Abstract
Background and objectives: Left ventricular hypertrophy (LVH) is an independent risk factor for premature cardiovascular death in hemodialysis (HD) patients and one of the three forms of uremic cardiomyopathy. Cardiovascular magnetic resonance (CMR) is a volume-independent technique to assess cardiac structure. We used CMR to assess the determinants of left ventricular mass (LVM) and LVH in HD patients.
Design, setting, participants, & measurements: A total of 246 HD patients (63.8% male; mean age 51.5 ± 12.1 yr) underwent CMR on a postdialysis day. LVM was measured from a stack of cine loops and indexed for body surface area (LVM index [LVMI]). Demographic, past biochemical, hematologic, and dialysis data were collected by patient record review. Results up to 180 d before CMR were collected. LVH was defined as LVMI >84.1 g/m2 (male) or >76.4 g/m2 (female).
Results: A total of 157 (63.8%) patients had LVH. LVH was more common in patients with higher predialysis systolic BP, predialysis pulse pressure, and calcium-phosphate product (Ca × PO4). Furthermore, LVH was significantly associated with higher end-diastolic and systolic volumes and lower ejection fraction. There were positive correlations with LVMI and end-diastolic and systolic volumes. There were weak positive correlations among LVMI, mean volume of ultrafiltration, and Ca × PO4. Using multivariate linear and logistic regression (entering one BP and cardiac variable), the independent predictors of LVMI and LVH were end-diastolic volume, predialysis systolic BP, and Ca × PO4.
Conclusions: The principal determinants of LVM and LVH in HD patients are end-diastolic LV volume, predialysis BP, and Ca × PO4.
Patients with ESRD, particularly those who require hemodialysis (HD), have an increased risk for premature cardiovascular disease (CVD) (1). Left ventricular hypertrophy (LVH) is a common feature of patients with ESRD, a component of uremic cardiomyopathy and an independent risk factor for sudden cardiac death, heart failure, and cardiac arrhythmias in the general population and HD patients (2–4).
Studies that have assessed independent predictors of left ventricular mass index (LVMI; corrected for body surface area [BSA]) and LVH in patients with ESRD have used echocardiography and implicated factors such as hypertension, reduced blood vessel compliance, anemia, phosphate control, and dosage of dialysis (5–7); however, accurate echocardiographic estimation of LVMI in patients with ESRD is difficult because of large variation in intravascular (and hence intraventricular) volume during the interdialytic period and during dialysis. Geometric assumptions made during calculation of LVMI from conventional M-mode echocardiography dimensions result in greater inaccuracies as a result of geometric LV distortion in patients with LVH and ESRD.
Cardiac magnetic resonance (CMR) imaging provides more detailed, volume-independent measurement of cardiac structure and has been thoroughly validated using human autopsy and animal specimens (8,9). This technique has been established as the most accurate noninvasive method of assessing ventricular dimensions in patients, including those with stage 5 chronic kidney disease (10,11). In particular, measurements obtained by echocardiography tend to overestimate LVMI, particularly at higher values, when compared with CMR (12). Furthermore, pilot studies in patients with ESRD, using CMR to identify myocardial changes of uremic cardiomyopathy, have so far shown a significant reduction in long-term survival similar to previous echocardiography findings (13 and unpublished data). Thus, the aim of this study was to assess by CMR the determinants of LVMI and LVH in a cohort of HD patients.
Materials and Methods
Patients
Since 2002 (10,11,13), we have used CMR as part of the standard assessment of patients for renal transplantation. The renal transplant unit at the Western Infirmary (Glasgow, Scotland, UK) provides transplant services to a population of 2.8 million people in the west of Scotland. The transplant waiting list has 300 to 400 patients at any given time; approximately 100 to 120 new patients are placed on the waiting list, and approximately 70 adult transplants are performed annually. We assessed 246 patients who had been receiving thrice-weekly maintenance HD for at least 3 mo.
