Abstract
In the newest implementation of cochlear implant surgery, electrode arrays of 10 or 20 mm are inserted into the cochlea with the aim of preserving hearing in the region apical to the tip of the electrode array. In the current study two measures were used to assess hearing preservation: changes in audiometric threshold and changes in psychophysical estimates of nonlinear cochlear processing. Nonlinear cochlear processing was evaluated at signal frequencies of 250 and 500 Hz using Schroeder phase maskers with various indices of masker phase curvature. A total of 15 normal-hearing listeners and 13 cochlear implant patients (7 with a 10 mm insertion and 6 with a 20 mm insertion) were tested. Following surgery the mean low-frequency threshold elevation was 12.7 dB (125–750 Hz). Nine patients had postimplant thresholds within 5–10 dB of preimplant thresholds. Only one patient, however, demonstrated a completely normal nonlinear cochlear function following surgery—although most retained some degree of residual nonlinear processing. This result indicates (i) that Schroeder phase masking functions are a more sensitive index of surgical trauma than audiometric threshold and (ii) that preservation of a normal cochlear function in the apex of the cochlea is relatively uncommon but possible.
INTRODUCTION
In one of the newest applications of cochlear implants, electrode arrays ranging in length from 10 to 20 mm are inserted into the scala tympani of individuals with bilateral low-frequency hearing with the aim of preserving residual hearing apical to the tip of the array. A successful surgical outcome allows for an electric stimulation of basal neural tissue without damaging the apical cochlear structures that transmit low-frequency acoustic information (e.g., von Ilbert et al., 1999; Skarzynski et al., 2004, 2006, 2007; Gantz and Turner, 2003, 2004; Gstoettner et al., 2004; Gantz et al., 2005, 2006; Kiefer et al., 2005; Leutje et al., 2007). The mean hearing loss following this procedure ranges from 10 to 20 dB depending on the electrode array and the nature of the surgical technique (Gantz and Turner, 2004; Gantz et al., 2005; Skarzynski et al., 2003, 2006, 2007; Gstoettner et al., 2004, 2005; Kiefer et al., 2005). The combination of binaural low-frequency acoustic hearing and monaural high-frequency electric hearing—termed combined electric and acoustic stimulation (EAS)—has been shown to improve speech understanding in quiet and in noise beyond that achieved by aided acoustic or electric hearing alone (Wilson et al., 2002; Brill et al., 2002; Gantz et al., 2005; Kiefer et al., 2005; Gstoettner et al., 2004). Gantz et al. (2005) reported a mean consonant nucleus consonant (CNC) (Peterson & Lehiste, 1962) score in the combined EAS condition (implant plus binaural hearing aids) of 67% correct for 11 hybrid recipients (10 mm electrode), and Gstoettner et al. (2006) reported a mean CNC score (also for the combined EAS condition) of 75% correct for 20 mm, EAS patients. In both studies, the postoperative combined EAS scores represented a significant improvement in speech perception performance relative to the best aided scores obtained preoperatively. These EAS scores represent an above average performance relative to the mean 55%–60% monosyllabic word recognition typically reported for conventional implant recipients of newer generation technology (e.g., Baumgartner et al., 2007; Balkany et al., 2007; Gifford et al., 2008).
The research reviewed above makes it clear that hearing can be preserved to within 10–20 dB of preimplant levels following the insertion of a short electrode array. What is not clear is whether the presence of an electrode array in the scala tympani affects cochlear mechanics and function beyond that which is revealed by the audiogram. To date, the only pre- and postimplant measures of auditory function with EAS patients have been clinical assessments of the audiometric threshold. To provide a broader view, the current study assesses the effect of electrode insertion on a fundamental aspect of normal cochlear function—nonlinear cochlear processing. Nonlinear cochlear processing was assessed because in the normal cochlea it is responsible for high sensitivity, broad dynamic range, sharp frequency tuning, and enhanced spectral contrasts via suppression (e.g., Oxenham and Bacon, 2004).
Psychophysical estimates of nonlinear cochlear processing can be obtained by measuring masked thresholds for Schroeder phase harmonic complexes. Positive-Schroeder (m+) and negative-Schroeder (m−) phase complexes have identical amplitude spectra but different phase spectra (Schroeder, 1971). Although m+ and m− complexes have identical flat envelopes, m+ complexes tend to produce less masking. This difference in masking is often referred to as the phase effect. Several researchers have hypothesized that the phase effect results from the m+ complexes producing a more peaked response along the basilar membrane (BM) coupled with fast-acting compression (e.g., Kohlrausch and Sander, 1995; Carlyon and Datta, 1997; Summers and Leek, 1998; Summers, 2000). Consistent with the possibility that the phase effect is influenced by the cochlear nonlinearity are results showing that it is reduced in subjects with cochlear hearing loss (Summers and Leek, 1998; Summers, 2000; Oxenham and Dau, 2004). Given that hearing impairment likely involves some degree of outer hair cell destruction and∕or dysfunction, a reduction in psychophysical estimates of compression is a plausible outcome.
Physiological evidence supporting the relationship between the Schroeder phase effect and cochlear compression has been provided by Recio and Rhode (2000), who measured BM responses to m+ and m− complexes in chinchilla cochleas. Their findings corresponded well with psychophysical data in that the BM responses to the m+ complexes were much peakier than those to the m− complexes. Additionally, the BM response difference between m+ and m− complexes was reduced or absent in peripherally damaged cochleaes for which BM compression was substantially reduced or absent.
Summers (2001) examined the effects of phase for Schroeder phase harmonic complexes by measuring the overshoot for listeners with normal hearing and listeners with sensorineural hearing loss. Overshoot refers to the increase in threshold as a brief signal is moved from the temporal center to the onset of a broadband masker. Overshoot is known to be reduced in individuals with both permanent sensorineural hearing loss (Bacon and Takahashi, 1992) and temporary aspirin-induced hearing loss affecting the outer hair cell function (McFadden and Chamlin, 1990). Summers (2001) found that the overshoot with the Schroeder phase maskers was greatest for listeners with normal hearing and for positive phase (m+) Schroeder maskers. Summers (2001) concluded that the findings were consistent with previous reports that the masking effectiveness of positive phase Schroeder harmonic complexes is more influenced by nonlinear cochlear processing.
