Abstract
The mechanisms by which estrogens modulate PTH are controversial, including whether or not estrogen receptors (ERs) are present in the parathyroid glands. To explore these mechanisms, we combined a rat model of CKD with ovariectomy and exogenous administration of estrogens. We found that estrogen treatment significantly decreased PTH mRNA and serum levels. We did not observe ERα or ERβ mRNA or protein in the parathyroids, suggesting an indirect action of estrogens on PTH regulation. Estrogen treatment significantly decreased serum 1,25(OH)2 vitamin D3 and phosphorus levels. In addition, estrogens significantly increased fibroblast growth factor 23 (FGF23) mRNA and serum levels. In vitro, estrogens led to transcriptional and translational upregulation of FGF23 in osteoblast-like cells in a time- and concentration-dependent manner. These results suggest that estrogens regulate PTH indirectly, possibly through FGF23.
Estrogen deficiency is the main factor implicated in bone loss in postmenopausal osteoporosis.1 As a consequence of the lack of estrogens, bone turnover increases, leading to an imbalance between bone formation and bone resorption, favoring the latter.2,3 This imbalance affects calcium–phosphate metabolism and may increase serum parathyroid hormone (PTH) levels.4
Estrogen replacement therapy prevents bone loss and fractures,5,6 acting directly on bone cells through their specific estrogen receptors (ERs): α and β.7,8 In addition, in postmenopausal women, estrogens can also reduce PTH serum levels4,9 through an as of yet poorly understood mechanism.
A possible direct effect of estrogens reducing PTH acting through ERα and ERβ located in the parathyroid cells has been suggested, but the existence of ERα and ERβ in parathyroid tissue is still a controversial issue.10–13 Estrogens may also decrease PTH secretion by acting on other factors such as calcium,14,15 1,25(OH)2 D3 (calcitriol),15 and phosphorus,16,17 among others. Recently, fibroblast growth factor 23 (FGF23), involved in phosphorus and vitamin D metabolism,18 has been suggested to influence PTH synthesis and secretion.19
In women with chronic kidney disease (CKD), little is known about the role that estrogen deficiency plays in the pathogenesis and progression of bone disease.20,21 Understanding the mechanism through which estrogens act on PTH is also a subject of interest in these patients, because of the high prevalence of secondary parathyroid disorders.22 Because several aspects of the effects of estrogens on PTH remain unclear, the objective of this study was to investigate the factors and mechanisms involved in the likely effect of estrogens on the parathyroid gland.
Results
In Vivo Study
Renal Function, Estrogen Replacement, and Bone Mass.
Five different groups of rats were studied: CKD without ovariectomy (OVX), CKD+OVX treated with placebo, CKD+OVX treated with 17β-estradiol (E2) at doses of 15 and 45 ng/kg/d, and a group of rats with normal renal function without OVX of the same age. No differences in renal function (serum urea and creatinine) were observed among all groups with CKD (Table 1). As expected, the placebo group showed significantly lower estrogen serum levels, uterus weight (UW), and UW/body weight (BW) ratio than the CKD-control group with no OVX. However, with the administration of E2 (E2-15 and E2-45), significantly higher values of UW and UW/BW were observed compared with the placebo group (Table 1).
Table 1.
Serum biochemical markers and BMD in all groups at the end of the study
| Normal Group | CKD Control | CKD + OVX |
|||
|---|---|---|---|---|---|
| Placebo | E2-15 | E2-45 | |||
| Creatinine (mg/dl) | 0.6 ± 0.00 | 1.03 ± 0.08 | 1.01 ± 0.08 | 1.06 ± 0.10 | 0.90 ± 0.04 |
| Urea (mg/dl) | 30.60 ± 5.41 | 69.50 ± 11.03 | 67.33 ± 3.50 | 72.00 ± 14.15 | 65.00 ± 6.04 |
| Uterus weight (mg) | 496.00 ± 161.43 | 494.33 ± 65.58 | 181.83 ± 122.61a | 352.38 ± 98.20ab | 300.00 ± 44.22ab |
| Body weight (g) | 310.00 ± 26.05 | 320.50 ± 24.42 | 353.00 ± 36.99 | 312.50 ± 18.63b | 333.40 ± 9.24 |
| Uterus weight/body weight (mg/g) | 1.60 ± 0.48 | 1.58 ± 0.24 | 0.53 ± 0.38a | 1.12 ± 0.27ab | 0.90 ± 0.14ab |
| 17 ß-E2 (pg/ml) | 45.2 ± 22.6 | 32.03 ± 13.07 | 12.63 ± 3.16a | 23.64 ± 13.52b | 35.86 ± 3.28b |
| Proximal tibia BMD (g/cm2) | 0.28 ± 0.01 | 0.28 ± 0.02 | 0.25 ± 0.02a | 0.26 ± 0.02 | 0.28 ± 0.02b |
aP < 0.05 compared with the CKD-control group.
bP < 0.05 compared with the placebo group.
