Abstract
Dysproteinemias that result in monoclonal glomerular deposits of IgG are relatively uncommon. Here, we report the largest series of proliferative glomerulonephritis with monoclonal IgG deposits, a form of renal involvement by monoclonal gammopathy that mimics immune-complex glomerulonephritis. We retrospectively identified 37 patients, most of whom were white (81%), female (62%), or older than 50 yr (65%). At presentation, 49% had nephrotic syndrome, 68% had renal insufficiency, and 77% had hematuria. In 30% of the patients, we identified a monoclonal serum protein with the same heavy- and light-chain isotypes as the glomerular deposits (mostly IgG1 or IgG2), but only one patient had myeloma. Histologic patterns were predominantly membranoproliferative (57%) or endocapillary proliferative (35%) with membranous features. Electron microscopy revealed granular, nonorganized deposits, and immunofluorescence demonstrated glomerular deposits that stained for a single light-chain isotype and a single heavy-chain subtype, most commonly IgG3κ (53%). During an average of 30.3 mo of follow-up for 32 patients with available data, 38% had complete or partial recovery, 38% had persistent renal dysfunction, and 22% progressed to ESRD. Correlates of ESRD on univariate analysis were higher creatinine at biopsy, percentage of glomerulosclerosis, and degree of interstitial fibrosis but not immunomodulatory treatment or presence of a monoclonal spike. On multivariate analysis, higher percentage of glomerulosclerosis was the only independent predictor of ESRD. Only one patient lacking a monoclonal spike at presentation subsequently developed a monoclonal spike and no patient with a monoclonal spike at presentation subsequently developed a hematologic malignancy. We conclude that proliferative glomerulonephritis with monoclonal IgG deposits does not seem to be a precursor of myeloma in the vast majority of patients.
Among dysproteinemia-related renal diseases, those manifesting monoclonal glomerular deposits of IgG are relatively uncommon. Renal diseases caused by monoclonal IgG deposition include light- and heavy-chain deposition disease (LHCDD),1 type 1 cryoglobulinemic glomerulonephritis,2 immunotactoid glomerulonephritis (IT),3 light- and heavy-chain amyloidosis,4 and rarely fibrillary glomerulonephritis (FGN).3 LHCDD is characterized by the presence of nodular sclerosing glomerulopathy by light microscopy (LM); diffuse, linear staining of glomerular basement membranes (GBMs) and tubular basement membranes (TBMs) for a single heavy chain and a single light chain by immunofluorescence (IF); and nonfibrillar, “powdery” electron-dense deposits in GBMs and TBMs by electron microscopy (EM).1 Type 1 cryoglobulinemic glomerulonephritis exhibits a membranoproliferative or diffuse proliferative glomerulonephritis pattern on LM, usually with prominent intracapillary infiltrating monocytes and large, glassy intraluminal immune deposits.2 Ultrastructurally, the deposits commonly show an annular-tubular or fibrillar substructure. The glomerular deposits in IT are composed of microtubular structures with a diameter of 30 to 50 nm and a tendency for parallel alignment, whereas in FGN they are composed of Congo red–negative, randomly oriented fibrils measuring 16 to 24 nm in diameter.3 Light- and heavy-chain amyloidosis is extremely rare and, similar to light chain amyloidosis, is characterized by the presence of Congo red–positive deposits composed of haphazardly oriented fibrils that measure 8 to 14 nm in diameter.4
In 2004, we reported 10 patients with a novel form of glomerular injury related to monoclonal IgG deposition that could not be assigned to any of these conditions, which we termed “proliferative glomerulonephritis with monoclonal IgG deposits” (PGNMID).5 On IF, the glomerular deposits were monoclonal, staining for a single light-chain isotype and a single γ heavy-chain subclass. LM exhibited endocapillary proliferative or membranoproliferative glomerulonephritis, and EM revealed granular electron-dense deposits, mimicking ordinary immune-complex glomerulonephritis. Clinical presentations included proteinuria in 100% of patients (mean 24-h urine protein 5.8 g/d), renal insufficiency in 80% (mean serum creatinine 2.8 mg/dl), and micohematuria in 60%. A monoclonal serum or urine protein was identified in 50% of patients, although none of them had evidence of multiple myeloma (MM) or B cell lymphoproliferative disorder.5 The follow-up on these 10 patients was of short duration, and the treatment details were limited.
This report enlarges our experience with PGNMID to 37 cases, representing the largest series to date. Our aim was to define better the natural history, presenting features, treatment, and outcome of this enigmatic disease.
Results
Clinical Features
The majority of patients were white (81.1%) and female (62.2%; Table 1). All patients were adults and had a mean age of 54.5 yr (range 20 to 81). Close to two thirds of patients were older than 50 yr, and 16.2% were older than 70 yr.
Table 1.
Demographic characteristics
| Characteristic | Value |
|---|---|
| Female/male (n [%]) | 23/14 (62.2/37.8) |
| Age (yr; mean [range]) | 54.5 (20 to 81) |
| ≤40 (n [%]) | 7 (18.9) |
| 41 to 50 (n [%]) | 6 (16.2) |
| 51 to 60 (n [%]) | 10 (27.0) |
| 61 to 70 (n [%]) | 8 (21.6) |
| >70 (n [%]) | 6 (16.2) |
| Race (n [%]) | |
| white | 30 (81.1) |
| Hispanic | 4 (10.8) |
| black | 3 (8.1) |
On standard serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with immunofixation electrophoresis (IFE), seven patients had a monoclonal spike (M-spike) in both serum and urine, and four patients had an M-spike detectable in the serum only (Table 2). The M-spike was detected at presentation in 10 of these 11 patients but not until repeat testing 3 yr after presentation in the remaining patient. In all of these 11 patients, the monoclonal proteins in both serum and urine had the same heavy- and light-chain isotypes as the monoclonal Ig deposited in the kidney. IgG heavy-chain subclass analysis of glomerular deposits, performed in nine of the 11 patients, showed IgG1 in five patients, IgG2 in two, and IgG3 in two. Of the remaining 26 patients who had negative SPEP, UPEP, and IFE, four underwent serum free light-chain assay; of these, three were found to have normal κ:λ ratio, and one (who had glomerular monoclonal IgG3 κ deposition) had an elevated κ:λ ratio. Bone marrow examination, performed in 22 patients (including 10 of the 11 patients with a positive serum M-spike), revealed <5% plasma cells in 20. Of the remaining two patients, one had 5% λ-restricted B cell population (corresponding to IgGλ serum M-spike and IgGλ1 deposits in the kidney) and one had sheets of atypical plasma cells with intranuclear Dutcher body inclusions and κ restriction. The latter patient had carried a diagnosis of MM for several years and had undergone stem cell transplantation 1 yr before renal presentation. None of the patients had lymphadenopathy, hepatosplenomegaly, or lymphoma. One patient had primary renal amyloidosis diagnosed concomitantly with PGNMID on renal biopsy.5 On IF evaluation of this biopsy, the coexistent Congo red–positive renal amyloid deposits were composed of λ only and exclusively involved vessels (with sparing of glomeruli), and the nonamyloid glomerular deposits were composed of IgG2 λ. The bone marrow biopsy in this patient was negative for plasma cell dyscrasia and amyloid.