Further evidence linking the Schroeder phase effect with nonlinear cochlear processing was provided by Oxenham and Dau (2001). They used a temporal window model that incorporated a static nonlinearity, similar to that seen with BM compression. With a normal amount of compression, the output of the model was similar to behavioral results from normal-hearing subjects (Oxenham and Dau, 2001). Without compression, the phase effect was completely eliminated.
In the current study, estimates of audiometric threshold and nonlinear cochlear function were obtained before and after surgery for patients implanted with 10 and 20 mm electrode arrays. The following questions were asked: (i) What is the average change in audiometric threshold following surgery? (ii) Is the psychophysical measure of nonlinear cochlear function more sensitive to cochlear damage than audiometric threshold? (iii) Is it possible to preserve cochlear function within the range of normal following electrode insertion? If so, how common is this outcome?
THE EFFECTS OF SHORT ELECTRODE IMPLANTATION ON MASKER PHASE CURVATURE
Subjects
Two subject groups were evaluated for the present study. The first group included 15 listeners with normal hearing to be used as the control group representing a normal cochlear function. All 15 listeners with normal hearing were female with a mean age of 22.6 years (range of 19–31). The second group of subjects included 13 individuals with various degrees of hearing loss who had been identified as candidates for EAS. Of the 13 individuals, 6 were recipients of a 20 mm electrode array (MED EL, EAS) and 7 were recipients of a 10 mm electrode array (Cochlear, Nucleus Hybrid). Of the 13 EAS recipients, 10 individuals were available for both pre- and postimplant assessments of nonlinear cochlear processing. The remaining three subjects were only available for a postimplant evaluation. Postimplant evaluation of an audiometric threshold and Schroeder masking functions was obtained 2–13 months postoperatively (with a mean duration of 8 months). The mean age of the 13 EAS subjects was 46.0 years (range of 28–75 years) with 11 female and 2 male participants.
For the six subjects receiving the 20 mm MED EL EAS electrode array, preoperative audiometric thresholds gave ranges of from 10–55 dB Hearing Level (HL) at 500 Hz, 20–50 dB HL at 750 Hz, 55 to >120 dB HL at 1000–1500 Hz, and 80 to >120 dB HL at 2000 Hz and above. All six subjects were implanted using a round window surgical technique described by Skarzynski et al. (2007).
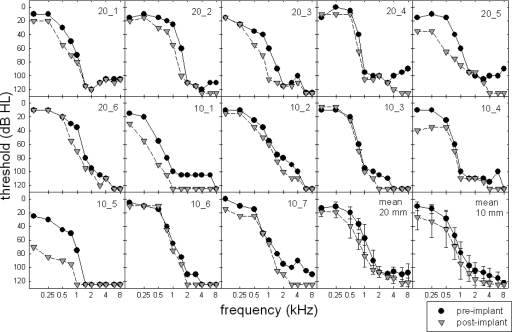
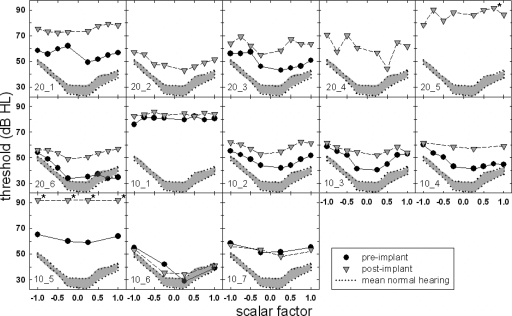
For the seven subjects receiving the 10 mm Nucleus Hybrid electrode array, preoperative audiometric thresholds—obtained using standard audiometric techniques—gave ranges of 0–30 dB HL at 500 Hz, 20–50 dB HL at 750 Hz, 25–115 dB HL at 1000–1500 Hz, and 95 dB HL and higher at 2000 Hz and above. All seven subjects were implanted via standard implantation techniques with the use of a cochleostomy described by Gantz et al. (2005). Figure 1 displays individual and mean audiometric thresholds for all 13 subjects for the preimplant (filled circles) and postimplant (shaded triangles) conditions. The error bars represent ±2 standard error. For those frequencies where no behavioral responses were obtained, a symbol was placed at 125 dB HL—which is beyond the limits of the audiometer for all frequencies tested. Further details will be provided in Sec. 2C.
Figure 1.
Individual and mean audiometric thresholds (in dB HL) obtained preoperatively (filled circles) and postoperatively (shaded inverted triangles).
Methods
Psychophysical estimates of nonlinear cochlear processing were measured by obtaining thresholds for pure-tone signals, 250 and 500 Hz, in the presence of positively and negatively scaled Schroeder phase harmonic complexes. The phases of the masker components were selected according to an equation originally proposed by Schroeder (1971) and more recently modified by Lentz and Leek (2001),
where C is a scalar factor and N is the number of components in the harmonic complex. A true positive (m+) or negative (m−) Schroeder phase complex is achieved when the scalar factor C is equal to 1 or −1, respectively. A sine-phase complex is produced when C is equal to 0. By varying the scalar value C from −1 to 1, a range of frequency sweep rates, or masker phase curvatures, can be produced (Lentz and Leek, 2001). When the phase curvature of the masker is equal, but opposite in sign, to that of the auditory filter centered at fs, masker effectiveness will be at a minimum. Thus, for the first ten subjects in the present study, masked thresholds were obtained for scalar factors, C, ranging from −1 to 1 in increments of 0.25. For the last three subjects enrolled, masked thresholds for scalar factors of −1, +1, −0.25, and +0.25 were obtained (more details are provided in Sec. 2C). Based on the findings of Oxenham and Dau (2001), the spectral range of the masker encompassed the frequency range between 0.4fs and 1.6fs. The fundamental frequency of the masker complex was 25 Hz. The overall level of the masker was fixed at 75 dB sound pressure level (SPL), and the signal level was varied adaptively. The durations of the masker and the signal were 400 and 200 ms, respectively (including 10 ms cos2 rise∕fall times). The signal was placed in the temporal center of the masker.
