Abstract
A recent report suggested that female recipients of male deceased-donor kidneys are at increased risk for graft failure because of H-Y antigen mismatch. In an attempt to confirm and extend these results, we studied all adult recipients of deceased-donor kidney transplants from 1990 through 2004 in the US Renal Data System. Compared with all other gender combinations, female recipients of male donor kidneys had a 12% increased risk for graft failure at 1 yr (hazard ratio 1.12; 95% confidence interval 1.05 to 1.19) but no excess risk at 10 yr (hazard ratio 1.03; 95% confidence interval 0.98 to 1.07). We observed a similar pattern of short- and long-term risk for both death-censored graft failure and mortality. The main results were consistent across several prespecified patient subgroups and were robust to sensitivity analyses. In conclusion, compared with other recipient-donor gender combinations, female recipients of male donor kidney transplants in the United States have an increased short-term risk but not long-term risk for adverse outcomes.
Allorecognition of human major histocompatibility antigens (i.e., HLA) and the associated immune response has important short- and long-term implications for successful kidney transplantation. Although much effort has been placed on trying to understand and control the immune response to these major histocompatibility antigens, relatively little is known about the role of minor histocompatibility antigens as determinants of outcome. H-Y antigens, derived from the Y chromosome, are a group of minor histocompatibility antigens that are widely expressed in human tissues1 and have been found to be prognostically important for the survival of recipient–donor gender-mismatched hematopoietic stem cell transplants.2–9
Several studies of heart, lung, liver, and corneal transplantation have suggested that female recipients of male donor organs/tissues are at increased risk for adverse graft outcomes10–13; however, other studies have failed to show this effect.14–18 Recently, a report by Gratwohl et al.,19 using data from the Collaborative Transplant Study (CTS), suggested that, after adjustment for the independent effects of recipient and donor gender, female recipients of male deceased-donor kidney transplants (versus all other recipient–donor gender combinations) were significantly associated with a 6 to 8% increase in the risk for graft failure and a 10 to 11% increase in the risk for death-censored graft failure during 10-yr of follow-up. They termed this phenomenon the “H-Y effect”; however, only 18.6% of the patients in the study by Gratwohl et al. were from North America, and a previous report failed to show an H-Y effect in zero-mismatched, US living-donor kidney transplants.20 In addition, the impact of adjusting for a more extensive set of covariates (including recipient and donor weight) was not assessed.
Given their greater susceptibility to unmeasured or residual confounding (as compared with randomized, controlled trials), it is important to reproduce the results of observational studies in different patient populations from different data sources.21 In an attempt to confirm the results of Gratwohl et al. and to determine the relevance of the H-Y effect in deceased-donor kidney transplants performed in the United States, we undertook a retrospective cohort study using data from the US Renal Data System (USRDS). We used a modeling strategy similar to that of Gratwohl et al. and then extended it to include additional covariate data, an evaluation of prespecified subgroups, and various sensitivity analyses.
Results
The median follow-up time for graft failure and patient mortality were 4.16 and 5.45 yr, respectively. This resulted in 548,904 and 660,602 total person-years at risk for graft failure and patient mortality, respectively. In total, there were 16,135 graft failures (6878 of which were deaths) during the first year of follow-up and 35,084 graft failures (22,566 of which were deaths) from the second to the 10th years of follow-up.
Baseline recipient, donor, and transplant characteristics across the four recipient–donor gender combinations are shown in Table 1. Female recipients were more likely to be highly sensitized and have “other” causes of ESRD; however, there were no major differences in the distribution of other recipient characteristics. Female deceased donors tended to be white and older and had strokes as the most common cause of death. In light of the last two characteristics, a greater proportion of female kidneys were deemed expanded-criteria donors. Transplant characteristics, including the number of HLA mismatches, cold ischemia time, and transplant era, were similarly distributed across recipient–donor gender strata.
Table 1.
