Abstract
Transgenic mice that overexpress cyclooxygenase-2 (COX-2) selectively in podocytes are more susceptible to glomerular injury by adriamycin and puromycin (PAN). To investigate the potential roles of COX-2 metabolites, we studied mice with selective deletion of prostanoid receptors and generated conditionally immortalized podocyte lines from mice with either COX-2 deletion or overexpression. Podocytes that overexpressed COX-2 were virtually indistinguishable from wild-type podocytes but were significantly more sensitive to PAN-induced injury, produced more prostaglandin E2 and thromboxane B2, and had greater expression of prostaglandin E2 receptor subtype 4 (EP4) and thromboxane receptor (TP). Treatment of COX-2-overexpressing podocytes with a TP antagonist reduced apoptosis, but treatment with an EP4 antagonist did not. In contrast, podocytes from COX-2-knockout mice exhibited increased apoptosis, markedly decreased cell adhesion, and prominent stress fibers. In vivo, selective deletion of podocyte EP4 did not alter the increased sensitivity to adriamycin-induced injury observed in mice overexpressing podocyte COX-2. In contrast, genetic deletion of TP in these mice prevented adriamycin-induced injury, with attenuated albuminuria and foot process effacement. These results suggest that basal COX-2 may be important for podocyte survival, but overexpression of podocyte COX-2 increases susceptibility to podocyte injury, which is mediated, in part, by activation of the thromboxane receptor.
Podocytes play a crucial role in regulation of glomerular function, and podocyte injury is an essential feature of progressive glomerular diseases. Although our understanding of podocyte biology has dramatically increased in recent years, mechanisms underlying functional and structural podocyte disturbances in renal diseases are still incompletely understood.1 Recent studies indicate that local podocyte damage can spread to induce injury in otherwise healthy podocytes and further affect both glomerular endothelial and mesangial cells, implying that even limited podocyte injury might initiate a vicious cycle of progressive glomerular damage.2
Mice with cyclooxygenase-2 (COX-2) gene deletion exhibit impaired glomerulogenesis and renal cortical development.3 However, increased expression of COX-2 in podocytes has been reported in various experimental models of progressive glomerular injury4,5 and in cultured podocytes stimulated by mechanical stress.6 Furthermore, in models of renal ablation, diabetic nephropathy, and salt-sensitive hypertension, inhibition of COX-2 activity by selective COX-2 inhibitors significantly decreases proteinuria and progressive renal injury.7–11 To determine if increased podocyte COX-2 expression plays a pathogenic role in glomerular injury, we recently generated COX-2 transgenic mice driven by a nephrin promoter, successfully inducing selective upregulation of COX-2 expression in podocytes. Although glomerular structure and function were normal at baseline in these transgenic mice, administration of either adriamycin or puromycin (PAN) led to significantly increased albuminuria compared with wild-type mice and induced further upregulation of COX-2 and downregulation of the slit diaphragm molecule nephrin. These studies suggested that increased podocyte COX-2 in response to injury may predispose the podocyte to further injury.12,13 In cultured podocytes, mechanical stress induced COX-2 and expression of the prostaglandin E2 (PGE2) receptor subtype 4 (EP4), and PGE2 stimulation of stretched podocytes resulted in a loss of actin stress fiber organization.6 These results suggest that enhanced prostanoid signaling may be a facilitating event for morphologic changes and may directly influence podocyte function under pathophysiological conditions that promote synthesis of COX-2 metabolites. To investigate potential roles of COX-2 metabolites in podocyte function, we generated conditionally immortalized podocyte lines with either deficiency or overexpression of COX-2. In further in vitro and in vivo studies, we identified a role for COX-2-derived prostanoids as potential mediators of podocyte injury.
Results
Activation of Podocyte Prostaglandin Production in Response to Injury
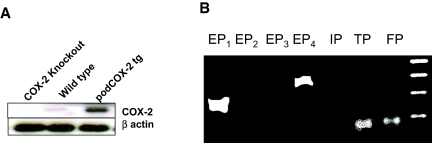
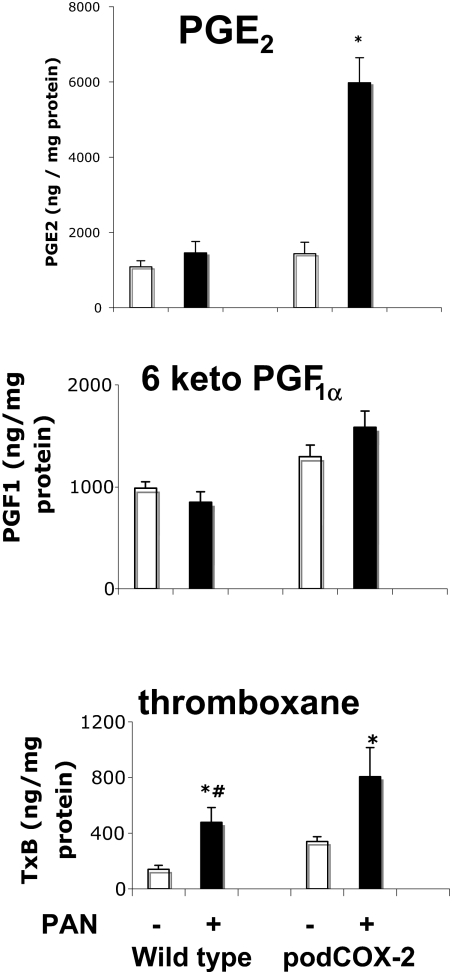
To investigate the potential role of prostanoids in podocyte injury, we generated conditionally immortalized cultured podocytes from wild-type mice and mice with increased podocyte COX-2 expression (podCOX-2-tg). Immunoreactive COX-2 expression is indicated in Figure 1A. As expected, the podocytes from podCOX-2-tg mice exhibited a high basal level of immunoreactive COX-2, whereas a low level of expression was detectable in podocytes from wild-type mice. We previously showed that podCOX-2-tg mice exhibited significantly increased glomerular injury in response to adriamycin. To determine the prostanoid receptor(s) that might be involved in this podocyte injury response, we initially determined prostanoid receptor mRNA expression in cultured wild-type mouse podocytes. In agreement with previous findings,14 cultured wild-type podocytes expressed mRNA for the PGE2 receptor subtypes EP1 and EP4, the thromboxane A2 receptor (TP), and the prostaglandin F2α (PGF2α) receptor (FP), but did not express detectable EP2, EP3, or prostacyclin (intraperitoneally) receptor mRNA (Figure 1B). Similar to wild-type podocytes, podCOX-2-tg podocytes also expressed only EP1 and EP4, TP, and FP. We exposed cells to PAN (50 μg/ml) for 24 h and measured production of PGE2, prostacyclin (6-keto-PGF1α), and thromboxane B2 (TxB2) in PAN-treated podCOX-2-tg and wild-type podocytes. In podCOX-2-tg podocytes, PAN significantly increased PGE2 production (fourfold basal levels, P < 0.05) and TxB2 production (2.4-fold basal levels, P < 0.05) but only minimally increased prostacyclin production (1.2-fold basal levels, NS) (Figure 2). In podocytes from wild-type mice, PAN also stimulated TxB2 production (threefold basal levels, P < 0.05).
