Abstract
Breast density, the percentage of glandular breast tissue, has been identified as an important yet underutilized risk factor in the development of breast cancer. A quantitative method to measure breast density with dual energy imaging was investigated using a computer simulation model. Two configurations to measure breast density were evaluated: the usage of monoenergetic beams and an ideal detector, and the usage of polyenergetic beams with spectra from a tungsten anode x-ray tube with a detector modeled after a digital mammography system. The simulation model calculated the mean glandular dose necessary to quantify the variability of breast density to within 1∕3%. The breast was modeled as a semicircle 10 cm in radius with equal homogenous thicknesses of adipose and glandular tissues. Breast thicknesses were considered in the range of 2–10 cm and energies in the range of 10–150 keV for the two monoenergetic beams, and 20–150 kVp for spectra with a tungsten anode x-ray tube. For a 4.2 cm breast thickness, the required mean glandular doses were 0.183 μGy for two monoenergetic beams at 19 and 71 keV, and 9.85 μGy for two polyenergetic spectra from a tungsten anode at 32 and 96 kVp with beam filtrations of 50 μm Rh and 300 μm Cu for the low and high energy beams, respectively. The results suggest that for either configuration, breast density can be precisely measured with dual energy imaging requiring only a small amount of additional dose to the breast. The possibility of using a standard screening mammogram as the low energy image is also discussed.
Keywords: breast density, dual energy, mammography
INTRODUCTION
According to the WHO, as of 2005, breast cancer is the fifth most common cause of cancer death, responsible for over half a million deaths per year worldwide and the leading cause of all cancer deaths in women.1 Breast density, the percentage of glandular breast tissue, has been shown to be a strong indicator of breast cancer risk.2, 3, 4, 5, 6, 7, 8, 9, 10 The positive association between breast density and subsequent breast cancer risk originally reported by Wolfe used a qualitative classification scheme where images of the breast were visually assessed and assigned to one of four empirically derived categories: N1–primarily fat, P1≤25% prominent ducts, P2≥25% prominent ducts, and DY dense fibroglandular tissue. He reported a progressive stepwise increase in future breast cancer risk: N1–lowest risk, P1–low risk, P2–high risk, and DY–highest risk.2, 3
Since the Wolfe report, a variety of approaches for measuring breast density, both areal and volumetric, have subsequently been described. Areal-based techniques have included qualitative and quantitative classification schemes,2, 11, 12 and also quantitative numerical estimations derived from manual and automatic segmentation of a digital image histogram and pixel values.11, 13, 14, 15, 16, 17, 18, 19 Although most of these quantitative measures provide a continuous measure of breast density, a notable limitation is the binary classification of a pixel into either 100% fibroglandular or 100% adipose tissue. Additionally, an areal measurement ignores the physical 3D character of a real breast. Breasts of different thicknesses can potentially all yield the same measurement of areal breast density yet correspond to widely varying volumetric breast density values. Volume-based techniques are able to overcome these limitations. These techniques have included attempts to standardize20, 21 and calibrate22, 23, 24 mammographic image data. However, these techniques require certain assumptions to be made in order to measure two independent quantities (e.g., breast density, and thickness) from a single image. A common limitation of such techniques is the assumption of uniform breast thickness.
Dual energy imaging can also be used to quantify breast density and has the advantage of measuring thicknesses of adipose and glandular tissues separately. While the female breast is composed largely of glandular and adipose tissue, dual energy imaging in mammography25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 has primarily focused on the imaging of a third material such as calcium,25, 26, 28, 32, 33, 34, 37, 40, 41 iodine,29, 30, 35, 42 or neoplastic breast tissue.27, 38, 39, 43 In the above cases, information about the two primary tissues is often ignored, either by eliminating their contrast,26, 27, 38, 39, 43 considering them as a single tissue,33 or shifting focus to a third material.25, 31, 44 There are a small number of reports on the use of dual energy imaging to measure breast density. Early simulation work by Breitenstein and Shaw36 predicted dual energy SNR tissue composition values at a fixed level of exposure for selected dual kVp and single kVp techniques with spectra from a molybdenum target x-ray tube. Dual energy x-ray absorptiometry (DXA) has previously been applied to the measurement of breast density.6 However, the clinical application of this technique was hampered by the beam spectra of the DXA system, which are not optimal for measuring breast density. Dual energy mammography is not currently being used for breast density measurement.
The purpose of this simulation study is to investigate the use of dual energy imaging for the quantification of breast density. Two cases were evaluated. The first case considered the use of monoenergetic beams and an ideal photon counting detector, designed to predict the peak performance possible when using dual energy imaging to quantify breast density. The second modeled x-ray spectra from an investigational mammography system with an energy integrating detector, a tungsten target, and rhodium and copper beam filters for the low and high energy images. The two techniques were studied and the effects of varying beam energies, dose ratios, and prepatient filtration on breast density are presented for a range of breast thicknesses.
