Abstract
The authors investigated the performance of the iterative Steiglitz–McBride (SM) algorithm on an autoregressive moving average (ARMA) model of signals from a fast, sparsely sampled, multiecho, chemical shift imaging (CSI) acquisition using simulation, phantom, ex vivo, and in vivo experiments with a focus on its potential usage in magnetic resonance (MR)-guided interventions. The ARMA signal model facilitated a rapid calculation of the chemical shift, apparent spin-spin relaxation time , and complex amplitudes of a multipeak system from a limited number of echoes (≤16). Numerical simulations of one- and two-peak systems were used to assess the accuracy and uncertainty in the calculated spectral parameters as a function of acquisition and tissue parameters. The measured uncertainties from simulation were compared to the theoretical Cramer–Rao lower bound (CRLB) for the acquisition. Measurements made in phantoms were used to validate the estimates and to validate uncertainty estimates made from the CRLB. We demonstrated application to real-time MR-guided interventions ex vivo by using the technique to monitor a percutaneous ethanol injection into a bovine liver and in vivo to monitor a laser-induced thermal therapy treatment in a canine brain. Simulation results showed that the chemical shift and amplitude uncertainties reached their respective CRLB at a signal-to-noise ratio (SNR)≥5 for echo train lengths (ETLs)≥4 using a fixed echo spacing of 3.3 ms. estimates from the signal model possessed higher uncertainties but reached the CRLB at larger SNRs and∕or ETLs. Highly accurate estimates for the chemical shift (<0.01 ppm) and amplitude (<1.0%) were obtained with ≥4 echoes and for (<1.0%) with ≥7 echoes. We conclude that, over a reasonable range of SNR, the SM algorithm is a robust estimator of spectral parameters from fast CSI acquisitions that acquire ≤16 echoes for one- and two-peak systems. Preliminary ex vivo and in vivo experiments corroborated the results from simulation experiments and further indicate the potential of this technique for MR-guided interventional procedures with high spatiotemporal resolution ∼1.6×1.6×4 mm3 in ≤5 s.
Keywords: Multigradient echo acquisition, chemical shift imaging (CSI), autoregressive moving average (ARMA), MR-guided interventions
INTRODUCTION
Many fast chemical shift imaging (CSI) acquisitions use echo-planar1, 2 or spiral techniques3 to sample 32–128 echoes and the spectra from these acquisitions are analyzed by means of filtered or unfiltered fast Fourier transformation (FFT).4, 5 For simple two-peak systems, such as fat and water, spatiotemporal resolution can be improved by decreasing the number of echoes to 2 (Refs. 6, 7) or 3,8, 9 which typically incorporates assumptions about certain parameters, such as the chemical shift and the apparent spin-spin relaxation time , to measure a specific spectral parameter, such as amplitude.
Two-dimensional (2D) multiple fast gradient-recalled echo (MFGRE) acquisitions that rapidly collect a limited number of echoes (2-32) are now standard on many magnetic resonance (MR) platforms because they are useful for mapping,10 chemical shift encoding for fat-water separation,11 and fast cardiac applications.12 With the limited sampling window provided by so few echoes, applying the FFT to these echoes for fast spectral analysis becomes suboptimal owing to the extensive filtering required to control truncation artifacts and the subsequent trade-off in spectral resolution.13
Various time-domain analysis techniques have been investigated as alternatives or complements to the FFT technique for quantitative determination of the chemical shift, relaxation time, and amplitude of each MR-detectable chemical specimen with a focus on maintaining the standard high spectral resolution CSI (Ref. 14) at low spatiotemporal resolutions. The autoregressive moving average (ARMA) approach models the MR signal as a sum of damped complex exponentials in noise. This is a suitable model of the observed free induction decay because it allows spectral characterization without truncation artifacts or extensive prefiltering of the data.15, 16
Our recent feasibility study applied an ARMA model to a MFGRE acquisition for a CSI-based temperature estimation technique that relied on the temperature sensitive proton resonance frequency (PRF) shift.17 This work described here extends that research to investigate the use of the iterative Steiglitz–McBride (SM) algorithm to provide precise estimates of multiple spectral parameters in the presence of noise. We describe the generalized approach for a multipeak system and compare the algorithm’s precision to the Cramer–Rao lower bound (CRLB)18, 19, 20 for simulated one- and two-peak systems, assuming tissue parameters similar to those for water and fat. Last, we present potential applications and advantages of this fast CSI technique for real-time MR-guided interventional procedures.
BACKGROUND
Autoregressive moving average model of the signal and spectral parameter determination
If a discrete set of signals from a gradient echo train is collected, then the MR signal, y(t), can be modeled in the time domain discretely as a sum of damped complex exponentials, that is,
| (1) |
where N is the number of MR-detectable chemical species (water, fat, etc.), TE0 is the minimum echo time, n is the echo train length (ETL), ESP is the spacing between echoes, C, f, and are the complex amplitude, chemical shift, and apparent spin-spin relaxation time of each chemical specimen, respectively, w(t) is white noise with zero mean, t is time, and k is the chemical specimen. Generally, an ARMA model describes how an observation, such as y(t), can be generated as a filtered input signal with white noise.21 Note that x(t) can be written as
| (2) |
where α(0)=1 and α(m) is in the form
| (3) |
This essentially means that the frequency and information of a noiseless signal are in the α(m) coefficients. This is a representative of an autoregressive process. However, the measured signal contains noise, w(t). Therefore, to measure the signal x(t),
| (4) |
where β(m) are also complex-valued coefficients. This is a general form of an autoregressive moving average process. Using the z-transform defined as
| (5) |
Eq. 4 can be written as
| (6) |
In this work, we used the iterative SM algorithm22 initialized with the results of Prony’s method23 to calculate α and β in MATLAB (MathWorks, Inc., Natick, MA). Details on these algorithms are described by Jackson.24 Once α and β are known, the poles can be calculated which are the roots of the denominator in Eq. 6. If ρk denotes the pole for chemical specimen k, then the chemical shift of that specimen can be expressed as
| (7) |
where ESP is the echo spacing, γ is the gyromagnetic ratio, and B0 is the static magnetic field.
In addition, can be expressed as
| (8) |
Finally, the complex amplitudes of water and fat can be computed separately using the Cauchy residue theorem,25, 26 where
| (9) |
and
| (10) |
(see Ref. 17).