Figure 1.
Expression of COX-2 and EP receptor expression in differentiated cultured podocytes. (A) Expression of COX-2 in differentiated cultured podocytes. Low levels of immunoreactive COX-2 could be detected in cultured wild-type podocytes but not in COX-2-knockout podocytes. Higher levels of COX-2 expression were seen in COX-2 transgenic podocytes. (B) Prostaglandin receptor expression in differentiated podocytes. RT-PCR with specific primers (sequences in Table 2) indicated that EP1, EP4, TP, and FP were expressed in differentiated podocytes, whereas EP2, EP3, and prostacyclin (intraperitoneally) receptor mRNA were not detectable.
Figure 2.
Puromycin-induced production of (top) PGE2, (middle) PGF1α, and (bottom) TxB2 in podocytes from podCOX-2-tg mice. Differentiated podocytes were cultured as described in Concise Methods. Puromycin (50 μg/ml) was added to wild-type and podCOX-2-tg podocytes 24 h before the measurement of prostaglandin production. Results were normalized to total cellular protein content (n = 4; *P < 0.05 compared with basal level; #P < 0.05, between wild type + PAN and podCOX-2-tg + PAN).
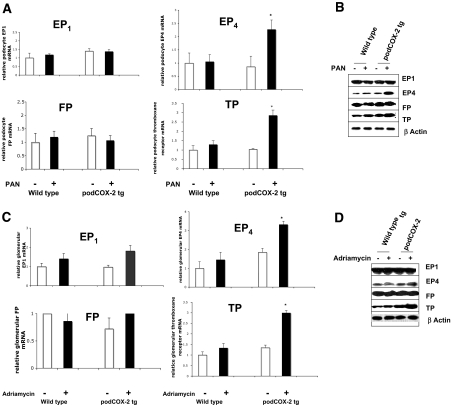
Puromycin induced EP4 and TP mRNA expression in the podCOX-2-tg podocytes but not in wild-type podocytes (2.3 ± 0.4 versus 1.1 ± 0.3 and 2.9 ± 0.3 versus 1.3 ± 0.2 fold control, respectively, n = 4, P < 0.05 compared with wild-type and podCOX-2-tg basal levels or wild type + PAN), whereas EP1 and FP mRNA levels did not increase (Figure 3A). Similarly, protein levels of EP4 and TP increased in the PAN-treated podCOX-2-tg podocytes (Figure 3B).
Figure 3.
Activation of EP receptors in podocyte injury. (A) Puromycin stimulated EP4 and TP mRNA expression in podocytes from podCOX-2-tg mice. RNA was isolated from cultured differentiated podocytes in each group with or without PAN administration: EP1, EP4, TP, and FP (PGF2α receptor). mRNA expression were measured by real-time RT-PCR as described in Concise Methods (n = 4; *P < 0.05 compared with basal level or wild type + PAN). (B) Puromycin-induced EP4 and TP expression in cultured podocytes from podCOX-2-tg mice. EP1, EP4, FP, and TP from differentiated podocytes were measured with their specific antibodies by immunoblotting (detail in Concise Methods). The photographs are representative of three separate experiments. (C) Adriamycin upregulated glomerular EP4 and TP mRNA in podCOX-2-tg mice. Glomeruli were isolated from wild-type and podCOX-2-tg mice with or without adriamycin injection as described in Concise Methods. Expression of EP1, EP4, TP, and FP mRNA were tested as in (A) (n = 4; *P < 0.05 compared with basal level or wild type + adriamycin). (D) Adriamycin stimulated glomerular EP4 and TP expression in podCOX-2-tg mice. EP1, EP4, FP, and TP from isolated glomeruli were measured with their specific antibodies by immunoblotting as in (B). The photographs are representative of three separate experiments.
Prostanoid receptor mRNA and protein expression also were determined in glomeruli isolated from wild-type and podCOX-2-tg mice under basal conditions or 6 wk after administration of adriamycin. mRNA levels of EP4 and TP increased significantly in glomeruli isolated from adriamycin-treated podCOX-2-tg mice (3.3 ± 0.2 and 3.0 ± 0.1 fold control of podCOX-2-tg, n = 4, P < 0.05 compared with basal levels of wild type, podCOX-2-tg, or wild type + adriamycin) (Figure 3C). Protein levels of EP4 and TP also increased in glomeruli from podCOX-2-tg mice in response to adriamycin (Figure 3D).