THEORY
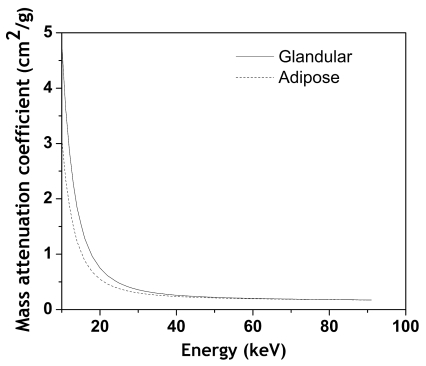
The basis for dual energy imaging is the differential attenuation of glandular and adipose tissues as a function of energy. Mass attenuation curves for the two tissues are shown in Fig. 1. The figure shows that in order to maximize the differential attenuation, the mean energy of the low energy beam needs to be as low as possible and the mean energy of the high energy beam needs to be as high as possible. An analytical computer simulation model modeling x-ray photon transport was developed to investigate the technique feasibility over the range of expected breast densities and thicknesses encountered in clinical mammography.
Figure 1.
Plot showing the energy dependence of x-ray attenuation for glandular and adipose tissue.
Calculation of breast density
The low and high energy detector signals are functions of glandular and adipose tissue thicknesses:
| (1) |
The tissue thicknesses can be determined by inverting the system of equations:
| (2) |
Each pixel provides a measurement of each tissue thickness. The breast density d for a pixel can be expressed
| (3) |
The total breast density, D, is then defined as the average over all pixel measurements within the breast shadow (N):
| (4) |
Breast density measurement uncertainty
The measurement uncertainty in breast density for the whole breast (D) can be written using propagation of error:
| (5) |
After substituting in expressions for the partial derivatives, the uncertainty can be written as
| (6) |
Expressions for , , and σtgta are functions of the detector signals Sl and Sh. Their derivation follows from a similar analysis, presented in the Appendix, and can be written as
| (7) |
| (8) |
| (9) |
where is the squared signal to noise ratio of the detector signal Si and is the mean attenuation of material j for detector signal i. Combining all of the above terms, the breast density measurement uncertainty can be written in final form as
| (10) |
Given a known breast density, D, it is possible to express the percent relative standard deviation (%RSD) as
| (11) |
MATERIALS AND METHODS
Simulation input functions and parameters
Detector signals
The detector signal S is given by
| (12) |
where f is the aperture size. The square of the aperture is the effective measurement area and was set at 0.0049 mm2 to correspond with an aperture size of 70 μm, which is the same as one aperture used in digital mammography (Selenia, Hologic Inc., Bedford, MA). The fill factor was set to be unity.
N0(E) is the incident x-ray spectrum (photon fluence per energy). For the case of two monoenergetic beams, beam spectra were simulated from 10 to 150 keV.
For the case of two polyenergetic beams, the XCOMP5R code of Nowotny and Hoofer45 was used to simulate x-ray photons generated from a tungsten anode x-ray tube. A typical effective anode angle in mammography is 24 deg.46 This effective angle was also used as the inherent anode angle for simulations. A comparison of the XCOMP5R, at an anode angle of 24 deg, and TASMIP (Ref. 47) spectral models showed only a small difference (<3%) in incident mean beam energy. Typically, however, the inherent anode angle is between 0 and 16 deg with the remaining angulation provided by titling the x-ray tube. The x-ray tube filtration was set at 1 mm Be. Beam spectra were simulated from 20 to 150 kVp.
The term e−(μf(E)tf) corresponds to the prepatient filtration. Spectra from the tungsten anode x-ray tube were filtered by 50 μm rhodium for the low energy image and 300 μm copper for the high energy image. The filter in the low energy image corresponds to what would be expected in a screening mammogram. The purpose of a high energy filter is to increase the mean beam energy. Numerous options spanning the periodic table are available yielding similar increases. The best choice is a compromise between increasing beam energy and minimizing tube loading. The selection of a 300 μm copper filter provides excellent beam filtration and does not increase the resultant tube loading to prohibitively high levels.
μj(Ei) is the attenuation coefficient of material j at energy E. Elemental attenuation coefficients were obtained from a previous report by Boone.48 Values spanned the full periodic table and energies extended below and above the range necessary for applications in medical imaging. The chemical compositions of glandular and adipose tissues were from the data of Hammerstein.49 Combined, the data defined the functions μg(E) and μa(E).
tj is the corresponding tissue thickness. Breast thicknesses were considered in a range of 2–10 cm.
W(E) is an energy weighting factor used to describe the detector type, and Q(E) is the quantum detection efficiency of the detector. An ideal photon counting detector was simulated in the first case and, for this case, W(E) and Q(E) were both set to 1. The second case simulated the detection properties of the amorphous selenium photoconductor in a digital mammography system. W(E) in this case was set to E and Q(E) was calculated for a 200 μm selenium absorber (mass thickness of 85.6 mg∕cm2).
G(E) is the absorption due to the presence of the cellular x-ray scatter grid. The transmission of the grid was measured at several energies and the data were empirically fit and extrapolated for all remaining higher energies. The grid has 30 μm copper septa with 640 μm air interspaces.50, 51
Noise sources
Noise was considered similar to other cascaded system models52 but due to the size of the whole breast, frequency dependent effects were ignored and the propagation of noise was considered in the spatial domain. When considering the ideal detector, only quantum x-ray noise was included. When considering the detector in the digital mammography system, variances of quantum x-ray noise, gain (i.e., Swank) noise, digitization error, additive electronics noise, and the additional noise imparted due to x-ray scatter were included:
| (13) |
where is the quantum x-ray noise. The quantum noise was calculated from the mean number of photons per unit area per energy. After transmission through material and absorption in the detector, the noise is expressed as
| (14) |
The gain noise, , was modeled with an energy dependent Swank factor with data from previous work on amorphous selenium.53 The gain noise is itself proportional to the quantum noise and not a completely independent term.