Table 2.
Clinical characteristics at presentation
| Characteristic | Value |
|---|---|
| Peripheral edema (n [%]) | 23 (62.2) |
| 24-h urine protein (g/d; mean [range]) | 5.70 (0.36 to 17.00) |
| Proteinuria <1 g/24 h (n [%]) | 1/35 (2.9) |
| Proteinuria 1–3g/24 h (n [%]) | 10/35 (28.6) |
| Proteinuria >3g/24 h (n [%]) | 24/35 (68.6) |
| Full nephrotic syndrome (n [%]) | 17/35 (48.6) |
| Serum albumin (g/dl; mean [range]) | 3.1 (1.1 to 4.9) |
| Hematuria (n [%]) | 27/35 (77.1) |
| Serum creatinine at biopsy (mg/dl; mean [range]) | 2.77 (0.70 to 17.00) |
| Renal insufficiency at presentation (n [%]) | 25 (67.6) |
| Evidence of dysproteinemia (n [%])a | 11/37 (29.7) |
| Serum paraprotein only | 4 |
| Serum and urine paraprotein | 7 |
| Multiple myeloma | 1 |
| AL-amyloid | 1 |
| Low C3 (n [%]) | 3 (8.1) |
| Low C4 (n [%]) | 3 (8.1) |
| Low C3 and C4 (n [%]) | 4 (10.8) |
| Positive serum cryoglobulin (n [%])b | 0 (0.0) |
| Positive hepatitis C antibody (n [%]) | 1/30 (3.3) |
| Positive rheumatoid factor (n [%]) | 1/18 (5.5) |
aIn one patient, M-spike was not detected until 3 yr after presentation.
bPerformed repeatedly.
Four patients had a history of carcinoma (colon carcinoma in one, anal carcinoma in two, and breast and bladder carcinoma in one). One patient with a serum IgGλ M-spike had had an upper respiratory tract infection 5 d before presentation with renal failure. Another patient had a history of HIV infection. None of the remaining 35 patients had a history of recent or chronic infection. One patient carried a diagnosis of autoimmune hemolytic anemia. None of the patients had a history of systemic lupus erythematosus, rheumatoid arthritis, mixed connective tissue disease, or Sjögren syndrome. Chronic hypertension was present in 14 (37.8%) patients and diabetes in five (13.5%; including one patient who had evidence of diabetic glomerulosclerosis on biopsy).
Serum cryoglobulin titers were negative in all patients (performed repeatedly in many patients), and none of the patients had any systemic manifestations of cryoglobulinemia. Serum complement was depressed in 10 (27%) patients (including depressed C3 and C4 in four, depressed C4 alone in three, and depressed C3 alone in three; Table 2). Of the 10 patients with hypocomplementemia, seven had IgG3 glomerular deposits and three had IgG1 glomerular deposits. Rheumatoid factor, tested in 18 patients, was negative in 17 and positive in one. Hepatitis C antibody, tested in 30 patients, was negative in 29 and positive in one (who had MM and normal serum complements).
At presentation, all patients had proteinuria (Table 2). The mean 24-h urine protein was 5.70 g (range 0.36 to 17.00 g). Proteinuria was in the nephrotic range in 68.6% of patients, and 48.6% developed full nephrotic syndrome. Microhematuria was documented in 77.1% of patients, whereas gross hematuria was present in only one patient. Two thirds of patients had renal insufficiency, including three who were on hemodialysis. The mean serum creatinine was 2.77 mg/dl (range 0.70 to 17.00 mg/dl). The mean serum albumin was 3.1 g/dl (range 1.1 to 4.9 g/dl), and peripheral edema was present in 62.2% of patients.
Pathologic Findings
Light Microscopy
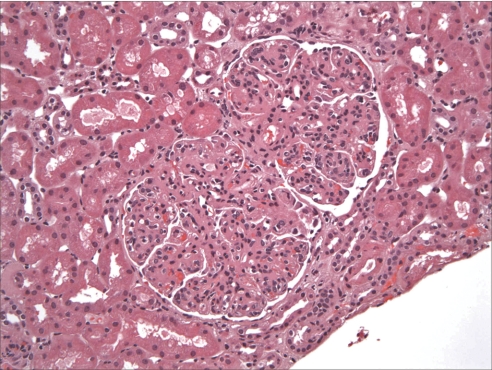
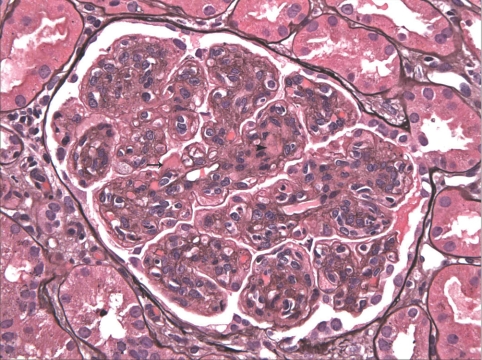
Sampling for LM included mean 17.7 glomeruli (range 2 to 62 glomeruli). A mean of 17.5% of glomeruli were globally sclerotic (Table 3). The glomerular alterations were heterogeneous, with the majority of biopsies displaying variable degrees of endocapillary hypercellularity and duplication of the GBM (Figures 1 and 2). The most common histologic pattern, seen in 21 (56.8%) cases, was predominantly membranoproliferative glomerulonephritis characterized by diffuse and global double-contoured glomerular capillary walls (GCWs) with mesangial cell interposition and mesangial expansion by increased mesangial cell number and matrix (Figure 2). Of these 21 cases, 17 also showed endocapillary hypercellularity (focal in 10 and diffuse in seven), including focal macrophage infiltration, and five showed segmental membranous features. The second most common pattern, seen in 13 (35.1%) cases, was predominantly endocapillary proliferative glomerulonephritis, characterized by endocapillary hypercellularity (diffuse in 10 and focal in three) and leukocyte infiltration causing luminal occlusion. Of these 13 cases, six had associated segmental membranoproliferative features, two had moderate neutrophil infiltration, and one had segmental membranous features. The third histologic pattern, seen in two (5.4%) cases only, was predominantly membranous glomerulonephritis characterized by GCW thickening and global subepithelial deposits. Both cases also showed focal endocapillary hypercellularity and segmental membranoproliferative features. The fourth and rarest pattern, observed in one case only, was pure mesangial proliferative glomerulonephritis characterized by diffuse mesangial hypercellularity.
