines are also applicable to African-American (AA) hemodialysis patients. Differences in mineral metabolism between AA and non-AA individuals without CKD have been demonstrated previously in relation to calcium balance (8,9) and bone histomorphometry (10,11). These differences have been seen in the nondialysis CKD population as well, with AA CKD patients demonstrating differences in their levels of bone and mineral parameters that would suggest a more severe degree of secondary hyperparathyroidism (12). A recent study involving AA hemodialysis patients suggests that a higher serum iPTH threshold may be necessary in this ethnic group to avoid the development of adynamic bone disease (ABD) (13), which has been associated with adverse outcomes such as vascular calcifications, (14,15) fractures (16,17), and increased mortality (5,18). In particular, a iPTH of less than 200 pg/ml or a combination of a iPTH of less than 178 pg/ml with a higher serum phosphorus and calcium were associated with increased mortality in hemo- and peritoneal dialysis patients (5,18).
African Americans constitute a significant proportion of patients on hemodialysis in the United States, approaching 32% in 2005 (19). Therefore, it is important to explore any potential differences in the assessment and treatment of bone and mineral disorders in this population. Although the work of Sawaya et al. and Gupta et al. has led to the recognition that there are differences between AA and non-AA hemodialysis subjects in relation to iPTH, the optimal serum levels of iPTH across the histologic spectrum of bone and mineral disorders have yet to be established in this population (13,20). The purpose of this study was to evaluate the relationship of bone histology across the spectrum of ROD to classical biochemical markers of bone and mineral metabolism in prevalent AA hemodialysis patients receiving the current standard of care for CKD-MBD.
Materials and Methods
We prospectively evaluated the relationship between standard biochemical and clinical measurements to bone biopsy findings in prevalent hemodialysis patients. The study was conducted at three urban hemodialysis units located in southeastern Michigan from March 2005 to May 2007. The institutional review board approved this study, signed informed consent was obtained from each participating subject, and the study was conducted in adherence to the Declaration of Helsinki.
Patients
AA hemodialysis subjects 18 yr of age or older with a dialysis vintage of 3 mo or more and no substantial change in active vitamin D therapy (initiating, discontinuing, or >50% change in dosage) in the previous 3 mo were eligible for enrollment. Exclusion criteria were: history of parathyroidectomy, receipt of bisphosphonates, calcimimetics or steroids in the previous 3 mo, and women of child bearing age (if pregnant or planning to become pregnant during the course of the study). During the screening process, serum iPTH levels were measured on eligible subjects. The purpose of this screening was to identify potential subjects across a broad range of iPTH values to ensure representation of the varying degrees of ROD. Thereafter, patients were scheduled to undergo transiliac bone biopsies.
Clinical Data Collection
Clinical information on demographics, height, weight, use and type of phosphate binders, active vitamin D exposure, dialysate calcium, and hemodynamic measurements (mean arterial pressure, pulse pressure) were collected from review of the electronic medical record, dialysis treatment logs, and patient interview. Radiology procedures, obtained as part of routine patient care before the date of bone biopsy, if available, were reviewed for an explicit statement about the presence of vascular calcification. Radiology procedures evaluated included digitized x-rays and computed tomography scans.
Biochemical Data Collection
Biochemical values were determined using automated methods. Routine biochemical values (monthly and bimonthly) were recorded for 6 mo before biopsy and at the time of biopsy. These included traditional bone and mineral markers [corrected calcium, phosphorus, calcium-phosphorus (Ca × PO4) product, iPTH and/or Bio-iPTH, serum bicarbonate, total alkaline phosphatase], liver enzymes (aspartate aminotransferase and alanine transaminase), nutritional markers (albumin, serum creatinine, protein catabolic rate normalized), and markers of anemia (ferritin, hemoglobin)]. We also evaluated measurements of dialysis adequacy (urea reduction ratio, single-pooled Kt/V). Two of the three dialysis units routinely utilized the Bio-Intact PTH Assay (Nichols Institute Diagnostics, San Clemente, CA; reference range 12.6 to 53.5 pg/ml) in the 6 mo before bone biopsy. The third unit utilized the Total Intact PTH Assay (Scantibodies Labs, Santee, CA; reference range 14 to 66 pg/ml). Assessment of cumulative and average active vitamin D exposure was based on the dose of its analog, paricalcitol, with a conversion factor of 3:1 used for the three subjects on intravenous calcitriol (21). On the day of bone biopsy, total iPTH along with cyclase activating PTH (CAP; reference range 5 to 39 pg/ml), cyclase inhibitory PTH (CIP), the CAP/CIP ratio, and bone-specific alkaline phosphatase (BS-AP) were measured. Serum BS-AP levels were measured using an immunocapture enzyme activity assay (Metra BSAP EIA kit, San Diego, CA). The normal range of the assay is 11.6 to 29.6 ng/ml for premenopausal women, 14.2 to 42.7 ng/ml for postmenopausal women, and 15.0 to 41.3 ng/ml for men. The intra- and interassay coefficients of variation are less than 6% and less than 8%, respectively.
On the basis of biochemical values at the time of biopsy, the attainment of the K/DOQI goals for phosphorus, Ca × PO4, and iPTH were evaluated in relation to biopsy findings. Subjects were categorized by iPTH groups and compared in relation to biopsy findings. iPTH groups were chosen to demonstrate findings below goal (<150 pg/ml), at goal (150 to 300 pg/ml), above the iPTH value previously associated with higher mortality (>600 pg/ml) and the range between these cutoffs (301 to 600 pg/ml) (5).