The digitization error, , was estimated given the mean entrance exposure necessary to generate one gray level. The additive electronics noise, , was determined by a logarithmic fit of noise as a function of signal. The offset of the fit corresponded to the additive electronics noise in pixel counts. These last two values were determined, in part, with data provided by the manufacturer of our digital mammography system.54
The noise due to x-ray scatter, , was estimated from measurements of scatter-to-primary ratio (SPR) with the digital mammography system using lead beam stops at BR12 phantom thicknesses of 0.5, 1, 2, 4, 6, and 8 cm at three different energies. The data were empirically fit and extrapolated for all higher energies. The added noise variance due to x-ray scatter was equal to the calculated SPR.
Mean glandular dose
Values for the mean glandular dose (MGD) per photon were calculated with Monte Carlo simulations.55, 56 Dose data were available for breast thicknesses of 2–10 cm, densities of 0%, 50%, and 100%, and energies of 5–150 keV.
THE OPTIMIZATION PROBLEM
The breast was modeled as a semicircle 10 cm in radius and composition was set to 50% glandular and 50% adipose tissue by volume (homogenous equal thicknesses of adipose and glandular tissues), consistent with the FDA definition of the standard breast.46 A thickness of 4.2 cm was selected to be representative of the set. The size of the breast is inversely related to the total required dose due to statistical pixel averaging and occupies an area equivalent to 3 205 704 pixels (at a pixel pitch of 70 μm). We require the precision of greater than 99% of all breast density measurements to fall within 1%. Thus, for a normal distribution, a particular simulation was considered a success if the %RSD for the whole breast was less than 1∕3%. For a given beam pair and breast thickness, the %RSD was calculated at a particular dose and dose ratio (i.e., the relative allocation of available dose for the low and high energy images). The dose ratio was varied from 0 to 1 with a step size of 0.1. If no ratio passed the tested %RSD criteria, the particular dose was incremented and the process repeated until success. In addition, to test the effect of glandularity on the required dose, compositions of 10% and 90% glandularity were also simulated at 4.2 cm for the tungsten anode beam. To test the effect of heterogeneity, two samples, each 1000 points in length, were compared. The first had a heterogeneous distribution, uniformly distributed, of glandularities evenly spaced from 0% to 100% with an effective mean glandularity of 50%. The second was a completely homogenous distribution, with the glandularity fixed at 50%. The mean variances were compared for the two distributions at an equal fixed mean glandular dose for the tungsten anode beam.
RESULTS
Two monoenergetic beams
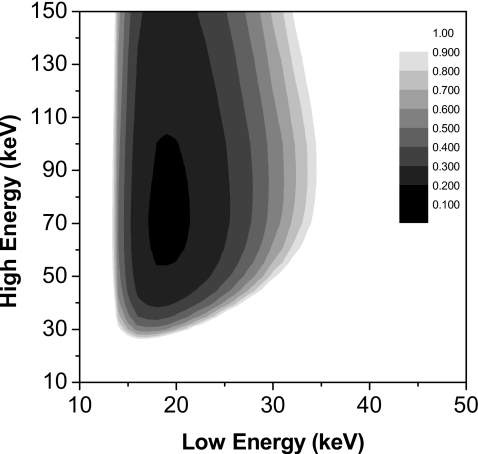
Shown in Fig. 2 is a dose contour plot for the two monoenergetic beams at a breast thickness of 4.2 cm. The minimal required MGD of 0.183 μGy was obtained at low and high beam energies of 19 and 71 keV. The optimal relative dose allocation between the low and high energy images was 0.6:0.4.
Figure 2.
Dose contour plot expressing the required MGD as a function of the two monoenergetic beams and a 4.2 cm breast. Minimal MGD was 0.183 μGy at 19 and 71 keV.
Tungsten anode spectra
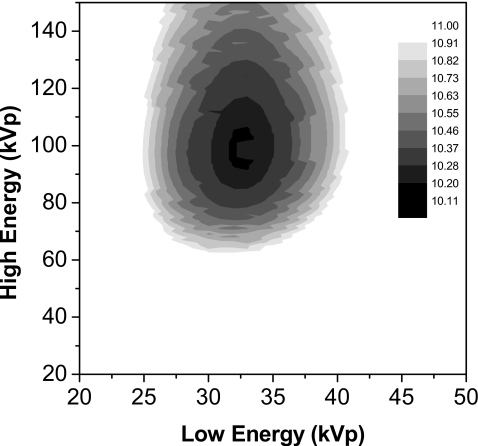
The dose contour plot for the dual energy case using x-ray beam spectra from a tungsten anode for a 4.2 cm breast is shown in Fig. 3. The minimal required MGD was 9.85 μGy and obtained at beam spectra of 32 and 96 kVp. The optimal dose allocation ratio was 0.7:0.3.
Figure 3.
Dose contour plot expressing the required MGD as a function of the two polyenergetic tungsten anode beam spectra and a 4.2 cm breast. Minimal MGD was 9.85 μGy at 32 and 96 kVp.
Additionally, the minimal required MGD for glandularities of 10% and 90% were 39.90 and 7.52 μGy, respectively. The relative increase in the mean variance for the heterogeneous data set relative to the homogenous data set was 6.6%.
