Abstract
Background and objectives: Hepatitis C virus (HCV) infection is prevalent in hemodialysis patients and causes excess mortality. Interferon (IFN) treatment of chronic HCV infection in hemodialysis patients results in high sustained virological response (SVR) rates 6 mo after treatment. The authors aimed to identify factors associated with SVR in hemodialysis patients through analysis of individual patient data obtained from systematic review of published literature.
Design, setting, participants & measurements: Medline was searched from 1966 through February 2009, and prospective studies describing IFN treatment of hemodialysis patients with chronic HCV infection with published individual patient data were included. To identify factors associated with SVR, logistic regression was applied with adjustment for study.
Results: Twenty studies of IFN treatment provided data on 428 patients. Overall SVR was 45% and in univariate analyses was higher with: 1) three million units or higher three times weekly of IFN; 2) treatment for at least 6 mo; 3) treatment completion; 4) lower baseline HCV RNA; 5) female gender; and 6) early virological negativity. Although limited by missing data, these relationships persisted in multivariate regression.
Conclusions: SVR is more likely with larger IFN dose, longer treatment duration, treatment completion, female gender, lower HCV RNA and early virological negativity. For appropriate treatment candidates, regimens should consist of three million units of IFN three times weekly for at least 6 mo, with patients encouraged to complete the full course.
Hepatitis C virus (HCV) infects an estimated 170 million people worldwide (1). The prevalence of HCV in hemodialysis (HD) patients ranges from 3 to 23% in developed countries (2) and exceeds 50% in some developing countries (3). HCV-infected HD patients have higher mortality rates than noninfected HD patients, with reported relative risks from 1.25 to 1.57 (4,5). Untreated, spontaneous viral clearance occurs in only 0.5% of chronic HCV-infected patients per year (6). The standard measure of treatment success, sustained virological response (SVR), is defined as achieving HCV RNA negativity six months after treatment completion. In non-HD patients, interferon (IFN) monotherapy achieves SVR in 9 to 22% of patients (7–9) but combination pegylated IFN and ribavirin achieves SVR in 50 to 60% (10,11). However, IFN and ribavirin are associated with significant toxicity including influenza-like symptoms, anemia and depression with IFN (7–9), and hemolytic anemia with ribavirin (7,8).
Most studies of HCV-infected HD patients have investigated IFN monotherapy. Only recently, studies have explored pegylated IFN or ribavirin (12–18). IFN treatment after kidney transplantation is associated with increased rates of allograft rejection (19,20), so treatment before transplantation is advised (21,22). Our recent meta-analysis of summary data in HCV-infected HD patients demonstrated an overall SVR rate of 41% with IFN (22), higher than rates in IFN-treated non-HD patients (7,8), but rates of treatment discontinuation due to adverse events were also higher (22).
Identifying factors associated with a higher likelihood of SVR among HD patients has important implications for selecting treatment candidates and the optimal treatment regimen. In non-HD patients, higher SVR rates are associated with younger age, female gender, lower patient weight, HCV genotypes other than 1, lower baseline HCV RNA, and absence of cirrhosis on liver biopsy (7–11). Early virological response (EVR), defined as a 2-log10 or larger decrease in HCV RNA by the 12th week of treatment, is a powerful predictor of SVR (23,24).
We investigated whether these previously identified factors associated with SVR in non-HD patients could be validated in the HD population. The majority of studies of IFN in HD patients reported individual patient data, allowing us to extend our prior subgroup analysis and meta-regression of summary data (22) to identify factors associated with SVR.
Materials and Methods
Study Selection
The literature search strategy and study selection process for our systematic review have been described previously (22). We included prospective studies of IFN-based treatment of HD patients with chronic HCV infection and with reported SVR at 5 mo or more after treatment completion. Studies involving kidney transplant recipients, CKD stages 1 to 5 not on dialysis, or not reporting SVR were excluded as were studies with sample sizes less than 10. Studies of pegylated IFN were excluded because pharmacokinetic differences may result in different SVR rates than with standard IFN. The literature search was updated on February 1, 2009.
Individual Patient Data Analysis
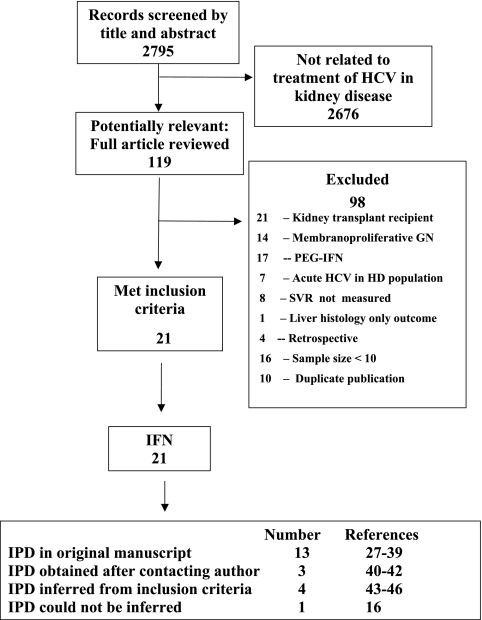
All studies specified treatment protocols such as dose and duration, but only 65% contained individual patient data. A single investigator (Craig E. Gordon) extracted published individual patient data and contacted the authors of all included studies directly to request additional unpublished data. If contact was unsuccessful, we inferred data from study inclusion and exclusion criteria (e.g., transaminase elevation or presence of cirrhosis) when able to do so with certainty. When data were unclear or required adjudication, additional authors were consulted until consensus was achieved. Based on these criteria, 20 studies of IFN treatment of HCV-infected HD patients met inclusion criteria (Figure 1) and one was excluded (16).
