Abstract
Background and objectives: Cyclosporin A (CsA) is a well-established treatment for steroid-dependent nephrotic syndrome (SDNS) that may, however, cause chronic ischemic renal lesions. The objective of the study was to assess the prevalence of CsA nephrotoxicity (CsAN) in protocol biopsies of children with SDNS.
Design, settings, participants, & measurements: From 1990 through 2008, we performed 71 renal biopsies in 53 patients with SDNS. The mean CsA C2 levels were 466 ± 134 ng/ml, and the mean duration of treatment was 4.7 ± 2.0 yr before biopsy (range 2.9 to 12.7 yr).
Results: CsAN was observed in 22 (31%) of 71 renal biopsies. Of these, 11 corresponded to isolated vascular or tubular lesions, and 11 corresponded to combined vascular and tubular lesions. The majority of CsAN lesions were mild (17 of 22). In no cases were lesions graded as severe. By regression analysis, CsAN was positively associated with the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) and with hyperuricemia and negatively associated with minimal-change lesions. By multivariate analysis, only association with the use of ACEIs or ARBs retained significance. Stratification of the population according to CsA C2 levels showed increased risk for CsAN for C2 levels >600 ng/ml.
Conclusions: Mild to moderate CsAN occurs in approximately one third of patients who have SDNS and are treated with CsA for >3 yr. Our data suggest that patients who require high dosages of CsA or treatment for hypertension, in particular when ACEIs/ARBs are used, are at higher risk for CsAN.
The efficacy of cyclosporin A (CsA) in reducing the relapse rate of patients with steroid-dependent nephrotic syndrome (SDNS) and inducing remissions in a substantial number of patients with nongenetic forms of steroid-resistant nephrotic syndrome is well established (1–4). CsA has immunosuppressive effects that decrease disease activity and acts directly on podocytes to stabilize their cytoskeleton (5,6).
As new treatments for SDNS become available, indications for CsA therapy need to be balanced against its adverse effects. Among these, chronic CsA nephrotoxicity (CsAN) is one of the most concerning. CsA causes vasoconstriction of afferent arterioles that is mediated by various mechanisms, including direct stimulation of smooth muscles cells, endothelial activation, and upregulation of local vasoconstrictive mediators (4).
There are no reliable clinical and biologic markers for CsAN, and performing renal biopsies is the only approach to detect early signs of ischemic renal damage caused by CsA. No consensus exists on the indication of performing protocol biopsies in patients who have SDNS and are treated with CsA.
Since 1990, we have performed systematic biopsies in all patients who are treated with prolonged cycles of CsA. In this work, we retrospectively analyzed these biopsies to assess the prevalence of CsAN lesions, the risk factors for the development of CsAN, and the clinical benefits of performing systematic biopsies in patients who have SDNS and are treated with CsA.
Materials and Methods
Patients
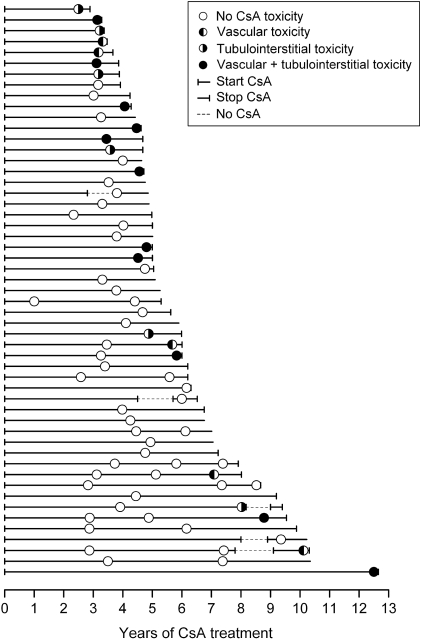
Medical records and renal pathology of 53 patients who were treated in our division with CsA for SDNS since 1990 and underwent biopsy to monitor CsAN were retrospectively evaluated. Their clinical characteristics are summarized in Table 1. At the time when CsA was started, all patients met the criteria for SDNS (3). CsA treatment was continued without intermission until biopsy in most patients (Figure 1). In five children, CsA therapy was stopped, but treatment was restarted within 12 to 18 mo as a result of rapid relapses of NS (Figure 1).
Table 1.