The effect of dose allocation
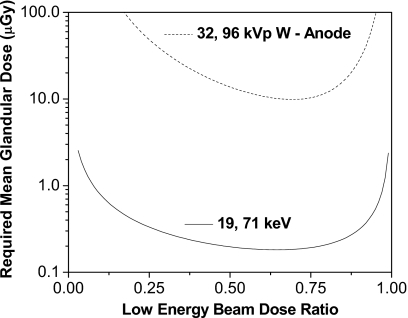
The effect of varying the dose allocation ratio is seen in Fig. 4, where the required dose is shown as a function of the low energy dose ratio for the two configurations: at 19 and 71 keV and at 32 and 96 kVp beam pair for a 4.2 cm breast.
Figure 4.
Required MGD as a function of the relative low energy dose for the two configurations and a 4.2 cm breast. Energies were 19 and 71 keV, and 32 and 96 kVp.
Summary of results
Optimal beam energies, dose ratios, and required MGD data for each technique and all thicknesses are shown in Table 1.
Table 1.
Minimal dose values, dose allocation ratios and energies for each technique, and thickness.
| Breast thickness (cm) | Beam configuration | |||||
|---|---|---|---|---|---|---|
| Monoenergetic beams | Polyenergetic beams (W anode) | |||||
| Beam energy (keV) (low, high) | Dose ratio (low:high) | MGD (μGy) | Beam spectra (kVp) (low, high) | Dose ratio (low:high) | MGD (μGy) | |
| 2 | 16, 70 | 0.6:0.4 | 0.301 | 29, 87 | 0.6:0.4 | 10.39 |
| 3 | 17, 70 | 0.6:0.4 | 0.218 | 29, 86 | 0.7:0.3 | 9.33 |
| 4 | 19, 71 | 0.6:0.4 | 0.186 | 31, 94 | 0.7:0.3 | 9.59 |
| 5 | 20, 74 | 0.6:0.4 | 0.172 | 33, 102 | 0.7:0.3 | 10.36 |
| 6 | 21, 74 | 0.7:0.3 | 0.167 | 35, 110 | 0.7:0.3 | 12.10 |
| 7 | 22, 79 | 0.7:0.3 | 0.167 | 37, 116 | 0.7:0.3 | 13.64 |
| 8 | 23, 79 | 0.7:0.3 | 0.172 | 37, 103 | 0.7:0.3 | 16.12 |
| 9 | 24, 84 | 0.7:0.3 | 0.179 | 37, 104 | 0.7:0.3 | 19.48 |
| 10 | 25, 85 | 0.7:0.3 | 0.189 | 41, 137 | 0.7:0.3 | 22.66 |
DISCUSSIONS
The major limitation of dual energy application for microcalcification detection has been the low signal to noise ratio of the desired signal in the subtracted image.57 This is in part due to the small area occupied by the object of interest. On the other hand, this is not the case for breast density quantification as the whole breast is used for measurement. This suggests that dual energy imaging is well suited to quantify breast density with respect to mean glandular dose.
Of the cases considered, as expected, the use of two monoenergetic beams showed the lowest dose requirement. The simulation of monoenergetic beams also provides physical insight and sets upper limits on the theoretical peak performance. In Fig. 2, for the 4.2 cm breast thickness, the ideal low energy beam was centered at 19 keV, coinciding with the first simulation study of dual energy mammography for imaging calcium by Johns and Yaffe25 while the ideal high energy of 71 keV was slightly higher than the previously reported 68 keV. However, as the imaging tasks were different, it was not expected that the energies would be the same. The result for the ideal low beam energy is convenient as it is near the mean energy of most clinical mammographic spectra and suggests that a dual energy measurement of breast density can make use of an existing mammogram as the low energy image. This is further substantiated by the fact that the optimal low energy beam for tungsten anode is 32 kVp, which is within the clinical range. The tungsten anode beam spectra, however, did require substantially more dose than the monoenergetic beams. It is clear, however, that regardless of the technique, the overall predicted required dose is relatively low when compared to the dose of 1.6 mGy for a standard screening mammogram.58 As an example, consider the case of spectra from the tungsten anode and a 4.2 cm breast thickness. The required dose is only 9.85 μGy, which is a small fraction of the dose from a standard screening mammogram. Furthermore, it is possible to use the standard screening mammogram itself as the low energy image. In this case, the relative dose allocation between the low and high energy image would not be optimal as the dose in the low energy image would far exceed what is required. However, this is not a hindrance as the required additional dose from the high energy image would remain low. The added dose for the tungsten spectra would be 2.96 μGy. Currently, the optimal kVp predictions are outside the range of current clinical mammography systems. However, the x-ray tube simulated in this study is based on a tungsten anode. Standard tungsten anode x-ray tubes are capable of imaging at high kVps and it is not difficult to conceive that a tungsten tube designed for mammography could be modified for usage at higher kVps. Alternatively, it is still possible to measure breast density if the system is limited to a certain maximum kVp, which will increase the required dose. Our digital mammography system is limited to maximum beam energy of 49 kVp. In this case the added dose from the high energy image would increase to 3.93 μGy for a 4.2 cm breast. In either case, the expected added dose from the additional high energy image is less than 1% of the dose for one view in the case of standard mammography (1.6 mGy).
The first breast DXA reported that the technique was highly repeatable. The mean energies for the 100 and 140 kVp broad beam spectra used in the DXA system were estimated to be approximately 52 and 64 keV, respectively.59 The 100 kVp low energy beam is far above the optimal beam energy predicted in this study for quantifying breast density.