Relatively low signal frequencies were chosen so that quiet thresholds across the range of the masker spectrum were likely either normal or near normal. Also, Oxenham and Dau (2001) examined masker phase effectiveness at 250 Hz and found a large phase effect (>20 dB) in normal-hearing listeners. Furthermore, a 250 Hz signal was chosen so that it was possible to obtain EAS subjects with normal or near-normal hearing for a (limited) range of frequencies above fs. The reason that normal or near-normal hearing above the test frequencies is important is that the cochlear location of the nonlinearity generally ranges from 1∕3 to 1∕2 of an octave above that of the cochlear place corresponding to the characteristic frequency of the test stimulus (e.g., Davis, 1983; Chatterjee and Zwislocki, 1997; Rhode and Recio, 2000). Thus, it seems reasonable that to obtain a true estimate of the nonlinear cochlear function in the low-frequency cochlear region, one must ensure that cochlear functioning above fs is also relatively normal. Gifford et al. (2007) examined the effects of masker phase curvature in subjects meeting audiologic candidacy for EAS and reported that nonlinear cochlear processing was present in the majority of subjects tested. Given that subjects were required to have hearing thresholds less than 55 dB HL at 500 Hz (see Sec. 2A), the nonlinear properties of the cochlea would be expected to be present (though perhaps slightly reduced) at the test frequency (Neely and Kim, 1986). However, it is the comparison between the pre- and postimplant phase effects that was of interest, not simply the magnitude of the phase effect.
Thresholds for Schroeder phase masking were measured in an adaptive three-interval forced-choice paradigm with a 3-down, 1-up stepping rule to track 79.4% correct (Levitt, 1971). A run consisted of eight reversals. The first two reversals were discarded, and the threshold was determined using the average signal level at the remaining six reversal points. The initial step size of 5 dB was decreased to 2 dB after the second reversal. On the rare occasion that an estimate had a standard deviation greater than 5 dB, that run was discarded. All reported thresholds represent the mean of at least two estimates. If the difference between the two thresholds was greater than 3 dB, one additional run was completed and averaged. A third run was required for just nine of the individual thresholds obtained in the current study. The maximum level for the dynamically varying stimulus was fixed at 92 dB SPL. During a run, it was permissible for the threshold track to reach the ceiling value; however, if the tracking procedure called for a higher level, that run was discarded. If two runs for a particular condition were discarded on this basis, it was concluded that a threshold for that condition could not be achieved. Subjects were provided with a minimum of 1 h training on simultaneous masking with the Schroeder phase maskers. This amount of training was found to be sufficient for the majority of subjects in order to achieve stable threshold estimates. One subject, however, required nearly 2 h in order to become fully acquainted with the task, as evidenced by threshold stabilization.
All stimuli were generated and produced digitally at a 20 kHz sampling rate. Stimuli were routed monaurally to one channel of Sennheiser HD250 Linear II stereo headphones via an Echo Indigo input∕output laptop soundcard. Subjects were tested in a double-walled sound-attenuating booth. Observation intervals were signaled by the highlighting of visual stimuli on a laptop computer monitor. The signal was presented randomly in one of three intervals. Subjects responded by either pressing a button on the number keypad or using a mouse. Visual feedback was provided.
Results
Audiometry
Figure 1 displays individual pre- and postoperative audiometric thresholds for frequencies from 125 to 8000 Hz in the implanted ear. An absent behavioral response to sound was denoted by a threshold of 125 dB HL, which was beyond the limits of the audiometer for all frequencies tested. A two-way, repeated-measures analysis of variance (ANOVA) was completed with frequency and time of measurement (pre-versus postimplant) as the variables. There was a significant effect of time (F(1,12)=57.73, p<0.001). That is, at the group level, postimplant thresholds were found to be significantly poorer than preimplant thresholds. Mean thresholds that collapsed across frequency were 73.6 and 86.3 dB HL for the pre- and postimplant conditions, respectively. There was also a significant effect of frequency, which was expected given the sloping nature of the hearing losses (F(12,10)=193.547, p<0.001). A statistical analysis also revealed that there was no significant interaction between the frequency and the time of the threshold assessment (F(1,10)=1.80, p=0.067). In other words, the degree of threshold change between the pre- and postimplant assessments was not found to be significantly different across the range of signal frequencies tested.
The statistical analysis revealed significantly elevated thresholds following the surgical insertion of an electrode array; however, if we were to use a ±10 dB test-retest variability as the clinical estimate of a nonsignificant difference in behavioral thresholds for clinical audiometric procedures (Stuart et al., 1991), there were five subjects for whom no difference in threshold was observed at 500 Hz, postoperatively. Of these five subjects, two were recipients of the 20 mm array (20̱4 and 20̱6) and three were recipients of the 10 mm array (10̱2, 10̱3, and 10̱6).
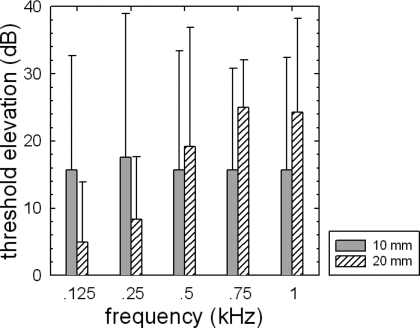
Mean postoperative threshold elevation (in decibels) as a function of frequency is plotted in Fig. 2. The shaded and hatched bars represent the 10 and 20 mm recipients, respectively. The error bars represent a +2 standard error. An ANOVA was completed, analyzing the effect of the electrode array length on the degree of threshold elevation. Only results from thresholds at 125–750 Hz were entered into the analysis because hearing losses at higher frequencies tended to be near the ceiling. The results of the statistical analysis revealed no main effect of the electrode array length on the degree of threshold elevation (F(1,11)=0.071, p=0.795). The mean threshold elevation for the 10 mm group was 16.4 dB. The mean threshold elevation for the 20 mm group was 14.4 dB. There was an effect of frequency (F(3,11)=4.276, p=0.012) and a significant interaction between the array length and frequency (F(1,3)=5.549, p=0.003). An inspection of Fig. 2 suggests that the threshold elevation for the 10 mm group tended to be constant across frequencies, whereas the threshold elevation for the 20 mm group increased with frequency. The degree of threshold elevation, however, was not significantly different across the 10 and 20 mm groups.
Figure 2.
Mean degree of audiometric threshold elevation for the subjects receiving 10 mm (shaded bars) and 20 mm (hatched bars) electrode arrays. The error bars represent a ±2 standard error.
