Abstract
Background: The wearable artificial kidney (WAK) has been a holy grail in kidney failure for decades. Described herein are the breakthroughs that made possible the creation of the WAK V1.0 and its advanced versions V 1.1 and 1.2.
Design: The battery-powered WAK pump has a double channel pulsatile counter phase flow. This study clarifies the role of pulsatile blood and dialysate flow, a high-flux membrane with a larger surface area, and the optimization of the dialysate pH. Flows and clearances from the WAK pump were compared with conventional pumps and with gravity steady flow.
Results: Raising dialysate pH to 7.4 increased adsorption of ammonia. Clearances were higher with pulsatile flow as compared with steady flow. The light WAK pump, geometrically suitable for wearability, delivered the same clearances as larger and heavier pumps that cannot be battery operated. Beta2 microglobulin (β2M) was removed from human blood in vitro. Activated charcoal adsorbed most β2M in the dialysate. The WAK V1.0 delivered an effective creatinine clearance of 18.5 ± 3.2 ml/min and the WAK V1.1 27.0 ± 4.0 ml/min in uremic pigs.
Conclusions: Half-cycle differences between blood and dialysate, alternating transmembrane pressures (TMP), higher amplitude pulsations, and a push-pull flow increased convective transport. This creates a yet undescribed type of hemodiafiltration. Further improvements were achieved with a larger surface area high-flux dialyzer and a higher dialysate pH. The data suggest that the WAK might be an efficient way of providing daily dialysis and optimizing end stage renal disease (ESRD) treatment.
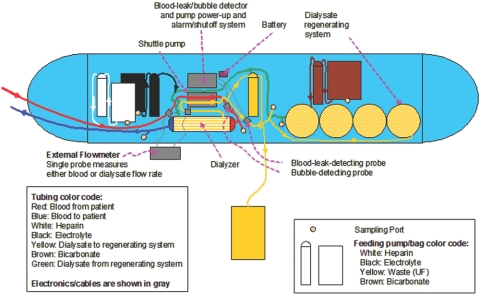
Dialysis treatment outcomes in ESRD patients remain disappointing. Growing evidence indicates that longer and more frequent dialysis may be conducive to biochemical as well as clinical improvements. However, implementation of daily dialysis is largely impractical (1–7). The development of a wearable artificial kidney (WAK) that would allow for longer and more frequent dialysis has been ongoing for decades, but has resulted in failure due almost entirely to insurmountable technical problems (8,9). A previously described (10–17) miniaturized WAK (Figure 1) intended to be worn continuously was analyzed in vitro and in vivo. The overall weight of this initial prototype is 10 pounds. The operational characteristics of the WAK (V1.1) were examined as well as the roles of the oscillating flows and pressures of dialysate and blood at half-cycle different pulsation waves, as compared with those of conventional pumps and steady flow. The removal of β2M in vitro by the WAK, the effects of alkalinizing the dialysate, and using a larger surface area high-flux dialyzer were studied (10–17). Technical breakthroughs, which made the WAK possible, are described here.
Figure 1.
Schematics of the WAK. Blood drawn from a double lumen catheter (red) is anticoagulated with heparin from a reservoir (white) using a commercially available, battery-operated micro pump (ambIT, Sorenson, Salt Lake City, UT) and circulated through the blood channel of the WAK pump (gray) and into the dialyzer (AN-69 0.6 m2. Hospal, France). The blood returns to the venous side of the double lumen catheter (blue). Clean dialysate (green) enters the dialyzer after an ambIT pump infuses a solution containing potassium, calcium, and magnesium from another reservoir (black). The dialysate circulates in countercurrent flow to the blood and exits (yellow) into the dialysate channel of the WAK pump. Another ambIT pump removes a predetermined amount of the spent dialysate (yellow) into a collection bag. The rest of the dialysate goes through a series of sorbent (yellow)- containing canisters (designed and built in our laboratory) containing urease, zirconium phosphate, hydrous zirconium oxide, and activated carbon. An ambIT pump infuses a solution containing sodium bicarbonate from a reservoir (brown) into the dialysate. The dialysate then returns to the dialyzer (green).
Materials and Methods
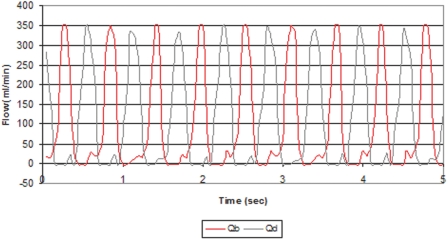
The WAK pump uses a 3-Watt Faulhaber DC motor, (Faulhaber GmbH & Co. KG, Schönaich, Germany). The motor causes an intermittent mechanism with two metal arms to alternatively compress two elastic chambers. At the entry and exit of these chambers, valves open and close to allow alternating pulsatile flow of both blood and dialysate into the dialyzer. When one chamber is propelling fluid out of the pump (as in systole), the other one is filling fluid into the pump (as in diastole), creating peak flow in one channel while the flow in the other channel is at its trough (Figure 2).
Figure 2.