CMR Technique and Analysis
Non–gadolinium-based contrast CMR was performed using a 1.5 Tesla magnetic resonance imaging scanner (Sonata; Siemens, Erlangen, Germany). Scans were consistently performed 24 h after the end of the last dialysis session. Long-axis pilot scans were obtained through the apex of the left ventricle, aligning it with the center of the mitral valve, and then 8-mm-thick short-axis images were obtained. A fast imaging with steady-state precession sequence was then used to acquire cine images in long-axis planes (vertical long axis, horizontal long axis, LV outflow tract) followed by sequential short-axis LV cine loops (8-mm-slice thickness, 2-mm gap between slices) from the atrioventicular ring to the apex. Slices were contiguous (i.e. with no gap). Imaging parameters, which were standardized for all patients, included repetition time/echo time/flip angle/voxel size/field of view of 3.14 ms/1.6 ms/60°/2.2 × 1.3 × 8.0 mm/340 mm. LVM was analyzed by two observers, who were blinded to patient clinical characteristics, from short-axis cine loops using manual tracing of epicardial and endocardial end-systolic and end-diastolic contours. End-systolic volume (ESV) and end-diastolic volume (EDV) and LVM were calculated using analysis software (Argus; Siemens). Values were compared with published normal values: LVH was defined as LVMI (LVM/BSA) >84.1 g/m2 (male) or >76.4 g/m2 (female), and LV systolic dysfunction was defined as LV ejection fraction (LVEF) <55%. LV dilation was defined as EDV/BSA >111.7 ml/m2 (male) or 99.3 ml/m2 (female) or ESV/BSA >92.8 ml/m2 (male) or 70.3 ml/m2 (female) (14).
Clinical and Blood Result Data Collection
Demographic information and clinical (including drug) history were recorded at the time of CMR. Retrospective review of the electronic patient record was performed to retrieve HD information, including immediate pre-HD and post-HD systolic and diastolic BP (SBP and DBP, respectively), dosage of ultrafiltration (UF), dialysis adequacy using urea reduction ratio (URR), and biochemical and hematologic blood results. Information was collected at 30 d intervals up to 180 d before CMR and mean results were calculated as described previously (15). Calcium-phosphate product (Ca × PO4) was also calculated from blood results collected. URR was calculated using an established protocol (16).
Statistical Analysis
Statistical analyses were performed using SPSS 15.0 (SPSS, Chicago, IL). Graphs were drawn using GraphPad Prism 4.00 (GraphPad Software, San Diego, CA). Data are expressed as mean ± SD. Correlations between LVMI and other factors were evaluated by Pearson correlation coefficient. Variables that were identified by univariate analysis as significant predictors were entered into a backward stepwise linear and logistic regression model to identify independent predictors of LVMI and LVH, respectively. Because of significant interdependence, SBP and DBP were entered separately into the model. Only one cardiac parameter (EDV/BSA, ESV/BSA, or LVEF) was entered for the same reason.
Results
Clinical and Cardiac Parameters
A total of 246 HD patients were assessed by CMR between 2002 and 2008. LVH was present in 157 (63.8%) of patients. The demographic, HD, blood, drug, and cardiac data are presented in Table 1.
Table 1.