Figure 1.
Systematic review of the literature. GN, glomerulonephritis; IPD, individual patient data; PEG-IFN, pegylated IFN.
Statistical Analyses
Using individual patient data, we investigated the relationship between SVR and patient, viral, and treatment characteristics based on factors previously identified in non-HD patients (7–9), HD patients (25,26), and our previous meta-analysis of summary data (22). Patient factors included age, gender, duration of HD, and previous kidney transplantation. Viral factors included HCV genotype, baseline quantitative HCV RNA, cirrhosis or bridging fibrosis on liver biopsy, duration of HCV infection, and elevation of transaminases. Treatment characteristics included intended dose and duration of IFN and measures of treatment completion, including achieved treatment duration and the proportion of intended treatment completed. Because studies did not uniformly report 2-log10 decrease in quantitative HCV RNA at 3 mo, the standard EVR definition, we instead defined “early virological negativity” (EVN) as HCV RNA clearance following 1 to 3 mo of treatment. We analyzed HCV RNA as a continuous (log10 HCV RNA) and a categorical variable (cutoff of < or > = 400,000 IU/ml) based on exploratory analysis of the data. As in the original studies, we defined elevated transaminases as greater than 40 IU/L. Selecting lower cutoff values did not affect the relationship between this variable and SVR.
We applied univariate logistic regression using SAS version 9.1 (Cary, NC) to investigate the relationship between predictors and SVR. To account for potential confounding by study, we reanalyzed these relationships using study as a categorical set of dummy variables. We performed multivariate logistic regression using factors associated with SVR in univariate regression with p-values <0.05 and with clinically important factors. Multivariate analysis was limited because individual variables were often missing for entire studies. We also found unanticipated clustering of data; e.g., all patients identifiable by age, gender, HCV RNA and genotype were treated with 3 MU of IFN or higher and for 6 mo or longer. This clustering precluded multiple imputation and necessitated multiple sets of multivariate analyses using alternate subsets of studies.
To analyze the relationship of the above covariates and treatment discontinuation due to adverse events, we applied identical methods. We focused on treatment discontinuation as opposed to adverse events alone because it is more consistently reported and a marker of serious adverse events.
Results
Study Characteristics
Twenty studies met inclusion criteria (Figure 1) with individual patient data published in 13 studies (27–39) and obtained for three additional studies after contacting authors (40–42). Limited data based on inclusion criteria were imputed for the remaining four studies (43–46), but no further data were provided despite multiple attempts to contact authors.
The 20 studies of IFN treatment comprised 428 patients with varying completeness of data for specific covariates (Table 1). Patients had a mean age of 46 yr and 60% were male. The mean duration of HD was 72 mo and of HCV infection was 73 mo. Mean pretreatment HCV RNA was 363,307 IU/ml. The prevalence of HCV genotype 1 was 65%; 4% had cirrhosis, and 17% had bridging fibrosis on liver biopsy.
Table 1.
Characteristics of all patients and those subsets with data on HCV RNA, gender, or early virological negativity
| Characteristic | Complete Dataset (n = 428) |
HCV RNA Data (n = 112) |
Gender Data (n = 275) |
Early Virological Negativity Data (n = 196) |
||||
|---|---|---|---|---|---|---|---|---|
| Number with Data on Characteristica | Mean or Proportion | Number with Data on Characteristica | Mean or Proportion | Number with Data on Characteristica | Mean or Proportion | Number with Data on Characteristica | Mean or Proportion | |
| Patient Characteristics | ||||||||
| Age (yr) | 275 | 46 | 86 | 41 | 275 | 46 | 148 | 46 |
| Female gender (%) | 275 | 40 | 86 | 33 | 275 | 40 | 148 | 41 |
| Duration of hemodialysis (mo) | 210 | 72 | 80 | 71 | 210 | 72 | 107 | 89 |
| Prior kidney transplantation (%) | 126 | 31 | 60 | 32 | 126 | 31 | 75 | 23 |
| HCV Characteristics | ||||||||
| HCV RNA (IU/ml) | 112 | 363,307b | 112 | 363,307 | 86 | 395,505 | 59 | 219,891 |
| HCV genotype (% genotype 1) | 220 | 65 | 110 | 51 | 135 | 56 | 113 | 67 |
| Duration of HCV infection (mo) | 175 | 73 | 78 | 62 | 153 | 74 | 129 | 77 |
| LFTs, pre-treatment (% elevated) | 219 | 78 | 63 | 62 | 160 | 76 | 87 | 67 |
| Liver biopsy | ||||||||
| Cirrhosis (%) | 242c | 4 | 70 | 4 | 172 | 4 | 141 | 4 |
| Cirrhosis or bridging fibrosis (%) | 188c | 21 | 59 | 7 | 146 | 25 | 113 | 19 |
| Treatment Characteristics | ||||||||
| Interferon dose | ||||||||
| 1 to 1.5 MU (%) | 24 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 MU (%) | 342 | 80 | 112 | 100 | 213 | 77 | 149 | 76 |
| 5 to 6 MU (%) | 62 | 14 | 0 | 0 | 62 | 23 | 47 | 24 |
| Duration | ||||||||
| 4 mo (%) | 37 | 9 | 0 | 0 | 37 | 14 | 37 | 19 |
| 6 mo (%) | 129 | 31 | 41 | 37 | 74 | 28 | 45 | 23 |
| 12 mo (%) | 253 | 60 | 71 | 63 | 155 | 58 | 114 | 58 |
| Treatment complete (%) | 419 | 75 | 112 | 85 | 266 | 76 | 196 | 80 |
| Treatment length (mo) | 419 | 8 | 103 | 9 | 267 | 8 | 196 | 8 |
| Treatment Outcomes | ||||||||
| Early virological negativity (%) | 182 | 60 | 59 | 81 | 138 | 56 | 182 | 60 |
| Sustained virological response (%) | 411 | 45 | 98 | 32 | 271 | 49 | 182 | 50 |
Number of patients with data on presence or absence of characteristic (e.g., number with data on any HCV genotype or results of liver biopsy).