Effect of TP or EP4 Receptor Deletion on Adriamycin-Induced Podocyte Injury in podCOX-2-tg Mice
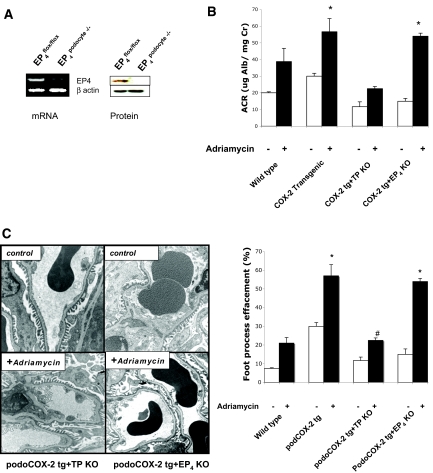
We have demonstrated previously that the increased adriamycin-induced glomerular injury seen in podCOX-2-tg mice was prevented by a selective COX-2 inhibitor.12 To determine whether the alterations in podocyte structure and function seen in vivo were due to alterations in either EP4 or TP activation, we crossed podCOX-2-tg mice with mice with selective deletions of these receptors. Because global EP4 knockout mice have decreased viability due to patent ductus arteriosus,15 patent foramen ovale,16 or both, we selectively deleted EP4 from podocytes by crossing floxed EP4 mice with podocin-Cre transgenic mice and then crossing the EP4podocyte −/− mice with podCOX-2-tg mice. Because EP4 is not readily detectable in glomeruli by immunochemistry in wild-type mice (data not shown), we confirmed successful podocyte EP4 receptor targeting by reverse transcription PCR (RT-PCR) and immunoblotting of isolated glomeruli (Figure 4A).
Figure 4.
The effect of prostanoid receptor deletion on adriamycin nephropathy in podCOX-2-tg mice. (A) Characterization of EP4podocyte−/− mice. Top panel indicated results of real-time RT-PCR from isolated glomeruli, indicating decreased EP4 mRNA in EP4podocyte−/− mice. The right panel demonstrated undetectable EP4 protein in EP4podocyte−/− glomeruli. β-Actin was used as a loading control. (B) Thromboxane receptor deficiency prevented adriamycin-induced albuminuria in podCOX-2-tg mice (n = 6 to 11; *P < 0.05). (C) Thromboxane receptor deletion prevented adriamycin-induced foot process effacement and mesangial sclerosis in podCOX-2-tg mice. The photographs are representative of three separate experiments. The graph indicates semiquantitative data of foot process enfacement (n = 3 to 5; *P < 0.05 compared with basal level in wild type or transgenic; #P < 0.05 compared with transgenic + adriamycin).
There were no apparent structural abnormalities detectable by light or electron microscopy or increased albuminuria at baseline in podCOX-2-tg mice with either TP deletion or podocyte EP4 deletion. In response to adriamycin, podCOX-2-tg x EP4 podocyte−/− mice exhibited comparable increases to podCOX-2-tg mice in albuminuria, which began to increase by 2 wk (Supplemental Figure 1) and had similar levels of albuminuria at 6 wk (50.4 ± 7.0 versus 55.0 ± 11.7 μg albumin (Alb)/mg creatinine, NS) (Figure 4B) and structural glomerular abnormalities (moderate mesangial sclerosis and foot process effacement, 54.0 ± 1.8% versus 56.7 ± 7.9%, NS) (Figure 4C). In contrast, in response to adriamycin, podCOX-2-tg x T−/− mice had significantly less albuminuria (26.8 ± 4.8 μg Alb/mg creatinine, n = 6, P < 0.05) (Figure 4B) and foot process effacement (15.0 ± 1.7%, P < 0.05) (Figure 4C), although no significant differences were detectable by light microscopy.
Pathologic Impact of COX-2 Overexpression in PAN-Induced Podocyte Injury
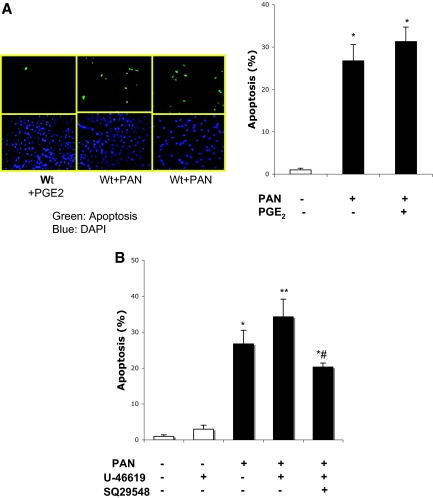
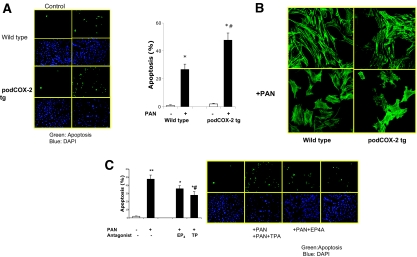
There were no apparent differences in basal attachment spreading and survival of podCOX-2-tg podocytes compared with those of wild-type podocytes (Figure 6A). However, PAN induced significantly more apoptosis in podCOX-2-tg podocytes than in wild-type podocytes (Figure 5A), although cytoskeleton reorganization was observed in both cell types (Figure 5B). L-161982, a specific EP4 receptor antagonist, did not significantly decrease apoptosis in response to PAN administration (Figure 5C). In contrast, the TP antagonist SQ29548 significantly reduced PAN-induced apoptosis (from 47.8 ± 5.1% to 28.0 ± 4.4%, n = 4, P < 0.05) (Figure 5C).
Figure 6.