Study population characteristics by recipient–donor gender statusa
| Study Population Characteristics | Male Recipient (n [%]) |
Female Recipient (n [%]) |
||
|---|---|---|---|---|
| Male Donor(n = 43,243) | Female Donor(n = 28,029) | Male Donor(n = 27,706) | Female Donor(n = 18,899) | |
| Recipient age (yr) | ||||
| 18.0 to 34.9 | 8310 (19.2) | 5184 (18.5) | 6314 (22.8) | 3970 (21.0) |
| 35.0 to 49.9 | 16,592 (38.4) | 10,348 (36.9) | 10,362 (37.4) | 6888 (36.5) |
| 50.0 to 64.9 | 14,618 (33.8) | 9746 (34.8) | 8983 (32.4) | 6486 (34.3) |
| ≥65.0 | 3723 (8.6) | 2751 (9.8) | 2047 (7.4) | 1555 (8.2) |
| Recipient race | ||||
| white | 29,080 (67.3) | 19,035 (67.9) | 18,459 (66.6) | 12,456 (65.9) |
| black | 11,934 (27.6) | 7413 (26.5) | 7424 (26.8) | 5092 (26.9) |
| other | 2229 (5.1) | 1581 (5.6) | 1823 (6.6) | 1351 (7.1) |
| Cause of ESRD | ||||
| glomerulonephritis | 11,576 (26.8) | 7601 (27.1) | 7015 (25.3) | 4675 (24.7) |
| diabetes | 11,599 (26.8) | 7545 (26.9) | 7030 (25.4) | 4848 (25.7) |
| hypertension | 9903 (22.9) | 6295 (22.5) | 4693 (16.9) | 3224 (17.1) |
| cystic disease | 3535 (8.2) | 2264 (8.1) | 2746 (9.9) | 2001 (10.6) |
| other | 4818 (11,1) | 3120 (11.1) | 4934 (17.8) | 3373 (17.9) |
| unknown/missing | 1812 (4.2) | 1204 (4.3) | 1288 (4.7) | 778 (4.1) |
| Peak PRA (%) | ||||
| <10 | 28,943 (66.9) | 18,994 (67.8) | 14,643 (52.9) | 10,072 (53.3) |
| 10 to 49 | 6093 (14.1) | 3966 (14.1) | 5196 (18.7) | 3512 (18.6) |
| ≥50 | 3183 (7.4) | 2023 (7.2) | 4931 (17.8) | 3361 (17.8) |
| unknown/missing | 5024 (11.6) | 3046 (10.9) | 2936 (10.6) | 1954 (10.3) |
| Time on dialysis (mo) | ||||
| no dialysis (preemptive) | 2206 (5.1) | 1372 (4.9) | 1698 (6.1) | 1080 (5.7) |
| 0 to 6 | 2716 (6.3) | 1746 (6.2) | 1742 (6.3) | 1181 (6.3) |
| 6 to 12 | 4495 (10.4) | 2840 (10.1) | 2653 (9.6) | 1814 (9.6) |
| 12 to 24 | 9859 (22.8) | 6334 (22.6) | 5828 (21.0) | 4036 (21.4) |
| 24 to 36 | 7521 (17.4) | 5124 (18.3) | 4712 (17.0) | 3202 (16.9) |
| 36 to 48 | 5271 (12.2) | 3560 (12.7) | 3438 (12.4) | 2244 (11.9) |
| ≥48 | 11,175 (25.8) | 7053 (25.2) | 7635 (27.6) | 5342 (28.3) |
| Transplant number | ||||
| first transplant | 37,628 (87.0) | 24,368 (86.9) | 23,962 (86.5) | 16,425 (86.9) |
| retransplant | 5615 (13.0) | 3661 (13.1) | 3744 (13.5) | 2474 (13.1) |
| Donor age (yr) | ||||
| 0 to 9 | 1873 (4.3) | 1246 (4.5) | 1762 (6.4) | 1154 (6.1) |
| 10 to 39 | 24,013 (55.5) | 10,670 (38.1) | 15,473 (55.9) | 6967 (36.9) |
| 40 to 59 | 11,550 (26.7) | 11,138 (38.7) | 6953 (25.1) | 7389 (39.1) |
| ≥60 | 2384 (5.5) | 2722 (9.7) | 1397 (5.0) | 1884 (10.0) |
| unknown/missing | 3423 (7.9) | 2253 (8.0) | 2121 (7.7) | 1505 (8.0) |
| Donor race | ||||
| white | 35,255 (81.5) | 23,900 (85.3) | 22,504 (81.2) | 16,053 (84.9) |
| black | 5108 (11.8) | 2506 (8.9) | 3252 (11.7) | 1718 (9.1) |
| other | 782 (1.8) | 658 (2.3) | 542 (2.0) | 448 (2.4) |
| unknown/missing | 2098 (4.9) | 965 (3.4) | 1408 (5.1) | 680 (3.6) |
| Donor cause of death | ||||
| anoxia | 3302 (7.6) | 3065 (10.9) | 2305 (8.3) | 2109 (11.2) |
| CVA/stroke | 12,094 (28.0) | 15,200 (54.2) | 7319 (26.4) | 10,139 (53.7) |
| head trauma | 22,637 (52.3) | 7619 (27.2) | 14,559 (52.5) | 5190 (27.5) |
| CNS tumor | 263 (0.6) | 254 (0.9) | 160 (0.6) | 202 (1.1) |
| other | 4947 (11.4) | 1891 (6.7) | 3363 (12.1) | 1259 (6.7) |
| Deceased-donor type | ||||
| standard criteria | 36,231 (83.8) | 21,649 (77.2) | 23,479 (84.7) | 14,587 (77.2) |
| expanded criteria | 7012 (16.2) | 6380 (22.8) | 4227 (15.3) | 4312 (22.8) |
| Cold ischemia time (h) | ||||
| 0 to 12 | 6028 (13.9) | 3770 (13.5) | 3725 (13.4) | 2557 (13.5) |
| 12 to 24 | 20,049 (46.4) | 12,927 (46.1) | 12,963 (46.8) | 8780 (46.5) |
| 24 to 36 | 11,150 (25.8) | 7333 (26.2) | 7276 (26.3) | 4933 (26.1) |
| ≥36 | 2688 (6.2) | 1754 (6.3) | 1572 (5.7) | 1066 (5.6) |
| unknown/missing | 3328 (7.7) | 2245 (8.0) | 2170 (7.8) | 1563 (8.3) |
| No. of HLA mismatches | ||||
| 0 to 1 | 6358 (14.7) | 4233 (15.1) | 4659 (16.8) | 3156 (16.7) |
| 2 to 4 | 23,761 (54.9) | 15,268 (54.5) | 14,927 (53.9) | 10,063 (53.3) |
| 5 to 6 | 12,285 (28.4) | 7942 (28.3) | 7532 (27.2) | 5276 (27.9) |
| unknown/missing | 839 (1.9) | 586 (2.1) | 588 (2.1) | 404 (2.1) |
| Transplant era | ||||
| 1990 to 1994 | 13,867 (32.1) | 8303 (29.6) | 8984 (32.4) | 5448 (28.8) |
| 1995 to 1999 | 13,785 (31.9) | 9450 (33.7) | 8775 (31.7) | 6247 (33.1) |
| 2000 to 2004 | 15,591 (36.1) | 10,276 (36.7) | 9947 (35.9) | 7204 (38.1) |
aCNS, central nervous system; CVA, cerebrovascular accident.