The instantaneous blood flow waves of the WAK pump as recorded by flow-meter probes (Transonic Systems, Ithaca, NY) placed at the blood and dialysate tubing before the entrance to the pump and connected to Lab View virtual instruments (National Instruments, Austin, TX). Alternating peak and troughs flows of blood (red) and dialysate (green) are generated in opposite phase at 108 cycles/min.
We compared three flow systems (WAK, conventional pumps, and gravity-generated steady continuous flow). Heparinized porcine blood was dialyzed with the following dialyzers: Hemophan® (100 HG, 0.22 m2, Kuf 1.6, Dialysatoren, Dransfeld, Germany), Multiflow 60, (0.6 m2, Kuf 1.5, Hospal Industrie, Meyziu, France), and F3® (0.4 m2, Kuf 1.7, Fresenius, Bad Hamburg, Germany). When using conventional pumps, dialysate was circulated with a centrifugal pump (Powerhead, Meiko, Taichung, Taiwan).
The dimensions, weight, and performance of the WAK pump for both blood and dialysate were compared with those of conventional roller pumps used in dialysis (Table 1). Urea, creatinine, and potassium were added to the blood to achieve concentrations of blood urea nitrogen (BUN) to 60 mg/dl, creatinine to 10 mg/dl, and potassium to 6 mEq/L, respectively. Temperature was kept at 37°C. All pumps were run at several flow rates. After stabilization at each flow rate, cycles per minute, flows, and pressures were recorded using flow meters (Transonic Systems, Ithaca, NY) and in-line pressure sensors (Merit Medical, South Jordan, UT), connected to LabView virtual instruments (National Instruments, Austin, TX), recording 40 points per second. Samples were analyzed in duplicate using an i-STAT® Portable Clinical Analyzer (i-STAT, East Windsor, IL) and a 990 Hitachi® Autoanalyzer (Hitachi Ltd, Chiyoda, Tokyo, Japan). Each experiment was performed five times.
Table 1.
Main pump specifications
| Pump (flow = 95 ml/min) | WAK (blood & dialysate) | Minipump (blood) | Masterflex (blood) | Profile (dialysate) |
|---|---|---|---|---|
| Stroke volume | 0.8 | 8 | 0.7 | - |
| Cycles/min | 110 | 13 | 125 | - |
| Peak Inst. Flow (ml/min) | 350 | 120 | 140 | - |
| Size (cm) | 10 × 7 × 5 | 30 × 15 × 15 | 30 × 15 × 15 | 8 × 7 × 7 cm |
| Weight (grams) | 380 | 4650 | 2950 | 65 |
Specifications of pumps used in this study. WAK, wearable artificial kidney.
For the dialysate clearance studies, fresh spent dialysate was collected from ESRD patient high-flux dialysis treatments and modified by the addition of urea, creatinine, potassium, and calcium to achieve levels of BUN 50 mg/dl, creatinine 4 mg/dl, potassium 4 mEq/L, and Ca 9 mg/dl. This dialysate was circulated through the sorbent containers for 6 h with sampling at the exit site of each sorbent container. All experiments were performed five times. Clearances at different flow rates were compared. We measured urea removal from dialysate per gram of sorbent at 15 pH levels. Also, we compared continuous flow (n = 6) and pulsatile flow with both the WAK (n = 13) and conventional pumps (n = 9) for 6 h and measured the average effective velocity of the dialysate in contact with the sorbent particles. Average flow velocity (Vavg) was calculated as (18):
Vavg = Flow Rate / Cross-Sectional Area.
To assess β2M removal, heparinized normal human blood was dialyzed in vitro with the WAK for seven hours. Blood and dialysate samples were drawn every 30 min. β2M was assayed by microparticle enzyme immunoassay (Abbott Laboratories, Abbott Park, IL).
In vivo studies were performed in five bilateral ureterally ligated anesthetized 81.8 ± 7.4 kg pigs. The next day they were dialyzed for 8 h with a WAK V1.1. Samples of blood and dialysate at both entry and exit ports of the dialyzer were drawn hourly. Removal rates and plasma clearance of urea, creatinine, and potassium were compared for the WAK V1.0 (n = 12) (data from reference 11) and the WAK V1.1 (n = 6, present studies), using pooled estimates of variance in the t-statistics calculation of t-values and p-values. These studies were approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Hospital and conducted in observance of NIH guidelines.
Total blood clearances were calculated as follows:
Total Blood Clearance = Qbf × (Cin − Cout) / Cin where Qbf is blood flow rate, Cin is solute concentration entering, and Cout is solute concentration exiting.
Urea creatinine and β2M clearances were calculated as follows:
Clearance = Qbf × (Cin − Cout) × [1-Hct] / Cin.
The amount of solute removed was calculated as follows:
Total solute removed = (Cin − Cout) × Qbf × t for urea, potassium, and
Total solute removed = (Cin − Cout) × Qbf × [1-Hct] × t for β2M, creatinine and phosphorus.
