Abstract
Cytokines and nitric oxide (NO) stimulate rat mesangial cells to synthesize and secrete inflammatory mediators. To understand better the signaling pathways that contribute to this response, we exposed rat mesangial cells to the prototypic inflammatory cytokine IL-1β and analyzed the changes in the pattern of gene expression. IL-1β downregulated the gene encoding the matricellular glycoprotein secreted modular calcium-binding protein 1 (SMOC-1) in mesangial cells. Inflammatory cytokines attenuated SMOC-1 mRNA and protein expression through endogenous production of NO, which activated the soluble guanylyl cyclase. Silencing SMOC-1 expression with small interfering RNA decreased the formation of TGF-β, reduced SMAD binding to DNA, and decreased mRNA expression of genes regulated by TGF-β. In a rat model of anti–Thy-1 glomerulonephritis, glomerular SMOC-1 mRNA and protein decreased and inducible NO synthase expression increased simultaneously. Treatment of nephritic rats with the inducible NO synthase–specific inhibitor l-N6-(1-iminoethyl)-lysine prevented SMOC-1 downregulation. In summary, these data suggest that NO attenuates SMOC-1 expression in acute glomerular inflammation, thereby limiting TGF-β–mediated profibrotic signaling.
Inflammatory glomerular disorders are accompanied by an exaggerated formation of inflammatory mediators such as cytokines or nitric oxide (NO).1–3 Under inflammatory conditions, glomerular mesangial cells produce high amounts of inflammatory mediators such as cytokines, reactive oxygen species, and NO; therefore, these smooth muscle cell–like pericytes are commonly regarded as key players in glomerular inflammation.4–6 Huge amounts of NO are produced by cultured rat mesangial cells after exposure to cytokines such as IL-1β and TNF-α by the induction of the inducible NO synthase (iNOS). Remarkably, this process results in a positive regulatory loop based on a further activation of the iNOS transcriptional machinery by NO and reactive oxygen species.7,8 Subsequently, these high amounts of NO induce cell death by apoptosis or necrosis in mesangial cells.9,10 As shown in several rat and mouse models of glomerulonephritis (GN), blocking or silencing NOS activity by suitable inhibitors or by a dietary reduction of l-arginine reduced neutrophil influx and matrix deposition during glomerular inflammation. These results clearly suggest a detrimental role for NO also in vivo.11,12 In contrast, as exemplified in a chronic model of GN, administration of the iNOS-specific inhibitor l-N6-(1-iminoethyl)-lysine (L-NIL) markedly enhanced proteinuria and fibronectin deposition, indicating that activity of iNOS may also exert protective effects during inflammation.13 These seemingly conflicting results might be explained by the high diversity of molecular targets of NO that trigger further downstream deleterious or protective signaling pathways. To evaluate such different actions of NO on mesangial cells, we thought it worthwhile to analyze the effects of NO on the transcription or protein patterns that constitute the end points of NO-modulated signaling cascades. In previous experiments, we and others were able to identify various genes that are under expressional control by NO.11,14–16 To analyze the effects of NO on mRNA and protein expression, we used the mRNA-based fingerprinting method RNA arbitrarily primed reverse transcriptase–PCR (RAP-PCR)17 and two-dimensional protein gel electrophoresis. Further analysis of the mechanisms of NO-modulated gene expression revealed a tendency that NO shifts the transcription pattern of mesangial cells to a more protective antiapoptotic direction when soluble guanylyl cyclase (sGC) transduces its signaling effects or into a more deleterious direction when NO acts independent from sGC by affecting the cellular redox state. This has been documented by a cGMP-dependent upregulation of the manganese superoxide dismutase and of the PDGF receptor α as well as by a cGMP-dependent downregulation of the superoxide-producing NADPH oxidase subunit Nox1.18–20 In contrast, the expression of the chemokine macrophage inflammatory protein 2 is upregulated by NO in rat mesangial cells in a cGMP-independent manner, and blocking of iNOS activity in a rat model of anti–Thy-1 GN by L-NIL resulted in a clear reduction of glomerular neutrophil infiltration accompanied by reduced levels of macrophage inflammatory protein 2 mRNA and protein expression.11
A protective role of the iNOS/cGMP pathway in vivo was further corroborated by reports that described an adverse effect of NOS inhibition and a beneficial effect of Bay 41-2272, an activator of the sGC in the rat models of anti–Thy-1 GN.13,21,22 We compared mRNA expression patterns of rat glomerular mesangial cells treated with the NO donor (Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NO) or vehicle using the RAP-PCR method. We could identify secreted modular calcium binding protein 1 (SMOC-1) to be downregulated at the mRNA level. SMOC-1 was first described as a member of the BM-40 (also referred to as SPARC, or osteonectin) family of matricellular proteins.23 It is worth mentioning that in previous experiments, we could identify BM-40 as an NO-regulated gene; therefore, we postulate that NO generally affects signaling processes mediated by matricellular proteins.16 SMOC-1 is primarily localized in basement membranes and seems to play a role in embryogenesis, but its biologic function is so far not understood.24 Here we show the regulation of SMOC-1 mRNA and protein by NO in renal mesangial cells and in an animal model of glomerular inflammation. Furthermore, it seems that SMOC-1 is involved in TGF-β–mediated signaling processes, and this suggests that NO acts in an antifibrotic manner by downregulating SMOC-1 expression.
Results
Identification of SMOC-1 as a Cytokine-Regulated Gene by RAP-PCR
To analyze the effects of IL-1β on the transcription pattern, we treated rat mesangial cells with vehicle or IL-1β (1 nM) for 16 h. Thereafter, mRNA was isolated and reverse-transcribed to cDNA. We then performed RAP-PCR in the presence of both primers Rap1 and Rap2. The radioactively labeled PCR products were separated by gel electrophoresis and analyzed using the BAS-1500 system. Comparison of the intensities of the resulting cDNA patterns revealed an obvious difference between vehicle-treated control and IL-1β–treated cells (data not shown). The respective fragment was excised, amplified using primers Rap1 and Rap2 under stringent conditions, cloned into a PCR2.1 vector, and sequenced. Computer-aided sequence analysis revealed that the cloned fragment represented unambiguously a cDNA for rat SMOC-1 deposited in the GENEMBL library under the accession number NM_001002835.23
Effects of IL-1β on SMOC-1 mRNA and Protein Levels Involve NO
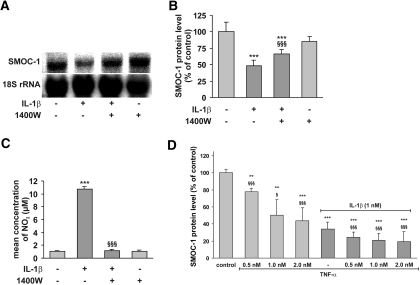
To confirm the results obtained by RAP-PCR, we treated mesangial cells with IL-1β (1 nM) for 24 h. To evaluate the effect of endogenously produced NO by mesangial cells after IL-1β–mediated induction of the iNOS, we added the iNOS-specific inhibitor 1400 W (1 mM) to block endogenous NO synthesis. Thereafter, we analyzed SMOC-1 mRNA expression and SMOC-1 protein levels in conditioned media by Northern blotting and ELISA, respectively. IL-1β markedly reduced SMOC-1 mRNA and protein levels (Figure 1, A and B) and augmented iNOS activity as the nitrite content in conditioned media (Figure 1C). Expectedly, 1400W completely blocked IL-1β–evoked nitrite formation (Figure 1C), but, remarkably, it also reversed the cytokine-mediated effect on both SMOC-1 mRNA and protein levels, suggesting a role for NO in modulating SMOC-1 expression (Figure 1, A and B). Similar results were obtained using the nonspecific NOS inhibitor NG-monomethyl-l-arginine (L-NMMA) to block endogenous NO synthesis (Supplemental Figure 1).