Reported value is mean HCV RNA. Median value was 100,952 IU/ml.
Number of patients with data on cirrhosis greater because cirrhosis variable included studies where presence of cirrhosis was inclusion or exclusion criterion. Relaxing this assumption did not affect results of univariate or multivariate analyses.
LFT, liver function tests; MU, million units.
Because we identified a significant proportion of missing individual patient data, in particular for important factors such as HCV RNA, gender, and EVN, we examined baseline characteristics in the cohorts with data for these covariates and in the overall cohort. Most characteristics were similar both in the subpopulations and the overall cohort, and not surprisingly, the largest discrepancies occurred in the smaller subsets (those with data for HCV RNA and EVN).
Treatment Response
Overall SVR was 45% (95% CI, 40 to 50%). SVR was similar in the subpopulations with data on gender and EVN but was somewhat lower in patients with data on HCV RNA (Table 1). EVN was achieved in 60% (95% CI, 55 to 69%).
Univariate Logistic Regression of SVR
Univariate logistic regression (Table 2) demonstrated that 3 MU or higher of IFN (OR 3.3, 95% CI 1.2 to 9.1) and intended treatment duration of 6 mo or longer (OR 2.0, 1.1 to 3.9) both were associated with higher SVR. Treatment length, whether defined by months of actual treatment (OR 1.13, 1.07 to 1.19 per month), completion of the intended treatment (OR 4.1, 2.4 to 6.8), or proportion completed of intended treatment (OR 1.3, 1.2 to 1.4 per 10% of intended treatment), was associated with higher SVR. Lower baseline HCV RNA was associated with higher likelihood of SVR, whether analyzed continuously (OR 3.6, 1.9 to 6.7 per log10 decrease in HCV RNA) or categorically (OR 11.1, 1.4 to 100.0 for HCV RNA < = 400,000 IU/ml). Female gender (OR 2.1, 1.3 to 3.5) and EVN (OR 5.1, 2.6 to 10.0) were also associated with increased odds of SVR. Patient age, duration of HCV or HD, HCV genotype, cirrhosis/bridging fibrosis, and other covariates were not statistically significantly associated with SVR. Limiting the analysis to the 16 studies with individual patient data had minimal or no effect on the results (results not shown).
Table 2.
Odds ratio of sustained virological response after interferon (univariate and bivariate logistic regression)
| Unadjusted |
Adjusted for Study |
|||
|---|---|---|---|---|
| Number with Covariate | Odds Ratio (95% CI) | Odds Ratio (95% CI) | ||
| Patient Characteristics | ||||
| Age (per additional 10 yr) | 271 | 1.1 (0.9 to 1.3) | 1.0 (0.8 to 1.3) | |
| Female gender | 271 | 2.1 (1.3 to 3.5) | 2.4 (1.4 to 4.2) | |
| Duration of hemodialysis (per month) | 210 | 1.0 (0.9 to 1.1) | 1.0 (0.9 to 1.1) | |
| Prior kidney transplantation (versus none) | 126 | 0.9 (0.4 to 2.0) | 0.7 (0.3 to 1.3) | |
| HCV Characteristics | ||||
| HCV RNA (per log10 decrease) | 98 | 3.6 (1.9 to 6.7) | 3.1 (1.6 to 6.3) | |
| HCV RNA (<= versus >400,000 IU/ml) | 98 | 11.1 (1.4 to 100.0) | 11.1 (1.3 to 100.0) | |
| HCV genotype 1 (versus others) | 207 | 1.1 (0.6 to 2.1) | 0.9 (0.4 to 1.8) | |
| Duration of HCV infection (per month) | 162 | 1.0 (0.99 to 1.01) | 1.0 (0.99 to 1.01) | |
| Elevated transaminases, pretreatment | 218 | 0.9 (0.5 to 1.7) | 0.7 (0.3 to 1.6) | |
| Cirrhosis | 230 | 1.9 (0.5 to 7.4) | 1.7 (0.4 to 7.4) | |
| Cirrhosis or bridging fibrosis | 177 | 0.9 (0.5 to 1.9) | 0.6 (0.3 to 1.3) | |
| Treatment Characteristics | ||||
| Interferon dose: 3 MU or higher | 411 | 3.3 (1.2 to 9.1) | 3.2 (0.8 to 12.5) | |
| Duration: 6 mo or longer | 411 | 2.0 (1.1 to 3.9) | 5.3 (0.8 to 36.3) | |
| Treatment completed | 402 | 4.1 (2.4 to 6.8) | 6.4 (3.4 to 12.0) | |
| Proportion completing Treatment (per 10%) | 399 | 1.3 (1.2 to 1.4) | 1.4 (1.3 to 1.6) | |
| Treatment duration (per month) | 403 | 1.13 (1.07 to 1.19) | 1.3 (1.2 to 1.5) | |
| Early Virological Negativity | ||||
| Early virological negativity | 172 | 5.1 (2.6 to 10.0) | 13.1 (5.3 to 32.3) | |
Logistic Regression of SVR with Adjustment for Study
To account for possible unmeasured differences between studies that might confound the relationship between candidate variables and SVR, we performed logistic regression of SVR with adjustment for study (Table 2) and did not identify any additional factors. As might be expected, because of less power after adjusting for study, the effects of treatment dose and duration, while remaining large, were no longer statistically significant. Although the strong relationships with SVR for treatment completion and EVN remained statistically significant, estimates became less precise. The relationship between patient factors (gender, HCV RNA) and SVR remained virtually unchanged in magnitude and statistical significance.