Patient characteristics, treatment, and renal biopsy findingsa
| Patient Characteristics | Value | N |
|---|---|---|
| Male gender (n [%]) | 36 (68.0) | 53 |
| Ethnicity (white:Asian; n [%]) | 51:2 (96.2:3.8) | 53 |
| Age of onset of NS (yr; median [range]) | 3.5 (0.7 to 13.2) | 53 |
| Weight (SDS; mean ± SD) | 0.9 ± 1.7 | 71 |
| Height (SDS; mean ± SD) | −0.2 ± 1.2 | 71 |
| BMD (z score; mean ± SD) | −1.2 ± 1.2 | 33 |
| SBP (SDS; mean ± SD) | 0.0 ± 0.6 | 71 |
| DBP (SDS; mean ± SD) | 0.3 ± 0.4 | 71 |
| CsA treatment | ||
| Age at CsA initiation (yr; median [range]) | 6.5 (2.2 to 14.2) | 53 |
| Years of NS before CsA (yr; median [range]) | 1.1 (0.4 to 11.2) | 53 |
| Total time on CsA (yr; median [range]) | 5.9 (2.9 to 12.5) | 53 |
| No. of relapses on CsA | 0.5 (0.0 to 3.0) | 71 |
| CsA dosage (mean ± SD) | ||
| mg/kg per d | 4.2 ± 1.2 | 71 |
| mg/m2 per d | 125 ± 28 | 71 |
| CsA C2 levels (ng/ml; mean ± SD) | 454 ± 122 | 71 |
| Other treatments | ||
| prednisone dosage (mg/m2 per d; mean [range]) | 5.0 (0.0 to 15.5) | 71 |
| amlodipine (n [%]) | 15 (21.1) | 71 |
| ramipril or losartan (n [%]) | 11 (15.5) | 71 |
| aldactone (n [%]) | 3 (4.2) | 71 |
| labetolol (n [%]) | 1 (1.4) | 71 |
| Renal biopsy | ||
| age at biopsy (yr; median [range]) | 11.5 (5.6 to 20.3) | 71 |
| biopsies per patient (n [%]) | ||
| 1 | 40 (75.5) | 53 |
| 2 | 8 (15.1) | 53 |
| 3 | 5 (9.4) | 53 |
| time on CsA before biopsy (yr; mean ± SD) | ||
| first biopsy | 3.9 ± 1.7 | 53 |
| second biopsy | 6.1 ± 1.0 | 13 |
| third biopsy | 8.4 ± 1.3 | 5 |
| Renal pathology (n [%]) | ||
| MCD | 29 (40.8) | 71 |
| mesangial proliferation | 12 (17.0) | 71 |
| FGGS | 14 (19.7) | 71 |
| FSGS | 16 (22.5) | 71 |
When data refer to individual patients the total number is 53, and when data refer to the 2 yr before each biopsy, the total number is 71. CsA, cyclosporin A; BMD, bone mineral density; DBP, diastolic BP; FGGS, focal global glomerular sclerosis; FSGS, focal segmental glomerular sclerosis; MCD, minimal-change disease; NS, nephrotic syndrome; SBP, systolic BP; SDS, SD score.
Figure 1.
Timing of renal biopsies in individual patients, cyclosporin A (CsA) treatment, and CsA nephrotoxicity. Each line represents an individual patient. Each circle represents a renal biopsy and is filled to indicate the presence of CsA nephrotoxicity as indicated in the legend. Hashed lines indicate transient discontinuation of CsA treatment. Lines that end with a vertical bar indicate that the patient stopped CsA treatment; otherwise, each line ends at the time of the last follow-up.
We do not routinely perform a renal biopsy before starting CsA for SDNS. Since 2005, we have been performing a first biopsy after 3 yr of treatment and every 2 yr thereafter. Before then, we had a similar policy but with less defined rules (Figure 1). Biopsies were performed only in patients who had a clinical indication to continue CsA for at least 1 yr.
Altogether, 71 biopsies were performed. Forty patients underwent a single renal biopsy after 3.9 ± 1.7 yr of CsA treatment. Two and three biopsies were performed in eight and five patients, respectively (Table 1, Figure 1).
The medical records were reviewed to calculate the mean value of relevant data in the 2 yr before biopsies (Tables 1 and 2). In five cases, CsA was discontinued for a variable length of time during the 2 yr before the biopsy. In these cases, data collection was limited to the months of CsA therapy within the 2-yr period before the biopsy. Weight and height were expressed as SD score (SDS) using the Tanner-Whitehouse reference charts (7), bone mineral density was expressed as z score using locally defined normal values, and BP measurements were expressed as SDS (8).
Table 2.