Figure 1.
Effects of IL-1β, 1400W, and TNF-α on SMOC-1 mRNA and protein expression. Quiescent mesangial cells were treated as indicated for 24 h with IL-1β (1 nM) in the presence (+) or absence (−) of the iNOS inhibitor 1400W (2 mM). (A) Representative Northern blot of three independent experiments for SMOC-1 mRNA. (B) The data obtained from the analysis of SMOC-1 protein levels in conditioned media by direct ELISA for SMOC-1. (C) Nitrite levels as analyzed in conditioned media. (D) Quiescent mesangial cells were treated with the indicated concentrations of TNF-α in absence or presence of IL-1β as indicated for 24 h, and conditioned media were subjected to direct ELISA for SMOC-1. Data are means ± SD (n = 3). ***P < 0.001, **P < 0.01 versus vehicle-treated controls; §§§P < 0.001, §P < 0.05 versus IL-1β (1 nM).
Next we tested whether TNF-α also modulates SMOC-1 protein expression and whether TNF-α alters the effects of IL-1β. To this end, we treated mesangial cells with various concentrations of TNF-α or TNF-α + IL-1β (1 nM) for 24 h and determined SMOC-1 protein levels by ELISA analysis. As depicted in Figure 1D, TNF-α markedly reduced SMOC-1 protein levels (to approximately 50% compared with controls when administered at higher dosages), but the combination of both cytokines was much more effective, resulting in a reduction of SMOC-1 expression to approximately 20% compared with vehicle-treated controls.
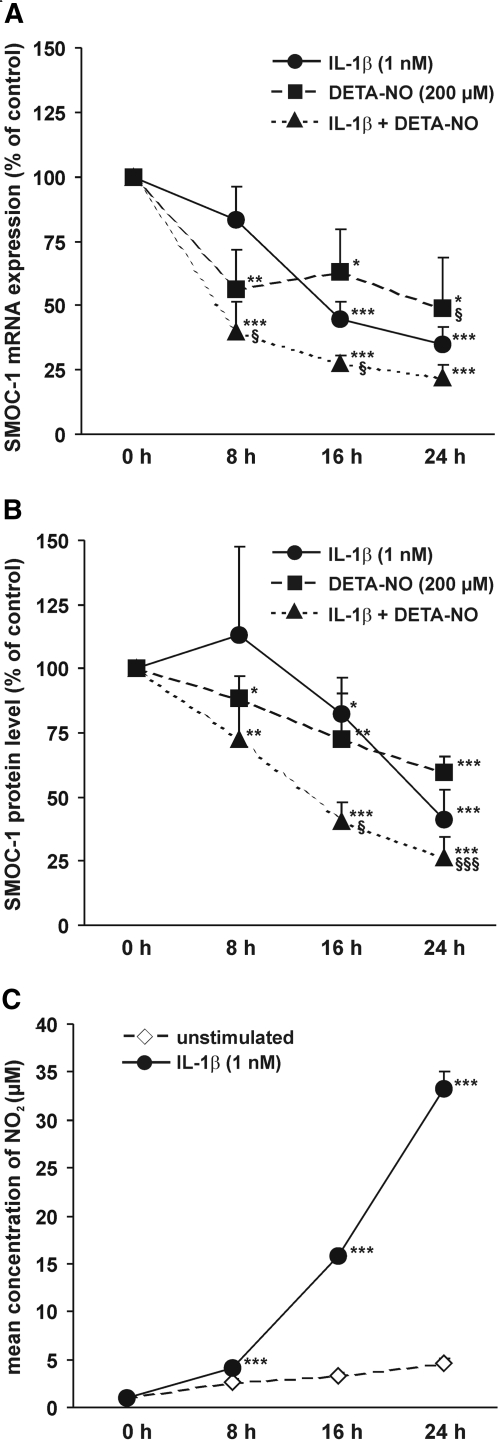
To analyze the time dependence of cytokine- and NO-mediated downregulation of SMOC-1 mRNA and protein levels, we treated mesangial cells with IL-1β (1 nM), the NO donor DETA-NO (200 μM), or a combination of both for 8, 16, and 24 h. IL-1β and DETA-NO significantly reduced SMOC-1 mRNA levels in a comparable manner. A combination of IL-1β and DETA-NO further reduced SMOC-1 mRNA expression (Figure 2A). As determined by ELISA, subsequent to the changes in SMOC-1 mRNA levels, IL-1β and DETA-NO treatment reduced SMOC-1 protein levels (Figure 2B). Not unexpected, the onset of IL-1β– and DETA-NO–mediated reduction of SMOC-1 protein levels was less prominent at the early time points, but it finally ended up in a comparable reduction of mRNA and protein levels after 24 h (Figure 2, A and B). In contrast to IL-1β, DETA-NO affected SMOC-1 expression already after 8 h, which is due to the delay in cytokine-stimulated mesangial cell production of endogenous NO (Figure 2C).
Figure 2.
Effects of IL-1β and DETA-NO on SMOC-1 mRNA and protein expression. Quiescent mesangial cells were treated with IL-1β and the NO donating reagent DETA-NO as indicated for 8, 16, or 24 h. Total RNA (20 μg each) was subjected to Northern blotting with probes specific for SMOC-1 mRNA and 18S rRNA. (A) Densitometric evaluation of SMOC-1/18S ratios. (B) Conditioned media were subjected to direct ELISA using an antibody specific for SMOC-1. Data are expressed as percentage of SMOC-1 protein level in untreated cells (control). (C) Time course of nitrite formation in mesangial cells. Data are means ± SD (n = 3 to 6). ***P < 0.001, **P < 0.01, *P < 0.05 versus vehicle-treated controls; §§§P < 0.001, §P < 0.05 versus IL-1β (1 nM).
IL-1β Affects SMOC-1 Expression by Activation of sGC
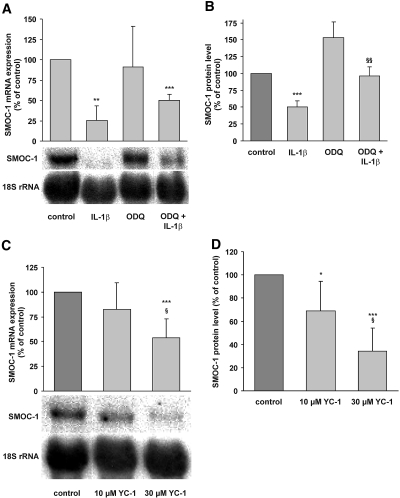
To examine whether NO formed after the induction of iNOS expression by IL-1β acts via activation of sGC on SMOC-1 expression, we treated mesangial cells with IL-1β with or without the sGC inhibitor 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1–1 (ODQ; 200 μM). SMOC-1 mRNA and protein analysis revealed that administration of ODQ at least partly reversed cytokine/NO-mediated downregulation of SMOC-1 mRNA and protein expression (Figure 3, A and B). Similar results were obtained when NS-2028 (5 μM) was used to inhibit sGC activity (data not shown). Moreover, activation of sGC by 3-(5-hydroxymethyl-2-furyl)-1-benzylindazole (YC-1) clearly diminished SMOC-1 mRNA and protein levels, corroborating a role for sGC in the downregulation of SMOC-1 expression (Figure 3, C and D).