Clinical, drug, and cardiac data for patients
| Parameter | All Patients (n = 246) | No LVH (n = 89) | LVH (n = 157) | P |
|---|---|---|---|---|
| Age (yr; mean ± SD) | 51.4 ± 12.1 | 51.8 ± 12.2 | 51.2 ± 12.1 | 0.20 |
| Male gender (n [%]) | 157 (63.8) | 55 (61.8) | 102 (65.0) | 0.62 |
| BMI (kg/m2; mean ± SD) | 25.6 ± 4.6 | 25.2 ± 4.2 | 25.8 ± 4.8 | 0.45 |
| Ischemic heart disease (n [%]) | 51 (20.7) | 21 (23.6) | 30 (19.1) | 0.40 |
| Diabetes (n [%]) | 156 (63.4) | 58 (65.2) | 98 (62.4) | 0.67 |
| Chronic heart failure (n [%]) | 15 (6.1) | 5 (5.6) | 10 (6.4) | 0.81 |
| Cerebrovascular disease (n [%]) | 20 (8.1) | 10 (11.2) | 10 (6.4) | 0.18 |
| Peripheral vascular disease (n [%]) | 18 (7.3) | 7 (7.9) | 11 (7.0) | 0.80 |
| Hypertension (n [%]) | 223 (90.7) | 79 (88.8) | 144 (91.4) | 0.44 |
| Duration on HD (yr; mean ± SD) | 3.0 ± 3.0 | 2.9 ± 3.1 | 3.0 ± 2.9 | 0.89 |
| UF volume (L; mean ± SD) | 2.2 ± 1.1 | 2.1 ± 0.9 | 2.3 ± 1.2 | 0.08 |
| Pre-HD SBP (mmHg; mean ± SD) | 142.3 ± 21.8 | 138.1 ± 24.3 | 144.6 ± 20.0 | 0.02 |
| Post-HD SBP (mmHg; mean ± SD) | 132.3 ± 23.1 | 129.2 ± 24.8 | 133.8 ± 21.9 | 0.14 |
| Pre-HD DBP(mmHg; mean ± SD) | 79.8 ± 14.0 | 79.1 ± 16.4 | 80.3 ± 12.4 | 0.52 |
| Post-HD DBP(mmHg; mean ± SD) | 73.3 ± 13.8 | 72.3 ± 15.9 | 73.9 ± 12.5 | 0.36 |
| Pre-HD PP (mmHg; mean ± SD) | 62.5 ± 17.1 | 59.1 ± 17.6 | 64.4 ± 16.5 | 0.02 |
| Post-HD PP (mmHg; mean ± SD) | 58.9 ± 17.6 | 57.1 ± 18.9 | 60.0 ± 16.8 | 0.22 |
| URR (%; mean ± SD) | 70.8 ± 7.5 | 67.6 ± 11.9 | 70.7 ± 6.4 | 0.64 |
| Hemoglobin (g/dl; mean ± SD) | 11.3 ± 1.3 | 11.3 ± 1.5 | 11.3 ± 1.3 | 0.95 |
| Adjusted Ca (mmol/L; mean ± SD) | 2.31 ± 0.20 | 2.29 ± 0.20 | 2.31 ± 0.20 | 0.61 |
| Phosphate (mmol/L; mean ± SD) | 1.27 ± 0.40 | 1.20 ± 0.40 | 1.32 ± 0.50 | 0.06 |
| Ca × PO4 (mean ± SD) | 2.93 ± 1.00 | 2.72 ± 0.90 | 3.05 ± 1.00 | 0.03 |
| Albumin (mean ± SD) | 37.3 ± 5.9 | 37.9 ± 6.1 | 36.9 ± 5.8 | 0.24 |
| PTH (mean ± SD) | 32.5 ± 28.7 | 34.3 ± 28.3 | 31.4 ± 29.2 | 0.54 |
| Aspirin (n [%]) | 97 (39.4) | 38 (42.7) | 59 (37.6) | 0.43 |
| Warfarin (n [%]) | 11 (4.5) | 3 (3.8) | 8 (5.1) | 0.53 |
| ACEI/ARB (n [%]) | 61 (24.8) | 23 (25.8) | 38 (24.2) | 0.78 |
| Diuretic (n [%]) | 50 (20.3) | 23 (25.8) | 27 (17.2) | 0.11 |
| CCA (n [%]) | 73 (29.7) | 25 (28.1) | 48 (30.6) | 0.68 |
| α Adrenoceptor blocker (n [%]) | 17 (6.9) | 9 (10.1) | 8 (5.1) | 0.14 |
| β Adrenoceptor blocker (n [%]) | 106 (43.1) | 36 (40.4) | 70 (44.6) | 0.53 |
| ESA (n [%]) | 196 (79.7) | 74 (83.1) | 122 (77.7) | 0.46 |
| Vitamin D analogue (n [%]) | 99 (40.2) | 35 (39.3) | 64 (40.8) | 0.83 |
