Abstract
Background and objectives: This study investigated whether the slope of estimated GFR is different between nonproteinuric subjects with and without diabetes, and what clinical factors are associated with the GFR slope.
Design, setting, participants, & measurements: An observational cohort study was performed in 923 subjects, and the predictive value of baseline variables on the GFR slope was investigated.
Results: On the basis of the median 3-yr follow-up and 7 measurements of GFR, GFR slope (%/yr, median and interquartile range) was significantly larger in subjects with diabetes (−2.39 (−4.86 to 0.15), n = 729) than in those without diabetes (−1.02 (−4.28 to 1.37), n = 194), and this difference remained significant with or without presence of hypertension. After adjustments for confounding factors, predictors of GFR decline were found to be baseline high values of glycosylated hemoglobin A1C (HbA1C), GFR, systolic blood pressure, and low plasma total protein in subjects with diabetes, whereas only the latter two were significant in subjects without diabetes. In subjects with diabetes, the high GFR was accounted for by high HbA1C at baseline, and the predictors of GFR decline differed between those with and without hypertension, or with high and low baseline GFR. Any combination of the predictors showed increased risk for GFR decline.
Conclusions: GFR slope is substantially affected by multiple factors at various stages. The degree of chronic hyperglycemia is likely to play a crucial role in elevating GFR and accelerating the decline in patients with type 2 diabetes even from the normoalbuminuric stage.
Once proteinuria occurs and/or chronic renal failure develops, the rate of decline of renal function in diabetic nephropathy is considerably higher than the rate in other renal diseases (1–3). We believed that patients with diabetes suffer a faster decline of renal function than subjects without diabetes, explaining why the number of patients with diabetic end-stage renal failure is increasing (1). However, is this true in subjects without proteinuria? Sufficient data are not available from studies that investigated individuals without proteinuria and compared the rate of decline of renal function between subjects with and without diabetes.
Progressive loss of GFR in patients with diabetes has been considered to occur after development of micro- and macroalbuminuria. However, recent studies have identified that among patients with type 2 diabetes and normoalbuminuria, some already have renal insufficiency (4–7) and some will develop renal insufficiency without any development of albuminuria (8). A recent paper indicated the risk of early progressive renal function decline in nonproteinuric patients with type 1 diabetes (9). Putative predictors for renal function loss are known to include proteinuria, dietary protein intake, systemic blood pressure (BP), glomerular hypertension, hypoproteinemia (decreased oncotic pressure), anemia, smoking, hyperlipidemia, and glycemic control (1,10). But it is not certain in normoalbuminuric patients what factors would affect the decline in renal function.
We investigated (1) whether the slope of estimated GFR based on serial measurements of serum creatinine is different between subjects with and without diabetes in individuals without proteinuria, and (2) what clinical factors are associated with the GFR slope in these subjects.
Patients and Methods
Study Population
An observational cohort study was performed. All consecutive patients who visited the outpatient clinic of Jiyugaoka Internal Medicine were enrolled between 2004 and 2006. Most of the patients had type 2 diabetes and/or hypertension, because the clinic is specialized in diabetes care. Individuals who had been already treated for diabetes or hypertension were included in the study in April 2004. Individuals whose treatments for diabetes and/or hypertension were newly started after April 2004 were included in the study after the 5 visits, at least more than 3 mo, when their BP control and/or blood glucose control were stabilized. Subjects without type 2 diabetes and without hypertension visited the clinic because of common diseases other than diabetes or hypertension, such as hyperlipidemia, hyperuricemia, gastric ulcer, or neurosis. All subjects that fulfilled the following inclusion and exclusion criteria participated in the study. Individuals who attended the clinic for more than 1 yr and had more than at least three measurements of serum creatinine after 2004 were eligible for inclusion. Patients with type 1 diabetes were not included. For individuals with type 2 diabetes, those with normoalbuminuria, defined by an urinary albumin to creatinine ratio (ACR) less than 30 mg/g creatinine, were eligible, and those with an ACR of 30 mg/g creatinine or more were excluded. For individuals without diabetes, ACR was unfortunately not measured. Alternatively, we confined individuals without diabetes to those whose three consecutive measurements for proteinuria by dipstick were all negative. Subjects were followed up to March 2008. The study was approved by the local ethical committee and was carried out in accordance with the Helsinki Declaration II.
Type 2 diabetes was diagnosed according to the Japan Diabetes Society (JDS) criteria (11,12); that is, principally fasting blood glucose of 7.0 mmol/L (126 mg/dl) or more or casual blood glucose of 11.1 mmol/L (200 mg/dl) or more. Classification as not having diabetes was made when any measurement (median number of seven measurements during the follow-up) of fasting or casual blood glucose revealed no greater than the above levels. Hypertension was defined by a systolic BP (SBP) of more than 140 mmHg or diastolic BP (DBP) above 90 mmHg, or both, or patients already being treated with antihypertensive drugs. Hyperlipidemia was defined as serum concentrations of total cholesterol (TC) of more than 220 mg/dl, triglycerides (TG) of more than 150 mg/dl, HDL cholesterol (HDL) of less than 40 mg/dl, or patients already being treated by lipid lowering agents. LDL cholesterol level was calculated by Friedewald's formula.