Blood and urinary testsa
| Test | Value | N |
|---|---|---|
| Blood tests | ||
| cholesterol (mg/dl; mean ± SD) | 204 ± 64 | 69 |
| triglycerides (mg/dl; mean ± SD) | 109 ± 56 | 69 |
| total proteins (g/dl; mean ± SD) | 7.05 ± 0.54 | 71 |
| albumin (g/dl; mean ± SD) | 4.04 ± 0.69 | 71 |
| Mg (mmol/L; mean ± SD) | 0.97 ± 0.43 | 31 |
| Ca (mmol/L; mean ± SD) | 2.32 ± 0.09 | 67 |
| P (mmol/L; mean ± SD) | 1.39 ± 0.23 | 67 |
| Na (mmol/L; mean ± SD) | 139.8 ± 1.8 | 71 |
| K (mmol/L; mean ± SD) | 4.3 ± 0.2 | 71 |
| uric acid (mg/dl; mean ± SD) | 5.1 ± 1.3 | 65 |
| CRP (mg/dl; median [range]) | 0.1 (0.0 to 0.7) | 55 |
| glucose (mg/dl; mean ± SD) | 85.5 ± 7.5 | 66 |
| RBC (×1,000,000; mean ± SD) | 4.54 ± 0.73 | 71 |
| hemoglobin (g/dl; mean ± SD) | 13.0 ± 1.2 | 71 |
| hematocrit (%; mean ± SD) | 36.6 ± 4.2 | 71 |
| WBC (×1000; mean ± SD) | 8.47 ± 2.51 | 71 |
| platelets (×100,000; mean ± SD) | 3.23 ± 0.81 | 71 |
| Creatinine clearance (Shwartz) | ||
| baseline (before CsA; ml/min per 1.73 m2; mean ± SD) | 121.4 ± 21.6 | 53 |
| 1 yr after CsA (% from baseline; mean ± SD) | −10.2 ± 15.5 | 53 |
| at biopsy (mean ± SD) | −7.3 ± 22.4 | 71 |
| Urine | ||
| protein-creatinine ratio (mg:mg; median [range]) | 0.09 (0.00 to 0.70) | 65 |
| specific gravity (mean ± SD) | 1.020 ± 0.004 | 69 |
| pH (mean ± SD) | 5.8 ± 0.5 | 69 |
Data refer to the 2 yr before each biopsy. See text for details. CRP, C-reactive protein; RBC, red blood cells; WBC, white blood cells.
CsA was administered in two separate oral doses. Blood levels were obtained 2 h after the morning dose (C2 levels). The initial target dosage was 400 to 450 ng/ml and was thereafter adapted according to the clinical evolution. Most patients were followed at our center and underwent monthly blood tests during the first year of treatment and, when stable, every 2 mo thereafter.
The mean prednisone dosage during the 2 yr before biopsy was computed and expressed as mg/m2. Prescription of antihypertensive drugs, mainly calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin receptor blockers (ARBs), was recorded when the drug was taken for >6 mo before biopsy (Table 1).
Relapses were defined as proteinuria >3+ by dipstick for 3 consecutive days. The GFR was estimated using the Schwartz formula (9).
Renal Pathology
Renal biopsies were analyzed after standard hematoxylin-eosin, periodic acid-Schiff, and Masson trichrome staining. Immunofluorescence was performed for IgG, IgA, IgM, fibrinogen, C3, and C1q. All specimens were reviewed by one pathologist.
CsAN was defined as the presence of tubulointerstitial lesions and/or CsA-associated arteriolopathy. CsA-associated tubular atrophy with interstitial fibrosis were classified as mild, moderate, or severe when they involved <7, 7 to 30, or >30% of the renal parenchyma, respectively (10). CsA-associated arteriolar hyalinosis was similarly defined as mild, moderate, or severe according to Mihatsch and colleagues (10,11). Briefly, mild lesions are characterized by focal hyaline depositions in one arteriole without circumferential and transmural involvement, moderate lesions are similar but involve more than one arteriole, whereas severe lesions are characterized by circumferential deposits that extend across the entire arterial wall, independent from the number of involved vessels.
For subsequent analysis, CsAN lesions were defined as “limited” when they involved only tubular or vascular lesions and “overt” when vascular and tubular lesions coexisted in the same biopsy. The grading of overt lesions corresponded to the highest grading of the vascular and tubular scores.
Statistical Analysis
Statistical analysis was performed with SPSS 11.0 software (SPSS Inc., Chicago, IL). Logistic regression was used to analyze risk factors for CsAN. The analysis was first conducted in univariate mode. Multivariate analysis was performed using independent variables that reached a statistical significance with P < 0.1 at the univariate level. Continuous data that passed normality tests are expressed as mean ± SD. Data are otherwise expressed as median and range. When indicated, normal data were compared with t test, and data that were not normally distributed were compared using the Mann-Whitney U test. Categorical data are expressed in percentage. All P values are two-sided and considered significant for values <0.05.
Results
CsA and BP Treatments
The mean CsA C2 levels were 454 ± 122 ng/ml. Under CsA, the number of relapses per year decreased from 2.0 ± 1.1 (range 1.0 to 6.0) to 0.5 ± 0.5 (range 0.0 to 3.0; P < 0.0001). Likewise, the median steroid dosage decreased from 11.6 (range 6.5 to 22.5) to 5.0 mg/m2 per d (range 0.0 to 15.5 mg/m2 per d; P < 0.0001). All patients achieved complete remission of their proteinuria between relapses.