| Ejection fraction (%; mean ± SD) | 64.7 ± 13.3 | 68.5 ± 7.9 | 62.6 ± 15.1 | 0.001 |
| LVMI (g/m2; mean ± SD) | 99.4 ± 30.0 | 67.2 ± 10.8 | 116.9 ± 31.3 | 0.001 |
| EDV/BSA (ml/m2; mean ± SD) | 77.8 ± 31.1 | 58.2 ± 18.3 | 88.7 ± 34.7 | 0.001 |
| ESV/BSA (ml/m2; mean ± SD) | 29.6 ± 21.9 | 19.7 ± 10.8 | 36.5 ± 27.5 | 0.001 |
| LVSD (n [%]) | 45 (18.3) | 6 (6.7) | 39 (24.8) | 0.001 |
| LV dilation (n [%]) | 39 (15.8) | 2 (2.2) | 37 (23.6) | 0.001 |
Comparisons between patients with and without LVH are shown. Tests of significance are t test and χ2 test. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor II blocker; BMI, body mass index; CCA, calcium channel antagonist; ESA, erythropoiesis-stimulating agent; LVSD, left ventricular systolic dysfunction; MAP, mean arterial pressure; PP, pulse pressure.
Factors Associated With LVH
Higher pre-HD SBP, pre-HD pulse pressure (PP), and Ca × PO4 were significantly associated with LVH (pre-HD SBP 144.6 ± 20.0 [LVH] versus 138.1 ± 24.3 mmHg [no LVH; P = 0.02]; pre-HD pulse pressure 64.4 ± 16.5 mmHg [LVH] versus 59.1 ± 17.6 mmHg [no LVH; P = 0.02]; and Ca × PO4 3.05 ± 1.00 [LVH] versus 2.72 ± 1.00 mmol2/L2 [no LVH; P = 0.03], respectively; Table 1). Serum phosphate and mean UF dosage were higher in the LVH group of patients, but these were not statistically significant. When comparing both groups, there were no significant differences in age, gender, body mass index, cardiovascular history, duration on HD, mean post-HD SBP and DBP, and mean pre-HD DBP and URR. Furthermore, LVH was not significantly associated with a difference in mean hemoglobin, albumin, adjusted serum calcium, or parathyroid hormone (PTH) measured during the 180 d before CMR. There was no significant difference in drug use between both groups.
Table 1 also shows LV cardiac parameters measured: Ejection fraction, EDV/BSA, and ESV/BSA. LVH was significantly associated with lower LVEF, higher end-diastolic and systolic LV volumes, LV systolic dysfunction, and LV dilation.
Correlations with LVMI
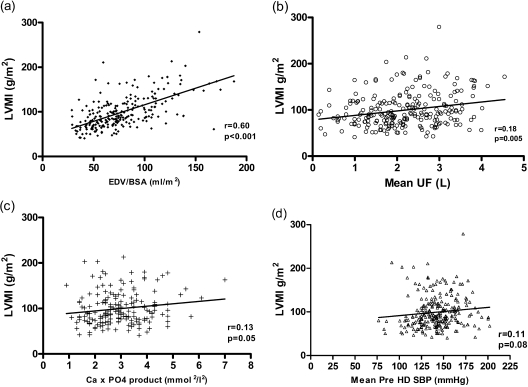
There were strong positive correlations between LVMI and EDV/BSA (r = 0.60, P < 0.001; Figure 1a) and ESV/BSA (r = 0.54, P < 0.001) and negative correlation with LVEF (r = −0.37, P < 0.001). In addition, there were weaker but significant correlations with mean dosage of UF (r = 0.18; P = 0.005; Figure 1b) and Ca × PO4 (r = 0.13, P = 0.05; Figure 1c). Factors that were close to statistical significance were post-HD DBP (r = 0.13, P = 0.06) and mean pre-HD SBP (r = 0.11, P = 0.08; Figure 1d). Mean UF correlated significantly with EDV/BSA (r = 0.22, P = 0.001; Table 2).