Table 3.
Light microscopic findingsa
| Pathologic Findings | Value |
|---|---|
| No. of glomeruli (mean) | 17.7 |
| % of globally sclerotic glomeruli (mean) | 17.5 |
| Predominant histologic pattern | |
| membranoproliferative GNb | 21 (56.8) |
| endocapillary proliferative GNb | 13 (35.1) |
| mesangial proliferative GN | 1 (2.7) |
| membranous GN | 2 (5.4) |
| Crescentsc | 12 (32.4) |
| focal | 10 |
| diffuse | 2 |
| Interstitial inflammation: None/focal/diffused | 4/28/5 (10.8/75.7/13.5) |
| Tubular atrophy and interstitial fibrosis: None/mild/moderate/severee | 2/25/6/4 (5.4/67.6/16.2/10.8) |
| Arteriosclerosis and arteriolar hyalinosis: None/mild/moderate/severe | 9/16/11/1 (24.3/43.2/29.7/2.7) |
| Concurrent vascular amyloid | 1 (3) |
aGN, glomerulonephritis.
bWith or without membranous features.
cFocal, <50% of glomeruli; diffuse, ≥50% of glomeruli.
dFocal <50% of cortical surface area; diffuse ≥50%.
eMild, 0 to 25% of cortical surface area; moderate, 26 to 50%; severe, >50%.
Figure 1.
Glomerular capillary lumina are globally narrowed by mesangial and endocapillary proliferation including abundant infiltrating monocytes. Magnification, ×200 (hematoxylin and eosin).
Figure 2.
There are widespread double contours of the GBMs. Segmental subendothelial (arrow) and mesangial (arrowhead) nonargyrophilic deposits are seen. Magnification, ×400 (Jones methenamine silver).
Crescents were present in 12 (32.4%) cases and were purely cellular in 10 and mixed cellular and fibrous in two (Table 3). When present, the crescents affected a mean of 20% of glomeruli, but in two cases they involved ≥50% of glomeruli. Focal glomerular necrosis was observed in only three (8.1%) cases. One patient had moderate underlying diabetic glomerulosclerosis. Thirty-three (89.2%) cases showed interstitial inflammation, which was predominantly focal. The degree of tubular atrophy and interstitial fibrosis ranged from absent (5.4% of cases) to mild (67.6%) to moderate (16.2%) to severe (10.8%). Arteriosclerosis ranged from absent (24.3% of cases) to mild (43.2%) to moderate (29.7%) to severe (2.7%).
Immunofluorescence
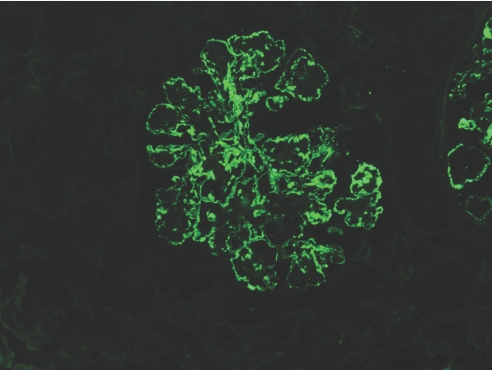
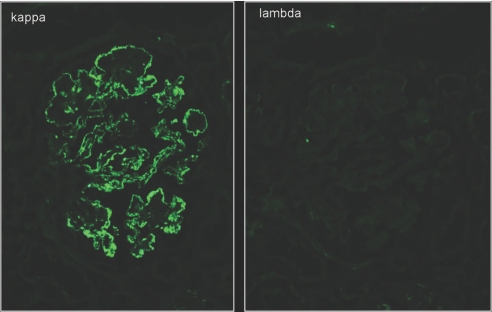
By IF, deposits were identified exclusively in the glomeruli (with the exception of the single case with associated vascular amyloidosis). The texture of the deposits was granular in 32 (86.5%) cases, semilinear in three (8.1%), and smudgy in two (5.4%). The deposits localized to the GCW and mesangium in 30 (81.1%) cases, to the GCW alone in five (13.5%) cases, and to the mesangium alone in two (5.4%) cases. IgG was the only Ig deposited, with a mean intensity of 2.4+ (on a scale of 0 to 3; Table 4, Figure 3). All cases showed light-chain isotype restriction, including 27 (73%) cases with sole positivity for κ (mean intensity 2.4+; Figure 4) and 10 (27%) with sole positivity for λ (mean intensity 2.8+). In a similar distribution to the IgG deposits, glomerular deposition of C3 (mean intensity 2.3+) was detected in 36 (97.3%) cases and C1q (mean intensity 1.2+) in 23 (63.9% of 36) cases tested. None of the cases showed glomerular staining for IgA, specific glomerular staining for IgM, or granular IgG staining in TBMs or interstitium.
Table 4.