Measurements
The BP was measured with an appropriately sized cuff in the sitting position after resting for more than 5 min. Three measurements on different days were recorded, and the average was used for the analysis. Nonfasting blood samples were obtained for measurements of glycosylated hemoglobin A1C (HbA1C), plasma concentrations of glucose and total protein (TP), serum concentrations of creatinine and lipids, and blood cell counts at baseline and 1-yr follow-up. HbA1C was measured by HPLC (normal range, 4.3 to 5.8%) and was certified by the American National Glycohemoglobin Standardization Program (NGSP = 1.019 × JDS + 0.30). Serum and urinary concentrations of creatinine were measured by an enzymatic method with isotope-dilution mass spectrometry (IDMS)-traceable calibrator (N-assay L Creatinine Kit, Nittoubo Medical Co., Tokyo, Japan). The method was consistent throughout the study period with interassay variation coefficients of 0.38 and 0.43% at creatinine concentrations of 1.030 and 4.100 mg/dl, respectively. Urinary albumin was measured by a turbidimetric immunoassay. The urinary albumin excretion rate (AER) was measured using the ACR in random urine samples. The GFR was estimated using the following equation recently generated by the Japanese Society of Nephrology (JSN): GFR (ml/min/1.73 m2) = 194 × serum creatinine−1.094 × Age−0.287 × 0.739 (if female) (13). In a Japanese general population study by JSN (personal communication with Professor E. Imai and Professor M. Horio), the proportions of GFR (> = 90/60 to 89/<60) estimated by this equation were 18/75/8% at the age of 50 to 59 yr, and 13/71/16% at the age of 60 to 69 yr. Serum concentration of creatinine was measured every 4 to 6 mo in each individual. The first two values of GFR from the entry into the study were recorded and the average was used as the baseline GFR value in consideration of the physiologic variations for serum concentrations of creatinine.
Statistical Analyses
For each subject, a linear regression model of time on GFR (least-squares method) was created, and the slope of the regression line was used to estimate the subject's change in GFR over time. Then the GFR slope was expressed as percent per year by dividing the slope by the baseline GFR value. Because no definite criteria for GFR decliner exist, a GFR slope of less than −4.0%/yr, which was obtained from the control subjects aged 50 to 70 yr in the Baltimore Aging Study (14), was used as the threshold to define GFR decliner. Results are given as the mean ± SD unless otherwise stated. The significance of differences between the two groups was determined by χ2 tests for categorical variables and the unpaired t test for continuous variables. Multiple linear regression was used to analyze the associations of variables with GFR slope values (or baseline GFR values) controlling for potential confounders. Multiple logistic regression was used to explore the determinants of GFR decliners and the interaction effects of potential risk factors controlling for potential confounders. The validity of the models was confirmed by conducting the likelihood-ratio test (Hosmer–Lemeshow test). P values under 5% (two-tailed) were considered significant. All analyses were performed with the statistical software package Dr. SPSS II (SPSS Japan Inc., Tokyo, Japan).
Results
Summary of Study Subjects
For all subjects, the median follow-up time was 3.1 yr. The median number of GFR measurements per subject was seven. Clinical characteristics of the subjects at baseline according to the presence of diabetes are shown in Table 1. Subjects without diabetes were more likely to have hypertension and hyperlipidemia. Subjects with diabetes were more likely to be young and male and to have higher values of body mass index, HbA1C, GFR, hemoglobin, and length of follow-up, and lower values of SBP, DBP, HDL, LDL, and platelet.
Table 1.
Baseline characteristics of subjects according to the presence of diabetes.
| Characteristic | Subjects with Diabetes | Subjects without Diabetes | P |
|---|---|---|---|
| n | 729 | 194 | |
| Age (yr) | 58 ± 12 | 61 ± 12 | 0.0098 |
| Male (%) | 504 (69.1) | 79 (40.7) | <0.0001 |
| BMI (kg/m2) | 25.7 ± 4.1 | 24.8 ± 3.9 | 0.0042 |
| HbA1C (%) | 6.7 ± 1.1 | 5.3 ± 0.3 | <0.0001 |
| Serum Cr (mg/dl) | 0.75 ± 0.20 | 0.75 ± 0.25 | 0.9861 |
| Baseline GFR (ml/min/1.73 m2)a | 78.9 (68.4 to 90.8) | 72.3 (63.4 to 82.1) | <0.0001 |
| SBP (mmHg) | 124 ± 14 | 131 ± 17 | <0.0001 |
| DBP (mmHg) | 68 ± 11 | 73 ± 12 | <0.0001 |
| Hypertension (%) | 375 (51.4) | 148 (76.3) | <0.0001 |
| RASI user (%) in patients with hypertension | 287 (76.5) | 97 (65.5) | 0.0141 |
| HDL (mg/dl) | 53 ± 13 | 58 ± 15 | <0.0001 |
| LDL (mg/dl) | 109 ± 30 | 114 ± 33 | 0.0312 |
| TG (mg/dl) | 158 ± 124 | 161 ± 118 | 0.7841 |
| Hyperlipidemia (%) | 485 (66.5) | 148 (76.3) | 0.0119 |
| Plasma TP (g/dl) | 7.1 ± 0.6 | 7.3 ± 0.5 | 0.0002 |
| Hemoglobin (g/dl) | 14.1 ± 1.5 | 13.7 ± 1.7 | 0.0015 |
| Platelet (104/mm3) | 22.5 ± 5.8 | 23.7 ± 5.4 | 0.0080 |
| Length of follow-up (yr)a | 3.1 (2.3 to 3.6) | 2.9 (2.0 to 3.3) | 0.0001 |
| Number of Cr measurementsa | 7 (6 to 8) | 6 (4 to 7) | 0.0598 |
BMI, body mass index; Cr, creatinine; RASI, renin-angiotensin system inhibitor.