The placebo group showed significantly lower bone mineral density (BMD) than the CKD-control group. BMD loss was partially prevented with the dose of 15 μg/kg body weight/d of E2 and totally prevented with the dose of 45 μg/kg body weight/d (Table 1).
Effect of Estrogens on PTH mRNA and Serum Levels.
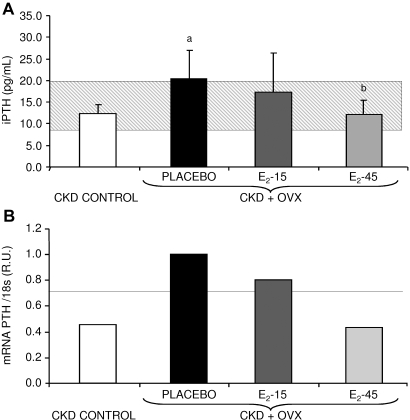
The placebo group showed a significant increase in the serum intact PTH (iPTH) levels compared with the CKD-control group. The PTH increase was partially and totally blunted with 15 and 45 μg/kg body weight/d doses of E2, respectively (Figure 1A). Similar results were obtained at the transcriptional level by quantitative real-time RT-PCR (qRT-PCR). A decreasing trend in the PTH mRNA levels with E2 treatment was observed, achieving similar values as the CKD-control group with the high E2 dose (45 μg/kg body weight/d; Figure 1B).
Figure 1.
(A) Serum iPTH levels of rats treated with placebo, E2-15 μg/kg body weight/d, E2 −45 μg/kg body weight/d, and the CKD-control group. aP < 0.05 compared with CKD-control group and bP < 0.05 compared with placebo group. The gray horizontal bar represents the range (mean ± SD) for the normal group. (B) PTH mRNA levels measured by quantitative real-time RT-PCR. R.U., relative units referred to the placebo group. The horizontal line represents PTH gene expression in the normal group.
Evaluation of the Likely Direct Effect of E2 on PTH Regulation: Study of ERα and ERβ on Parathyroid Tissue.
To evaluate the putative direct effect of E2 on PTH regulation, the presence of ERα and ERβ was analyzed by qRT-PCR in each pool of parathyroid glands from the CKD-control, placebo, E2-15, and E2-45 groups. No signal for ERα and ERβ was observed in any of the four studied groups.
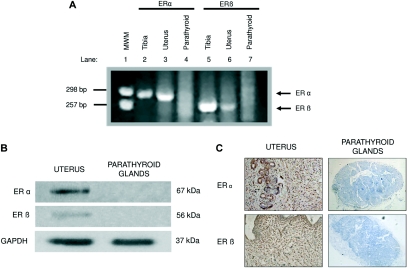
To double check the absence of ERs on parathyroid tissue, both ERα and ERβ were also assessed using parathyroid glands extracted from normal rats (no CKD, no OVX) at mRNA and protein levels. At the transcriptional level, three independent qRT-PCR experiments and two independent RT-PCR experiments showed no expression of either ERα or ERβ genes in normal parathyroid tissue. As expected, ERα and ERβ transcripts were observed in the uterus, used as a positive control tissue in the qRT-PCR experiments (data not shown) and also in the uterus and tibia used in RT-PCR experiments (Figure 2A).
Figure 2.
ERα and ERβ detection in parathyroid tissue. (A) Rat ERα (lanes 2, 3, and 4) and ERβ (lanes 5, 6, and 7) RT-PCR in different tissues resolved on agarose gel electrophoresis. Molecular weight markers (MWM; pUC18/HaeIII) (lane 1). (B) Western blot analysis of ERα and ERβ proteins in parathyroid glands and uterus. Anti-GAPDH was used as a loading control. (C) Immunohistochemical staining of the ERα and ERβ in parathyroid glands and uterus. Antibodies dilution: 1:50 for parathyroid tissue and 1:1000 for uterus). Hematoxylin counterstaining (magnification: ×20).
At the protein level, parathyroid glands did not show ERα and ERβ expression as depicted in the Western blot and immunohistochemistry analyses, despite that, in the latter, we applied a primary antibody concentration for ERα and ERβ 20 times higher than in the uterus tissue to the parathyroid glands. On the contrary, both receptors were highly expressed in the uterus (Figure 2B and C).
Evaluation of the Putative Indirect Effect of E2 on PTH Regulation.
To study the likely indirect mechanisms involved in the effect of E2 on PTH mRNA and serum levels, other serum biochemical parameters related to PTH regulation were studied.
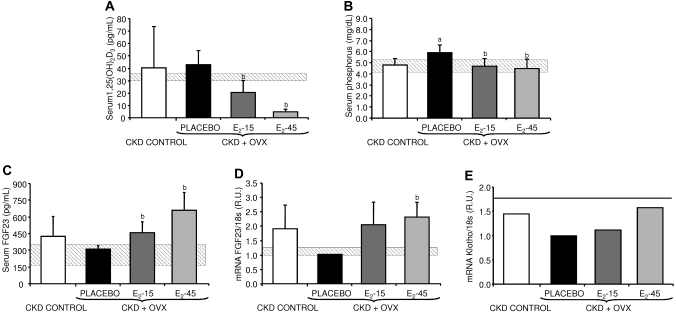
E2 was able to significantly decrease 1.25(OH)2D3 levels in a dose-dependent manner, reaching lower values than the CKD-control group with both doses of E2 (Figure 3A). E2 also decreased serum phosphorus compared with the placebo group (Figure 3B), with a similar trend to that observed in the serum PTH results (Figure 1A).