Where Cin is the concentration of the solute going into the dialyzer, Cout is the concentration of the solute coming out of the dialyzer, Qbf is blood flow, Htc is hematocrit and t is time. Kt/V was calculated as follows:
Kt/V = Effective Clearance × Time / Total Body Water
where [△ Solute] is the difference between concentrations in and out of the dialyzer, time was 480 min. Total body water was estimated as 60% of body weight.
Statistical Analyses
ANOVA analysis and Tukey-Kramer post hoc comparisons were used for potassium, urea, and creatinine clearances of different dialyzer with the different pump flows. An ANOVA model with individual regression models expressing the linear relationship of flow velocity on Urea/ZrP resulted on strong linear relationships, including terms for pulse frequency in beats per minute (bp.m), flow, and their interaction. The residuals of the ANOVA model indicated that their distribution was reasonably normal and that the linear fit was appropriate.
The results of the animal studies, in particular the removal rates and plasma clearance of urea, creatinine, and potassium compared for the WAK V1.0 (n = 12) and the WAK V1.1 (n = 6), were analyzed with t test using pooled estimates of variance in calculating the t statistics.
Results
Pumps and flows.
The WAK pump provides simultaneous pulsatile flows of both blood and dialysate at a half-cycle phase difference and at higher amplitudes and pulse frequency than roller pumps (Figure 2). At a blood flow rate (Qb) of 95 ml/min, the peak flow was 349.5 ml/min (95% confidence), while at a dialysate flow (Qd) of 90 ml/min, the peak flow was 342.5 ml/min (95% confidence).
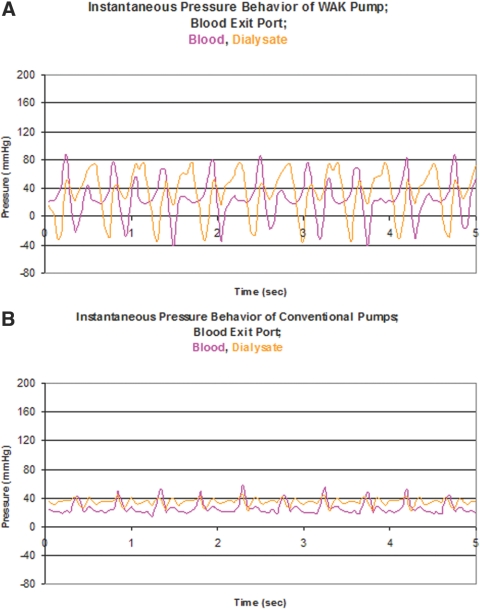
The effects of the pulsatile nature of the WAK and conventional pumps are displayed in Figures 3 (blood entry) and 4 (blood exit). Pressure time tracings at the blood entrance and dialysate exit of the dialyzer are shown at the flow rates described in Figure 2. The single WAK pump generates both blood and dialysate flows in opposite directions at 120 bp.m, and in Figure 3, upper panel, one appreciates △ TMP of −10 to +150 mmHg with pressure amplitudes higher than those of conventional pumps (lower panel) where the △ TMP is only 0 to +60 mmHg. At the blood exit port as seen in Figure 4, the WAK (upper panel) generated a △ TMP of −50 to +50 mmHg with pressure amplitudes higher than those of conventional pumps (lower panel), where the △ TMP is only −10 to +20 mmHg. Analysis of the pressure waves thus shows that with the WAK pump the △ TMP reaches at each cycle a peak of 160 mmHg at the proximal port (Figure 3). At the distal port, the peak △ TMP is 100 mmHg (Figure 4).
Figure 3.
Instantaneous pressures at the blood and dialysate entrance ports for the WAK pump (upper panel) and conventional pump (lower panel). Pressure transducers were placed at the entrance and exit ports of both blood and dialysate in the dialyzers and hooked to the Lab View virtual instruments (National Instruments, Austin, TX).
Figure 4.
Instantaneous pressures at the blood and dialysate exit ports for the WAK pump (upper panel) and conventional pump (lower panel).
Battery life in the present bench tests was approximately 3 d.
Small solute clearances.
Urea and creatinine in vitro clearances (K) are lower in continuous steady flow from gravity as compared with either the WAK pump or the conventional pump, as described in Table 2. ANOVA analysis determined that Kurea was significantly determined by both flow rate (P < 0.0001) and flow type (P < 0.0001). Tukey-Kramer post hoc comparisons indicated that differences among all flow types were significantly different (conventional versus gravity P < 0.0001, conventional versus WAK P = 0.0323, gravity versus WAK P < 0.0001), with the WAK and conventional pump being least different. ANOVA run on creatinine yielded similar findings. Kcrea was significantly determined by both flow rate (P < 0.0001) and flow type (P < 0.0001). Tukey-Kramer post hoc comparisons indicated that differences among all pumps were significantly different (conventional versus gravity P < 0.0001, conventional versus WAK P = 0.0024, gravity versus WAK P < 0.0001), with the WAK and conventional pump being least different.
Table 2.