Effect of exogenous prostanoids on PAN-stimulated podocyte injury. (A) Exogenous PGE2 did not further increase PAN-induced podocyte apoptosis. Preincubation of PGE2 (1 μM) did not significantly affect PAN-induced apoptosis in wild-type differentiated podocytes (n = 4; *P < 0.05 compared with wild-type control). (B) Exogenous TxA2 analogue augmented PAN-stimulated podocyte apoptosis. A TxA2 analogue, U-46619 (1 μM), augmented PAN-induced apoptosis, which was prevented by the TP-specific receptor antagonist SQ29548 (n = 4; *P < 0.05 compared with wild-type control; **P < 0.05 compared with wild type + PAN; #P < 0.05 compared with wild type + U-46619).
Figure 5.
Effect of COX-2 overexpression in PAN-induced podocyte injury. (A) Puromycin induced more severe apoptosis in podCOX-2-tg podocytes. Left: a representative photograph from four experiments. Top: podocytes in wild type. Bottom: podocytes from podCOX-2-tg mice. Green or blue staining as indicated in Figure 3B. Graph is quantitative results of apoptosis (n = 4; *P < 0.05 compared with basal level; #P < 0.05 compared with wild type + PAN). (B) Puromycin-induced cytoskeleton reorganization. Puromycin altered cytoskeleton organization in wild-type and podCOX-2-tg podocytes. The compressed filaments are appreciated in both types after PAN stimulation. The photos represent three repeated experiments. (C) Thromboxane receptor antagonist attenuated PAN-induced apoptosis in podCOX-2-tg podocytes (n = 4; **P < 0.01 compared with basal levels). Thromboxane receptor antagonist SQ29548 (10 μM) significantly reduced PAN-induced apoptosis (n = 4; *P < 0.05 compared with basal levels; #P < 0.05 compared with COX-2 transgenic podocytes + PAN). The EP4 receptor antagonist L-161982 (10 μM) did not significantly decrease apoptosis compared with podCOX-2-tg podocytes + PAN.
Impact of Exogenous PGE2 and Thromboxane Analogues on Cultured Podocytes
Prostaglandin E2 has been reported to promote podocyte cytoskeleton disorder induced in response to mechanical stretching.6 However, when exogenous PGE2 was administered to wild-type podocytes 10 min before PAN, there was no change in the amount of apoptosis (Figure 6A). In contrast, a thromboxane A2 (TxA2) analogue, U-46619, significantly increased PAN-induced podocyte apoptosis, which could be inhibited by the TP receptor antagonist SQ29548 (Figure 6B).
Effect of COX-2 Deletion in Podocytes
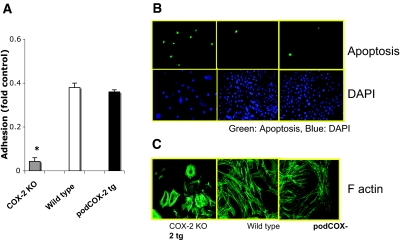
We also developed conditionally immortalized podocyte cell lines from COX-2−/− mice. As expected, these podocytes expressed no immunoreactive COX-2 (Figure 1A). The COX-2-knockout podocytes had markedly decreased cell adhesion to type IV collagen (Figure 7A) and laminin (data not shown). When grown on glass coverslips, the COX-2-knockout podocytes demonstrated cytoskeleton disorganization and displayed prominent cortical actin rings (Figure 7B). Furthermore, they had an increased basal rate of apoptosis (65.3 ± 5.9% in COX-2-knockout mice versus 1.0 ± 0.4% in wild-type mice, P < 0.05) (Figure 7C). Puromycin administration resulted in virtually complete detachment of COX-2-knockout podocytes.
Figure 7.
Critical role of basal COX-2 in podocyte survival and cytoskeleton structure. (A) COX-2 deletion led to the detachment of differentiated podocytes. Adhesion to collagen IV was measured as described in Concise Methods (n = 4; *P < 0.05 compared with wild type or COX-2 transgenic group). There was no significant difference between these two groups. (B) COX-2 deletion increased basal podocyte apoptosis. Green (FITC) staining indicated the cell apoptosis in the top panel; blue (4′,6-diamidino-2-phenylindole) staining indicated the total podocytes. (C) COX-2 deletion led to disruption of the normal podocyte actin cytoskeleton. Actin filaments were stained by phalloidin binding assay. In COX-2-knockout podocytes, the stress fibers could be easily appreciated; some cells displayed a prominent cortical actin ring.
Discussion
Cyclooxygenases are rate-limiting enzymes that metabolize arachidonic acid to prostaglandin G2 and subsequently to prostaglandin H2. Prostaglandin H2 then serves as a common substrate for further synthesis of specific prostanoids (prostaglandins and thromboxane) by specific synthases. Prostaglandins mediate many important physiologic processes, such as regulation of vascular tone, salt and water homeostasis, and mediation or modulation of hormonal actions. Unlike the constitutive isoform COX-1, expression and activity of COX-2 are induced in response to a variety of stimuli, and COX-2 metabolites mediate inflammation and pain. Prostanoids are bioactive lipids that exert autocrine and paracrine functions by binding to specific G-protein-coupled receptors to activate intracellular signaling and gene transcription. Of note, PGE2 can interact with four G-protein-coupled receptor subtypes: EP1, EP2, EP3, and EP4. Of interest for the present studies, EP1 is coupled to Gq and is a vasoconstrictor, whereas EP4 is coupled to Gs and induces vasodilation. PGF2α and TxA2 are also Gq-coupled receptors that induce increases in intracellular calcium and vasoconstriction. Of note, thromboxane receptors are activated not only by TxA2 but also by the common prostanoid precursor prostaglandin H2.
COX-2 expression increases in podocytes in response to a variety of glomerular injuries,4,5 and our previous studies demonstrated that increased podocyte COX-2 expression predisposes to further podocyte injury in response to adriamycin or PAN.12 In the present studies, we found that adriamycin selectivity increased glomerular expression of the PGE2 receptor subtype EP4 and the thromboxane receptor TP. When we crossed the podocyte COX-2-overexpressing mice to TP knockout mice, the increased sensitivity to adriamycin injury was reversed, whereas podCOX-2-tg mice with selective podocyte EP4 deletion were not protected.