Median and interquartile range are given.
GFR Slope and Characteristics of the GFR Decliners
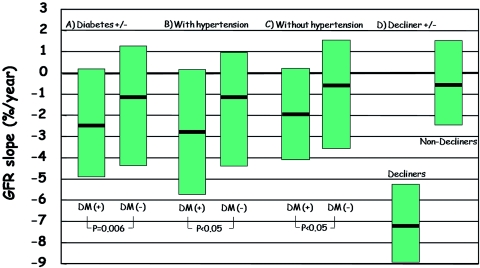
GFR slope (%/yr) was significantly larger in subjects with diabetes than in those without diabetes, and this difference remained significant in subgroups with or without hypertension (Figure 1). To investigate the association of GFR slope with clinical variables, subjects were classified into GFR decliners and nondecliners according to the presence of diabetes (Table 2). The proportion of decliners was slightly but not significantly higher in subjects with diabetes than in those without diabetes (30.1% versus 26.8%, P = 0.3618). In subjects with diabetes, GFR decliners were more likely to be female, to have hypertension, and to have higher baseline values of GFR and HbA1C and a lower value of plasma TP. In subjects without diabetes, GFR decliners had only higher values of SBP and DBP and a lower value of plasma TP. At second follow-up, GFR decliners with diabetes had higher HbA1C and SBP, but variables such as lipid profiles and others were similar between the two groups (data not shown). AER was investigated only in subjects with diabetes. At baseline the ACR value was higher in decliners than in nondecliners, but it was similar at the end of follow-up and the number who developed microalbuminuria (ACR ≥ 30 mg/g creatinine) was also similar between the two groups.
Figure 1.
Comparison of GFR slope (%/yr) (A) between subjects with and without diabetes, according to the presence of diabetes in individuals (B) with and (C) without hypertension, and (D) between decliners and nondecliners. Median and interquartile ranges are given. DM, type 2 diabetes.
Table 2.
Comparison of clinical variables between GFR decliners and nondecliners in subjects with and without diabetesa
| Variable | Subjects with Diabetes |
Subjects without Diabetes |
||||
|---|---|---|---|---|---|---|
| Decliner (n = 222) | Nondecliner (n = 507) | P | Decliner (n = 52) | Nondecliner (n = 142) | P | |
| Age (yr) | 59 ± 12 | 58 ± 11 | 0.7054 | 63 ± 11 | 60 ± 12 | 0.0957 |
| Male (%) | 139 (62.6) | 365 (72.0) | 0.0149 | 21 (40.4) | 58 (40.8) | 0.9999 |
| BMI (kg/m2) | 26.0 ± 4.4 | 25.6 ± 4.0 | 0.2797 | 24.7 ± 3.8 | 24.8 ± 4.0 | 0.7998 |
| ACR | ||||||
| at baseline | 12.8 (8.2 to 19.4)c) | 11.1 (7.8 to 17.0)c) | 0.0115 | |||
| at end of follow-up | 11.2 (7.4 to 20.5)c) | 11.0 (7.2 to 18.7)c) | 0.4993 | |||
| Microalbuminuria at end of follow-up (%) | 32 (14.4) | 70 (13.8) | 0.9190 | |||
| Serum Cr (mg/dl) | ||||||
| at baseline | 0.70 ± 0.18 | 0.77 ± 0.20 | <0.0001 | 0.70 ± 0.17 | 0.77 ± 0.27 | 0.0814 |
| at end of follow-up | 0.87 ± 0.28 | 0.78 ± 0.19 | <0.0001 | 0.83 ± 0.19 | 0.74 ± 0.20 | 0.0067 |
| GFR (ml/min/1.73 m2)b | ||||||
| at baseline | 81.9 (70.8 to 98.0) | 77.8 (67.8 to 88.5) | 0.0016 | 74.9 (66.3 to 81.8) | 71.2 (62.4 to 82.7) | 0.3887 |
| at end of follow-up | 66.8 (55.6 to 80.3) | 75.4 (65.5 to 85.3) | <0.0001 | 62.8 (54.3 to 70.1) | 71.7 (63.8 to 84.2) | <0.0001 |
| change (follow-up − baseline) | −13.9 (−19.6 to −8.5) | −2.3 (−6.8 to 1.8) | <0.0001 | −10.0 (−15.6 to −6.5) | 0.5 (−3.9 to 5.0) | <0.0001 |
| GFR slope per year (ml/min/1.73 m2)b | −5.06 (−7.67 to −3.18) | −0.32 (−1.48 to 1.20) | <0.0001 | −4.36 (−6.79 to −3.52) | 0.26 (−0.78 to 2.00) | <0.0001 |
| GFR slope (%/yr)b | −6.13 (−9.32 to −4.07) | −0.50 (−1.92 to 1.67) | <0.0001 | −5.97 (−9.32 to −4.56) | 0.36 (−1.04 to 3.13) | <0.0001 |
| HbA1C (%) | ||||||
| at baseline | 6.9 ± 1.2 | 6.5 ± 0.9 | <0.0001 | 5.2 ± 0.4 | 5.3 ± 0.3 | 0.4594 |
| at second follow-upc | 6.8 ± 1.0 | 6.6 ± 0.9 | 0.0438 | 5.3 ± 0.3 | 5.3 ± 0.3 | 0.4429 |
| Hypertension (%) | 127 (57.2) | 248 (48.9) | 0.0476 | 48 (76.9) | 108 (76.1) | 0.9999 |
| SBP (mmHg) | ||||||
| at baseline | 126 ± 14 | 124 ± 14 | 0.0537 | 136 ± 18 | 129 ± 16 | 0.0195 |