Bone Biopsy
Three weeks before bone biopsy, all patients received tetracycline hydrochloride for in vivo double labeling at a dose of 250 mg orally 3 times daily for 3 d followed by an 11-d interval then a second label of either oral tetracycline hydrochloride (250 mg 3 times daily) or demeclocycline hydrochloride (300 mg twice daily) for the next 3 d. Phosphate binders and other antacids were not taken on days of tetracycline administration. A transiliac bone biopsy was performed in all patients 3 to 4 d after receiving the second label using a Jamshidi 8G (3 to 4 mm in diameter) and 11G (4 to 5 mm in diameter) needle, and the two samples were processed as described previously (22). Bone histomorphometry was performed by one of the authors (H.M.), who was blinded to patient characteristics and biochemical data. Bone samples were processed as described previously by the reference laboratory at the University of Kentucky (22–25).
Qualitative histologic classification was made using the conventional classification (22). In addition, histomorphometric parameters were measured using the Osteoplan System II (Kronton, Munich, Germany) as described previously (26,27). Data were also analyzed on the basis of the turnover, mineralization, and bone volume “TMV” classification system (1,6). The two classifications were compared.
Statistical Analyses
Descriptive variables are presented as mean with SD or median with interquartile range where appropriate. Dichotomous or categorical variables were compared using χ2 analysis. Continuous variables were compared using t test, one-way ANOVA with Bonferroni post hoc test or Kruskal–Wallis for nonparametric data where appropriate. A P value of less than 0.05 was considered statistically significant. Pearson correlation coefficients (r) were obtained among iPTH levels, biochemical values at the time of biopsy, and bone histomorphometric parameters. Spearman's rank correlation analysis was used to evaluate nonparametric data. Data were analyzed with SPSS software (version 15.0; SPSS).
Results
Patients
A total of 43 AA hemodialysis subjects were enrolled into the study. Subject characteristics and individual values of bone and mineral characteristics are summarized in Tables 1 and 2. There were no differences in demographic characteristics and iPTH and CAP circulating levels between male and female patients. Thirty percent of the patients were diabetic. iPTH (379 versus 636 pg/ml, P = 0.06) and CAP (211 versus 358 pg/ml, P = 0.06) levels demonstrated a trend toward lower levels in diabetes versus nondiabetics. Most subjects were receiving intravenous vitamin D (95%) and phosphate binder therapy (93%). Nearly half of the patients demonstrated radiologic evidence of vascular calcification (49%) in the period before biopsy.
Table 1.
Population characteristicsa
| All Subjects (n = 43) | |
|---|---|
| Demographics | |
| age (yr) [mean (SD)] | 53.7 (11.6) |
| dialysis vintage (mo) [mean (SD)] | 40.4 (24.5) |
| male | 22 (51) |
| female | 21 (49) |
| diabetes | 13 (30) |
| history of fracture | 7 (16) |
| radiological signs of calcification | 21 (49) |
| intravenous vitamin D | 41 (95) |
| Phosphate binder | |
| no binder | 3 (7) |
| calcium acetate | 10 (23) |
| sevelamer | 28 (65) |
| >1 binder | 2 (5) |
| Biopsy result | |
| ABD | 7 (16) |
| mild HPT | 15 (35) |
| moderate HPT | 16 (37) |
| severe HPT | 5 (12) |
All results reported as (n, %) unless otherwise indicated. ABD, adynamic bone disease; HPT, hyperparathyroidism.
Table 2.
Individual characteristics of CKD-MBD at time of biopsya
| Patient # | Cacorr (mg/dl) | Phosphate (mg/dl) | Ca × PO4 (mg2/dl2) | iPTH (pg/ml) | CAP (pg/ml) | CIP (pg/ml) | CAP/CIP Ratio (pg2/ml2) | BS-AP (ng/ml) | Vitamin D Dose (μg)b | Binder | Biopsy Result |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.5 | 4.5 | 38 | 207.0 | 122.3 | 84.7 | 1.4 | 21.0 | 1 | CA + S | ABD |