Figure 3.
Effects of the sGC inhibitor ODQ and the sGC activator YC-1 on cytokine-mediated SMOC-1 mRNA and protein expression. Quiescent mesangial cells were treated with IL-1β, ODQ, or combinations of IL-1β plus ODQ as indicated for 24 h. (A and B) Thereafter, mRNA was isolated for Northern blotting against a SMOC-1 and 18S rRNA probe (A) and conditioned media were subjected to direct ELISA for SMOC-1 (B). Quiescent mesangial cells were treated with the indicated concentrations of YC-1 for 8 h. Total RNA (20 μg each) was subjected to Northern blotting with probes specific for SMOC-1 mRNA and 18S rRNA. (C) Representative Northern blot experiment with the densitometric evaluation of SMOC-1/18S ratios of seven experiments. Conditioned media were analyzed for SMOC-1 protein expression by ELISA. (D) SMOC-1 protein levels of six experiments. Data are means ± SD (n = 3). ***P < 0.001, **P < 0.01, *P < 0.05 versus vehicle-treated controls; §§P < 0.01 versus IL-1β alone; §P < 0.05 versus 10 μM YC-1.
Silencing of SMOC-1 Protein Expression with siRNA Reveals a Possible Role of SMOC-1 in TGF-β Signaling in Mesangial Cells
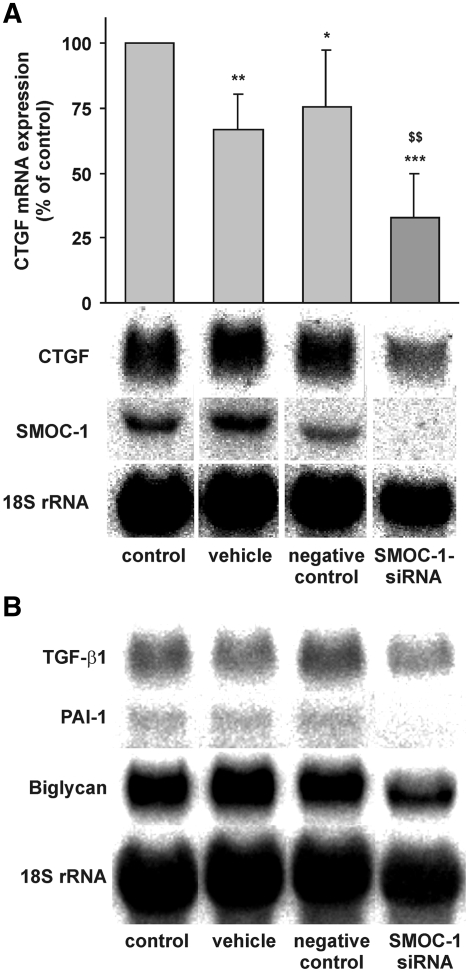
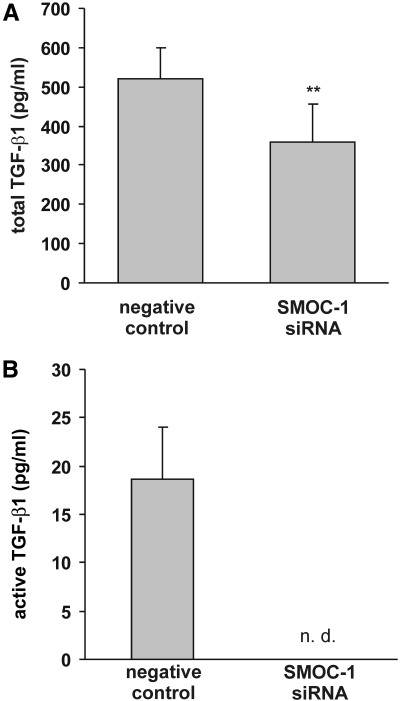
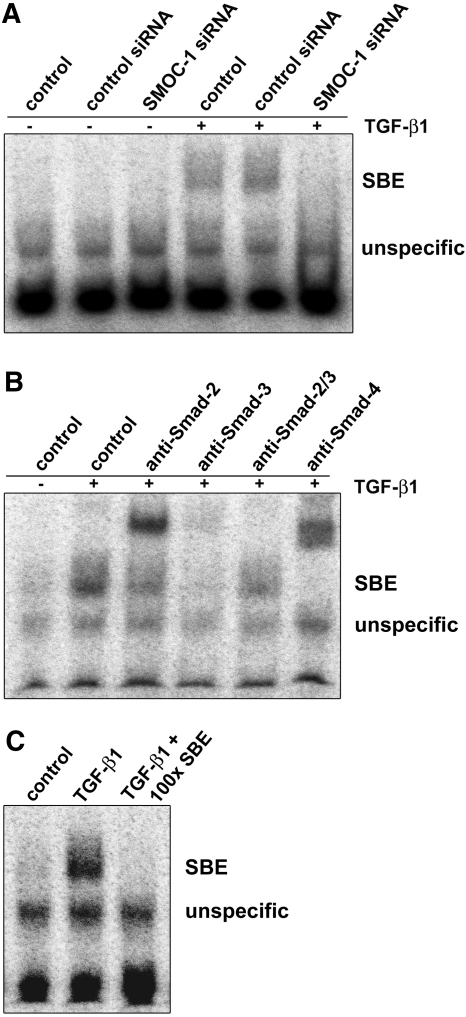
In contrast to other highly related matricellular proteins such as SPARC and SMOC-2 that mediate cell matrix interactions or play a role in cell growth or angiogenic signaling,25–27 nothing is known to the best of our knowledge about the biologic function of SMOC-1. To elucidate potential signaling cascades triggered by SMOC-1, we silenced SMOC-1 expression using a suitable siRNA and compared the behavior of mesangial cells depleted from SMOC-1 with intact controls. Because it is known that matricellular proteins act on growth factor signaling and endogenously produced NO by mesangial cells has an impact on TGF-β signaling,28–30 we speculated that this process might involve SMOC-1. To address this point, we silenced SMOC-1 expression and analyzed the mRNA expression of matrix-associated genes such as connective tissue growth factor (CTGF), TGF-β1, plasminogen activator inhibitor 1 (PAI-1), and the proteoglycan biglycan, all genes that are known to be under expressional control by NO and/or TGF-β in rat mesangial cells and that may therefore serve as a marker for NO/TGF-β–modulated signaling cascades.31,32 As depicted in Figure 4, silencing of SMOC-1 expression resulted not only in a clear reduction of SMOC-1 mRNA but also in a marked reduction of CTGF, TGF-β1, PAI-1, and biglycan, thereby indicating that SMOC-1 affects expression of these matrix-associated genes. To analyze further whether SMOC-1 affects expression and formation of total and active TGF-β, we analyzed conditioned media from mesangial cells treated with SMOC-1 siRNA for active and latent TGF-β by ELISA. Silencing of SMOC-1 resulted in a clear reduction of total TGF-β, indicating that SMOC-1 has an impact on TGF-β protein expression (Figure 5A). More interesting, knockdown of SMOC-1 completely blunted the content of active TGF-β, indicating that SMOC-1 regulates the availability of active TGF-β secreted by mesangial cells (Figure 5B). To analyze the effects of SMOC-1 on TGF-β signaling in more detail, we tested whether silencing of SMOC-1 affects binding of Smad proteins on a Smad-binding DNA element (SBE). To this end, mesangial cells were transfected with or without a nonspecific control siRNA and a siRNA directed against SMOC-1 and stimulated with TGF-β1 for 2 h to trigger SMAD signaling. We then analyzed binding of nuclear SMAD proteins by electrophoretic mobility shift assay (EMSA). Treatment of mesangial cells with SMOC-1–specific siRNA abolished the binding of SMAD complexes to its SBE, further corroborating a role of SMOC-1 in TGF-β signaling (Figure 6A). Specificity of the SMAD complexes (SMAD-2/SMAD-4 and SMAD-3/SMAD-4) bound to the SBE was verified by supershift analysis (Figure 6B) and in a competition assay with a 100-fold excess of a unlabeled SBE consensus oligonucleotide (Figure 6C).