| at second follow-upc | 125 ± 17 | 121 ± 14 | 0.0045 | 126 ± 20 | 125 ± 14 | 0.6167 |
| DBP (mmHg) | ||||||
| at baseline | 68 ± 11 | 68 ± 10 | 0.9131 | 77 ± 14 | 72 ± 12 | 0.0087 |
| at second follow-upc | 68 ± 12 | 67 ± 11 | 0.6028 | 68 ± 12 | 69 ± 10 | 0.4554 |
| RASI use in patients with hypertension (%) | ||||||
| at baseline | 96 (43.2) | 191 (37.7) | 0.1821 | 26 (50.0) | 71 (50.0) | 1.0000 |
| at second follow-upc | 110 (49.5) | 212 (41.8) | 0.0637 | 30 (57.7) | 76 (53.5) | 0.7233 |
| Hyperlipidemia (%) | 142 (64.0) | 343 (67.7) | 0.3756 | 40 (76.9) | 108 (76.1) | 0.9999 |
| HDL (mg/dl) | 54 ± 14 | 53 ± 13 | 0.3279 | 57 ± 15 | 59 ± 15 | 0.4021 |
| LDL (mg/dl) | 108 ± 33 | 110 ± 28 | 0.3809 | 110 ± 34 | 116 ± 32 | 0.2210 |
| TG (mg/dl) | 158 ± 114 | 158 ± 128 | 0.9894 | 186 ± 140 | 152 ± 108 | 0.0744 |
| Plasma TP (g/dl) | ||||||
| at baseline | 7.0 ± 0.7 | 7.1 ± 0.6 | 0.0248 | 7.2 ± 0.7 | 7.3 ± 0.4 | 0.0534 |
| at second follow-upc | 7.1 ± 0.4 | 7.1 ± 0.4 | 0.4436 | 7.1 ± 0.4 | 7.1 ± 0.4 | 0.4207 |
| Hemoglobin (g/dl) | 14.0 ± 1.6 | 14.1 ± 1.5 | 0.2625 | 13.8 ± 1.7 | 13.6 ± 1.7 | 0.5928 |
| Platelet (104/mm3) | 22.2 ± 6.0 | 22.6 ± 5.7 | 0.3576 | 23.7 ± 5.6 | 23.7 ± 5.3 | 0.9743 |
See Patients and Methods for definition.
Median and interquartile range are given.
One year after baseline.
Determinants of Baseline GFR
Because significantly higher baseline GFR values were found only in decliners with diabetes, we investigated the determinants of the baseline GFR in subjects with diabetes. In multiple linear regression analysis with the baseline GFR as the dependent variable and HbA1C, SBP; body mass index; serum concentrations of HDL, LDL, and TG; hemoglobin; platelet; and plasma TP as the independent variables, significant relationships for the baseline GFR values (shown as follows by milliliters of change in GFR by units of change in each risk factor as regression coefficient) were noted with HbA1C (2.8 per %, P < 0.0001), HDL (0.2 per mg/dl, P = 0.043), TG (−0.3 per mg/dl, P < 0.0001), hemoglobin (3.6 per g/dl, P < 0.0001), and platelet (0.5 per 104/mm3, P < 0.0001).
Comparison of GFR Values when the Casual Blood Glucose during the Follow-Up Was at the Maximum and the Minimum
We examined whether the GFR value could be affected by the simultaneously measured casual blood glucose level within a subject. GFR value obtained at the maximal blood glucose during the follow-up was significantly higher than that at the minimal blood glucose in subjects with diabetes (GFR at blood glucose; 78.0 ± 20.0 ml/min/1.73 m2 at 244 ± 81 mg/dl versus 76.0 ± 18.6 ml/min/1.73 m2 at 112 ± 31 mg/dl, P < 0.0001 by paired t test), but not in those without diabetes (72.5 ± 16.5 ml/min/1.73 m2 at 135 ± 31 mg/dl versus 73.5 ± 15.9 ml/min/1.73 m2 at 102 ± 15 mg/dl, NS).
Effect of Baseline Variables on GFR Slope
In multiple linear regression analysis with the GFR slope (%/yr) as the dependent variable and baseline factors described in Table 3 as the independent variables, significant relationships for the GFR slope were noted with baseline values of HbA1C, GFR, SBP, and plasma TP in all subjects and subjects with diabetes. The NS variables were not shown. HbA1C and baseline GFR were not related with the GFR slope in subjects without diabetes. In subjects with diabetes, the GFR slope was significantly larger in those with hypertension than in those without (P = 0.0251), and larger in those with high GFR than in those with low GFR (P = 0.0003). Baseline GFR was a significant predictor of GFR decline in each subgroup, whereas HbA1C was not associated with the GFR slope in a subgroup with low GFR. Baseline SBP was significant only in a subgroup with low GFR, and baseline TP was significant only in subgroups without hypertension and with high GFR.