| 2 | 9.9 | 4.0 | 40 | 40.7 | 23.9 | 16.8 | 1.4 | 14.6 | 8 | S | ABD |
| 3 | 7.8 | 3.9 | 30 | 384.1 | 267.0 | 117.1 | 2.3 | 17.3 | 0 | S | ABD |
| 4 | 8.6 | 4.7 | 40 | 301.6 | 159.4 | 142.2 | 1.1 | 12.6 | 10 | CA | ABD |
| 5 | 9.5 | 4.5 | 43 | 284.3 | 147.4 | 136.9 | 1.1 | 21.5 | 7 | S | ABD |
| 6 | 9.6 | 4.6 | 44 | 202.3 | 90.8 | 111.5 | 0.8 | 16.2 | 3 | CA | ABD |
| 7 | 9.5 | 5.9 | 56 | 157.5 | 61.2 | 96.3 | 0.6 | 12.9 | 8 | S | ABD |
| 8 | 8.3 | 6.6 | 55 | 235.0 | 158.0 | 77.0 | 2.1 | 50.8 | 3 | S | Mild HPT |
| 9 | 9.9 | 4.9 | 49 | 412.6 | 177.8 | 234.8 | 0.8 | 38.4 | 0 | S | Mild HPT |
| 10 | 7.5 | 9.6 | 72 | 380.9 | 158.0 | 222.9 | 0.7 | 23.2 | 2 | None | Mild HPT |
| 11 | 8.9 | 5.7 | 51 | 201.8 | 115.9 | 85.9 | 1.3 | 15.0 | 7 | S | Mild HPT |
| 12 | 9.9 | 4.9 | 49 | 75.1 | 41.0 | 34.1 | 1.2 | 29.0 | 6 | S | Mild HPT |
| 13 | 8.8 | 4.9 | 43 | 484.1 | 279.5 | 204.6 | 1.4 | 27.4 | 5 | S | Mild HPT |
| 14 | 9.8 | 6.2 | 61 | 2187.2 | 1248.3 | 938.9 | 1.3 | >64.0 | 3 | S | Mild HPT |
| 15 | 8.2 | 6.6 | 54 | 237.6 | 124.0 | 113.5 | 1.1 | 40.3 | 4 | S | Mild HPT |
| 16 | 9.1 | 5.4 | 49 | 1002.8 | 625.8 | 377.0 | 1.7 | >64.0 | 4 | S | Mild HPT |
| 17 | 7.9 | 5.8 | 46 | 245.0 | 149.0 | 96.0 | 1.6 | 23.2 | 1 | S | Mild HPT |
| 18 | 8.6 | 6.2 | 53 | 268.0 | 164.0 | 104.0 | 1.6 | – | 6 | S | Mild HPT |
| 19 | 8.7 | 4.7 | 41 | 784.0 | 543.0 | 241.0 | 2.0 | 54 | 2 | None | Mild HPT |
| 20 | 9.6 | 6.4 | 61 | 277.7 | 158.0 | 119.7 | 1.3 | 19.6 | 3 | CA | Mild HPT |
| 21 | 10.1 | 7.4 | 75 | 457.0 | 185.0 | 272.0 | 0.7 | 32.3 | 7 | CA + S | Mild HPT |
| 22 | 8.0 | 7.0 | 56 | 575.0 | 347.0 | 228.0 | 1.5 | 31.2 | 1 | S | Mild HPT |
| 23 | 9.5 | 5.3 | 50 | 241.0 | 147.0 | 94.0 | 1.6 | >64 | 7 | S | Moderate HPT |
| 24 | 9.5 | 6.7 | 64 | 485.9 | 271.0 | 214.9 | 1.3 | 41.8 | 5 | CA | Moderate HPT |
| 25 | 8.8 | 6.0 | 53 | 430.8 | 248.7 | 182.1 | 1.4 | 34.1 | 10 | S | Moderate HPT |
| 26 | 9.0 | 6.1 | 55 | 438.0 | 250.0 | 188.0 | 1.3 | 34.8 | 2 | CA | Moderate HPT |
| 27 | 8.3 | 5.7 | 47 | 216.0 | 141.0 | 75.0 | 1.9 | 19.1 | 3 | S | Moderate HPT |
| 28 | 8.9 | 6.3 | 56 | 408.0 | 220.0 | 188.0 | 1.2 | — | 4 | CA | Moderate HPT |
| 29 | 9.5 | 5.8 | 55 | 1105.7 | 646.5 | 459.3 | 1.4 | 62.0 | 5 | S | Moderate HPT |
| 30 | 8.9 | 4.0 | 36 | 699.0 | 404.0 | 295.0 | 1.4 | 51.5 | 3 | S | Moderate HPT |
| 31 | 9.9 | 5.7 | 56 | 649.3 | 516.8 | 132.5 | 3.9 | 23.3 | 3 | S | Moderate HPT |
| 32 | 8.5 | 5.6 | 48 | 840.0 | 447.0 | 393.0 | 1.1 | 29.1 | 5 | S | Moderate HPT |
| 33 | 8.5 | 9.2 | 78 | 1057.0 | 500.0 | 557.0 | 0.9 | 16.8 | 12 | S | Moderate HPT |
| 34 | 9.6 | 5.1 | 49 | 310.0 | 155.0 | 155.0 | 1.0 | — | 9 | CA | Moderate HPT |
| 35 | 8.8 | 9.0 | 79 | 1140.1 | 583.7 | 556.4 | 1.0 | 38.0 | 0 | S | Moderate HPT |
| 36 | 7.7 | 5.8 | 45 | 388.2 | 216.4 | 171.8 | 1.3 | 18.0 | 3 | S | Moderate HPT |
| 37 | 9.3 | 6.2 | 58 | 350.0 | 201.0 | 149.0 | 1.3 | — | 10 | S | Moderate HPT |
| 38 | 9.0 | 5.9 | 53 | 961.7 | 530.5 | 431.2 | 1.2 | 52.2 | 8 | CA | Moderate HPT |
| 39 | 9.2 | 7.1 | 65 | 755.8 | 528.0 | 227.0 | 2.3 | 63.8 | 6 | CA | Severe HPT |
| 40 | 9.2 | 6.0 | 55 | 1110.3 | 516.8 | 593.6 | 0.9 | >64 | 3 | CA | Severe HPT |
| 41 | 8.9 | 7.9 | 70 | 941.4 | 434.5 | 506.9 | 0.9 | >64 | 3 | none | Severe HPT |
| 42 | 9.8 | 4.8 | 47 | 1316.6 | 590.6 | 726.0 | 0.8 | >64 | 3 | S | Severe HPT |
| 43 | 10.0 | 7.5 | 75 | 752.0 | 598.0 | 154.0 | 3.9 | 46.1 | 3 | S | Severe HPT |
CAP, cyclase activating parathyroid hormone (PTH); CIP, cyclase inactive PTH; Ca × PO4, calcium-phosphorous product; BS-AP, bone-specific alkaline phosphatase; S, sevelamer; CA, calcium acetate.
