Abstract
Background & objectives: Chronic kidney disease (CKD) is commonly complicated by secondary hyperparathyroidism (SHPT), leading to increased risk of morbidity and mortality. SHPT is a progressive disease often requiring long-term therapy to control parathyroid hormone (PTH) and mineral imbalances. Vitamin D sterols and phosphate binders, used as traditional therapies to lower PTH and phosphorus, may provide inadequate long-term control for many dialysis patients. Cinacalcet, by simultaneously lowering PTH, calcium, phosphorus, and calcium-phosphorus levels, may maintain PTH and mineral balance in these individuals. However, as with traditional therapies, long-term data are limited.
Design, setting, participants, & measurement: Dialysis subjects from at least one of five lead-in studies (double-blind placebo-controlled, including one extension trial) completing up to 52 wk of either cinacalcet or placebo were eligible for this open-label extension study, including an 8-wk dose titration (initiated at 30 mg/d), followed by 24-wk maintenance and up to 132 wk of follow-up. Final efficacy analysis was at week 180.
Results: Three hundred thirty-four of 589 enrolled subjects received cinacalcet from the beginning of the lead-in study. Weekly median PTH values were ≤300 pg/ml (weeks 16 through 180) and median Ca×P values were ≤55 mg2/dl2 (weeks 4 through 180). Similar results were exhibited in the 255 subjects who initially received placebo. Among the patients exposed to cinacalcet from the beginning of the lead-in study, 3% of subjects exhibited treatment-related serious adverse events.
Conclusions: Cinacalcet effectively maintained PTH, Ca and P reductions in dialysis subjects for up to 180 wk.
Secondary hyperparathyroidism (SHPT) occurs often in chronic kidney disease (CKD). It is characterized by increases in the synthesis and secretion of parathyroid hormone (PTH) and by disturbances in calcium (Ca), phosphorus (P), and vitamin D metabolism (1). SHPT is a progressive disorder among those undergoing dialysis in whom PTH levels increase by an average of 6% per year (2). Elevated PTH, Ca, P, and Ca×P levels and alterations in vitamin D metabolism are associated with important adverse outcomes, including cardiovascular disease, in such patients (3–6).
The long-term efficacy and safety of traditional therapies for SHPT have not been critically examined. Despite many years of clinical use, results from prospective clinical trials describing therapeutic responses to any vitamin D sterol among dialysis patients with SHPT lasting more than 13 mo are available from only six patients after 2 yr of follow-up (20). Sustained treatment with vitamin D alone often proves inadequate for dialysis patients with SHPT (7–9). Disturbances in calcium and phosphorus metabolism frequently disrupt therapy (10), rendering consistent biochemical control of the disorder difficult (8).
Treatment with cinacalcet hydrochloride (cinacalcet) often concurrently lowers PTH, Ca, P, and Ca×P levels among dialysis patients with SHPT (11–14). Its use can provide sustained control of key biochemical parameters in those receiving traditional treatments for SHPT as judged by the achievement of values within the target ranges recommended by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQITM) (15). Unfortunately, a relatively small number of patients (n = 59) have been evaluated thus far, and efficacy results are available in only 16 subjects after 3 yr of follow-up (15). Confirmation of these preliminary findings is required.
To further assess the long-term therapeutic efficacy of cinacalcet for managing SHPT among patients receiving dialysis, we measured the adequacy of control of biochemical outcomes for SHPT as judged by the achievement of recommended values (16) after 180 wk of cinacalcet therapy.
Materials and Methods
Subjects
A total of 589 of 1184 subjects completing one or more lead-in double-blind, randomized controlled trials comparing cinacalcet with placebo as treatment for SHPT (study A [study 20000172 (11)], study B [study 20000183 (11)], study C [study 20000188 (13)], study D [study 200010240], or study E [study 20010141]) enrolled in this open-label extension study. The lead-in studies lasted 6 mo (studies A, B, and C) or 12 mo (study E). Study D was a 6-mo extension study for subjects completing studies A or B. Subjects thus received cinacalcet or placebo for either 26 or 52 wk before entering the current study.
Inclusion and exclusion criteria for the lead-in studies were described previously (11,13,17). Key entry criteria were age ≥18 yr, mean Ca ≥8.4 mg/dl, mean plasma intact PTH (iPTH) level ≥300 pg/ml, CKD stage 5 requiring hemodialysis ≥1 mo (study E) or ≥3 mo (studies A and B), or hemodialysis or peritoneal dialysis ≥1 mo (study C). All studies were conducted in accordance with the Declaration of Helsinki and in compliance with the Food and Drug Administration (FDA) and the International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) regulations/guidelines. All subjects gave written informed consent.
Design of the Open-label Extension
Immediately after completing a lead-in study, eligible subjects entered the open-label extension study. Cinacalcet doses were re-titrated from 30 mg/d without an intervening washout period. All subjects underwent 8 wk of dose titration to maintain the blind of the lead-in studies. Cinacalcet doses were increased if PTH was >250 pg/ml unless serum Ca was <7.8 mg/dl, symptoms of hypocalcemia developed, the maximum dose had been reached, or an adverse event (AE) precluded a dose increase. Doses were reduced if PTH was <100 pg/ml, Ca <7.5 mg/dl, or symptoms of hypocalcemia could not be managed by changes in concomitant therapy.