No patient had a GFR <90 ml/min per 1.73 m2. As reported in Table 2, GFR decreased by approximately 10% after CsA was initiated and remained stable thereafter. Twenty-two patients (25 biopsies) received BP medications (Table 1, Figure 2).
Figure 2.
(A) Distribution of CsA nephrotoxicity lesions according to antihypertensive treatment and CsA C2 levels. Circles are filled to indicate the presence of CsA nephrotoxicity as indicated in the legend. cPatients with combined treatment with calcium channel blocker (CCB) and angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). (B) Odds ratios for the development of overt CsA toxicity. The population was divided using various cutoffs of CsA C2 levels. CsA C2 levels were calculated for each patient as the average of all levels measured during a period of 2 yr before each biopsy. Odds ratios are adjusted for the use of ACEIs or ARBs. Horizontal bars indicate low and high 95% confidence intervals. *P < 0.03.
The mean dosage of ramipril was 0.10 ± 0.07 mg/m2 per d (range 0.02 to 0.22 mg/m2 per d), the mean dosage of losartan was 0.49 ± 0.25 mg/m2 per d (range 0.12 to 0.74 mg/m2 per d), and the mean dosage of amlodipine was 0.08 ± 0.05 mg/m2/d (range 0.02 to 0.18 mg/m2 per d). None of these medications was prescribed to reduce proteinuria. The mean systolic BP was 0.22 ± 0.62 (SDS) in patients who were taking antihypertensive agents and −0.22 ± 0.61 (SDS) in patients who did not need medications for BP control (P = 0.006). The diastolic BP was similar in both groups.
Renal Pathology
Renal pathology results are reported in Table 1. As indicated, 41% of samples corresponded to minimal-change disease (MCD). The remaining biopsies were equally distributed among focal segmental glomerular sclerosis (FSGS), focal global glomerular sclerosis (FGGS), and mesangial proliferation. Immunofluorescence studies were negative in 57 biopsies. Mesangial IgM deposits (1+ to 2+) were observed in five biopsies with mesangial proliferation, two biopsies with MCD, and one biopsy with FGGS. Mesangial C3 deposits (1+ to 2+) were observed in seven biopsies, and mesangial IgA deposits (2+) were found in two biopsies.
CsAN was observed in 22 (31%) of 71 specimens obtained from 22 patients. The underlying renal lesions corresponded to MCD in five cases, mesangial proliferation in five cases, FSGS in five cases, and FGGS in seven cases.
The extent of CsAN lesions was mild in the majority of specimens (17 of 22) and moderate in the remaining. Toxicity lesions were limited to vascular or tubular structures in 50% of samples (limited toxicity) and involved both type of structures in 50% of samples (overt toxicity; Table 3).
Table 3.
Prevalence of CsA nephrotoxicity
| CsA Toxicity | Mild | Moderate | Severe |
|---|---|---|---|
| Limited | |||
| vascular | 3/71 | 3/71 | – |
| tubulointerstitial | 5/71 | – | – |
| Overt | |||
| vascular + tubulointerstitial | 9/71 | 2/71 | – |
Risk Factors for CsA Toxicity
The risk for developing CsA-related lesions was analyzed by logistic regression (Table 4). By univariate analysis, the use of ACEIs or ARBs was associated with a significant risk for developing CsAN. This result was confirmed at the multivariate level. At the univariate level, MCD was protective against the development of CsAN, whereas high CsA C2 levels were marginally associated with an increased risk for combined vascular and tubulointerstitial lesions. These results were not statistically significant when adjusted for the use of ACEIs or ARBs.
Table 4.