Vitamin D exposure reported as microgram per treatment.
Bone Histologic Results
Qualitative bone histologic findings demonstrated that most subjects were classified within the mild (35%) or moderate (37%) hyperparathyroidism (HPT) category, with 16% classified as ABD and 12% as severe HPT. There were no cases of normal bone histology, osteomalacia, or mixed ROD. None of the bone samples showed stainable aluminum. Quantitative assessment of histomorphometric results according of the TMV nomenclature compared with the conventional classification are shown in Table 3. There were significant differences in parameters of bone turnover between the three histologic groups. Osteoid seams were significantly thinner in patients with ABD than the two other groups. There was no difference in bone volume/tissue volume between the three groups.
Table 3.
Histomorphometric parameters of bone turnover, mineralization, and volume in patients grouped according to conventional nomenclaturea
| ABD | Mild to Moderate HPT | Severe HPT | Normal Values | |
|---|---|---|---|---|
| Parameters of bone turnover (T) | ||||
| Activation frequency (yr−1) | 0.27 ± 0.11b | 0.89 ± 0.32c | 1.67 ± 0.20d | 0.49 to 0.72 |
| Bone formation rate/bone surface (mm3/cm2 per yr) | 1.66 ± 0.66b | 4.53 ± 1.94c | 8.67 ± 2.30d | 1.80 to 3.80 |
| Parameters of bone mineralization (M) | ||||
| Osteoid thickness (μm) | 8.56 ± 0.65b | 12.5 ± 3.54c | 14.2 ± 4.02c | <20 |
| Mineralization lag time (days) | 47.5 ± 24.6b | 56.0 ± 40.0b | 30.4 ± 11.7b | <50 |
| Parameter of bone volume (V) | ||||
| Cancellous bone volume/tissue volume (%) | 20.3 ± 6.50b | 26.9 ± 7.12b | 24.5 ± 11.3b | 16.8 to 22.9 |
Results are expressed as mean ± SD. Results with the same letter are not statistically different; one-way ANOVA with Bonferroni post hoc test.
Patient Characteristics by Histologic Category
Overall subject characteristics were compared by histologic category (Tables 4 and 5). Because there were no cases of normal histology, osteomalacia, or mixed osteodystrophy, subjects were grouped into either ABD, mild to moderate HPT, or severe HPT. Average values in the 6-mo period before biopsy and at the time of biopsy (±3 wk) were compared between groups. There were no significant differences detected when comparing age, dialysis vintage, weight, body mass index, presence of diabetes, binder therapy, fracture history, presence of vascular calcification, hospitalization rates, corrected calcium, CAP/CIP ratio, serum bicarbonate, aspartate aminotransferase, alanine transaminase, hemoglobin, measures of dialysis efficacy (single-pooled Kt/V and urea reduction ratio), predialysis mean arterial pressure, pulse pressure, aluminum levels, and dialysate calcium concentrations. Differences in classic biochemical indices of CKD-MBD were seen between groups with the exception of serum bicarbonate level. The lowest phosphorus, Ca × PO4, iPTH, CAP, CIP, alkaline phosphatase, BS-AP, ferritin and serum creatinine levels were found in the ABD group and rose as the severity of HPT increased on bone biopsy (P < 0.05). BS-AP demonstrated the strongest association with histologic diagnosis. All differences seen in the previous 6-mo period were also present at the time of biopsy with the exception of hemoglobin. Mean hemoglobin values in the previous 6 mo were similar between the groups; however, at the time of biopsy, the ABD group had a lower hemoglobin as compared with the other groups (P < 0.05).
Table 4.
Population characteristics by histologic categorya
| ABD n = 7 | Mild to Moderate HPT n = 31 | Severe HPT n = 5 | P Value | |
|---|---|---|---|---|
| Age (yr) | 63 (10) | 53 (12) | 47 (10) | 0.119 |
| Dialysis vintage (mo) | 36 (16) | 40 (27) | 47 (16) | 0.753 |
| Dry weight (kg) | 76 (24) | 83 (15) | 81 (11) | 0.585 |
| Body mass index (kg/m2) | 26 (8) | 29 (7) | 27 (5) | 0.535 |
| Diabetes | 43% | 32% | 0% | 0.252 |
| No binder | 0% | 6% | 20% | 0.467 |
| Calcium-based binder | 43% | 23% | 40% | 0.467 |
| Noncalcium-based binder | 57% | 71% | 40% | 0.467 |
| Hospitalization in previous year | 29% | 42% | 60% | 0.553 |
| Radiologic evidence of calcification | 71% | 45% | 40% | 0.416 |
| History of fracture | 14% | 19% | 0% | 0.547 |
Results reported as mean (SD) unless otherwise indicated.
Table 5.