METHODS AND MATERIALS
Simulation and the Cramer–Rao lower bound
The spectral modeling algorithm was tested on simulated signals for one- and two-peak systems. In the one-peak system, acquisition parameters included spin lattice relaxation time (T1), which was set at 500 ms, and . The TR was fixed at 70 ms to maintain consistent temporal resolution. The minimum TE was set to 2 ms, with an echo spacing of 3.3 ms. The two-peak system added a fat signal that had 25% of the amplitude of the water peak. For the fat peak, T1=300 ms and . A flip angle of 37° (the Ernst angle for fat signal27) was used. All other parameters were the same as those in the one-peak system. To simulate noise in both systems, Gaussian noise was added to real and imaginary channels of the complex time-domain signal. The signal-to-noise ratio (SNR) was defined as the amplitude of each spectral component divided by the standard deviation of the magnitude noise. To obtain the measurement uncertainty, we performed 20 000 random trials at each SNR value. The number of trials was determined by the number of measurements needed to obtain the uncertainty measurement at the lowest SNR=5. Accuracies and uncertainties of the spectral parameters (chemical shift, , and complex amplitude) were calculated using the SM algorithm for an ETL of up to 16 echoes to determine, as a function of SNR, how the number of acquired echoes at a fixed ESP affected the accuracy and precision of the measured spectral parameters.
The CRLB was used to provide a comparative theoretical basis of the uncertainty independent of the spectral processing technique. The CRLB is defined as the minimum achievable variance for an estimator.19, 20 The CRLB for this model (the sum of complex damped exponentials) has been analytically defined in the one-peak system and can be numerically determined for a multipeak system, as described by Kumaresan and Tufts.18 For example, if a MR signal with only one peak is present, the minimum uncertainty in the chemical shift given by the CRLB can be defined as
| (11) |
where
| (12) |
and SNRA is the SNR of the first echo.
The uncertainties of the amplitudes, chemical shifts, and values using the SM algorithm were compared to the CRLBs to ascertain the precision of the algorithm in the presence of noise. Correlation coefficients between simulation values and the CRLBs were calculated for each parameter in both the one- and two-peak systems.
The effects of ESP and were simulated in the one-peak system with ETL=16. The uncertainty values for the chemical shift, , and amplitude from 20 000 measurements were determined as a function of ESP and and represented on surface maps.
We also performed simulations to measure the sensitivity of detecting secondary peaks in noise. In these simulations, the signal parameters were the same as those in two-peak system with an ETL=16, but the amplitude of water was changed to correspond to SNR for water of 10–50. Measurements of sensitivity were made at different secondary peak SNR levels to determine at which SNR the secondary signal could be detected. This enabled the effects from water SNR, fat SNR, and fat∕water ratios to be analyzed.
Multiple, fast gradient-recalled echo (MFGRE) Imaging
A 2D MFGRE acquisition was modified to provide multiple echoes for each TR using unipolar readout gradient pulses, with time-optimized rewinding gradient pulses on a 1.5 T clinical MR scanner (Excite HD, GE Healthcare Technologies, Waukesha, WI). Each echo of the MFGRE sequence provided one phase-encoded readout line in k-space per TR with a specific TE. Parallel acquisition and processing techniques28 were used to lower the acquisition time, allowing a more liberal selection of TR values for contrast and to facilitate interleaved slice acquisitions (acceleration factor=2). The ESP was increased to accommodate longer readout times (lower bandwidths) for increased SNR at the cost of a smaller spectral bandwidth. This resulted in aliasing of peaks outside the window, such as fat. However, the ESP was always carefully selected so that primary peaks, such as fat and water, did not overlap.17
Phantom imaging
To test the performance of the SM algorithm on real CSI data from a MFGRE acquisition, measurements were made in water and fat-water phantoms and compared with the expected values of the chemical shift and . For the water phantom, an agarose gel (3% w∕v) was created using distilled, de-ionized water to minimize variation in the metal ion content of the water, which can lower relaxation times. To create the fat-water phantom, a mixture of 50% mayonnaise and 50% lemon juice (by volume)17, 29 was set in an agarose gel (3 % w∕v). Both phantoms were scanned with the same acquisitions parameters (16 echoes, TR∕TE0=70 ms∕1.9 ms, ESP=3.3 ms, flip angle=30°, receiver bandwidth=279 Hz∕pixel, acquisition matrix=128×128, acceleration factor=2, voxel size=1.6×1.6×5.0 mm3, and acquisition time=4.5 s∕image). The MR signal was detected using an eight-channel high resolution brain array (MRI Devices, Waukesha, WI).
For the water phantom, the measurements calculated by the SM algorithm were compared with a spoiled-gradient echo (SPGR) acquisition with exponential fitting of the signal at different TE values. A student’s t-test assuming equal variances (as determined through an F-test) was performed to test for statistical differences between the values from the MFGRE and SPGR acquisitions. Uncertainties in the chemical shift, , and amplitude were measured over ten acquisitions and compared with the CRLB.
These comparisons were also made for the fat-water phantom and, again, uncertainties in the water and fat chemical shifts, , and amplitude values for water and fat were calculated over ten consecutive acquisitions and compared with the CRLB. Spatial variations in the differences in chemical shift between water and fat were measured across the phantom. We also compared the values for water between the MFGRE acquisition and a SPGR acquisition with fat suppression.
Imaging of ethanol injection into ex vivo liver tissue
To demonstrate the power of the technique for providing unique information for MR-guided interventions such as chemical ablations, we used the MFGRE acquisition (16 echoes, TR∕TE0=70 ms∕2.1 ms, ESP=3.3 ms, flip angle=60°, receiver bandwidth=244 Hz∕pixel, acquisition matrix=128×128, acceleration factor=2, voxel size=1.6×1.6×4.0 mm3, and acquisition time=4.5 s∕image) and SM algorithm to monitor the injection of 2 ml ethanol in an ex vivo bovine liver sample. Ethanol is a chemical agent used in interventions because it induces coagulation necrosis of tissue by cellular dehydration, protein denaturation, and small vessel thrombosis.30 The location and the relative concentration of the ethanol were determined by locating the methyl (CH3) peak versus the water peak. Changes in the chemical shift, , and amplitude of both the water peak and the ethanol-methyl peak were measured over time in a region of interest (ROI).
Monitoring of thermal therapy in canine brain in vivo
We previously reported on the technique for monitoring thermal therapy using phantoms and small animals in vivo.17 Here, we examined the usefulness of the multiparametric nature of the technique for guiding MR thermal therapy interventions in a large animal model by using the MFGRE acquisition to guide the application of laser-induced thermal therapy in a canine brain in vivo using a 1.5 T scanner. The animal used in this experiment was handled in accordance with the Institutional Animal Care and Use Committee. A water-cooled applicator containing a laser fiber (Biotex Inc., Houston, TX) with a 1 cm diffusing tip (808 nm) was inserted into the right hemisphere of the canine brain using MR guidance. Treatment was delivered using an exposure of 3.5 W∕cm2 for 180 s under continuous MFGRE monitoring (16 echoes, TR∕TE0=69 ms∕2.1 ms, flip angle=30°, receiver bandwidth=244 Hz∕pixel, acquisition matrix=128×128, acceleration factor=2, voxel size=1.6×1.6×4.0 mm3, and acquisition time=4.4 s∕image). The ESP was 3.3 ms, giving a spectral bandwidth of 303 Hz. Using the frequency map from the SM algorithm, the shift in the PRF was measured to detect temperature changes31 using a temperature sensitivity of −0.0098 ppm∕°C.32 In addition, maps and -corrected T1-W images (signal extrapolated to TE=0 ms) were created from the data and analyzed to demonstrate the potential usefulness.