Figure 3.
(A) Serum 1,25(OH)2D3, (B) serum phosphorus, and (C) serum FGF23 levels in the CKD-control, placebo, E2-15, and E2-45 groups. aP < 0.05 compared with the CKD-control group and bP < 0.05 compared with the placebo group. (D) FGF23 mRNA levels measured by quantitative real-time RT-PCR from tibias of rats from the placebo, E2-15, E2-45, and CKD-control groups. R.U., relative units referred to the placebo group. aP < 0.05 compared with the CKD-control group and bP < 0.05 compared with the placebo group. The gray horizontal bars represent the range (mean ± SD) for the normal group. (E) Klotho mRNA levels measured by quantitative real-time RT-PCR. R.U., relative units referred to the placebo group. The horizontal line represents Klotho mRNA levels in the normal group.
Because of the known effect of FGF23 on phosphate metabolism and PTH function,18,19 serum FGF23 was also measured. FGF23 mRNA and serum levels decreased in the placebo group compared with the CKD-control group. Interestingly, rats treated with both E2 doses showed higher FGF23 mRNA and serum levels, achieving higher values than the CKD-control group (Figure 3C and D).
Because FGF23 requires Klotho as a coreceptor, the expression of this gene was measured in the pools of parathyroid glands and individual kidneys from all groups. An estrogen dose-dependent increase in Klotho mRNA levels in the parathyroid glands was observed (Figure 3E). However, no significant differences were found in kidney (data not shown).
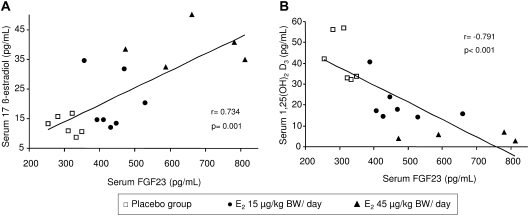
In addition, FGF23 serum levels positively correlated with serum E2 levels (r = 0.734, P = 0.001) and negatively correlated with serum 1.25(OH)2D3 (r = −0.791, P < 0.001) in the three groups with CKD+OVX (Figure 4).
Figure 4.
Correlation between (A) serum E2 versus serum FGF23 and (B) serum FGF23 versus serum 1.25(OH)2D3. r, Pearson coefficient.
In Vitro Study: Direct Effect of E2 on FGF23
To confirm the finding that E2 might directly increase FGF23, the effect of E2 on FGF23 mRNA and protein levels was evaluated using UMR-106 osteoblasts.
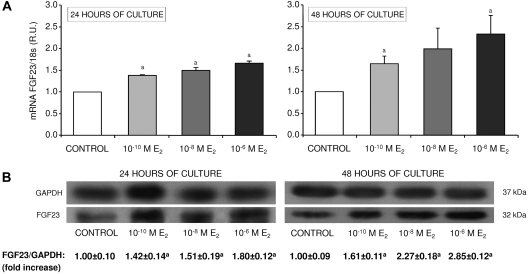
E2 significantly increased FGF23 mRNA levels in a concentration- and time-dependent manner achieving the highest mRNA levels when cells were cultured for 48 h (Figure 5A).
Figure 5.
In vitro effect of E2 on FGF23. (A) FGF23 mRNA levels measured by qRT-PCR from UMR-106 cells cultured with vehicle (control) and different concentrations of E2 (10−10, 10−8, and 10−6 M) for 24 and 48 h. R.U., relative units referred to the control group. (B) Representative image of Western blot analysis of FGF23 protein from UMR-106 cells treated with 10−10, 10−8, or 10−6 M of E2 for 24 and 48 h. Anti-GAPDH was used as loading control. Mean ± SD of three independent experiments are shown. aP < 0.05 compared with the CKD-control group.
To analyze FGF23 at the protein level, total extracts of proteins from cells cultured with E2 for 24 and 48 h were subjected to Western blot. A single band of 32 kD corresponding to intact FGF23 was detected in all samples. In addition, FGF23 protein levels increased when both E2 concentration and time of culture increased, following the same pattern seen in qRT-PCR (Figure 5B).
Discussion
Estrogen treatment prevents bone loss in postmenopausal women23 by a direct action on bone cells,8 but it might act also indirectly influencing the synthesis and secretion of calciotropic hormones, such as PTH,4 inhibiting PTH-dependent bone resorption.24 The effect of estrogens on PTH could be direct, acting on ERα and ERβ, in the parathyroids; or indirect, influencing calcium, phosphorus, or other mediators, and then secondarily, reducing PTH levels. In this study, we showed that the parathyroid tissue does not express both ERα and ERβ and that FGF23 is upregulated by estrogens. As a result, we suggest that estrogens would not act on PTH directly but likely indirectly by a mechanism which may involve FGF23.