We also generated cultured podocytes from wild-type and podCOX-2-tg mice and found that, similar to the in vivo observations, PAN administration increased PGE2 and thromboxane production and EP4 and TP expression in podCOX-2-tg podocytes. We also found that PAN-induced apoptosis in podCOX-2-tg podocytes was significantly decreased by a TP receptor antagonist but not by an EP4 receptor antagonist. Similarly, in wild-type podocytes, administration of a thromboxane analogue augmented PAN-induced apoptosis, whereas PGE2 administration had no effect. Therefore, these results suggest that podocyte injury may induce COX-2 expression and increase thromboxane production, which then sensitizes the podocyte to further injury. Previous studies in cultured podocytes indicated that mechanical stress induced increased COX-2-derived PGE2 production and alterations in the cytoskeleton.6 In preliminary studies, we did detect that podocyte adhesion was inhibited by PGE2 administration, which was reversed by the EP4 antagonist (data not shown). Recently, PGE2 was reported to initiate a positive feedback loop via EP4 in the podocyte signaling network, activated by increased glomerular capillary pressure, which could be detrimental to podocyte health and glomerular filtration barrier integrity.17 However, other studies have suggested that PGE2 serves a cytoprotective function in renal epithelial cells18,19 In other cell types, PGE2 has been shown to promote cell survival, cell growth, migration, invasion, and angiogenesis.20,21 In this regard, podocytes from COX-2-knockout mice were found to exhibit significant apoptosis even under basal conditions, suggesting that COX-2-derived prostaglandins also may serve a trophic cytoprotective function.
Although selective COX-2 inhibitors have been shown to decrease proteinuria and retard progressive glomerular injury in experimental models of kidney disease,22 the associated cardiovascular risks preclude the therapeutic use of COX-2 inhibitors in progressive renal injury. Thromboxane A2 and thromboxane receptor activation mediate renal vasoconstriction and contribute to the pathogenesis of angiotensin-II-dependent hypertension,23 progression of diabetic nephropathy,24–27 and renal failure in endotoxemic mice28 or rats with 5/6 nephrectomy.29 Older studies suggested that thromboxane synthase inhibitors or receptor antagonists were efficacious in streptozotocin-induced renal damage,30 lupus nephritis,31–33 cyclosporine nephrotoxicity,34–36 remnant kidney,37 and renal allograft rejection.38 Our current results strongly suggest that thromboxane receptor antagonism may serve as a viable potential therapeutic option to retard podocyte injury.
In summary, these studies indicate that COX-2 and its derived prostaglandins may mediate basal podocyte differentiation and survival, at least in vitro. Although increased podocyte COX-2 concentrations do not significantly affect basal physiologic function in response to renal insults, increased podocyte COX-2 expression does contribute to susceptibility to further injury, at least in part through increased thromboxane production and increased thromboxane receptor expression.
Concise Methods
Materials
Puromycin was purchased from Sigma Chemical (St. Louis, MO). Adriamycin was from Bedford Laboratories (Belford, OH). SC58236 was a gift from Pfizer (St. Louis, MO). The EP4 receptor antagonist L-161982 was a generous gift from Dr. John Obenchain (Merck Frosst Canada & Co., Kirkland, Quebec, Canada). The rabbit anti-murine COX-2 polyclonal antibody, PGE2, 6-keto-PGF1α, TxB2 enzyme immunoassay kits, specific TP receptor antagonist SQ29548, TxA2 analogue-U-46619, PGE2, specific EP1 receptor antagonist SC19920, and rabbit anti-EP1, EP4, FP, and TP polyclonal antibodies were purchased from Cayman Chemical (Ann Arbor, MI); goat anti-nephrin, rabbit anti-synaptopodin, and goat anti-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescence phallotoxin was from Molecular Probes/Invitrogen Detection Technologies (Eugene, OR), terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling apoptosis detection kit was from Upstate (Lake Placid, NY), and Dynabeads M-450 Tosylactivated were purchased from Dynal Biotec (Lake Success, NY). Nylon cell strainers were purchased from BD Biosciences (Bedford, MA). Albuwell and creatinine kits were from Exocell (Philiadaphia, PA). Other reagents were purchased from Sigma Chemical (St. Louis, MO).
Experimental Animals
Nephrin-driven COX-2 transgenic mice on the B6/D2 background (podCOX-2-tg) were genotyped by PCR and Southern blotting as described previously.12 The EP4flox/flox mice39 generated a podocyte-selective EP4 deletion (EP4podocyte−/−) after Cre-dependent excision by breeding to podocin-specific Cre mice, which were from Susan Quaggin40,41 Both thromboxane-receptor-deficient mice (TP−/−) (on a B6/D2 background)42 and EP4podocyte−/− mice (on a C57/B6 background) were further bred to podCOX-2-tg mice. Both B6/D212 and C57/B643 mice are resistant to adriamycin-induced renal injury.
To determine glomerular injury in response to adriamycin, age- and strain-matched (10- to 12-wk-old) male mice were administered adriamycin (10 mg/kg by retroorbital injection) or saline as controls. Urine was collected weekly in metabolic cages, and the mice were euthanized at the end of 6 wk. Glomerular injury was assessed histologically by light microscopy and electron microscopy.
All animal procedures were approved by the Animal Care and Use Committee of Vanderbilt University Medical Center.