Multivariate Logistic Regression of SVR
We performed multivariate logistic regression to determine whether factors identified in univariate analyses remained independently associated with SVR after adjustment for possible confounding variables (Table 3). Due to missing data, selection of a single model was not possible, so in separate analyses we demonstrated the independent association of increased SVR with 3 MU or higher of IFN and intended or achieved treatment duration (Models 1 to 3), as well as with female gender and intended or achieved treatment duration (Models 4 to 6).
Table 3.
Multivariate logistic regression of sustained virological response after interferon
| Odds Ratio (95% Confidence Interval) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Number of Patients | Dose 3 MU or Highera | Female Gendera | Intended Treatment Duration (≥6 mo) | Completed Intended Treatment | Actual Treatment Duration (per month) | Log10 HCV RNA (per log10 decrease)b | ||
| Models using interferon dose 3 MU or highera | ||||||||
| Model 1: | Interferon dose | 411 | 3.7 | a | 2.2 | |||
| Intended duration | (1.3 to 10.0) | (1.1 to 4.3) | ||||||
| Model 2: | Interferon dose | 402 | 3.9 | a | 4.2 | |||
| Completed treatment | (1.4 to 10.8) | (2.5 to 7.1) | ||||||
| Model 3: | Interferon dose | 403 | 3.2 | a | 1.1 | |||
| Actual duration | (1.2 to 8.9) | (1.1 to 1.2) | ||||||
| Models using female gendera | ||||||||
| Model 4: | Female gender | 271 | a | 2.2 | 2.7 | |||
| Intended duration | (1.3 to 3.6) | (1.4 to 5.4) | ||||||
| Model 5: | Female gender | 262 | a | 2.2 | 3.3 | |||
| Completed treatment | (1.3 to 3.7) | (1.8 to 6.1) | ||||||
| Model 6: | Female gender | 263 | a | 2.3 | 1.1 | |||
| Actual duration | (1.4 to 3.9) | (1.1 to 1.2) | ||||||
| Models using log10 HCV RNA | ||||||||
| Model 7: | HCV RNA | 98 | c | c | d | 1.2 | 3.9 | |
| Actual duration | (1.1 to 1.4) | (2.0 to 7.7) | ||||||
| Model 8: | HCV RNA | 83 | c | 2.0 | c | d | 3.6 | |
| Female gender | (0.7 to 6.3) | (1.8 to 7.1) | ||||||
| Model 9: | HCV RNA | 83 | c | 1.9 | c | d | 1.3 | 4.0 |
| Actual duration | (0.6 to 6.3) | (1.1 to .5) | (2.0 to 8.3) | |||||
| Female gender | ||||||||
All patients with data on dose or gender received 3 MU or higher of interferon. Multivariate models could not be developed with these variables.
Similar relationships existed when HCV RNA analyzed using 400,000 IU/ml as a cutoff.
All patients with data on HCV RNA were treated with 3 MU or higher of interferon and for an intended treatment course of 6 mo or longer.
Unable to evaluate models with HCV RNA and other measures of treatment completion due to nonconvergence of data.
Multivariate analysis of HCV RNA was limited because all patients with HCV RNA data received 3 MU or higher of IFN for 6 mo or longer, and because there were strong associations between treatment completion and reporting of HCV RNA. Actual treatment duration and HCV RNA were independently associated with SVR (Model 7), even after adjustment for gender (Model 9). Female gender maintained an OR of 2 when adjusted for HCV RNA (Model 8) and treatment duration (Model 9), but lost statistical significance. We did not investigate EVN in multivariate models because it is an intermediate outcome.
The relationship between SVR and clinically relevant but nonstatistically significant factors in univariate analyses remained nonsignificant in multivariate models. Because of their importance in non-HD patients, we forced HCV genotype and cirrhosis/bridging fibrosis into multivariate models, but they remained nonsignificant.
Treatment Discontinuation
All 20 studies provided data on treatment discontinuation for 428 patients. The rate of treatment discontinuation ranged from 0 to 50% with an overall rate of 26% (95% CI, 20 to 34%). We examined the same variables used in logistic regression of SVR with treatment discontinuation as the outcome (Table 4). Of these, longer duration of HD was associated with a reduced likelihood of treatment discontinuation (OR 0.88, 0.80 to 0.96) and previous kidney transplantation (OR 2.55, 1.03 to 6.31) with an increased likelihood. No other factors were associated with treatment discontinuation. Multivariate logistic regression demonstrated that the effect of previous kidney transplantation was of borderline significance (OR 2.5, 1.0 to 6.3), and duration of HD (OR 0.9, 0.8 to 1.0) was nonsignificant in two variable models.
Table 4.