Glomerular immunofluorescence staining
| Parameter | No. of Patients | % of Patients |
|---|---|---|
| IgGa | 37 | 100 |
| IgG1 κ | 7/32 | 21.9 |
| IgG1 λ | 2/32 | 6.3 |
| IgG2 λ | 2/32 | 6.3 |
| IgG3 κ | 17/32 | 53.1 |
| IgG3 λ | 4/32 | 12.5 |
| C3 | 36 | 97.3 |
| C1q | 23/36 | 63.9 |
aIgG subtype staining not available for five patients, including three with IgG-κ and two with IgG-λ.
Figure 3.
There is global granular to semilinear staining of GBMs for IgG. Fewer punctate granular deposits are also present in the mesangium. No staining is observed in Bowman's capsule or the TBMs. Magnification, ×200 (IF micrograph).
Figure 4.
There is strong global granular to semilinear staining for κ light chain outlining the GCWs, with fewer deposits in the mesangium. There is no staining of Bowman's capsule or TBMs. The stain for λ light chain is completely negative in glomeruli, as well as tubules. Magnification, ×400 (IF micrographs).
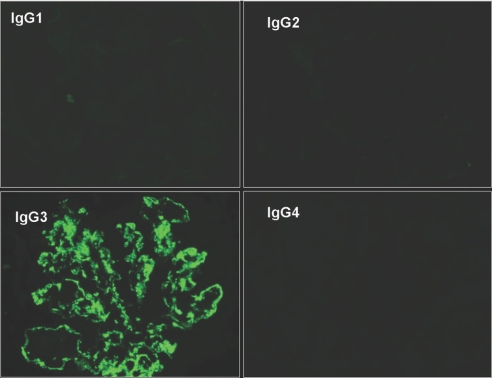
IF staining for IgG1 through 4 subclasses showed monotypic deposits in all 32 cases tested, including nine (28.1%) IgG1 (seven IgG1κ and two IgG1λ), two (6.3%) IgG2λ, and 21 (65.6%) IgG3 (17 IgG3κ and four IgG3λ; Figure 5). No case showed positivity for IgG4. On statistical analysis, IgG3 subtype correlated with the absence of M-spike (only two of 21 patients with IgG3 deposits had a positive M-spike compared with seven of 11 with IgG1 or IgG2; P = 0.0026).
Figure 5.
On IF staining for IgG subtypes, there is strong glomerular positivity for IgG3 with negative staining for IgG1, IgG2, and IgG4. Magnification, ×400.
Electron Microscopy
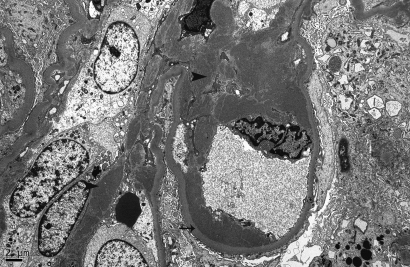
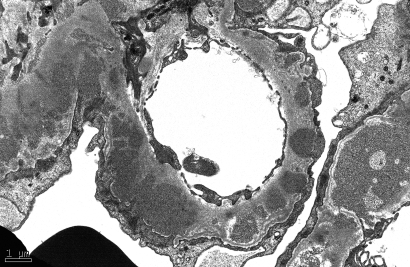
In all cases, granular electron-dense deposits were confined to the glomerular compartment. The glomerular immune deposits were primarily subendothelial (n in 100% of cases) and mesangial (n in 94.6% of cases) and were generally global (involving ≥50% of the total glomerular loops; Table 5, Figure 6). Subepithelial deposits were less frequent, identified in 56.8% of patients, and were segmental in most cases (Figure 7). Two of three cases with global subepithelial deposits had primarily membranous features by LM. Intramembranous deposits were present in 13.5% of cases.
Table 5.
Electron microscopic findings
| Location of Granular Electron-Dense Deposits | No. of Patients | % of Patients |
|---|---|---|
| Mesangial (segmental, global) | 35 (10, 25) | 94.6 |
| Subendothelial (segmental, global) | 37 (10, 27) | 100.0 |
| Subepithelial (segmental, global) | 21 (18, 3) | 56.8 |
| Intramembranous | 5 (4, 1) | 13.5 |
Segmental, involving <50% of the glomerular loops; global, involving ≥50% of the glomerular loops.
Figure 6.
There are abundant, large, granular electron-dense mesangial (arrowheads) and subendothelial (arrow) deposits. Magnification, ×4000 (electron micrograph).
Figure 7.
This micrograph shows abundant, medium-sized, granular electron-dense subepithelial and intramembranous deposits. Magnification, ×12,000 (electron micrograph).
In 25 (67.6%) cases, the electron-dense deposits had a finely granular texture throughout, without substructure. In the remaining 12 cases, the deposits were mostly granular but with focally variegated texture (without evidence of well-developed microtubules or fibrils) in seven cases, with rare ill-defined fibrils measuring <12 mm in diameter in three patients and 15 to 21 nm in diameter in one patient, and with focal organization into lattice-like arrays with a periodicity of 15 nm in one case. None of the cases showed predominantly fibrillar deposits typical of FGN; large organized microtubular deposits typical of IT; deposits with annular-tubular substructure commonly seen in cryoglobulinemic glomerulonephritis; or punctate, ribbon-like deposits along the GBM and TBM characteristic of Randall type LHCDD. The mean degree of foot process effacement was 68.3% (range 20 to 100%).
The single patient who had mostly granular deposits but with rare fibrils measuring 15 to 21 nm in diameter had a negative M-spike at presentation. Three years later, an M-spike was detected and a repeat biopsy was performed. The repeat biopsy showed a membranoproliferative pattern as seen in the first biopsy, but ultrastructurally there was evolution to more extensive fibrillar substructure involving 25% of the total deposits.