The study indicated that the clinical value of the DXA images was limited for quantifying breast tissue densities and choosing alternative (i.e., lower) beam energies might improve the tissue selectivity of the technique. As seen from Eqs. 7, 8, 9, 10 above, the noise variance in dual energy imaging is a strong function of the two materials’ combined attenuation coefficient separation and a reduction in dose for a given SNR is, in general, possible by increasing the spectral separation between the two beams.60 At energies of 52 and 64 keV, the combined differential attenuation was relatively low.
An important source of error in quantification of breast density is x-ray scatter. It causes both random and systematic error in breast density measurements. The random error, which is caused by the additive stochastic noise due to x-ray scatter, has been included in the noise analysis. However, the systematic error, which is caused by the offset to the pixel signal due to x-ray scatter, has not been included in this simulation. Previous simulation studies involving dose requirements in dual energy imaging have also not included x-ray scatter in the simulation.25, 31, 36 Therefore, scatter correction in the low and high energy images is necessary for accurate breast density measurement. There are a number of previously reported scatter correction techniques that can be used for this purpose.61, 62, 63 Future experimental implementation of the technique will address the systematic error caused by x-ray scatter. The current study has focused on the minimum required patient dose for reliable breast density measurement assuming that the systematic error due to x-ray scatter can be corrected.
For the model used in this study, the effect of heterogeneity was determined to yield a relatively small increase in the average variance, and hence required mean glandular dose. This increase is likely due to reduced x-ray transmission and a loss of quantum statistics in regions of high breast density. It will be useful to include further simulations of different mean glandularities and heterogeneous breast tissue distributions in future studies.
The data presented here suggest that a new technique can be developed to reliably measure breast density. The technique can optionally make use of a standard screening mammogram in conjunction with a second high energy image exposure. In either case, a relatively small amount of dose would be imparted to the patient in comparison to a standard screening mammogram. A recent report64 has called into question the validity of any areal measure of breast density and a technique like the one presented here is able to incorporate the needed three-dimensional information when determining breast density.
ACKNOWLEDGMENTS
The authors would like to thank Dr. John Boone for providing us with dose tables. This research was supported in part by Grant No. R01 EB000514 awarded by the NBIB, DHHS.
APPENDIX: DUAL ENERGY NOISE PROPAGATION
Using propagation of errors, the variance of function in Eq. 6 can be written as
| (A1) |
| (A2) |
| (A3) |
| (A4) |
| (A5) |
| (A6) |
where J2, the squared Jacobian determinant, is equal to
| (A7) |
The partial derivatives are calculated according to the following with an added step of defining the final quantity as the product of the mean attenuation and detector signal from Eq. 12:
| (A8) |
J2 can then be written as
| (A9) |
Combining the above terms and rewriting expressions with the form as , , , and σtatf reduce to
| (A10) |
| (A11) |
| (A12) |
Details here are consistent with previous derivations that have been presented elsewhere in the literature.60, 65, 66, 67, 68, 69, 70
References
- World Heatlh Organization Fact Sheet N297: Cancer (2006).
- Wolfe J. N., “Breast patterns as an index of risk for developing breast cancer,” AJR, Am. J. Roentgenol. 126(6), 1130–1137 (1976). [DOI] [PubMed] [Google Scholar]
- Sickles E. A., “Wolfe mammographic parenchymal patterns and breast cancer risk,” AJR, Am. J. Roentgenol. 188(2), 301–303 (2007). [DOI] [PubMed] [Google Scholar]
- Kerlikowske K., “The mammogram that cried Wolfe,” N. Engl. J. Med. 356(3), 297–300 (2007). [DOI] [PubMed] [Google Scholar]
- Boyd N. F., Guo H., Martin L. J., Sun L., Stone J., Fishell E., Jong R. A., Hislop G., Chiarelli A., Minkin S., and Yaffe M. J., “Mammographic density and the risk and detection of breast cancer,” N. Engl. J. Med. 10.1056/NEJMoa062790 356(3), 227–236 (2007). [DOI] [PubMed] [Google Scholar]
- Shepherd J. A., Kerlikowske K. M., Smith-Bindman R., Genant H. K., and Cummings S. R., “Measurement of breast density with dual X-ray absorptiometry: feasibility,” Radiology 10.1148/radiol.2232010482 223(2), 554–557 (2002). [DOI] [PubMed] [Google Scholar]
- Byrne C., “Studying mammographic density: implications for understanding breast cancer,” J. Natl. Cancer Inst. 10.1093/jnci/89.8.531 89(8), 531–533 (1997). [DOI] [PubMed] [Google Scholar]
- Boyd N. F., Byng J. W., Jong R. A., Fishell E. K., Little L. E., Miller A. B., Lockwood G. A., Tritchler D. L., and Yaffe M. J., “Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study,” J. Natl. Cancer Inst. 10.1093/jnci/87.9.670 87(9), 670–675 (1995). [DOI] [PubMed] [Google Scholar]