Odds ratio of treatment discontinuation due to adverse events after interferon (univariate regression)a
| Number with Covariate | Odds Ratio of Treatment Discontinuation | 95% Confidence Interval | |
|---|---|---|---|
| Patient Characteristics | |||
| Age (per year) | 275 | 1.0 | 0.9 to 1.1 |
| Female gender | 275 | 1.2 | 0.7 to 2.1 |
| Duration of hemodialysis (per month) | 215 | 0.88 | 0.80 to 0.96b |
| Prior kidney transplantation (versus none) | 131 | 2.55 | 1.03 to 6.31b |
| HCV Characteristics | |||
| Log10 HCV RNA (IU/ml(per log10 decrease) | 112 | 0.9 | 0.5 to 1.4 |
| HCV RNA <= 400,000 IU/ml | 112 | 0.7 | 0.2 to 2.3 |
| HCV genotype 1 (versus others) | 225 | 0.9 | 0.5 to 1.6 |
| Duration of HCV infection (per month) | 175 | 1.0 | 0.99 to 1.01 |
| Elevated transaminases, pretreatment | 219 | 0.8 | 0.4 to 1.7 |
| Cirrhosis | 242 | 2.3 | 0.6 to 9.1 |
| Cirrhosis + bridging fibrosis | 188 | 1.9 | 0.8 to 4.5 |
| Treatment Characteristics | |||
| Dose 3 MU or highera | 428 | 1.9 | 0.6 to 5.6 |
| Duration: 6 mo or longera | 428 | 0.6 | 0.28 to 1.1 |
Sensitivity analysis using adverse event as outcome did not reveal any statistically significant relationships except when compared with 3 MU and 1 to 1.5 MU dosing, 5 to 6 MU of interferon had an OR of an adverse event of 1.9 (95% confidence interval, 1.1 to 3.4) and 5.0 (1.5 to 16.3), respectively. The OR of an adverse event with 3 MU of interferon was 2.6 (0.9 to 7.7) when compared with 1 to 1.5 MU of interferon. Interestingly, the OR of 6 mo of intended treatment duration was 1.7 (1.1 to 2.6) when compared with 12 mo of treatment. We did not investigate treatment completion with adverse events because of a high likelihood of confounding.
Bivariate models showed prior kidney transplantation remained marginally statistically significantly associated (2.55, 1.03 to 6.31) with increased likelihood of treatment discontinuation after adjustment for duration of hemodialysis, but the latter relationship was nonsignificant.
We performed sensitivity analysis using the likelihood of an adverse event as the outcome without regard to treatment discontinuation. No patient or viral characteristic was associated with adverse events. However, IFN dose of 5 to 6 MU was associated with an increased likelihood of adverse events when compared with 3 MU (OR 1.9, 1.1 to 3.4) or 1 to 1.5 MU (OR 5.0, 1.5 to 16.3). The odds of an adverse event were higher with 3 MU of IFN (OR 2.6, 0.9 to 7.7), when compared with 1 to 1.5 MU. Surprisingly, intended treatment duration of 6 versus 12 mo was associated with a higher likelihood (OR 1.7, 1.1 to 2.6) of adverse events, but this effect did not persist after adjustment for dose.
Discussion
The decision to treat a HD patient for HCV infection is complex and requires weighing the benefits and risks of treatment, durability of treatment response, risks of untreated HCV infection, co-morbid conditions, and transplantation candidacy. IFN treatment of chronic HCV infection in HD patients results in a 45% SVR, significantly higher than the 10 to 20% rate reported for IFN monotherapy in non-HD patients (7–9,22). This difference may be related to lower IFN clearance in HD patients with fixed dosing, but differences in patient, viral, or treatment characteristics may also contribute. This meta-analysis of individual patient data extends the present understanding of factors associated with SVR in HD patients and provides information that clinicians should consider in deciding which patients to treat and how to treat them.
IFN dose of 3 MU or higher, intended treatment duration of 6 mo or longer, actual treatment duration, female gender, lower baseline HCV RNA levels, and EVN were associated with statistically significant increases in the likelihood of SVR. These relationships persisted after we adjusted for study. Multivariate analysis demonstrated independence of these relationships although, due to missing data, we were unable to establish independence of all these predictors in a single model. However, we could show that actual treatment duration remained independently associated with SVR in models adjusted for dose, gender, or HCV RNA.
In non-HD patients, HCV genotypes 1, 4 and cirrhosis/bridging fibrosis are associated with lower SVR (7–10) but, surprisingly, neither of these had a statistically significant association with SVR in HD patients. The wide confidence intervals surrounding estimates for these variables, however, highlight the uncertainty about their true effect size. These nonsignificant relationships may represent important clinical differences in HD patients or insufficient statistical power because of missing data for both variables.
Few of the studied patient, viral, or treatment factors were associated with treatment discontinuation except increasing duration of HD and previous kidney transplantation, but these did not persist in bivariate models. Although there was no relationship between IFN dose and treatment discontinuation, we observed a statistically significant association between higher dose and the odds of developing an adverse event. Our previous analysis found that specific adverse events had different rates of treatment discontinuation (22), so increasing doses of IFN may lead to adverse events (e.g., influenza-like symptoms or anemia) that do not result in treatment discontinuation.
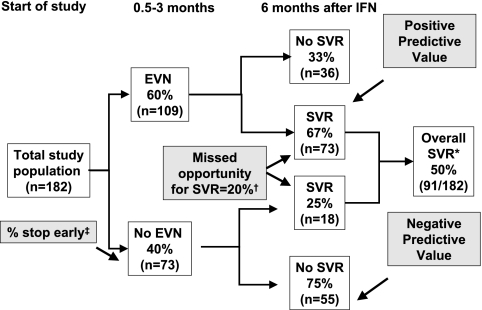
EVN was a strong predictor of SVR in univariate analysis. Achieving viral negativity anytime in the first 3 mo of treatment was associated with an SVR of 67% (positive predictive value), whereas 25% of HD patients who did not achieve EVN ultimately had an SVR yielding a negative predictive value of 75% (Figure 2).