Biochemical and treatment characteristics at time of biopsya
| Adynamic | Mild to Moderate HPT | Severe HPT | P Value | |
|---|---|---|---|---|
| Corrected calcium (mg/dl) | 9.1 (0.8)b | 8.9 (0.7)b | 9.4 (0.5)b | 0.344 |
| Phosphorus (mg/dl) | 4.6 (0.7)b | 6.2 (1.3)c | 6.7 (1.3)c | 0.005 |
| Ca × PO4 (mg2/dl2) | 42 (8)b | 55 (10)c | 62 (11)c | 0.002 |
| Serum bicarbonate (mmol/L) | 23.4 (2.8)b | 21.9 (2.7)b | 22.2 (3.7)b | 0.430 |
| iPTHa (pg/ml) | 225 (111)b | 566 (423)b,c | 975 (242)c | 0.006 |
| CAP (1 to 84) (pg/ml) | 125 (79)b | 321 (244)b,c | 534 (66)c | 0.009 |
| CIP (7 to 84) (pg/ml) | 101 (42)b | 245 (189)b,c | 442 (243)c | 0.010 |
| CAP/CIP (pg2/ml2) | 1.2 (0.6)b | 1.4 (0.6)b | 1.8 (1.3)b | 0.434 |
| Median BS-AP, IQR (ng/ml) | 16.2 (8.1) | 34.1 (28.3) | 64.0 (9.1) | <0.0001 |
| Total alkaline phosphatase (IU/L) | 75 (22)b | 150 (77)c | 246 (90)c | 0.001 |
| Dialysate calcium (mmol/L) | 2.6 (0.4)b | 2.6 (0.4)b | 2.4 (0.2)b | 0.565 |
| Cumulative vitamin D in previous 3 wk | 41 (27)b | 38 (27)b | 35 (14)b | 0.918 |
| Average vitamin D dosage (μg/treatment) | 5.3 (3.9)b | 4.6 (3.0)b | 3.6 (1.3)b | 0.620 |
| Aluminum (μg/L) | 1.7 (2.9) | 2.7 (3.4) | – | 0.547 |
| Albumin (g/dl) | 3.9 (0.3)b | 4.0 (0.3)b | 4.2 (0.3)b | 0.181 |
| Serum creatinine (mg/dl) | 7.9 (2.6)b | 10.8 (2.9)b | 13.3 (3.0)c | 0.007 |
| Protein catabolic rate normalized in previous 6 mo (g/kg per 24 h) | 1.04 (0.23)b | 1.05 (0.27)b | 1.07 (0.16)b | 0.982 |
| Aspartate aminotransferase (IU/L) | 24 (8)b | 25 (35)b | 14 (6)b | 0.750 |
| Alanine transaminase (IU/L) | 16 (5) | 23 (21) | – | 0.398 |
| Hemoglobin (g/dl) | 10.8 (1.0)b | 12.3 (1.2)c | 11.9 (2.2)c | 0.044 |
| Ferritin (ng/ml) | 257 (118)b | 566 (292)c | 981 (412)d | 0.001 |
| Kt/V | 1.6 (0.4)b | 1.6 (0.3)b | 1.9 (0.5)b | 0.283 |
| Urea reduction ratio (%) | 69 (9)b | 72 (9)b | 74 (11)b | 0.716 |
| Prehemodialysis mean arterial pressure (mmHg) | 105 (4)b | 109 (13)b | 118 (14)b | 0.221 |
| Prehemodialysis pulse pressure (mmHg) | 73 (14)b | 70 (14)b | 74 (11)b | 0.704 |
Results with the same letter are not statistically different, one-way ANOVA with Bonferroni post hoc test. iPTH, CAP, and CIP assay conducted by Scantibodies Laboratory in all study subjects. Results reported as mean (SD) unless otherwise indicated. IQR, interquartile range.
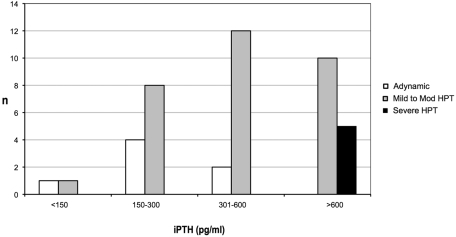
The results of the bone biopsy findings categorized by iPTH groups (<150, 150 to 300, 301 to 600, >600 pg/ml) is presented in Figure 1. Most (57%) ABD subjects were found in the 150- to 300-pg/ml range, whereas most of the mild to moderate HPT (40%) were found in the 301- to 600-pg/ml range. All of the severe HPT were found in the more than 600 pg/ml group.
Figure 1.
Bone histology of individual subjects categorized by intact parathyroid hormone (iPTH) values.
Correlations between PTH Peptides, BS-AP, and Vitamin D Doses and Histomorphometric Parameters
Coefficients of correlation between the various parameters are shown in Table 6. iPTH and CAP were correlated positively, although weakly, with bone volume. iPTH, CAP, and BS-AP correlated significantly with static, cellular, and dynamic parameters of bone turnover. The dose of vitamin D at time of biopsy was negatively associated with mineralization lag time (Table 6).
Table 6.