Figure 1.
Scatterplot demonstrating correlation between LVMI and (a) EDV/BSA, (b) Mean UF, (c) Ca × PO4 product, and (d) mean pre-HD SBP (Pearson's correlation coefficient).
Table 2.
Correlation between LVMI and clinical, blood, and cardiac parameters
| Parameter | r | P |
|---|---|---|
| EDV/BSA (ml/m2) | 0.60 | <0.001 |
| Mean pre-HD SBP (per mmHg) | 0.12 | 0.08 |
| Ca × PO4 (mmol2/L2) | 0.13 | 0.05 |
| Mean UF volume (L/dose) | 0.18 | 0.005 |
| ESV/BSA (ml/m2) | 0.54 | <0.001 |
| Stroke volume/BSA | 0.46 | <0.001 |
| Ejection fraction (%) | −0.37 | <0.001 |
| Age (yr) | −0.15 | 0.10 |
| BMI (kg/m2) | −0.07 | 0.23 |
| Duration on HD (yr) | 0.06 | 0.40 |
| Mean post-HD SBP (mmHg) | 0.06 | 0.34 |
| Mean pre-HD DBP (mmHg) | 0.10 | 0.11 |
| Mean post-HD DBP (mmHg) | 0.13 | 0.06 |
| Mean pre-HD PP (mmHg) | 0.06 | 0.38 |
| MAP post-HD PP (mmHg) | −0.02 | 0.76 |
| URR (%) | 0.07 | 0.78 |
| Hemoglobin (g/dl) | −0.01 | 0.92 |
| Adjusted Ca (mmol/L) | −0.11 | 0.12 |
| Phosphate (mmol/L) | −0.03 | 0.63 |
| Albumin (g/dl) | 0.02 | 0.78 |
| PTH (pmol/L) | −0.03 | 0.72 |
BMI, body mass index.
Determinants of LVH and LVMI
Univariate followed by multivariate logistic and linear regression analyses were performed to determine predictors of LVH and LVMI, respectively (Tables 3 and 4). Univariate analyses identified EDV/BSA, mean Ca × PO4 product, and mean pre-HD SBP as factors that were significantly associated with presence of LVH. Multivariate logistic regression modeling (only entering one BP variable and cardiac parameter because of their significant interdependence) similarly identified EDV/BSA, pre-HD SBP, and Ca × PO4 as the most robust independent predictors of LVH.
Table 3.
Multivariate linear regression entering only significant correlates into the (backward stepwise) model (adjusted R2 = 0.49)
| B | β | P | 95% CI | |
|---|---|---|---|---|
| Constant | −13.5 | 0.40 | −45.33 to 18.3 | |
| EDV/BSA | 0.80 | 0.66 | <0.001 | 0.66 to 0.91 |
| Mean pre-HD SBP | 0.24 | 0.13 | 0.01 | 0.05 to 0.42 |
| Ca × PO4 product | 5.38 | 0.16 | 0.003 | 1.53 to 9.24 |
B, unstandardized regression coefficient; β, standardized β coefficient; CI, confidence interval. Units of measurement as before.
Table 4.