Clinical Outcome
Clinical follow-up was available for 32 (86.5%) patients. The mean duration of follow-up for the entire cohort was 30.3 mo (range 1 to 114 mo). Outcomes are presented in Tables 6 and 7. On follow-up, four (12.5%) patients had complete recovery (CR), eight (25.0%) patients had partial recovery (PR), one (3.1%) patient had persistent microhematuria (with normal creatinine and no proteinuria), 12 (37.5%) patients had persistent renal dysfunction (PRD), and seven (21.9%) patients had progressed to ESRD. Five patients (four with ESRD and one with PR) died: Two of metastatic carcinoma, one (who had ESRD) of hyperkalemia, and two of undetermined cause. Only one of the patients with negative SPEP/UPEP at presentation subsequently developed M-spike, and none of those with M-spike at presentation subsequently developed MM or lymphoma.
Table 6.
Clinical follow-up (32 patients)
| Parameter | Value |
|---|---|
| Duration of follow-up (mo; mean [range]) | 30.3 (1.0 to 114.0) |
| Treatment | |
| none | 5 (15.6) |
| RAS blockade alone | 9 (28.1) |
| IM | 18 (56.3) |
| steroids | 11 |
| cyclophosphamide | 3 |
| cyclosporine | 2 |
| mycophenolate mofetil | 5 |
| rituximab | 4 |
| chlorambucil | 1 |
| thalidomide | 2 |
| bortezomib (Velcade) | 1 |
| Outcomea | |
| CR | 4 (12.5) |
| PR | 8 (25.0) |
| PRD | 12 (37.5) |
| Persistent hematuria (with normal creatinine and no proteinuria) | 1 (3.1) |
| ESRD | 7 (21.9) |
| Death | 5 (15.6) |
aCR: Remission of proteinuria to <500 mg/d with normal renal function; PR: Reduction in proteinuria by at least 50% and to <2 g/d with stable renal function (no more than a 20% increase in serum creatinine); PRD: Failure to meet criteria for either CR or PR but not reaching ESRD, including patients with unremitting proteinuria, or progressive chronic kidney disease.
Table 7.
Characteristics of patients with CR or PRa
| Characteristic | Serum Creatinine at Biopsy (mg/dl) | 24-H Urine Protein at Biopsy(g/d) | M-Protein in Serum | % of Glomeruli with Crescents | Degree of Tubular Atrophy and Interstitial Fibrosis | Treatment | Duration of Follow-up (mo) |
|---|---|---|---|---|---|---|---|
| Patients with CR | |||||||
| 1 | 3.7 | 9.0 | No | 0 | Mild | PRED | 25 |
| 2 | 1.9 | 7.8 | No | 0 | Mild | RAS blockade alone | 67 |
| 3 | 2.2 | 7.5 | No | 0 | Moderate | PRED/CYT with CR then relapse treated with PRED/MMF with CR | 30 |
| 4 | 0.8 | 3.8 | No | 3 | None | RAS blockade alone | 114 |
| Patients with PR | |||||||
| 1 | 1.7 | 3.5 | No | 0 | Mild | RAS blockade alone | 44 |
| 2 | 5.9 | 1.0 | No | 20 | Mild | PRED/CYT | 40 |
| 3 | 2.7 | 17 | No | 6 | Mild | Rituximab | 33 |
| 4 | 1.2 | 3.4 | No | 0 | Mild | RAS blockade alone | 4 |
| 5 | 1.3 | 5.0 | No | 4 | Mild | RAS blockade/MMF | 14 |
| 6 | 0.7 | 3.0 | No | 0 | Mild | Rituximab | 44 |
| 7 | 5.5 | 3.5 | IgG-λ | 0 | Mild | RAS blockade/PRED | 81 |
| 8 | 1.6 | 9.0 | IgG-κ | 0 | Mild | RAS blockade/PRED/chlorambucil | 93 |
| Patient with persistent microhematuria | 0.7 | 0.36 | No | 0 | None | None | 8 |
aCYT, cyclophosphamide; MMF, mycophenolate mofetil; PRED, prednisone.
On univariate analysis, the correlates of reaching ESRD were higher creatinine at biopsy (P = 0.002), higher percentage of global glomerulosclerosis (P < 0.001), greater degree of tubular atrophy and interstitial fibrosis (P = 0.009), and arteriosclerosis (P = 0.001). Patients who reached ESRD tended to be older (mean age 63.0 versus 52.5 yr; P = 0.065). Gender, the degree of proteinuria, the type of therapy, the presence of M-spike on SPEP or UPEP, and the glomerular pattern by LM did not correlate significantly with outcome.
By Cox regression, correlates of the rate of progression to ESRD on univariate analysis were higher creatinine at biopsy (P = 0.005), higher percentage of global glomerulosclerosis (P < 0.001) and crescents (P = 0.040), greater degree of tubular atrophy and interstitial fibrosis (P = 0.007), and arteriosclerosis (P = 0.006). Using the Cox proportional hazards model, the only independent predictor of the rate of progression to ESRD on multivariate analysis was higher percentage of global glomerulosclerosis (hazard ratio 1.08; 95% confidence interval 1.00 to 1.15; P = 0.039).
Treatment
Five (15.6%) patients were not treated (two had severe tubular atrophy and interstitial fibrosis on biopsy, one had microhematuria with minimal proteinuria and normal creatinine at presentation, one had diabetes and bladder and breast carcinomas, and one refused therapy). On follow-up, three of the five had developed ESRD (including the two with severe tubular atrophy and interstitial fibrosis), one continued to have persistent microhematuria, and one had PRD.
Nine (28.1%) patients were treated with renin-angiotensin system (RAS) blockade alone (angiotensin-converting enzyme inhibitor in seven patients and angiotensin II receptor blocker in two patients). Of these nine patients, two had CR, two had PR, four had PRD, and one (who had severe tubular atrophy and interstitial fibrosis) had progressed to ESRD.
Eighteen (56.3%) patients received immunomodulatory therapy (IM) either with or without concurrent RAS blockade (six patients and 12 patients, respectively). Of these 18 patients, two developed CR, six developed PR, seven developed PRD, and three developed ESRD. IM consisted of prednisone alone in six patients, one of whom developed CR, one PR, two PRD, and two ESRD. Prednisone and an alkylating agent (cyclophosphamide or chlorambucil) was given to three patients, two of whom had PR and one of whom developed ESRD. Thalidomide was given to one patient with prednisone and to one patient with Bortezomib (Velcade), both of whom developed PRD. Mycophenolate mofetil was given to three patients, as the sole agent in one, with prednisone and cyclophosphamide in one, and with cyclosporine in one. Among these three patients, one developed CR, one PR, and one PRD. Rituximab was used in combination with mycophenolate mofetil and/or cyclosporine in two patients and alone in two patients with resultant two PR and two PRD. These 18 patients included nine of the 11 patients who had a positive M-spike on SPEP/UPEP.