Schroeder phase masking
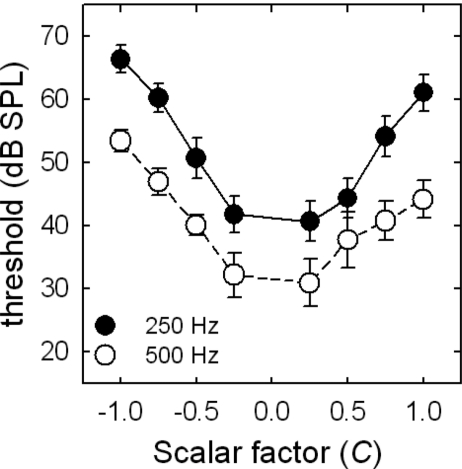
Figure 3 displays mean masked thresholds for the Schroeder phase maskers as a function of masker phase curvature, or scalar factor (C), for the normal-hearing listeners. The filled and unfilled symbols represent thresholds for the 250 and 500 Hz signals, respectively. The error bars represent a ±2 standard error. The Schroeder masking functions for the normal-hearing listeners exhibit the typical curved pattern that has been reported elsewhere (Oxenham and Dau, 2001, 2004). That is, the masked thresholds vary as a function of both masker phase curvature and signal frequency. The mean Schroeder phase effect, which was calculated as the threshold difference between the peak (C=−1) and valley (C=0.25) of the function, was 26.8 dB for 250 Hz and 24.1 dB for 500 Hz. Oxenham and Dau (2001, 2004) presented similar data with Schroeder phase effects of 22 dB at 250 Hz and 21 dB at 500 Hz. The phase curvature associated with the peak (C=−1) and valley (C=0.25) of the function was also similar to that reported by Oxenham and Dau (2001); however, Oxenham and Dau (2004) used a 50 Hz fundamental for a masker complex centered at 500 Hz in contrast to the 25 Hz fundamental used in the current study.
Figure 3.
Mean masked thresholds (in dB SPL) for the normal-hearing listeners as a function of masker phase curvature, or scalar factor (C). The filled and unfilled circles represent masked thresholds for the 250 and 500 Hz signals, respectively. The error bars represent a +2 standard error.
Signal frequency: 250 Hz
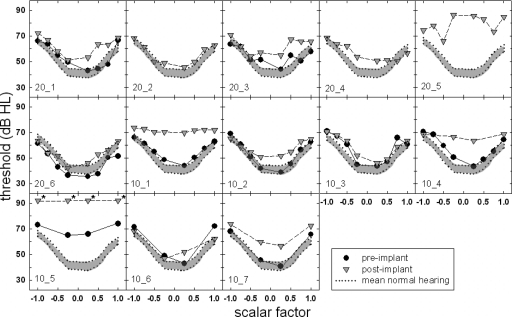
Figures 45 display the masking functions for the EAS subjects obtained both pre- and postoperatively for signal frequencies of 250 and 500 Hz. Preoperative masked thresholds are represented by the filled circles, and postoperative thresholds are represented by the shaded inverted triangles. The shaded area at the bottom of each figure represents mean masked thresholds for the normal-hearing listeners and a ±2 standard error about the mean. After data were collected from the first nine subjects, it was realized that the most important masker scalar factors for determining the shape of the masking function were +1, −1, +0.25, and −0.25. Thus, in an effort to save time during the time-intensive sessions, the last three subjects were run using only these four scalar factors for each signal frequency. If a masked threshold could not be obtained for a given condition (i.e., the signal level would have to be increased beyond the 92 dB SPL maximum), the “threshold” was plotted at 92 dB SPL and an asterisk was placed next to the symbol. This occurred for just one subject at 250 Hz and for two subjects at 500 Hz.
Figure 4.
Individual masked thresholds (in dB SPL) at 250 Hz for the implanted subjects. The filled symbols represent the preoperative condition, and the shaded inverted triangles represent the postoperative condition. The shaded area outlined by a dotted line encompasses the area of the mean masked thresholds and a +2 standard error for the normal-hearing listeners.
Figure 5.
Individual masked thresholds (in dB SPL) at 500 Hz for the implanted subjects. The filled symbols represent the preoperative condition, and the shaded inverted triangles represent the postoperative condition. The shaded area outlined by a dotted line encompasses the area of the mean masked thresholds and a +2 standard error for the normal-hearing listeners.
Examining the preimplant data for the 250 Hz signal (filled circles), there were nine subjects for whom the minima of the masking function were located in the shaded region—or near the mean thresholds for normal-hearing listeners. This included all three of the 20 mm subjects for whom we obtained preimplant data and six out of the seven 10 mm subjects (excluding 10̱5). This outcome should not be surprising given that the poorest audiometric threshold at 250 Hz obtained preoperatively for these subjects was 25 dB HL. That is, research has shown that the effects of cochlear damage on psychophysical tasks thought to reflect nonlinear cochlear processing tend to become evident once thresholds reach 30 dB HL (e.g., Laroche et al., 1992). Thus this group of nine subjects exhibited minima thresholds near the mean normal-hearing listeners’ minima thresholds. The one 10 mm subject who did not exhibit normal cochlear nonlinearity prior to surgery (10̱5) had preoperative thresholds in the range of 25–50 dB HL through 750 Hz. Thus, it is not surprising that she demonstrated little nonlinear cochlear processing even before surgery.
Postoperative results (inverted triangles) demonstrate a different pattern of results. For the 250 Hz signal shown in Fig. 4, none of the 10 nor 20 mm subjects’ minima thresholds were within the shaded region. This suggests that the surgical insertion of the electrode array resulted in a significant elevation of all subjects’ masked thresholds for the 0.25 scalar factor. The minima of masking function for subjects 20̱2, 20̱6, and 10̱3, however, were just marginally outside of the shaded region.
Recall that five subjects demonstrated no significant postoperative elevation in the audiometric threshold at 250 Hz (20̱4, 20̱6, 10̱2, 10̱3, and 10̱6). None of these five subjects, however, exhibited masked thresholds in the shaded region of normal hearing for both the pre- and postimplant conditions. Further examination of these data revealed that subject 10̱2 exhibited an abnormal postoperative masking function despite the fact that her postimplant audiometric thresholds increased by just 5 dB at 250 Hz and by 10 dB at 500 Hz. Subject 10̱6 also demonstrated abnormal masking functions postoperatively even though her pre- and postimplant audiometric thresholds were identical at 250 Hz and were within 5 dB of one another at 500 Hz. On the other hand, subjects 20̱5, 10̱1, 10̱4, and 10̱5 demonstrated considerably reduced or absent nonlinear cochlear processing evidenced by a flat or relatively flat postoperative masking function. Given that these subjects exhibited considerable postimplant elevations in audiometric thresholds, this outcome was not surprising. Subject 10̱7, however, demonstrated much reduced nonlinear function following surgery even though her pre- and postimplant audiometric thresholds varied by 15 dB at 250 Hz and by 10 dB at 500 Hz. These results suggest that while pre- and postimplant audiometric thresholds may differ by a value that is just outside the acceptable range for audiometric test-retest variability, the Schroeder masking pattern may be significantly altered postoperatively. Thus, Schroeder phase masking appears to be a more sensitive index of surgically related damage to the cochlea.