- Wolfe J. N., Saftlas A. F., and Salane M., “Mammographic parenchymal patterns and quantitative-evaluation of mammographic densities—A case-control study,” AJR, Am. J. Roentgenol. 148(6), 1087–1092 (1987). [DOI] [PubMed] [Google Scholar]
- Shepherd J. A., Herve L., Landau J., Fan B., Kerlikowske K., and Cummings S. R., “Clinical comparison of a novel breast DXA technique to mammographic density,” Med. Phys. 10.1118/1.2193691 33(5), 1490–1498 (2006). [DOI] [PubMed] [Google Scholar]
- Byng J. W., Boyd N. F., Fishell E., Jong R. A., and Yaffe M. J., “The quantitative-analysis of mammographic densities,” Phys. Med. Biol. 10.1088/0031-9155/39/10/008 39(10), 1629–1638 (1994). [DOI] [PubMed] [Google Scholar]
- American College of Radiology, ACR Breast Imaging Reporting and Data System (BI-RADS), 4th ed. (American College of Radiology, Reston, VA, 2003). [Google Scholar]
- Byng J. W., Boyd N. F., Fishell E., Jong R. A., and Yaffe M. J., “Automated analysis of mammographic densities,” Phys. Med. Biol. 10.1088/0031-9155/41/5/007 41(5), 909–923 (1996). [DOI] [PubMed] [Google Scholar]
- Heine J. J. and Velthuizen R. P., “A statistical methodology for mammographic density detection,” Med. Phys. 10.1118/1.1323981 27(12), 2644–2651 (2000). [DOI] [PubMed] [Google Scholar]
- Lu L. J., Nishino T. K., Khamapirad T., Grady J. J., M. H.Leonard, Jr., and Brunder D. G., “Computing mammographic density from a multiple regression model constructed with image-acquisition parameters from a full-field digital mammographic unit,” Phys. Med. Biol. 10.1088/0031-9155/52/16/013 52(16), 4905–4921 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., Wolverton D. E., Zhong W., Cumming S., and Olopade O. I., “Computerized analysis of mammographic parenchymal patterns for breast cancer risk assessment: Feature selection,” Med. Phys. 10.1118/1.598851 27(1), 4–12 (2000). [DOI] [PubMed] [Google Scholar]
- Castella C., Kinkel K., Eckstein M. P., Sottas P. E., Verdun F. R., and Bochud F. O., “Semiautomatic mammographic parenchymal patterns classification using multiple statistical features,” Acad. Radiol. 14(12), 1486–1499 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou C., Chan H. P., Petrick N., Helvie M. A., Goodsitt M. M., Sahiner B., and Hadjiiski L. M., “Computerized image analysis: Estimation of breast density on mammograms,” Med. Phys. 10.1118/1.1376640 28(6), 1056–1069 (2001). [DOI] [PubMed] [Google Scholar]
- Martin K. E., Helvie M. A., Zhou C., Roubidoux M. A., Bailey J. E., Paramagul C., Blane C. E., Klein K. A., Sonnad S. S., and Chan H. P., “Mammographic density measured with quantitative computer-aided method: comparison with radiologists’ estimates and BI-RADS categories,” Radiology 10.1148/radiol.2402041947 240(3), 656–665 (2006). [DOI] [PubMed] [Google Scholar]
- Highnam R., Brady M., and Shepstone B., “A representation for mammographic image processing,” Med. Image Anal. 1(1), 1–18 (1996). [DOI] [PubMed] [Google Scholar]
- Highnam R., Pan X., Warren R., Jeffreys M., Davey Smith G., and Brady M., “Breast composition measurements using retrospective standard mammogram form (SMF),” Phys. Med. Biol. 51(11), 2695–2713 (2006). [DOI] [PubMed] [Google Scholar]
- Kaufhold J., Thomas J. A., Eberhard J. W., Galbo C. E., and Trotter D. E., “A calibration approach to glandular tissue composition estimation in digital mammography,” Med. Phys. 10.1118/1.1493215 29(8), 1867–1880 (2002). [DOI] [PubMed] [Google Scholar]
- Pawluczyk O., Augustine B. J., Yaffe M. J., Rico D., Yang J., Mawdsley G. E., and Boyd N. F., “A volumetric method for estimation of breast density on digitized screen-film mammograms,” Med. Phys. 10.1118/1.1539038 30(3), 352–364 (2003). [DOI] [PubMed] [Google Scholar]
- Shepherd J. A., Herve L., Landau J., Fan B., Kerlikowske K., and Cummings S. R., “Novel use of single x-ray absorptiometry for measuring breast density,” Technol. Cancer Res. Treat. 4(2), 173–182 (2005). [DOI] [PubMed] [Google Scholar]
- Johns P. C. and Yaffe M. J., “Theoretical optimization of dual-energy x-ray imaging with application to mammography,” Med. Phys. 10.1118/1.595766 12(3), 289–296 (1985). [DOI] [PubMed] [Google Scholar]
- Johns P. C., Drost D. J., Yaffe M. J., and Fenster A., “Dual-energy mammography: Initial experimental results,” Med. Phys. 10.1118/1.595767 12(3), 297–304 (1985). [DOI] [PubMed] [Google Scholar]
- Taibi A., Fabbri S., Baldelli P., di Maggio C., Gennaro G., Marziani M., Tuffanelli A., and Gambaccini M., “Dual-energy imaging in full-field digital mammography: A phantom study,” Phys. Med. Biol. 10.1088/0031-9155/48/13/307 48(13), 1945–1956 (2003). [DOI] [PubMed] [Google Scholar]
- Kappadath S. C. and Shaw C. C., “Quantitative evaluation of dual-energy digital mammography for calcification imaging,” Phys. Med. Biol. 10.1088/0031-9155/49/12/007 49(12), 2563–2576 (2004). [DOI] [PubMed] [Google Scholar]
- Bornefalk H., Lewin J. M., Danielsson M., and Lundqvist M., “Single-shot dual-energy subtraction mammography with electronic spectrum splitting: Feasibility,” Eur. J. Radiol. 10.1016/j.ejrad.2006.08.004 60(2), 275–278 (2006). [DOI] [PubMed] [Google Scholar]
- Lewin J. M., Isaacs P. K., Vance V., and Larke F. J., “Dual-energy contrast-enhanced digital subtraction mammography: feasibility,” Radiology 10.1148/radiol.2291021276 229(1), 261–268 (2003). [DOI] [PubMed] [Google Scholar]