In our study, estrogen deprivation in CKD rats showed, as expected, a significant decrease in UW/BW ratio and serum estrogen levels, together with a significant decrease in BMD in the most trabecular area of the tibia, caused by the increased bone resorption from estrogen deprivation.3,5,7,25
To achieve a hormonal replacement equivalent to that used in postmenopausal women, two different estrogen concentrations were tested.25,26 Both doses were able to significantly increase the UW/BW ratio and the estrogen serum levels compared with the untreated group (Table 1). However, only the high estrogen dose completely reversed the estrogen insufficiency and totally avoided the loss of BMD (Table 1). Estrogen treatment was able not only to prevent BMD loss but also to avoid the increase in PTH mRNA and serum levels compared with the placebo group, achieving similar values to the CKD control group with no OVX (Figure 1). The latter is still a controversial issue, because previous experimental studies10,14 have shown contradictory results related to the effect of estrogens on PTH, likely explained by methodological differences such as renal function and the age of the rats.
The mechanisms by which estrogens may induce a reduction in PTH synthesis and secretion are still not well understood.4,27,28 If the parathyroid cells would possess ERs, estrogens might directly influence PTH; however, this aspect is still controversial. Some authors have described the lack of ERs in parathyroid glands,11–13 and others have reported that parathyroid glands are target organs for estrogens,10 showing that estrogens increased PTH gene expression. It is not easy to explain these controversial results. However, the different experimental approaches, doses of estrogens tested, periods of treatment, and procedures used for gland removal (parathyroidectomy11–13 or thyroparathyroidectomy10) may explain these conflicting results.
In this study, the presence of ERα and ERβ in parathyroid tissue from the different groups studied, including normal rats, was tested by four different experimental approaches. Despite this careful and meticulous research, the ERs were not found in parathyroid tissue, even when using a primary antibody concentration 20 times higher in parathyroids than in uterus in the immunohistochemistry analysis (Figure 2).
This negative finding supports the hypothesis that the parathyroid glands do not express ERs, and thus estrogens cannot reduce PTH by a direct mechanism. Other receptors, such as the retinoid X receptor, might play a role in the effect of estrogens on different genes,29–31 but the definitive estrogen signaling pathways seem necessarily to involve the ERs.32
Another possible factor involved in PTH regulation by estrogens could be calcitriol, but there is no clear evidence to support this hypothesis. Some experimental data suggest that estrogens do not influence calcitriol levels14,33,34; meanwhile, others have shown that estrogens might modulate vitamin D receptor expression34,35 and decrease serum calcitriol levels.35,36 In our study, a significant dose-dependent decrease of serum calcitriol and PTH levels was observed with the use of estrogens (Figures 1A and B and 3A); this decrease was strikingly higher in the case of calcitriol. The reduction in serum calcitriol levels should have been accompanied by higher PTH levels37 unless a third player, in this case estrogens, was interfering in calcitriol–PTH regulation, decreasing both calcitriol and PTH.
Another mechanism by which estrogens can influence PTH levels is decreasing serum phosphorus levels. Several works have described that estrogens can downregulate the kidney sodium–phosphate cotransporter (Na-Pi), increasing phosphorus in urine and causing hypophosphatemia.17,38,39 In agreement with this view, we found that estrogen administration significantly decreased serum phosphorus levels to values similar to the CKD-control group (Figure 3B). Because phosphorus is well known to increase the synthesis and secretion of PTH,40 the reduction of phosphorus, secondary to the use of estrogens, could have been at least partly responsible for the reduction in PTH levels.
Finally, another possible factor linking the changes observed in phosphorus and PTH is FGF23, which has been identified as one of the most potent phosphatonins able to increase urinary phosphorus41,42 by inhibiting Na-Pi–dependent phosphate reabsorption in the proximal tubule. In addition, FGF23 also inhibits 1α-hydroxylase, leading to a decrease in calcitriol levels,43 and it can act directly on the mitogen activating protein kinase (MAPK) pathway of the parathyroid gland, leading to a decrease in PTH synthesis and secretion.19
In our study, all of the previously described findings were present. The estrogen-treated rats showed significantly higher serum and bone FGF23 values and significantly lower serum calcitriol, phosphorus, and PTH values, the latter also confirmed by qRT-PCR.
Because the whole set of results strongly suggested that the estrogen effect on PTH may be at least partly driven by FGF23, and FGF23 requires Klotho as a coreceptor to suppress PTH expression and secretion,19,44 we also measured Klotho gene expression in parathyroid glands. A dose-dependent increase in Klotho mRNA levels was observed in the parathyroid glands from rats treated with estrogens, likely caused by the stimulatory effect of FGF23 on Klotho expression.19 However, previous findings suggest estrogens potentially suppress Klotho expression in estrogen target organs.45 The fact that estrogens did not suppress Klotho together with the finding of the lack of ERs in the parathyroid glands are in keeping with our findings that the parathyroid glands are not a direct target tissue for estrogens. No changes in kidney Klotho mRNA levels were found despite that the kidney is a target organ for estrogens. It may be speculated that the high levels of FGF23 may counterbalance or mask the estrogen effect in the kidney.