Comparison between continuous flow and pulsatile flow by both WAK and conventional pumps
| Hemophan Dialyzer |
Gravity |
WAK Pump |
Conventional Pump |
||||
|---|---|---|---|---|---|---|---|
| Qb | Qd | Kurea | Kcrea | Kurea | Kcrea | Kurea | Kcrea |
| 130 | 140 | 41.5 ± 13.0 | 31.7 ± 9.3 | 55.0 ± 8.3 | 41.3 ± 8.9 | 55.7 ± 5.9 | 44.9 ± 6.6 |
| 95 | 105 | 33.4 ± 10.3 | 23.6 ± 10.4 | 44.1 ± 6.7 | 34.6 ± 6.3 | 46.1 ± 3.5 | 38.9 ± 4.2 |
| 80 | 90 | 31.6 ± 10.3 | 21.5 ± 9.1 | 37.8 ± 6.1 | 30.0 ± 7.3 | 42.4 ± 3.9 | 35.0 ± 4.0 |
| 67 | 75 | 27.9 ± 8.3 | 17.4 ± 7.6 | 34.8 ± 7.0 | 26.8 ± 6.4 | 36.8 ± 2.3 | 31.1 ± 3.0 |
| 52 | 55 | 23.3 ± 5.9 | 15.5 ± 5.5 | 26.0 ± 6.3 | 22.2 ± 6.4 | 30.4 ± 2.5 | 26.1 ± 2.5 |
| 35 | 42 | 18.3 ± 4.2 | 13.0 ± 4.5 | 21.7 ± 5.2 | 17.7 ± 4.9 | 24.3 ± 0.7 | 20.3 ± 1.4 |
| 18 | 28 | 12.2 ± 2.2 | 9.3 ± 2.9 | 12.7 ± 2.5 | 10.8 ± 2.8 | 17.4 ± 1.5 | 14.4 ± 1.2 |
WAK, wearable artificial kidney; Qb, blood flow rate in ml/min; Qd, dialysate flow rate in ml/min; Kurea, clearance of urea in ml/min; Kcrea, creatinine clearance in ml/min.
Table 3 compares urea and creatinine clearances from two different dialyzers using the WAK pump. ANOVA indicated that flow rate (P < 0.0001), but not type of dialyzer (P = 0.0609), determined urea clearance. Similar analysis for creatinine indicated the effect of dialyzer was somewhat stronger (P = 0.0145), showing higher creatinine clearances for AN69 at higher flow rates. A similar analysis with conventional pumps (Table 4) also showed that flow rate (P < 0.0001), but not dialyzer type (P = 0.2291), determined urea clearance. Parallel analysis for creatinine indicated a significant effect only for flow rate (P = <0.0001) and not for dialyzer type (P = 0.4851).
Table 3.
Urea and creatinine clearance using AN 69 and F3 dialyzers using the WAK pump at various flow rates
| WAK Pump |
AN69 Dialyzer |
F3 Dialyzer |
|||
|---|---|---|---|---|---|
| Qb | Qd | Kurea | Kcrea | Kurea | Kcrea |
| 23 | 20 | 10.1 ± 1.7 | 10.4 ± 2.7 | 15.7 ± 1.6 | 14.4 ± 2.0 |
| 35 | 30 | 20.6 ± 8.2 | 19.1 ± 4.5 | 21.8 ± 2.6 | 20.1 ± 2.5 |
| 57 | 43 | 33.8 ± 0.2 | 32.3 ± 1.4 | 31.3 ± 4.7 | 27.8 ± 5.7 |
| 77 | 60 | 48.8 ± 0.0 | 44.3 ± 4.3 | 42.0 ± 1.9 | 37.0 ± 3.2 |
| 92 | 70 | 58.1 ± 4.0 | 52.2 ± 6.2 | 53.2 ± 7.1 | 44.7 ± 7.2 |
| 100 | 95 | 64.5 ± 6.3 | 57.5 ± 7.8 | 56.2 ± 3.7 | 47.6 ± 5.0 |
| 110 | 138 | 74.5 ± 0.1 | 66.3 ± 2.0 | 61.5 ± 11.5 | 53.5 ± 6.8 |
WAK, wearable artificial kidney; Qb, blood flow rate in ml/min; Qd, dialysate flow rate in ml/min; Kurea, clearance of urea in ml/min; Kcrea, creatinine clearance in ml/min.
Table 4.
Urea and creatinine clearance with AN 69 and F3 dialyzers using conventional pumps at various flow rates
| Conventional Pump |
AN69 Dialyzer |
F3 Dialyzer |
|||
|---|---|---|---|---|---|
| Qb | Qd | Kurea | Kcrea | Kurea | Kcrea |
| 22 | 28 | 17.9 ± 4.7 | 15.1 ± 4.7 | 17.3 ± 2.1 | 15.6 ± 0.1 |
| 35 | 40 | 22.5 ± 1.6 | 20.5 ± 4.0 | 26.8 ± 2.4 | 24.0 ± 0.5 |
| 57 | 45 | 30.1 ± 1.6 | 27.6 ± 4.3 | 35.0 ± 0.5 | 31.4 ± 3.4 |
| 76 | 60 | 43.5 ± 2.8 | 38.7 ± 0.8 | 41.5 ± 0.3 | 39.2 ± 1.8 |
| 92 | 70 | 52.7 ± 6.4 | 47.0 ± 1.8 | 49.7 ± 2.9 | 37.8 ± 6.8 |
| 100 | 95 | 56.0 ± 1.6 | 50.5 ± 1.9 | 55.6 ± 2.9 | 44.0 ± 4.1 |
| 110 | 139 | 63.3 ± 6.8 | 58.6 ± 4.4 | 75.1 ± 9.9 | 63.8 ± 8.9 |
Qb, blood flow rate in ml/min; Qd, dialysate flow rate in ml/min; Kurea, clearance of urea in ml/min; Kcrea, creatinine clearance in ml/min.