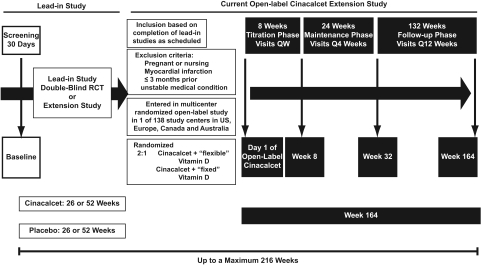
When the study was designed, the impact of concurrent vitamin D therapy on the efficacy of cinacalcet was unknown, and subjects were assigned randomly 2:1 to cinacalcet plus either “flexible” or “fixed” doses of vitamin D based on the initial study protocol (Figure 1). The type and dose of vitamin D brand was adjusted in the flexible group at the discretion of the physician or kept constant in the fixed group according to safety guidelines in the protocol. Vitamin D doses were reduced if Ca ≥ 11 mg/dl, P ≥ 6.5 mg/dl, or Ca×P ≥ 70 mg2/dl2, or doses were increased if symptoms of hypocalcemia occurred or Ca <8.4 mg/dl. Randomization was stratified by region, previous treatment group, and baseline PTH. Flexible vitamin D therapy was permitted during the follow-up phase. For the current analysis, pooled results for patients given flexible or constant doses of vitamin D were used because the doses of vitamin D in each group did not differ materially and results from post hoc analyses of phase 3 clinical trials indicated that the efficacy of cinacalcet was unaffected by vitamin D use (18).
Figure 1.
Study schema. Subjects entering this study had completed one or more prior lead-in studies: study A (study 20000172), study B (study 20000183), study C (study 20000188), study D (study 20010240), or study E (study 20010141). Studies A, B, and C were each 6-mo randomized, double-blind, placebo-controlled trials comparing cinacalcet therapy with placebo; study E was a 12-mo randomized, double-blind, placebo-controlled trial also comparing cinacalcet with placebo; study D was a 6-mo extension study of subjects who completed study A or study B. Thus, subjects may have received either 26 or 52 wk of treatment with either cinacalcet or placebo before entering this study. The current study consisted of three phases totaling 164 wk: (1) an 8-wk dose-titration phase, beginning at cinacalcet 30 mg p.o. daily, with weekly visits and sequential dose increases to 60, 90, 120, and 180 mg p.o. daily every 2 wk; (2) a 24-wk maintenance phase and visits every 4 wk; and (3) a maximum 132-wk follow-up phase with visits every 12 wk, during which flexible vitamin D therapy was permitted for all subjects. All total, subjects could have received up to a maximum of 216 wk of cinacalcet therapy. RCT, randomized controlled trial.
At each study visit, blood samples were collected to measure PTH, Ca, and P, and AE safety assessments were done. Central laboratories (Covance Central Laboratory Services, Inc., Indianapolis, IN, and Geneva Switzerland; Sonic Clinical Trials, New South Wales, Australia) were used for biochemical determinations.
Statistical Analyses
Long-term efficacy analyses included the lead-in studies with results analyzed according to prior treatment, either originally cinacalcet or originally placebo. Efficacy was determined relative to lead-in study baseline values. Final efficacy analyses used data collected up to and including week 180 after the lead-in study baseline.
Descriptive statistics at each time point included number of subjects (n), mean, SD (SD), standard error (SE), median, first quartile (Q1), third quartile (Q3), minimum and maximum for continuous variables, and counts and percentages for discrete variables. Reductions in PTH were calculated against the mean PTH over the 6-mo period. Data were analyzed using SAS version 8.2.
Results
Disposition of Subjects
A total of 589 subjects were enrolled into the open-label extension study with four (<1%) subjects enrolled from study A, 145 (25%) from study B, 237 (40%) from study C, 145 (25%) from study A followed by study D, 36 (6%) from study B followed by study D, and 22 (4%) from study E (Table 1). Among all subjects, 334 (57%) received cinacalcet in the lead-in studies for either 6 mo, n = 236 (71%), or 12 mo, n = 98 (29%), whereas 255 subjects (43%) received placebo for 6 mo, n = 150 (59%), or 12 mo, n = 105 (41%). Four (<1%) subjects did not receive study medication in this extension study.
Table 1.
Disposition of subjects during the extension study
| Original cinacalceta (n = 334) | Original placebo (n = 255) | |
|---|---|---|
| Enrolled in extension study, n | 334 | 255 |
| Did not receive study medication, n (%) | 1 (<1) | 3 (1) |
| Received study medication, n (%) | 333 (100) | 252 (99) |
| Titration phase—weeks 1–8, n (%) | ||
| Started | 333 (100) | 252 (99) |
| Discontinued | 15 (4) | 18 (7) |
| Completed | 318 (95) | 234 (92) |
| Maintenance phase—weeks 9–32, n (%) | ||
| Started | 318 (95) | 234 (92) |
| Discontinued | 56 (17) | 50 (20) |
| Completed | 262 (78) | 184 (72) |
| Follow-up phase—weeks 33–164, n (%) | ||
| Startedb | 261 (78) | 182 (71) |
| Discontinued | 136 (41) | 103 (40) |
| Completed | 125 (37) | 79 (31) |
| Total discontinued | 209 (63) | 176 (69) |
| Subjects rolled over to extension study, n (%) | ||
| After 6 moc | 236 (71) | 150 (59) |
| After 12 mod | 98 (29) | 105 (41) |
All subjects who had 26 to 52 wk of cinacalcet therapy prior to study entry.
One original cinacalcet and two original placebo subjects completed maintenance phase and did not start follow-up phase.
Subjects completing studies A, B, and C.
Subjects completing studies D and E.
Demographics and Baseline Characteristics
Mean PTH levels (±SD) were modestly but not significantly higher in the original cinacalcet group than in the original placebo group (774 ± 651 pg/ml versus 694 ± 392 pg/ml) as were median values (613 pg/ml versus 583 pg/ml) (Table 2). At baseline, 348 (59%) subjects used vitamin D and 552 (94%) subjects used at least one phosphate binder. There were no differences in demographic features, medical history, or serum Ca, serum P, and Ca×P levels between subjects given cinacalcet or placebo originally.