To further study the direct effect of estrogens on FGF23 metabolism, we performed in vitro experiments using osteoblast-like cells. The results showed that estrogens, in the presence of a constant concentration of phosphorus, increase FGF23 levels in a concentration- and time-dependent manner, measured at transcriptional and translational levels (Figure 5A and B). The mechanism by which estrogens signaling stimulate FGF23 is unknown. According to the classical estrogen signaling pathway,46 the nuclear ERs may bind putative estrogen response elements (EREs) in the gene promoter, acting as transcription factors; however, further studies are needed to fully understand the mechanisms by which estrogens may upregulate FGF23.
Therefore, taking all our experiments together, we postulate that PTH regulation by estrogens is mainly indirect, and FGF23 (a new factor never described as part of this axis until now) may be a candidate for potential factors linking estrogens and PTH. However, further studies are needed to confirm whether FGF23 stimulated by estrogens directly suppresses parathyroid function.
Concise Methods
In Vivo Study: Animals, Drugs, and Experimental Design
Six-month-old female sexually mature Sprague-Dawley rats with a mean BW at the beginning of study of 325 ± 32 g (n = 24) were used. The animals were fed with a standard rodent chow containing 0.6% calcium and 0.6% phosphorus (Panlab, Barcelona, Spain) and housed in wire cages. Water and food administration was ad libitum.
E2 (Innovative Research of America, Sarasota, FL) was dissolved in ethanol and diluted with corn oil to a final volume of 0.8 ml corn oil/kg body weight/injection. The final doses of E2 administered to rats were 15 and 45 μg/kg body weight/d. This treatment was administered intraperitoneally 5 d/wk for 8 wk. Placebo (0.8 ml corn oil/kg body weight/d) was administered following the same procedure.
CKD was surgically induced using the modified technique by Ormrod and Miller (equivalent to 7/8 nephrectomy).47 Estrogen deprivation was surgically induced performing bilateral OVX. Both procedures were done in the same intervention using 42 mg/kg of intraperitoneally ketamine (Ketolar; Warner Lambert) and 0.16 mg/kg of medetomidine (Dontor; Orion, Espoo, Finland) as anesthetics. One week after surgery, a total of 20 animals with CKD+OVX were divided into three experimental groups. Group 1 (E2-15, n = 8) received intraperitoneal E2 (15 μg/kg body weight/d). Group 2 (E2-45, n = 5) received intraperitoneal E2 (45 μg/kg body weight/d). Group 3 (placebo, n = 7) received vehicle (corn oil at 0.8 ml/kg body weight/d) administered through intraperitoneal injections as described. A fourth group with CKD (same procedure) and no OVX was used as the CKD-control group (n = 4). A group of rats (n = 5) with normal renal function without OVX (normal group) was also included in the study.
After 8 wk of treatment, all rats were killed by exsanguination. Blood samples, tibias, uteri, parathyroid glands, and kidneys were removed and stored frozen at −80 °C until analysis. Blood samples were drawn for serum analyses, the right tibia was removed to perform BMD analyses, and uteri were collected to be weighed and used as a tissue marker of estrogen replacement. Because of the small size of each individual gland and to obtain enough total RNA for quantitation, the parathyroid glands from each group studied were pooled (8, 14, 16, 10, and 10 glands were pooled from the CKD-control, placebo, E2-15, E2-45, and normal groups, respectively), and each individual's left tibia and kidney were used to extract total RNA. Parathyroid glands, left tibias, and uteri from normal rats were used both to extract total RNA and proteins. In addition, parathyroid glands and uteri were also embedded in paraffin. The protocol was approved by the Laboratory Animal Ethics Committee of Oviedo University.
In Vitro Study
To study the in vitro regulation of FGF23 by E2, a rat osteosarcoma cell line UMR-106 (Health Protection Agency Culture Collections, Salisbury, UK) was used. UMR-106 cells were grown in phenol red–free αMEM (Sigma-Aldrich, St. Louis, MO) containing 1 mM phosphorus with 10% charcoal-stripped FBS (Sigma-Aldrich), 100 U/ml penicillin, and 100 μg/ml streptomycin-sulfate (Biochrom, Berlin, Germany) at 37 °C in a humidified atmosphere with 5% CO2. Cells were grown to subconfluence and were cultured in phenol red–free αMEM containing 0.25% (wt/vol) BSA (culture medium) for 24 h. At the end of this adaptation period, cells were exposed to vehicle (ethanol) or E2 at 10−10, 10−8, or 10−6 M concentrations for 24 or 48 h in culture medium. After the period of exposure, cells were collected to extract total RNA and proteins to measure FGF23.
Analytical and Technical Procedures
Serum Markers and BMD Analysis.