Discussion
Most forms of immune complex–mediated glomerulonephritis, including endocapillary proliferative glomerulonephritis, membranoproliferative glomerulonephritis, and membranous glomerulonephritis, are due to localization in glomeruli of antibodies complexed to endogenous or exogenous antigens, which at the ultrastructural level appear as granular electron-dense deposits. In these situations, the deposited Igs are typically polyclonal, staining for both κ and λ light chains. Renal parenchymal deposition of monoclonal Igs or their subunits may also occur and lead to a diverse spectrum of renal injury, including amyloidosis, Randall-type monoclonal Ig deposition disease, IT, and type 1 cryoglobulinemic glomerulonephritis.
In cases of endocapillary proliferative or membranoproliferative glomerulonephritis in which the deposits stain for IgG and a single light chain, diagnostic considerations would include PGNMID, type 1 cryoglobulinemic glomerulonephritis, and IT. The diagnosis of IT is established when the majority of deposits on EM are composed of microtubules with a diameter of 30 to 50 nm and hollow centers.3 Because not all cases of type 1 cryoglobulinemic glomerulonephritis show the characteristic intraluminal “immune thrombi” and annular-tubular substructure of deposits on EM, type 1 cryoglobulinemia should be carefully excluded on clinical grounds before rendering a diagnosis of PGNMID. In our experience, PGNMID is far more common than type 1 cryoglobulinemic glomerulonephritis and IT. The clinical and pathologic differences among these three conditions are summarized in Table 8.2,3 Importantly, patients with PGNMID have a lower incidence of dysproteinemia (30% of patients in our cohort) than those with type 1 cryoglobulinemic glomerulonephritis (76%) and IT (67%).2,3 In contrast to light-chain Fanconi syndrome, in which Fanconi syndrome typically precedes the development of MM or amyloid,6 PGNMID does not seem to represent a premyelomatous condition, because only one of our 26 patients lacking M-spike on SPEP/UPEP at presentation subsequently developed M-spike during the follow-up period (mean 30.3 mo, up to 114 mo), and none of those with an M-spike at presentation subsequently developed MM or lymphoma.
Table 8.
Clinical and pathologic differences among PGNMID, type 1 cryoglobulinemic glomerulonephritis, and IT
| Parameter | PGNMIDa | Type 1 Cryoglobulinemic Glomerulonephritis2 | IT3 |
|---|---|---|---|
| Hypocomplementemia | 27% | 58% | 33% |
| Evidence of serum or urine monoclonal protein | 30% | 76% | 67% |
| Underlying MM | Very rare | Very rare | Very rare |
| Underlying lymphoma/leukemia | Very rare | 33% | 17% |
| Renal insufficiency at presentation | 60% | 76% | 83% |
| Nephrotic syndrome | 53% | 38% | 50% |
| Intracapillary monocyte infiltration | + | +++ | + |
| Intracapillary protein thrombi | No | Yes | No |
| Most common IgG subclass | IgG3 | IgG3 | IgG1 |
| Texture of deposits on EM | Granular | Focal annular-tubular or fibrillar | Microtubular with a diameter of 30 to 50 nm and hollow centers in parallel stacks |
aData compiled from our cohort and the other cases reported in the literature.
Twenty-one additional patients with PGNMID have been reported in the literature.7–12 Ten of these are reported in abstract form,11,12 and most lack detailed clinical and pathologic descriptions or follow-up. The clinical and pathologic features of these patients, when described, share some similarities to our patients. Clinical presentation included hematuria and proteinuria. Nephrotic syndrome and renal failure were present in 10 (63%) and nine (56%) of the 16 patients, respectively, with available data. Only three (19%) of the 16 patients with available data had a monoclonal protein in serum or urine, two of whom were identified only by immunoblotting. One patient reported by Evans et al.8 had PGNMID with monoclonal IgG-κ subepithelial deposits as well as follicular B cell lymphoma infiltrating the kidney and expressing surface IgG-κ. In that patient, κ Bence Jones protein was detected in the urine, whereas serum immunofixation showed a monoclonal IgM protein. None of the remaining 20 patients had any hematologic malignancy. Most of the 16 patients with available follow-up data were treated with prednisone with or without other immunosuppressive agents. Five (31.3%) of the 16 subsequently progressed to ESRD.
We were unable to show a statistical benefit of treatment in this uncontrolled retrospective study, probably in part because of the small sample size and the tendency for patients with a higher creatinine and crescents to be offered IM. Moreover, multiple immunosuppressive drug regimens were used over time in this population. Prospective, multicenter, controlled study of a larger cohort of patients with PGNMID is needed to determine the optimal therapeutic regimen. Until such a study is performed, treatment recommendations must be made purely on the basis of clinical experience with small numbers of patients. We recommend that all patients with proteinuria be treated with RAS blockade. In those with nephrotic-range proteinuria, a decreased GFR, or biopsy features suggestive of progression (crescents, glomerulosclerosis, and interstitial fibrosis), a trial of IM is reasonable but should be individualized to the patient's profile and potential risks of therapy. At present, it seems reasonable to include an alkylating agent (e.g., cyclophosphamide) in patients with significant percentages of crescents. Because the pathogenesis of PGNMID presumably involves hypersecretion of monoclonal IgG by a clonal proliferation of B cells or plasma cells, use of a chimeric mAb against the CD20 antigen on the surface of B cells is a rational but unproven approach. Treatment with rituximab could potentially spare the adverse effects of prednisone and other traditional immunosuppressive agents. Recent data from uncontrolled trials indicated that rituximab is effective in the treatment of idiopathic membranous glomerulopathy,13 systemic lupus erythematosus,14,15 ANCA vasculitis,16 type II cryoglobulinemic glomerulonephritis,17 and FGN.18 Only four of our patients were treated with rituximab, given alone in two who achieved PR (Table 7) and in combination with other agents in two who had PRD. The effectiveness of rituximab in this population deserves further study.