Univariate and multivariate logistic regression analyses determining presence of LVH
| Variable | Univariate Analyses |
Multivariate Analyses |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| EDV/BSA (per ml/m2) | 1.08 | 1.04 to 1.13 | 0.001 | 1.06 | 1.04 to 1.08 | <0.001 |
| Mean Ca × PO4 (per mmol2/L2) | 1.37 | 1.01 to 1.87 | 0.04 | 1.74 | 1.17 to 2.57 | 0.006 |
| Mean pre-HD SBP (per mmHg) | 1.02 | 1.01 to 1.05 | 0.04 | 1.02 | 1.01 to 1.04 | 0.01 |
| Age (per year) | 0.99 | 0.99 to 1.02 | 0.70 | |||
| Gender (male versus female) | 1.55 | 0.91 to 2.65 | 0.11 | |||
| BMI (per kg/m2) | 0.96 | 0.89 to 1.03 | 0.27 | |||
| HD duration (per year) | 1.01 | 0.85 to 1.20 | 0.88 | |||
| Ischemic heart disease | 1.34 | 0.67 to 2.60 | 0.38 | |||
| Diabetes | 1.20 | 0.69 to 2.60 | 0.52 | |||
| Chronic heart failure | 0.88 | 0.28 to 2.72 | 0.82 | |||
| Cerebrovascular disease | 2.76 | 0.96 to 6.93 | 0.09 | |||
| Peripheral vascular disease | 0.83 | 0.29 to 2.42 | 0.74 | |||
| Hypertension | 0.71 | 0.30 to 1.70 | 0.45 | |||
| UF volume (per L) | 1.26 | 0.95 to 1.66 | 0.11 | |||
| URR (per %) | 1.06 | 0.91 to 1.23 | 0.45 | |||
| Mean post-HD SBP (per mmHg) | 0.99 | 0.97 to 1.02 | 0.55 | |||
| Mean pre-HD DBP (per mmHg) | 0.98 | 0.95 to 1.01 | 0.21 | |||
| Mean post-HD DBP (per mmHg) | 1.01 | 0.98 to 1.04 | 0.49 | |||
| Mean pre-HD PP (per mmHg) | 1.02 | 1.01 to 1.05 | 0.05 | |||
| Mean post-HD PP (per mmHg) | 0.99 | 0.97 to 1.01 | 0.47 | |||
| Ejection fraction (per %) | 0.95 | 0.88 to 1.03 | 0.21 | |||
| ESV/BSA (per ml/m2) | 0.93 | 0.85 to 1.02 | 0.12 | |||
| Mean hemoglobin (per g/dl) | 0.86 | 0.60 to 1.23 | 0.41 | |||
| Mean adjusted Ca (per mmol/L) | 1.61 | 0.35 to 7.48 | 0.54 | |||
| Mean serum PO4 (per mmol/L) | 2.10 | 1.00 to 4.29 | 0.06 | |||
| Mean albumin (per g/dl) | 1.00 | 0.96 to 1.05 | 0.91 | |||
| Mean PTH (per pmol/L) | 0.99 | 0.99 to 1.01 | 0.54 | |||
BMI, body mass index; CI, confidence interval; OR, odds ratio; PP, pulse pressure.
Linear regression analysis was performed to create the most robust predictive model for LVMI (Table 3). Factors considered in the model were those that were identified as significant (or close) correlates with LVMI. As before, because of their close interdependence, BP variables and cardiac parameters were entered separately into the multivariate model. Using a backward stepwise model, the most significant model (R2 = 0.49) generated included EDV/BSA, mean pre-HD SBP, and Ca × PO4 as significant predictors of LVMI.