Serum urea, creatinine, calcium, and phosphorus levels were measured using a multichannel autoanalyzer (Hitachi 717; Boehringer Mannheim, Berlin, Germany), serum E2 levels were measured by RIA (Diagnostic Systems Laboratories, Webster, TX), serum iPTH by IRMA (Rat PTH kit Immunotopics, San Juan Capistrano, CA), serum 1.25(OH)2D3 by RIA (IDS, Boldon, Tyne & Wear, UK), and serum FGF23 with a sandwich ELISA kit (Kainos Laboratories, Tokyo, Japan), following the manufacturer's protocol in all cases.
BMD was measured at the proximal one eighth of the right tibia using dual-energy x-ray absorptiometry (QDR-100; Hologic, Bedford, MA) with software specifically prepared and adapted to small animals.48
RNA Extraction, cDNA Synthesis, and Quantitative Real-Time RT-PCR.
Total RNA extraction was performed by the method of Chomczynski.49 Total RNA concentration and purity were quantified by spectrophotometry UV-Vis (NanoDrop Technologies, Wilmington, DE), measuring the absorbance at 260 and 280 nm. RNA integrity was corroborated using formaldehyde/agarose gels. All RNA samples were stored in RNase-free tubes at −80 °C until analysis.
RT-PCR to synthesize cDNA was performed from 1 μg of total RNA previously extracted from parathyroid, tibia, uterus, kidney, and cell culture samples using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions. The cDNAs obtained were stored at −20 °C until required for analysis.
qRT-PCR was performed in the in vivo and in vitro studies on an ABI Prism 7000 Sequence Detection System using TaqMan Universal PCR Master Mix (Applied Biosystems). PTH, ERα, ERβ, FGF23, and Klotho genes and rRNA 18s as endogenous control were analyzed using TaqMan pre-Developed assay reagents (TaqMan Gene Expression Assays-On-Demand; Applied Biosystems). All reactions were performed in triplicate, amplifying endogenous and target genes in the same plate. Relative quantitative evaluation of target genes was performed by comparing threshold cycles using ΔΔCT method, as described previously.50,51
Detection by RT-PCR of ERα and ERβ.
Because of the controversy related to the existence of ERα and ERβ in parathyroid glands, the presence of mRNA corresponding to the receptors in parathyroid tissue was also analyzed amplifying cDNA from normal rats with specific oligonucleotides for ERα (forward: 5′-GCA CAA GCG TCA GAG AGA TG-3′; reverse: 5′-GCA CTC TCT TTG CCC AGT TG-3′) and ER β (forward: 5′-GGT GTG GGT ACC GTA TAG TG-3′; reverse: 5′-ATC ATG TGC ACC AGT TCC TTG-3′). The cDNA from normal tibia was used as positive control.
Protein Extraction and Western Blot Analysis.
To enrich the nuclear protein fraction to test the presence of ERα and ERβ, total proteins from a pool of 10 parathyroid glands and uteri (used as a positive control) from normal rats were extracted using a high-salts buffer containing 500 mM NaCl, 50 mM HEPES (pH 7.0), and 1× Protease Inhibitor Cocktail (Complete Mini; Roche Diagnostics, Mannheim, Germany).
To analyze the in vitro effect of E2 on FGF23 protein, total proteins from UMR-106 cells exposed for 24 or 48 h to different E2 concentrations were extracted using a standard RIPA buffer with protease inhibitors.
All samples of proteins were quantified by Bradford's method (Bio-Rad, Hercules, CA).
For the study of ERs proteins, aliquots of 20 μg of protein from the parathyroids and uterus were electrophoresed on SDS-PAGE minigels and transferred to a Hybond P membrane (GE Healthcare UK, Buckinghamshire, UK) following standard protocols.52 ERα and ERβ proteins were detected with a mouse anti-ER α monoclonal IgG1 antibody (dilution 1:1000; Acris Antibodies, Hiddenhausen, Germany) and a rabbit anti-ER β polyclonal IgG antibody (dilution 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), respectively. Rabbit anti-GAPDH polyclonal antibody (dilution 1:25,000; Santa Cruz Biotechnology) was used as a loading control.
For the in vitro FGF23 protein assay, three independent experiments were performed using aliquots containing 30 μg of total proteins from E2-treated UMR-106 cells. Total proteins were loaded on SDS-PAGE according to the same protocol described for the study of ERs with Western blot. FGF23 protein was detected using a goat anti-FGF23 polyclonal antibody (dilution 1:100; Santa Cruz Biotechnology) and rabbit anti-GAPDH polyclonal antibody (1:20,000; Santa Cruz Biotechnology) was used as load control. In both cases, chromogenic detection was performed with Pierce ECL Western Blotting Substrate (Thermo Scientific, Rockford, IL). For relative Western blot quantification, Quantity One 1-D Analysis Software v4 (Bio-Rad) and a GS-800 Calibrated Densitometer (Bio-Rad) were used.
Immunohistochemistry.