Table 3.
Results of multiple linear regression analysis to assess the significance of baseline variables on GFR slope in all subjects and those with and without diabetesab
| Characteristic GFR slope (%/yr)d | All Subjects n = 923 |
Subjects with Diabetes |
Subjects without Diabetes (n = 194) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Subjects with Diabetes (n = 729) |
Hypertension |
Baseline GFR (ml/min/1.73 m2) |
||||||||||||
| With (n = 375) |
Without (n = 354) |
≥77.4 (n = 390) |
<77.4 (n = 339) |
|||||||||||
| −2.19 (−4.72 to 0.52) | −2.39 (−4.86 to 0.15) | −2.81 (−5.88 to 0.11) | −2.00 (−4.34 to 0.28) | −2.70 (−5.27 to −0.73) | −1.65 (−4.36 to 0.99) | −1.02 (−4.28 to 1.37) | ||||||||
| RCc (0.11) | P | RC (0.11) | P | RC (0.09) | P | RC (0.18) | P | RC (0.19) | P | RC (0.13) | P | RC (0.15) | P | |
| Baseline HbA1C, per % | −0.832 | 0.000 | −0.791 | 0.000 | −0.776 | 0.041 | −0.691 | 0.001 | −0.971 | 0.000 | −0.437 | 0.224 | −0.408 | 0.779 |
| Baseline GFR, per 10 ml/min/1.73 m2 | −0.712 | 0.000 | −0.755 | 0.000 | −0.825 | 0.000 | −0.720 | 0.000 | −0.487 | 0.025 | −0.925 | 0.003 | −0.556 | 0.059 |
| Baseline SBP, per 10 mmHg | 0.460 | 0.000 | −0.386 | 0.009 | −0.401 | 0.083 | −0.247 | 0.235 | −0.264 | 0.218 | −0.432 | 0.048 | −0.672 | 0.016 |
| Baseline plasma TP, per g/dl | 1.176 | 0.000 | 0.969 | 0.004 | 0.621 | 0.225 | 1.443 | 0.001 | 2.016 | 0.000 | 0.217 | 0.623 | 2.209 | 0.011 |
Subjects with diabetes were subsequently analyzed according to the presence of hypertension and baseline values of GFR.
The independent variables were baseline factors such as age; BMI; gender; HbA1C; SBP; RASI use; baseline GFR; serum concentrations of HDL, LDL, and TG; plasma TP; hemoglobin; and platelet.
Regression coefficient (RC) means milliliters of change in GFR by units of change in the risk factors; parentheses indicate the R2 value.
Median and interquartile range are given. GFR value of 77.4 ml/min/1.73 m2 was the median in all subjects.
Determinants of GFR Decliners
A multiple logistic regression analysis with GFR decliner as the dependent variable and the baseline factors listed in Table 3 as the independent variables revealed that high values of HbA1C and SBP, a low value of plasma TP, and female gender increased the risk for GFR decline in all subjects and subjects with diabetes, and other independent variables were NS (Table 4). High baseline GFR was significant only in subjects with diabetes. The multiple logistic model was associated with a χ2 in a log-likelihood test of 54.3 (P < 0.0001). Further investigation of HbA1C at baseline and second follow-up indicated baseline HbA1C as a more important determinant of GFR decline, because median GFR slopes (categorized by baseline HbA1C/follow-up HbA1C) were −3.86 (≥7.0/≥7.0, n = 113), −3.92 (≥7.0/<7.0, n = 95), −1.84 (<7.0/≥7.0, n = 81), and −2.32 (<7.0/<7.0, n = 606). In subjects without diabetes, low TP was the only risk for GFR decliner with borderline significance. Interaction effects of two risk factors from high HbA1C, high GFR, high SBP, low TP, and female gender were all significant (Table 5).
Table 4.
Results of the multiple logistic regression analysis for determinants of GFR decliner in all subjects and those with and without diabetesa
| Determinantb | All Subjects |
Subjects with Diabetes |
Subjects without Diabetes |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Baseline GFR (≥77.4 versus <77.4 ml/min/1.73 m2) | 1.34 (0.76 to 1.86) | 0.087 | 1.49 (1.03 to 2.18) | 0.036 | 0.87 (0.41 to 1.87) | 0.723 |
| Baseline HbA1C | ||||||
| <6.0% | 1.0 (reference) | 1.0 (reference) | 0.72 (0.23 to 2.27)c | 0.571 | ||
| 6.0 to 6.9% | 1.31 (0.91 to 1.87) | 0.143 | 1.42 (0.89 to 2.27) | 0.141 | ||
| ≥7.0% | 2.64 (1.77 to 3.94) | 0.000 | 2.93 (1.76 to 4.87) | 0.000 | ||
| Baseline SBP (≥127 versus <127 mmHg) | 1.56 (1.17 to 2.18) | 0.003 | 1.50 (1.06 to 2.13) | 0.023 | 1.94 (0.86 to 4.34) | 0.109 |
| Baseline plasma TP (≤7.1 versus >7.1 g/dl) | 1.51 (1.10 to 2.06) | 0.010 | 1.44 (1.02 to 2.01) | 0.041 | 2.04 (0.99 to 4.23) | 0.055 |
| Gender (female versus male) | 1.63 (1.10 to 2.40) | 0.015 | 1.59 (1.02 to 2.45) | 0.040 | 1.50 (0.61 to 3.70) | 0.582 |
The analysis was performed with the GFR decliner as the dependent variable and age; BMI; gender; HbA1C; SBP; RASI use; baseline GFR; serum concentrations of HDL, LDL and TG; plasma TP; hemoglobin; and platelet as the independent variables.