Isolation of Glomeruli
Glomeruli were isolated immediately after euthanasia, using a modification of the Dynabeads method of Takemoto et al.44 Briefly, mice were anesthetized by an intraperitoneal injection of Nembutal (0.05 mg/g body wt) and perfused through the descending aorta with 8 × 107 Dynabeads (Dynal Biotech, ASA, Oslo, Norway) diluted in PBS. The kidneys were removed, minced into 1-mm3 pieces, and digested in collagenase (1 mg/ml collagenase A and 100 U/ml deoxyribonuclease I in HBSS) at 37°C for 30 min with gentle agitation. The collagenase-digested tissue was gently pressed through a 100-μm cell strainer using a flattened pestle, and the cell strainer was then washed with 5 ml of cold HBSS. The filtered cells were passed through a new cell strainer without pressing, and the cell strainer was washed with 5 ml of cold HBSS. The cell suspension was then centrifuged at 200 × g for 5 min at 4°C. The supernatant was discarded, and the cell pellet was resuspended in 2 ml of cold HBSS. Finally, glomeruli containing Dynabeads were gathered by a magnetic particle concentrator and washed three times with cold HBSS. During the procedure, kidney tissues were kept at 4°C except for the collagenase digestion at 37°C. The preparation consisted of >90% glomeruli.
Culture of Mouse Podocytes
To generate immortal podocyte cell lines, the COX-2-knockout mice3 or podCOX-2-tg mice were bred to ImmortoMouse (Charles River, Wilmington, MA), and only mice expressing both the SV40 gene and either COX-2 deficiency or increased podocyte COX-2 expression were selected. Primers used for genotyping are summarized in Table 1.
Table 1.
Primer sequences for genotyping
| Genotype | Upper Primer | Lower Primer | Fragment Size (bp) |
|---|---|---|---|
| EP4flox/flox | 5′-GTT AGA TGG GGG GAG GGG ACA ACT | 5′-TCT GTG AAG CGA GTC CTT AGG CT | Wild type: 243 EP4flox/flox: 344 |
| Podocin-Cre | 5′-GCA TAA CCA GTG AAA CAG CAT TGC TG | 5′-GGA CAT GTT CAG GGA TCG CCA GGC G | Cre: 275 |
| TP−/− | 5′-TGG GGG TAG CTA TGG TGT TC | 5′-GTG AGA AGG GCC GTG TGA T | Wild type: 150 TP−/−: 700 |
| 5′-CTT CCT CGT GCT TTA CGG TA | |||
| ImmortoMouse (SV40) | 5′-CCT GGA ATA GTC ACC ATG | 5′-CAA TGC CTG TTT CAT GCC | ImmortoMouse: 420 |
| CCOX-2 KO | 5′-ACC TCT GCG ATG CTC TTC C | 5′-CAC CAT TGA ATC CAG TCC GG | Wild type: 857 |
| 5′-CTT GGG TGG AGA GGC TAT TC | 5′-AGG TGA GAT GAC AGG AGA TC | COX-2 KO: 280 | |
| podCOX-2-tg | 5′-ACA GAA AGA CTG CGA CAG TCA CAG AC | 5′-GCT CAT ACA TTC CCC CAC GGT TTT GAC | podCOX-2-tg: 972 |
Conditionally immortalized mouse podocyte cells were generated as described previously.45,46 Briefly, epithelial cells from decapsulated glomeruli were isolated and grown at high density in RPMI 1640 + 10% FCS. After 5 to 7 d, the cultures were slightly trypsinized to remove glomerular remnants and immunodissected with an anti-nephrin antibody. Podocytes were maintained in the medium with mouse recombinant IFN-γ (Sigma, St. Louis, MO) (with gradually reduced concentrations from 100 to 10 U/ml) for permissive conditions (33°C). Dishes were coated with type I collagen (Sigma, St. Louis, MO), and cells were plated at a density of 1 × 105 cells/cm2. Podocytes were cloned and characterized by specific markers (e.g., nephrin and Wilms Tumor 1 for podocytes and synaptopodin for differentiated podocytes). Cells between passage 10 and 15 were used for all experiments. Differentiation was induced by switching the incubation temperature to 37°C (nonpermissive conditions) and removing IFN-γ from the culture media for 10 to 14 d.
RNA Extraction and Real-Time RT-PCR
Total glomerular and podocyte RNA were extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH) and chloroform and further purified with an RNeasy kit (Qiagen, Valencia, CA). Real-time RT-PCR was performed with specific primers (Table 2) and IQ SYBR Green Supermix Kit (Bio-Rad Laboratories, Hercules, CA) at 95°C for 3 min and then 95°C for 20 s, 62°C for 20 s, and 72°C for 60 s in 40 cycles. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. Comparative CT and statistical analysis were calculated as per instructions of User Bulletin #2 from ABI (Applied Biosystems, Hammonton, NJ).
Table 2.
Primer sequence for real-time PCR
| Upper Primer | Lower Primer | |
|---|---|---|
| EP1 | 5′-CGG TGG AAG GAC AAC AAG CAG | 5′-CCA TGC AGC CAC CCA GGA AAT G |
| EP4 | 5′-AGA CGC TCA TGT GCT GGA AG | 5′-TGG ACG CAT AGA CTG CAA AG |
| TP | 5′-GGC CAC ACT CTG CCG GGT CTA | 5′-AGC AAG GGC ATC CAA CAC ACC GTG |
| FP | 5′-CAC ATA TCC TCA GTG GTT AGG | 5′-GTT CTG GAC GCT TGG ATT TG |
| IP | 5′-CGG GCA CGA GAG GAT GAA GT | 5′-CGG GCA CAC AGG CAA CAC AAC |
Immunoblotting
Cultured cells or isolated glomeruli were homogenized as described previously.47 Proteins were resuspended in SDS sample buffer, diluted in SDS buffer containing 2-mercaptoethanol (Sigma Chemical, St. Louis, MO), and boiled for 10 min before loading. The samples were run on 8% SDS-PAGE gels under reducing conditions and transferred onto a polyvinylidene fluoride membrane (Immobilion-P; Millipore, Bedford, MA). After being blocked with 5% nonfat milk in Tween®/Tris-buffered salt solution, the membranes were exposed to the primary antibody overnight at 4°C, followed by horseradish-peroxidase-conjugated secondary antibodies. The horseradish peroxidase signal was enhanced using the europium-sensitized luminescence method, and the images were developed on high-performance autoradiography film (Hyperfilm MP; Amersham Biosciences, Buckinghamshire, UK). Membranes were rehybridized with goat anti-β-actin antibody (Santa Cruz, CA) to normalize protein loading.