RESULTS
Simulation and the Cramer–Rao lower bound
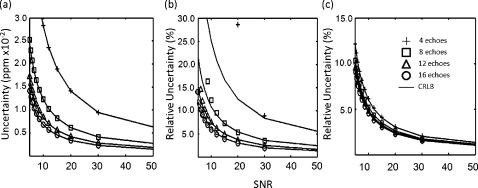
Performance of the SM algorithm on simulated data demonstrated a high correlation between the uncertainties in chemical shift and amplitude and the CRLB values for SNR≥5 and ETL≥4. When uncertainties approached the CRLB, the algorithm performed near optimum at the theoretical lower bound of the uncertainty for the given model. Figure 1 displays the uncertainties in the signal-peak model using the SM algorithm for the chemical shift, , and amplitude estimates as a function of SNR for ETL of 4–16 echoes. Solid lines represent the CRLBs for each simulation. The uncertainty of the estimates decreased as the SNR and the number of echoes increased. measurements generally showed lower correlation (slightly higher uncertainty than the CRLBs), primarily at lower SNR values, than other spectral parameters. This effect was expectedly exacerbated when using truncated ETLs (<8) which have a reduced TEmax. For example, the uncertainty of the measured from simulations began to exceed the CRLBs at SNR<10 for ETL=8 and SNR<30 for ETL=4 (Fig. 1). Table 1 shows the correlation coefficients between the simulated data and the CRLBs. In agreement with Fig. 1, the chemical shift and amplitude showed very high correlation coefficients (Pearson’s R2>0.9990), with the correlation of the measurements between simulation and the CRLBs decreasing as the number of echoes decreased.
Figure 1.
Simulation and CRLB results for 4 (crosses), 8 (squares), 12 (triangles), and 16 (circles) echoes. This simulation used 20 000 trials. Estimates for chemical shift achieved an uncertainty at the CRLB for SNR≥5 for each ETL (4–16 echoes) (a). The uncertainties in for ETL=16 achieved the CRLB for all SNRs≥5 (b). As the ETL decreases, the uncertainty diverges from the CRLB. For instance, at ETL=8, the uncertainty is higher than the CRLB at SNR<10.Compared with the chemical shift (a) and (b), the uncertainties of the amplitudes in the simulation and their CRLBs are less dependent on the number of echoes (c).
Table 1.
Correlation coefficients between simulation and the CRLB in a water-only signal over multiple echo-train lengths. The uncertainties in chemical shift and amplitude have a very high correlation with the CRLB, as does the uncertainty in at ETL=16. However, correlation between the uncertainties in and the CRLB decreases as the number of echoes decreases.
| No. of Echoes | Chemical Shift | Amplitude | |
|---|---|---|---|
| 4 | 0.9997 | 0.7919 | 0.9996 |
| 8 | 0.9997 | 0.8886 | 0.9998 |
| 12 | 0.9996 | 0.9944 | 0.9999 |
| 16 | 0.9994 | 0.9996 | 0.9999 |
The measured uncertainty of the chemical shift and estimates demonstrated an inverse proportionality to the ETL, as expected from the derivation of the CRLBs.18 For SNR=20, the dependence of the chemical shift and on the number of echoes had a relationship of N−1.01 (Pearson’s R2=0.990) and N−1.02 (Pearson’s R2=0.980), respectfully, where N is the number of echoes. The amplitude estimates exhibited less dependence on the number of echoes with a N−0.22 relationship (Pearson’s R2=0.982).
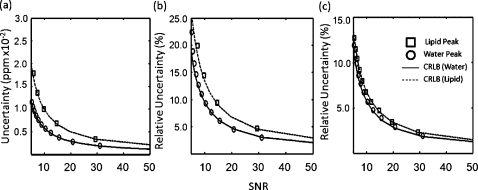
Simulations also showed agreement between the spectral parameter uncertainties and the CRLBs in the two-peak model representing water and fat. Figure 2 is a plot of the uncertainties and the corresponding CRLBs for a simulated two-peak model of water and fat for ETL=16. Corresponding correlation coefficients are shown in Table 2. Uncertainties in the chemical shifts of both water and fat signals approached the CRLBs and maintained high correlation coefficients. As observed in the one-peak simulation of water, values diverged more from the CRLBs than did other spectral parameters. Uncertainties in the fat chemical shift and values were demonstrably higher even for the same SNR. Uncertainty in the amplitudes showed little difference between water and fat. This result was consistent in a range of water and fat concentrations. Further, as seen in the one-peak model, the uncertainty of each peak’s spectral parameters increased as the number of echoes decreased.
Figure 2.
Simulation and CRLB results for a two-peak signal of water and fat with ETL=16. This simulation used 20 000 trials. Estimates for the chemical shift of both water and fat achieved an uncertainty at the CRLB (a). The uncertainties in for both peaks reached their CRLBs. The of fat (30 ms) was set to half of that of water (60 ms) (b). The CRLB for the amplitudes of both water and fat were similar when represented as relative uncertainties (c). The uncertainties provided by the algorithm had a similar result.
Table 2.
Correlation coefficients between simulation and the CRLB in a fat-water signal over multiple echo-train lengths. As with the one-peak (water only) signal, the uncertainties in chemical shift and amplitude have a high correlation with the CRLB for both water and fat. The uncertainties in correlate less with the CRLB as the number of echoes decreases.
| No. of Echoes | Water | Fat | ||||
|---|---|---|---|---|---|---|
| PRF | Amplitude | PRF | Amplitude | |||
| 4 | 0.9753 | 0.5813 | 0.9997 | 0.953 | 0.4811 | 0.9968 |
| 8 | 0.9758 | 0.6667 | 0.9999 | 0.9566 | 0.5495 | 0.9984 |
| 12 | 0.981 | 0.7294 | 0.9999 | 0.9721 | 0.6811 | 0.9994 |
| 16 | 0.9886 | 0.9638 | 0.9999 | 0.9551 | 0.8435 | 0.9999 |
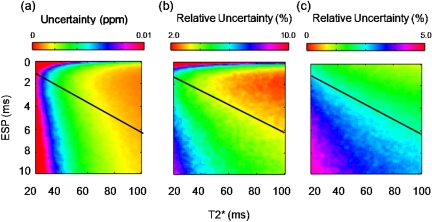
Surface maps illustrated the impact of and ESP on the one-peak model with ETL=16 (Fig. 3). Generally, the uncertainties were lower as increased and the ESP decreased. Areas below the black line in Figs. 3a, 3b, 3c indicate areas where the TE of the last echo (TEmax) was larger than the simulated . As expected, if collected echoes had TE values greater than the value of , then the uncertainty of that estimate increased. Therefore, in tissues such as fat, which have a low value, the ESP needs to be decreased to maintain precision.
