Abstract
Background and objectives: The optimal donor age for transplanting a single pediatric kidney in an adult recipient remains unknown. En block kidney transplantation is usually performed when the donor age is <5 yr.
Design, setting, participants, & measurements: We compared the outcomes of adult patients who underwent transplantation with single pediatric kidneys from donors who were younger than 5 yr (group 1, n = 40) and from donors who were aged 5 to 10 yr of age (group 2, n = 39) in our center.
Results: The donor kidney sizes were significantly smaller in group 1 than in group 2 (P < 0.001), and group 1 required more ureteral stents than group 2 (73 versus 38%). The surgical complications, delayed graft function, and development of proteinuria were similar in both groups. Group 1 had slightly higher rejection episodes than group 2 (25 versus 18%; P = 0.67), and graft function was comparable in both groups. There were no statistical differences between the two groups in patient (P = 0.73) or death-censored graft (P = 0.68) survivals over 5 yr.
Conclusions: Single pediatric kidney transplants from donors who are younger than 5 yr can be used with acceptable complications and long-term outcomes as those from older donors.
Transplantation of en block pediatric kidneys into an adult was first performed in 1972 (1). Giving both pediatric kidneys instead of one theoretically provides sufficient nephron mass to an adult body. Good long-term graft survival has been demonstrated (2,3). Splitting en block kidneys and transplanting a single pediatric kidney into each recipient could potentially increase kidney transplants. Mixed results have been reported (4–9). The technical concerns of vascular and ureteral complications (4,5,10,11) and the medical concerns of delayed graft function (DGF), rejection, and hyperfiltration injury have been raised (4,8,12).
The minimum donor age or body weight that allows successfully splitting en block kidneys for adult recipients remains controversial. Registry data reported worse outcomes in single pediatric kidney transplants than en block transplants from donors who were younger than 5 yr or weighed <21 kg (2,3). As a result, en block transplantation has generally been considered the “preferred” method when donor age is <5 yr (2). In this study, we summarize our experience using single pediatric kidneys from donors who were younger than 5 yr. We compare the posttransplantation complications and the long-term outcomes of adult patients who underwent transplantation with single pediatric kidneys from donors who were younger than 5 yr with those who underwent transplantation with single kidneys from donors who were older than 5 but younger than 10 yr.
Materials and Methods
Study Population
We reviewed all adult recipients who underwent transplantation with single pediatric kidneys from deceased donors aged ≤10 yr between January 1996 and June 2007. Our Organ Procurement Organization protocol was to offer all pediatric kidneys en block when the donor was younger than 5 yr. The majority of kidneys from donors aged 5 to 10 yr were also offered en block. Our center's protocol was to use a single pediatric kidney when the anatomy was acceptable for splitting. This practice was discussed at the initial transplantation evaluation, and informed consent was obtained for listing. The patient was well informed and consented again at the time of surgery. One or two more patients were brought in as “backup,” and the cross-matches with all patients were performed. As soon as en block kidneys were split, a second kidney was offered back to the United Network for Organ Sharing (UNOS) for reallocation. If not placed, then the second kidney would be transplanted into a backup patient, which usually occurred immediately after the first transplantation.
Surgical Technique
En block splitting was performed when the kidney had no obvious damage and had good arterial anatomy with an aortic cuff. A suitable carrel patch was created to facilitate the arterial anastomosis. The right renal vein was extended with a vena cava cuff. The kidney was placed in a “natural” position without tacking down, and a Doppler ultrasound was performed to verify good blood flow and kidney position after closure of the abdominal wall. Ureteral stents were placed for small donor ureters or multiple ureters on an individual basis. Ureteral stents were usually removed at 6 wk.
Immunosuppressive Therapy
Our standard triple immunosuppression regimen of steroids, tacrolimus, and mycophenolic acid was used. Before 2002, high-risk patients, defined as six-antigen mismatch or panel-reactive antibody >25%, received basiliximab induction at the time of transplantation and again on postoperative day 4. Beginning in 2002, all recipients received basiliximab induction therapy. Intravenous methylprednisolone was administrated before reperfusion and tapered to maintenance oral prednisone. Tacrolimus dosages were adjusted to keep the 12-h trough levels between 10 and 12 ng/ml for the first 3 mo, 7 to 10 ng/ml for the remainder of the first year, and 4 to 7 ng/ml thereafter. Each patient received either mycophenolate mofetil (MMF) at 1 g or enteric coated sodium mycophenolate (Myfortic; Novartis, East Hanover, NJ) at 720 mg twice daily.
