Abstract
Podocyte dysfunction, one of the major causes of proteinuria, leads to glomerulosclerosis and end stage renal disease, but its underlying mechanism remains poorly understood. Here we show that Wnt/β-catenin signaling plays a critical role in podocyte injury and proteinuria. Treatment with adriamycin induced Wnt and activated β-catenin in mouse podocytes. Overexpression of Wnt1 in vivo activated glomerular β-catenin and aggravated albuminuria and adriamycin-induced suppression of nephrin expression, whereas blockade of Wnt signaling with Dickkopf-1 ameliorated podocyte lesions. Podocyte-specific knockout of β-catenin protected against development of albuminuria after injury. Moreover, pharmacologic activation of β-catenin induced albuminuria in wild-type mice but not in β-catenin-knockout littermates. In human proteinuric kidney diseases such as diabetic nephropathy and focal segmental glomerulosclerosis, we observed upregulation of Wnt1 and active β-catenin in podocytes. Ectopic expression of either Wnt1 or stabilized β-catenin in vitro induced the transcription factor Snail and suppressed nephrin expression, leading to podocyte dysfunction. These results suggest that targeting hyperactive Wnt/β-catenin signaling may represent a novel therapeutic strategy for proteinuric kidney diseases.
Proteinuria, resulting from defects in glomerular filtration, is an early pathologic feature of many common forms of chronic kidney diseases (CKDs) such as diabetic nephropathy (DN) and focal segmental glomerulosclerosis (FSGS). As CKD inevitably progresses into end-stage kidney failure, a devastating condition that necessitates renal replacement therapy and is growing at a rate of 6 to 7% annually, this poses an enormous challenge to the medical community and society worldwide.1,2 The glomerular filtration barrier is composed of three layers: inner fenestrated endothelium, glomerular basement membrane, and outer glomerular visceral epithelium (podocyte). Increasing evidence indicates that podocyte dysfunction, as manifested by foot process effacement and phenotypic alteration, plays a crucial role in conferring the defective glomerular filtration and onset of proteinuria.3,4 Recent studies have established that mutations or deletion of several slit diaphragm (SD)-associated proteins (e.g., nephrin and podocin) contribute to podocyte dysfunction, leading to the development of massive proteinuria and congenital nephrotic syndrome in animal models and patients.5–7 However, how podocyte dysfunction is triggered in many acquired forms of proteinuric kidney diseases including DN, in which mutations of SD proteins are rare,8,9 remains ambiguous.
Wnts are a family of secreted glycoproteins that play an essential role in organogenesis, stem cell homeostasis, and tumor formation.10–13 Upon binding to their cell membrane receptor Frizzled and coreceptors LRP5/6, Wnts induce a series of downstream signaling events involving Disheveled, axin, adenomatosis polyposis coli, and glycogen synthase kinase-3β (GSK-3β), resulting in dephosphorylation of β-catenin. This causes the stabilization of β-catenin, leading to its translocation into the nuclei, where it binds to T cell factor/lymphoid enhancer-binding factor to stimulate the transcription of Wnt target genes.14 This canonical Wnt/β-catenin signaling in vivo is tightly regulated in multiple ways. There are several secreted antagonists of Wnt signaling, including soluble Frizzled-related protein, Wnt inhibitory factor, and Dickkopf (DKK).10,14 Of them, DKK is quite unique in that it specifically inhibits the canonical Wnt signaling pathway by binding to the LRP5/6 component of the receptor complex.15,16
Wnt/β-catenin signaling is implicated in kidney development and diseases. In metanephric kidney, Wnt4 and Wnt9b are highly expressed in the branching ureteric bud and in nephrogenic mesenchyme, and canonical Wnt signaling is functionally important for renal epithelial cell lineage specification and mesenchymal-epithelial transition during metanephric kidney development.17–19 However, in adult kidney, Wnt signaling appears silenced.17,20,21 Re-activation of Wnt/β-catenin signaling occurs in certain types of kidney diseases, including obstructive nephropathy.22–25 However, whether Wnt/β-catenin signaling is implicated in podocyte homeostasis and dysfunction, is unknown.
Here we report that Wnt/β-catenin signaling is activated in glomerular podocytes after injury in vivo and in vitro. Ectopic expression of exogenous Wnt1 gene exacerbates podocyte injury and albuminuria in mice induced by adriamycin (ADR), whereas blockade of Wnt signaling with DKK1 ameliorates podocyte dysfunction. Furthermore, genetic ablation of β-catenin in podocytes protects against the development of proteinuria. These studies underscore a critical role of hyperactive Wnt/β-catenin signaling in mediating podocyte dysfunction and proteinuria. Our findings suggest that Wnt/β-catenin signaling could be a novel target for therapeutic intervention of a variety of proteinuric kidney diseases.
Results
Wnt/β-Catenin Signaling Is Activated in Podocytes after Injury In vivo and In vitro
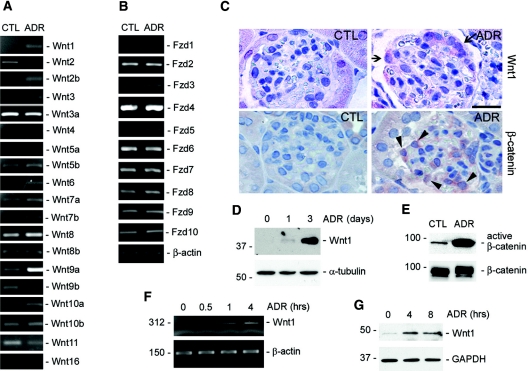
We first examined Wnt expression in ADR nephropathy, a widely used and well characterized model of podocyte injury and proteinuria in mice.26,27 As shown in Figure 1A, a comprehensive survey of all 19 Wnts in mouse by reverse transcriptase(RT)-PCR showed that the mRNA expression of several Wnts, including Wnt1, Wnt2b, Wnt6, and Wnt9a, was significantly induced in the isolated glomeruli as early as day 1 after ADR injection. Moderate induction of Wnt4 and Wnt10a and slight inhibition of Wnt2 and Wnt11 were also evident. We further examined the expression of various Frizzled receptors in the isolated glomeruli. Among ten different Fzd receptors, most were expressed in mouse glomeruli, and only Fzd5 mRNA was induced after ADR injury (Figure 1B). Consistent with the mRNA induction, Wnt1 protein expression in the glomeruli was also induced starting at day 1 and reached the peak at day 3 (Figure 1, C and D), indicating that Wnt1 induction is an early event that significantly precedes or parallels with the onset of proteinuria in this model. Immunohistochemical staining revealed that Wnt1 appeared to be specifically induced in glomerular podocytes after ADR injection (Figure 1C). To examine the biologic consequence of Wnt induction in this model, we next investigated the activation of β-catenin, the canonical pathway of Wnt signaling in the glomeruli after ADR treatment. As shown in Figure 1C, increased cytoplasmic and nuclear β-catenin staining was observed in the glomeruli, presumably in podocytes, on the basis of the location of the positive cells, after ADR injection. Similarly, Western blot analysis showed a dramatic increase in dephosphorylated, active β-catenin in the glomeruli isolated from ADR-treated mice, as compared with vehicle controls (Figure 1E).