Table 2.
Lead-in study baseline demographics, key laboratory values, and medical history of subjects of the extension study
| Characteristic | Original Cinacalcet (n = 334) | Original Placebo (n = 255) |
|---|---|---|
| Sex, n (%) | ||
| Male | 210 (63) | 166 (65) |
| Female | 124 (37) | 89 (35) |
| Race, n (%) | ||
| White | 159 (48) | 157 (62) |
| Black | 123 (37) | 70 (27) |
| Other | 52 (16) | 28 (11) |
| Age | ||
| Mean (SD), years | 50.9 (14.1) | 53.3 (14.9) |
| Median (Q1, Q3), years | 51.0 (40.0, 61.0) | 53.0 (41.0, 65.0) |
| <65 yr, n (%) | 273 (82) | 189 (74) |
| ≥65 yr, n (%) | 61 (18) | 66 (26) |
| Duration of dialysisa, months | ||
| Mean (SD) | 69.6 (62.4) | 71.7 (69.2) |
| Median (Q1, Q3) | 51.0 (23.0, 94.0) | 47.0 (23.0, 90.0) |
| PTH, pg/ml | ||
| Mean (SD) | 773.50 (651.34) | 693.82 (392.20) |
| Median (Q1, Q3) | 613.08 (435.20, 870.78) | 583.00 (425.96, 794.00) |
| Serum calcium, mg/dl | ||
| Mean (SD) | 9.83 (0.80) | 9.88 (0.81) |
| Median (Q1, Q3) | 9.80 (9.27, 10.27) | 9.83 (9.24, 10.49) |
| Serum phosphorus, mg/dl | ||
| Mean (SD) | 6.23 (1.70) | 6.11 (1.50) |
| Median (Q1, Q3) | 6.00 (5.10, 7.27) | 6.12 (5.00, 7.03) |
| Ca × Pb, mg2/dl2 | ||
| Mean (SD) | 61.01 (16.45) | 60.27 (15.00) |
| Median (Q1, Q3) | 58.99 (48.78, 71.46) | 60.18 (50.72, 69.91) |
| PTH ≤300 pg/ml + Ca×P ≤55 mg2/dl2, n (%) | 1 (<1) | 0 (0) |
| Co-existing diabetes, n (%) | ||
| Yes | 93 (28) | 74 (29) |
| No | 241 (72) | 181 (71) |
| Hypertension, n (%) | ||
| Yes | 317 (95) | 242 (95) |
| No | 17 (5) | 13 (5) |
| Coronary artery disease, n (%) | ||
| Yes | 91 (27) | 65 (25) |
| No | 243 (73) | 190 (75) |
| Congestive heart failure, n (%) | ||
| Yes | 77 (23) | 39 (15) |
| No | 257 (77) | 216 (85) |
| Myocardial infarction, n (%) | ||
| Yes | 43 (13) | 36 (14) |
| No | 291 (87) | 219 (86) |
| Peripheral vascular disease, n (%) | ||
| Yes | 55 (16) | 47 (18) |
| No | 279 (84) | 208 (82) |
| Cerebrovascular accident, n (%) | ||
| Yes | 28 (8) | 30 (12) |
| No | 306 (92) | 225 (88) |
Data unavailable for 19 subjects in original cinacalcet group and two subjects in original placebo group.
Data unavailable for one subject in original cinacalcet group.
Long-term Efficacy
PTH.
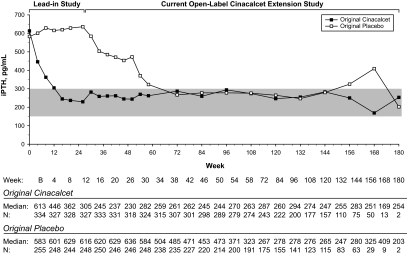
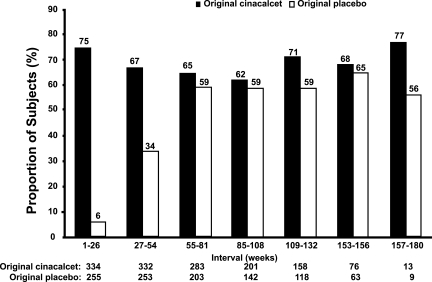
Median PTH values decreased to ≤300 pg/ml by week 72 and remained below this level through week 180, except for weeks 156 and 168, among patients originally given placebo. In contrast, median PTH levels were ≤300 pg/ml by week 16 and at each interval of follow-up among subjects originally given cinacalcet (Figure 2). Mean PTH levels were below baseline values from the lead-in studies at each 6-mo evaluation among patients given cinacalcet originally but did not decline below baseline until week 54 among patients given placebo originally, reflecting the later transition to cinacalcet therapy (Table 3). Among patients given cinacalcet originally, the proportion of subjects achieving a reduction in PTH ≥30% from mean baseline values for each 6-mo period was similar throughout the study (range, 62 to 77%) but increased during the first 12 mo, reaching a plateau between weeks 55 to 84 and the end of study (range, 56 to 65%) in the group given placebo originally and cinacalcet thereafter (Figure 3).
Figure 2.
Median PTH values recorded at each scheduled visit for subjects who originally received cinacalcet or placebo during lead-in studies. Shaded area is the NKF-KDOQITM–recommended range for serum PTH levels.
Table 3.