Figure 2.
Impact of early virological negativity on sustained virological response. Data on early virological negativity and SVR were available for 182 patients, of whom 109 (60%) achieved early virological negativity. Seventy-three of the 109 patients who achieved EVN had SVR, equal to a positive predictive value of 67%. Of 73 patients who did not achieve EVN, 18 achieved SVR and the remainder were HCV RNA positive 6 mo after treatment. Thus, the negative predictive value of not achieving SVR after not achieving early virological negativity was 75%. If treatment was discontinued in patients not achieving EVN, 18 patients (20% of the total achieving SVR) would have missed the opportunity to become HCV RNA negative. *Value different from overall SVR because EVN data only available in 182 of 428 patients (see Table 1). †If treatment was discontinued in patients who did not achieve EVN, 18 (20%) of 91 total SVR would have been missed. ‡Proportion of patients who would stop treatment early because they did not achieve EVN.
Because of limitations in reporting within the original studies, our definition of EVN differs from that used in non-HD patients, where EVR is defined as a 2 log10 or larger decrease in HCV RNA or viral negativity after 3 mo of treatment. Nonetheless, it is similar to the recent complete EVR definition (HCV RNA negative at 12 wk) (47) in non-HD patients, and our results suggest that EVN in HD patients is also associated with a higher likelihood of SVR. In non-HD patients, however, the absence of an EVR implies a less than 3% likelihood of SVR with continued treatment (negative predictive value of 97 to 100%) (23,24), so treatment may be stopped for patients with HCV genotype 1 to avoid morbidity associated with further treatment (23). In contrast, the negative predictive value of EVN in HD patients is only 75%, so continued treatment could still achieve SVR in 25%. The 1- to 3-mo virological negativity definition used in studies of HD patients should not be used to stop treatment early. Instead, the more conservative 2-log10 decrease in quantitative HCV RNA after 12 wk of treatment definition used in non-HD patients (23) could be considered for HD patients, but further research is needed. Determining the optimal definition for EVR in HD patients has important implications for kidney transplant candidates because transplantation is delayed during IFN treatment due to the risk of allograft rejection in IFN-treated patients who undergo deceased donor kidney transplantation (21).
Publication bias is always a concern in meta-analysis. We addressed this by searching the proceedings of recent nephrology and hepatology meetings, but did not identify additional studies of IFN for HCV-infected HD patients. Second, the generalizability of our results may be limited by selection bias because studies typically enrolled kidney transplantation candidates and excluded patients with psychiatric or hematologic diseases. Missing data were a particular problem for this analysis and limited our ability to investigate whether statistically significant univariate relationships persisted in complete multivariate models. Additionally, missing data may have decreased the statistical power to detect univariate relationships. Other important factors associated with SVR in non-HD patients, such as race and insulin resistance (47), were also unreported. Most of the included studies involved IFN monotherapy; but recent studies suggest that combined pegylated IFN and low-dose ribavirin, with careful monitoring of ribavirin plasma levels, may result in higher SVR rates (48). Finally, although SVR rates appear higher in IFN-treated HD patients, SVR is only a surrogate outcome. Future research should investigate whether achieving SVR in HD patients translates into improved clinical outcomes.
Despite these limitations, this study is the first to provide important information on predictors of SVR in IFN-treated HD patients using individual patient data. There appears to be a threshold dose of 3 MU three times weekly, below which SVR is less likely to be achieved. When compared with 3 MU of IFN, higher doses do not appear to increase the likelihood of SVR but result in higher adverse event rates. We did not identify a significant difference between 6 and 12 mo of treatment, but less than 6 mo of IFN resulted in lower SVR. Our analysis did not have the statistical power to study interactions between HCV genotype and treatment duration. Current guidelines in non-HD patients recommend that treatment lengths be tailored by genotype (47). In the absence of specific data, this may be extrapolated to HD patients.
In HD patients, there appears to be a strong effect of treatment completion. Patients should be encouraged to complete the intended treatment course, regardless of the selected treatment length. Achieving viral negativity in the first 3 mo of treatment appears to be a strong predictor of ultimately achieving SVR. However, EVN, as reported in included studies, had insufficient negative predictive value to guide decisions to terminate treatment early. Clinicians may consider adopting the 2-log10 definition of EVR from the non-HD literature until further data become available in HD patients.
The combined treatment data suggest that HD patients with chronic HCV infection, who are appropriate treatment candidates, should be treated with 3 MU of IFN three times weekly for at least 6 mo. Treated patients, particularly those who achieve EVN, should be encouraged to complete the planned treatment. Female gender and low pretreatment HCV RNA level are two factors that predict higher likelihood of SVR. Future research is needed to determine the persistence of viral negativity in patients who achieve SVR, a particularly important issue for viral negative patients awaiting kidney transplantation.
Disclosures
None.
Acknowledgments
We are indebted to the following individuals who provided individual patient data not available in the original publication: Dr. Ali Akcay, Baskent University Faculty of Medicine, Ankara, Turkey; Dr. Mahdia Buargub, Tripoli Central Hospital, Tripoli, Libya; Dr. TM Chan, University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong; Dr. Jacques Chanard, Centre-Hospitalier et Universitaire, Reims, France; Dr. Maria Lucia Ferraz, Hospital Sao Paulo, Sao Paulo, Brazil; Dr. Jacques Izopet and Dr. Florence Nicot, Hopital Purpan, Toulouse, France; Dr. Dujanaj Mousa and Dr. Abdullah Al Waili, Riyadh Armed Forces Hospital, Riyadh Kingdom of Saudi Arabia; Dr. Maria Raptopolou-Gigi, University of Thessaloniki, Thessaloniki, Greece.