The presence of ERα and ERβ in parathyroid tissue was also determined by immunohistochemistry in 5-μm-thick serial sections from paraffin-embedded parathyroid glands and uteri from normal rats using the same specific antibodies used for Western blot and hematoxylin counterstaining (Dako REAL EnVision; Dako, Carpinteria, CA) following the manufacturer's instructions. For ER detection in uterus tissue, a dilution of 1:1000 of both antibodies was used; however, to increase the sensitivity of the detection of ERα and ERβ in parathyroid tissue, the dilution of both antibodies was 1:50.
Statistical Analysis
Biochemical markers, UW, BW, proximal tibia BMD, qRT-PCR, and Western blot quantitation were statistically analyzed using t test. Correlations between serum FGF23, serum E2, and 1.25(OH)2D3 were performed using the Pearson correlation coefficient (r).
The results are expressed as mean ± SD. Differences were considered significant when P < 0.05. All statistical analyses were performed using SPSS 12.0 for Windows (SPSS, Chicago, IL).
Disclosures
None.
Acknowledgments
This work was supported by Fondo de Investigaciones Sanitarias (FIS 02/0688 and FIS 02/0613), ISCIII-Retic-RD06, REDinREN (16/06), and Fundación Renal Íñigo Álvarez de Toledo. N.C.-L. was supported by FICYT and by ISCIII-Retic-RD06, REDinREN (16/06) and P.R.-G. by Fundación Renal Íñigo Álvarez de Toledo and FICYT.
The authors thank Dr. Socorro Braga and Dr. Teresa Fernández-Coto for their assistance in the biochemical analyses and Dr. Daniel Alvarez-Hernández, Dr. Aranzazu Rodríguez-Rodríguez, and Angeles González-Carcedo for their help. We also thank Marino Santirso for the language review.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Weitzmann MN, Pacifici R:Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest 116: 1186–1194, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raisz LG:Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin Invest 115: 3318–3325, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manolagas SC:Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Atkinson EJ, Melton LJ, III, Riggs BL:Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: A population-based study. J Clin Endocrinol Metab 82: 1522–1527, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Rodriguez A, Naves M, Rodriguez-Rebollar A, Gomez C, Braga S, Cannata-Andia JB:Hormonal replacement therapy in an animal model with chronic renal failure and ovariectomy: Biochemical and densitometric study. Kidney Int 63[ Suppl 85]: 57–61, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Pinkerton JV, Dalkin AC:Combination therapy for treatment of osteoporosis: A review. Am J Obstet Gynecol 197: 559–565, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Riggs BL, Khosla S, Melton , III:Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23: 279–302, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S:Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130: 811–823, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Stock JL, Coderre JA, Mallette LE:Effects of a short course of estrogen on mineral metabolism in postmenopausal women. J Clin Endocrinol Metab 61: 595–600, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Naveh-Many T, Almogi G, Livni N, Silver J:Estrogen receptors and biologic response in rat parathyroid tissue and C cells. J Clin Invest 90: 2434–2438, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince RL, MacLaughlin DT, Gaz RD, Neer RM:Lack of evidence for estrogen receptors in human and bovine parathyroid tissue. J Clin Endocrinol Metab 72: 1226–1228, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Saxe AW, Gibson GW, Russo IH, Gimotty P:Measurement of estrogen and progesterone receptors in abnormal human parathyroid tissue. Calcif Tissue Int 51: 344–347, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Lim SK, Won YJ, Lee HC, Huh KB, Park YS:A PCR analysis of ERalpha and ERbeta mRNA abundance in rats and the effect of ovariectomy. J Bone Miner Res 14: 1189–1196, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Liel Y, Shany S, Smirnoff P, Schwartz B:Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology 140: 280–285, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Ten Bolscher M, Netelenbos JC, Barto R, Van Buuren LM, Van der vijgh WJ: Estrogen regulation of intestinal calcium absorption in the intact and ovariectomized adult rat. J Bone Miner Res 14: 1197–1202, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Uemura H, Irahara M, Yoneda N, Yasui T, Genjida K, Miyamoto KI, Aono T, Takeda E:Close correlation between estrogen treatment and renal phosphate reabsorption capacity. J Clin Endocrinol Metab 85: 1215–1219, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Faroqui S, Levi M, Soleimani M, Amlal H:Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int 73: 1141–1150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T:Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J:The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holley JL, Schmidt RJ:Hormone replacement therapy in postmenopausal women with end-stage renal disease: A review of the issues. Semin Dial 14: 146–149, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Weisinger JR, Gonzalez L, Alvarez H, Hernandez E, Carlini RG, Capriles F, Cervino M, Martinis R, Paz-Martinez V, Bellorin-Font E:Role of persistent amenorrhea in bone mineral metabolism of young hemodialyzed women. Kidney Int 58: 331–335, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Felsenfeld AJ, Rodriguez M, Aguilera-Tejero E:Dynamics of parathyroid hormone secretion in health and secondary hyperparathyroidism. Clin J Am Soc Nephrol 2: 1283–1305, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Delmas PD:Treatment of postmenopausal osteoporosis. Lancet 359: 2018–2026, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Cosman