Figure 1.
Wnt/β-catenin signaling is activated in podocytes after injury in vivo and in vitro. (A and B) RT-PCR demonstrates an altered expression of various (A) Wnt and (B) Frizzled receptor (Fzd) mRNA in the glomeruli isolated from mice at 1 d after ADR injection. (C) Immunohistochemical staining shows Wnt1 and β-catenin induction in podocytes after ADR injury. Arrows indicate the Wnt1-positive cells. Arrowheads show the cytoplasmic and nuclear staining of β-catenin. Scale bar, 30 μm. (D) Western blot demonstrates an induction of Wnt1 protein in the glomeruli isolated from mice at different time points as indicated after ADR injection. (E) Activation of β-catenin signaling in the isolated glomeruli after ADR injection. Glomerular lysates were immunoblotted with antibodies against active and total β-catenin, respectively. (F and G) RT-PCR and Western blot demonstrate upregulation of Wnt1 (F) mRNA and (G) protein expression in podocytes after injury in vitro. Mouse podocytes were treated with ADR (10 μg/ml) for various periods of time as indicated.
We sought to investigate the regulation and function of Wnt1 in the subsequent experiments, because it is the prototype member of the Wnt family and its signal is known to be mediated through β-catenin-dependent canonical pathway.12,14 Furthermore, the de novo induction of Wnt1 (Figure 1, A and C) after ADR may suggest a unique role for it in podocyte injury in this model. Using cultured mouse podocytes in vitro, we found that ADR could directly induce Wnt1 expression. As illustrated in Figure 1F, Wnt1 mRNA was rapidly induced as early as 0.5 h after ADR treatment. Similarly, Wnt1 protein expression was also induced (Figure 1G). Together, it appears clear that Wnt signaling is activated in the early stage during podocyte injury induced by ADR in vivo and in vitro.
A Pivotal Role of Wnt Signaling in Podocyte Dysfunction and Albuminuria In vivo
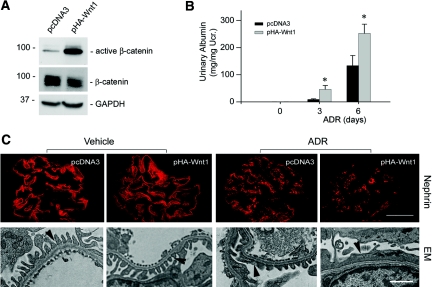
To assess the potential role of Wnt/β-catenin activation in podocyte dysfunction and albuminuria, we intended to express exogenous Wnt1 in vivo by use of hydrodynamic-based gene delivery into mice.28,29 As shown in Figure 2A, dephosphorylated, active β-catenin levels were increased in the isolated glomeruli at 16 h after Wnt1 plasmid injection, indicating glomerular β-catenin signaling activation. We found that overexpression of exogenous Wnt1 in vivo significantly exacerbated albuminuria in mice at different time points after ADR injection (Figure 2B). Urine albumin levels were substantially elevated in mice that received Wnt1 plasmid injection, compared with pcDNA3 controls (Figure 2B). Immunofluorescence staining for nephrin illustrated that exogenous Wnt1 expression did not significantly affect nephrin abundance and distribution under normal conditions (Figure 2C). Nephrin protein was reduced, and its distribution was altered from a linear to granular pattern after ADR injection. In agreement with albuminuria data (Figure 2B), mice with ectopic expression of Wnt1 gene manifested more severe podocyte injury, as illustrated by less nephrin abundance and a more distorted distribution, compared with the pcDNA3 controls (Figure 2C). Electron microscopy (EM) also displayed more severe foot process effacement after ADR administration in mice that received Wnt1 plasmid injection, compared with pcDNA3 controls (Figure 2C).
Figure 2.
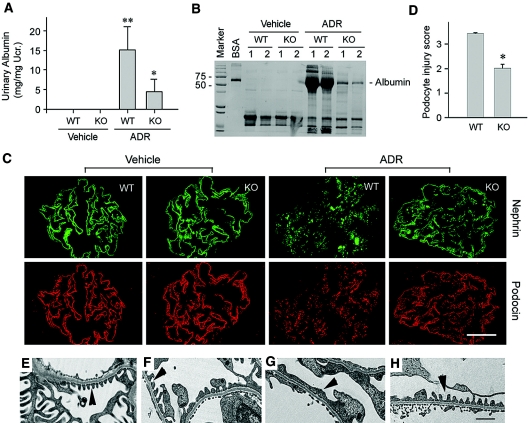
Exogenous Wnt1 aggravates podocytes dysfunction and albuminuria in vivo. BALB/c mice were administrated with either Wnt1 expression vector (pHA-Wnt1) or control plasmid (pcDNA3) through a hydrodynamics-based tail vein injection. (A) Wnt1 gene delivery induces active β-catenin accumulation in the glomeruli. Glomerular lysates from the mice injected with either pcDNA3 or pHA-Wnt1 expression vector were immunoblotted with antibodies against active β-catenin, total β-catenin, and glyceraldehyde 3-phosphate dehydrogenase, respectively. (B) Exogenous Wnt1 exacerbates albuminuria in mice after ADR injection. *P < 0.05 versus pcDNA3 controls (n = 6). (C) Representative micrographs showing nephrin staining and ultrastructure of podocyte foot processes in different groups as indicated. Immunofluorescence staining shows nephrin protein expression and distribution in different groups. Scale bar = 30 μm. Podocyte foot processes integrity was revealed by EM. Arrowheads indicate secondary foot process and SD. Scale bar, 1 μm.