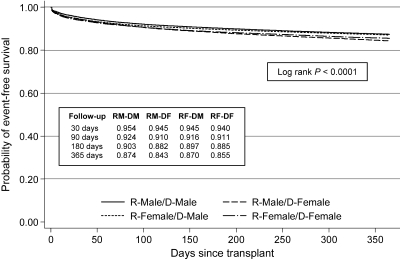
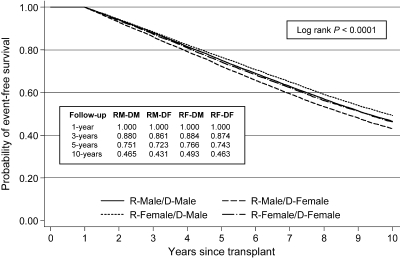
Figures 1 and 2 depict unadjusted Kaplan-Meier survival curves for the outcome of graft failure at 1 yr and 10 yr (conditioning on 1-yr survival), respectively, stratified by recipient–donor gender categories. At 1 yr, recipients of female deceased-donor kidneys had inferior survival to recipients of male deceased-donor kidneys, regardless of recipient gender. Conditional 10-yr event-free survival curves showed greater separation, with male recipients of female donors (RM-DF) having the lowest survival (43.1% at 10 yr) and female recipients of male donors (RF-DM) having the highest survival (49.3% at 10 yr). Interestingly, the outcomes of gender-concordant pairs (RF-DF and RM-DM) were highly comparable and intermediate relative to the other two groups (46.3 to 46.5% at 10 yr). A similar pattern was noted for death-censored graft failure and patient mortality (data not shown).
Figure 1.
Kaplan-Meier curves for 1-yr graft survival stratified by recipient–donor gender status. RM-DM, recipient male–donor male; RM-DF, recipient male–donor female; RF-DM, recipient female–donor male; RF-DF, recipient female–donor female.
Figure 2.
Kaplan-Meier curves for 1-yr graft survival (conditional 1-yr survival) stratified by recipient–donor gender status.
Table 2 displays the results of multivariable Cox proportional hazards models for the three primary outcomes at 1 and 10 yr after transplantation. At both time points, female recipient gender was independently associated with a decreased risk for adverse graft and patient outcomes, whereas female donor gender exhibited the opposite pattern of association. After adjustment for these and other covariates, the RF-DM group showed a modest but significantly increased risk for graft failure and death-censored graft failure at 1 yr as compared with all other recipient–donor gender combinations (adjusted hazard ratio [HR] 1.12 [95% confidence interval (CI) 1.05 to 1.19] and 1.14 [95% CI 1.06 to 1.24], respectively); however, RF-DM status did not portend a significantly worse conditional graft or death-censored graft failure at 10 yr of follow-up (adjusted HR 1.03 [95% CI 0.98 to 1.07] and 1.04 [95% CI 0.99 to 1.11], respectively). The relative hazard of mortality was higher at 1 than 10 yr, but neither reached statistical significance.
Table 2.
HRs from Cox proportional hazards models for graft failure, death-censored graft failure, and patient mortality
| Follow-up Duration and Outcome | Total Patients | Total Events | Recipient Gender (HR [95% CI])a | Donor Gender (HR [95% CI])b | Recipient Female Donor Male (HR [95% CI])c |
|---|---|---|---|---|---|
| Follow-up to 1 yrd | |||||
| graft failure | 117,877 | 16,135 | 0.90 (0.86 to 0.95) | 1.17 (1.13 to 1.22) | 1.12 (1.05 to 1.19) |
| death-censored graft failure | 117,877 | 10,224 | 0.92 (0.86 to 0.98) | 1.23 (1.16 to 1.29) | 1.14 (1.06 to 1.24) |
| patient mortality | 117,877 | 6878 | 0.87 (0.81 to 0.94) | 1.08 (1.02 to 1.15) | 1.10 (0.99 to 1.21) |
| Follow-up to 10 yre | |||||
| graft failure | 97,706 | 35,084 | 0.92 (0.89 to 0.95) | 1.05 (1.02 to 1.08) | 1.03 (0.98 to 1.07) |
| death-censored graft failure | 97,706 | 19,422 | 0.98 (0.94 to 1.03) | 1.07 (1.03 to 1.11) | 1.04 (0.99 to 1.11) |
| patient mortality | 97,706 | 22,566 | 0.89 (0.85 to 0.93) | 1.04 (1.00 to 1.07) | 1.03 (0.97 to 1.08) |
aReferent is male recipient.
bReferent is male donor.
cReferent is all other recipient–donor gender combinations.
dCox model including the following covariates: Recipient age, gender, race, cause of ESRD, peak PRA, and time on dialysis; donor variables (including donor gender); and HLA mismatches, cold ischemia time, and transplant era.
eTen-year survival analyses conditional on 1-yr survival.