Phallodin Binding Assay
Cultured differentiated podocytes on glass coverslips were washed in PBS, fixed in 3.7% paraformaldehyde in PBS for 20 min, and extracted with acetone at −20°C for 3 to 5 min. After being blocked with 1% BSA, podocytes were incubated with 0.1% saponin in PBS containing a saturating amount (0.4 μM) of Oregon Green 488 phalloidin (Invitrogen Corporation, Carlsbad, CA) for 30 min at room temperature in darkness and examined at 500 to 520 nm.
Cell Adhesion Assay
We utilized modifications of a previously described protocol.48 Briefly, 100 μl of single-cell podocyte suspensions (1 × 106 cells/ml) in serum-free medium containing 0.1% BSA were added to 96-well plates coated with collagen IV or laminin and blocked with 0.1% BSA, followed by incubation for 60 min at 37°C. Nonadherent cells were removed by washing the wells with PBS. Cells were then fixed with 4% paraformaldehyde and stained with 1% crystal violet, and the OD of the cell lysates was read at 570 nm. Cells bound to wells coated with 100% FCS were used to indicate 100% adhesion (control), and cells bound to wells coated with 1% BSA were used to evaluate background. Each sample was performed in triplicate and expressed as fold of control. Because the data for adhesion to collagen IV and laminin produced similar results, we only show the former in this paper.
Apoptosis Detection
Measurements utilized a terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling apoptosis detection kit (Upstate, Lake Placid, NY). 4′,6-Diamidino-2-phenylindole was used for counterstain. The percentage of apoptotic cells in 400 total cells from the same field was determined for quantification.
Urinary Albumin and Creatinine
Urinary albumin levels were determined by ELISA using a murine microalbuminuria ELISA kit (AlbuwellM; Exocell, Philadelphia, PA). The urine creatinine concentration was measured with a microplate assay kit (Creatinine Companion; Exocell, Philadelphia, PA). All measurements were performed in duplicate, and albuminuria was determined as the ratio of urinary albumin (μg/ml) to creatinine (mg/ml).
Statistical Analysis
All values are presented as mean ± SEM. ANOVA and Bonferroni t tests were used for statistical analysis, and differences were considered significant when P < 0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. John Obenchain (Merck Frosst Canada & Co., Kirkland, Quebec, Canada) for the EP4 receptor antagonist L-161982 and Pfizer (St. Louis, Missouri) for SC58236. This work was supported by funds from the Department of Veterans Affairs and National Institutes of Health Grants DK51265, DK62794, and DK79341.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Shankland SJ:The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa I, Ma J, Motojima M, Matsusaka T:Podocyte damage damages podocytes: autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens 14: 205–210, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM:Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Wang JL, Cheng HF, Zhang MZ, McKanna JA, Harris RC:Selective increase of cyclooxygenase-2 expression in a model of renal ablation. Am J Physiol 275: F613–F622, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S:Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest 107: 889–898, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martineau LM, McVeigh LI, Jasmin BJ, Kennedy CR:p38 MAP kinase mediates mechanically-induced cyclooxygenase-2 and prostaglandin EP4 receptor expression in podocytes: Implications for the actin cytoskeleton. Am J Physiol Renal Physiol 286: F693–F701, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Wang JL, Cheng HF, Shappell S, Harris RC:A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int 57: 2334–2342, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Sanchez PL, Salgado LM, Ferreri NR, Escalante B:Effect of cyclooxygenase-2 inhibition on renal function after renal ablation. Hypertension 34: 848–853, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Horiba N, Kumano E, Watanabe T, Shinkura H, Sugimoto T, Inoue M:Subtotal nephrectomy stimulates cyclooxygenase 2 expression and prostacyclin synthesis in the rat remnant kidney. Nephron 91: 134–141, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Kong WX, Ma J, Gu Y, Yang HC, Zuo YQ, Lin SY:[Renal protective effects of specific cyclooxygenase-2 inhibitor in rats with subtotal renal ablation]. Zhonghua Nei Ke Za Zhi 42: 186–190, 2003 [PubMed] [Google Scholar]
- 11.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ:A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Cheng H, Wang S, Jo YI, Hao CM, Zhang M, Fan X, Kennedy C, Breyer MD, Moeckel GW, Harris RC:Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol 18: 551–559, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Jo YI, Cheng H, Wang S, Moeckel GW, Harris RC:Puromycin induces reversible proteinuric injury in transgenic mice expressing cyclooxygenase-2 in podocytes. Nephron Exp Nephrol 107: e87–e94, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bek M, Nusing R, Kowark P, Henger A, Mundel P, Pavenstadt H:Characterization of prostanoid receptors in podocytes. J Am Soc Nephrol 10: 2084–2093, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Trivedi DB, Sugimoto Y, Loftin CD:Attenuated cyclooxygenase-2 expression contributes to patent ductus arteriosus in preterm mice. Pediatr Res 60: 669–674, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Chamberlin M, Lozynski J:To go against nature: Manipulating the neonatal ductus arteriosus with prostaglandin. Newborn Infant Nurs Rev 6: 158–162, 2006 [Google Scholar]