Signal frequency: 500 Hz
Figure 5 displays masking data for the 500 Hz signal. Examining the preimplant data at 500 Hz (circles), there was just one subject (10̱6) for whom the minima of the masking function was located within the shaded region representing the normal-hearing data. There were other subjects, however, whose preoperative masking functions may have been elevated but still exhibited clear maxima and minima (20̱3, 20̱6, 10̱2, 10̱3, and 10̱4).
Examining postoperative results at 500 Hz, there were no subjects for whom pre and postimplant masking functions were both within the shaded region depicting the mean data (+2 standard error) for the normal-hearing listeners. Examining those subjects whose preoperative masking function was either normal or elevated but still exhibited curvature with a definitive maximum and minimum (20̱3, 20̱6, 10̱2, 10̱3, 10̱4, and 10̱6), five of these individuals (excluding 10̱6) had nearly lost all evidence of nonlinear cochlear processing at 500 Hz following surgery—based on the masked thresholds displayed in Fig. 5. This was a surprising outcome—particularly for subjects 10̱2 and 10̱3—as their postoperative audiometric thresholds increased by just 5–15 dB at 500 and 750 Hz. Thus one might have hypothesized that there would have been a postimplant reduction in the Schroeder phase effect but not a near elimination of the effect.
The threshold functions shown in Figs. 45 do not necessarily convey whether the phase effects may have been within the range expected for normal-hearing listeners. Furthermore, the masked thresholds also do not necessarily demonstrate whether a postoperative reduction in the phase effect—or a psychophysical estimate of nonlinearity—was observed. That is, an individual may demonstrate higher than normal masked thresholds but a normal or near-normal phase effect. Thus the effects of masker phase curvature, or the Schroeder phase effect, were calculated for each EAS subject by determining the difference in masked threshold between the maximum and the minimum of each function for each signal frequency. These data are shown in Figs. 67.
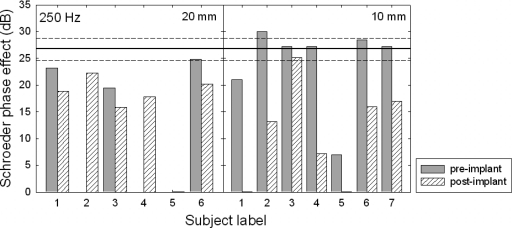
Figure 6.
Schroeder phase effect (in dB) at 250 Hz for the preoperative (shaded bars) and postoperative (hatched bars) conditions. The 20 and 10 mm subjects’ data are shown in the left and right panels, respectively. The solid line at 26.8 dB represents the mean phase effect for the 15 normal-hearing subjects, and the dashed lines represent a ±2 standard error.
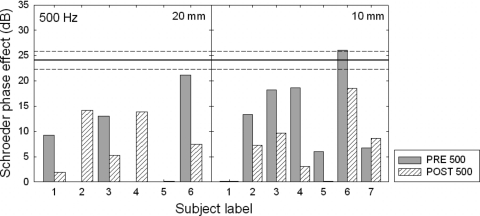
Figure 7.
Schroeder phase effect (in dB) at 500 Hz for the preoperative (shaded bars) and postoperative (hatched bars) conditions. The 20 and 10 mm subjects’ data are shown in the left and right panels, respectively. The solid line at 24.1 dB represents the mean phase effect for the 15 normal-hearing subjects and the dashed lines represent a ±2 standard error.
For those instances where there may have been a negative-Schroeder phase effect (the masked threshold for a scalar factor of 0.25 was higher than that for −1.0), a value of zero was entered, indicating the lack of nonlinear cochlear function. Figures 67 display the Schroeder phase effect (in decibels) at 250 and 500 Hz for each of the individual subjects both preoperatively (shaded bars) and postoperatively (hatched bars). The six 20 mm subjects’ data are shown in the left panel, and the seven 10 mm subjects’ data are shown in the right panel. The solid horizontal line represents the mean phase effect for the 15 normal-hearing listeners, and the dashed lines above and below represent a ±2 standard error.
Examining the 250 Hz data in Fig. 6, one of the 20 mm subjects (20̱6) and five of the 10 mm subjects (20̱2, 20̱3, 20̱4, 20̱6, and 20̱7) had preoperative phase effects in the range expected for normal-hearing listeners. Postoperatively, however, only one subject, 10̱3, retained a phase effect that was within a ±2 standard error of the mean for normal-hearing listeners. This suggests that all but a single subject demonstrated an abnormal degree of nonlinear cochlear processing at 250 Hz following surgery. There were several subjects, however, who did exhibit a considerable phase effect following surgery—albeit not within the expected range for a normal cochlear function. Thus, EAS surgery resulted in the preservation of some residual cochlear nonlinearity, but not necessarily within the range of normal.
Examining the 500 Hz data in Fig. 7, just one subject (10̱6) exhibited a normal phase effect preoperatively. Postoperatively, none of the 13 subjects retained a normal degree of nonlinear cochlear processing. There were several subjects, however, who exhibited a postoperative phase effect, which suggests that these implant recipients retained some degree of nonlinear cochlear processing. Nevertheless, the effects of surgical trauma appeared to be more apparent at 500 Hz.