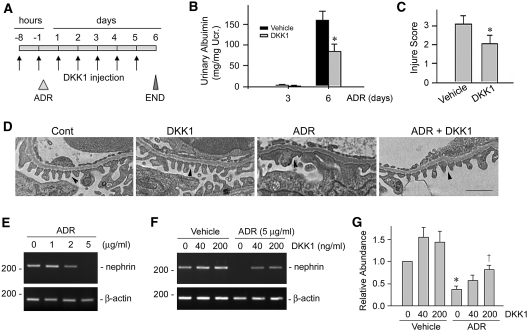
To ascertain the role of Wnt signaling in the pathogenesis of albuminuria, we next blocked endogenous Wnt signaling in mice by using Wnt antagonist. To this end, mice were injected with recombinant DKK1 protein, a natural inhibitor of the canonical Wnt signaling by the virtue of its ability to bind to and block the LRP5/6 coreceptor.15 As shown in Figure 3A, mice were injected intravenously with recombinant mouse DKK1 protein and ADR in different intervals as indicated. At day 6 after ADR injection, severe albuminuria was induced in the vehicle control group. However, albuminuria in the DKK1-treated group was significantly reduced (Figure 3B). Podocyte injury as defined by nephrin reduction was also ameliorated after DKK1 treatment (Figure 3C). EM studies also revealed an improvement of the integrity of podocyte foot process and SD after DKK1 administration (Figure 3D). We further investigated the protective role of Wnt antagonist on podocyte after injury in vitro. As demonstrated in Figure 3E, ADR suppressed nephrin mRNA expression in cultured mouse podocytes in a dose-dependent manner. However, incubation with recombinant DKK1 significantly restored nephrin expression (Figure 3, F and G), providing direct evidence for the involvement of Wnt signaling in podocyte dysfunction and proteinuria.
Figure 3.
Blockade of Wnt signaling attenuates podocyte injury and proteinuria. (A) Experimental design showing the strategy of recombinant DKK1 protein and ADR injections in mice. (B and C) DKK1 reduces (B) ADR-induced albuminuria and (C) podocyte injury (as defined by nephrin loss and altered distribution) in mice. *P < 0.05 versus vehicle group (n = 8). (D) Representative micrographs showing podocyte foot processes in different groups as indicated. Arrowheads indicate secondary foot process and SD. Scale bar, 1 μm. (E through G) DKK1 restores nephrin expression after ADR treatment in cultured mouse podocytes. (E) ADR suppressed nephrin expression in a dose-dependent manner in podocytes. (F and G) DKK1 restored nephrin expression after ADR treatment. (F) Representative RT-PCR result and (G) relative abundances of nephrin mRNA (with value in control group = 1.0) are shown. *P < 0.05 versus controls; †P < 0.05 versus the group without DKK1 treatment (n = 3).
Mice with Podocyte-Specific Ablation of β-Catenin Are Protected against Podocyte Dysfunction and Albuminuria
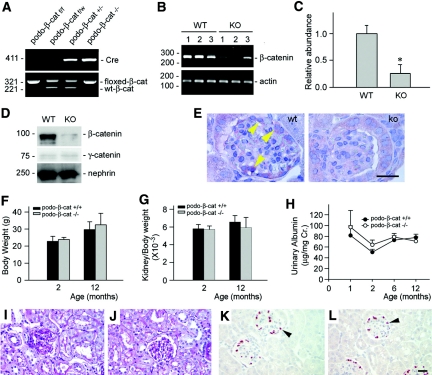
We next investigated the role of β-catenin, the principal downstream effector of canonical Wnt signaling, in mediating podocyte injury and proteinuria. To this end, we created conditional knockout mice in which β-catenin gene was selectively disrupted in glomerular podocytes by use of the Cre-LoxP system (Figure 4A). RT-PCR results demonstrated that β-catenin mRNA level was substantially reduced in the isolated glomeruli from the mice with podocyte-specific knockout of β-catenin (designated as podo-β-cat−/−), compared with those from control littermates (Figure 4, B and C). Similarly, β-catenin protein was also remarkably diminished in the isolated glomeruli from podo-β-cat−/− mice (Figure 4D), whereas glomerular γ-catenin abundance was not significantly changed. Immunohistochemical staining showed that β-catenin was largely localized in podocytes in the glomeruli of wild-type mice after ADR injury, but was absent in the glomerular podocytes of podo-β-cat−/− mice (Figure 4E). There was no difference in body weight, kidney/body weight ratio, and urine albumin between podo-β-cat−/− mice and their wild-type littermates up to 12 mo after birth (Figure 4, F through H). Kidney histology was normal in podo-β-cat−/− mice (Figure 4, I and J). Podocyte numbers and density as shown by WT-1 staining were similar in podo-β-cat−/− mice and their control littermates (Figure 4, K and L). Nephrin, podocin expression and distribution, and the ultrastructure of SD were intact in podo-β-cat−/− mice (data not shown). In short, β-catenin appears dispensable for podocyte development, survival, and function in vivo.
Figure 4.
Mice with podocyte-specific deletion of β-catenin are healthy. (A) Genotyping of the mice by PCR analysis of genomic DNA. (B and C) RT-PCR analysis shows the glomerular β-catenin mRNA abundance in podo-β-cat−/− mice and their control littermates. WT, wild-type mice; KO, podo-β-cat−/− mice. Numbers (1, 2, and 3) denote each individual animal in a given group. *P < 0.05 versus WT (n = 3). (D) Western blot analysis of glomerular β-catenin protein in podo-β-cat−/− mice. Glomerular lysates were prepared from WT or KO mice and immunoblotted with antibodies against β-catenin, γ-catenin, and nephrin, respectively. (E) Immunohistochemical staining shows loss of β-catenin in glomerular podocytes. Arrowhead indicates positive staining for β-catenin in the glomeruli of WT mice at 1 d after ADR injection. No β-catenin staining was observed in the glomeruli of KO mice at the same conditions. (F through L) Mice with podocyte-specific ablation of β-catenin are phenotypically normal. There was little difference in (F) body weight, (G) kidney weight index, and (H) urinary albumin between KO mice and control littermates at different time points (n = 4 to 6). Kidney histology as shown by (I and J) periodic-acid-Schiff staining and (K and L) podocyte by WT-1 staining are normal in (J and L) KO mice, compared with (I and K) control littermates.