The pathogenesis of PGNMID remains elusive. The absence of underlying infectious, autoimmune, or other systemic disease in the vast majority of patients and the light-chain and heavy-chain subclass restriction argue against antigen-antibody immune complex deposition and, instead, favor that monoclonal IgG is deposited as a free, noncomplexed Ig that has the ability to aggregate to form definable electron dense deposits. Because up to two thirds of patients have no detectable M protein (by standard SPEP/UPEP/IFE) even after long follow-up, in these patients we propose that this unique glomerulonephritis may arise in the course of normal immune responses. It is possible that during an immune response (to extrinsic or intrinsic antigens), one or more clones of B cells proliferate and produce monoclonal IgG molecules (particularly IgG3) with ability to self-aggregate and rapidly deposit in glomeruli through entrapment and/or interaction with negatively charged glomerular constituents. The small quantity of this monoclonal IgG may elude detection by SPEP/UPEP/IFE because of its high avidity for the glomeruli and rapid aggregability favored by its intrinsic physical properties and glomerular sieving itself. The possibility of an oligoclonal response with the same IgG light- and heavy-chain isotype cannot be excluded because monoclonality can be proved only by immunoblotting/immunofixation techniques.
Human IgG is divided into four subclasses that differ in their heavy-chain structure, molecular weight, concentration in the serum, isoelectric point (pI), and immunogenicity. Of the four subclasses, IgG3, which composes only 8% of IgG in the circulation, has several properties that allow it to be intrinsically “nephritogenic.”19,20 (1) It is the most positively charged subclass (pI 8.2 to 9.0), favoring affinity for intrinsic anionic sites in the GCW. (2) It has the highest molecular weight (170,000 Da), making it more size-restricted by the glomerular filtration barrier. Thus, in the course of filtration, the intracapillary concentration of circulating IgG3 would be predicted to rise, promoting the potential for intraglomerular aggregation. (3) In fact, IgG3 has the unique physicochemical property of self-aggregability via Fc–Fc interactions and is known to be selectively enriched in murine and human cryoglobulinemia, murine lupus nephritis,2,21,22 and human IgG myeloma hyperviscosity syndrome.20 (4) It has the greatest complement-fixing capacity, which in turn could activate downstream inflammatory mediators that promote glomerular leukocyte infiltration and proliferation, leading to glomerulonephritis.
These special properties of IgG3 may explain the predominance of this relatively uncommon serum subtype in our patients. Monoclonal IgG3 was identified in the glomeruli of two thirds of our patients, particularly those without detectable M-spikes, and was accompanied by co-deposition of C3 in all patients and by hypocomplementemia in one third of patients. Glomerular C3 activation and resultant hypocomplementemia are also known to occur in other glomerular diseases associated with monoclonal IgG3 deposition, such as type 1 cryoglobulinemic glomerulonephritis,2 attesting to its ability to activate complement even in the absence of circulating immune complexes.
In contrast to heavy-chain deposition disease in which the CH1 constant domain is deleted, using mAbs to epitopes of the constant domains of IgG heavy chains, we found no detectable deletion in any of the constant domains in PGNMID.5 The intact CH2 domain is essential for complement fixation. Amino acid sequencing of glomerular deposits in PGNMID is needed to determine whether there are unique amino acid substitutions in the heavy or light chains that may increase the pI or hydrophobicity of IgG molecules, which could promote the propensity for self-aggregation and glomerular deposition, as has been reported in Randall-type light-chain deposition disease.23
In summary PGNMID is a novel form of glomerulonephritis that mimics immune-complex type glomerulonephritis on LM and EM; however, by IF, the glomerular deposits are monoclonal, staining for a single light-chain isotype and a single γ heavy-chain subclass, most commonly IgG3 κ. Despite the monoclonality, only 30% of patients have a detectable serum M-spike, and hematologic malignancy is rare. The disease affects adults and is more common in white and female individuals. Most patients present with nephrotic-range proteinuria and hematuria with or without renal insufficiency. Prognosis is variable, with nearly one quarter of patients progressing to ESRD within 2.5 yr despite IM. Because the majority of patients do not have M-spike or plasma cell dyscrasia, renal biopsy with careful attention to light-chain and IgG isotype staining is essential for diagnosis. Larger multicenter studies of PGNMID will be required to define the optimal therapeutic approach.
Concise Methods
Thirty-seven patients with PGNMID were identified by retrospective review of all native renal biopsies received and accessioned at Columbia University Medical Center (34 patients, biopsy incidence 0.17%) and Ohio State University Medical Center (three patients) from November 1999 through November 2008. These included 27 newly identified cases and extended follow-up in our 10 previously reported cases.5 Previously defined diagnostic criteria for PGNMID5 include renal biopsy findings of glomerulonephritis with the following: (1) Glomerular immune deposits staining positive for γ heavy chain (IgG), with negativity for α (IgA) and μ (IgM) heavy-chains, indicating restriction to a single (γ) Ig class; (2) positive staining for a single γ (IgG) subclass (IgG1, IgG2, IgG3, or IgG4); (3) positive staining for a single light-chain isotype (κ or λ), indicating monoclonality; (4) predominantly granular electron-dense deposits in mesangial, subendothelial, and/or subepithelial locations by EM, resembling immune complex glomerulonephritis; and (5) no clinical or laboratory evidence of cryoglobulinemia. Renal biopsy samples were processed by standard techniques for LM, IF, and EM. IF was performed on 3-μm cryostat sections using polyclonal FITC-conjugated antibodies to IgG, IgM, IgA, C3, C1q, and κ and λ light chains (Dako Corp., Carpinteria, CA). Determination of the IgG subclass was performed on 3-μ cryostat sections using monoclonal FITC-conjugated antibodies to IgG1 (clone 8c/6-39), IgG2 (clone HP6014), IgG3 (clone HP6050), and IgG4 (clone HP6023; The Binding Site, Birmingham, UK). IF staining intensity was graded 0 to 3+ on a semiquantitative scale.