Table 3 shows the relative hazard for graft failure at 1 and 10 yr (conditional on 1-yr survival) for the RF-DM group (versus all others) across various prespecified patient subgroups. The association was similar to the main results or null at both time points for all categories of recipient race, donor race, and HLA mismatch, with no evidence of statistical interaction. Of note, recipient–donor pairs with zero HLA mismatches showed a similar relation between RF-DM and graft failure as the total study population. Although there was evidence of heterogeneity across HRs within categories of peak panel-reactive antibody (PRA) and cause of ESRD, there were no consistent patterns of association across these subgroups. The more recent eras of kidney transplantation showed a null effect of RF-DM versus all other recipient–donor gender combinations, but the test of interaction was only marginally significant at the 1-yr time point.
Table 3.
Subgroup analyses for the association of recipient female–donor male (versus all other recipient–donor gender combinations) and graft failure
| Patient Subgroup | Follow-up to 1 yr (HR [95% CI]) | Pa | Follow-up to 10 yr (HR [95% CI])b | Pa |
|---|---|---|---|---|
| Recipient race | ||||
| white | 1.13 (1.06 to 1.21) | 0.715 | 1.04 (0.99 to 1.09) | 0.364 |
| black | 1.10 (1.01 to 1.20) | 1.01 (0.95 to 1.07) | ||
| other | 1.07 (0.89 to 1.27) | 0.99 (0.87 to 1.11) | ||
| Donor race | ||||
| white | 1.12 (1.05 to 1.20) | 1.02 (0.98 to 1.07) | ||
| black | 1.09 (0.97 to 1.22) | 0.895 | 1.03 (0.95 to 1.12) | 0.515 |
| other | 1.11 (0.85 to 1.45) | 1.11 (0.92 to 1.35) | ||
| unknown/missing | 1.17 (0.99 to 1.39) | 1.10 (0.98 to 1.23) | ||
| Cause of ESRD | ||||
| glomerulonephritis | 1.09 (0.99 to 1.19) | 1.03 (0.97 to 1.09) | ||
| diabetes | 1.15 (1.05 to 1.25) | 1.08 (1.02 to 1.15) | ||
| hypertension | 1.10 (0.99 to 1.22) | 0.923 | 1.02 (0.95 to 1.09) | 0.0005 |
| cystic disease | 1.16 (1.01 to 1.33) | 0.85 (0.77 to 0.94) | ||
| other | 1.12 (1.01 to 1.24) | 1.02 (0.95 to 1.10) | ||
| unknown/missing | 1.14 (0.95 to 1.36) | 1.17 (1.01 to 1.35) | ||
| Peak PRA (%) | ||||
| 0 to 9 | 1.14 (1.06 to 1.23) | 0.99 (0.94 to 1.04) | ||
| 10 to 49 | 1.21 (1.10 to 1.34) | 0.008 | 1.06 (0.99 to 1.13) | 0.037 |
| 50 to 100 | 0.99 (0.89 to 1.09) | 1.08 (1.00 to 1.16) | ||
| unknown/missing | 1.15 (1.03 to 1.30) | 1.07 (0.99 to 1.17) | ||
| HLA mismatches | ||||
| 0 to 1 | 1.26 (1.12 to 1.41) | 1.05 (0.97 to 1.13) | ||
| 2 to 4 | 1.09 (1.01 to 1.17) | 0.080 | 1.04 (0.99 to 1.09) | 0.598 |
| 5 to 6 | 1.11 (1.02 to 1.21) | 1.00 (0.94 to 1.06) | ||
| unknown/missing | 1.20 (0.94 to 1.52) | 1.01 (0.84 to 1.20) | ||
| Transplant era | ||||
| 1990 to 1994 | 1.18 (1.09 to 1.28) | 1.04 (0.99 to 1.09) | ||
| 1995 to 1999 | 1.08 (0.99 to 1.17) | 0.058 | 1.03 (0.97 to 1.09) | 0.605 |
| 2000 to 2004 | 1.08 (0.99 to 1.18) | 1.00 (0.92 to 1.08) | ||
| Overall | 1.12 (1.05 to 1.19) | <0.0001 | 1.03 (0.98 to 1.07) | 0.192 |
aP value for interaction except for overall measure of association.
bTen-year survival analyses conditional on 1-yr survival.
Table 4 shows sensitivity analyses assessing the robustness of the primary results. The HR estimates did not materially change at either time point for most of the strategies used to reanalyze the data. Of note, the 10-yr graft and patient outcomes for scenario C incorporate the first year of follow-up, in which the risk has been shown to be the highest and thus exhibits significantly elevated HRs for the total follow-up.