Coefficients of correlation between PTH peptides, BS-AP, vitamin D doses, and histomorphometric parameters of bonea
| iPTH | CAP | BS-AP | Vitamin D Dose | Cumulative Vitamin D Dose | |
|---|---|---|---|---|---|
| BV/TV | 0.34b | 0.30b | 0.12 | 0.06 | 0.12 |
| OV/BV | 0.44c | 0.36 | 0.56c | −0.23 | −0.06 |
| OS/BS | 0.45c | 0.35b | 0.48c | −0.19 | −0.02 |
| O.Th | 0.43c | 0.39b | 0.55c | −0.17 | −0.01 |
| NOb/BPm | 0.49c | 0.47c | 0.43c | −0.19 | −0.08 |
| ES/BS | 0.53c | 0.44c | 0.60c | 0.01 | 0.18 |
| E.De | 0.50c | 0.43c | 0.50c | −0.03 | 0.05 |
| NOc/BPm | 0.60c | 0.51c | 0.50c | 0.03 | 0.13 |
| FbS/BS | 0.55c | 0.50c | 0.54c | −0.01 | 0.09 |
| MARd | 0.64c | 0.54c | 0.30 | −0.32 | −0.18 |
| BFR/BS | 0.60c | 0.54c | 0.49c | 0.21 | 0.12 |
| MLt | −0.09 | −0.11 | 0.10 | −0.37b | −0.32 |
| Ac.f | 0.50c | 0.50c | 0.39b | 0.09 | 0.10 |
BV/TV, bone volume/tissue volume; OV/BV, osteoid volume/bone volume; OS/BS, osteoid surface/bone surface; O.Th, osteoid thickness; NOb/BPm, number of osteoblasts/bone perimeter; ES/BS, erosion surface/bone surface; E.De, erosion depth; NOc/BPm, number of osteoclasts/bone perimeter; FbS/BS, fibrosis surface/bone surface; MARd, mineral apposition rate/d; BFR/BS, bone formation rate/bone surface; MLt, mineralization lag time; Ac.f, activation frequency.
P < 0.05.
P < 0.01.
Correlations between Bone and Mineral Markers to iPTH
Correlations between iPTH and biochemical parameters at the time of bone biopsy were evaluated. Significant positive correlations were demonstrated between iPTH and CAP (r = 0.97; P < 0.001), CIP (r = 0.95; P < 0.001), BS-AP (r = 0.64; P < 0.001), serum creatinine (r = 0.52; P < 0.001), alkaline phosphatase (r = 0.51; P = 0.001), Ca × PO4 (r = 0.34; P = 0.026), and albumin (r = 0.32; P = 0.040).
Analysis of Achievement of K/DOQI Goals for ROD
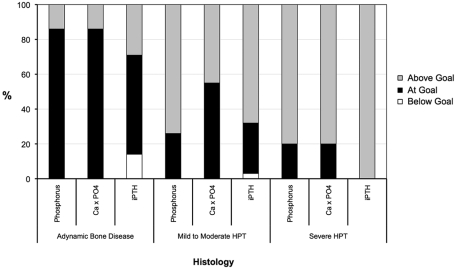
We evaluated overall attainment of K/DOQI goals for phosphorus (3.5 to 5.5 mg/dl), Ca × PO4 (<55 mg2/dl2), and iPTH (150 to 300 pg/ml) at the time of biopsy in each of the histologic groups (Figure 2). Most subjects in the ABD category had achieved the K/DOQI goals. Only 9.5% of the study population had simultaneously achieved the three K/DOQI goals, of which 75% demonstrated ABD on biopsy.
Figure 2.
Percentage of subjects at Kidney Disease Outcomes Quality Initiative (K/DOQI) goals for phosphorus (3.5 to 5.5 mg/dl), calcium-phosphate product (Ca × PO4; <55 mg2/dl2), and iPTH (150 to 300 pg/ml) by histologic category at time of biopsy.
Discussion
This study provides the most comprehensive analysis of CKD-MBD in AA hemodialysis subjects to date across the spectrum of ROD. Gupta et al. previously demonstrated that in AA hemodialysis patients, iPTH levels tend to be 1.5 to 2 times that of their non-AA counterparts (20); however, this study was limited by the lack of bone histology. Sawaya et al. (13) found that non-AA subjects with low bone turnover disease had lower iPTH (160 ± 41 pg/ml) as compared with AA patients (460 ± 115 pg/ml, P < 0.01). The study presented here extends these previous reports by evaluating the relationships of both traditional and nontraditional biomarkers to bone histology across the spectrum of ROD. This study confirms that AA subjects have iPTH levels that are variable and higher than expected in relation to bone histology. Although iPTH and PTH peptides correlated well with histomorphometric measurements of bone turnover, this study also found that BS-AP had a strong direct correlation. These relationships were also seen when subjects were grouped by histologic diagnosis, with BS-AP demonstrating the strongest association with histologic diagnosis. Despite this relationship, BS-AP remains a remarkably underutilized marker of bone turnover by the nephrology community. BS-AP is a useful, noninvasive, and readily available laboratory test that can be used to assist the clinician in making a more comprehensive and noninvasive assessment of bone turnover in AA subjects.