Patients' medical records were reviewed for demographic information, presenting clinical and laboratory findings, presence of serum and urine M-spike, treatment, and outcome. The following definitions were applied: Nephrotic-range proteinuria, 24-h urine protein ≥3 g/d; hypoalbuminemia, serum albumin ≤3.5 g/dl; renal insufficiency, serum creatinine >1.2 mg/dl; and hematuria, >5 red blood cells per high-power field on microscopic examination of the urinary sediment. Nephrotic syndrome was defined as nephrotic-range proteinuria, hypoalbuminemia, and peripheral edema. For the purpose of outcome analysis, the following definitions were used: (1) CR: Remission of proteinuria to <500 mg/d with normal renal function; (2) PR: Reduction in proteinuria by at least 50% and to <2 g/d with stable renal function (no more than a 20% increase in serum creatinine); (3) PRD: Failure to meet criteria for either CR or PR but not reaching ESRD, including patients with unremitting proteinuria or progressive chronic kidney disease; and (4) ESRD: Requiring renal replacement therapy.
Statistical analysis was performed using SPSS 15 for Windows (SPSS, Chicago, IL). Continuous variables are reported as means ± SD. Analysis was performed using nonparametric exact methods, including the Fisher exact test, the Mann-Whitney U test, and the Kruskal-Wallis test, as appropriate for variable type. Multivariate analysis was performed using the Cox proportional hazards model. Statistical significance was assumed at P < 0.05.
The study was approved by the institutional review boards of Columbia University Medical Center and Ohio State University Medical Center.
Disclosures
None.
Acknowledgments
We thank Dr. Robert Winchester for helpful discussions.
The study was supported in part by the Glomerular Center at Columbia University.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D'Agati VD:Renal monoclonal immunoglobulin deposition disease: The disease spectrum. J Am Soc Nephrol 12: 1482–1492, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Nasr SH, Markowitz GS, Reddy BS, Maesaka J, Swidler MA, D'Agati VD:Dysproteinemia, proteinuria, and glomerulonephritis. Kidney Int 69: 772–775, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, D'Agati VD:Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int 63: 1450–1461, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Nasr SH, Colvin R, Markowitz GS:IgG1 lambda light and heavy chain renal amyloidosis. Kidney Int 70: 7, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D'Agati VD:Proliferative glomerulonephritis with monoclonal IgG deposits: A distinct entity mimicking immune-complex glomerulonephritis. Kidney Int 65: 85–96, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Maldonado JE, Velosa JA, Kyle RA, Wagoner RD, Holley KE, Salassa RM:Fanconi syndrome in adults: A manifestation of a latent form of myeloma. Am J Med 58: 354–364, 1975 [DOI] [PubMed] [Google Scholar]
- 7.Alpers CE, Tu WH, Hopper J, Jr, Biava CG:Single light chain subclass (kappa chain) immunoglobulin deposition in glomerulonephritis. Hum Pathol 16: 294–304, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Evans DJ, Macanovic M, Dunn MJ, Pusey CD:Membranous glomerulonephritis associated with follicular B-cell lymphoma and subepithelial deposition of IgG1-kappa paraprotein. Nephron Clin Pract 93: c112–c118, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Lee JG, Moon KC, Lee JE, Kim P, Lee JG, Kim JH, Lee KY:A case of proliferative glomerulonephritis with monoclonal IgG deposits. Korean J Nephrol 23: 987–991, 2004 [Google Scholar]
- 10.Komatsuda A, Masai R, Ohtani H, Togashi M, Maki N, Sawada K, Wakui H:Monoclonal immunoglobulin deposition disease associated with membranous features. Nephrol Dial Transplant 23: 3888–3894, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Bridoux F, Zanetta G, Mougenot B, Goujon JM, Vanhille P, Bauwens M, Chevet D, Ronco P, Preud'homme JL, Touchard G:Glomerulopathy with non-organized and non-Randall type monoclonal immunoglobulin deposits: A rare entity [Abstract]. J Am Soc Nephrol 12: 94A, 2001 [Google Scholar]
- 12.Geldenhuys L, Jones B:Glomerulonephritis with monoclonal immunoglobulin deposits [Abstract]. J Am Soc Nephrol 19: 671A, 2008 [Google Scholar]
- 13.Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, Remuzzi G:Rituximab in idiopathic membranous nephropathy: A one-year prospective study. J Am Soc Nephrol 14: 1851–1857, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I:B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis Rheum 50: 2580–2589, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Jayne D:Role of rituximab therapy in glomerulonephritis. J Am Soc Nephrol September17, 2008. [ epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Keogh KA, Wylam ME, Stone JH, Specks U:Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 52: 262–268, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Roccatello D, Baldovino S, Rossi D, Mansouri M, Naretto C, Gennaro M, Cavallo R, Alpa M, Costanzo P, Giachino O, Mazzucco G, Sena LM:Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinemic glomerulonephritis. Nephrol Dial Transplant 19: 3054–3061, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Collins M, Navaneethan SD, Chung M, Sloand J, Goldman B, Appel G, Rovin BH:Rituximab treatment of fibrillary glomerulonephritis. Am J Kidney Dis 52: 1158–1162, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Grey HM, Hirst JW, Cohn M:A new mouse immunoglobulin: IgG3. J Exp Med 133: 289–304, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capra JD, Kunkel HG:Aggregation of gamma-G3 proteins: Relevance to the hyperviscosity syndrome. J Clin Invest 49: 610–621, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdelmoula M, Spertini F, Shibata T, Gyotoku Y, Luzuy S, Lambert PH, Izui S:IgG3 is the major source of cryoglobulins in mice. J Immunol 143: 526–532, 1989 [PubMed] [Google Scholar]
- 22.Alpers CE, Smith KD:Cryoglobulinemia and renal disease. Curr Opin Nephrol Hypertens 17: 243–249, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Vidal R, Goñi F, Stevens F, Aucouturier P, Kumar A, Frangione B, Ghiso J, Gallo G:Somatic mutations of the L12a gene in V-kappa (1) light chain deposition disease: Potential effects on aberrant protein conformation and deposition. Am J Pathol 155: 2009–2017, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]