Photodynamic therapy comprises a photosensitising agent, which accumulates in malignant tissue, and a light source, which activates the photosensitiser, causing it to generate highly reactive oxygen species that destroy malignant cells. Temoporfin is a second generation photosensitiser that has a shorter half life than its predecessors and is thought to be more selective towards tumours. These two factors should decrease the incidence of photosensitivity, one of the main side effects of photodynamic therapy. We report on a group of patients who received a single dose of temoporfin (Foscan, Scotia Pharmaceuticals) and developed partial thickness burns after minimal exposure to light.
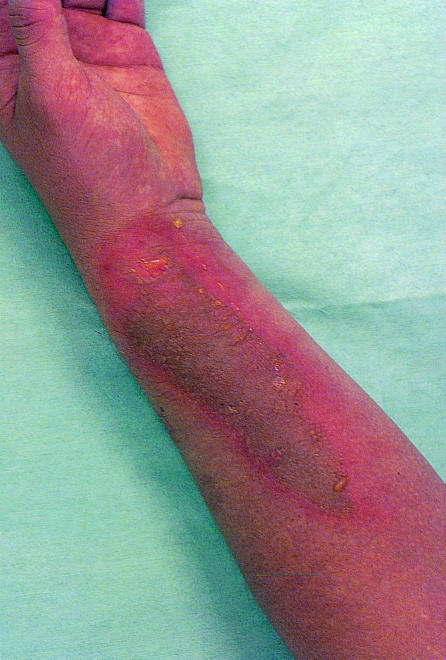
Fourteen healthy men aged between 20 and 26 were given a single dose (0.100-0.129 mg/kg) of temoporfin as part of a pharmacokinetic study. The dosage was a standard therapeutic one.1 After two weeks they were exposed to a test dose of sunlight. Twelve men showed no photosensitivity and were told to avoid prolonged exposure to bright sunlight for three months. Within 48 hours of discharge six of the 12 men had developed partial thickness burns on the left forearm (figure) and more superficial burns on other body areas (about 1% of total body surface area) after transient exposure to daylight. They were referred to the regional burns centre where they were treated conventionally with paraffin dressings and reviewed at five day intervals. Healing was much slower than with conventional thermal injury (28 versus 14 days), with prominent scarring in several men. As the men had signed disclaimers before the trial, they were not automatically entitled to compensation.
Photodynamic therapy is used in the treatment of various malignancies,2,3 and photosensitivity is a recognised complication. There is only one report of a skin burn,4 which was caused by a pulse oximeter that probably activated the photosensitiser. There are no previous published reports of burns after exposure to environmental light.
The use of photodynamic therapy is increasing and this may result in more adverse events as described here. Burns associated with photodynamic therapy may need referral to a specialist burns unit.
Figure.
Partial thickness burn on left forearm
References
- 1.Kubler AC, Haase T, Staff C, Kahle B, Rheinwald M, Muhling J. Photodynamic therapy of primary nonmelanomatous skin tumours of the head and neck. Lasers Surg Med. 1999;25:60–68. doi: 10.1002/(sici)1096-9101(1999)25:1<60::aid-lsm8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.McCaughan JS, Jr, Williams TE. Photodynamic therapy for endobronchial malignant disease: a prospective fourteen-year study. J Thorac Cardiovasc Surg. 1997;114:940–946. doi: 10.1016/S0022-5223(97)70008-4. [DOI] [PubMed] [Google Scholar]
- 3.Dilkes MG, De Jode ML, Gardiner Q, Kenyon GS, McKelvie P. Treatment of head and neck cancer with photodynamic therapy: results after one year. J Laryngol Otol. 1995;109:1072–1076. doi: 10.1017/s0022215100132050. [DOI] [PubMed] [Google Scholar]
- 4.Farber NE, McNeely J, Rosner D. Skin burn associated with pulse oximetry during perioperative photodynamic therapy. Anesthesiology. 1996;84:983–985. doi: 10.1097/00000542-199604000-00028. [DOI] [PubMed] [Google Scholar]