Regression analysis of risk factors and markers for CsA nephrotoxicitya
| Parameter | Limited or Overt Toxicity |
Overt Toxicity |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Univariate analysis | ||||||
| patient characteristics | ||||||
| male gender | 1.42 | 0.47 to 4.29 | 0.54 | 2.42 | 0.48 to 12.26 | 0.28 |
| age of onset of NS (yr) | 0.94 | 0.78 to 1.19 | 0.54 | 1.04 | 0.84 to 1.29 | 0.70 |
| age at CsA (yr) | 1.02 | 0.88 to 1.18 | 0.78 | 1.05 | 0.88 to 1.26 | 0.58 |
| years of NS before CsA | 1.09 | 0.92 to 1.30 | 0.33 | 1.04 | 0.83 to 1.29 | 0.75 |
| years of CsA at biopsy | 0.85 | 0.67 to 1.06 | 0.15 | 0.90 | 0.68 to 1.20 | 0.48 |
| no. of relapses | 0.51 | 0.20 to 1.33 | 0.17 | 0.57 | 0.16 to 2.01 | 0.38 |
| weight (SDS) | 1.08 | 0.81 to 1.46 | 0.60 | 1.08 | 0.75 to 1.57 | 0.67 |
| height (SDS) | 0.89 | 0.58 to 1.35 | 0.58 | 0.85 | 0.49 to 1.45 | 0.54 |
| BMD (z score) | 1.42 | 0.76 to 2.68 | 0.27 | 1.04 | 0.54 to 2.01 | 0.90 |
| SBP (SDS) | 0.89 | 0.40 to 1.99 | 0.78 | 1.22 | 0.44 to 3.40 | 0.70 |
| DBP (SDS) | 1.67 | 0.46 to 6.10 | 0.44 | 1.58 | 0.31 to 7.96 | 0.58 |
| treatment variables | ||||||
| CsA dosage (mg/kg per d; mean) | 0.84 | 0.55 to 1.28 | 0.41 | 0.83 | 0.48 to 1.42 | 0.49 |
| CsA T2 levels (ng/ml; mean) | 1.01 | 1.01 to 1.01 | 0.26 | 1.01 | 1.00 to 1.01 | 0.06 |
| steroid dosage (mg/m2 per d; mean) | 0.99 | 0.87 to 1.14 | 0.90 | 1.08 | 0.90 to 1.28 | 0.41 |
| CCB | 2.39 | 0.74 to 7.74 | 0.15 | 2.55 | 0.63 to 10.24 | 0.19 |
| ACEI or ARB | 8.76 | 2.04 to 7.56 | 0.003 | 13.2 | 2.95 to 59.09 | 0.001 |
| renal pathology | ||||||
| MCD | 0.31 | 0.01 to 0.96 | 0.04 | 0.33 | 0.12 to 2.03 | 0.49 |
| FSGS | 1.02 | 0.31 to 3.38 | 0.98 | 0.80 | 0.15 to 4.19 | 0.79 |
| FGGS | 2.80 | 0.84 to 9.32 | 0.10 | 1.50 | 0.35 to 6.52 | 0.59 |
| mesangial proliferation | 2.59 | 0.66 to 0.09 | 0.17 | 2.84 | 0.61 to 13.29 | 0.19 |
| markers of CsA toxicity | ||||||
| uric acid (mg/dl) | 1.64 | 1.02 to 2.62 | 0.04 | 2.81 | 1.39 to 5.69 | 0.01 |
| Multivariate analysis | ||||||
| MCD | 0.38 | 0.11 to 1.28 | 0.12 | |||
| ACEI or ARB | 7.40 | 1.68 to 2.65 | 0.008 | 11.35 | 2.40 to 53.80 | 0.002 |
| CsA T2 levels (ng/ml; mean) | 1.00 | 0.99 to 1.01 | 0.19 | |||
CI, confidence interval; OR, odds ratio.
The impact of ACEI/ARB treatment is best illustrated in Figure 2A. As shown, the majority of patients who were not receiving BP medications had no CsAN lesion as opposed to six of 11 patients who had overt lesions of CsA toxicity and were treated with ACEIs or ARBs.
Patients with CsAN tended to have higher C2 levels. This was further tested by analyzing biopsy results using various cutoffs for CsA C2 levels. Results were adjusted for the use of ACEIs or ARBs and are reported in Figure 2B. As shown, odds ratios for developing CsA-related vascular and tubulointerstitial lesions increased progressively from 2.5 to 6.5 as the cutoff for CsA C2 levels was increased from 460 to 600 ng/ml. Results became significant for levels >600 ng/ml.
Biologic Markers of CsAN
To identify potential biologic markers of CsAN, we also performed the same logistic regression analysis using all variables reported in Table 2 (full results not shown). A significant association was found only between the development of CsA lesions and serum uric acid levels as indicated in Table 4. Serum uric acid levels were 5.6 ± 1.3 mg/dl in samples with CsAN lesions and 4.9 ± 1.3 mg/dl in the absence of CsAN (P = 0.03). When the analysis was restricted to samples with overt CsAN, mean uric acid levels were 6.4 ± 1.2 mg/dl (P = 0.004).
Treatment Changes in Patients with CsAN
CsA was stopped within 6 mo in 15 cases, within 1 yr in four cases, and within 18 mo in the remaining three cases (Figure 1). In most cases, children were treated with alternate-day steroids at higher dosages or, in recent years, with mycophenolate mofetil.
Discussion
CsA efficacy in reducing the number of relapses in patients with SDNS is well established (1,2). Nonetheless, treatment with CsA can have significant adverse effects. Of these, CsAN is a major concern, particularly when patients have a significant probability of total recovery.