Discussion
Premature CVD is the most common cause of mortality in patients with ESRD. Although the risk of accelerated coronary artery disease and myocardial infarction is increased in this population, the major increased cardiovascular risk is attributable to heart failure and to sudden, presumed arrhythmic cardiac death (17). The atypical nature of CVD in this population is underscored by the futility of interventions, such as statin therapy, that are effective in other high-risk populations (18). This has prompted the identification of alternative risk factors and therapeutic targets. Of these, LVH is common in patients with ESRD and may contribute to arrhythmias as a result of impaired subendocardial perfusion and increased myocardial fibrosis (19). These changes cause chaotic, nonuniform, cardiac myocyte depolarization and repolarization, which, in turn, increase the risk for re-entrant tachyarrhythmias. Thus, identification of factors that are linked to LVH may lead to new treatment strategies to address cardiovascular risk in ESRD.
Several studies have used echocardiography to study the determinants of LVMI and LVH. They identified factors that increase cardiac preload (e.g., salt and water retention, presence of arteriovenous fistulas and anemia), afterload (hypertension, calcific arteriosclerosis), and humoral factors (including PTH and catecholamines) (20,21); however, there are conflicting results, presumably as a result, in part, of the unreliability of echocardiographic measurement of LVMI in HD patients (12). This is the first study to identify independent determinants of LVMI and LVH in HD patients using CMR. The intravascular volume-dependent inaccuracies of echocardiography are avoided by CMR scanning as a result of direct characterization of the LV borders. Our study also differs from previous published data because we obtained serial blood and dialysis data during 180 d as opposed to single results obtained at the time of imaging. Our data show that both LVMI and presence of LVH are associated with and are independently predicted by high end-diastolic LV volumes, higher pre-HD SBP, and Ca × PO4.
EDV: Increased Cardiac Preload
In our study, EDV/BSA was the strongest parameter that predicted LVMI and LVH in multivariate analyses. This reflects the notion that development of LVH in ESRD is due, in part, to the adaptive response of the left ventricle to long-term volume overload. LV mechanical stretch stimulates cardiac sarcomere production and LV wall hypertrophy in an attempt to reduce wall stress (19); however, as wall thickening worsens, capillary vessels fail to develop adequately and perfuse the myocardium, particularly in the subendocardium. This results in myocyte ischemia and death, followed by myocardial fibrosis, systolic dysfunction, and further ventricular dilation. Our data support this mechanism: LVMI correlates positively with EDV/BSA and ESV/BSA and negatively with LVEF.
Pre-HD BP and Ca × PO4: Increased Afterload
The other major factors that were associated with LVH in our study are those that have been associated with increased afterload: hypertension and Ca × PO4. These observations are consistent with many reports of an association between BP and LVH in HD patients (20,22,23). Although measurement of BP before starting and immediately at the end of dialysis is highly variable, dependent on fluid volume status and the hemodynamic effects of dialysis, it remains the clinical standard. Twenty-four-hour ambulatory BP monitoring studies have confirmed these associations (24), but this method is not universally available, is time-consuming, and is uncomfortable for dialysis patients.
Elevated Ca × PO4 is a risk factor for arterial calcification and, as a consequence, contributes to BP and the development of LVH. It has been shown to be an independent predictor of cardiovascular and sudden death in patients with ESRD (25). Other measurements and markers of vascular calcification, such as in pulse wave velocity and electron-beam computed tomography, have demonstrated an association with LVH in patients with ESRD (26,27). Previous studies demonstrated a potential role of PTH in promoting myocardial fibrosis (8), but our data showed no correlation between LVMI and PTH.
Anemia as a predictor of LVMI?
Our study did not demonstrate a predictive role of hemoglobin concentration for LVMI. This contrasts with other published data that demonstrated a strong correlation between LVM and anemia (11,28), and the most likely explanation is the higher values and narrower range in our cohort (mean hemoglobin 11.3 g/dl; SD 1.3) compared with other studies.
Conclusions
Although previous studies have examined the determinants of LVH using echocardiography, the use of which is inaccurate in patients with ESRD, this study has confirmed that the main determinants of LVH in ESRD are preload and afterload. We accept these data are limited by the relatively short period (180 d) of collection and absence of repeat CMR scanning of patients. Nonetheless, given the futility of conventional CV risk management (e.g. with statins) in this population, LVH offers a therapeutic target and improved BP, fluid volume, and bone mineral control offer interventions with potential to prevent or regress LVH. In addition, CMR offers a high fidelity assessment of LVMI that will allow the use of small populations to power studies of LVH in this population.