Figure 3.
Surface maps demonstrate the effects ESP and on a single-peak signal at SNR=20 have on the uncertainties for chemical shift (a), (b), and amplitude (c). Areas below the black line represent where the ETL . This causes an increase in uncertainty, which is more evident on (b) and (c). ESP>1.0 ms can provide low uncertainties as long as the ESP is short enough not to sample above the of the tissue.
In addition to precision, we also used simulation to test the accuracy of the SM algorithm. Table 3 summarizes the measured accuracy of the estimates provided by the SM algorithm in a simulated signal containing water at SNR=20. These estimates are presented as the root-mean squared error (RMSE). The algorithm maintains high accuracy for the chemical shifts (<0.01 ppm) and amplitudes (<1.0%) for an ETL of 4–16. This was consistent down to SNR=5. values also maintained high accuracy but increased to ≥1% (0.6 ms) for ETL≤5 at SNR=20. For each parameter, the accuracy decreased with decreasing ETL and SNR, as expected. Accuracies in the two-peak signals for the fat-water simulation are summarized in Table 4. The RMSE values were higher for the water parameters in the fat-water signal than those for the parameters of water in the water-only signal. However, accuracy was high for both chemical shift and amplitude, ≤0.01 ppm and ≤1%, respectfully, for ETLs of 4–16. At ETL≤6, the RMSE of was ≥6% for the longer peak (water). As expected, measurements required a larger ETL to lower the bias when the ESP was fixed.
Table 3.
Accuracies (root-mean squared error) determined through simulation over 20 000 independent trials in a water signal at a SNR of 20. High accuracy is achieved for the chemical shifts and amplitudes from four to sixteen echoes. values also has high accuracy but increases to >1% (0.6 ms) at ≤5 echoes.
| No. of Echoes | Water PRF (ppm) | Water (% error) | Water amplitude (% error) |
|---|---|---|---|
| 4 | 1.85×10−4 | 5.65 | 4.02×10−2 |
| 5 | 1.36×10−4 | 1.03 | 4.02×10−2 |
| 6 | 1.05×10−4 | 0.493 | 3.82×10−2 |
| 7 | 8.44×10−5 | 0.246 | 3.64×10−2 |
| 8 | 7.24×10−5 | 0.194 | 3.47×10−2 |
| 12 | 4.29×10−5 | 0.107 | 3.01×10−2 |
| 16 | 3.16×10−5 | 0.076 | 2.82×10−2 |
Table 4.
Accuracies (root-mean squared error) determined through simulation over 20 000 independent trials in a water∕fat signal at a water SNR of 20. The accuracy of chemical shift is within 0.01 ppm and that of amplitude is within 1% RMSE for ETLs of 4–16. At ETL≤6, the RMSE for both values is high, especially for the longer peak (water).
| No. of Echoes | Water PRF (ppm) | Water T2* (% error) | Water amplitude (% error) | Fat PRF (ppm) | Fat T2* (% error) | Fat amplitude (% error) |
|---|---|---|---|---|---|---|
| 4 | 8.28×10−3 | 42.1 | 0.425 | 7.70×10−3 | 16.6 | 0.932 |
| 5 | 6.74×10−3 | 38.4 | 0.322 | 6.60×10−3 | 0.426 | 0.785 |
| 6 | 4.69×10−3 | 6.52 | 0.227 | 4.7×10−3 | 0.347 | 0.478 |
| 7 | 2.71×10−3 | 0.474 | 0.136 | 2.7×10−3 | 0.302 | 0.281 |
| 8 | 2.1×10−3 | 0.223 | 0.106 | 2.1×10−3 | 0.263 | 0.219 |
| 12 | 4.70×10−4 | 0.113 | 3.95×10−2 | 4.84×10−4 | 0.166 | 8.87×10−2 |
| 16 | 1.79×10−4 | 8.02×10−2 | 2.93×10−2 | 2.05×10−4 | 0.131 | 7.15×10−2 |
The sensitivity of second peak detection in noise was also investigated during the simulations. Using fat as a model for the second peak, it was observed that the sensitivity of locating the secondary peak depended on the relative SNR between the peaks as well as the individual SNR of each peak. In a model with a simulated water signal and SNRs of 10–50, the simulated fat peak was consistently detectable down to a SNR of 2.64±0.48 for fat∕water amplitude ratios of ≥0.05.
Phantom imaging
In the water-agar phantom (SNR=88.6), the mean value was 34.8±0.2 ms in a ROI using the MFGRE acquisition with the SM algorithm and 34.6±0.2 ms using a simple SPGR acquisition in which each echo was acquired separately. The lack of statistical difference between the two techniques (p=0.093) indicated that the two methods of acquisition were equivalent for this measurement. Noise estimates in the phantom were 0.00105±0.0004 ppm, 0.356±0.149 ms, and 0.28±0.16% for the chemical shift, , and amplitude, respectively. Each noise measurement encompassed the calculated CRLBs (0.0010 ppm for chemical shift, 0.419 ms for , and 0.16 % for amplitude) at the 95% confidence level.
The mean values in the fat-water phantom were 25.0±0.2 and 12.7±1.1 ms for water and fat, respectively. The water measured from a standard fat-suppressed SPGR acquisition was 24.6±0.5 ms, which, despite the different acquisition techniques, did not statistically differ with the for water from the MFGRE acquisition (p=0.124). Table 5 shows the tabulated uncertainties in the chemical shift, , and amplitude estimates for water and fat in the fat-water phantom. Fat had a higher uncertainty than water in all three parameters.
Table 5.
Chemical shift, and amplitude uncertainties in a fat-water phantom. The SNRs for water and fat were 34.5 and 25.4, respectively. Using these values, the generated CRLBs for each parameter are calculated within the 95% confidence levels.