This project was not specifically funded, but the National Kidney Foundation supported a clinical research fellowship for Craig E. Gordon.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.World Health Organization: Weekly Epidemiological Record 1999;49: 10, December 1999 [Google Scholar]
- 2.Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW: Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int 65: 2335–2342, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Huraib S, al-Rashed R, Aldrees A, Aljefry M, Arif M, al-Faleh FA: High prevalence of and risk factors for hepatitis C in haemodialysis patients in Saudi Arabia: A need for new dialysis strategies. Nephrol Dial Transplant 10: 470–474, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G: Meta-analysis: Effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther 20: 1271–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjertson DW, Kopple JD, Greenland S: Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 18: 1584–1593, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Watanabe H, Saito T, Shinzawa H, Okumoto K, Hattori E, Adachi T, Takeda T, Sugahara K, Ito JI, Saito K, Togashi H, Suzuki R, Hayashi M, Miyamura T, Matsuura Y, Kawata S: Spontaneous elimination of serum hepatitis C virus (HCV) RNA in chronic HCV carriers: A population-based cohort study. J Med Virol 71: 56–61, 2003 [DOI] [PubMed] [Google Scholar]
- 7.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK: Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 339: 1485–1492, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J: Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352: 1426–1432, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Zeuzem S, Teuber G, Naumann U, Berg T, Raedle J, Hartmann S, Hopf U: Randomized, double-blind, placebo-controlled trial of interferon alfa2a with and without amantadine as initial treatment for chronic hepatitis C. Hepatology 32: 835–841, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 358: 958–965, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347: 975–982, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Kokoglu OF, Ucmak H, Hosoglu S, Cetinkaya A, Kantarceken B, Buyukbese MA, Isik IO: Efficacy and tolerability of pegylated-interferon alpha-2a in hemodialysis patients with chronic hepatitis C. Journal of Gastroenterology and Hepatology 21: 575–580, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Russo MW, Ghalib R, Sigal S, Joshi V: Randomized trial of pegylated interferon alpha-2b monotherapy in haemodialysis patients with chronic hepatitis C. Nephrol Dial Transplant 21: 437–443, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Sporea I, Popescu A, Sirli R, Golea O, Totolici C, Danila M, Vernic C: Pegylated-interferon alpha 2a treatment for chronic hepatitis C in patients on chronic haemodialysis. World J Gastroenterol 12: 4191–4194, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R: Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. Journal of Viral Hepatitis 13: 316–321, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Liu CH, Liang CC, Lin JW, Chen SI, Tsai HB, Chang CS, Hung PH, Kao JH, Liu CJ, Lai MY, Chen JH, Chen PJ, Kao JH, Chen DS: Pegylated interferon alpha-2a versus standard interferon alpha-2a for treatment-naive dialysis patients with chronic hepatitis C: A randomised study. Gut 57: 525–530, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Carriero D, Fabrizi F, Uriel AJ, Park J, Martin P, Dieterich DT: Treatment of dialysis patients with chronic hepatitis C using pegylated interferon and low-dose ribavirin. The International Journal of Artificial Organs 31: 295–302, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Ucmak H, Kokoglu OF, Hosoglu S, Dogan E, Sayarlioglu H, Kuzhan N, Isik IO: Long-term efficacy of pegylated interferon alpha-2a in HCV-positive hemodialysis patients. Renal Failure 30: 227–232, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D: Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation 59: 1426–1431, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Rostaing L, Modesto A, Baron E, Cisterne JM, Chabannier MH, Durand D: Acute renal failure in kidney transplant patients treated with interferon alpha 2b for chronic hepatitis C. Nephron 74: 512–516, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Kidney disease: Improving global outcomes (KDIGO) Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int 73 [Suppl 109]: S1–S99, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB: Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: A systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis 51: 263–277, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J: Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology 38: 645–652, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Goncales FL, Jr., Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Chaneac M, Reddy KR: Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol 43: 425–433, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Russo MW, Goldsweig CD, Jacobson IM, Brown RS, Jr.: Interferon monotherapy for dialysis patients with chronic hepatitis C: An analysis of the literature on efficacy and safety. Am J Gastroenterol 98: 1610–1615, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P: Meta-analysis: Interferon for the treatment of chronic hepatitis C in dialysis patients. Aliment Pharmacol Ther 18: 1071–1081, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Campistol JM, Esforzado N, Martinez J, Rosello L, Veciana L, Modol J, Casellas J, Pons M, de Las Cuevas X, Piera J, Oliva JA, Costa J, Barrera JM, Bruguera M: Efficacy and tolerance of interferon-alpha(2b) in the treatment of chronic hepatitis C virus infection in haemodialysis patients. Pre- and post-renal transplantation assessment. Nephrol Dial Transplant 14: 2704–2709, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Casanovas-Taltavull T, Baliellas C, Benasco C, Serrano TT, Casanova A, Perez JL, Guerrero L, Gonzalez MT, Andres E, Gil-Vernet S, Casais LA: Efficacy of interferon for chronic hepatitis C virus-related hepatitis in kidney transplant candidates on hemodialysis: Results after transplantation. Am J Gastroenterol 96: 1170–1177, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Buargub M, El Huni S, Tagdi M: Tolerance and efficacy of interferon-alpha in hemodialysis patients in Tripoli. Saudi J Kidney Dis Transpl 17: 338–343, 2006 [PubMed] [Google Scholar]