Median values were 77.4 ml/min/1.73 m2 for GFR, 127 mmHg for SBP, and 7.1 g/dl for plasma TP. OR, odds ratio; CI, confidence interval.
HbA1C was not categorized but used as a continuous variable.
Table 5.
Interaction effects of two risk factors from high HbA1C, high GFR, high SBP, low TP, and female on determining GFR decliner in subjects with diabetesa
| Determinantb | OR (95% CI) | P |
|---|---|---|
| High HbA1C + high GFR | 3.54 (2.14 to 5.85) | 0.000 |
| High HbA1C + high SBP | 3.19 (1.88 to 5.40) | 0.000 |
| High HbA1C + low TP | 3.12 (1.81 to 5.37) | 0.000 |
| High HbA1C + female | 3.47 (1.89 to 6.37) | 0.000 |
| High GFR + high SBP | 2.34 (1.38 to 3.97) | 0.002 |
| High GFR + low TP | 2.33 (1.39 to 3.93) | 0.001 |
| High GFR + female | 2.60 (1.45 to 4.67) | 0.001 |
| High SBP + low TP | 2.19 (1.30 to 3.71) | 0.003 |
| High SBP + female | 2.38 (1.30 to 4.34) | 0.005 |
| Low TP + female | 2.42 (1.34 to 4.36) | 0.003 |
The interaction effects of two risk factors were explored by the presence/absence of the two factors with a reference as having both absences, after adjustment for other variables listed in Table 4.
High HbA1C, high GFR, high SBP, and low TP were defined as HbA1C ≥ 7.0, GFR ≥ 77.4 ml/min/1.73 m2, SBP ≥ 127 mmHg, and TP ≤ 7.1 g/dl according to the results in Table 4.
Discussion
This study showed that the GFR slope was larger in subjects with type 2 diabetes and normoalbuminuria than in those without diabetes without proteinuria. This result remained significant whether or not the subjects also had hypertension. High SBP and low plasma TP were found to be predictors of GFR decline in subjects without diabetes. In subjects with diabetes, high baseline GFR and high HbA1C were also found to be significant predictors. To our knowledge this is the first study to report that chronic hyperglycemia elevates baseline GFR values and accelerates subsequent GFR decline in subjects with diabetes.
Variables Affecting the GFR Slope: Baseline GFR and HbA1C
The high baseline GFR, potentially being elevated by the high HbA1C, significantly affected the subsequent greater rate of decline in GFR only in subjects with diabetes. Lack of significance in subjects without diabetes supports the important role in diabetes. The effect of hyperglycemia on increasing GFR was indicated in patients with type 1 (15,16) and type 2 diabetes (17) with normal renal function. Jin et al. indicated a similar finding to ours; glomerular hyperfiltrators exist among Japanese subjects with type 2 diabetes without proteinuria or hypertension, and they were characterized by higher HbA1C than normofiltrators (18). A significant linear correlation of HbA1C with estimated/measured GFR was shown (19,20). It is likely that chronic hyperglycemia may act through a mechanism that involves increased nitric oxide generation and/or action, leading to an increase in GFR (21). We found that a GFR value estimated by serum creatinine was higher at high blood glucose levels than at low blood glucose levels within a subject with diabetes. This is of particular interest and supports the above findings.
It is interesting to find that, in subjects with diabetes, the rate of progression to microalbuminuria in decliners was similar to that in nondecliners, and decliners were characterized by significantly higher levels of baseline GFR, baseline ACR, and HbA1C at baseline and follow-up, and a greater fall of GFR. This may support a notion that decline in GFR could precede the onset of microalbuminuria (4–8,22), being accelerated by elevated GFR and HbA1C. The effect of elevated GFR on accelerating renal function loss in type 2 diabetes was somewhat controversial (23). Although one report supported the association of high GFR with greater subsequent GFR decline, it failed to clarify the association between the baseline HbA1C and the GFR decline because of the small numbers (24). Estimation of GFR slope allows an investigation to deal with a large sample size as in our study.
Whether the association of high GFR with greater GFR decline was due to the regression-to-the-mean principle should be considered. This is the principle that with repeated measurements, when subjects are selected at the first measurement for having a high value for GFR, these subjects tend to subsequently show a lower value that lies closer to the population mean than their measured value at the first measurement and vice versa (25). We therefore used the average of the first two GFR values as a baseline GFR value, corrected the slope for baseline GFR as has been demonstrated by others (9), and compared the annual change in renal function of subjects with comparable renal function. We investigated the slope and intercept of GFR decline in the model where the second, third, fourth, or fifth measurement of GFR was used instead of the baseline GFR. In subjects with diabetes, those with high baseline GFR significantly and persistently showed greater values of the slope and intercept than those with low GFR (data not shown). Thus high GFR is likely a risk predictor of renal function decline in subjects with diabetes, independent of the regression-to-the-mean phenomenon.