| Water (CRLB) | Fat (CRLB) | |
|---|---|---|
| Chemical shift (ppm) | 0.0043±0.0018 (0.0031) | 0.0049±0.0011 (0.0038) |
| (ms) | 2.20±0.80 (2.29) | 1.09±0.37 (1.40) |
| Amplitude (%) | 3.04±0.43 (2.83) | 3.91±0.95 (3.65) |
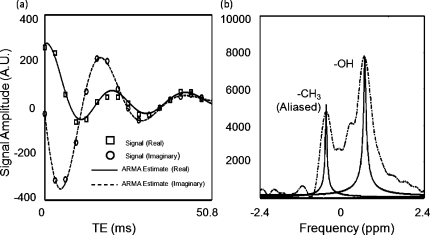
The real and imaginary components of the time-domain signal in this single voxel in the fat-water phantom were compared to those in a synthesized signal with parameters calculated by the SM algorithm as shown in Fig. 4a. A difference of 1.2% was seen between the real component of the acquisition and SM algorithm and a difference of 2.0% was seen in the imaginary components. Figure 4b shows the plotted results of the SM algorithm versus an unfiltered FFT (zero-padded to 1024 points) for a spectrum taken from one 1.6×1.6×5 mm3 voxel in the fat-water phantom. Note that the fat peak is aliased because ESP=3.3 ms. Aliasing of fat typically occurs at ESP≥2.2 ms at 1.5 T. Despite the aliasing, the peak location was still recovered accurately. Across the entirety of the phantom (3704 voxels), the water chemical shift had a standard deviation of 0.237 ppm and the fat had a standard deviation of 0.234 ppm, indicating that these deviations may be due in part to magnetic field changes across the sample. The mean difference between the fat and water chemical shift across the phantom was 3.4853±0.0301 ppm. This was an 87% reduction in the standard deviation and illustrated the power of the technique when fat is used as an internal reference for susceptibility correction in applications such as temperature imaging.
Figure 4.
The real and imaginary signals and fits of an MFGRE acquisition of the fat-water phantom with ETL=16 (a). A difference of 1.2% was seen between the real component of the signal and those calculated with the SM algorithm and a difference of 2.0% was seen between the imaginary signal components. The FFT of the signal from (a) (dashed line) compared with spectral parameters estimated with the SM algorithm (b) (solid lines). Note how the spectral peaks can be separated and plotted in the correct position even in the occurrence of aliasing.
Imaging of ethanol injection into ex vivo liver tissue
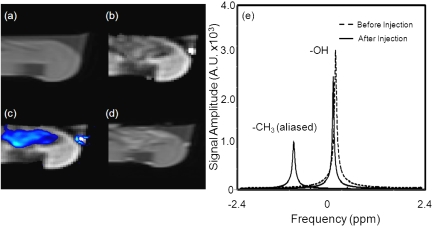
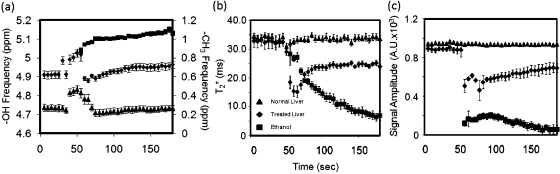
The spectral parameters of the injected ethanol in bovine liver ex vivo were measured using continuous MFGRE monitoring (acquisition time=4.5 s∕image). Figure 5 contains the pre- and post-T1-W images obtained with a fast spin echo (FSE) sequence in addition to T1-W amplitude and chemical shift images made from measurements taken during real-time monitoring of the percutaneous injection. Figure 5a shows the T1-W FSE image used to localize the needle. Figure 5b displays the water amplitude image 60 s into the ethanol injection, while Fig. 5c illustrates a false color map of the amplitude of the ethanol peak overlaid on a T1-W amplitude image of water at the same time point. The region where the ethanol signal was located by the SM algorithm corresponds well with intratreatment, -corrected, T1-W images calculated using the measurements from the SM algorithm [Fig. 5b] and the post-treatment T1-W FSE image [Fig. 5d]. Figure 5e illustrates the spectrum provided by our algorithm for an averaged signal over an ROI before and after the ethanol was percutaneously injected into the ex vivo liver sample. The methyl (CH3) peak from the ethanol is located 3.72 ppm away from water. Note the similar aliasing as seen with the fat-water phantom. Figure 6 illustrates the observed changes in the chemical shift (a), (b), and amplitude (c) of the untreated liver, treated liver, and the methyl peak of ethanol over time. The frequency difference between the water and methyl group had an uncertainty of 0.021 ppm. The chemical shifts in all three ROIs moved in the same direction after the injection, indicating changes from field drifts or susceptibility. The value for ethanol steadily decreased [Fig. 6b], whereas the of the liver tissue decreased to a value of approximately 24 ms, which is 27% lower than the value before the injection. Figure 6c shows that a 39% reduction in the T1-weighted amplitude for water recovered by 16% as the ethanol diffused from the region.
Figure 5.
T1-weighted FSE acquisition used to localizing the injection needle (a). Image of the amplitude of water provided by the SM algorithm demonstrates a decrease in signal where the ethanol was injected (b). Map of the amplitude of the ethanol peak overlaid by a T1-weighted image for the amplitude of water (c). Ethanol was located by detecting the presence of the methyl peak. Good correlation exists between areas of damage shown by a post-treatment T1-weighted FSE acquisition (d) and measurements calculated with the SM algorithm for amplitude (b) and chemical shift (c). Spectral parameters in the liver before (dashed line) and after (solid line) ethanol was percutaneously injected (e).
Figure 6.
The evolution of the chemical shift (a), (b), and amplitude (c) for water (triangles) and ethanol’s methyl peak (squares) over the entire treatment time. A susceptibility shift can be seen in the water chemical shift in (a). The for water decreased by 27% after the introduction of ethanol (b). A 39% reduction in the T1-weighted amplitude for water recovered by 16% as ethanol diffused from region (c).
Noise estimates in the liver tissue were 0.0022±0.0005 ppm, 0.861±0.272 ms, and 1.46±0.24% for the chemical shift, and amplitude, respectively. The chemical shift and noise measurements, which encompassed the calculated CRLBs at the 95% confidence level, were calculated as 0.0023 ppm for the chemical shift and 0.902 ms for . At the measured SNR (40.0) and (30.7 ms), the CRLB of the amplitude measurement was 0.34%. The mean amplitude noise estimates did not match the CRLB at the 95% confidence level.