Table 4.
Sensitivity analyses comparing female recipient–male donor kidney transplants with all other recipient–donor gender combinations
| Follow-up Duration and Outcome | Scenario A (HR [95% CI])a | Scenario B (HR [95% CI])b | Scenario C (HR [95% CI])c | Scenario D (HR [95% CI])d | Scenario E (HR [95% CI])e |
|---|---|---|---|---|---|
| Follow-up to 1 yrf | |||||
| graft failure | 1.12 (1.05 to 1.19) | 1.12 (1.05 to 1.19) | 1.12 (1.05 to 1.19) | 1.12 (1.05 to 1.21) | 1.08 (1.00 to 1.17) |
| death-censored graft failure | 1.14 (1.05 to 1.24) | 1.15 (1.06 to 1.24) | 1.14 (1.06 to 1.24) | 1.15 (1.05 to 1.25) | 1.11 (1.01 to 1.22) |
| patient mortality | 1.10 (0.99 to 1.21) | 1.09 (0.99 to 1.21) | 1.10 (0.99 to 1.21) | 1.10 (0.99 to 1.23) | 1.04 (0.92 to 1.17) |
| Follow-up to 10 yrf,g | |||||
| graft failure | 1.03 (0.99 to 1.08) | 1.03 (0.98 to 1.08) | 1.05 (1.02 to 1.09) | 1.02 (0.98 to 1.07) | 1.04 (0.99 to 1.09) |
| death-censored graft failure | 1.04 (0.98 to 1.11) | 1.05 (0.99 to 1.11) | 1.08 (1.03 to 1.13) | 1.04 (0.98 to 1.11) | 1.06 (0.99 to 1.13) |
| patient mortality | 1.03 (0.98 to 1.09) | 1.02 (0.97 to 1.08) | 1.05 (1.00 to 1.10) | 1.02 (0.96 to 1.08) | 1.03 (0.97 to 1.10) |
aContinuous covariates modeled as continuous and imputed median/modal values for missing continuous/categorical data. HR (95% CI) for recipient female–donor male versus all other donor–recipient gender combinations
bComprehensive covariate adjustment including recipient weight, donor weight, time on dialysis, donor race, cause of death, and extended-criteria donor status.
cAnalysis during entire 10-yr follow-up period (i.e., not conditioning on 1-yr survival).
dFirst kidney transplants only.
eCase-wise deletion of observations with missing covariate data.
fBaseline Cox model with recipient age, gender, race, cause of ESRD, peak PRA, and regraft status; donor age and gender; and HLA mismatches, cold ischemia time, and transplant era.
gTen-year survival analyses conditional on 1-yr survival (unless otherwise specified).
Discussion
This study revealed that, after adjustment for recipient and donor gender, RF-DM deceased-donor kidney transplants in the United States are associated with a modestly increased short-term (i.e., 1 yr) risk for adverse graft and patient outcomes as compared with all other recipient–donor gender combinations; however, RF-DM was not associated with a significant increase in the long-term (i.e., 10 yr) risk for graft failure and patient mortality. These findings were generally consistent across various patient subgroups and robust to several sensitivity analyses. Our short-term findings are consistent with Gratwohl et al.,19 who found that RF-DM had a significantly increased risk for graft failure and death-censored graft failure at 1 yr; however, unlike Gratwohl et al., we found no significantly increased risk during the 10-yr follow-up.
Minor histocompatibility antigens, including the protein products of H-Y, have been shown in murine models to be important in eliciting an immune response to the allograft, independent of HLA mismatches.22–24 In human models, the importance of the H-Y effect has been most clearly demonstrated in hematopoietic stem cell transplantation. Female recipients exposed to H-Y antigens from male donors were more likely to experiences graft rejection,2,3,9 whereas male recipients of female donors had a higher risk for graft-versus-host disease and graft-versus-leukemia reaction.4,6–8 Although H-Y antigens are widely distributed in various human tissues,1 the degree of expression of the H-Y peptide (which is relevant for the immune response) seems to vary as a function of the type of tissue. H-Y peptide expression seems to be highest in the cells of hematopoietic lineage.25 This may account for the importance of the H-Y effect in the outcomes of hematopoietic stem cell transplantation. Interestingly, the expression of H-Y peptides was considerably lower in the kidney.25
Some studies of nonkidney solid-organ transplants have shown a detrimental impact of the male donor–female recipient pairing on outcomes,10–13 but other reports have not confirmed these findings.14–18,26 Similar studies of kidney transplantation have ranged from single case reports to registry analyses.2,20,27 Although there is evidence that H-Y antigens can cause acute rejection in RF-DM HLA-identical kidney transplants27 and also lead to an H-Y antigen-specific antibody response,28 the population-level impact of this phenomenon on graft and patient outcomes is uncertain.