Figure 4.
Silencing of SMOC-1 decreases mRNA expression of TGF-associated genes. Mesangial cells were left untreated (control) or transfected with vehicle, 10 nM of specific siRNA targeting SMOC-1, or control siRNA. After transfection, serum-starved mesangial cells were kept in serum-free medium for 24 h. Total RNA (20 μg each) was subjected to Northern blotting with probes specific for CTGF mRNA, SMOC-1 mRNA, TGF-β1 mRNA, PAI-1 mRNA, biglycan mRNA, and 18S rRNA. (A) Densitometric evaluation of CTGF/18S ratios is displayed as means ± SD (n = 5). (B) Representative blots for TGF-β1, PAI-1, and biglycan. ***P < 0.001, **P < 0.01, *P < 0.05 versus untransfected controls; $$P < 0.01 versus nonspecific control siRNA.
Figure 5.
Silencing of SMOC-1 decreases active and total TGF-β1 protein in conditioned media. Mesangial cells were transfected with specific siRNA targeting SMOC-1 (10 nM) or negative control siRNA. After transfection, serum-starved mesangial cells were kept in serum-free medium for 24 h. Conditioned media were analyzed for active and—after acidic activation of TGF-β—total TGF-β using a commercially available TGF-β1 ELISA kit. Data are means ± SD (n = 4). **P < 0.01 versus nonspecific control siRNA.
Figure 6.
Silencing of SMOC-1 decreases binding of Smad proteins on a consensus SBE. (A) Quiescent mesangial cells were transfected with or without control siRNA or siRNA directed against SMOC-1. Twenty-four hours after transfection, media were removed and cells were treated with or without TGF-β1 (10 ng/ml) for 2 h. Nuclear extracts were prepared, and formation of SMAD-SBE complexes was analyzed by EMSA. (B) Supershift analysis with nuclear extracts of TGF-β1–treated mesangial cells. The indicated antibodies were added 60 min before the addition of the labeled oligonucleotides. (C) Competition analysis with unlabeled SMAD-SBE oligonucleotide. Thirty minutes before the addition of the labeled oligonucleotides, nuclear extracts of TGF-β1–treated mesangial cells were preincubated with a 100-fold excess of the unlabeled competitor oligonucleotide. The data shown are representative of three similar experiments.
Inhibition of iNOS Enhances Glomerular Expression of SMOC-1 in Anti–Thy-1 GN
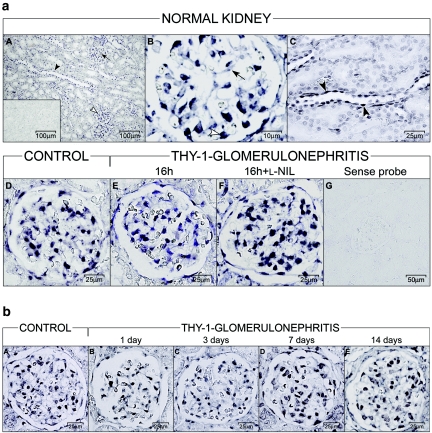
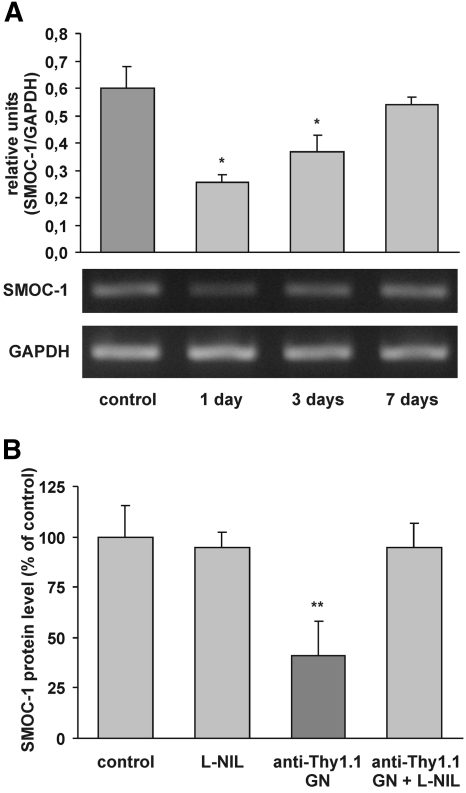
To test further the relevance of our in vitro findings in an appropriate animal model, we examined renal expression of SMOC-1 in normal rat kidney and under inflammatory conditions in anti–Thy-1 GN. In the normal kidney, in situ hybridization revealed distinct expression of SMOC-1 in glomeruli and in the tubulointerstitium, in all probability being located in mesangial cells and podocytes and in distal tubular epithelial cells (Figure 7a, A through C). Given the low expression of SMOC-1 mRNA and the lack of antibodies suitable for detecting SMOC-1 in tissue sections, this proposition could not be further confirmed by using specific cellular markers together with in situ hybridization or immunostaining. Induction of anti–Thy-1 GN had no influence on the cellular pattern of SMOC-1 expression between 2 h and 14 d after injection of the antibody (Figure 7). Quantification of RT-PCR revealed a reduction of SMOC-1 expression in isolated glomeruli at days 1 and 3, with normalization to control level at day 7 (Figure 8A). We performed further experiments to explore whether NO might influence glomerular expression of SMOC-1 in anti–Thy-1 GN at 16 h after induction of disease. This point in time closely correlates with the expression of iNOS and with the maximal inflammatory response in the glomerulus.11 Using a specific ELISA, we could confirm the expression of SMOC-1 in normal glomeruli on the protein level as well (Figure 8B). Anti–Thy-1 GN caused a marked reduction of SMOC-1 in isolated glomeruli (Figure 8B); however, this effect was completely prevented when nephritic rats were treated with the iNOS-specific inhibitor L-NIL (Figure 8B), demonstrating the importance of NO in modulating SMOC-1 expression in vivo. L-NIL had no effect on the expression of SMOC-1 in healthy controls (Figure 8B).
Figure 7.
In situ hybridization for SMOC-1 in normal and Thy-1 nephritic kidneys (16 h). (a, A through C) In the normal kidney, SMOC-1 is expressed in mesangial cells (A and B, black arrow), podocytes (A and B, white arrowhead), and in distal tubular epithelial cells (A and C, black arrowhead). (A, insert) Sense riboprobe. (E) In Thy-1 GN, the expression of SMOC-1 was slightly reduced 16 h after the induction of nephritis. (F versus E) Pretreatment with the specific iNOS inhibitor L-NIL prevented the decline in SMOC-1 expression in glomeruli from Thy-1 nephritic rats. (D) Expression of SMOC-1 in the normal kidney. (G) Sense riboprobe. There was no difference in controls receiving L-NIL or vehicle (data not shown). Bars indicate magnification. (b) Time course of SMOC-1 expression in glomeruli from control and anti–Thy-1 nephritic kidneys by in situ hybridization. Reduction of SMOC-1 expression was observed at days 1 (B) and 3 (C) after anti–Thy-1–induced GN compared with control kidneys (A). (D and E) Levels of SMOC-1 expression 7 (D) and 14 (E) days after induction of disease were comparable with control kidneys.