- Lemacks M. R., Kappadath S. C., Shaw C. C., Liu X., and Whitman G. J., “A dual-energy subtraction technique for microcalcification imaging in digital mammography—A signal-to-noise analysis,” Med. Phys. 10.1118/1.1494832 29(8), 1739–1751 (2002). [DOI] [PubMed] [Google Scholar]
- Brandan M. E. and Ramirez R. V., “Evaluation of dual-energy subtraction of digital mammography images under conditions found in a commercial unit,” Phys. Med. Biol. 10.1088/0031-9155/51/9/014 51(9), 2307–2320 (2006). [DOI] [PubMed] [Google Scholar]
- Bliznakova K., Kolitsi Z., and Pallikarakis N., “Dual-energy mammography: Simulation studies,” Phys. Med. Biol. 10.1088/0031-9155/51/18/004 51(18), 4497–4515 (2006). [DOI] [PubMed] [Google Scholar]
- Mou X. and Chen X., “Error analysis of calibration materials on dual-energy mammography,” Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv (2007), Vol. 10(Pt 2), pp. 596–603. [DOI] [PubMed]
- Saito M., “Dual-energy approach to contrast-enhanced mammography using the balanced filter method: spectral optimization and preliminary phantom measurement,” Med. Phys. 10.1118/1.2790841 34(11), 4236–4246 (2007). [DOI] [PubMed] [Google Scholar]
- Breitenstein D. S. and Shaw C. C., “Comparison of three tissue composition measurement techniques using digital mammograms—A signal-to-noise study,” J. Digit Imaging 11(3), 137–150 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone J. M., Shaber G. S., and Tecotzky M., “Dual-energy mammography: A detector analysis,” Med. Phys. 10.1118/1.596548 17(4), 665–675 (1990). [DOI] [PubMed] [Google Scholar]
- Marziani M., Taibi A., Tuffanelli A., and Gambaccini M., “Dual-energy tissue cancellation in mammography with quasi-monochromatic x-rays,” Phys. Med. Biol. 10.1088/0031-9155/47/2/309 47(2), 305–313 (2002). [DOI] [PubMed] [Google Scholar]
- Asaga T., Chiyasu S., Mastuda S., Mastuura H., Kato H., Ishida M., and Komaki T., “Breast imaging: Dual-energy projection radiography with digital radiography,” Radiology 164(3), 869–870 (1987). [DOI] [PubMed] [Google Scholar]
- Brettle D. S. and Cowen A. R., “Dual-energy digital mammography utilizing stimulated phosphor computed radiography,” Phys. Med. Biol. 10.1088/0031-9155/39/11/012 39(11), 1989–2004 (1994). [DOI] [PubMed] [Google Scholar]
- Chakraborty D. P. and Barnes G. T., “An energy sensitive cassette for dual-energy mammography,” Med. Phys. 10.1118/1.596406 16(1), 7–13 (1989). [DOI] [PubMed] [Google Scholar]
- Baldelli P., Bravin A., Di Maggio C., Gennaro G., Sarnelli A., Taibi A., and Gambaccini M., “Evaluation of the minimum iodine concentration for contrast-enhanced subtraction mammography,” Phys. Med. Biol. 10.1088/0031-9155/51/17/008 51(17), 4233–4251 (2006). [DOI] [PubMed] [Google Scholar]
- Asaga T., Masuzawa C., Yoshida A., and Matsuura H., “Dual-energy subtraction mammography,” J. Digit Imaging 8(1 Suppl 1), 70–73 (1995). [DOI] [PubMed] [Google Scholar]
- Shaw E., Kline R., Gillin M., Souhami L., Hirschfeld A., Dinapoli R., and Martin L., “Radiation Therapy Oncology Group: Radiosurgery quality assurance guidelines,” Int. J. Radiat. Oncol., Biol., Phys. 27(5), 1231–1239 (1993). [DOI] [PubMed] [Google Scholar]
- Nowotny R. and Höfer A., “Ein Programm fur die Berechnung von diagnostischen Röntgenspektren,” Fortschr Röntgenstr 142, 685–689 (1985). [DOI] [PubMed] [Google Scholar]
- Bushberg J. T., Seibert J. A., Leidholdt E. M., and Boone J. M., The Essential Physics of Medical Imaging, 2nd Ed. (Lippincott Williams & Wilkins, Philadelphia, 2002), pp. 278–279. [Google Scholar]
- Boone J. M., Fewell T. R., and Jennings R. J., “Molybdenum, rhodium, and tungsten anode spectral models using interpolating polynomials with application to mammography,” Med. Phys. 10.1118/1.598100 24(12), 1863–1874 (1997). [DOI] [PubMed] [Google Scholar]
- Boone J. M. and Chavez A. E., “Comparison of x-ray cross sections for diagnostic and therapeutic medical physics,” Med. Phys. 10.1118/1.597899 23(12), 1997–2005 (1996). [DOI] [PubMed] [Google Scholar]
- Hammerstein G. R., Miller D. W., White D. R., Masterson M. E., Woodard H. Q., and Laughlin J. S., “Absorbed radiation-dose in mammography,” Radiology 130(2), 485–491 (1979). [DOI] [PubMed] [Google Scholar]
- Boone J. M., Seibert J. A., Tang C. M., and Lane S. M., “Grid and slot scan scatter reduction in mammography: comparison by using Monte Carlo techniques,” Radiology 10.1148/radiol.2222010491 222(2), 519–527 (2002). [DOI] [PubMed] [Google Scholar]
- Rezentes P. S., de Almeida A., and Barnes G. T., “Mammography grid performance,” Radiology 210(1), 227–232 (1999). [DOI] [PubMed] [Google Scholar]
- Zhao W. and Rowlands J. A., “Digital radiology using active matrix readout of amorphous selenium: theoretical analysis of detective quantum efficiency,” Med. Phys. 10.1118/1.598097 24(12), 1819–1833 (1997). [DOI] [PubMed] [Google Scholar]
- Blevis I. M., Hunt D. C., and Rowlands J. A., “X-ray imaging using amorphous selenium: Determination of Swank factor by pulse height spectroscopy,” Med. Phys. 10.1118/1.598245 25(5), 638–641 (1998). [DOI] [PubMed] [Google Scholar]
- Smith A. P., Hologic, Inc., Bedford, MA (personal communication).