Disclosures
None.
Acknowledgments
The study was supported by the Darlinda's Charity for Renal Research. R.K.P. (FS/08/030/24993) and P.B.M. are funded by the British Heart Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Baigent C, Burbury K, Wheeler D: Premature cardiovascular disease in chronic renal failure. Lancet 356: 147–152, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285, 1996 [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP: Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray D, Barre PE: Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int 49: 1428–1434, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 49: 1379–1385, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: Role of calcium-phosphorus metabolism. J Am Soc Nephrol 16: 2796–2803, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chow KM, Szeto CC, Kum LC, Kwan BC, Fung TM, Wong TY, Leung CB, Li PK: Improved health-related quality of life and left ventricular hypertrophy among dialysis patients treated with parathyroidectomy. J Nephrol 16: 878–885, 2003 [PubMed] [Google Scholar]
- 8.Myerson SG, Bellenger NG, Pennell DJ: Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension 39: 750–755, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Katz J, Milliken MC, Stray-Gundersen J, Buja LM, Parkey RW, Mitchell JH, Peshock RM: Estimation of human myocardial mass with MR imaging. Radiology 169: 495–498, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG: Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int 69: 1839–1845, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Stewart GA, Mark PB, Johnston N, Foster JE, Cowan M, Rodger RS, Dargie HJ, Jardine AG: Determinants of hypertension and left ventricular function in end stage renal failure: A pilot study using cardiovascular magnetic resonance imaging. Clin Physiol Funct Imaging 24: 387–393, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Stewart GA, Foster J, Cowan M, Rooney E, McDonagh T, Dargie HJ, Rodger RS, Jardine AG: Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int 56: 2248–2253, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Patel RK, Mark PB, Johnston N, McGeoch R, Lindsay M, Kingsmore DB, Dargie HJ, Jardine AG: Prognostic value of cardiovascular screening in potential renal transplant recipients: A single-center prospective observational study. Am J Transplant 8: 1673–1683, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU: Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 17: 323–329, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Santos SF, Mendes RB, Santos CA, Dorigo D, Peixoto AJ: Profile of interdialytic blood pressure in hemodialysis patients. Am J Nephrol 23: 96–105, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Owen WF, Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Herzog CA: Cardiac arrest in dialysis patients: Approaches to alter an abysmal outcome. Kidney Int Suppl S197–S200, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F: Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 19.London GM, Parfrey PS: Cardiac disease in chronic uremia: Pathogenesis. Adv Ren Replace Ther 4: 194–211, 1997 [DOI] [PubMed] [Google Scholar]
- 20.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME: Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol 12: 2759–2767, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH: Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114: 345–352, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Fagugli RM, Pasini P, Quintaliani G, Pasticci F, Ciao G, Cicconi B, Ricciardi D, Santirosi PV, Buoncristiani E, Timio F, Valente F, Buoncristiani U: Association between extracellular water, left ventricular mass and hypertension in haemodialysis patients. Nephrol Dial Transplant 18: 2332–2338, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C: Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 47: 62–68, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Peixoto AJ, Santos SF, Zoccali C: Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 1: 389–398, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Yildiz A, Memisoglu E, Oflaz H, Yazici H, Pusuroglu H, Akkaya V, Erzengin F, Tepe S: Atherosclerosis and vascular calcification are independent predictors of left ventricular hypertrophy in chronic haemodialysis patients. Nephrol Dial Transplant 20: 760–767, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Nitta K, Akiba T, Uchida K, Otsubo S, Otsubo Y, Takei T, Ogawa T, Yumura W, Kabaya T, Nihei H: Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res 27: 47–52, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]