Figure 8.
Analysis of SMOC-1 mRNA and protein expression in the course of anti–Thy-1 GN. (A) Total mRNA from glomeruli obtained at days 1, 3, and 7 after induction of anti–Thy-1 GN was subjected to RT-PCR using primers SMOC-1 and SMOC-2 for 30 cycles (40 s at 94°C, 2 min at 53°C, and 2 min at 72°C). Transcripts were separated on a 1% agarose gel. The intensity of the bands was analyzed densitometrically and corrected for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels. Data are means ± SD, P < 0.05 versus vehicle-treated controls (n = 4). (B) Glomerular proteins from control animals and anti–Thy-1 nephritic rats (16 h) treated with or without L-NIL were subjected to direct ELISA for SMOC-1. Data are means ± SD (n = 3). **P < 0.01 versus vehicle-treated control animals.
Discussion
NO has been shown to play a prominent role in inflammatory diseases such as several forms of GN.1,11,33–35 NO produced in high amounts by iNOS is a potent mediator of redox signaling and triggers deleterious effects,36 but it also activates sGC, resulting predominantly in protective cellular mechanisms. In fact, total blockade of iNOS activity in experimental models of GN revealed conflicting results,13,37,38 which questions the use of NOS inhibitors in the treatment of inflammatory processes. Intriguing, treatment of inflammatory kidney diseases with small molecules that activate sGC could constitute a promising attempt for mimicking protective NO actions.21,22
To understand better cytokine/NO-modulated signaling pathways, we exposed cultured mesangial cells to the prototypic inflammatory cytokine IL-1β and analyzed the changes in the gene expression pattern by RAP-PCR. In this setting, we were able to identify among others a cDNA encoding the matricellular protein SMOC-1 to be downregulated under inflammatory conditions. This regulatory process was mediated by the cytokine-induced activation of the NO/cGMP signaling cascade. SMOC-1 was first characterized by the screening of a human fetal brain cDNA library using EST cDNA clones as probes with a high sequence homology to cDNAs derived from already known matricellular proteins. Sequence analysis and recombinant protein expression revealed high protein similarities to conserved follistatin-like and extracellular calcium-binding domains defining SMOC-1 undoubtedly as a matricellular protein.23 In the same series of experiments, Vannahme et al.39 identified a further matricellular protein referred to as SMOC-2. Meanwhile, a biologic role for SMOC-2 in synergistically augmenting signaling processes triggered by vascular endothelial growth factor, basic fibroblast growth factor, or PDGF has been established, indicating that SMOC-2 acts as a promitogenic and proangiogenic factor.26,27 In contrast, even if great success has been made to decipher the genomic structure and localization of human and bovine SMOC-1 genes and the tissue distribution of SMOC-1 protein,23,24,40 so far, only limited information has been reported regarding the biologic activity of SMOC-1. Nevertheless, two recent reports suggested a participation of SMOC-1 in cardiovascular disease as well as in carcinogenesis. Within the framework of the HyperGEN study, Sherva et al.41 demonstrated a linkage between the chromosomal localization of the SMOC-1 gene and the incidence of hereditary arterial stiffness in black individuals. Another report described SMOC-1 to be associated with astrocytoma. In that study, Boon et al.42 performed the serial analysis of gene expression method to analyze the gene expression patterns in astrocytic tumor samples and observed a clear upregulation of SMOC-1 at least in more advanced states of tumor manifestation. Here, we report that SMOC-1 mRNA and protein expression are downregulated by cytokines and NO in mesangial cells and that this effect is at least partially mediated by activation of sGC.
TGF-β amplifies its signaling effects by inducing its own gene expression.43 We demonstrate that silencing of SMOC-1 expression at least partially interrupts this positive regulatory loop, resulting in (1) decreased mRNA expression of typical TGF-β–regulated genes involved in matrix turnover and signaling, (2) attenuated formation of active and latent TGF-β, and (3) decreased binding of SMAD complexes to a consensus SBE site. This clearly indicates a role for SMOC-1 in amplifying TGF-β signaling devices, but the molecular mechanisms by which SMOC-1 enhances TGF-β signaling processes have to be elucidated in further studies. Moreover, as depicted in Supplemental Figure 2, TGF-β downregulates SMOC-1 expression and thus establishes a further facet for a feedback regulation affecting its own signaling device. This indeed may be required for a fine-tuning of TGF-β activity.
We could also demonstrate downregulation of SMOC-1 mRNA and protein in a model of acute GN, at the same time when maximal iNOS expression and inflammation occurred in the glomerulus.11 Importantly, in line with our observations made in vitro, blocking NO synthesis completely prevented the downregulation of SMOC-1 protein in experimental GN, indicating that NO when endogenously produced in the course of glomerular inflammation reduces SMOC-1 expression. So far, we cannot provide data showing SMOC-1 protein expression by immunohistochemistry. Antibodies raised against rat SMOC-1 protein are not commercially available, and our antibodies, even if valuable for Western blot and ELISA analysis, do not recognize SMOC-1 protein in either frozen or paraffin-embedded tissues as tested under various procedures of demasking tissue antigens.
Regarding the biologic function of SMOC-1, we suggest that NO counteracts SMOC-1 expression in GN, and this may limit TGF-β–mediated signaling cascades that favor profibrotic processes and loss of glomerular function. On the basis of our findings, its tempting to speculate that total blockade of iNOS enzyme activity in the course of GN, followed by an increase in matricellular proteins such as SMOC-1 or SPARC, could favor a profibrotic setting within the glomerulus,16 and this would perfectly fit the observations made by Westenfeld et al.,13 who described enhanced fibrin deposition when iNOS activity was blocked in a chronic rat model of anti–Thy-1 GN. We clearly have much more work to do to dissect the exact role of SMOC-1 in profibrotic TGF-β–driven signaling processes, but the results are likely to be very exciting.
Concise Methods
Reagents
Human recombinant IL-1β was from Cell Concept (Umkirch, Germany), and TNF-α was a gift from Knoll AG (Ludwigshafen, Germany). Media were from Invitrogen (Karlsruhe, Germany), FCS was from Biochrom AG (Berlin, Deutschland), and tissue culture plastic was from Greiner BioOne (Frickenhausen, Germany). α-32P-dCTP, α-33P-dCTP, and “ready prime” DNA labeling kit were obtained from GE Healthcare (München, Germany). Nylon blotting membranes were purchased from Perkin Elmer (Überlingen, Germany), and Immobilon-P (polyvinylidene difluoride) membranes were from Millipore (Eschborn, Germany). DETA-NO, ODQ, 4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1–1 (NS-2028), YC-1, L-NIL, L-NMMA, and 1400W were purchased from Alexis (Grünberg, Germany).