Effect of dialysate flow.
Figure 5 demonstrates that increasing the frequency of the pulses (bp.m) augmented the extracted amount of urea per gram of zirconium phosphate by 20%. Removal of urea by the WAK system is shown to be a linear function of both average flow velocity and frequency of pulsation.
Figure 5.
Removal of urea by WAK system is shown to be a linear function of both parameters of dialysate flow: Average flow velocity and frequency of pulsation.
Individual regression models expressing the linear relationship of flow velocity on urea (grams)/ZrP (grams) resulted in strong linear relationships:
0 bp.m urea/ZrP = 0.04532 + 0.08678 × velocity, P = 0.0047, r2 = 0.8902;
70 bp.m urea/ZrP = 0.03186 + 0.13145 × velocity, P < 0.0001, r2 = 0.9772;
140 bp.m urea/ZrP = 0.06007 + 0.10817 × velocity, P < 0.0001, r2 = 0.9337.
ANOVA analysis determined that the three slopes were sufficiently close that a single slope of 0.1020, P = <0.001, was adequate to fit the linear relationship. The model indicated that although the slopes were parallel, the three bp.m lines did require separate intercepts to properly fit the data.
Effect of dialysate pH.
Shown in Figure 6, as dialysate pH increased due to bicarbonate addition, there was enhanced extraction of urea from the dialysate as the urea is adsorbed onto the zirconium phosphate more effectively, increasing from 5 to 55 mg (per gram of zirconium phosphate). The nonlinear model converged in 7 iterations urea = 0.00120 × pH5.3077. The model predicted 0.8249 of the variance of urea, P < 0.0001.
Figure 6.
As dialysate pH increases due to bicarbonate addition, there is enhanced extraction of urea from the dialysate as it is adsorbed onto the zirconium phosphate more effectively.
β2M removal.
Table 5 shows the concentration of β2M in the dialysate after each of the sorbents in the WAK, over time. In 7 h the total WAK sorbent system (in order of exposure: urease, zirconium phosphate, hydroxyl zirconium oxide, and charcoal) removed 9.7 ± 2.0 mg of β2M from spent dialysate, which amounts to 73.7 ± 11.3% of the amount of β2M entering the system. Most β2M was adsorbed by the charcoal.
Table 5.
Concentration of β2M in dialysate exiting various WAK sorbent beds (μg/L)
| Time into tests (hr) | Post-urease | Post-zirconium-phosphate | Post-hydroxy-zirconium-oxide | Post-activated-charcoal | Overall Removal, % |
|---|---|---|---|---|---|
| 0.5 | 401.4 | 213.3 | 14.8 | 0.0 | 100.0 |
| 1.0 | 492.3 | 397.7 | 304.6 | 35.3 | 93.6 |
| 1.5 | 559.0 | 496.6 | 393.1 | 65.7 | 88.0 |
| 2.0 | 587.2 | 588.9 | 446.6 | 101.3 | 81.5 |
| 2.5 | 576.3 | 622.9 | 511.6 | 116.7 | 78.7 |
| 3.0 | 550.8 | 643.1 | 484.4 | 124.9 | 77.2 |
| 3.5 | 594.9 | 584.1 | 516.9 | 137.6 | 74.9 |
| 4.0 | 521.9 | 530.8 | 515.4 | 119.8 | 78.1 |
| 4.5 | 521.4 | 576.7 | 520.3 | 146.6 | 73.2 |
| 5.0 | 536.9 | 546.1 | 548.6 | 185.9 | 66.1 |
| 5.5 | 505.1 | 512.0 | 527.4 | 167.5 | 69.4 |
| 6.0 | 501.7 | 521.3 | 510.5 | 179.4 | 67.3 |
| 6.5 | 553.0 | 545.7 | 553.7 | 163.1 | 70.2 |
| 7.0 | 530.7 | 587.4 | 496.8 | 171.8 | 68.7 |
Concentration of β2M in dialysate exiting various WAK sorbent beds (μg/L;) initial batch concentration = 548 μg/L, average flow rate = 72.6 ml/min. Concentration of β2M in dialysate exiting various WAK sorbent containers (μg/L;) initial concentration = 548 μg/L, average flow rate = 72.6 ml/min, total dialysate in seven hours (420 min) was 30.49 liters.