The statistical analysis using a repeated-measures ANOVA was computed comparing phase effects for the preoperative 10 and 20 mm recipients to the normal-hearing listeners at 250 and 500 Hz. The 10 and 20 mm data were combined into one group termed EAS. A significant effect of subject group was found such that the Schroeder phase effect was found to be larger for the normal-hearing listeners relative to the preimplant subjects (F(1,23)=14.36, p<0.001). This trend is more apparent in Fig. 7 for the 500 Hz signal. The mean Schroeder phase effects that collapsed across frequencies were 18.3 dB for the preoperative EAS subjects and 25.5 dB for the normal-hearing subjects. A significant effect of signal frequency was also found (F(1,1)=33.26, p<0.001); the mean Schroeder phase effects were 25.2 dB for 250 Hz and 18.6 dB for 500 Hz. There was also a significant interaction between the frequency and the subject group (F(1,1)=11.42, p=0.003). An all pairwise multiple comparison using the Tukey test revealed that there was a significant difference between the preimplant EAS and the normal-hearing data at 500 Hz (q=7.06) but not at 250 Hz (q=2.10). This is another finding that is easily observed in Figs. 67. Clearly more subjects achieved normal phase effects preoperatively at 250 Hz than at 500 Hz. The post hoc analysis also revealed that the Schroeder phase effect was not significantly different across signal frequencies for the normal-hearing listeners (q=2.67) but was significantly different across frequencies for the EAS subjects (q=8.35). This was not unexpected given the sloping nature of the audiometric thresholds in the preimplant subjects, resulting in a reduced effect of masker phase curvature at 500 Hz.
Another statistical analysis was completed examining the effect of time of testing (pre—versus postimplant) for the EAS Schroeder phase effect. There was a significant main effect of time (pre—versus postimplant) on the Schroeder phase effect (F(1,9)=43.6, p<0.001). That is, the preimplant Schroeder phase effect was found to be significantly greater than the postimplant effect. The phase effects that collapsed across frequencies were 18.34 and 9.9 dB for the pre- and postimplant conditions, respectively. The mean preoperative phase effects were 23.6 and 13.1 dB at 250 and 500 Hz. The mean postoperative phase effects were 13.5 and 6.4 dB at 250 and 500 Hz. This confirms the fact that the surgical insertion of the electrode array significantly reduced nonlinear cochlear processing in the lower-frequency region.
DISCUSSION
It is reasonable to suppose that the insertion of a relatively stiff foreign body (an electrode array) into the scala tympani could alter cochlear mechanics or cochlear function. In the Introduction it was noted that the audiogram provides only a very narrow view of changes in cochlear function. To broaden that view the current study assessed changes in both audiometric thresholds and low-frequency nonlinear cochlear processing following the insertion of electrode arrays 10 and 20 mm into the cochlea.
All of the 13 patients tested had some degree of hearing preservation. The electrode insertion depth was related in a frequency-specific manner to the preservation of audiometric thresholds. Hearing loss with a 10 mm insertion was relatively constant across a frequency range of 125–750 Hz. For a 20 mm insertion hearing loss increased over a range of 125–750 Hz. Thus although no significant differences were noted in the degree of hearing preservation across the 10 and 20 mm groups, it is reasonable to expect that a deeper electrode insertion will be associated with greater hearing loss at higher frequencies within the 125–750 Hz range.
Nine subjects had postoperative audiometric thresholds that were within 10 dB of the preoperative estimates at the signal frequencies tested, which is considered within the limits of acceptable test-retest variability (Stuart et al., 1991). Out of these nine subjects, only one demonstrated a normal nonlinear cochlear function, as evidenced by the Schroeder phase effect following surgery at 250 Hz (Fig. 6), and none of the subjects demonstrated a normal cochlear function postoperatively at 500 Hz (Fig. 7). However, there were subjects for whom the postoperative Schroeder phase effect was clearly abnormal but who still exhibited some phase effect and thus retained some degree of nonlinear cochlear processing. Based on these findings, we conclude that the measure used in the current study is a more sensitive index of postsurgical alterations to cochlear function than the standard audiometric threshold.
In the light of this more sensitive index, is it possible to preserve a normal cochlear function at the apical end of the cochlea following the insertion of electrodes arrays 10 and 20 mm into the cochlea? The answer is “yes” for the 10 mm insertion—for a single subject (10̱3) at 250 Hz. This is likely due to the higher degree of threshold elevation just above 500 Hz than above 250 Hz. Although only one subject demonstrated a normal nonlinear cochlear function postoperatively, most subjects still exhibited a postimplant phase effect (more so at 250 Hz than at 500 Hz). Thus, although retention of normal nonlinear cochlear processing is not the most common outcome of hearing preservation surgery, preservation of some cochlear nonlinearity (with postoperative phase effects >5 dB) was achieved for ten subjects at 250 Hz and for seven subjects at 500 Hz.
If the preservation of low-frequency cochlear function is possible following surgery, does it make a difference for speech recognition? Speech recognition and psychophysical data were available for only 7 of the 13 patients—the native English speaking patients (see Table 1). The European clinical trial of EAS did not require a evaluation of postoperative acoustic only nor of ipsilateral EAS speech perception for the implanted ear. Thus, these data were not available for the six Polish speaking 20 mm EAS subjects. Table 1 displays CNC monosyllabic word recognition data for the seven 10 mm English speaking subjects for the pre- and postimplant acoustic only (A) conditions (for the implanted ear), postimplant electric only (E), and postimplant ipsilateral EAS (A+E). For the most part, the 10 mm subjects demonstrated considerable preservation of acoustic only speech perception in the implanted ear following surgery. Only one subject (10̱5) demonstrated a significant decrement in acoustic only word recognition postoperatively using a binomial distribution statistic for 50-item word lists (Thornton and Raffin, 1978). Subject 10̱5, however, was the only subject with a near complete loss of hearing.
Table 1.
Individual and mean CNC word recognition scores, in percent correct, for the acoustic only (A) both pre- and postimplant, electric only (E), and ipsilateral electric (E) plus acoustic (A) conditions.
| Subjects | A only implanted ear preimplant | A only implanted ear postimplant | E only | Ipsilateral E+A |
|---|---|---|---|---|
| 10̱1 | 16 | 10 | 70 | 66 |
| 10̱2 | 38 | 44 | 76 | 78 |
| 10̱3 | 20 | 20 | 32 | 38 |
| 10̱4 | 16 | 16 | 6 | 30 |
| 10̱5 | 8 | 0 | 48 | 58 |
| 10̱6 | 44 | 44 | 54 | 76 |
| 10̱7 | 40 | 38 | 0 | 52 |
| Mean (Standard deviation) | 26.0 (14.3) | 24.6 (17.5) | 40.9 (29.6) | 56.9 (18.3) |
For the seven English speaking 10 mm subjects, there was a significant correlation between the magnitude of the threshold elevation and the postoperative acoustic-only speech perception. The greater the elevation, the poorer the understanding (r=−0.82, p=0.02). This is not surprising. There was no evidence that the Schroeder data provided predictive information about speech understanding that was not supplied by audiometric thresholds. However, a much larger data set is needed to assess this rigorously. The ease of the Schroeder masking task and the need for just two to four thresholds per frequency provides motivation to use this measure on a much larger sample of hearing preservation patients.