Rejection
Acute rejection was confirmed by kidney biopsy. Biopsy was done by an open technique during the first 2 to 3 mo. After this, allograft tended to reach adult size and could be biopsied percutaneously under the real-time guidance of ultrasound. The severity of rejection was defined according to Banff criteria. Rejection of grade 1 or below was initially treated with intravenous methylprednisolone. Thymoglobulin was used for steroid-resistant rejection or as initial therapy for any rejection of Banff grade 2 or higher. Plasmapheresis and intravenous Ig were used for antibody-mediated rejection.
Other Interventions
All recipients received a 24-h infusion of low molecular weight Dextran-40 that was started intraoperatively. Postoperatively, the patients were placed on 81 mg/d aspirin. Standard antifungal, antibacterial, and cytomegalovirus prophylaxis were administered per protocol. Angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) was started when proteinuria was detected.
Statistical Analysis
Outcome measures included (1) patient and death-censored graft survivals over 5 yr, (2) quality of graft function as assessed by serum creatinine (SCr) and estimated GFR (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation, (3) incidence of biopsy-confirmed and treated acute rejection, and (4) surgical complications and medical events. Proteinuria was detected by routine urinalysis and then confirmed by a 24-h urine protein >250 mg or a spot urine protein-creatinine ratio >250 mg/g. Renal graft loss was defined by primary nonfunction, loss of renal function, and patient death. Patient death included all mortalities from the time of kidney transplantation. Statistical analyses were performed using SAS 9.1.3 software (SAS Institute, Cary, NC). χ2 test was used for count data, t test for continuous measures, and two-way ANOVA for adjusting effects. Odds ratios were calculated using a stepwise multivariable logistic regression model. Product-limit estimates of survival curves were generated by the Kaplan-Meier method.
Results
Seventy-nine adult patients received single pediatric kidneys from 50 pediatric donors aged 9 mo (0.75 yr) to 10 yr during this study period. All of them underwent transplantation >1 yr before this study. Mean follow-up was 5.30 yr (range 1.17 to 12.58 yr) as of June 30, 2008. Patients were separated into two groups according to donor age: Group 1, <5 yr; group 2, ≥5 yr. Group 1 had 40 adults who received single pediatric kidneys from 21 donors who were younger than 5 yr. The other two kidneys were not transplanted because of obvious damage during procurement. None of these single kidneys from donors who were younger than 5 yr was taken away from us when offered back to UNOS for reallocation. Group 2 had 39 adults who received single kidneys from 29 donors of 5 to 10 yr. Seven single kidneys had been split during procurement and were offered to us directly. Nine kidneys were initially offered to us en block but were taken away after split when they were offered back to UNOS for reallocation. Three kidneys had had severe damage and were not transplanted.
Table 1 summarizes the demographic characteristics of both recipients and donors in the two groups. The donor age, body weight, kidney length, and kidney surface (length × width) were significantly lower in group 1 than in group 2 (P < 0.001). The cold ischemia times were similar. There were no differences in recipient age, gender, race, body weight, body mass index, and peak panel-reactive antibodies between the two groups. There was approximately one additional HLA mismatch in group 1 than in group 2.
Table 1.