We further investigated the role of β-catenin in podocyte dysfunction by challenging podo-β-cat−/− mice with ADR. As shown in Figure 5A, wild-type control mice developed severe albuminuria at day 3 after intravenous injection of ADR at a dose of 25 mg/kg body wt. However, under the same conditions, podo-β-cat−/− mice were largely protected against the development of albuminuira (Figure 5A). SDS-PAGE analysis of urine samples revealed that albumin was the major constituent of urine proteins in mice after ADR injury (Figure 5B). Compared with wild-type controls, much less urine albumin was detected in podo-β-cat−/− mice after ADR injection (Figure 5B). Of note, genetically modified mice with mixed genetic backgrounds were relatively resistant to ADR-induced albuminuria, compared with BALB/c mice. Even using a high-dose protocol (ADR at 25 mg/kg body wt), the urinary albumin levels in these mice only reached to 10 to 20% of that in BALB/c mice produced by a low dose of ADR (10 mg/kg body wt). Immunofluorescence staining illustrated that nephrin and podocin levels were reduced and their distribution was changed from a linear to granular pattern in control mice after ADR injury (Figure 5C). However, both nephrin and podocin levels and their staining pattern were largely preserved in podo-β-cat−/− mice under the same conditions (Figure 5, C and D). We further examined the ultrastructure of podocyte foot processes and SD by EM. As shown in Figure 5, E through H, foot process effacement was evident and SD disappeared in most areas of the glomeruli in wild-type mice after ADR injection, whereas these ultrastructural lesions were largely prevented in podo-β-cat−/− mice (Figure 5H).
Figure 5.
Podocyte-specific ablation of β-catenin protects against albuminuria and podocyte injury induced by ADR in mice. (A) Urinary albumin concentration in WT and KO mice at 3 d after ADR injection. **P < 0.01 versus vehicle control, *P < 0.05 versus WT mice after ADR injection (n = 4 to 9). (B) Representative SDS-PAGE shows the urine proteins in different groups of mice as indicated. Numbers (1 and 2) denote each individual animal in a given group. (C and D) Immunofluorescence staining demonstrates the abundance and distribution pattern of nephrin and podocin in different groups as indicated. (C) Representative micrographs and (D) semiquantitative determination of podocyte injury (as defined by nephrin loss and altered distribution) are given. *P < 0.05 versus WT group (n = 4 to 9). (E through H) EM shows the alterations of podocyte foot processes after ADR injection in WT and KO mice. (E) WT control, (F) KO control, (G) WT after ADR, and (H) KO after ADR. (G) Foot process effacement is evident in WT podocytes after ADR. Scale bar, 1 μm.
Activation of β-Catenin in Podocytes Is Sufficient for Inducing Albuminuria In vivo
To further explore the role of β-catenin in podocyte dysfunction, we used an opposite strategy by inducing its activation. As shown in Figure 6A, treatment of mouse podocytes with lithium chloride (LiCl) caused β-catenin activation and induced its nuclear translocation in cultured mouse podocytes. β-catenin was predominantly localized at cell-cell adhesion sites in podocytes as shown by an indirect immunofluorescence staining (Figure 6A); however, it clearly underwent nuclear translocation after LiCl treatment (Figure 6C, arrowheads), indicating its activation.
Figure 6.
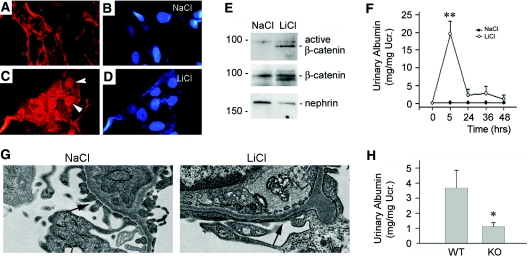
Activation of β-catenin signaling induces podocyte dysfunction and albuminuria in vivo. (A through D) Incubation with LiCl (30 mM) induces β-catenin activation and nuclear translocation in cultured podocytes. (A and B) NaCl control, (C and D) LiCl (30 mM), (A and C) β-catenin staining, and (B and D) DAPI. Arrowheads indicate nuclear staining of β-catenin after LiCl treatment. (E) Activation of β-catenin signaling in the glomeruli by LiCl in vivo. Glomerular lysates from the mice injected with either LiCl or control NaCl were immunoblotted with antibodies against active β-catenin, total β-catenin, and nephrin, respectively. (F) Activation of β-catenin by LiCl causes heavy, albeit transient, albuminuria in mice. **P < 0.01 versus NaCl control (n = 4 to 10). (G) EM shows podocyte foot process effacement in mice after LiCl injection. (H) Podocyte-specific ablation of β-catenin protects mice from development of albuminuria after LiCl injection. *P < 0.05 versus WT controls (n = 7).
We next examined the effect of β-catenin activation by LiCl on podocyte dysfunction and albuminuria in vivo. As shown in Figure 6E, BALB/c mice injected with LiCl displayed an increased level of dephosphorylated, active β-catenin in the isolated glomeruli, compared with those injected with sodium chloride (NaCl) control. Interestingly, heavy, albeit transient, albuminuria was detected at 5 h after LiCl injection in mice (Figure 6F). Consistent with albuminuria, podocyte foot process effacement was evident in mouse glomeruli at 5 h after LiCl injection, as revealed by EM studies (Figure 6G). To clarify the involvement of podocyte β-catenin activation in lithium-induced albuminuria, we treated podo-β-cat−/− mice and their control littermates with LiCl. As shown in Figure 6H, albuminuria was induced in control littermates at 5 h after injection, whereas the urine albumin level was reduced in podo-β-cat−/− mice. These data suggest that podocyte-specific β-catenin activation is primarily responsible for the development of albuminuria in mice after LiCl treatment. Of note, urinary albumin levels in genetically modified mice with mixed genetic backgrounds (Figure 6H) were lower than that in BALB/c mice (Figure 6F), indicating the effects of different genetic backgrounds on the susceptibility of mice in response to lithium injury.