CsAN is primarily caused by chronic ischemic insult to the kidney, resulting in arteriolar hyalination and tubulointerstitial changes, including striped interstitial fibrosis, tubular vacuolization, and atrophy (11). Because these lesions may be difficult to distinguish from the underlying renal disease, criteria have been developed to stage CsA-induced renal lesions (11,12); however, even when using these criteria, the diagnosis of CsAN may be uncertain. Progressive interstitial fibrosis can develop with time in patients who have NS and are not treated with CsA, although less frequently and to a lesser extent (4,13,14). Conversely, arteriolar lesions are generally regarded as more reliable indicators of CsA toxicity, particularly when moderate or severe (4,11).
In our study, no severe CsAN lesions were observed, and moderate signs of toxicity were found only in five of 22 positive biopsies. Numerous studies have documented the prevalence of CsAN in patients who were treated for idiopathic nephrotic syndrome. Since 1986, >200 follow-up biopsies in patients who were treated with CsA for SDNS have been reported in the literature (1,12–27). These studies are difficult to compare because they vary in the number of biopsies, underlying renal pathology, duration of follow-up, dosages of CsA, and criteria to define CsAN. Overall, the prevalence of CsAN varies between 15 and 60% after 2 to 5 yr of CsA treatment. Lesions are mild in most cases.
Our data constitute one of the largest series of follow-up biopsies in patients who had SDNS and were treated with CsA for a prolonged period. Unlike previous studies, which did not identify biologic markers of CsAN, we observed higher uric acid levels in patients with toxic lesions. CsA is known to cause hyperuricemia in children with renal transplantation by limiting their renal tubular excretion of uric acid (28). In children who have received a liver transplant, this effect has been shown to be more frequent with CsA- than FK506-based immunosuppression regimens (29). Conversely, hyperuricemia can be associated with hypertension, chronic tubulointerstitial renal lesions, afferent arteriolopathy, and increased renal vascular resistance, raising the hypothesis that hyperuricemia is a co-factor in salt-sensitive hypertension and may exacerbate CsA-induced nephropathy (30). It is currently debated whether uric acid is a marker or a culprit in the development of renal lesions. Experimental data obtained in rat models of CsAN have confirmed that CsA increases serum uric acid levels but have also shown that increasing serum uric acid exacerbates CsA-induced nephropathy (31).
From a clinical standpoint, the significant overlap of serum uric acid levels between patients with and without CsAN limits its usefulness as a biologic marker for CsAN. Nevertheless, our results indicate that it is prudent to monitor the development of hyperuricemia in patients who receive CsA for SDNS.
Various clinical and pathology risk factors for the development of CsAN have been inconsistently reported in the literature (12,14,17,18,27). These include steroid resistance, low GFR, high proteinuria, FSGS lesions, high relapse rate, prolonged CsA treatment, and CsA serum levels. Some of these factors clearly do not apply to our population, and our analysis may carry an increased risk for type 1 errors related to the sample size. The vast majority of our patients experienced very few and short relapses under CsA treatment. A link between CsAN and the number of relapses or the duration of proteinuria was therefore unlikely. Likewise, it is generally accepted that longer treatments with CsA increase the risk for nephrotoxicity (4,12,16). This was not observed in our cohort. The timing of most follow-up biopsies, however, was clustered around 3 to 5 yr (Figure 1), which decreased considerably the power of the analysis. Conclusions of this study should therefore not be extended to treatments that exceed 5 yr.
A similar consideration probably applies to discrepancies between studies that have attempted to correlate renal toxicity and CsA levels, because these have generally been kept within a relatively narrow interval. CsA C2 levels >700 ng/ml were reported by Nakamura et al. (17) to be associated with a higher risk for CsAN in a cohort that included 18 children with idiopathic nephrotic syndrome but also five children with lupus nephritis. Our analysis indicates that a positive association probably exists also for lower CsA C2 levels. On the basis of these results, it seems prudent to avoid C2 levels that are >550 ng/ml.
The most concerning finding of this study was the strong association between CsAN and the use of ACEIs or ARBs. These drugs are increasingly used for their renoprotective effects in proteinuric renal diseases but reduce the glomerular perfusion pressure (32). Cumulative vascular effects of renal angiotensin II inhibition and CsA may therefore exacerbate renal hypoperfusion. Recent clinical studies in elderly patients with atherosclerotic vascular disease suggested, in fact, that excessive angiotensin II inhibition in kidneys that are vascularly compromised may have detrimental effects (33). Conversely, experimental models of CsA-induced interstitial fibrosis have shown that CsA renal toxic effects are mediated in part by angiotensin II and that, despite more pronounced renal hypoperfusion, the addition of an ACEI to CsA limits tubulointerstitial changes (34,35).