Mean percent change from lead-in study baseline at 6-mo intervals for PTH, Ca×P, Ca, and P levels
| Week | PTH |
Ca × P |
Ca |
P |
||||
|---|---|---|---|---|---|---|---|---|
| Original cinacalcet mean (SE) [n] | Original placebo mean (SE) [n] | Original cinacalcet mean (SE) [n] | Original placebo mean (SE) [n] | Original cinacalcet mean (SE) [n] | Original placebo mean (SE) [n] | Original cinacalcet mean (SE) [n] | Original placebo mean (SE) [n] | |
| Week 26 | −51.2 (2.1) [318] | 9.9 (2.8) [246] | −14.3 (1.7) [311] | 2.1 (1.8) [242] | −5.9 (0.6) [316] | 1.3 (0.4) [246] | −9.0 (1.7) [312] | 1.0 (1.8) [242] |
| Week 54 | −38.2 (2.9) [279] | −12.3 (4.8) [200] | −10.1 (2.0) [273] | −10.3 (2.2) [196] | −4.6 (0.6) [275] | −5.5 (0.8) [201] | −6.1 (1.8) [274] | −5.0 (2.1) [197] |
| Week 84 | −28.8 (4.6) [222] | −25.5 (5.2) [155] | −11.1 (2.5) [221] | −12.3 (2.5) [153] | −6.1 (0.8) [225] | −5.2 (0.8) [159] | −6.0 (2.2) [221] | −7.9 (2.3) [153] |
| Week 108 | −32.7 (4.4) [177] | −25.8 (6.5) [123] | −12.3 (2.2) [179] | −13.0 (2.6) [124] | −6.0 (0.9) [185] | −3.7 (0.8) [131] | −6.7 (2.1) [179] | −10.4 (2.5) [124] |
| Week 132 | −35.0 (5.7) [110] | −38.4 (5.7) [83] | −7.7 (3.2) [112] | −20.1 (2.9) [82] | −5.3 (1.0) [116] | −4.0 (1.2) [84] | −4.1 (3.0) [113] | −16.0 (3.0) [83] |
| Week 156 | −32.8 (9.0) [50] | −24.0 (11.3) [29] | −12.2 (4.7) [54] | −14.6 (4.4) [34] | −5.9 (1.3) [56] | −4.0 (1.7) [35] | −7.7 (4.5) [54] | −9.8 (5.2) [34] |
| Week 180 | −49.7 (38.8) [2] | −55.9 (35.3) [2] | −9.6 (9.5) [9] | −17.8 (10.9) [8] | −5.7 (3.5) [10] | 0.4 (2.9) [8] | −1.2 (13.5) [9] | −19.7 (10.7) [9] |
Figure 3.
Proportion of patients in original cinacalcet and original placebo groups who achieved ≥30% reduction in PTH from baseline at each 6-mo period. Reductions in PTH values were calculated against the mean PTH value over the 6-mo period; % is incremental. Some scheduled visits were shifted up to a maximum of 2 wk to align with the closest week indicated in the table. N, number of subjects entering period.
Acheivement of biochemical targets.
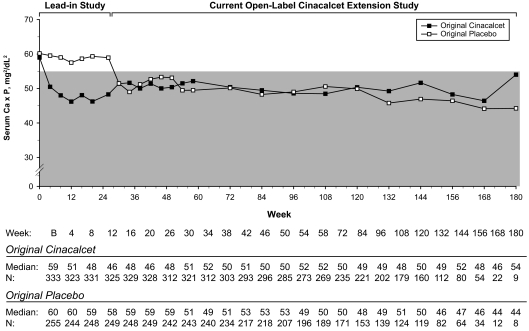
Median Ca levels were maintained within the recommended range of 8.5 to 9.5 mg/dl through week 168 beginning at week 4 in the original cinacalcet group and at week 30 in the original placebo group (Tables 4 and 5). Median P levels were lower than parent study baseline at each study visit and during the maintenance and follow-up phases were maintained within or just above the recommended range of 3.5 to 5.5 mg/dl (Table 4). Median serum P levels ranged from 5.00 to 5.90 mg/dl in the original cinacalcet group and from 4.20 to 6.00 mg/dl in the original placebo group (Table 5). In both groups, median Ca×P level was maintained ≤55 mg2/ml2 through week 180 with a decrease from 59 mg2/dl2 at baseline to 51 mg2/dl2 at week 4 in the original cinacalcet group and from 60 mg2/dl2 at baseline to 51 mg2/dl2 at week 30 following cinacalcet initiation in the original placebo group (Figure 4). As with PTH levels, mean Ca×P levels were decreased at week 26 to end of study for the original cinacalcet group and at week 54 to end of study for the original placebo group (Table 3).
Table 4.