MR thermometry in canine brain in vivo
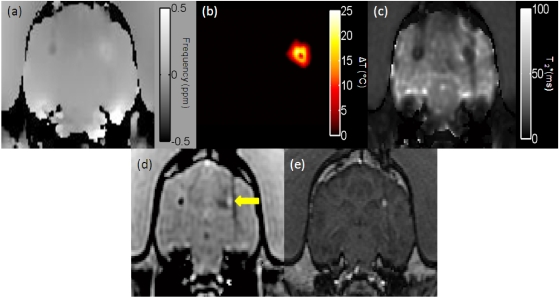
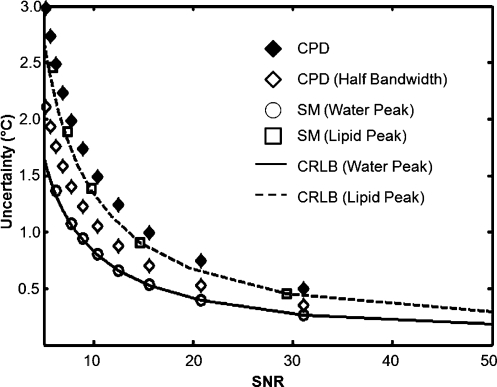
The MFGRE (ETL=16) with processing of the SM algorithm was applied to monitor a laser-induced thermal therapy in canine brain in vivo. Figure 7a shows the water proton field map, which is the source of the temperature images, and Fig. 7b displays the temperature map. The SM algorithm calculated the noise in the temperature map as 0.34±0.09 °C in an unheated region contralateral to the therapy site. This is in excellent agreement with the calculated CRLB (SNR=39.5, ) of 0.35 °C. Using the complex phase difference (CPD) of a single echo from the same acquisition at (which is the optimal value for CPD), the uncertainty approximately doubled to 0.69±0.18 °C. Unlike the CPD technique, the sensitivity of the CSI technique is inherently driven by the SNR of the acquisition and is independent of the echo time used (apart from the impact on SNR). Figure 7d is the T1-W image, corrected for decay from our knowledge of the , which was also calculated at each time point. During treatment delivery, an anomalous artifact arose in the temperature images that correlated with a hyperintense area seen near the laser source on the T1-W images. A suspected treatment-induced hemorrhage was confirmed by a post-treatment T1-W SPGR acquisition [Fig. 7e]. An image of the calculated for water [Fig. 7c] shows a clearly hypointense lesion.
Figure 7.
The water PRF map of a canine brain used to create the temperature maps had a temperature sensitivity coefficient of −0.0098 ppm∕°C (a). The temperature map for the thermal therapy in the canine brain (b). The noise calculated in an unheated region contralateral to the therapy site was 0.34±0.09 °C. Using the CPD at , the uncertainty increased to a mean of 0.69±0.18 °C. A map generated by the SM algorithm (c). The T1-weighted images with correction simultaneously calculated at each acquisition along with the temperature maps (d). During treatment, a hyperintense area developed near the laser source, raising the suspicion of a treatment-induced hemorrhage (indicated by an arrow). The hyperintense lesion, clearly shown on (c), was confirmed as a treatment-induced hemorrhage on a post-treatment T1-weighted SPGR acquisition (e).
DISCUSSION
The observed robust performance of the SM algorithm in accuracy, uncertainty, and secondary peak detection across a range of SNR values for a very low number of echoes and was corroborated by phantom, ex vivo, and in vivo imaging results. The use of minimal samples represented as a rational polynomial in the z-domain, which has been shown to converge exponentially as the number of echoes increases,16 is important because this allows the acquisition either to run faster with less computational overhead or to facilitate the acquisition of multiple slices within the same TR period.
In addition to choosing a low number of echoes, the choice of other acquisition parameters, such as the ESP, can affect the accuracy and precision of the measurements. We have shown that choosing ESPs that extend TEmax past the of the tissue increases the uncertainty in the measurements (Fig. 3). This was demonstrated in our estimates for the spectral parameters of fat in two-peak systems where , which displayed higher uncertainties compared with tissues that had higher values. In this work, we limited ourselves to acquisition parameters conducive to imaging ≤16 echoes every 5 s with a fixed ESP (3.3 ms at 1.5 T) such that the lipid peaks would not alias into the water. Thus, we did not investigate the use of a variable ESP, at low ETLs, which could possibly improve the estimates in tissues with low tissues.
Theoretical calculation of the CRLB demonstrates that the uncertainty is inversely proportional to the SNR, a relationship corroborated by simulation measurements. Therefore, MR acquisition parameters, such as the flip angle, should be chosen to increase the SNR of the peak of interest. For instance, in the one-peak system, the flip angle should provide maximum SNR at the Ernst angle. For two-peak systems in which information from both peaks is desired, acquisition parameters should be tailored to help increase the SNR of the secondary peak, which was assumed here to be the smaller of the two. If TR is fixed for optimal timing, the flip angle could be used to provide optimal modulation of the secondary peak signal.
The measurements we made from the MFGRE acquisition of the fat-water phantom reinforced simulation results and further demonstrated that the algorithm can measure the values from each chemical species in the two-peak system with a single acquisition. A large error (23.0%) observed in the tissue-based measurement for water (60 ms) in the presence of a 25% fat signal using exponential fitting of the magnitude signal was avoided when the SM algorithm was used to calculate the for each chemical species separately. Given adequate SNR, the acquisition used in this work could provide maps of eight to ten slices in a single breathhold (≤20 s) using ETL=8, while also providing inherent fat suppression. A recent study in liver constrained the solution of the estimated tissue-based for water by assuming equivalent values for water and fat, owing to a heavy iron overload.11 The authors concluded that this assumption may not be reasonable. The technique investigated here could potentially be used to separate these values directly, without any assumptions on the chemical shift or equivalency, thereby allowing identification of separate values for water and fat. Another potential application is quantitating the distribution of superparamagnetic iron oxides (SPIOs).33 These particles have been suggested for use in thermal therapy, and such therapies could potentially benefit from the temperature imaging capabilities of our technique as well.34
We demonstrated the potential for monitoring chemical ablations, such as an ethanol injection, with an ex vivo experiment. Conventional methods of guidance rely on signal changes provided by ultrasound35 or CT,36 while some investigations have used MR imaging.37, 38, 39, 40 Our results demonstrated the ability of this method to accurately detect the chemical shift of the methyl group in ethanol dynamically and to separate it from the water component at 1.5 T using the two-peak model. The methylene protons, which are J-coupled with the methyl protons,39 were also detectable at 1.5 T by using a higher-order (three-peak) model. The reason the algorithm works so well in this case is that if the model does not encompass all peaks, the most dominant peaks are found first. In the cases where there is clear distinction between peaks, there is no significant loss of accuracy or precision in peak parameter estimation (i.e., a single peak model will return an excellent estimation of the dominant peak from the two-peak system). Characterization of the dynamics of these lower SNR peaks using higher-order models is currently under investigation.
Dynamic T1-W images and images provided additional information that correlated with the agent location as observed on postinjection T1-weighted images. The changes in the and amplitude of water in treated liver could possibly be due to cellular dehydration effects. Sironi et al.40 suggested this as a possible effect when their study reported similar signal changes in hepatocellular carcinoma lesions treated with ethanol on T2-W images from a spin-echo acquisition. However, correlations between the signal changes and the underlying physiological mechanisms are not clear and will be a focus of future work. It is also important to note that if a reference standard of known concentration is kept in the image, the amplitude of each chemical shift could possibly provide a quantitative means to monitor the progress of therapy and better relate therapy to outcome. In addition to ablative chemicals such as ethanol or acetic acid, drugs suspended in lipid emulsifications can potentially be monitored as well.