Alternatively, the association between ACEIs/ARBs and CsAN may be indirect if patients with CsA-induced vascular toxicity develop more hypertension. In this hypothesis, a similar association with the use of other antihypertensive drugs, such as CCBs, would be expected. Although our analysis did not reveal a significant association, five of 12 biopsies that were performed in patients who were receiving CCBs before the biopsy showed signs of CsAN, as opposed to nine of 49 biopsies that were performed in patients with normotension (Figure 2). In addition, two patients who had overt CsA nephrotoxicity and were receiving ACEIs or ARBs were also treated with a CCB. In this respect, the number of observations is probably too small to draw definitive conclusions.
Conclusions
As new treatments such as mycophenolate mofetil and rituximab become available (2,36,37), indications for follow-up renal biopsies aimed at detecting early signs of CsAN are rapidly decreasing. Nonetheless, our data suggest that if patients' disease is well controlled with low dosages of CsA and if they do not develop hypertension, then a renal biopsy can probably be postponed to after 4 to 5 yr of treatment. If, however, patients develop hypertension, require high dosages of CsA, or develop significant hyperuricemia, then a renal biopsy should be performed sooner. Prospective studies are required to evaluate the best antihypertensive medication that should be used in conjunction with CsA.
Disclosures
None.
Acknowledgments
This work was performed with the financial support of the E-RARE Project “PodoNet: EU Consortium for Clinical, Genetic and Experimental Research into Hereditary Diseases of the Podocyte.”
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Niaudet P, Habib R, Tete MJ, Hinglais N, Broyer M: Cyclosporin in the treatment of idiopathic nephrotic syndrome in children. Pediatr Nephrol 1: 566–573, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Hodson EM, Willis NS, Craig JC: Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev (23): CD002290, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Ehrich JH, Geerlings C, Zivicnjak M, Franke D, Geerlings H, Gellermann J: Steroid-resistant idiopathic childhood nephrosis: Overdiagnosed and undertreated. Nephrol Dial Transplant 22: 2183–2193, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Niaudet P, Habib R: Cyclosporine in the treatment of idiopathic nephrosis. J Am Soc Nephrol 5: 1049–1056, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Mathieson PW: Proteinuria and immunity: An overstated relationship? N Engl J Med 359: 2492–2494, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner JM, Whitehouse RH: Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Intern Med 51: 170–179, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A working group report from the National High Blood Pressure Education Program: National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics 98: 649–658, 1996 [PubMed] [Google Scholar]
- 9.Schwartz GJ, Gauthier B: A simple estimate of glomerular filtration rate in adolescent boys. Pediatrics 106: 522–526, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Mihatsch MJ, Antonovych T, Bohman SO, Habib R, Helmchen U, Noel LH, Olsen S, Sibley RK, Kemény E, Feutren G: Cyclosporine A nephropathy standardization of the evaluation of kidney biopsies. Clin Nephrol 41: 23–32, 1994 [PubMed] [Google Scholar]
- 11.Jennette JC, Olson JL, Schwartz MM, Silva FG: Pathology of the Kidney, 6th Ed., Philadelphia, Lippincott Williams & Wilkins, 2007, pp 1427–1439 [Google Scholar]
- 12.Fujinaga S, Kaneko K, Muto T, Ohtomo Y, Murakami H, Yamashiro Y: Independent risk factors for chronic cyclosporine induced nephropathy in children with nephritic syndrome. Arch Intern Med 91: 666–670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seikaly MG, Prashner H, Nolde-Hurlbert B, Browne R: Long-term clinical and pathological effects of cyclosporine in children with nephrosis. Pediatr Nephrol 14: 214–217, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kranz B, Vester U, Büscher R, Wingen AM, Hoyer PF: Cyclosporine-A-induced nephrotoxicity in children with minimal-change nephrotic syndrome: Long-term treatment up to 10 years. Pediatr Nephrol 23: 581–586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikura K, Ikeda M, Hattori S, Yoshikawa N, Sasaki S, Iijima K, Nakanishi K, Yata N, Hond M: Effective and safe treatment with cyclosporine in nephrotic children: A prospective, randomized multicenter trial. Kidney Int 73: 1167–1173, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gregory MJ, Smoyer WE, Sedman A, Kershaw DB, Valentini RP, Johnson K, Bunchman TE: Long-term cyclosporine therapy for pediatric nephrotic syndrome: A clinical and histologic analysis. J Am Soc Nephrol 7: 