Demographic characteristics of pediatric donors and adult recipients between group 1 (donor age ≤5 yr) and group 2 (donor age >5 yr)a
| Characteristic | Group 1 (n = 40) | Group 2 (n = 39) | P |
|---|---|---|---|
| Pediatric donors | |||
| age (yr) | |||
| mean ± SD | 2.7 ± 1.3 | 7.9 ± 1.4 | 0.0001 |
| range | 0.75 to 5.00 | 5.20 to 10.00 | |
| gender (%) | |||
| male | 63 | 59 | 0.93 |
| female | 37 | 41 | |
| body weight (kg) | |||
| mean ± SD | 13.4 ± 3.1 | 27.8 ± 5.6 | 0.0001 |
| range | 8 to 20 | 18 to 36 | |
| kidney | |||
| length (cm; mean ± SD) | 6.4 ± 0.7 | 7.9 ± 0.9 | 0.0001 |
| length range | 5 to 8 | 7 to 9 | |
| surface (cm2; mean ± SD) | 25.0 ± 4.7 | 30.3 ± 7.1 | 0.0009 |
| CIT (h; mean ± SD) | 18.5 ± 6.9 | 19.2 ± 6.0 | 0.65 |
| Adult recipients | |||
| age (yr; mean ± SD) | 44.0 ± 16.5 | 45.5 ± 16.2 | 0.51 |
| gender (%) | |||
| male | 45 | 56 | 0.43 |
| female | 55 | 44 | |
| race (%) | |||
| black | 55 | 59 | 0.89 |
| nonblack | 45 | 41 | |
| body weight (kg; mean ± SD) | 76.6 ± 18.0 | 78.2 ± 16.0 | 0.20 |
| BMI (kg/m2; mean ± SD) | 26.3 ± 5.4 | 27.7 ± 5.6 | 0.06 |
| peak PRA (%; mean ± SD) | 18 ± 31 | 11 ± 22 | 0.21 |
| HLA mismatch (mean ± SD) | 3.9 ± 1.2 | 2.8 ± 1.9 | 0.004 |
BMI, body mass index; CIT, cold ischemia time; PRA, panel-reactive antibodies.
A similar percentage of patients in group 1 (55%) received basiliximab induction as compared with group 2 (59%). The 12-h trough levels of tacrolimus and daily dosages of MMF and prednisone were similar between the two groups (Table 2). For statistical purpose, each 360 mg of Myfortic was considered equivalent to 500 mg of MMF.
Table 2.
Summary of immunosuppressive medications, renal function, and survival rates in group 1 and group 2a
| Parameter | Year 1 |
Year 3 |
Year 5 |
|||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | |
| Medications (mean ± SD) | ||||||
| tacrolimus (ng/ml) | 8.1 ± 1.8 | 7.9 ± 2.0 | 7.4 ± 2.1 | 7.6 ± 1.7 | 6.4 ± 2.1 | 6.2 ± 2.3 |
| MMF (g/d) | 1.6 ± 0.5 | 1.8 ± 0.6 | 1.4 ± 0.7 | 1.5 ± 0.6 | 1.2 ± 0.5 | 1.4 ± 0.7 |
| prednisone (mg/d) | 6.2 ± 1.8 | 5.9 ± 1.6 | 5.4 ± 1.7 | 5.5 ± 1.8 | 4.8 ± 1.1 | 4.5 ± 1.3 |
| Renal function | ||||||
| SCr (mg/dl) | 1.5 ± 0.7 | 1.4 ± 0.4 | 1.4 ± 0.5 | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.6 ± 1.2 |
| eGFR (ml/min)b | ||||||
| mean ± SD | 62 ± 30 | 62 ± 17 | 63 ± 25 | 66 ± 17 | 62 ± 22 | 59 ± 24 |
| P | 0.97 | 0.63 | 0.98 | |||
| adjusted eGFRc | ||||||
| mean ± SD | 63 ± 25 | 63 ± 20 | 63 ± 22 | 67 ± 23 | 60 ± 26 | 60 ± 25 |
| P | 0.97 | 0.63 | 0.97 | |||
| Survival rates | ||||||
| no. at risk | 40 | 39 | 33 | 34 | 26 | 28 |
| patient (%) | 98 | 93 | 87 | 87 | 84 | 87 |
| graft (%) | 80 | 94 | 74 | 78 | 74 | 71 |
eGFR, estimated GFR; MMF, mycophenolate mofetil; SCr, serum creatinine.
Nonadjusted means.
Adjusted for gender.
The posttransplantation events and causes of graft loss are shown in Table 3. Significantly more patients in group 1 had a ureteral stent placed than in group 2 (P = 0.001). The incidences of surgical complications that required further surgical intervention (urine leak, ureteral stricture, lymphocele, vascular thrombosis) were similar in both groups (15 versus 13%). There were no statistical differences in the incidence of DGF, development of proteinuria, or treatment with ACEI or ARB.
Table 3.