Wnt/β-Catenin Signaling Is Activated in Human Proteinuric Kidney Diseases
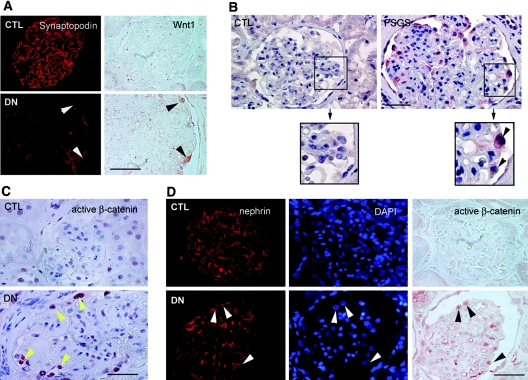
To investigate the relevance of Wnt/β-catenin activation in mediating podocyte dysfunction in proteinuric kidney diseases other than ADR nephropathy, we studied Wnt1 expression and β-catenin activation in the most common forms of proteinuric kidney disorders such as DN and FSGS. As reported previously,28 administration of streptozotocin in uninephrectomized CD1 mice induced heavy albuminuria and glomerular injury in 3 mo, with renal lesions that imitate the early pathology in human DN. As shown in Supplementary Figure 1A, Wnt1 protein was upregulated in the glomerular cells in this model, compared with the controls. Likewise, Wnt1 protein was also induced in the glomerular cells of human kidney biopsies from patients with DN, whereas Wnt1 was essentially undetectable by immunohistochemical staining in normal human kidney glomeruli (Supplementary Figure 1B). Double staining for Wnt1 and synaptopodin revealed that the Wnt1-positive cells in human DN biopsies were podocytes (Figure 7A, arrowheads). Wnt1 also markedly induced in the glomerular cells of human biopsies from patients with FSGS (Figure 7B). In view of their localization, these Wnt1-positive cells in human FSGS samples also appeared to be podocytes (Figure 7B, arrowheads in the boxed area). Of five human DN and four FSGS samples, four and three of them displayed an increased expression of Wnt1, respectively. Compared with normal controls, active β-catenin was also markedly increased in human proteinuric kidney diseases and predominantly localized in the nuclei of glomerular podocytes (Figure 7C). The nuclear localization of active β-catenin in podocytes in human CKD was further confirmed by triple staining for nephrin, nuclei, and active β-catenin (Figure 7D, arrowheads). Therefore, Wnt/β-catenin signaling is activated, and likely implicated, in the pathogenesis of common forms of human proteinuric kidney diseases such as DN and FSGS.
Figure 7.
Wnt/β-catenin signaling is activated in human proteinuric nephropathies. Human kidney specimens were immunostained with specific antibodies against Wnt1, synaptopodin, nephrin, and active β-catenin, respectively. (A) Representative micrographs of double staining for Wnt1 and synaptopodin in human kidney biopsy from patients with DN. Arrowheads indicate colocalization of Wnt1 and synaptopodin. Scale bar, 40 μm. (B) Representative micrographs demonstrate Wnt1 induction in the glomerular cells in human kidney biopsy from the patients with FSGS. Arrowheads within the boxed area indicate Wnt1-positive cells. Scale bar, 40 μm. (C) Representative micrographs show active β-catenin staining in the nuclei of glomerular cells in human kidney biopsy from patients with DN. Arrowheads (yellow) indicate active β-catenin-positive nuclei. Scale bar, 40 μm. (D) Representative micrographs show the nuclear staining of active β-catenin in podocytes in human kidney biopsy from the patients with DN. Arrowheads indicate the nephrin-positive podocytes with nuclear staining for active β-catenin. Scale bar, 40 μm. CTL, control.
Wnt/β-Catenin Signaling Induces Snail and Suppresses Nephrin Expression In vitro
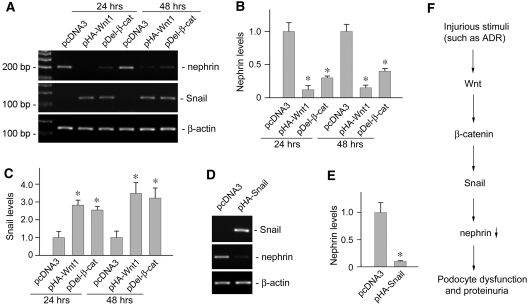
To provide a mechanistic link of Wnt/β-catenin signaling to podocyte dysfunction, we investigated its potential downstream targets that are relevant to podocyte biology. To this end, we first examined the expression of nephrin, a key SD protein that is essential for maintaining the integrity of podocyte structure and function. As shown in Figure 8, transient transfection of mouse podocytes with either HA-tagged Wnt1 expression vector (pHA-Wnt1) or Flag-tagged N-terminal truncated, stabilized β-catenin expression vector (pDel-β-cat) was sufficient to suppress nephrin expression. Interestingly, Wnt1 and stabilized β-catenin also induced the expression of Snail (Figure 8, A and C), a key transcription factor that is implicated in mediating podocyte epithelial to mesenchymal transition (EMT) and dysfunction.30 Furthermore, forced expression of Snail by transfection in cultured podocytes was sufficient for repressing nephrin expression (Figure 8, D and E), suggesting that Snail is an intermediate effector that is responsible for mediating nephrin suppression by Wnt/β-catenin signaling. Therefore, it appears clear that a cascade of pathogenic pathway exists in which Wnt/β-catenin signaling targets key molecules that are vital for podocyte function, thereby promoting podocyte injury and proteinuria (Figure 8F).
Figure 8.
Wnt/β-catenin signaling induces Snail expression and suppresses nephrin in podocytes. (A through C) Wnt1 and β-catenin are sufficient for triggering nephrin suppression and Snail induction in podocytes. Mouse podocytes were transiently transfected with Wnt1 expression vector (pHA-Wnt1), expression plasmid for the stabilized β-catenin with N-terminal deletion (pDel-β-cat), or empty vector pcDNA3, respectively, followed by analyzing nephrin and Snail expression. Representative results on (A) nephrin and Snail mRNA expression and quantitative data on relative (B) nephrin or (C) Snail levels (with value in pcDNA3 control group = 1.0) are presented. *P < 0.05 versus controls (n = 3). (D and E) Snail is sufficient for suppressing nephrin expression in podocytes. Mouse podocytes were transiently transfected with pHA-Snail or pcDNA3 plasmids, followed by analyzing nephrin expression. (D) Representative RT-PCR results and (E) quantitative determination of relative nephrin mRNA levels (with the value in the pcDNA3 control group = 1.0) are presented. *P < 0.05 versus controls (n = 4). (F) Simplified diagram depicts the molecular pathway by which Wnt/β-catenin signaling mediates podocyte dysfunction and proteinuria.
Discussion
The results presented in this study demonstrate that Wnt/β-catenin signaling is a critical player in the pathogenesis of podocyte dysfunction and albuminuria. Several lines of evidence support this conclusion. First, Wnt/β-catenin is activated in the podocytes of mouse and human kidneys with primary glomerular disorders such as ADR nephropathy, DN, and FSGS. Second, ectopic expression of Wnt1 gene in mice exacerbates podocyte injury and albuminuria, whereas blockade of Wnt signaling with its antagonist DKK1 ameliorates podocyte lesions. Third, mice with podocyte-specific ablation of β-catenin are protected against the development of albuminuria after ADR injury. Finally, activation of β-catenin is able to induce albuminuria in a podocyte-specific fashion. Together, these gain- and loss-of-function experiments in whole animal at Wnt and β-catenin levels provide clear evidence for a pivotal role of the hyperactive Wnt/β-catenin signaling in mediating podocytopathy. Our findings also underscore that the Wnt/β-catenin pathway might represent a promising target in developing new classes of therapeutic modalities for the treatment of a variety of proteinuric kidney diseases in humans.