- 17.Faour WH, Gomi K, Kennedy CR:PGE2 induces COX-2 expression in podocytes via the EP4 receptor through a PKA-independent mechanism. Cell Signal 20: 2156–2164, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Weber TJ, Monks TJ, Lau SS:PGE2-mediated cytoprotection in renal epithelial cells: evidence for a pharmacologically distinct receptor. Am J Physiol 273: F507–F515, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Grekas D, Kalekou H, Tourkantonis A:Effect of prostaglandin E2 (PGE2) in the prevention of acute renal failure in anesthetized dogs. In situ renal preservation. Ren Fail 11: 27–31, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Stenson WF:Prostaglandins and epithelial response to injury. Curr Opin Gastroenterol 23: 107–110, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Aoudjit L, Potapov A, Takano T:Prostaglandin E2 promotes cell survival of glomerular epithelial cells via the EP4 receptor. Am J Physiol Renal Physiol 290: F1534–F1542, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hermann M, Shaw S, Kiss E, Camici G, Buhler N, Chenevard R, Luscher TF, Grone HJ, Ruschitzka F:Selective COX-2 inhibitors and renal injury in salt-sensitive hypertension. Hypertension 45: 193–197, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM:Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension 43: 364–369, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Okumura M, Imanishi M, Okamura M, Hosoi M, Okada N, Konishi Y, Morikawa T, Miura K, Nakatani T, Fujii S:Role for thromboxane A2 from glomerular thrombi in nephropathy with type 2 diabetic rats. Life Sci 72: 2695–2705, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA:The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes 55: 110–119, 2006 [PubMed] [Google Scholar]
- 26.Sebekova K, Eifert T, Klassen A, Heidland A, Amann K:Renal effects of S18886 (Terutroban), a TP receptor antagonist, in an experimental model of type 2 diabetes. Diabetes 56: 968–974, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Michel F, Simonet S, Vayssettes-Courchay C, Bertin F, Sansilvestri-Morel P, Bernhardt F, Paysant J, Silvestre JS, Levy BI, Feletou M, Verbeuren TJ:Altered TP receptor function in isolated, perfused kidneys of nondiabetic and diabetic ApoE-deficient mice. Am J Physiol Renal Physiol 294: F120–F129, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Boffa JJ, Just A, Coffman TM, Arendshorst WJ:Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J Am Soc Nephrol 15: 2358–2365, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lariviere R, Moreau C, Rodrigue ME, Lebel M:Thromboxane blockade reduces blood pressure and progression of renal failure independent of endothelin-1 in uremic rats. Prostaglandins Leukot Essent Fatty Acids 71: 103–109, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Uriu K, Kaizu K, Hashimoto O, Komine N, Etoh S:Acute and chronic effects of thromboxane A2 inhibition on the renal hemodynamics in streptozotocin-induced diabetic rats. Kidney Int 45: 794–802, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Spurney RF, Fan PY, Ruiz P, Sanfilippo F, Pisetsky DS, Coffman TM:Thromboxane receptor blockade reduces renal injury in murine lupus nephritis. Kidney Int 41: 973–982, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Salvati P, Lamberti E, Ferrario R, Ferrario RG, Scampini G, Pugliese F, Barsotti P, Patrono C:Long-term thromboxane-synthase inhibition prolongs survival in murine lupus nephritis. Kidney Int 47: 1168–1175, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Yoshida T, Kameda H, Ichikawa Y, Tojo T, Homma M:Improvement of renal function with a selective thromboxane A2 synthetase inhibitor, DP-1904, in lupus nephritis. J Rheumatol 23: 1719–1724, 1996 [PubMed] [Google Scholar]
- 34.Perico N, Rossini M, Imberti O, Malanchini B, Cornejo RP, Gaspari F, Bertani T, Remuzzi G:Thromboxane receptor blockade attenuates chronic cyclosporine nephrotoxicity and improves survival in rats with renal isograft. J Am Soc Nephrol 2: 1398–1404, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Spurney RF, Mayros SD, Collins D, Ruiz P, Klotman PE, Coffman T:Thromboxane receptor blockade improves cyclosporine nephrotoxicity in rats. Prostaglandins 39: 135–146, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Smith SR, Creech EA, Schaffer AV, Martin LL, Rakhit A, Douglas FL, Klotman PE, Coffman TM:Effects of thromboxane synthase inhibition with CGS 13080 in human cyclosporine nephrotoxicity. Kidney Int 41: 199–205, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Zoja C, Perico N, Corna D, Benigni A, Gabanelli M, Morigi M, Bertani T, Remuzzi G:Thromboxane synthesis inhibition increases renal prostacyclin and prevents renal disease progression in rats with remnant kidney. J Am Soc Nephrol 1: 799–807, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Coffman TM, Ruiz P, Sanfilippo F, Klotman PE:Chronic thromboxane inhibition preserves function of rejecting rat renal allografts. Kidney Int 35: 24–30, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Schneider A, Guan Y, Zhang Y, Magnuson MA, Pettepher C, Loftin CD, Langenbach R, Breyer RM, Breyer MD:Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis 40: 7–14, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB:Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Quaggin SE:A “molecular toolbox” for the nephrologist. J Am Soc Nephrol 13: 1682–1685, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Thomas DW, Mannon RB, Mannon PJ, Latour A, Oliver JA, Hoffman M, Smithies O, Koller BH, Coffman TM:Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest 102: 1994–2001, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ:Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C:A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mundel P, Reiser J, Kriz W:Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8: 697–705, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Shankland SJ, Pippin JW, Reiser J, Mundel P:Podocytes in culture: Past, present, and future. Kidney Int 72: 26–36, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Cheng HF, Harris RC:Cyclooxygenase-2 expression in cultured cortical thick ascending limb of Henle increases in response to decreased extracellular ionic content by both transcriptional and post-transcriptional mechanisms. Role of p38-mediated pathways. J Biol Chem 277: 45638–45643, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Cai S, Bulus N, Fonseca-Siesser PM, Chen D, Hanks SK, Pozzi A, Zent R:CD98 modulates integrin β1 function in polarized epithelial cells. J Cell Sci 118: 889–899, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.