This study demonstrates that the K/DOQI iPTH cutoff of less than 150 pg/ml may not be the optimal cutoff in AA hemodialysis patients for identification of those with low bone turnover, because many patients with ABD were found above this level. This finding is also supported by a recent study conducted by Baretto et al. demonstrating that 88% of study subjects who had achieved K/DOQI iPTH targets demonstrated low bone turnover on bone biopsy (28). This lower cutoff of iPTH was defined based on several histormorphometric studies conducted in the previous 3 decades (7), in addition to outcomes data demonstrating increased mortality seen at both low (≤200 pg/ml) and higher levels of iPTH (>511 pg/ml) (2,3,18,29,30). However, the last decade has seen a paradigm shift in the management of CKD-MBD that may limit the modern day use of this cutoff, particularly in AA hemodialysis patients. In the referenced studies, most of the patients were white, were not on intravenous vitamin D therapy, and phosphate binding was achieved with aluminum compounds (7). The limited armamentarium of yesteryear (aluminum, oral calcitriol) has been replaced by various effective agents (intravenous vitamin D, calcium and noncalcium, nonaluminum-containing phosphate binders, and calcimimetics) that can now be utilized in the treatment of CKD-MBD. There are few data on the effects of these treatment modalities alone or in combination on bone histology (31–34) and overall outcomes, especially in AA subjects.
The study presented here also demonstrates that only 9% of our study population simultaneously attained all K/DOQI goals. This study was not designed to evaluate a generally controlled population; rather, AA subjects across a spectrum of iPTH values. However, this low proportion of patients is similar to the one observed in the data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) in over 4000 study subjects that demonstrated that only 8% of subjects were able to simultaneously achieve all K/DOQI goals at 6 mo (J Am Soc Nephrol 14: 269A–270A, 2003). Our similar results are likely explained, at least in this study population, by the higher iPTH values found in AA subjects. However, one important observation was that in the seven subjects with ABD, six (86%) had both controlled phosphorus and Ca × PO4, albeit in most patients a higher iPTH than expected. This is in contrast to the subjects with mild to moderate HPT, who as a group more commonly (23 of 31) had at least one of the parameters above the goal range, with varying iPTH values. In patients that appear best controlled, suspicion of ABD may be warranted. Conducting more invasive testing such as bone biopsy in these subjects may help determine the exact nature of bone histology and guide optimal treatment.
An intriguing observation from this study is that there were no patients presenting with osteomalacia or mixed uremic osteodystrophy. The lack of mineralization defect could most probably be attributed to the avoidance of aluminum exposure and the widespread use of vitamin D compounds. Also, none of the patients presented with normal bone histology, which is similar to more recent histomorphometric studies that have demonstrated low prevalence of normal bone histology (28,31). In the mild form of HPT, the rate of bone turnover may be in the normal range. However, the presence of abnormalities such as woven osteoid, presence of peritrabecular fibrosis and/or osteoclasts of increased size and containing numerous nuclei, which reflect the effects of iPTH on bone, cannot be classified as normal. Positive correlations between iPTH, CAP, BS-AP, and bone histomorphometric parameters (static and dynamic) were shown and there was little difference in the correlation coefficients for bone parameters between iPTH, CAP, and BS-AP.
Traditional biochemical markers of CKD-MBD correlated well with biopsy findings; however, the sample was likely not large enough to determine additional markers that may be useful in the noninvasive assessment of bone histology. A relationship between the CAP/CIP ratio and bone histology was not detected in this sample; however, the study population was likely not powered for evaluation of this parameter. Increased total alkaline phosphatase and BS-AP were associated with higher iPTH levels and more severe HPT on bone biopsy and may provide additional noninvasive insight for the clinician.
There were some other interesting relationships demonstrated in this AA population. Serum creatinine and ferritin levels were lowest in patients with ABD and demonstrated an association with iPTH and histologic diagnosis. The ferritin observation may be explained by an inflammatory component or perhaps factors related to anemia and its management. The association of serum creatinine to both iPTH (r = 0.52) and bone histology was rather strong in this population. This observation is in keeping with previous studies that have demonstrated a direct relationship between serum creatinine and phosphorus with iPTH values (18,29). A possible explanation may be a decreased muscle mass and/or malnutrition in the ABD group, although relevant data related to nutritional status and muscle mass were not ascertained. However, there were no differences between the histologic groups in relation to the nutritional parameters collected, although the sample size was small. It is still unclear how creatinine may relate to bone histology, and it may indeed involve factors associated with the malnutrition-inflammation cachexia syndrome in these patients (35). Despite our observation that dry weight was similar across the groups, it may also be possible that a change in distribution of fat exists in the low turnover group (decreased muscle/fat ratio) that may have accounted for similar weight yet lower creatinine. These interesting observations warrant further study.
Our study provides important and useful information for the management of AA hemodialysis patients; however, it may be limited by a relatively small sample size. There was a low prevalence of ABD in the study presented here. However, it is difficult to define the exact prevalence of ABD in the hemodialysis population, because studies have used various definitions (i.e., defined by iPTH or defined by histomorphometry), have different racial composition, and different treatment modalities. One of the largest studies evaluating ABD by biopsy found an approximate 20% prevalence of low bone turnover in dialysis patients, which is consistent with the results of this study (36).
In conclusion, this study describes the biochemical and treatment characteristics across a range of histologic diagnoses in AA hemodialysis subjects undergoing standard of care treatment for CKD-MBD. This study confirms that AA subjects have iPTH levels that are variable and higher than expected in relation to bone histology. AA subjects with ABD had iPTH values higher than expected but appeared to have well controlled calcium and phosphorus. iPTH, PTH peptides, and BS-AP correlated well with histomorphometric measurements of bone turnover and when subjects were grouped by histologic diagnosis. BS-AP may be an additional useful marker in AA hemodialysis patients for the noninvasive assessment of bone histology. Only 9.5% of subjects were within suggested K/DOQI ranges for Ca × PO4, phosphorus, and iPTH simultaneously, of which 75% demonstrated ABD on biopsy.