Overall summary of serum Ca, serum P, and Ca × P values at selected visitsa
| Time Period | Ca (mg/dl) |
P (mg/dl) |
Ca×P (mg2/dl2) |
|||
|---|---|---|---|---|---|---|
| Original Cinacalcet | Original Placebo | Original Cinacalcet | Original Placebo | Original Cinacalcet | Original Placebo | |
| Baseline (lead-in study) | ||||||
| Mean (SE) | 9.83 (0.04) | 9.88 (0.05) | 6.23 (0.09) | 6.11 (0.09) | 61.01 (0.90) | 60.27 (0.94) |
| Median (Q1, Q3) | 9.80 | 9.83 | 6.00 | 6.12 | 58.99 | 60.18 |
| (9.27, 10.27) | (9.24, 10.49) | (5.10, 7.27) | (5.00, 7.03) | (48.78, 71.46) | (50.72, 69.91) | |
| [n] | [334] | [255] | [334] | [255] | [333] | [255] |
| Week 34 (end of titration)b | ||||||
| Mean (SE) | 9.17 (0.05) | 9.24 (0.07) | 5.72 (0.11) | 5.61 (0.11) | 52.44 (1.03) | 51.73 (1.08) |
| Median (Q1, Q3) | 9.20 | 9.20 | 5.60 | 5.50 | 51.66 | 49.00 |
| (8.50, 9.80) | (8.45, 9.95) | (4.45, 6.90) | (4.40, 6.70) | (39.72, 62.64) | (39.33, 62.89) | |
| [n] | [318] | [243] | [312] | [240] | [312] | [240] |
| Week 58 (end of maintenance)c | ||||||
| Mean (SE) | 9.26 (0.06) | 9.06 (0.07) | 5.70 (0.11) | 5.58 (0.13) | 52.70 (1.00) | 50.37 (1.19) |
| Median (Q1, Q3) | 9.20 | 9.10 | 5.50 | 5.50 | 52.14 | 49.50 |
| (8.70, 9.80) | (8.43, 9.70) | (4.40, 6.70) | (4.20, 6.60) | (40.04, 63.19) | (38.54, 58.00) | |
| [n] | [273] | [192] | [269] | [189] | [269] | [189] |
| End follow-up phase (week 180) | ||||||
| Mean (SE) | 9.43 (0.32) | 9.58 (0.28) | 5.99 (0.42) | 5.21 (0.76) | 56.02 (4.06) | 52.72 (8.01) |
| Median (Q1, Q3) | 9.75 | 9.65 | 5.90 | 4.20 | 54.02 | 44.25 |
| (8.90, 10.20) | (8.95, 10.20) | (5.80, 6.90) | (3.50, 7.20) | (49.56, 61.95) | (37.03, 69.60) | |
| [n] | [10] | [8] | [9] | [9] | [9] | [8] |
Some scheduled visits were shifted up to a maximum of 2 wk to align with the closest week indicated in the table.
Week 34 corresponds to end-of-dose-titration phase for subjects whose lead-in study duration was 26 wk.
Week 58 corresponds to end of maintenance phase for subjects whose lead-in study duration was 26 wk.
Table 5.
Overall summary of serum Ca and serum P values at each visit
| Serum Calcium (mg/dl) Median (Q1, Q3) [N] |
Serum Phosphorus (mg/dl) Median (Q1, Q3) [N] |
|||
|---|---|---|---|---|
| Original Cinacalcet | Original Placebo | Original Cinacalcet | Original Placebo | |
| Baseline | 9.80 (9.27, 10.27) | 9.83 (9.24, 10.49) | 6.00 (5.10, 7.27) | 6.12 (5.00, 7.03) |
| [334] | [255] | [334] | [255] | |
| Week 4 | 9.10 (8.44, 9.70) | 9.82 (9.20, 10.50) | 5.50 (4.50, 6.80) | 5.90 (4.82, 7.20) |
| [325] | [244] | [324] | [244] | |
| Week 8 | 8.90 (8.30, 9.48) | 9.84 (9.28, 10.40) | 5.40 (4.40, 6.53) | 6.00 (5.00, 6.88) |
| [331] | [249] | [331] | [250] | |
| Week 12 | 8.80 (8.20, 9.50) | 9.98 (9.30, 10.50) | 5.27 (4.30, 6.60) | 5.79 (4.74, 6.90) |
| [330] | [250] | [326] | [251] | |
| Week 16 | 8.90 (8.20, 9.60) | 9.96 (9.32, 10.40) | 5.40 (4.40, 6.50) | 5.89 (4.90, 6.90) |
| [331] | [249] | [329] | [248] | |
| Week 20 | 8.93 (8.20, 9.60) | 9.90 (9.40, 10.50) | 5.30 (4.30, 6.66) | 5.90 (4.90, 6.80) |
| [328] | [249] | [329] | [249] | |
| Week 26 | 9.20 (8.50, 9.80) | 10.00 (9.44, 10.56) | 5.27 (4.20, 6.53) | 5.90 (5.00, 7.00) |
| [316] | [246] | [312] | [242] | |
| Week 30 | 9.20 (8.60, 9.90) | 9.40 (8.70, 10.00) | 5.50 (4.20, 6.90) | 5.50 (4.50, 6.50) |
| [321] | [245] | [322] | [243] | |
| Week 34 | 9.20 (8.50, 9.80) | 9.20 (8.45, 9.95) | 5.60 (4.45, 6.90) | 5.50 (4.40, 6.70) |
| [318] | [243] | [312] | [240] | |
| Week 38 | 9.10 (8.48, 9.80) | 9.40 (8.60, 10.20) | 5.50 (4.30, 6.80) | 5.50 (4.50, 6.70) |
| [308] | [236] | [303] | [234] | |
| Week 42 | 9.20 (8.50, 9.80) | 9.40 (8.70, 10.20) | 5.60 (4.40, 7.20) | 5.60 (4.30, 6.80) |
| [300] | [226] | [293] | [218] | |
| Week 46 | 9.20 (8.60, 9.80) | 9.40 (8.80, 10.00) | 5.50 (4.30, 6.90) | 5.70 (4.40, 6.70) |
| [300] | [222] | [296] | [218] | |