The study by Gratwohl et al.19 is the first large-scale, epidemiologic analysis to suggest an H-Y effect (i.e., RF-DM deceased-donor kidney transplants) causes an increased risk for both short- and long-term graft failure and death-censored graft failure versus all other recipient–donor gender combinations. Our findings in US deceased-donor kidney transplant recipients suggest an elevated risk in the first year after transplantation but no detectable excess risk thereafter during 10 yr of follow-up. The reasons for the differences in long-term results may relate to the populations studied (including restrictions on entry into the study cohort) and variations in clinical practice patterns. Only 18.6% of the patients studied by Gratwohl et al. were from North America, with the vast majority of patients from Europe (69%). Although speculative, the degree of male donor to female recipient size mismatch may, on average, differ in the two continental populations, with US patients having a more favorable ratio.29–31 This overall size mismatch may not overshadow the short-term adverse impact of the H-Y effect, but it may have a long-term salubrious effect on graft outcomes.
We restricted our study population to January 1, 1990, through December 31, 2004, because the completeness of the USRDS data elements that were important to this analysis was less than optimal in 1988 and 1989. The cohort of Gratwohl et al. was assembled from January 1, 1985, through December 31, 2004. For the exclusion of earlier patients to explain the discordant long-term findings of the two studies, most of the detrimental effect of RF-DM deceased-donor kidney transplants would have to occur in the patients who underwent transplantation from 1985 to 1989. Unfortunately, a subgroup analysis by transplant era was not presented by Gratwohl et al., but our data suggest there may have been improvements in the adjusted risk for graft failure for RF-DM kidney transplants over time. Importantly, differences in clinical practice patterns related to the intensity of immunosuppression may lead to variable expression of the H-Y effect in European versus US patients.
It is interesting to note that, although 1-yr graft survival outcomes slightly favored the USRDS cohort, conditional 10-yr graft survival estimates favored the CTS cohort by up to 10%.19 Again, this may reflect differences in the study cohorts (e.g., recipient subgroups with inferior graft survival; e.g., black patients are more highly prevalent in the USRDS) and variations in clinical practice patterns (e.g., more widespread use of induction therapy in US patients). It is possible that the presence of numerous competing causes for graft failure may have reduced the likelihood of detecting a modest but significant long-term adverse impact of unfavorable H-Y mismatches in the USRDS cohort.
Other limitations of our study deserve note. First, the actual immune response to H-Y antigens in RF-DM deceased-donor kidney transplants and the impact of different immunotherapy regimens could not be ascertained. Although the H-Y effect is thought to be due to an MHC-restricted cell-mediated immune response,5,24,27 there is also evidence of humoral responses to these antigens.28 Prospective human studies measuring the H-Y–specific immune response in RF-DM kidney transplants, along with treatment and histologic data, may further elucidate the importance of this effect in the current era of transplantation.
Second, missing data were minimal for the primary exposure and outcomes of interest, but information on confounders were variably complete. Because all covariates were categorized in the main analysis, a missing value indicator was used to account for these incomplete data elements; however, data were reanalyzed after imputation of missing continuous/categorical variables with median/modal values, as well as case-wise deletion of observations with any missing data. The results of these sensitivity analyses were consistent with the main findings. Finally, residual confounding is always a concern in the setting of observational studies of registry data. We performed more comprehensive covariate adjustment in sensitivity analyses and found similar results to the main analysis. Although not definitive, this provides some reassurance that important confounders were likely not missed.
In conclusion, after adjustment for recipient and donor gender, female recipients of male deceased-donor kidneys in the United States exhibited a small but significantly increased risk for graft failure and patient mortality in the first year after transplantation as compared with all other recipient-donor gender combinations. This finding may be related to an alloimmune response to H-Y antigens in RF-DM kidney transplants; however, unlike the analysis by Gratwohl et al.,19 a significant increase in long-term risk could not be detected. Future research should examine the potential mechanisms underlying the H-Y effect and potential therapeutic measures to reduce the increased risk for adverse allograft outcomes.
Concise Methods
Study Population
All patients who received a deceased-donor kidney transplant from January 1, 1990, to December 31, 2004 (with follow-up until June 30, 2005), and were captured in the USRDS were eligible for inclusion in the study.32 Exclusion criteria were (1) age <18 yr at the time of transplantation, (2) multiorgan transplant recipients (including kidney-pancreas transplants), and (3) living-donor kidney transplants. Among the 118,230 kidney transplant recipients satisfying the study entry criteria, 353 were excluded because they were missing data on recipient gender, recipient race, donor gender, and/or donor cause of death, resulting in an analytical cohort of 117,877 patients.
Exposures and Outcomes
The primary exposure of interest was female recipients of male deceased-donor kidneys versus all other recipient–donor gender combinations. Recipient and donor gender were also entered as individual covariates into the multivariable models. The outcomes of interest included graft failure, death-censored graft failure, and patient mortality. Graft failure was defined as return to dialysis after transplant failure, preemptive retransplantation, or death with a functioning graft. Death-censored graft failure was similarly defined, except death with a functioning graft was considered a censoring event. All deaths before and after transplant failure were included in the patient mortality outcome. These outcomes were assessed during 1 and 10 yr after transplantation. The 10-yr follow-up analysis was conditional on patient survival at 1 yr after transplantation as per Gratwohl et al.19
Confounders
Along with recipient–donor gender status, covariates that may confound the relation between exposure and outcome were incorporated into multivariable models. The covariates chosen for the primary analysis reflected those used by Gratwohl et al. in their report19: (1) Recipient factors (age, gender, race, cause of ESRD, peak PRA titer, and regraft status); (2) donor factors (age and gender); and (3) transplant factors (degree of HLA mismatch, cold ischemia time, and transplant era). All continuous variables were grouped into predefined categories for the primary analysis; however, they were also modeled continuously in sensitivity analyses (the Sensitivity Analysis section).