Disclosures
None.
Acknowledgments
A reference in this article was previously published in abstract form (J Am Soc Nephrol 14:269A-270A, 2003). This study was sponsored in part by Scantibodies, Inc., Santee, California, and the Kentucky Nephrology Research Trust. The authors thank Juliana Van Willigen for technical support and Dr. Anatole Besarab for his critical review of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Lowrie EG, Lew NL: Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15: 458–482, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A: Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: Evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol 15: 770–779, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Malluche HH, Monier-Faugere MC: Renal osteodystrophy: What's in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol 65: 235–242, 2006 [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]:S1–S201, 2003 [PubMed] [Google Scholar]
- 8.Abrams SA, O'Brien KO, Liang LK, Stuff JE: Differences in calcium absorption and kinetics between black and white girls aged 5–16 years. J Bone Miner Res 10: 829–833, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM: Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr 85: 1657–1663, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Parisien M, Cosman F, Morgan D, Schnitzer M, Liang X, Nieves J, Forese L, Luckey M, Meier D, Shen V, Lindsay R, Dempster DW: Histomorphometric assessment of bone mass, structure, and remodeling: A comparison between healthy black and white premenopausal women. J Bone Miner Res 12: 948–957, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Weinstein RS, Bell NH: Diminished rates of bone formation in normal black adults. N Engl J Med 319: 1698–1701, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez OM, Isakova T, Andress DL, Levin A, Wolf M: Prevalence and severity of disordered mineral metabolism in blacks with chronic kidney disease. Kidney Int 73: 956–962, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Sawaya BP, Butros R, Naqvi S, Geng Z, Mawad H, Friedler R, Fanti P, Monier-Faugere MC, Malluche HH: Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney Int 64: 737–742, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 15.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC: Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15: 1943–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Atsumi K, Kushida K, Yamazaki K, Shimizu S, Ohmura A, Inoue T: Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis 33: 287–293, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Coco M, Rush H: Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36: 1115–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Avram MM, Mittman N, Myint MM, Fein P: Importance of low serum intact parathyroid hormone as a predictor of mortality in hemodialysis and peritoneal dialysis patients: 14 years of prospective observation. Am J Kidney Dis 38: 1351–1357, 2001 [DOI] [PubMed] [Google Scholar]
- 19.U.S. Renal Data System 2006 Annual Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases, 2006 [Google Scholar]
- 20.Gupta A, Kallenbach LR, Zasuwa G, Divine GW: Race is a major determinant of secondary hyperparathyroidism in uremic patients J Am Soc Nephrol 11:330–334, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Llach F, Yudd M: Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis 38: S45–S50, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Malluche HH, Faugere MC: Atlas of Mineralized Bone Histology, New York, Karger, 1986 [Google Scholar]
- 23.Goldner J: A modification of the Masson trichrome technique for routine laboratory purposes. Am J Pathol 14: 237–243, 1938 [PMC free article] [PubMed] [Google Scholar]
- 24.Lillie PD, Fullmer HM: Histopathologic Technique and Practical Histochemistry, New York, McGraw Hill, 1976 [Google Scholar]
- 25.Denton J, Freemont AJ, Ball J: Detection of distribution of aluminum in bone. J Clin Pathol 37: 136–142, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malluche HH, Sherman D, Meyer W, Massry SG: A new semiautomatic method for quantitative static and dynamic bone histology. Calcif Tissue Int 34: 439–448, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Manaka RC, Malluche HH: A program package for quantitative analysis of histologic structure and remodeling dynamics of bone. Comput Programs Biomed 13: 191–202, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Baretto FC, Baretto DV, Moyses RMA, Neves KR, Canziani MEF, Draibe SA, Joregetti V, Carvalho AB: K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int 73: 771–777, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Avram MM, Sreedhara R, Fein P, Oo KK, Chattopadhyay J, Mittman N: Survival on hemodialysis and peritoneal dialysis over 12 years with emphasis on nutritional parameters. Am J Kidney Dis 37: S77–S80, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Ferreira A, Frazao JM, Monier-Faugere MC, Gil C, Galvao J, Oliveira C, Baldaia J, Rodrigues I, Santos C, Ribeiro S, Hoenge RRM, Duggal A, Malluche HH: Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol 19: 405–412, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malluche HH, Monier-Faugere MC, Wang G, Fraza OJ, Charytan C, Coburn JW, Coyne DW, Kaplan MR, Baker N, McCary LC, Turner SA, Goodman WG: An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol 69: 269–278, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Malluche HH, Siami GA, Swanepoel C, Wang GH, Mawad H, Confer S, Smith M, Pratt RD, Monier-Faugere MCOn behalf of the Spd405–307 Lanthanum Carbonate Study Group: Improvements in renal osteodystrophy in patients treated with lanthanum carbonate for two years. Clin Nephrol 70:284–295, 2008 [PubMed] [Google Scholar]
- 34.Malluche HH, Mawad H, Monier-Faugere MC: Effects of treatment of renal osteodystrophy on bone histology. Clin J Am Soc Nephrol 3[Suppl 3]: S157–S163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaf J: Adynamic bone disease and malnutrition-inflammation-cachexia syndrome. Kidney Int 71: 1326; author reply 1327, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Malluche HH, Monier-Faugere MC: Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl 38: S62–S67, 1992 [PubMed] [Google Scholar]