As we previously demonstrated,17 the ability to measure multiple chemical shifts rapidly makes this technique extremely attractive for use in MR thermometry. Our current in vivo data in the canine brain highlight some of the potential of multiparametric monitoring for thermal therapy procedures because both physiologic (temperature) and anatomic (T1-W, maps) information are available. In this case, a treatment-induced hemorrhage was monitored during treatment. In CPD techniques, the optimal echo time is given by the of the signal.41 Magnitude images are therefore contaminated by the -weighting of the signal which can obscure T1 changes in the image. This would make it difficult to observe the hemorrhage and, more importantly, determine when it occurred during treatment.
Additionally, the ability to separate and measure the chemical shift in temperature-insensitive fat quickly and accurately is useful for providing inherent fat suppression and for internal referencing in thermometry. Currently, it takes too much time to acquire the needed images at high resolution.42 Our technique provides these benefits without sacrificing the spatiotemporal resolution of current CPD techniques.17 However, our simulation results show that a higher SNR is needed when taking advantage of this property to uncertainty in the temperature measurement on par with the unreferenced technique. This attempt to maintain high SNR is one of the reasons we used a higher ESP (by means of lower receiver bandwidth) in our acquisition sequence. With this technique, simulations predict that uncertainties in temperature of <1 °C can be achieved with SNR>34 using fat as an internal reference (assuming that water and fat component of 60 and 30 ms, respectfully, and ETL=16). A similar uncertainty is achievable using the signal peak for water only with SNR≥10.
Our simulation results also demonstrate that for the same imaging time and parameters, this technique is more sensitive than current CPD techniques as a function of SNR because it maintains better accuracy and uncertainty across a variety of values and improves sensitivity of temperature imaging (Fig. 8). However, there is always a trade-off. Within the same TR, we could have collected up to three interleaved slices and expanded our coverage. We demonstrated here that this technique has the ability to use fewer echoes (≥4) and still provide reasonably accurate and precise estimates of the chemical shift, which allows time to collect multiple slices.
Figure 8.
The uncertainty in temperature versus imaging SNR for a signal containing 25% fat. The simulation (n=20 000 samples) for evaluating the uncertainty in the signal for water using the SM algorithm (circles) is compared with CRLB results (solid line). Note that our algorithm stays close to the CRLB over a wide range of SNRs. For reference, the CPD results are shown for an optimal TE at the same bandwidth as the 16-echo sequence (black diamonds) and at one half this bandwidth (white diamonds) as would more likely be used. This case also assumes that the CPD can perfectly remove the influence of the fat signal, which is unlikely; thus, it stands as a “best case” scenario for CPD against our technique. As can be seen, in addition to providing fat-water separation, the fast CSI approach easily outperforms the CPD approach across a large range of imaging SNR values for the same imaging time.
CONCLUSION
We investigated the performance of the SM algorithm using a limited number of echoes (≤16) returned from a fast CSI sequence to obtain precise estimates of spectral parameters in one- and two-peak systems in the presence of noise. Using this technique in simulation, we showed that the calculated values for the chemical shift and amplitude are highly accurate and precise, reaching their respective CRLBs over a wide range of SNR values and ETLs. We also showed that the measurements are accurate and precise at higher SNR and ETL values. Simulation results were corroborated by phantom, ex vivo, and in vivo measurements. All these findings make this technique attractive for monitoring MR-guided interventions, which require rapid, accurate, and precise knowledge of major chemical species such as water and fat.
ACKNOWLEDGMENTS
The authors would like to thank Kimberly Herrick from Scientific Publications at the University of Texas M.D. Anderson Cancer Center for her help in preparing this article. This work was supported in part by NIH training grant 5T32CA119930.
References
- Mansfield P., “Spatial mapping of the chemical shift in NMR,” Magn. Reson. Med. 1, 370–386 (1984). [DOI] [PubMed] [Google Scholar]
- Oshio K., Kyriakos W., and Mulkern R. V., “Line scan echo planar spectroscopic imaging,” Magn. Reson. Med. 44, 521–524 (2000). [DOI] [PubMed] [Google Scholar]
- Mayer D., Kim D. H., Spielman D. M., and Bammer R., “Fast parallel spiral chemical shift imaging at 3T using iterative SENSE reconstruction,” Magn. Reson. Med. 59, 891–897 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourg S. and Nuzillard J. M., “Influence of noise on peak integrals obtained by direct summation,” J. Magn. Reson. 134, 184–188 (1998). [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Fisher M. J., Nelson S. J., and Brown T. R., “Evaluation of manual methods for integration of in vivo phosphorus NMR spectra,” NMR Biomed. 1, 131–135 (1988). [DOI] [PubMed] [Google Scholar]
- Dixon W. T., “Simple proton spectroscopic imaging,” Radiology 153, 189–194 (1984). [DOI] [PubMed] [Google Scholar]
- Ma J., Son J. B., Bankson J. A., Stafford R. J., Choi H., and Ragan D., “A fast spin echo two-point Dixon technique and its combination with sensitivity encoding for efficient T2-weighted imaging,” Magn. Reson. Imaging 23, 977–982 (2005). [DOI] [PubMed] [Google Scholar]
- Glover G. H. and Schneider E., “Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction,” Magn. Reson. Med. 10.1002/mrm.1910180211 18, 371–383 (1991). [DOI] [PubMed] [Google Scholar]
- Reeder S. B., Pineda A. R., Wen Z., Shimakawa A., Yu H., Brittain J. H., Gold G. E., Beaulieu C. H., and Pelc N. J., “Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): Application with fast spin-echo imaging,” Magn. Reson. Med. 10.1002/mrm.20624 54, 636–644 (2005). [DOI] [PubMed] [Google Scholar]
- Positano V., Salani B., Pepe A., Santarelli M. F., De Marchi D., Ramazzotti A., Favilli B., Cracolici E., Midiri M., Cianciulli P., Lombardi M., and Landini L., “Improved T2* assessment in liver iron overload by magnetic resonance imaging,” Magn. Reson. Imaging (in press). [DOI] [PubMed]