Cell Culture
Rat glomerular mesangial cells were cultivated as described previously44 and grown in RPMI 1640 medium supplemented with 10% FCS, 2 mM glutamine, 5 ng/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin. Quiescent cells were obtained by incubating mesangial cells for 24 h in serum-free DMEM supplemented with 0.1 mg/ml essentially fatty acid–free BSA. Stimulation of mesangial cells with cytokines or NO donors was performed with fresh serum-free culture medium. The NO donor DETA-NO used to analyze the effects of NO on SMOC-1 expression was applied at a concentration of 200 μM in most experiments, and we measured a nitrite concentrations of 287 μM after a 24-h stimulation period and this—on the basis that one molecule of DETA-NO releases two molecules of NO—fits our previous observations that DETA-NO is decomposed with a half-life of 16.5 h.45 Mesangial cells were used between passages 12 and 21. To exclude cytotoxic effects of the different stimulation and transfection protocols, we assessed cytotoxicity by LDH assay (Roche Diagnostics, Mannheim, Germany). None of the various stimulation procedures exerted significant cytotoxic effects (Supplemental Figure 3). Viability of cells transfected with siRNA was additionally analyzed by trypan blue staining (Supplemental Table 1).
Nitrite Analysis
Nitrite (NO2−), the stable end product of NO, was measured in the culture medium with the Griess method using a ready-to-use reagent (Merck, Darmstadt, Germany).46
Northern Blot Analysis
Isolation of total RNA from mesangial cells and Northern blotting was performed as described previously.47,48 Filter-bound RNA was hybridized to the radiolabeled SMOC-1 cDNA fragment that was generated by cloning an RT-PCR product representing 528 bp of rat SMOC-1 cDNA into the vector pCR2.1 (Invitrogen). The RT-PCR product was amplified using the following primers related to the SMOC-1 cDNA sequence deposited in the GENEMBL libraries (accession no. NM_001002835): SMOC-1 5′-AAT CCT CGA GAG GGC ATT GTG AT-3′ (Pos. 718 through 740) and SMOC-2 5′-AGA TGG CCT TAT CCT TGT TCA GG-3′ (Pos. 1246 through 1224).
Equivalent loading of the RNA probes was corrected after rehybridization of the filter with a cDNA probe for 18S RNA (Ambion, Applied Biosystems, Darmstadt, Germany). SMOC, TGF-β1, PAI-1, biglycan mRNA, and 18S RNA levels were analyzed by an automated detector system BAS 1500 of Fujifilm (Raytest, Straubenhardt, Germany). mRNA levels were corrected for 18S RNA and were expressed as percentage of vehicle-treated controls.
Generation of a SMOC-1–Specific Antibody for Western Blot and ELISA Analysis
A polyclonal antibody raised against a SMOC-1–derived peptide (SVEVDDPFKDRELPGC, representing amino acids 299 through 314 from rat SMOC-1 protein; SwissProt accession no. NP_001002835.1) in rabbit was generated and affinity-purified by Eurogentec (Köln, Germany). The antibodies were tested by Western blotting of cellular proteins as well as proteins from conditioned media precipitated with TCA. Specificity of the antibodies was demonstrated by preincubation with various concentrations of the peptide used for the immunization protocol. Western blot analysis showed bands with the expected size of 60 kD in lysates and conditioned media from mesangial cells that disappeared in the presence of the SMOC-1–related peptide (Supplemental Figure 4A). Remarkably, SMOC-1 protein was detected predominantly in conditioned media, confirming rat SMOC-1 as an extracellular protein.23 Further experiments for the analysis of SMOC-1 protein expression were performed by direct ELISA using conditioned media from mesangial cells. For further assessment of the specificity of the SMOC-1 antibodies, mesangial cells were transfected with a siRNA directed against SMOC-1 mRNA. This treatment reduced SMOC-1 protein levels in the conditioned media to <20% compared with untreated and vehicle- and control siRNA–treated mesangial cells (Supplemental Figure 4B). This marked effect was considered to be sufficient to examine the influence of SMOC-1 in cell signaling.
For Western blot analysis, homogenates or precipitated proteins from conditioned media of mesangial cells were subjected to SDS-PAGE (12% acrylamide gel). After transfer to a polyvinylidene difluoride membrane, SMOC-1 protein was detected using the affinity-purified SMOC-1–specific antibody. The blot was then incubated with a secondary horseradish peroxidase–conjugated antibody (goat anti-rabbit IgG-HRP; Santa Cruz Biotechnology, Heidelberg, Germany) and developed with the ECL detection system (Amersham, Braunschweig, Germany).
For ELISA, immunoplates (Nunc, Wiesbaden, Germany) were incubated overnight with 100 μl of conditioned medium per well at room temperature. Thereafter, the medium was removed and plates were further incubated with 200 μl of BSA (2% in PBS) per well for 60 min at 37°C. Wells were washed two times with PBS; subsequently, 75 μl of SMOC-1 antibody (0.2 μg/ml in 2% BSA in PBS) per well was added. After incubation for 90 min at 37°C, wells were washed two times with PBS and 75 μl of a secondary antibody (goat anti-rabbit, 1:10,000 in 2% BSA in PBS) was applied for 60 min at 37°C. Afterward, wells were washed three times with PBS and incubated with 100 μl of TMB for up to 30 min at room temperature. The colorimetric reaction was stopped by addition of 50 μl of 1 M H2SO4, and the plates were analyzed using a microplate reader at 450 nm. ELISA analysis of TGF-β was performed using a commercially available Quantikine kit (R&D Systems, Wiesbaden-Nordenstadt, Germany). As mentioned in the user manual provided by the manufacturer, the antibodies are highly specific for the active form of TGF-β. The cross-reactivity with the latent TGF-β complex is <1%. This allows discrimination between active TGF-β and total TGF-β by modifications of the protocol. For measurement of total TGF-β in conditioned media, latent TGF-β was converted into the activated form by acidification followed by a neutralization step. For the detection of active TGF-β, ELISA was performed with conditioned media that were not subjected to a previous acidification step.30
RAP-PCR
mRNA was prepared from mesangial cells using a NucleoTrap mRNA-Kit (Macherey-Nagel, Düren, Germany), and 80 ng of poly(A) mRNA was reverse-transcribed to cDNA at 42°C using reverse transcriptase (Invitrogen) in the presence of oligo(dT) primers.
Low-stringency RT-PCR was then performed in accordance with the instructions provided with the RAP-PCR kit (Stratagene, Amsterdam, Netherlands) using primers Rap1 (5′-TAAAATCTACCTTATAAAGTTC-3′) and Rap2 (5′-AGTGGCCTGTGAGCATACTGT-3′) for a three-step PCR protocol (the first cycle at 3 min at 90°C, 3 min at 35°C, and 5 min at 72°C and 40 additional cycles at 40 s at 94°C, 2 min at 53°C, and 2 min at 72°C) using a Taq-Polymerase (MBI-Fermentas, St. Leon-Rot, Germany). To enable detection and quantification of the PCR products, we performed the reaction in the presence of [α-33P]dCTP.
The radiolabeled PCR products were separated on a 6% sequencing gel and exposed to an x-ray film overnight. Bands that represented cDNAs of apparently differentially regulated genes were marked on the x-ray film and the corresponding regions of the gel were excised. Subsequently, the cDNA was eluted from the gel slices in 100 μl of TE buffer (1 mM EDTA and 10 mM Tris HCl [pH 7.8]) by heating at 60°C for 1 h.
A total of 2 μl of each sample was then reamplified under stringent conditions. The PCR products were separated on a 1% agarose gel and extracted from the gel using a kit from Macherey-Nagel (NucleoSpin, Düren, Germany). Thereafter, DNA fragments were ligated into the cloning vector pCR2.1 (TA Cloning Kit; Invitrogen) and sequenced using an automated sequence analyzer A310 (Perkin Elmer Applied Biosystems, Weiterstadt, Germany). Computer-aided sequence comparison with the GENEMBL databases was performed with the BLASTN program (NCBI, Bethesda, MD)
Transfection of Mesangial Cells with siRNA
Mesangial cells were grown in Petri dishes (60 × 15 mm) and transfected for 24 to 36 h with 10 nM unspecific siRNA or 10 nM siRNA directed against SMOC-1 using HiPerfect transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. Afterward cells were incubated in serum-free medium for 8 to 16 h.