Wnt/β-catenin is an evolutionarily conserved signal pathway that participates in the regulation of a diverse array of cellular processes.10,12 Members of the Wnt family of proteins are highly expressed and of critical importance for nephron formation,17,18 whereas their expression and signaling are largely silenced in the adult kidney. Wnts are able to transmit their signal through both canonical, which is mediated by β-catenin, and noncanonical, β-catenin-independent, pathways.10,14 It appears, however, that the signaling elicited by Wnt in podocytes under pathologic conditions is likely mediated by the canonical pathway. This notion is consistent with the observations that β-catenin is activated in podocytes after Wnt induction. It should be noted that several Wnts are induced simultaneously in the glomeruli after ADR administration (Figure 1A), and they may potentially work in a coordinated fashion to trigger podocyte dysfunction and proteinuria. Accordingly, ectopic expression of Wnt1 alone is not sufficient to initiate podocyte dysfunction in normal mice but aggravates podocyte injury induced by ADR (Figure 2). Interestingly, pharmacologic activation of β-catenin by LiCl is able to induce albuminuira in vivo. It is worthwhile to point out that lithium administration in humans also induces proteinuria, nephrotic syndrome, and progressive nephropathy,31–33 and the severity of renal lesions is closely associated with the duration of lithium administration and cumulative dose.32
Although exactly how β-catenin activation leads to podocyte dysfunction remains elusive, it could be related to its ability to induce cell phenotypic changes in podocytes. Wnt/β-catenin signaling is known to play a central role in cell fate determination11,13 and is implicated in the regulation of EMT, a phenotypic conversion that occurs in embryonic development, cancer metastasis, and tissue fibrosis.34,35 We have recently shown that podocytes also undergo EMT in vitro after TGF-β1 stimulation and in vivo in DN, leading to loss of their characteristic features and acquisition of mesenchymal markers.30 Consistent with this, overexpression of either Wnt1 or stabilized β-catenin by transient transfection is sufficient to suppress nephrin expression in cultured podocytes (Figure 8). In considering that the efficiency of transient transfection in podocytes only reaches about 30 to 60% (Supplementary Figure 2), the magnitude of nephrin suppression in these experiments is quite impressive. Of interest, Wnt1 overexpression and β-catenin activation in podocytes also induces the expression of Snail, a key transcription factor that is generally regarded as a “master gene” to initiate EMT in different circumstances.36–38 Recent studies show that Snail also directly represses nephrin expression in podocytes39 and therefore can function as an mediator that confers β-catenin signaling to nephrin suppression. It is thus becoming clear that Wnt/β-catenin activation may cause podocyte dysfunction by inhibiting nephrin expression, promoting cell dedifferentiation, and phenotypic conversion.
It is important to emphasize that Wnt/β-catenin activation is not limited to a particular form of proteinuric kidney disease such as ADR nephropathy, but appears to occur in many common human CKDs, including DN and FSGS, in which proteinuria and podocyte dysfunction are the primary features of kidney pathology. Recent studies also demonstrate an induction of Wnt2 expression in a rat model of podocyte injury and proteinuria induced by puromycin aminonucleoside, although the status of β-catenin activation was not examined in that study.39 Hence, our data presented here may provide a mechanistic linkage of Wnt/β-catenin activation, Snail induction, nephrin suppression, podocyte dysfunction, and the onset of proteinuria (Figure 8F) and could offer important insights into the pathogenesis of many common forms of proteinuric kidney diseases.
Wnt/β-catenin is a unique cell signaling regulator, because the key component of this signal pathway, β-catenin, also functions as a component of the cadherin complex, which controls cell-cell adhesion.40 As a structural protein, β-catenin interacts with P-cadherin and α-catenin, which links the SD to the actin cytoskeleton network in podocytes. However, podocyte-specific knockout of the β-catenin gene appears to have little effect on podocyte maturation, survival, and function, suggesting that it is functionally redundant in podocyte biology. The function of β-catenin as a structural protein is likely substituted in podo-β-cat−/− mice by γ-catenin, a structurally related protein that also binds to cadherin and α-catenin, and by several other proteins that link the SD to the actin cytoskeleton in podocytes.
In summary, our studies provide a proof of principle that hyperactive Wnt/β-catenin signaling is detrimental, resulting in podocyte dysfunction and proteinuria. Therefore, targeting this signaling may represent a novel strategy for developing therapeutic modalities for proteinuric kidney diseases, disorders that devastate the lives of millions of people worldwide.
Concise Methods
Cell Culture and Treatment
The conditionally immortalized mouse podocyte cell line was kindly provided by Dr. Peter Mundel (Mount Sinai School of Medicine, New York, NY), as described previously.30,41 Cells were cultured at 33°C in RPMI-1640 medium supplemented with 10% fetal bovine serum and recombinant IFN-γ (Invitrogen, Carlsbad, CA). To induce differentiation, podocytes were grown under nonpermissive conditions at 37 °C in the absence of IFN-γ. After serum starvation for 16 h, cells were treated with ADR (Doxorubicin hydrochloride; Sigma, St. Louis, MO) for various periods of time as indicated. For some experiments, podocytes were also transiently transfected with either HA-tagged Wnt1 expression vector (pHA-Wnt1; Upstate biotechnology, Lake Placid, NY) or Flag-tagged N-terminal truncated, stabilized β-catenin expression vector (pDel-β-cat), by using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), as described previously.30 Transfection efficiency in the podocytes typically arranged from 30 to 60% according to estimation after transfection with enhanced GFP expression vector, as shown in Supplementary Figure 2.