| Week 50 | 9.20 (8.50, 9.80) | 9.50 (8.80, 10.30) | 5.50 (4.20, 7.10) | 5.60 (4.50, 6.60) |
| [290] | [213] | [285] | [210] | |
| Week 54 | 9.40 (8.70, 9.90) | 9.20 (8.60, 9.80) | 5.70 (4.30, 6.70) | 5.40 (4.40, 6.60) |
| [275] | [201] | [274] | [197] | |
| Week 58 | 9.20 (8.70, 9.80) | 9.10 (8.43, 9.70) | 5.50 (4.40, 6.70) | 5.50 (4.20, 6.60) |
| [273] | [192] | [269] | [189] | |
| Week 72 | 9.20 (8.70, 9.80) | 9.20 (8.60, 9.90) | 5.60 (4.40, 6.65) | 5.50 (4.10, 6.60) |
| [243] | [174] | [236] | [171] | |
| Week 84 | 9.30 (8.50, 9.90) | 9.30 (8.70, 9.70) | 5.50 (4.30, 6.70) | 5.30 (4.35, 6.60) |
| [225] | [159] | [221] | [153] | |
| Week 96 | 9.20 (8.60, 9.70) | 9.30 (8.70, 9.90) | 5.20 (4.30, 6.30) | 5.10 (4.40, 6.10) |
| [204] | [146] | [202] | [139] | |
| Week 108 | 9.20 (8.50, 9.80) | 9.30 (8.70, 10.00) | 5.30 (4.20, 6.70) | 5.30 (4.20, 6.05) |
| [185] | [131] | [179] | [124] | |
| Week 120 | 9.30 (8.60, 9.90) | 9.20 (8.70, 9.90) | 5.60 (4.40, 7.00) | 5.20 (4.00, 6.40) |
| [163] | [123] | [160] | [119] | |
| Week 132 | 9.30 (8.60, 9.85) | 9.30 (8.60, 10.00) | 5.20 (4.37, 6.60) | 4.90 (3.90, 6.20) |
| [116] | [84] | [113] | [83] | |
| Week 144 | 9.40 (8.80, 9.80) | 9.20 (8.50, 9.80) | 5.60 (4.40, 7.00) | 5.10 (4.10, 6.70) |
| [81] | [65] | [81] | [65] | |
| Week 156 | 9.30 (8.60, 9.80) | 9.40 (8.70, 9.80) | 5.25 (4.10, 6.20) | 5.25 (4.20, 6.25) |
| [56] | [35] | [54] | [34] | |
| Week 168 | 9.50 (9.20, 10.00) | 9.25 (8.70, 9.70) | 5.00 (3.50, 6.80) | 4.70 (4.00, 5.60) |
| [23] | [14] | [23] | [13] | |
| Week 180 | 9.75 (8.90, 10.20) | 9.65 (8.95, 10.20) | 5.90 (5.80, 7.40) | 4.20 (3.50, 7.20) |
| [10] | [8] | [9] | [9] | |
Figure 4.
Median Ca×P values recorded at each scheduled visit for subjects who originally received cinacalcet or placebo during lead-in studies. Shaded area is the NKF-KDOQITM–recommended range for Ca×P.
Simultaneous achievement of goals for PTH and Ca×P.
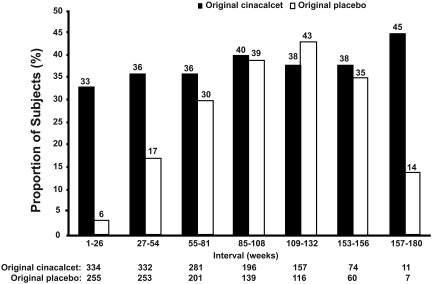
The proportion of patients that simultaneously achieved PTH ≤300 pg/ml and Ca×P ≤55 mg2/dl2 was maintained >33% throughout the study for the original cinacalcet group and exceeded 40% from weeks 85 to 108 (Figure 5). For the original placebo group, the proportion reaching this combined target increased from 4% initially to 30% during weeks 55 to 84, remaining above 30% thereafter as subjects were converted from placebo to cinacalcet therapy (Figure 5).
Figure 5.
Proportion of subjects achieving PTH ≤300 pg/ml and Ca×P ≤ 55 mg2/dl2 at each 6-mo period. Reductions in PTH and Ca×P values were calculated against the mean PTH or Ca×P values, respectively, over the 6-mo period. Some scheduled visits were shifted up to a maximum of 2 wk to align with the closest week indicated in the table. N, number of subjects entering period.
Cinacalcet Dosing
At the end of the titration phase, daily doses of cinacalcet were 30 mg in 159 (27%) subjects, 60 mg in 128 (22%), 90 mg in 124 (21%), 120 mg in 168 (29%), and 180 mg in four (1%). Doses at the end of the maintenance phase were 30 mg in 144 (25%) subjects, 60 mg in 84 (14%), 90 mg in 66 (11%), 120 mg in 79 (14%), and 180 mg in 171 (29%); 41 (7%) subjects terminated before the maintenance phase, or data were not available. At the end of the follow-up phase, the daily cinacalcet dose was 30 mg in 129 (22%) subjects, 60 mg in 75 (13%), 90 mg in 50 (9%), 120 mg in 60 (10%), and 180 mg in 139 (24%); 132 (23%) patients terminated study before completion.
Concomitant Vitamin D and/or Phosphate Binder Use
After the first dose of study medication during the titration phase, 130 (22%) subjects began treatment with vitamin D and 20 (3%) began treatment with a phosphate-binding agent. Paricalcitol was the most commonly used vitamin D sterol and calcium-based compounds were the most frequently used phosphate-binding agent (Table 6).
Table 6.