The reason for associations of baseline GFR with HDL, TG, hemoglobin, and platelet is unknown. A recent paper indicated that higher hemoglobin levels were related with glomerular hyperfiltration in subjects with type 2 diabetes (26), which is consistent with our observations.
Variables Affecting the GFR Slope: Baseline SBP, Plasma TP, and Female Gender
Because baseline HbA1C and GFR were significant only in subjects with diabetes, and baseline SBP and plasma TP were significant in subjects with and without diabetes but differed between subgroups in diabetes, it is likely that GFR is affected by multiple factors at various stages. The relationship of baseline SBP with the decline of GFR was previously indicated in patients with diabetes and with elevated albuminuria and higher SBP values (27). Our study indicates that slightly high levels of SBP of approximately 130 mmHg have deleterious adverse effects on renal function loss in subjects with and without diabetes. The effect was cancelled in subjects without hypertension. Lack of the effect in those with high GFR was because of the small proportion (37%) of hypertensive patients.
The significant effect of low plasma TP on the GFR slope was observed in subjects with and without diabetes. It is known that plasma TP and plasma albumin are important factors for maintaining oncotic pressure (28), which is a determinant of GFR and is decreased in subjects with nephrotic syndrome (29). Decreased plasma concentrations of TP and albumin could accelerate the progression of renal function loss in subjects with type 2 diabetes and renal insufficiency (1). However, few data are available on the effect in subjects with preserved GFR. Our data may indicate that a preserved level of plasma TP or oncotic pressure may be important even at the early stage in the course of renal function loss, although we should acknowledge the lack of measurements of plasma albumin and oncotic pressure. The reason for female gender as a risk for GFR decline is unknown; however, it is compatible with a finding that GFR is more reduced in women among subjects with type 2 diabetes and normoalbuminuria (4,7).
Interaction effects of two risk factors evidently increased the risk. Furthermore, high values of HbA1C and SBP tended to persist in subjects with diabetes. These findings emphasize more intensive treatment of modifiable risk factors.
Perkins et al. indicated high HbA1C as a risk of early renal function decline (9), which is consistent with our finding. Their study and ours indicated an existence of progressive renal function loss among patients with diabetes without proteinuria. Different from our study, baseline SBP and GFR were not the risk factors in their study, and the proportion of GFR decliner in patients with normoalbuminuric type 1 diabetes was 9%. Their subjects included those with type 1 diabetes, microalbuminuria, younger age, and higher HbA1C; they investigated risk of GFR decline only by a category of decliner/nondecliner; and a continuous variable of GFR slope was not used. Definition of decliner could be arbitrary, and above differences might be the explanations for the inconsistent findings.
Study Limitations
Some limitations of our study need to be mentioned. First, the validity for using estimated GFR is controversial. The new Japanese equations used in this study appear to estimate reasonably accurate GFR for the Japanese population; more accurate than the modified Modification of Diet in Renal Disease equation refitted for the Japanese overcoming the underestimation of GFR at the high values up to 110 ml/min/1.73 m2 (13). Because misclassification can occur when subjects are divided by a cutoff level of estimated GFR (30), we used continuous and categorical variables. The slope of measured GFR was very closely correlated with the slope of estimated GFR (r = 0.93) in longitudinal studies (31), thus the slope based on multiple measurements with a few years of follow-up may be worthwhile (31,32), overcoming the drawbacks of consuming time, high cost, and limited availability with measured GFR. Second, we should acknowledge that subjects without diabetes had clinical characteristics different from subjects with diabetes, and their ACR was not measured. Higher proportions of hypertension and hyperlipidemia in subjects without diabetes would have worsened the GFR slope and they may have microalbuminuria in the absence of proteinuria. However these would not affect our conclusion that subjects with diabetes, even with normoalbuminuria, have increased risk of renal function loss. Finally, future studies with longer follow-up and sufficient numbers of subjects would be necessary to elucidate the effects of predictors found in this study on GFR decline.