Posttransplantation events and causes of graft loss in the study perioda
| Parameter | Group 1 (n = 40) | Group 2 (n = 39) | P |
|---|---|---|---|
| Events (n [%]) | |||
| ureteral stent | 29 (73) | 15 (38) | 0.001 |
| DGF | 10 (25) | 8 (21) | 0.95 |
| acute rejection | 10 (25) | 7 (18) | 0.67 |
| surgical complication | 6 (15) | 5 (13) | 0.96 |
| proteinuria | 17 (43) | 14 (36) | 0.89 |
| ACEI/ARB treatment | 20 (50) | 21 (54) | 0.55 |
| Total graft loss (n [%]) | 14 (35) | 14 (36) | 0.87 |
| Causes of graft loss | 0.83 | ||
| DWFG | 5 | 5 | |
| FSGS | 2 | 3 | |
| CAN | 1 | 3 | |
| rejection | 2 | 1 | |
| infection | 1 | 1 | |
| TMA | 1 | 1 | |
| thrombosis | 2 | 0 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAN, chronic allograft nephropathy; DGF, delayed graft function; DWFG, death with functioning graft; TMA, thrombotic microangiopathy.
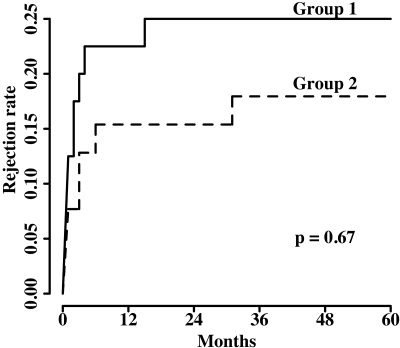
Group 1 had a slightly higher incidence of acute rejection than group 2 (25 versus 18%), but the difference was not statistically significant (P = 0.67). The time course of cumulative episodes of rejection are shown in Figure 1. No significant risk factor was identified by multivariable logistic regression analysis for the higher incidence of rejection in group 1 (Table 4). The 1-yr rejection rates were 22.7% (five of 22) with antibody induction versus 22.2% (four of 18) without antibody in group 1 (P = 1.0) and 13% (three of 23) versus 18.8% (three of 16) in group 2 (P = 0.97). There were no severe complications from biopsies (e.g., graft loss, bleeding that required surgical intervention or blood transfusion). The total graft loss was 35% in group 1 and 36% in group 2 (P = 0.87) during the study period. Death with functioning graft was the leading cause of graft loss in both groups. Two grafts were lost as a result of vascular thrombosis in group 1.
Figure 1.
Cumulative incidence of biopsy-confirmed and clinically treated graft rejection.
Table 4.
Multivariable analysis of risk factors for acute rejectiona
| Parameter | OR | 95% CI | Pb |
|---|---|---|---|
| Donor | |||
| age (group 1 versus 2) | 3.30 | 0.33 to 32.90 | 0.31 |
| body weight | 1.05 | 0.91 to 1.21 | 0.53 |
| kidney length | 1.05 | 0.50 to 2.21 | 0.89 |
| CIT | 0.99 | 0.91 to 1.09 | 0.84 |
| Recipient | |||
| race (nonblack versus black) | 0.37 | 0.10 to 1.37 | 0.13 |
| body weight | 1.03 | 0.97 to 1.10 | 0.29 |
| BMI | 0.97 | 0.80 to 1.18 | 0.75 |
| peak PRA | 0.99 | 0.98 to 1.02 | 0.83 |
| HLA mismatch | 0.83 | 0.54 to 1.28 | 0.40 |
None of the covariates is significant at 0.10 level. CI, confidence interval; OR, odds ratio.
A stepwise variable selection method is performed.
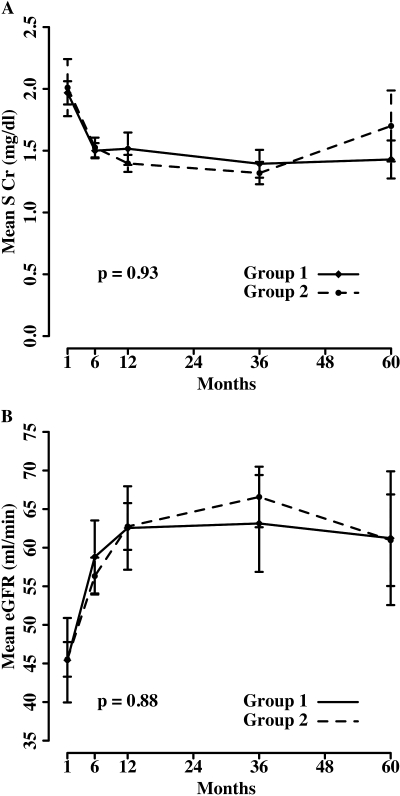
The kidney function in both groups improved dramatically in the first year after transplantation as reflected by a steadily decreasing SCr and increasing eGFR (Figure 2). The improvement of graft function continued to the third year. There were no differences in graft function as measured by SCr levels (P = 0.93) and unadjusted eGFR (P = 0.88) during 5 yr in both groups. The mean SCr and eGFR (both gender adjusted and unadjusted) are also summarized in Table 2. Neither unadjusted eGFR nor adjusted eGFR for gender factor between the two groups was significantly different at 1, 3, or 5 yr (Table 2).