Missing Data
As previously mentioned, observations with missing data on recipient gender, recipient race, donor gender, and/or donor cause of death were excluded from the analytical cohort because these individuals composed <1% of the total study population. A missing value indicator was assigned for other covariates with ≥1% missingness in the data set. In sensitivity analyses, the median and modal values for continuous and categorical variables (respectively) were imputed for missing data as described by Gratwohl et al.19 Finally, case-wise deletion of observations with any missing covariate data were also undertaken to examine the impact of this approach on the relation between exposure and outcome (Sensitivity Analyses).
Subgroup Analyses
Specific patient subgroups were examined in exploratory analyses to determine whether the relation between exposure and outcome significantly differed across levels of the subgroup variable. These subgroups were specified before the analysis phase of the study. Subgroups of interest included recipient race, donor race, cause of ESRD, peak PRA, HLA mismatches, and transplant era. These variables were chosen on the premise that the effect of H-Y incompatibility may differ across patients of varying genetic makeup, immunologic risk, and cause of kidney disease.
Sensitivity Analyses
More comprehensive adjustment for recipient and donor characteristics was pursued to obtain the most unbiased estimate of the measure of association between exposure and outcome. These models included covariates for time on dialysis, donor race, expanded-criteria donor status,33 and the recipient/donor weight. Additional analyses that included data from the entire 10-yr follow-up period (i.e., did not condition on 1-yr survival), recipients of first kidney transplants only, and case-wise deletion of observations that were missing any of the covariate data elements used in the primary analysis were performed.
Statistical Analysis
Baseline characteristics were compared across strata defined by recipient–donor gender combinations using the ANOVA or Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. The survival functions associated with the three primary outcomes were nonparametrically estimated using the Kaplan-Meier product limit method, and differences in these functions were tested using the log-rank statistic. The relation between female recipient–male donor gender status and the main outcomes were subsequently evaluated in multivariable Cox proportional hazards models to account for the influence of confounding variables. The proportional hazards assumption was examined using scaled Schoenfeld residuals and log(cumulative hazard) plots. No important departures from proportionality were detected. Analyses were also performed to determine the existence of effect measure modification across prespecified subgroups. All statistical analyses were performed using Stata/MP 10.1 for Windows (StataCorp, College Station, TX). A two-sided P < 0.05 was considered statistically significant. The study was approved by the research ethics board of the Toronto General Hospital, University Health Network.
Disclosures
None.
Supplementary Material
Acknowledgments
S.J.K. is supported by the Canadian Institutes of Health Research, Clinician-Scientist Award; J.S.G. is supported by the Michael Smith Foundation for Health Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
These data were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the author and in no way should be seen as an official policy or interpretation of the US government, the National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
References
- 1.de BM, Bakker A, Van Rood JJ, Van der WF, Goulmy E:Tissue distribution of human minor histocompatibility antigens: Ubiquitous versus restricted tissue distribution indicates heterogeneity among human cytotoxic T lymphocyte-defined non-MHC antigens. J Immunol 149: 1788–1794, 1992 [PubMed] [Google Scholar]
- 2.Goulmy E, Bradley BA, Lansbergen Q, Van Rood JJ:The importance of H-Y incompatibility in human organ transplantation. Transplantation 25: 315–319, 1978 [DOI] [PubMed] [Google Scholar]
- 3.Voogt PJ, Fibbe WE, Marijt WA, Goulmy E, Veenhof WF, Hamilton M, Brand A, Zwann FE, Willemze R, Van Rood JJ:Rejection of bone-marrow graft by recipient-derived cytotoxic T lymphocytes against minor histocompatibility antigens. Lancet 335: 131–134, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Gratwohl A, Hermans J, Niederwieser D, van BA, van Houwelingen HC, Apperley J:Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J 2: 363–370, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Spierings E, Vermeulen CJ, Vogt MH, Doerner LE, Falkenburg JH, Mutis T, Goulmy E:Identification of HLA class II-restricted H-Y-specific T-helper epitope evoking CD4+ T-helper cells in H-Y-mismatched transplantation. Lancet 362: 610–615, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR:Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood 103: 347–352, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C, Ho V, Soiffer RJ, Antin JH, Ritz J:Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood 105: 2973–2978, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gahrton G, Iacobelli S, Apperley J, Bandini G, Bjorkstrand B, Blade J, Boiron JM, Cavo M, Cornelissen J, Corradini P, Kroger N, Ljungman P, Michallet M, Russell NH, Samson D, Schattenberg A, Sirohi B, Verdonck LF, Volin L, Zander A, Niederwieser D:The impact of donor gender on outcome of allogeneic hematopoietic stem cell transplantation for multiple myeloma: Reduced relapse risk in female to male transplants. Bone