Concomitant vitamin D and phosphate binder use during titration and maintenance phases
| Vitamin and phosphate binder use during titration and maintenance phases | Total (n = 589) n (%) |
|---|---|
| Vitamin D use | |
| Started using vitamin D after first dose of study drug | 130 (22) |
| Never used/started vitamin D during time period | 109 (19) |
| Used/started vitamin D during the time period | 476 (81) |
| Calcitriol, i.v. only | 78 (13) |
| Calcitriol, p.o. only | 98 (17) |
| Paricalcitol only | 143 (24) |
| Doxercalciferol only | 17 (3) |
| Alfacalcidol, i.v. only | 17 (3) |
| Alfacalcidol, p.o. only | 58 (10) |
| Other or multiple vitamin D use | 65 (11) |
| Phosphate binder | |
| Started using phosphate binder after the first dose of study drug | 20 (3) |
| Never used/started phosphate binder during the time period | 14 (2) |
| Used/started phosphate binder during the time period | 571 (97) |
| Aluminum-containing only | 13 (2) |
| Calcium-containing only | 205 (35) |
| Sevelamer HCl only | 117 (20) |
| Other or multiple phosphate binder use | 236 (40) |
Long-term Safety
As this open-label extension study lacked a comparator group, concrete analysis of treatment-related AEs was not possible; however, observed AEs were consistent with the dialysis patient population and the treatment-specific AEs previously reported for the lead-in studies (11,13,17). Among all 585 subjects evaluable for safety, 565 (97%) reported at least one AE with the most frequently reported AEs being nausea (37%), vomiting (35%), and diarrhea (30%).
Serious adverse events (SAEs) were reported for 328 (56%) subjects. Of the SAEs that might be considered treatment related, the most frequently reported were vomiting (n = 7), nausea (n = 5), hypocalcemia (n = 5), and hypotension (n = 3). There were 73 (12%) deaths during the study and one additional death, due to cerebral hemorrhage, 1 wk after discontinuation from the study. Three deaths during the study were considered by investigators to be possibly related to study treatment. Two subjects with significant cardiovascular history died of myocardial infarction, and one subject with a history of diabetes and congestive heart failure died of cardiopulmonary arrest and bacteraemia. All three subjects' serum calcium levels were normal. Although hypocalcemia was reported in 32 (5%) subjects, 90% were of mild-to-moderate severity. Three subjects experienced severe hypocalcemia that resolved and subjects were restarted on cinacalcet therapy. One subject experienced life-threatening hypocalcemia with tetany and withdrew from the study, after which the tetany resolved. No trends were noted indicative of treatment-related effects in clinical chemistry (other than PTH, serum Ca and P) or hematology. All adverse events and adverse events leading to death were consistent with demographics and baseline characteristics of the patient population, as well as 3.2 yr of follow-up of patients with ESRD.
Discussion
This open-label, multicenter extension study suggests that cinacalcet can effectively exert long-term control of PTH and Ca×P within recommended guidelines in stage 5 CKD patients on dialysis. These results appreciably add to existing data on the efficacy and safety of cinacalcet, including data from several double-blind randomized, placebo-controlled studies following dialysis patients for 26 to 52 wk (11,13,17), as well as a small study on long-term efficacy following 59 subjects for up to 2 yr and 16 patients for up to 3 yr (15). All these studies have consistently shown that cinacalcet effectively reduces PTH, Ca, P, and Ca×P levels in subjects with stage 5 CKD receiving dialysis. This current study suggests that this efficacy can be maintained for up to 180 wk (3.5 yr) without any attenuation of effect and without requiring cinacalcet dosing escalation over time to sustain the effect.
Treatment with cinacalcet in this study maintained median PTH levels ≤300 pg/ml, per recommended clinical guidelines, for up to 180 wk (3.5 yr). Cinacalcet maintained median Ca×P within recommended targets for up to 180 wk, and median Ca and P levels for up to 168 wk. Up to 45% of subjects maintained recommended targets for both PTH and Ca×P levels through week 180. In the original cinacalcet group, PTH levels transiently increased between the conclusion of the lead-in studies and enrollment in the open-label study reflecting the required re-titration or possible discontinuation of cinacalcet between the lead-in and this extension study. In the original placebo group, declines in PTH andCa×P levels were observed beginning at week 54 after lead-in study baseline, reflecting the placebo phase of the lead-in study and initiation of cinacalcet in this open-label extension study. Both groups exhibited similar reductions in actual and percent changes in 6-mo intervals for PTH, Ca×P, Ca, and P levels following cinacalcet treatment.
The efficacy data of the current study is consistent with analysis of pooled data from the four double-blind, placebo-controlled lead-in studies of cinacalcet in patients with SHPT and stage 5 CKD on dialysis and traditional therapies (vitamin D sterols and oral phosphate binder), showing cinacalcet to be significantly more effective than placebo at maintaining recommended levels of PTH, Ca×P, Ca, and P (19). Treatment-related AEs reported in the double-blind placebo-controlled studies among subjects randomized to cinacalcet were limited to nausea and vomiting, which were generally mild to moderate in severity and transient in duration. Incidence of serious AEs was similar in both groups. In the prior extension study, approximately 55% of 59 subjects with SHPT on dialysis completing 100 wk of cinacalcet therapy achieved a PTH concentration within recommended levels and 60% had ≥30% reduction in PTH levels from baseline, with reported AEs mostly mild to moderate and largely restricted to nausea and vomiting (15).
Results from the present study, which has a larger sample size of 589 subjects treated with cinacalcet for up to 3.5 yr (180 wk), provide more robust and expanded long-term efficacy and safety data. Cinacalcet sustained PTH reductions without increases in Ca, P, or Ca×P for up to 3.5 yr and was well tolerated with only 8% of subjects discontinuing due to an AE. All AEs were consistent with demographics and baseline characteristics of the patient population, as well as 3.2 yr of follow-up of patients with ESRD. Of the AEs that might be considered treatment-related, most could be resolved with therapeutic adjustments allowing for continuation of or re-administration of cinacalcet.