Figure 2.
(A and B) Mean serum creatinine (SCr) (A) and estimated GFR (eGFR; unadjusted for gender) by Modification of Diet in Renal Disease (MDRD) equation (B).
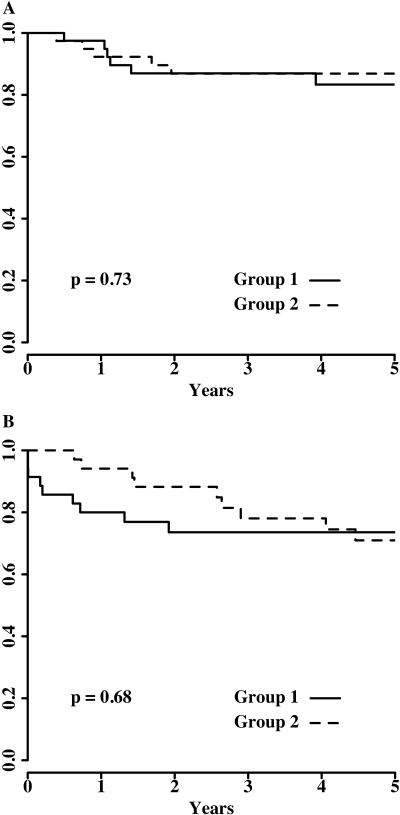
Figure 3 shows the Kaplan-Meier patient survival (Figure 3A) and death-censored graft survival (Figure 3B). No significant difference was found between the two groups in either patient survival (P = 0.73) or death-censored graft survival (P = 0.68) during 5 yr. The numbers of patients at risk and patient and death-censored graft survival rates at 1, 3, and 5 yr are also summarized in Table 2.
Figure 3.
(A and B) Kaplan-Meier estimated survivals of patients (A) and death-censored grafts (B) during 5 yr.
We also analyzed our data on the basis of donor body weight <12 versus ≥12 kg. There were 18 split kidneys from donors <12 kg and 61 split kidneys from donors ≥12 kg. The cumulative rejection rates were 27.8 versus 19.7% (P = 0.41). Kaplan-Meier estimated patient survivals at 1, 3, and 5 yr were 96, 89, and 85% for donors <12 kg and 94, 86, and 86% for donors ≥12 kg (log-rank P = 0.53). The death-censored graft survivals at 1, 3, and 5 yr were 83, 72, and 72% for donors <12 kg and 93, 79, and 72% for donors ≥12 kg (log-rank P = 0.46).
Discussion
The ever-widening disparity of supply and demand of renal allografts has led us to extend our criteria of acceptable donors, including the use of pediatric donor kidneys. The graft survival of en block kidney transplants has been shown to be similar to that of adult deceased-donor kidneys (3) and probably even live-donor kidneys (13). As a result, pediatric en block kidneys are routinely transplanted together into one recipient.
Splitting en block kidneys and transplanting a solitary pediatric kidney into one recipient could double the number of kidney transplants from one donor. Worse graft survivals with single pediatric kidney transplants compared with en block pair have been reported by several centers (4–7,10,12) as well as the registry data (2,3,11). High incidences of surgical complications, DGF, rejection, and hyperfiltration injury were reported as an argument against using small solitary kidneys (4,5,8,10–12). In one study, a 10% incidence of vascular thrombosis and an 11% incidence of ureteral leak were reported among 66 single pediatric kidney transplants (10). UNOS data from 1987 through 2003 noticed that the graft survival of en block kidneys at 1, 3, and 5 yr was superior (85, 76, and 71%) to that of single-kidney transplants (81, 68, and 63%) from donors aged <5 yr (P < 0.001) (2). Scientific Registry of Transplant Recipients data from 1993 through 2002 were remarkable for a 78% higher risk for graft loss among recipients of single kidneys compared with en block transplants from donors who weighed <21 kg (3); therefore, the current practice of using en block versus single pediatric kidney is usually based on the donor age of 5 yr or body weight of 20 kg.