- 30.Huraib S, Iqbal A, Tanimu D, Abdullah A: Sustained virological and histological response with pretransplant interferon therapy in renal transplant patients with chronic viral hepatitis C. Am J Nephrol 21: 435–440, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kamar N, Toupance O, Buchler M, Sandres-Saune K, Izopet J, Durand D, Rostaing L: Evidence that clearance of hepatitis C virus RNA after alpha-interferon therapy in dialysis patients is sustained after renal transplantation. J Am Soc Nephrol 14: 2092–2098, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Izopet J, Rostaing L, Moussion F, Alric L, Dubois M, That HT, Payen JL, Duffaut M, Durand D, Suc JM, Puel J: High rate of hepatitis C virus clearance in hemodialysis patients after interferon-alpha therapy. J Infect Dis 176: 1614–1617, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Espinosa M, Rodriguez M, Martin-Malo A, Alvarez de Lara MA, Gonzalez R, Lopez-Rubio F, de la Mata M, Aljama P: Interferon therapy in hemodialysis patients with chronic hepatitis C virus infection induces a high rate of long-term sustained virological and biochemical response. Clin Nephrol 55: 220–226, 2001 [PubMed] [Google Scholar]
- 34.Hanrotel C, Toupance O, Lavaud S, Thiefin G, Brodard V, Ingrand D, Diebold MD, Wynckel A, Chanard J: Virological and histological responses to one year alpha-interferon-2a in hemodialyzed patients with chronic hepatitis C. Nephron 88: 120–126, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Mousa DH, Abdalla AH, Al-Shoail G, Al-Sulaiman MH, Al-Hawas FA, Al-Khader AA: Alpha-interferon with ribavirin in the treatment of hemodialysis patients with hepatitis C. Transplant Proc 36: 1831–1834, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Okuda K, Hayashi H, Yokozeki K, Kondo T, Kashima T, Irie Y: Interferon treatment for chronic hepatitis C in haemodialysis patients: Suggestions based on a small series. J Gastroenterol Hepatol 10: 616–620, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Pol S, Thiers V, Carnot F, Zins B, Romeo R, Berthelot P, Brechot C: Efficacy and tolerance of alpha-2b interferon therapy on HCV infection of hemodialyzed patients. Kidney Int 47: 1412–1418, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Ozdemir FN, Akcay A, Sezer S, Boyacioglu S, Ozdemir BH, Arat Z, Haberal M: A six-year follow-up after interferon-alpha monotherapy for chronic hepatitis C infection in hemodialysis patients. Ren Fail 26: 583–588, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Koenig P, Vogel W, Umlauft F, Weyrer K, Prommegger R, Lhotta K, Neyer U, Stummvoll HK, Gruenewald K: Interferon treatment for chronic hepatitis C virus infection in uremic patients. Kidney Int 45: 1507–1509, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Rocha CM, Perez RM, Ferreira AP, Carvalho-Filho RJ, Pace FH, Silva IS, Pestana JO, Lanzoni VP, Silva AE, Ferraz ML: Efficacy and tolerance of interferon-alpha in the treatment of chronic hepatitis C in end-stage renal disease patients on hemodialysis. Liver Int 26: 305–310, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Chan TM, Wu PC, Lau JY, Lok AS, Lai CL, Cheng IK: Interferon treatment for hepatitis C virus infection in patients on haemodialysis. Nephrol Dial Transplant 12: 1414–1419, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Raptopoulou-Gigi M, Spaia S, Garifallos A, Xenou P, Orphanou H, Zarafidou E, Petridou P, Vrettou H, Vagionas G, Galaktidou G, Mavroudi I, Efkarpidou A, Kortsaris A: Interferon-alpha 2b treatment of chronic hepatitis C in haemodialysis patients. Nephrol Dial Transplant 10: 1834–1837, 1995 [PubMed] [Google Scholar]
- 43.Degos F, Pol S, Chaix ML, Laffitte V, Buffet C, Bernard PH, Degott C, Carnot F, Riffaud PC, Chevret S: The tolerance and efficacy of interferon-alpha in haemodialysis patients with HCV infection: A multicentre, prospective study. Nephrol Dial Transplant 16: 1017–1023, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Huraib S, Tanimu D, Romeh SA, Quadri K, Al Ghamdi G, Iqbal A, Abdulla A: Interferon-alpha in chronic hepatitis C infection in dialysis patients. Am J Kidney Dis 34: 55–60, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Benci A, Caremani M, Menchetti D, Sasdelli M, Giusti PB: Low-dose leukocyte interferon-alpha therapy in dialysed patients with chronic hepatitis C. Curr Med Res Opin 14: 141–144, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Fernandez JL, Rendo P, del Pino N, Viola L: A double-blind controlled trial of recombinant interferon-alpha 2b in haemodialysis patients with chronic hepatitis C virus infection and abnormal aminotransferase levels. Nephrologists' Group for the Study of HCV infection. J Viral Hepat 4: 113–119, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Ghany MG, Strader DB, Thomas DL, Seeff LB: Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 49: 1335–1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Leusen R, Adang RP, de Vries RA, Cnossen TT, Konings CJ, Schalm SW, Tan AC: Pegylated interferon alfa-2a (40 kD) and ribavirin in haemodialysis patients with chronic hepatitis C. Nephrol Dial Transplant 23: 721–725, 2008 [DOI] [PubMed] [Google Scholar]