SUMMARY
The average changes in threshold up to 750 Hz following surgery were 16.4 and 14.4 dB for the 10 and 20 mm insertions, respectively. The psychophysical estimate of nonlinear cochlear function, i.e., Schroeder phase effect, was more sensitive to the effects of surgery than audiometric thresholds. The most common outcome of surgery was elevation of audiometric thresholds and decreases or elimination of nonlinear cochlear function. It is possible, but not common, to preserve a normal auditory function at 250 Hz following electrode insertion. Preservation of some residual nonlinear cochlear functions was common for both the 10 and 20 mm subjects, more so at 250 Hz. If claims of “better” surgical techniques for hearing preservation are made in the future, then Schroeder phase masking patterns could be used to evaluate those claims.
ACKNOWLEDGMENTS
This work was supported by NIDCD Grant No. DC006538 to R.H.G. and by NIDCD Grant No. RO1 DC00654-15 to M.F.D. A portion of the results were presented at the 2006 International Conference on Cochlear Implants and Other Implantable Auditory Technologies, in Vienna, Austria, the 2005 Hearing Preservation Workshop in Warsaw, Poland, and the 2006 Hearing Preservation Workshop in Raleigh, NC. The authors would like to thank Dr. Chris Brown at Arizona State University for his assistance with programing as well as Sharon McKarns, Andrea Kosko, and Arkadiusz Wasowski for their help with data collection. This study was conducted in strict accordance with approved Arizona State University and International Center of Hearing and Speech IRB protocols.
References
- Bacon, S. P., and Takahashi, G. (1992). “Overshoot in normal-hearing and hearing-impaired subjects,” J. Acoust. Soc. Am. 10.1121/1.402967 91, 2865–2871. [DOI] [PubMed] [Google Scholar]
- Balkany, T., Hodges, A., Menapace, C., Hazard, L., Driscoll, C., Gantz, B., Kelsall, D., Luxford, W., McMenomy, S., Neely, G., Peters, B., Pillsbury, H., Roberson, J., Schramm, D., Telian, S., Waltzman, S., Westerberg, B., and Payne, S. (2007). “Nucleus freedom north american clinical trial,” Otolaryngol.-Head Neck Surg. 136, 757–762. [DOI] [PubMed] [Google Scholar]
- Baumgartner, W. D., Jappel, A., Morera, C., Gstoettner, W., Muller, J., Kiefer, J., Van De Heyning, P., Anderson, I., and Nielson, S. B. (2007). “Outcomes in adults implanted with the FLEXsoft electrode,” Acta Oto-Laryngol. 127, 579–586. [DOI] [PubMed] [Google Scholar]
- Brill, S., Lawson, D. T., Wolford, R. D., and Schatzer, R. (2002). “Speech processors for auditory prostheses.” 11th quarterly progress report on NIH Project N01—DC-8–2105.
- Carlyon, R. P., and Datta, J. (1997). “Excitation produced by Schroeder-phase complexes: Evidence for fast-acting compression in the auditory system,” J. Acoust. Soc. Am. 10.1121/1.418324 101, 3636–3647. [DOI] [PubMed] [Google Scholar]
- Chatterjee, M., and Zwislocki, J. J. (1997). “Cochlear mechanisms of frequency and intensity coding. I. The place code for pitch,” Hear. Res. 10.1016/S0378-5955(97)00089-0 111, 65–75. [DOI] [PubMed] [Google Scholar]
- Davis, H. (1983). “An active process in a cochlear mechanics,” Hear. Res. 10.1016/0378-5955(95)00172-7 9, 79-90. [DOI] [PubMed] [Google Scholar]
- Gantz, B. J., and Turner, C. W. (2003). “Combining acoustic and electrical hearing,” Laryngoscope 10.1097/00005537-200310000-00012 113, 1726–1730. [DOI] [PubMed] [Google Scholar]
- Gantz, B. J., and Turner, C. W. (2004). “Combining acoustic and electrical speech processing: Iowa∕Nucleus Hybrid implant,” Acta Oto-Laryngol. 124, 334–347. [DOI] [PubMed] [Google Scholar]
- Gantz, B. J., Turner, C. W., and Gfeller, K. E. (2006). “Acoustic plus electric speech processing: Preliminary results of a multicenter clinical trial of the Iowa∕Nucleus Hybrid implant,” Audiol. Neuro-Otol. 10.1159/000095616 11, 63–68. [DOI] [PubMed] [Google Scholar]
- Gantz, B. J., Turner, C. W., Gfeller, K. E., and Lowder, M. (2005). “Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing,” Laryngoscope 10.1097/01.MLG.0000157695.07536.D2 115, 796–802. [DOI] [PubMed] [Google Scholar]
- Gifford, R. H., Dorman, M. F., Spahr, A. J., and Bacon, S. P. (2007). “Auditory function and speech understanding in listeners who qualify for EAS surgery,” Ear Hear. 28, 114S–118S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H., Shallop, J. K., and Peterson, A. M. (2008). “Speech recognition materials and ceiling effects: Considerations for cochlear implant programs,” Audiol. Neuro-Otol. 13, 193–205. [DOI] [PubMed] [Google Scholar]
- Gstoettner, W. K., Helbig, S., Maier, N., Kiefer, J., Radeloff, A., and Adunka, O. (2006). “Ipsilateral electric acoustic stimulation of the auditory system: Results of long-term hearing preservation,” Audiol. Neuro-Otol. 11, 49–56. [DOI] [PubMed] [Google Scholar]
- Gstoettner, W., Kiefer, J., Baumgartner, W. D., Pok, S., Peters, S., and Adunka, O. (2004). “Hearing preservation in cochlear implantation for electric acoustic stimulation,” Acta Oto-Laryngol. 124, 348–352. [DOI] [PubMed] [Google Scholar]
- Gstoettner, W., Pok, S. M., Peters, S., Kiefer, J., and Adunka, O. (2005). “Cochlear implantation with preservation of residual deep frequency hearing,” HNO 53, 784–791. [DOI] [PubMed] [Google Scholar]