In this study, we directly compared the posttransplantation complications and the long-term outcomes of single pediatric kidney transplants from very young donors (group 1, age <5 yr) with those from more optimal ages (group 2, aged 5 to 10 yr). More (73%) patients in group 1 had ureteral stent placed than did patients in group 2 (38%). The incidences of DGF and surgical complications that required further intervention were similar in both groups. Only two patients in group 1 (none in group 2) lost kidneys as a result of vascular thrombosis. The first kidney, which was from a 4-yr-old donor who weighed 16 kg, was found to be twisted and thrombosed. The other kidney was from a 3-yr-old donor who weighed 19 kg, and the graft thrombosis was likely caused by a preexisting lupus anticoagulant antibody in the recipient. These complications are considerably lower compared with the previous reports (4,5,10,11). The youngest donor in our study was a 9-mo-old girl, who weighed 8 kg and had kidney length of only 5 cm. Both grafts remain functional after >6 yr of transplantation.
A high incidence of acute rejection has been reported after pediatric-donor kidney transplantation (4,8,12). This may be related to the higher incidence of DGF and hyperfiltration injury, which can upregulate the expression of HLA and/or other antigens in pediatric kidneys. We observed a slightly higher incidence of rejection in group 1 (25%) compared with group 2 (18%); however, the majority of rejection episodes responded to treatments, and only two grafts in group 1 and one graft in group 2 were lost from acute rejection. Before 2002, few high-risk patients (approximately 15% in each group) received antibody induction. To minimize the need for open biopsy in the early posttransplantation period, we have used antibody induction with basiliximab in all recipients since 2002. Our data did not show any significant difference in reducing rejection by basiliximab induction. Lack of difference was likely due to the small sample size as well as because only high-risk patients were treated with antibody induction before 2002. More potent induction with T cell–depleting antibodies, such as thymoglobulin, could potentially decrease the incidence of rejection and the need for open kidney biopsy after pediatric-donor kidney transplants.
Hyperfiltration injury is common after pediatric kidney transplantation (6–8). Functional and hemodynamic adaptation of small pediatric kidneys in adult recipients can lead to hyperfiltration injury, development of proteinuria, and, ultimately, FSGS. This might be particularly true in solitary kidney transplants from very young donors. Group 1 had a slightly higher incidence of proteinuria (43%) than group 2 (36%). ACEIs and/or ARBs were commonly started when patients developed proteinuria (14). Approximately half of our patients in each group received such treatment. Two grafts in group 1 and three in group 2 were lost as a result of FSGS. It is our routine practice now to start ACEI or ARB early, as soon as the graft function becomes stable and before proteinuria is detected.
Patient and death-censored graft survivals were not statistically different between the two groups. The graft survival at the first 3 yr trended lower in group 1 than in group 2. This was largely due to the two graft losses (5%) from vascular thrombosis in group 1. Despite these two initial graft losses, the 5-yr graft survival was similar in both groups. When our data are analyzed on the basis of donor body weight <12 versus ≥12 kg, none of the outcomes, including cumulative incidence of rejection, long-term patient survival, or death-censored graft survival, was significantly different between the split kidneys from donors with body weight <12 and ≥12 kg.
Other reports have supported the use of single pediatric-donor kidneys. Csapo et al. (15) compared 30 single pediatric kidneys from donors who were younger than 5 yr with 117 adult kidney transplants. The death-censored graft survivals at 1 and 4 yr were comparable in the two groups. Despite the higher incidences of DGF, rejection, and proteinuria, similar 5-yr patient and graft survivals between adult kidney transplants and single pediatric kidney transplants from donors who were ≤6 yr of age were reported by Modlin et al. (8). El-Sabrout and Buch (16) compared 39 single pediatric kidneys with 59 en block pairs transplanted in adults. All donors were younger than 5 yr. There was no difference in either patient or graft survival at 1 yr. Borboroglu et al. (17) reported good graft survival and excellent kidney function in 15 single kidney transplants from donors who were younger than 2 yr, as long as the donor body weight was >14 kg and kidney length was >6 cm.