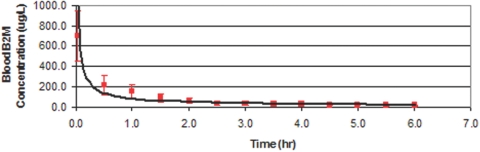
In a different experiment using healthy human blood dialyzed with the WAK, the β2M concentration fell from 698.9 ± 249.5 to 39.8 ± 19.9. in 2.5 h (Figure 7). Removal of β2M is most effective in the first half-hour. The nonlinear model converged in 9 iterations as β2M (ug/L) = 84.1880 × (Time,hr)−0.3075. The model predicted 0.7950 of the variance of β2M concentration, P < 0.0001.
Figure 7.
Removal of β2M from healthy human blood by WAK system is shown to be most effective in the first half-hour.
Animal studies.
Table 6 compares the small solute clearances from two sets of experiments involving WAK V1.0, as described in earlier publications (10,11), versus the WAK V1.1. Greater urea, creatinine and potassium clearances were achieved with the WAK V1.1.
Table 6.
Small solute clearances in uremic pigs WAK V1.0 vs. WAK V1.1
| Results | V 1.0 (n = 12) | V 1.1 (n = 6) | Units | P value |
|---|---|---|---|---|
| Effective urea clearance | 24.5 ± 9.8 | 37.0 ± 7.3 | [ml/min] | 0.0140 |
| Effective creatinine clearance | 18.5 ± 3.2 | 27.0 ± 4.0 | [ml/min] | 0.0001 |
| Effective potassium clearance | 32.6 ± 7.1 | 50.3 ± 14.0 | [ml/min] | 0.0024 |
| Effective phosphate clearance | 13.9 ± 1.8 | 23.1a | [ml/min] | N/A |
| Effective urea removal | 22.0 ± 8.2 | 31.3 ± 7.9 | [mg/min] | 0.0357 |
| Effective creatinine removal | 1.5 ± 0.2 | 2.4 ± 0.3 | [mg/min] | 0.0005 |
| Effective phosphate removal | 1.4 ± 0.2 | 2.5a | [mg/min] | N/A |
| Effective potassium removal | 1.9 ± 0.4 | 11.0 ± 2.9 | [mg/min] | <0.0001 |
| Hourly Kt/V | 0.040 ± 0.004 | 0.045 ± 0.002 | N/A | N/A |
8 h of WAK dialysis, in anesthetized uremic pigs. WAK, wearable artificial kidney.
Data available for one pig only, with average concentration in/out 11.01/5.83 mg/dl, average hematocrit 33%, average blood.
Discussion
A WAK must be comfortable and independent of stationary water and electricity sources. The WAK V1.1 operates with two 9-volt batteries, 375 ml of dialysate, and is small and light enough to be wearable. Previous attempts at building a WAK were overwhelmed by technical problems (8,9). This study describes the solute-removal capabilities of the sorbent dialysate regenerating WAK, demonstrating that the technical problems of the past are being addressed successfully.
The WAK pump propels simultaneous pulsatile flows of blood and dialysate with a half-cycle phase difference, such that when the blood flow peaks, the dialysate flow is at trough and vice versa (Figure 2). The frequent and intermittent pressure and flow changes are conducive to considerable differences in the magnitude and directions of the TMP along the dialyzer fibers (Figures 3 and 4) and have the potential to greatly influence solute clearance and mass transfer between blood and dialysate by increasing the fluid flux across the membrane pores. The pressure waves generated by the WAK pump show a different and unique pattern from the pressure and ▵ TMP patterns in conventional roller pumped machines (13,14). Three conditions are in concert to create a high degree of convective transport within the WAK. First, reverse flow is not permitted by the WAK pump valves, such that pressures in the compartments favor ultrafiltration from blood to dialysate. Second, the hollow fibers are not elastic, hence the same consequence. Third, liquids such as blood are noncompressible, with, again, the same consequence. Thus, the volume cycles drive a large tidal wave of blood into the hollow fibers with each cycle, which in turn generates higher magnitudes of instant pressures against the wall of the fiber. A significant increase in convection occurs. These intermittent flow changes create a push-pull mechanism across the dialyzer membrane.
Push–pull flows have been previously described (19,25). The push-pull of pulsatile flow has been shown to increase convective mass transfer across the dialyzer membrane, a desirable attribute (20–23). Previously, the acu-men® hemofiltration device utilized a pulsatile blood pump in a much larger collapsible chamber (24). The Pulsar pump propels blood by pulsatile flow (25), but there is no dialysis device currently used that implements such a mechanism for both blood and dialysate movement. The frequent and intermittent changing in TMP, as a result of the opposite phase flow cycles in the WAK, impedes the accumulation of blood cells and solutes within the pores of the membrane and prevent film formation (protein concentration polarization) along the inner walls of the hollow fibers. The increased amplitude and peak TMP also enhance convection.
The direction of the TMP gradient at the blood entry part of the dialyzer is opposite that at the blood exit side. Consequently, the fresh dialysate back filters into the blood compartment in the distal portion of the hollow fibers (Figures 3 and 4). This intermittent change in gradient and the bidirectional flux across the membrane suggest that this device may perform a different type of hemodiafiltration, similar in some aspects to that described by Fiore et al. and called “internal hemodiafiltration” (26,27). However, the WAK pump pulsatile flow pattern causes intermittent opposite phase changes in TMP and thus differs from other types of hemodiafiltration described (27). We name this “pulsatile push-pull internal hemodiafiltration.” An increase in convective mass transfer of middle molecular weight molecules in hemodiafiltration may be the preferable way of treating ESRD (28,29).