F, Shen V, Xie F, Seibel M, Ratcliffe A, Lindsay R:Estrogen protection against bone resorbing effects of parathyroid hormone infusion. Assessment by use of biochemical markers. Ann Intern Med 118: 337–343, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Naves Diaz M, Rodriguez Rodriguez A, Fernandez Martin JL, Serrano Arias M, Menendez Rodriguez P, Cannata Andia JB:Effects of estradiol, calcitriol and both treatments combined on bone histomorphometry in rats with chronic kidney disease and ovariectomy. Bone 41: 614–619, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Verhaeghe J, Oloumi G, van Herck E, van Bree R, Dequeker J, Einhorn TA, Bouillon R:Effects of long-term diabetes and/or high-dose 17 beta-estradiol on bone formation, bone mineral density, and strength in ovariectomized rats. Bone 20: 421–428, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Selby PL, Peacock M:Ethinyl estradiol and norethindrone in the treatment of primary hyperparathyroidism in postmenopausal women. N Engl J Med 314: 1481–1485, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Marcus R, Madvig P, Crim M, Pont A, Kosek J:Conjugated estrogens in the treatment of postmenopausal women with hyperparathyroidism. Ann Intern Med 100: 633–640, 1984 [DOI] [PubMed] [Google Scholar]
- 29.Segars JH, Marks MS, Hirschfeld S, Driggers PH, Martinez E, Grippo JF, Brown M, Wahli W, Ozato K:Inhibition of estrogen-responsive gene activation by the retinoid X receptor beta: Evidence for multiple inhibitory pathways. Mol Cell Biol 13: 2258–2268, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunez SB, Medin JA, Braissant O, Kemp L, Wahli W, Ozato K, Segars JH:Retinoid X receptor and peroxisome proliferator-activated receptor activate an estrogen responsive gene independent of the estrogen receptor. Mol Cell Endocrinol 127: 27–40, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Celli G, Darwiche N, De Luca LM:Estrogen induces retinoid receptor expression in mouse cervical epithelia. Exp Cell Res 226: 273–282, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Marino M, Galluzzo P, Ascenzi P:Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7: 497–508, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz B, Smirnoff P, Shany S, Liel Y:Estrogen controls expression and bioresponse of 1,25-dihydroxyvitamin D receptors in the rat colon. Mol Cell Biochem 203: 87–93, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Lai WP, Wu CF, Favus MJ, Leung PC, Wong MS:Ovariectomy worsens secondary hyperparathyroidism in mature rats during low-Ca diet. Am J Physiol Endocrinol Metab 292: E723–E731, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Noland KA, Kalu DN:Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech Ageing Dev 99: 109–122, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Colin EM, Van Den Bemd GJ, Van Aken M, Christakos S, De Jonge HR, Deluca HF, Prahl JM, Birkenhager JC, Buurman CJ, Pols HA, Van Leeuwen JP:Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the rat. J Bone Miner Res 14: 57–64, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Goodman WG:The flavors of vitamin D: Tasting the molecular mechanisms. Kidney Int 66: 1286–1287, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Dick IM, Prince RL:The effect of estrogen on renal phosphorus handling in the rat. Am J Nephrol 21: 323–330, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Dick IM, Devine A, Beilby J, Prince RL:Effects of endogenous estrogen on renal calcium and phosphate handling in elderly women. Am J Physiol Endocrinol Metab 288: E430–E435, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L, Campistol JM, Torres A, Rodriguez M:High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9: 1845–1852, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Bai X, Miao D, Li J, Goltzman D, Karaplis AC:Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145: 5269–5279, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T:Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98: 6500–6505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perwad F, Zhang MY, Tenenhouse HS, Portale AA:Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293: F1577–F1583, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS:In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J 23: 433–441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oz OK, Hajibeigi A, Howard K, Cummins CL, van Abel M, Bindels RJ, Word RA, Kuro-o M, Pak CY, Zerwekh JE:Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice. J Bone Miner Res 22: 1893–1902, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Parker MG:Transcriptional activation by oestrogen receptors. Biochem Soc Symp 63: 45–50, 1998 [PubMed] [Google Scholar]
- 47.Ormrod D, Miller T:Experimental uremia. Description of a model producing varying degrees of stable uremia. Nephron 26: 249–254, 1980 [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Alonso C, Menendez-Rodriguez P, Virgos-Soriano MJ, Fernandez-Martin JL, Fernandez-Coto MT, Cannata-Andia JB:Aluminum-induced osteogenesis in osteopenic rats with normal renal function. Calcif Tissue Int 64: 534–541, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Chomczynski P:A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15: 532–534, 536–537, 1993 [PubMed] [Google Scholar]
- 50.Livak KJ, Schmittgen TD:Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Carrillo-Lopez N, Alvarez-Hernandez D, Gonzalez-Suarez I, Roman-Garcia P, Valdivielso JM, Fernandez-Martin JL, Cannata-Andia JB:Simultaneous changes in the calcium-sensing receptor and the vitamin D receptor under the influence of calcium and calcitriol. Nephrol Dial Transplant 23: 3479–3484, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Maniatis T, Fistch EF, Sambrook J: Molecular Cloning, New York, Cold Spring Harbor Laboratory Press, 1989 [Google Scholar]