Generation of the Podocyte-Specific β-Catenin Knockout Mice and Genotyping
Homozygous floxed β-catenin mice (C57BL/6J strain) were obtained from Jackson Laboratories (Bar Harbor, ME). Transgenic mice expressing Cre recombinase under the control of a 2.5-kb fragment of the human podocin promoter (2.5P-Cre mice) were reported elsewhere.42 By mating β-catenin floxed mice with podocin-Cre transgenic mice, mice that were heterozygous for the β-catenin floxed allele were generated (genotype: β-catfl/+, Cre). These mice were crossbred to inactivate both β-catenin alleles by Cre-mediated excision, thereby creating conditional knockout mice in which β-catenin gene was specifically disrupted in glomerular podocytes (genotype: β-catfl/fl, Cre). The breeding protocol also generated heterozygous littermates (genotype: β-catfl/+, Cre), as well as wild-type and several control groups with different genotype (β-cat+/+; β-catfl/fl; β-catfl/+; β-cat+/+, Cre; referred to as controls). A routine PCR protocol was used for genotyping of tail DNA samples with the following primer pairs: Cre transgene, 5′-AGG-TGT-AGA-GAA-GGC-ACT-TAG-C-3′ and 5′-CTA-ATC-GCC-ATC-TTC-CAG-CAG-G-3′, which generated a 411-bp fragment; and β-catenin genotyping, 5′-AAG-GTA-GAG-TGA-TGA-AAG-TTG-TT-3′ and 5′-CAC-CAT-GTC-CTC-TGT-CTA-TTC-3′, which yielded 324- and 221-bp bands for the floxed and wild-type alleles, respectively. All animals were born normally at the expected Mendelian frequency. All control mice displayed normal phenotype. The Institutional Animal Care and Use Committee at the University of Pittsburgh approved animal experiments.
Animal Models of Podocyte Injury and Proteinuria
The mouse model of podocyte injury and proteinuria was established by intravenous injection of ADR.26,27 Different genetic backgrounds and strains of mice displayed significant divergences in response to ADR injury. All transgenic mice with C57BL/6J or mixed backgrounds were injected with a high dose of ADR protocol (25 mg/kg body wt), whereas BALB/c mice received a low dose of the drug (10 mg/kg body wt). Briefly, podo-β-cat−/− mice and their control littermates were injected with ADR at the dosage of 25 mg/kg body wt in saline solution through tail vein. In some experiments, male BALB/c mice weighing 20 to 22 g were obtained from Harlan Sprague-Dawley (Indianapolis, IN) and injected via the tail vein with ADR at 10 mg/kg body wt. At different time points after ADR injection, mice were sacrificed. Urine, kidney tissue, and glomeruli were collected. For activating endogenous β-catenin signaling in vivo, BALB/c mice, as well as podo-β-cat−/− and their control littermates were intravenously injected with either LiCl or control NaCl at 16 mmol/kg body wt. At 5, 24, and 48 h after injection, mice were sacrificed, and urine and kidney samples were collected. For introducing exogenous Wnt1 in vivo, BALB/c mice were administrated with Wnt1 expression vector (pUSE-Wnt1; Upstate Biotechnology, Lake Placid, NY) at 1 mg/kg by a hydrodynamic-based gene transfer technique via rapid injection of a large volume of DNA solution through the tail vein, as described previously.28 For inhibition of Wnt signaling in vivo, BALB/c mice were injected intravenously with recombinant mouse DKK1 protein (R&D Systems) at 50 μg/kg body wt, as indicated.
Urinary Albumin and Creatinine Assay
Urine albumin was measured by using a mouse Albumin ELISA quantitation kit, according to the manufacturer's protocol (Bethyl Laboratories, Inc., Montgomery, TX). Serum and urine creatinine was determined by a routine procedure as described previously.43
Glomerular Isolation
Glomerular isolation was performed according to the method described elsewhere.43,44 Briefly, mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg) and perfused with 8 × 107 Dynabeads M-450 (Dynal Biotech ASA, Oslo, Norway) diluted in 5 ml of PBS by the abdominal artery. After perfusion, kidneys were removed and cut into 1-mm3 pieces and digested in collagenase A (1 mg/ml) at 37°C for 30 min with gentle shaking. The tissue was then pressed gently through a 100-μm cell strainer (BD Falcon, Bedford, MA). Glomeruli containing Dynabeads were then gathered by using a magnetic particle concentrator. The isolated glomeruli were washed three times with cold PBS and used for subsequent studies.
RNA Extraction and RT-PCR
Total RNA isolation and RT-PCR for detecting Wnt1, nephrin, β-catenin, and Snail mRNA expression were carried out by the procedures described previously.30 Briefly, the first strand of cDNA synthesis was carried out by using a reverse transcription system kit according to the instructions of the manufacturer (Promega, Madison, WI). PCR amplification was performed using HotStar Taq Master Mix Kit (Qiagen, Valencia, CA). The sequences of the primers for 19 different Wnts, 10 Frizzled receptors, and β-actin were described previously.24 The sequences of other primer pairs were as follows: nephrin, 5′-CCC-AAC-ACT-GGA-AGA-GGT-GT-3′ (sense) and 5′-CTG-GTC-GTA-GAT-TCC-CCT-TG-3′ (antisense); β-catenin, 5′-GTC-AGC-TCG-TGT-CCT-GTG-AA-3′ (sense) and 5′-AGT-GGC-TGA-CAG-CAG-CTT-TT-3′ (antisense); and Snail, 5′-AGC-CCA-ACT-ATA-GCG-AGC-TG-3′ (sense) and 5′-CCA-GGA-GAG-AGT-CCC-AGA-TG-3′ (antisense).
Western Blot Analysis
The isolated glomeruli were pooled and lysed with radioimmunoprecipitation assay buffer containing 1% NP40, 0.1% SDS, 100 μg/ml phenylmethylsulfonyl fluoride, 1% protease inhibitor cocktail, and 1% phosphatase I and II inhibitor cocktail (Sigma) in PBS on ice. The supernatants were collected after centrifugation at 13,000 × g at 4°C for 20 min. Cultured mouse podocytes were lysed in SDS sample buffer. Protein expression was analyzed by Western blot analysis as described previously.43 The primary antibodies used were as follows: anti-β-catenin (BD Transduction, catalog no.: 610154), anti-dephosphorylated, active β-catenin (Upstate, catalog no.: 05-665), anti-γ-catenin (BD Transduction, catalog no.: 610253), anti-glyceraldehyde 3-phosphate dehydrogenase and anti-Wnt1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-nephrin (Progen, Heidelberg, Germany), and anti-α-tubulin (Sigma).