EMSA
Quiescent mesangial cells transfected with SMOC-1 siRNA or unspecific control siRNA were treated for 2 h with TGF-β1 (10 ng/ml), and nuclear extracts were prepared according to the method of Schreiber et al.49 For allowing for binding of SMAD proteins to their respective binding site, nuclear extracts were incubated with a double-stranded radioactively labeled SBE consensus sequence (5′GTC TAG ACCA 3′; Santa Cruz Biotechnology). SMAD/SBE complexes were separated on a 4.5% acrylamide gel and visualized using an automated detector system BAS 1500 of Fujifilm (Raytest). Competition experiments were done by preincubation of the DNA-binding reaction for 30 min with a 100-fold excess of the unlabeled double-stranded oligonucleotide. Supershift analyses were done by preincubation of 1 μg of supershift antibody to the binding reaction 30 min before the addition of the radioactively labeled oligonucleotides. Antibodies raised against Smad-2, Smad-2/-3, and Smad-3 were derived from Cell Signaling Technology (Frankfurt am Main, Germany). Antibodies against Smad-4 were obtained from Santa Cruz Biotechnology.
In Vivo Model of Anti–Thy-1 GN
Anti–Thy-1 GN was induced in adult male Wistar rats that weighed 180 to 200 g (Charles-River, Sulzfeld, Germany) by a single intravenous injection of mouse anti-rat Thy-1.1 IgG, clone OX-7 (BioTrend, Cologne, Germany) dissolved in 18 mM sodium phosphate (pH 7.4)/0.15 M NaCl (PBS), at a dosage of 1 mg/kg body wt. Control rats received a single intravenous injection of PBS only.
L-NIL, a selective inhibitor of iNOS, was administered intravenously at a dosage of 5 mg/kg body wt to control and nephritic rats 45 min before anti–Thy-1 GN was induced. Kidneys were harvested (n = 6 rats per group) 16 h after injection of the anti–Thy-1.1 antibody or PBS. Previous results from our group showed expression of iNOS in isolated glomeruli from anti–Thy-1 nephritic rats between 1 and 16 h after injection of the antibody.11 For determination of the maximal effect of NO inhibition on the expression of SMOC-1, kidneys were harvested at 2, 4, 8, 16, and 24 h (data not shown), and all additional experiments were performed 16 h after induction of the disease. The time course of renal SMOC-1 expression was determined 1, 3, 7, and 14 d after induction of anti–Thy-1 GN. Systolic BP was monitored by tail plethysmography. Rats were anesthetized with hexobarbital (150 mg/kg). Kidneys were removed, and glomeruli were isolated essentially as described by Krakower and Greenspon50 by sequential sieving using stainless steel meshes of the following pore sizes: 250, 150, 106, and 63 μm. Glomeruli from each kidney preparation were washed three times, examined by light microscopy (purity of isolates >95%), and processed for RNA isolation or ELISA for SMOC-1. All animal work was done in accordance with the German Animal Protection Law and was approved by the Ethics Review Committee for laboratory animals of the District Government of Muenster and Darmstadt, Germany.
In Situ Hybridization
A DNA probe comprising nucleotides 303 to 563 of rat SMOC-1 cDNA was cloned in pCR4-TOPO (Invitrogen Life Technologies, Karlsruhe, Germany). After linearization with SpeI and NotI, the sense and antisense riboprobes were transcribed in vitro using digoxygenin-labeled UTP and the DIG RNA Labeling Kit (Roche). In situ hybridization experiments on formaldehyde-fixed and RNAse-free paraffin-embedded kidney sections were performed as described previously51 except that probes were hybridized at 52°C for 16 h using 10% poly(vinyl alcohol)/150 mM NaCl/25 mM MgCl2/100 mM Tris/HCl (pH 9.5; Sigma-Aldrich) to amplify the reaction. After washing and blocking with 2% normal sheep serum, alkaline phosphatase–conjugated Fab fragments of the anti-digoxygenin antibody (Roche) were applied to tissue sections to visualize hybridized riboprobes at a dilution of 1:1000. Unbound antibody was removed by washing, and endogenous alkaline phosphatase was blocked with 5 mM levamisole before the reaction with 0.15 mg/ml 5-bromo-4-chloro-3-indolyl phosphate/0.3 mg/ml nitroblue tetrazolium (FAST BCIP/NBT; Sigma). Then the slides were dehydrated and observed without counterstain. All sections were hybridized with sense and antisense riboprobes in parallel and under the same conditions.
Statistical Analyses
When not otherwise indicated, data are expressed as percentage of vehicle-treated controls (mean ± SD). Significance was tested by t test or ANOVA, and P < 0.05 was considered to be statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The work of the authors' laboratory was supported by the German Research Foundation (SFB 553, SFB 815, FOG 784, GRK 757, GRK 880/1, GRK 1172, EXC 147, PF361/6-1, and SCHA 1082/2-1) and by the European Union (grant LSHM-CT-2004-005033, EICOSANOX).