- Boone J. M., “Glandular breast dose for monoenergetic and high-energy x-ray beams: Monte Carlo assessment,” Radiology 213(1), 23–37 (1999). [DOI] [PubMed] [Google Scholar]
- Boone J. M., “Normalized glandular dose (DgN) coefficients for arbitrary x-ray spectra in mammography: Computer-fit values of Monte Carlo derived data,” Med. Phys. 10.1118/1.1472499 29(5), 869–875 (2002). [DOI] [PubMed] [Google Scholar]
- Shaw C. C. and Gur D., “Comparison of three different techniques for dual-energy subtraction imaging in digital radiography: a signal-to-noise analysis,” J. Digit Imaging 5(4), 262–270 (1992). [DOI] [PubMed] [Google Scholar]
- Gennaro G. and di Maggio C., “Dose comparison between screen∕film and full-field digital mammography,” Eur. Radiol. 16(11), 2559–2566 (2006). [DOI] [PubMed] [Google Scholar]
- Hologic Inc., “QDR Series X-ray Bone Densitometers Technical Specifications Manual,” (Bedford Mass, 2005).
- Alvarez R. E., Seibert J. A., and Thompson S. K., “Comparison of dual energy detector system performance,” Med. Phys. 10.1118/1.1645679 31(3), 556–565 (2004). [DOI] [PubMed] [Google Scholar]
- Ersahin A., Molloi S., and Yao-Jin Q., “A digital filtration technique for scatter-glare correction based on thickness estimation,” IEEE Trans. Med. Imaging 10.1109/42.414624 14(3), 587–595 (1995). [DOI] [PubMed] [Google Scholar]
- Molloi S., Zhou Y., and Wamsely G., “Scatter-glare estimation for digital radiographic systems: Comparison of digital filtration and sampling techniques,” IEEE Trans. Med. Imaging 10.1109/42.746621 17(6), 881–888 (1998). [DOI] [PubMed] [Google Scholar]
- Seibert J. A. and Boone J. M., “X-ray scatter removal by deconvolution,” Med. Phys. 10.1118/1.596208 15(4), 567–575 (1988). [DOI] [PubMed] [Google Scholar]
- Kopans D. B., “Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk,” Radiology 246(2), 348–353 (2008). [DOI] [PubMed] [Google Scholar]
- Mourtada F. A., Beck T. J., and Links J. M., “Theoretical analysis of error propagation in triple-energy absorptiometry: application to measurement of lead in bone in vivo,” Med. Phys. 10.1118/1.597986 24(6), 925–927 (1997). [DOI] [PubMed] [Google Scholar]
- Gauntt D. M. and Barnes G. T., “X-ray tube potential, filtration, and detector considerations in dual-energy chest radiography,” Med. Phys. 10.1118/1.597373 21(2), 203–218 (1994). [DOI] [PubMed] [Google Scholar]
- Morgan D. R., Sones R. A., and Barnes G. T., “Performance-characteristics of a dual-energy detector for digital scan projection radiography,” Med. Phys. 10.1118/1.596047 14(5), 728–735 (1987). [DOI] [PubMed] [Google Scholar]
- Alvarez R. E., “Active energy selective image detector for dual-energy computed radiography,” Med. Phys. 10.1118/1.597831 23(10), 1739–1748 (1996). [DOI] [PubMed] [Google Scholar]
- Alvarez R. E. and Macovski A., “Energy-selective reconstructions in x-ray computerized tomography,” Phys. Med. Biol. 10.1088/0031-9155/21/5/002 21(5), 733–744 (1976). [DOI] [PubMed] [Google Scholar]
- Roessl E., Ziegler A., and Proksa R., “On the influence of noise correlations in measurement data on basis image noise in dual-energylike x-ray imaging,” Med. Phys. 10.1118/1.2514058 34(3), 959–966 (2007). [DOI] [PubMed] [Google Scholar]