Marrow Transplant 35: 609–617, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Stern M, Passweg JR, Locasciulli A, Socie G, Schrezenmeier H, Bekassy AN, Fuehrer M, Hows J, Korthof ET, McCann S, Tichelli A, Zoumbos NC, Marsh JC, Bacigalupo A, Gratwohl A:Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation 82: 218–226, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Candinas D, Gunson BK, Nightingale P, Hubscher S, McMaster P, Neuberger JM:Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet 346: 1117–1121, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Prendergast TW, Furukawa S, Beyer AJ, III, Browne BJ, Eisen HJ, Jeevanandam V:The role of gender in heart transplantation. Ann Thorac Surg 65: 88–94, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Roberts DH, Wain JC, Chang Y, Ginns LC:Donor-recipient gender mismatch in lung transplantation: Impact on obliterative bronchiolitis and survival. J Heart Lung Transplant 23: 1252–1259, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Bohringer D, Spierings E, Enczmann J, Bohringer S, Sundmacher R, Goulmy E, Reinhard T:Matching of the minor histocompatibility antigen HLA-A1/H-Y may improve prognosis in corneal transplantation. Transplantation 82: 1037–1041, 2006 [DOI] [PubMed] [Google Scholar]
- 14.De Santo LS, Marra C, De Feo M, Amarelli C, Romano G, Cotrufo M:The impact of gender on heart transplantation outcomes: a single center experience. Ital Heart J 3: 419–423, 2002 [PubMed] [Google Scholar]
- 15.Al-Khaldi A, Oyer PE, Robbins RC:Outcome analysis of donor gender in heart transplantation. J Heart Lung Transplant 25: 461–468, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Brooks BK, Levy MF, Jennings LW, Abbasoglu O, Vodapally M, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB:Influence of donor and recipient gender on the outcome of liver transplantation. Transplantation 62: 1784–1787, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Gutierrez C, Waddell TK, Liu M, Keshavjee S:Donor-recipient gender mismatch in lung transplantation: Impact on obliterative bronchiolitis and survival. J Heart Lung Transplant 24: 2000–2001, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Inoue K, Amano S, Oshika T, Tsuru T:Histocompatibility Y antigen compatibility and allograft rejection in corneal transplantation. Eye 14: 201–205, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gratwohl A, Dohler B, Stern M, Opelz G:H-Y as a minor histocompatibility antigen in kidney transplantation: A retrospective cohort study. Lancet 372: 49–53, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ellison MD, Norman DJ, Breen TJ, Edwards EB, Davies DB, Daily OP:No effect of H-Y minor histocompatibility antigen in zero-mismatched living-donor renal transplants. Transplantation 58: 518–520, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Peng RD, Dominici F, Zeger SL:Reproducible epidemiologic research. Am J Epidemiol 163: 783–789, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hirozane T, Matsumori A, Furukawa Y, Sasayama S:Experimental graft coronary artery disease in a murine heterotopic cardiac transplant model. Circulation 91: 386–392, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Koulack J, McAlister VC, MacAulay MA, Bitter-Suermann H, MacDonald AS, Lee TD:Importance of minor histocompatibility antigens in the development of allograft arteriosclerosis. Clin Immunol Immunopathol 80: 273–277, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Sonoda Y, Sano Y, Ksander B, Streilein JW:Characterization of cell-mediated immune responses elicited by orthotopic corneal allografts in mice. Invest Ophthalmol Vis Sci 36: 427–434, 1995 [PubMed] [Google Scholar]
- 25.Griem P, Wallny HJ, Falk K, Rotzschke O, Arnold B, Schonrich G, Hammerling G, Rammensee HG:Uneven tissue distribution of minor histocompatibility proteins versus peptides is caused by MHC expression. Cell 65: 633–640, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Zeier M, Schonherr R, Amann K, Ritz E:Effects of testosterone on glomerular growth after uninephrectomy. Nephrol Dial Transplant 13: 2234–2240, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer PF, Thorsby E:HLA-restricted cytotoxicity against male-specific (H-Y) antigen after acute rejection of an HLA-identical sibling kidney: Clonal distribution of the cytotoxic cells. Transplantation 33: 52–56, 1982 [DOI] [PubMed] [Google Scholar]
- 28.Tan JC, Wadia PP, Coram M, Grumet FC, Kambham N, Miller K, Pereira S, Vayntrub T, Miklos DB:H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation 86: 75–81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douverny JB, Baptista-Silva JC, Pestana JO, Sesso R:Importance of renal mass on graft function outcome after 12 months of living donor kidney transplantation. Nephrol Dial Transplant 22: 3646–3651, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Oh CK, Lee BM, Jeon KO, Kim HJ, Pelletier SJ, Kim SI, Kim YS:Gender-related differences of renal mass supply and metabolic demand after living donor kidney transplantation. Clin Transplant 20: 163–170, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Poggio ED, Hila S, Stephany B, Fatica R, Krishnamurthi V, del BC, Goldfarb D, Herts B, Dennis VW, Heeger PS, Braun W:Donor kidney volume and outcomes following live donor kidney transplantation. Am J Transplant 6: 616–624, 2006 [DOI] [PubMed] [Google Scholar]
- 32.US Renal Data System: USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 33.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ:Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 74: 1281–1286, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.