The current WAK uses the same sorbents as the REDY system (30–34). This sorbent based dialysate regeneration system has been effectively applied to the treatment of acute and chronic kidney failure patients for many years. The WAK uses only 375 ml of constantly regenerated and recirculated dialysate, hence the need for sorbents. The oscillating pump mechanism creates a high peak flow velocity that augments significantly the ability of the sorbents to remove urea. We speculate that the turbulence created by the pulsatile flow exposes more sorbent surface area, resulting in greater solute extraction.
The zirconium phosphate (34) cation exchanger is loaded with H+ and Na+ during manufacture. Free hydrogen ions compete with ammonium for binding to zirconium phosphate. By raising the dialysate pH to 7.4 (and neutralizing H+), more sites are opened for ammonium adsorption, optimizing urea removal (Figure 6).
The removal of β2M from blood is almost certainly confounded by the adsorption of β2M to the membrane. However, we have shown that the WAK sorbents effectively remove β2M from dialysate, mainly by charcoal (Table 5). Thus, convectively derived β2M may be effectively removed by the WAK. As discussed above, the hemodynamic characteristics of the WAK strongly favor convection. Since β2M is considered a marker of middle molecule uremic toxins and its removal is desirable, the present results further support the notion that the WAK may be effective in the treatment of uremic patients. Regarding small solutes, the in vivo results obtained with the WAK V 1.1 show improvement over those of the WAK V 1.0 (10–13). So far, we have shown that the WAK V1.1 has the potential to remove uremic solutes up to 8 h and is feasible in humans (15,16).
The question of cost is most pertinent. It seems obvious that widespread implementation of the WAK as the standard for renal replacement therapy would result in vast savings in labor and costs of operating a dialysis unit. This savings would be incremental on the savings in drug use and hospitalizations brought about already with daily dialysis (6). We are painfully aware that our efforts to build a WAK would be useless if the implementation of its use in practice is not affordable. The estimated cost of the WAK and disposables is expected to be less than that of current machines. Furthermore, we aim to keep the monthly cost of the disposables below the sums spent currently in operating a dialysis machine.
Upcoming questions are (1) Can the WAK be worn by humans over prolonged and continuous periods? and (2) would that improve outcomes in ESRD?
The results shown here encourage us to move ahead to answer them.
Disclosures
Victor Gura, MD, is the Chief Medical Officer of Xcorporeal Inc.
Acknowledgments
We are grateful to Drs. Hans Dietrich Pollaschegg, Eli Friedman, Claudio Ronco, Andrew Davenport, and Andre Kaplan for their generous mentoring and guidance, and to Gerard Smitts, PhD, for his assistance with statistical analysis.
Contents of this paper were presented in part in the American Society of Nephrology Meetings in November 2005 (13) and November 2006 (14).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Will Nephrologists Use a Wearable Artificial Kidney?” on pages 1401–1402.
References
- 1.Lockridge RS: The direction of end-stage renal disease reimbursement in the United States. Semin Dial 17: 125–130, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Lockridge RS, McKinney JK: Is HCFA's reimbursement policy controlling quality of care for end-stage renal disease patients? ASAIO J 47: 466–468, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Manns BJ, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C: Dialysis adequacy and health related quality of life in hemodialysis patients. ASAIO J 48: 565–569, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64: 339–349, 2003 [DOI] [PubMed] [Google Scholar]
- 5.McFarlane PA, Bayoumi AM, Pierratos A, Redelmeier DA: The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int 64: 1004–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Mohr PE, Neumann PJ, Franco SJ, Marainen J, Lockridge R, Ting G: The case for daily dialysis: Its impact on costs and quality of life. Am J Kidney Dis 37: 777–789, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Patel SS, Shah VS, Peterson RA, Kimmel PL: Psychosocial variables, quality of life, and religious beliefs in ESRD patients treated with hemodialysis. Am J Kidney Dis 40: 1013–1022, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Stephens RL, Jacobsen SC, Atkin-Thor E, Kolff W: Portable/wearable artificial kidney (WAK) – Initial evaluation. Proc Eur Dial Transplant Assoc 12: 511–518, 1976 [PubMed] [Google Scholar]
- 9.Murisasco A, Reynier JP, Ragon A, Boobes Y, Baz M, Durand C, Bertocchio P, Agenet C, el Mehdi M: Continuous arterio-venous hemofiltration in a wearable device to treat end-stage renal disease. Trans Am Soc Artif Intern Organs 32: 567–571, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Gura V, Ezon C, Beizai M, Polaschegg HD: CRRT for ESRD; the wearable artificial kidney; a feasible, safe, and cost effective way to provide daily dialysis. J Am Soc Nephrol 15: 639A, 2004 [Google Scholar]