- Kiefer, J., Pok, M., Adunka, O., Stuerzebecher, E., Baumgartner, W. D., and Schmidt, M. (2005). “Combined electric and acoustic stimulation of the auditory system: Results of a clinical study,” Audiol. Neuro-Otol. 10.1159/000084023 10, 134–144. [DOI] [PubMed] [Google Scholar]
- Kohlrausch, A., and Sander, A. (1995). “Phase effects in masking related to dispersion in the inner ear. II. Masking period patterns of short targets,” J. Acoust. Soc. Am. 10.1121/1.413097 97, 1817–1829. [DOI] [PubMed] [Google Scholar]
- Laroche, C., Quoc, H. T., Josserand, B., and Glasberg, B. (1992). “Frequency selectivity in workers with noise-induced hearing loss,” Hear. Res. 10.1016/0378-5955(92)90168-M 64, 61–72. [DOI] [PubMed] [Google Scholar]
- Lentz, J. J., and Leek, M. R. (2001). “Psychophysical estimates of cochlear phase response: Masking by harmonic complexes,” J. Assoc. Res. Otolaryngol. 2, 408–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 10.1121/1.1912375 49, 467–477. [DOI] [PubMed] [Google Scholar]
- Luetje, C. M., Thedinger, B. S., Buckler, L. R., Dawson, K. L., and Lisbona, K. L. (2007). “Hybrid cochlear implantation: Clinical results and critical review of 13 cases,” Otol. Neurotol. 28, 473–478. [DOI] [PubMed] [Google Scholar]
- McFadden, D., and Chamlin, C. A. (1990). “Reductions in overshoot during aspirin use,” J. Acoust. Soc. Am. 10.1121/1.399056 87, 2634–2642. [DOI] [PubMed] [Google Scholar]
- Neely, S. T., and Kim, D. O. (1986). “A model for active elements in cochlear biomechanics,” J. Acoust. Soc. Am. 10.1121/1.393674 79, 1472–1780. [DOI] [PubMed] [Google Scholar]
- Oxenham, A. O., and Bacon, S. P. (2004). “Psychophysical manifestations of compression: Normal-hearing listeners,” In Compression from Cochlea to Cochlear Implants, edited by Bacon S. P., Fay R. R., and Popper A. N. (Springer-Verlag, New York: ). [Google Scholar]
- Oxenham, A. O., and Dau, T. (2001). “Reconciling frequency selectivity and phase effects in masking,” J. Acoust. Soc. Am. 10.1121/1.1394740 110, 1525–1538. [DOI] [PubMed] [Google Scholar]
- Oxenham, A. O., and Dau, T. (2004). “Masker phase effects in normal-hearing and hearing-impaired listeners: Evidence for peripheral compression at low signal frequencies,” J. Acoust. Soc. Am. 10.1121/1.1786852 116, 2248–2257. [DOI] [PubMed] [Google Scholar]
- Peterson, G. E., and Lehiste, I. (1962). “Revised CNC lists for auditory tests,” J. Speech Hear Disord. 27, 62–70. [DOI] [PubMed] [Google Scholar]
- Recio, A., and Rhode, W. S. (2000). “Basilar membrane responses to broadband stimuli,” J. Acoust. Soc. Am. 10.1121/1.1318898 108, 2281–2298. [DOI] [PubMed] [Google Scholar]
- Rhode, W. S., and Recio, A. (2000). “Study of mechanical motions in the basal region of the chinchilla cochlea,” J. Acoust. Soc. Am. 10.1121/1.429404 107, 3317–3332. [DOI] [PubMed] [Google Scholar]
- Schroeder, M. R. (1971). “Synthesis of low peak-factor signal and binary sequences with low autocorrelation,” IEEE Trans. Inf. Theory 10.1109/TIT.1970.1054411 16, 85–89. [DOI] [Google Scholar]
- Skarzynski, H., Lorens, A., and Piotrowska, A. (2003). “A new method of partial deafness treatment,” Med. Sci. Monit. , 9, CS20–24. [PubMed] [Google Scholar]
- Skarzynski, H., Lorens, A., and Piotrowska, A. (2004). “Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach,” Acta Oto-Laryngol. 127, 41–48. [DOI] [PubMed] [Google Scholar]
- Skarzynski, H., Lorens, A., Piotrowska, A., and Anderson, I. (2006). “Partial deafness cochlear implantation provides benefit to a new population of individuals with hearing loss,” Acta Oto-Laryngol. 126, 934–940. [DOI] [PubMed] [Google Scholar]
- Skarzynski, H., Lorens, A., Piotrowska, A., and Anderson, I. (2007). “Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach,” Acta Oto-Laryngol. 127, 41–48. [DOI] [PubMed] [Google Scholar]
- Stuart, A., Stenstrom, R., Tompkins, C., and Vandenhoff, S. (1991). “Test-retest variability in audiometric threshold with supraaural and insert earphones among children and adults,” Audiology 30, 82–90. [DOI] [PubMed] [Google Scholar]
- Summers, V. (2000). “Effects of hearing impairment and presentation level on masking period patterns for Schroeder-phase harmonic complexes,” J. Acoust. Soc. Am. 10.1121/1.1318897 108, 2307–2317. [DOI] [PubMed] [Google Scholar]
- Summers, V. (2001). “Overshoot effects using Schroeder-phase harmonic maskers in listeners with normal hearing and with hearing impairment,” Hear. Res. 162, 1–9. [DOI] [PubMed] [Google Scholar]
- Summers, V., and Leek, M. R. (1998). “Masking of tones by Schroeder-phase harmonic complexes in normal hearing and hearing-impaired listeners,” Hear. Res. 10.1016/S0378-5955(98)00030-6 118, 139–150. [DOI] [PubMed] [Google Scholar]
- von Ilbert, C., Kiefer, J., Tillein, J., Pfennigdorff, T., Hartmann, R., Stuerzebecher, E., and Klinke, R. (1999). “Electric-acoustic stimulation of the auditory system,” ORL 10.1159/000027695 61, 334–340. [DOI] [PubMed] [Google Scholar]
- Wilson, B., Wolford, R., Lawson, D., and Schatzer, R. (2002). “Speech processors for auditory prostheses,” Third quarter progress report on NIH Project No. N01-DC-2—1002.