Although the larger population size of the current study allows for increased efficacy and safety analysis compared with the prior extension study, aspects of study design impose limitations. Slight differences in design of the lead-in studies and resulting differences in prior cinacalcet therapy duration, including possible interruption of cinacalcet treatment by some subjects, add possible heterogeneity to the subject population. The lack of a comparator group prevents concrete analysis of long-term treatment-related AEs.
In this study, the longest and most robust analysis of therapeutic options for SHPT, administration of cinacalcet in subjects receiving dialysis demonstrated effective, well-tolerated management of SHPT through simultaneous control of PTH, Ca, P, and Ca×P levels in accordance with recommended guidelines for up to 3.5 yr. Overall, these results suggest the potential for long-term cinacalcet therapy to stabilize or slow disease progression, thereby decreasing risk of bone and mineral metabolism sequelae and cardiovascular morbidity and mortality.
Disclosures
Dr. Sprague is a member of the Advisory Board for Amgen, Inc, and has received prior research funding from Amgen. Dr. Evenepoel is a member of the European Amgen Advisory Board and has received speakers' fees from Amgen GmbH. Drs. Curzi, Husserl, and Kopyt participate on Amgen's Speaker Bureau and Lehigh Valley Hospital (Dr. Kopyt) has received a research grant from Amgen. Drs. Sterling and Mix are employees of and stockholders in Amgen. Dr. Wong has received speakers' fees from Amgen. Dr. González has nothing to declare.
Acknowledgments
Funding for this study and the preparation of this manuscript was provided by Amgen, Inc. Writing support was provided by Dr. Jon Nilsen (an employee of Amgen, Inc) and Dr. Nelson Erlick, on behalf of Amgen, Inc. Editorial assistance was provided by Mandy Suggitt on behalf of Amgen, Inc. A general summary posting for this study (20020158) has been made available on www.clinicalstudyresults.org, a publicly available and free-access database.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Cinacalcet Hydrochloride in Chronic Kidney Disease–Mineral Bone Disorder,” on pages 1405–1408.
References
- 1.Torres PU: Cinacalcet HCl: A novel treatment for secondary hyperparathyroidism caused by chronic kidney disease. J Ren Nutr 16: 253–258, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG: Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int 57: 1176–1181, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca×PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Rostand SG, Drueke TB: Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 56: 383–392, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Brown AJ, Slatopolsky E: Drug insight: Vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nature Clinical Practice Endocrinology & Metabolism 3(2): 134–144, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Maung HM, Elangovan L, Frazao JM, Bower JD, Kelley BJ, Acchiardo SR, Rodriguez HJ, Norris KC, Sigala JF, Rutkowski M, Robertson JA, Goodman WG, Levine BS, Chesney RW, Mazess RB, Kyllo DM, Douglass LL, Bishop CW, Coburn JW: Efficacy and side effects of intermittent intravenous and oral doxercalciferol (1α-hydroxyvitamin D2) in dialysis patients with secondary hyperparathyroidism: A sequential comparison. Am J Kidney Dis 37(3): 532–543, 2001 [PubMed] [Google Scholar]
- 9.Steddon SJ, Schroeder NJ, Cunningham J: Vitamin D analogues: How do they differ and what is their clinical role? Nephrol Dial Transplant 16: 1965–1967, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Moe SM, Drueke TB: Management of secondary hyperparathyroidism: The importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium-phosphorus produce. Am J Nephrol 23: 369–379, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Shahapuni I, Mansour J, Harbouche L, Maouad B, Benyahia M, Rahmouni K, Oprisiu R, Bonne JF, Monge M, El Esper N, Presne C, Moriniere P, Choukroun G, Fournier A: How do calcimimetics fit into the management of parathyroid hormone, calcium, and phosphate disturbances in dialysis patients? Semin Dial 18: 226–238, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, Albizem MB, Coburn JW: Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: A randomized, double-blind, multicenter study. J Am Soc Nephrol 16: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Strippoli GF, Palmer S, Tong A, Elder G, Messa P, Craig JC: Meta-analysis of biochemical and patient-level effects of calcimimetic therapy. Am J Kidney Dis 47: 715–726, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Moe SM, Cunningham J, Bommer J, Adler S, Rosansky SJ, Urena-Torres P, Albizem MB, Guo MD, Zani VJ, Goodman WG, Sprague SM: Long-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrol Dial Transplant 20: 2186–2193, 2005 [DOI] [PubMed] [Google Scholar]
- 16.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 17.Sterrett JR, Strom J, Stummvoll H-K, Bahner U, Disney A, Soroka SD, Corpier C, Arruda JA, Schwanauer LE, Klassen PS, Olson KA, Block GA: Cinacalcet HCL (Sensipar/Mimpara) is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol 68: 10–17, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Goodman WG, Spiegel DM, Fadda GZ, Lien YH, Zeig S, Finkelstein FO, Mittman N, Olson KA, McMary LC, Klassen PS, Quarles LD: The magnitude of reductions in elevated serum calcium (Ca), Phosphorus (P), and CaxP levels with cinacalcet HCl is influenced by the dose of concurrent vitamin D sretols [Abstract F-PO753]. Am Soc Nephrol 16: 500A, 2005 [Google Scholar]
- 19.Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, Drüeke TB, Cunningham J, Sherrard DJ, McCary LC, Olson KA, Turner SA, Martin KJ: Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 67: 760–771, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Brandi L, Daugaard H, Tvedegaa E, Nielsen PK, Egmose C, Storm T, Olgaard K: Long-term suppression of secondary hyperparathyroidism by intravenous 1alpha-hydroxy vitamin D3 in patients on chronic hemodialysis. Am J Nephrology 12: 311, 1992 [DOI] [PubMed] [Google Scholar]