Histology and Immunohistochemical Staining
Human kidney specimens were obtained from diagnostic renal biopsies performed at the University of Pittsburgh Medical Center. Nontumor kidney tissue from the patients who had renal cell carcinoma and underwent nephrectomy was used as normal controls. The Institutional Review Board at the University of Pittsburgh approved all studies involving human tissues. Mouse model of DN was induced by injection of streptozotocin in the uninephrectomized CD1 mice, as described previously.28 Paraffin-embedded mouse and human kidney sections (3 μm thickness) were prepared by a routine procedure. Sections were stained with hematoxylin-eosin, periodic-acid-Schiff reagent by standard protocol. Immunohistochemical staining for Wnt1, β-catenin, and active β-catenin were performed using a routine protocol as described previously.30,43
Immunofluorescence Staining and Confocal Microscopy
Kidney cryosections were fixed with 3.7% paraformalin for 15 min at room temperature. After blocking with 10% donkey serum for 30 min, the slides were immunostained with primary antibodies against nephrin, podocin (Santa Cruz Biotechnology), and β-catenin. Slides were viewed under a Leica TCS-SL confocal microscope.43 For determination of the podocyte injury as defined by nephrin loss and altered distribution, a semiquantitative scoring method was used. A score of 0 represents no lesion, whereas 1, 2, 3, and 4 represent the nephrin loss and altered distribution, involving less than 25%, 25 to 50%, 50 to 75%, and more than 75% of the glomerular tuft area, respectively. At least five randomly chosen glomeruli were evaluated for each mouse, and an average composite score was calculated. For some human biopsy samples, a combined immunofluorescence and immunohistochemical double staining was performed according to the protocols described previously.45 Paraffin-embedded kidney sections were stained with a different combination of antibodies against Wnt1 (Santa Cruz Biotechnology; catalog no.: SC-5630) and synaptopodin (Research Diagnostics Inc., Flanders, NJ; catalog no.: PRO65194), and active β-catenin (Upstate; catalog no.: 05-665) and nephrin (Fitzgerald, Concord, MA; catalog no.: 20R-NP002), respectively. Cell nuclei were visualized by staining with 4′, 6-diamidino-2-phenylindole, HCl.
EM
EM of kidney samples was carried out by routine procedures as described previously.43 Briefly, mouse kidneys were perfusion-fixed with 2.5% glutaraldehyde in PBS by left cardiac ventricular injection and postfixed in aqueous 1% osmium tetroxide. Specimens were dehydrated through an ethanol series, infiltrated in a 1:1 mixture of propylene oxide-Polybed 812 epoxy resin (Polysciences, Warrington, PA), and then embedded. Ultrathin sections were stained with 2% uranyl acetate followed by 1% lead citrate. Sections were observed and photographed using a JEOL JEM 1210 transmission electron microscope (JEOL, Peabody, MA).
Statistical Analysis
Statistical analyses of the data were performed by using SigmaStat software (Jandel Scientific, San Rafael, CA). Comparison between groups was made using one-way ANOVA, followed by the Student's Newman–Keuls test. P < 0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grants DK061408, DK064005, and DK071040 (Y.L.); American Heart Association beginning Grant-in-Aid (0865392D); and the University of Pittsburgh Medical Center Health System Competitive Medical Research Fund (C.D.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Activation of Canonical Wnt Signaling Meets with Podocytopathy,” on pages 1864–1866.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS:Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, Locatelli F, MacLeod A, Vanholder R, Walker R, Wang H:The burden of kidney disease: Improving global outcomes. Kidney Int 66: 1310–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC:The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Shankland SJ:The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS:CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P:Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tryggvason K:Unraveling the mechanisms of glomerular ultrafiltration: Nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 10: 2440–2445, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Sako M, Nakanishi K, Obana M, Yata N, Hoshii S, Takahashi S, Wada N, Takahashi Y, Kaku Y, Satomura K, Ikeda M, Honda M, Iijima K, Yoshikawa N:Analysis of NPHS1, NPHS2, ACTN4, and WT1 in Japanese patients with congenital nephrotic syndrome. Kidney Int 67: 1248–1255, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Pettersson-Fernholm K, Forsblom C, Perola M, Groop PH:Polymorphisms in the nephrin gene and diabetic nephropathy in type 1 diabetic patients. Kidney Int 63: 1205–1210, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Clevers H:Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Pinto D, Clevers H:Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306: 357–363, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Thompson MD, Monga SP:WNT/beta-catenin signaling in liver health and disease. Hepatology 45: 1298–1305, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Fodde R, Brabletz T:Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19: 150–158, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Huang H, He X:Wnt/beta-catenin signaling: New (and old) players and new insights. Curr Opin Cell Biol 20: 119–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X:Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11: 951–961, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Niehrs C:Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25: 7469–7481, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR:Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol 293: F494–F500, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP:Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Ott KM, Barasch J:WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int 74: 1004–1008, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT:Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science 316: 1043–1046, 2007 [DOI] [PubMed] [Google Scholar]
- 21.He X:Cilia put a brake on Wnt signaling. Nat Cell Biol 10: 11–13, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Surendran K, Schiavi S, Hruska KA:Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Surendran K, McCaul SP, Simon TC:A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol 282: F431–F441, 2002 [DOI] [PubMed] [Google Scholar]
- 24.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y:Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS:Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol 17: 2812–2820, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Fogo AB:Animal models of FSGS: lessons for pathogenesis and treatment. Semin Nephrol 23: 161–171, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Wang YP, Tay YC, Harris DC:Progressive adriamycin nephropathy in mice: Sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Dai C, Yang J, Bastacky S, Xia J, Li Y, Liu Y:Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol 15: 2637–2647, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Dai C, Yang J, Liu Y:Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol 13: 411–422, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y:Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172: 299–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen CE, Amaral S, Frosch E:Lithium-induced nephrotic syndrome in a prepubertal boy. J Child Adolesc Psychopharmacol 18: 210–213, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Presne C, Fakhouri F, Noel LH, Stengel B, Even C, Kreis H, Mignon F, Grunfeld JP:Lithium-induced nephropathy: Rate of progression and prognostic factors. Kidney Int 64: 585–592, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D'Agati VD:Lithium nephrotoxicity: A progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11: 1439–1448, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Liu Y:Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Huber MA, Kraut N, Beug H:Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17: 548–558, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA:The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Tan X, Li Y, Liu Y:Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 17: 3382–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA:Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25: 5603–5613, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui I, Ito T, Kurihara H, Imai E, Ogihara T, Hori M:Snail, a transcriptional regulator, represses nephrin expression in glomerular epithelial cells of nephrotic rats. Lab Invest 87: 273–283, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Nelson WJ, Nusse R:Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483–1487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R:Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB:Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y:Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17: 2164–2175, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C:A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia JL, Dai C, Michalopoulos GK, Liu Y:Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol 168: 1500–1512, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.