In conclusion, type 2 diabetes may confer a substantially large decline in GFR although the subjects are normoalbuminuric and fairly controlled for blood glucose and BP. GFR is substantially affected by multiple factors at various stages. It is likely that the degree of chronic hyperglycemia plays a crucial role in elevating GFR and accelerating the decline in patients with type 2 diabetes even from the normoalbuminuric stage.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Yokoyama H, Tomonaga O, Hirayama M, Ishii A, Takeda M, Babazono T, Ujihara N, Takahashi C, Omori Y: Predictors of the progression of diabetic nephropathy and the beneficial effect of angiotensin-converting enzyme inhibitors in NIDDM patients. Diabetologia 40: 405–411, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Brazy PC, Stead WW, Fitzwilliam JF: Progressive renal disease: Role of race and antihypertensive medications. Kidney Int 37: 1113–1119, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Praga M, Hernández E, Montoyo C, Andrés A, Ruilope LM, Rodicio JL: Nephrotic proteinuria without hypoalbuminemia: clinical characteristics and response to angiotensin-converting enzyme inhibition. Am J Kidney Dis 17: 330–338, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Kramer HJ, Nguyen QD, Curhan G, Hsu CY: Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289: 3273–3277, 2003 [DOI] [PubMed] [Google Scholar]
- 5.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G: Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 27: 195–200, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Kramer CK, Leitão CB, Pinto LC, Silveiro SP, Gross JL, Canani LH: Clinical and laboratory profile of patients with type 2 diabetes with low glomerular filtration rate and normoalbuminuria. Diabetes Care 30: 1998–2000, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi MJapan Diabetes Clinical Data Management Study Group. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant 24: 1212–1219, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group: Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Parving HH, Mauer M, Ritz E: Diabetic nephropathy. In: The Kidney, 8th ed., edited by Brenner BM.Philadelphia, WB Saunders, pp 1265–1298, 2006 [Google Scholar]
- 11.Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto YCommittee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus: Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 55: 65–85, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Guideline Committee of the Japan Diabetes Society Japan Diabetes Society Evidence-Based Practice Guidelines for the Treatment of Diabetes in Japan, Nankodo, Tokyo, Japan, Japan Diabetes Society, 2004 [Google Scholar]
- 13.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida ACollaborators Developing the Japanese Equation for Estimated GFR: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Skøtt P, Vaag A, Hother-Nielsen O, Andersen P, Bruun NE, Giese J, Beck-Nielsen H, Parving HH: Effects of hyperglycaemia on kidney function, atrial natriuretic factor and plasma renin in patients with insulin-dependent diabetes mellitus. Scand J Clin Lab Invest 51: 715–727, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Soper CP, Barron JL, Hyer SL: Long-term glycaemic control directly correlates with glomerular filtration rate in early Type 1 diabetes mellitus before the onset of microalbuminuria. Diabet Med 15: 1010–1014, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Christensen PK, Lund S, Parving HH: The impact of glycaemic control on autoregulation of glomerular filtration rate in patients with non-insulin dependent diabetes. Scand J Clin Lab Invest 61: 43–50, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Jin Y, Moriya T, Tanaka K, Matsubara M, Fujita Y: Glomerular hyperfiltration in non-proteinuric and non-hypertensive Japanese type 2 diabetic patients. Diabetes Res Clin Pract 71: 264–271, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Vedel P, Obel J, Nielsen FS, Bang LE, Svendsen TL, Pedersen OB, Parving HH: Glomerular hyperfiltration in microalbuminuric NIDDM patients. Diabetologia 39: 1584–1589, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Rigalleau V, Lasseur C, Raffaitin C, Perlemoine C, Barthe N, Chauveau P, Combe C, Gin H: Glucose control influences glomerular filtration rate and its prediction in diabetic subjects. Diabetes Care 29: 1491–1495, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Chiarelli F, Cipollone F, Romano F, Tumini S, Costantini F, di Ricco L, Pomilio M, Pierdomenico SD, Marini M, Cuccurullo F, Mezzetti A: Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: Relation to glomerular hyperfiltration. Diabetes 49: 1258–1263, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Nosadini R, Carboni A, Manconi A, Angius F, Caria S, Cherchi S, Satta A, Faedda R, Obinu D, Nieddu M, Carraro A, Tonolo GC. The decline of glomerular function is not always associated with the development of micro- and macroalbuminuria in hypertensive patients with type 2 diabetes. Diabetologia [Epub ahead of print], 2008 [DOI] [PubMed] [Google Scholar]
- 23.Chaiken RL, Eckert-Norton M, Bard M, Banerji MA, Palmisano J, Sachimechi I, Lebovitz HE: Hyperfiltration in African-American patients with type 2 diabetes. Cross-sectional and longitudinal data. Diabetes Care 21: 2129–2134, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Silveiro SP, Friedman R, de Azevedo MJ, Canani LH, Gross JL: Five-year prospective study of glomerular filtration rate and albumin excretion rate in normofiltering and hyperfiltering normoalbuminuric NIDDM patients. Diabetes Care 19: 171–174, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Altman DG. Relation between two continuous variables. In: Practical Statistics for Medical Research, edited by Altman DG.London: Chapman and Hall/CRC, 1999, pp 277–324 [Google Scholar]
- 26.Rigalleau V, Raffaitin C, Perlemoine C, Gin H, Lasseur C, Chauveau P, Combe C, Barthe N: Higher hemoglobin levels in diabetic subjects with renal hyperfiltration. Am J Kidney Dis 49: 346, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Nielsen S, Schmitz A, Rehling M, Mogensen CE: Systolic blood pressure relates to the rate of decline of glomerular filtration rate in type II diabetes. Diabetes Care 16: 1427–1432, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Gong R, Dworkin LD, Brenner BM: The renal circulations and glomerular ultrafiltration. In: The Kidney, 8th ed., edited by Brenner BM.Philadelphia, WB Saunders, pp 91–129, 2006 [Google Scholar]
- 29.Canaan-Kuhl S, Venkatraman E, Ernst S, Olshen R, Myers B: Relationships among protein and albumin concentrations and oncotic pressure in nephrotic plasma. Am J Physiol Renal Physiol 264: F1052–F1059, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, Rahman M, Deysher AE, Zhang YL, Schmid CH, Levey AS: Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 18: 2749–2757, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Lewis J, Appel L, Cheek D, Contreras G, Faulkner M, Feldman H, Gassman J, Lea J, Kopple J, Sika M, Toto R, Greene T: Validation of creatinine-based estimates of GFR when evaluating risk factors in longitudinal studies of kidney disease. J Am Soc Nephrol 17: 2900–2909, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK; Treating to New Targets Investigators: Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2: 1131–1139, 2007 [DOI] [PubMed] [Google Scholar]