- Yu H., McKenzie C. A., Shimakawa A., Vu A. T., Brau A. C., Beatty P. J., Pineda A. R., Brittain J. H., and Reeder S. B., “Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation ,” J. Magn. Reson. Imaging 26, 1153–1161 (2007). [DOI] [PubMed] [Google Scholar]
- Positano V., Pepe A., Santarelli M. F., Scattini B., De Marchi D., Ramazzotti A., Forni G., Borgna-Pignatti C., Lai M. E., Midiri M., Maggio A., Lombardi M., and Landini L., “Standardized T2* map of normal human heart in vivo to correct T2* segmental artifacts,” NMR Biomed. 20, 578–590 (2007). [DOI] [PubMed] [Google Scholar]
- Bracewell R., The Fourier Transform and Its Applications (McGraw-Hill, New York, 1978. [Google Scholar]
- Vanhamme L., Sundin T., Hecke P. V., and Huffel S. V., “MR spectroscopy quantitation: A review of time-domain methods,” NMR Biomed. 10.1002/nbm.695 14, 233–246 (2001). [DOI] [PubMed] [Google Scholar]
- Koehl P., “Linear prediction spectral analysis of NMR data,” Prog. Nucl. Magn. Reson. Spectrosc. 34, 257–299 (1999). [Google Scholar]
- Belkic D., “Exponential convergence rate (the spectral convergence) of the fast Pade transform for exact quantification in magnetic resonance spectroscopy,” Phys. Med. Biol. 10.1088/0031-9155/51/24/014 51, 6483–6512 (2006). [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Hwang K. P., Elliott A. M., Shetty A., Hazle J. D., and Stafford R. J., “Dynamic chemical shift imaging for image-guided thermal therapy: Analysis of feasibility and potential,” Med. Phys. 10.1118/1.2831915 35, 793–803 (2008). [DOI] [PubMed] [Google Scholar]
- Kumaresan R. and Tufts D. W., “Estimating the parameters of exponentially damped sinusoids and pole-zero modeling in noise,” IEEE Trans. Acoust., Speech, Signal Process. 10.1109/TASSP.1982.1163974 30, 833–840 (1982). [DOI] [Google Scholar]
- Cramer H., Mathematical Methods of Statistics (Princeton University Press, Princeton, NJ, 1946). [Google Scholar]
- Rao C., “Information and the accuracy attainable in the estimation of statistical parameters,” Bull. Calcutta Math. Soc. 37, 81–89 (1945). [Google Scholar]
- Priestly M. B., Spectral Analysis and Time Series (Academic, London, 1981). [Google Scholar]
- Steiglitz K. and McBride L., “A technique for the identification of linear systems,” IEEE Trans. Autom. Control 10.1109/TAC.1965.1098181 10, 461–464 (1965). [DOI] [Google Scholar]
- Parks T. W. and Burrus C. S., Digital Filter Design (Wiley, New York, 1987. [Google Scholar]
- Jackson L. B., in Digital Filters and Signal Processing with Matlab Exercises, edited by Norwell M. A. (Kluwer, Dordrecht, 1996), p. 502. [Google Scholar]
- Williamson D. C., Hawesa H., Thacker N. A., and Williams S. R., “Robust quantification of short echo time 1H magnetic resonance spectra using the Pade approximant,” Magn. Reson. Med. 762–771 (2006). [DOI] [PubMed] [Google Scholar]
- Belkic D. and Belkic K., “The fast Pade transform in magnetic resonance spectroscopy for potential improvements in early cancer diagnostics,” Phys. Med. Biol. 10.1088/0031-9155/50/18/010 50, 4385–4408 (2005). [DOI] [PubMed] [Google Scholar]
- Haacke E. M., Brown R. W., Thompson M. R., and Venkatesan R., Magnetic Resonance Imaging: Physical Principles and Sequence Design (Wiley, New York, 1999). [Google Scholar]
- Bankson J. A., Stafford R. J., and Hazle J. D., “Partially parallel imaging with phase-sensitive data: Increased temporal resolution for magnetic resonance temperature imaging,” Magn. Reson. Med. 10.1002/mrm.20378 53, 658–665 (2005). [DOI] [PubMed] [Google Scholar]
- McDannold N., Hynynen K., Oshio K., and Mulkern R. V., “Temperature monitoring with line scan echo planar spectroscopic imaging,” Med. Phys. 10.1118/1.1350434 28, 346–355 (2001). [DOI] [PubMed] [Google Scholar]
- Poon R. T., Fan S. T., Tsang F. H., and Wong J., “Locoregional therapies for hepatocellular carcinoma: A critical review from the surgeon’s perspective,” Ann. Surg. 235, 466–486 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., “Non-invasive MR thermography using the water proton chemical shift,” Int. J. Hyperthermia 10.1080/02656730500204495 21, 547–560 (2005). [DOI] [PubMed] [Google Scholar]
- Chen L., Wansapura J. P., Heit G., and Butts K., “Study of laser ablation in the in vivo rabbit brain with MR thermometry,” J. Magn. Reson. Imaging 16, 147–152 (2002). [DOI] [PubMed] [Google Scholar]
- Akhlaghpoor S., Tomasian A., Arjmand Shabestari A., Ebrahimi M., and Alinaghizadeh M. R., “Percutaneous osteoid osteoma treatment with combination of radiofrequency and alcohol ablation,” Clin. Radiol. 62, 268–273 (2007). [DOI] [PubMed] [Google Scholar]
- Ji X., Shao R., Elliott A. M., Stafford R. J., Esparza-Coss E., Bankson J. A., Liang G., Luo Z. P., Park K., Markert J. T., and Li C., “Bifunctional gold nanoshells with a superparamagnetic iron oxide-silica core suitable for both MR imaging and photothermal therapy,” J. Phys. Chem. C 10.1021/jp0702245 111, 6245–6251 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbiati L., “New applications of ultrasonography: Interventional ultrasound,” Eur. J. Radiol. 27, S200–206 (1998). [DOI] [PubMed] [Google Scholar]
- Xiao Y. Y., Tian J. L., Li J. K., Yang L., and Zhang J. S., “CT-guided percutaneous chemical ablation of adrenal neoplasms,” AJR, Am. J. Roentgenol. 190, 105–110 (2008). [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Raman S. S., Yu N. C., and Lu D. S., “MR-guided percutaneous ethanol injection for hepatocellular carcinoma in a 0.2T open MR system,” J. Magn. Reson. Imaging 22, 566–571 (2005). [DOI] [PubMed] [Google Scholar]
- Roberts D. A., Rosen M. A., Clark T. W., Mondschein J., Soulen M. C., Siegelman E., and J. S.Leigh, Jr., “Chemical-shift MR imaging of acetic acid during percutaneous chemical ablation therapy: Preliminary work,” J. Vasc. Interv. Radiol. 13, 1055–1059 (2002). [DOI] [PubMed] [Google Scholar]
- Shinmoto H., Mulkern R. V., Oshio K., Silverman S. G., Colucci V. M., and Jolesz F. A., “MR appearance and spectral features of injected ethanol in the liver: Implication for fast MR-guided percutaneous ethanol injection therapy,” J. Comput. Assist. Tomogr. 21, 82–88 (1997). [DOI] [PubMed] [Google Scholar]
- Sironi S., Livraghi T., and DelMaschio A., “Small hepatocellular carcinoma treated with percutaneous ethanol injection: MR imaging findings,” Radiology 180, 333–336 (1991). [DOI] [PubMed] [Google Scholar]
- Ishihara Y., Calderon A., Watanabe H., Okamoto K., Suzuki Y., and Kuroda K., “A precise and fast temperature mapping using water proton chemical shift,” Magn. Reson. Med. 10.1002/mrm.1910340606 34, 814–823 (1995). [DOI] [PubMed] [Google Scholar]
- Rieke V. and Butts Pauly K., “MR thermometry,” J. Magn. Reson. Imaging 27, 376–390 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]