To our knowledge, this is the first study to compare directly long-term outcomes of using single pediatric kidneys from very young donors with those from older pediatric donors. Although there were higher incidences of acute rejection and vascular thrombosis that caused three more graft losses in the younger donor group, there were equivalent patient survival and long-term graft survival in both groups. The surgical complications and incidence of DGF were also similar. The risk for hyperfiltration injury and subsequent development of proteinuria and FSGS was not greater in the younger donor group. There was a continued progressive improvement in graft function over 3 yr in both groups after transplantation.
Conclusions
Single pediatric kidney transplants from donors who are younger than 5 yr can be used with acceptable complications and good long-term outcomes as those from older pediatric donors.
Disclosures
None.
Acknowledgments
We thank the members of Tulane University Abdominal Transplant Institute for maintaining the transplant database.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Meakins JL, Smith EJ, Alexander JW: En bloc transplantation of both kidneys from pediatric donors into adult patients. Surgery 71: 72–75, 1972 [PubMed] [Google Scholar]
- 2.Dharnidharka VR, Stevens G, Howard RJ: En-bloc kidney transplantation in the United States: An analysis of United Network of Organ Sharing (UNOS) data from 1987 to 2003. Am J Transplant 5: 1513–1517, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Pelletier SJ, Guidinger MK, Merion RM, Englesbe MJ, Wolfe RA, Magee JC, Sollinger HW: Recovery and utilization of deceased donor kidneys from small pediatric donors. Am J Transplant 6: 1646–1652, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Hayes JM, Novick AC, Streem SB, Hodge EE, Bretan PN, Graneto D, Steinmuller D: The use of single pediatric cadaver kidneys for transplantation. Transplantation 45: 106–110, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Harmon WE, Stablein D, Alexander SR, Tejani A: Graft thrombosis in pediatric transplant recipients: A report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation 51: 406–412, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Terasaki PI, Koyama H, Cecka J, Gjertson DW: The hyperfiltration hypothesis in human renal transplantation. Transplantation 57: 1450–1454, 1994 [PubMed] [Google Scholar]
- 7.Hayes JM, Steinmuller DR, Streem SB, Novick AC: The development of proteinuria and focal segmental glomerulosclerosis in recipients of pediatric donor kidneys. Transplantation 52: 813–817, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Modlin C, Novick AC, Goormastic M, Hodge E, Mastrioanni B, Myles J: Long term results with single pediatric donor kidney transplants in adult recipients. J Urol 156: 890–895, 1996 [PubMed] [Google Scholar]
- 9.Sharma AK, Meier S, Florman S, Nuhn MG, Slakey DP: Transplantation of adult recipients by single cadaveric kidneys from pediatric donors weighing < 25 kg can be a reliable option. Transpl Int 19: 67–71, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Satterwaithe R, Aswad S, Sunga V, Shidban H, Mendez RG, Bogaard T, Asai P, Khetan U, Magpayo M, Mendez R: Outcome of en bloc and single kidney transplantation from very young cadaveric donors. Tranplantation 63: 1405–1410, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Bresnahan BA, McBride MA, Cherikh WS, Hariharan S: Risk factors for renal allograft survival from pediatric cadaver donors: An analysis of United Network for Organ Sharing data. Transplantation 72: 256–261, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Arrazola L, Sozen H, Humar A, Papalois V, Uknis M, Matas AJ: Kidney transplant using pediatric donors: Effect on long term graft and patient survival. Transplant Proc 32: 1839–1841, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Sureshkumar KK, Reddy CS, Nghiem DD, Sandroni SE, Carpenter BJ: Superiority of pediatric en bloc renal allografts over living donor kidneys: A long-term functional study. Transplantation 82: 348–353, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Montanaro D, Gropuzzo M, Tulissi P: Renal allograft protection with early angiotensin-converting enzyme administration. Transplant Proc 36: 692–693, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Csapo Z, Knight RJ, Podder H, Kerman RH, Schoenberg L, Katz SM, Van Buren CT, Kahan BD: Transplantation of single pediatric kidneys into adult recipients. Transplant Proc 37: 697–698, 2005 [DOI] [PubMed] [Google Scholar]
- 16.El-Sabrout R, Buch K: Outcome of renal transplants from pediatric donors < 5 yr of age. Clin Transplant 19: 316–320, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Borboroglu PG, Foster CE, Philosphe B, Farney AC, Colonna JO, Schweitzer EJ, Bartlett ST: Solitary renal allografts from pediatric cadaver donors less than 2 years of age transplanted into adult recipients. Transplantation 77: 698–702, 2004 [DOI] [PubMed] [Google Scholar]