- 11.Gura V, Beizai M, Ezon C, Polaschegg HD. Continuous renal replacement therapy for end-stage renal disease: The wearable artificial kidney (WAK) in Ronco C, Brendolan A, Levin N W. (eds): Cardiovascular Disorders in Hemodialysis, Basel, Karger, Contrib Nephrol; 149: 325–333, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gura V, Beizai M, Ezon C: CRRT for CHF: The wearable continuous ultrafiltration system. ASAIO J 52: 59–61, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gura V, Beizai M, Ezon C, Polaschegg HD: Pulsatile blood and dialysate counter phase flows, increased sorbent capacity and a high flux membrane explain the high efficiency of the wearable artificial kidney (WAK). J Am Soc Nephrol 16: 38A–39A, 2005 [Google Scholar]
- 14.Rambod EM, Rosenfeld M, Beizai M, Gura V: The impact of pulsatile flow on the effectiveness of the WAK. J Am Soc Nephrol 17: 723A, 2006 [Google Scholar]
- 15.Gura V, Davenport A, Beizai M, Ezon C, and Ronco C: β2 microglobulin and phosphate clearance using a wearable artificial kidney: Pilot study. Am J Kidney Dis 2009April17 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Davenport A, Gura V, Ronco C, Beizai M, Ezon , Rambod E: A pilot study of a wearable artificial kidney in end stage renal failure patients. Lancet 370: 2005–2010, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gura V, Ronco C, Nalesso F, Brendolan A, Beizai M, Ezon C, Davenport A, Rambod E: A wearable hemofilter for continuous ambulatory ultrafiltration. Kidney Intl 73: 497–502, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Perry RH, Green DW, Maloney JO. (eds.): Perry's Chemical Engineers' Handbook, New York, McGraw-Hill, 1984, p 5–5 [Google Scholar]
- 19.Miwa M, Shinzato T: Push/pull hemodiafiltration: Technical aspects and clinical effectiveness. Artif Organs 23: 1123–1128, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Jaffrin MY, Ding LH, Gupta BB: Rationale of filtration enhancement in membrane plasmapheresis by pulsatile blood flow. Life Support Systems 5: 267–271, 1987 [PubMed] [Google Scholar]
- 21.Jaffrin MY, Ding LH, Laurent JM: Simultaneous convective and diffusive mass transfer in a hemodialyser. J Biomech Eng 112: 212–219, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Runge TM, Briceño JC, Sheller ME, Moritz CE, Sloan L, Bohls FO, Ottmers SE: Hemodialysis: Evidence of enhanced molecular clearance and ultrafiltration volume by using pulsatile flow. Int J Artif Organs 16: 645–652, 1993 [PubMed] [Google Scholar]
- 23.Ding LH, Laurent JM, Jaffrin MY: Dynamic filtration of blood: A new concept for enhancing plasma filtration. Int J Artif Organs 14: 365–370, 1991 [PubMed] [Google Scholar]
- 24.Manns M, Polaschegg HD, Schlaeper C, Steinbach B, Evering HG: The acu-men: A new device for continuous renal replacement therapy in acute renal failure. Kidney Int 54: 268–274, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Ash SR: The Allient dialysis system. Semin Dial 17: 164–166, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Fiore GB, Guadagni G, Lupi A, Ricci Z, Ronco C: A new semiempirical mathematical model for prediction of internal filtration in hollow fiber hemodialyzers. Blood Purif 24: 555–568, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ronco C, Cruz D: Hemodiafiltration history, technology and clinical results. Advances in Chronic Kidney Disease 14: 231–243, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Wizemann V, Lotz C, Techert F, Uthoff S: On-line haemodiafiltration versus low-flux haemodialysis. A prospective randomized study. Nephrol Dial Transplant 15 [Suppl 1]: 43, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Locatelli F, Mastrangelo F, Redaelli B, Ronco C, Marcelli D, La Greca G, Orlandini G: Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. The Italian Cooperative Dialysis Study Group. Kidney Int 50: 1293, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Marantz LB, Giorgianni MG. inventors. Urease in insoluble form for converting urea present in liquid. US patent 3989622, 11/2/1976 [Google Scholar]
- 31.Drukker W, Van Doomf ALW: Dialysate regeneration. In Replacement of Renal Function by Dialysis, 3rd Ed, edited by Maher JF, The Netherlands, Kluwer Academic Publishers, 1989, pp. 417–438 [Google Scholar]
- 32.Mansell MA, Wing AJ: Long term experience of home dialysis with sorbent regeneration of dialysate. Proc Eur Dial Transplant Assoc 13: 275, 1977 [Google Scholar]
- 33.Odell RA: Sorbent dialysis. In: Clinical Dialysis, 2nd Ed., edited by Nissenson AS, Fine RN, Gentile DE.Norwalk, CT, Appleton-Century-Crofts, 1990, pp 712–719 [Google Scholar]
- 34.Kobayashi E: A study of inorganic ion exchangers VII; the synthesis of γNH4ZrH(PO4)2 and ion-exchange properties of γ-Zr(HPO4)2. 2H2O. Bull Chem Soc Jpn 56: 3756–3760, 1983 [Google Scholar]