We thank Ute Schmidt for valuable technical support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Cattell V:Nitric oxide and glomerulonephritis. Kidney Int 61: 816–821, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Baud L, Ardaillou R:Involvement of reactive oxygen species in kidney damage. Br Med Bull 49: 621–629, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Pfeilschifter J, Beck KF, Eberhardt W, Huwiler A:Changing gears in the course of glomerulonephritis by shifting superoxide to nitric oxide-dominated chemistry. Kidney Int 61: 809–815, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Radeke HH, Meier B, Topley N, Flöge J, Habermehl GG, Resch K:Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int 37: 767–775, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Pfeilschifter J, Schwarzenbach H:Interleukin 1 and tumor necrosis factor stimulate cGMP formation in rat renal mesangial cells. FEBS Lett 273: 185–187, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Pfeilschifter J:Mesangial cells orchestrate inflammation in the renal glomerulus. News Physiol Sci 9: 271–276, 1994 [Google Scholar]
- 7.Mühl H, Pfeilschifter J:Amplification of nitric oxide synthase expression by nitric oxide in interleukin 1 beta-stimulated rat mesangial cells. J Clin Invest 95: 1941–1946, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck KF, Eberhardt W, Walpen S, Apel M, Pfeilschifter J:Potentiation of nitric oxide synthase expression by superoxide in interleukin 1 beta-stimulated rat mesangial cells. FEBS Lett 435: 35–38, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Hruby Z, Beck KF:Cytotoxic effect of autocrine and macrophage-derived nitric oxide on cultured rat mesangial cells. Clin Exp Immunol 107: 76–82, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mühl H, Sandau K, Brüne B, Briner VA, Pfeilschifter J:Nitric oxide donors induce apoptosis in glomerular mesangial cells, epithelial cells and endothelial cells. Eur J Pharmacol 317: 137–149, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Walpen S, Beck K-F, Schaefer L, Raslik I, Eberhardt W, Schaefer RM, Pfeilschifter J:Nitric oxide induces MIP-2 transcription in rat renal mesangial cells and in rat model of glomerulonephritis. FASEB J 15: 571–573, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Trachtman H:Nitric oxide and glomerulonephritis. Semin Nephrol 24: 324–332, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Westenfeld R, Gawlik A, de Heer E, Kitahara M, Abou-Rebyeh F, Floege J, Ketteler M:Selective inhibition of inducible nitric oxide synthase enhances intraglomerular coagulation in chronic anti-Thy 1 nephritis. Kidney Int 61: 834–838, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Pfeilschifter J, Eberhardt W, Beck KF:Regulation of gene expression by nitric oxide. Pflügers Arch 442: 479–486, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Bogdan C:Nitric oxide and the regulation of gene expression. Trends Cell Biol 11: 66–75, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Walpen S, Beck KF, Eberhardt W, Apel M, Chatterjee PK, Wray GM, Thiemermann C, Pfeilschifter J:Downregulation of SPARC expression is mediated by nitric oxide in rat mesangial cells and during endotoxemia in the rat. J Am Soc Nephrol 11: 468–476, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Welsh J, Chada K, Dalal SS, Cheng R, Ralph D, McClelland M:Arbitrarily primed PCR fingerprinting of RNA. Nucleic Acids Res 20: 4965–4970, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller T, Plesková M, McDonald MC, Thiemermann C, Pfeilschifter J, Beck KF:Identification of manganese superoxide dismutase as a NO-regulated gene in rat glomerular mesangial cells by 2D gel electrophoresis. Nitric Oxide 9: 183–193, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Beck KF, Güder G, Schaefer L, Pleskova M, Babelova A, Behrens MH, Mihalik D, Beck M, Schaefer RM, Pfeilschifter J:Nitric oxide upregulates induction of PDGF receptor-alpha expression in rat renal mesangial cells and in anti-Thy-1 glomerulonephritis. J Am Soc Nephrol 16: 1948–1957, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Plesková M, Beck KF, Behrens MH, Huwiler A, Fichtlscherer B, Wingerter O, Brandes RP, Mülsch A, Pfeilschifter J:Nitric oxide down-regulates the expression of the catalytic NADPH oxidase subunit Nox1 in rat renal mesangial cells. FASEB J 20: 139–141, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hohenstein B, Daniel C, Wagner A, Stasch JP, Hugo C:Stimulation of soluble guanylyl cyclase inhibits mesangial cell proliferation and matrix accumulation in experimental glomerulonephritis. Am J Physiol Renal Physiol 288: F685–F693, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Krämer S, Loof T, Martini S, Kron S, Kawachi H, Shimizu F, Neumayer HH, Peters H:Stimulation of soluble guanylate cyclase slows progression in anti-thy1-induced chronic glomerulosclerosis. Kidney Int 68: 47–61, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Vannahme C, Smyth N, Miosge N, Gösling S, Frie C, Paulsson M, Maurer P, Hartmann U:Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem 277: 37977–37986, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Gersdorff N, Müller M, Schall A, Miosge N:Secreted modular calcium-binding protein-1 localization during mouse embryogenesis. Histochem Cell Biol 126: 705–712, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lane TF, Sage EH:The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J 8: 163–173, 1994 [PubMed] [Google Scholar]
- 26.Liu P, Lu J, Cardoso WV, Vaziri C:The SPARC-related Factor SMOC-2 promotes growth factor-induced cyclin D1 expression and DNA synthesis via integrin-linked kinase. Mol Biol Cell 19: 248–261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C:The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem 281: 22855–22864, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Framson PE, Sage EH:SPARC and tumor growth: Where the seed meets the soil? J Cell Biochem 92: 679–690, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Alford AI, Hankenson KD:Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone 38: 749–757, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Akool el-S, Doller A, Müller R, Gutwein P, Xin C, Huwiler A, Pfeilschifter J, Eberhardt W:Nitric oxide induces TIMP-1 expression by activating the transforming growth factor beta-Smad signaling pathway. J Biol Chem 280: 39403–39416, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Keil A, Blom IE, Goldschmeding R, Rupprecht HD:Nitric oxide down-regulates connective tissue growth factor in rat mesangial cells. Kidney Int 62: 401–411, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, Martin F, Brady HR:Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem 274: 5830–5834, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Blantz RC, Munger K:Role of nitric oxide in inflammatory conditions. Nephron 90: 373–378, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Pfeilschifter J:Nitric oxide triggers the expression of proinflammatory and protective gene products in mesangial cells and the inflamed glomerulus. Nephrol Dial Transplant 17: 347–348, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Korhonen R, Lahti A, Kankaanranta H, Moilanen E:Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 4: 471–479, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Pfeilschifter J, Eberhardt W, Beck KF, Huwiler A:Redox signaling in mesangial cells. Nephron Exp Nephrol 93: e23–e26, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Narita I, Border WA, Ketteler M, Noble NA:Nitric oxide mediates immunologic injury to kidney mesangium in experimental glomerulonephritis. Lab Invest 72: 17–24, 1995 [PubMed] [Google Scholar]
- 38.Satriano J, Lortie MJ, Ishizuka S, Valdivielso JM, Friedman B, Munger KA:Inhibition of inducible nitric oxide synthase alters Thy-1 glomerulonephritis in rats. Nephron Physiol 102: p17–p26, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Vannahme C, Gösling S, Paulsson M, Maurer P, Hartmann U:Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J 373: 805–814, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava J, Premi S, Kumar S, Parwez I, Ali S:Characterization of SMOC-1 uncovers two transcript variants showing differential tissue and age specific expression in Bubalus bubalis. BMC Genomics 8: 436, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherva R, Miller MB, Lynch AI, Devereux RB, Rao DC, Oberman A, Hopkins PN, Kitzman DW, Atwood LD, Arnett DK:A whole genome scan for pulse pressure/stroke volume ratio in African Americans: The HyperGEN study. Am J Hypertens 20: 398–402, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boon K, Edwards JB, Eberhart CG, Riggins GJ:Identification of astrocytoma associated genes including cell surface markers. BMC Cancer 4: 39, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB:Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem 263: 7741–7746, 1988 [PubMed] [Google Scholar]
- 44.Pfeilschifter J, Vosbeck K:Transforming growth factor beta2 inhibits interleukin 1beta- and tumour necrosis factor α-induction of nitricoxide synthase in rat renal mesangial cells. Biochem Biophys Res Commun 175: 372–379, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Mühl H, Chang JH, Huwiler A, Bosmann M, Paulukat J, Ninic R, Nold M, Hellmuth M, Pfeilschifter J:Nitric oxide augments release of chemokines from monocytic U937 cells: Modulation by anti-inflammatory pathways. Free Radic Biol Med 29: 969–980, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR:Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131–138, 1982 [DOI] [PubMed] [Google Scholar]
- 47.Chomczynski P, Sacchi N:Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Beck KF, Mohaupt MG, Sterzel RB:Endothelin-1 inhibits cytokine-stimulated transcription of inducible nitric oxide synthase in glomerular mesangial cells. Kidney Int 48: 1893–1899, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Schreiber E, Matthias P, Müller MM, Schaffner W:Rapid detection of octamer binding proteins with ‘mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res 17: 6419, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krakower CA, Greenspon SA:The localization of the nephrotoxic antigen(s) in extraglomerular tissues: Observations including a measure of its concentration in certain locales. AMA Arch Pathol 66: 364–383, 1958 [PubMed] [Google Scholar]
- 51.Schaefer L, Raslik I, Gröne HJ, Schönherr E, Macakova K, Ugorcakova J, Budny S, Schaefer RM, Kresse H:Small proteoglycans in human diabetic nephropathy: Discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican and fibromodulin. FASEB J 15: 559–561, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.