543–549, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Nozu K, Iijima K, Yoshikawa N, Moriya Y, Yamamori M, Kako A, Matsuo M, Sakurai A, Okamura N, Ishikawa T, Okumura K, Sakaeda T: Association of cumulative cyclosporine dose with its irreversible nephrotoxicity in Japanese patients with pediatric-onset autoimmune diseases. Biol Pharm Bull 30: 2371–2375, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Iijima K, Hamahira K, Tanaka R, Kobayashi A, Nozu K, Nakamura H, Yoshikawa N: Risk factors for cyclosporine induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 61: 1801–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Rinaldi S, Sesto A, Barsotti P, Faraggiana T, Sera F, Rizzoni G: Cyclosporine therapy monitored with abbreviated area under curve in nephrotic syndrome. Pediatr Nephrol 20: 25–29, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Nakahata T, Tanaka H, Tsugawa K, Kudo M, Suzuki K, Ito E, Waga S: C1–C2 point monitoring of low-dose cyclosporin a given as a single daily dose in children with steroid-dependent relapsing nephrotic syndrome. Clin Nephrol 66: 219–220, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Iijima K, Nakamura H, Yoshikawa N: Two-year cyclosporine treatment in children with steroid-dependent nephritic syndrome. Pediatr Nephrol 13: 33–38, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Ganesan V, Milford DV, Taylor CM, Hulton SA, Parvaresh S, Ramani P: Cyclosporine-related nephrotoxicity in children with nephrotic syndrome. Pediatr Nephrol 17: 225–226, 2002 [DOI] [PubMed] [Google Scholar]
- 23.El-Husseini A, El-Basuony F, Mahmoud I, Sheashaa H, Sabry A, Hassan R, Taha N, Hassan N, Sayed-Ahmad N, Sobh M: Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: A single-centre experience. Nephrol Dial Transplant 20: 2433–2438, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Hino S, Takemura T, Okada M, Murakami K, Yagi K, Fukushima K, Yoshioka K: Follow up study of children with nephritic syndrome treated with a long-term moderate dose of cyclosporine. Am J Kidney Dis 31: 932–939, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Webb KL, Sargent P, Burke JR: Cyclosporin therapy in steroid-dependent nephrotic syndrome. J Paediatr Child Health 29: 188–191, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Wyszyńska T, Ksiazek J, Wieteska-Klimczak A, Gorzkowska-Paczwa M, Dorywalski T, Tysarowska A: Evaluation of the efficacy of cyclosporine A treatment in idiopathic nephrotic syndrome in children. Pol Merkur Lekarski 11: 140–143, 2001 [PubMed] [Google Scholar]
- 27.Niaudet P, Broyer M, Habib R: Treatment of idiopathic nephrotic syndrome with cyclosporine A in children. Clin Nephrol 35[Suppl 1]: S31–S36, 1991 [PubMed] [Google Scholar]
- 28.Hoyer PF, Lee IK, Oemar BS, Krohn HP, Offner G, Brodhel J: Renal handling of uric acid under cyclosporine A treatment. Pediatr Nephrol 2: 18–21, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Tumgor G, Arikan C, Kilic M, Aydogdu S: Frequency of hyperuricemia and effect of calcineurin inhibitors on serum uric acid levels in liver transplanted children. Pediatr Transplant 10: 665–668, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Menè P, Punzo G: Uric acid: Bystander or culprit in hypertension and progressive renal disease? J Hypertens 26: 2085–2092, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Mazzali M, Kim YG, Suga S, Gordon KL, Kang DH, Jefferson JA, Hughes J, Kivlighn SD, Lan HY, Johnson RJ: Hyperuricemia exacerbates chronic cyclosporine nephropathy. Transplantation 71: 900–905, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Abuelo JG: Normotensive ischemic acute renal failure. N Engl J Med 357: 797–805, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf SONTARGET investigators: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Kon V, Hunley TE, Fogo A: Combined antagonism of endothelin A/B receptors links endothelin to vasoconstriction whereas angiotensin II effects fibrosis: Studies in chronic cyclosporine nephrotoxicity in rats. Transplantation 60: 89–95, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Ramírez C, Olmo A, O'Valle F, Masseroli M, Aguilar M, Gómez-Morales M, Revelles F, García-Chicano MJ, Arrebola F, Reguero ME, del Moral RG: Role of intrarenal endothelin 1, endothelin 3, and angiotensin II expression in chronic cyclosporin A nephrotoxicity in rats. Exp Nephrol 8: 161–172, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, Van Der Heijden AJ: Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 23: 2013–2020, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas B, Niaudet P, Nivet H, Raynaud N, Taque S, Ronco P, Bouissou F: Rituximab treatment for severe steroid- or cyclosporine-dependent nephritic syndrome: A multicentric series of 22 cases. Pediatr Nephrol 23: 1269–1279, 2008 [DOI] [PubMed] [Google Scholar]