Abstract
Fabry disease is a lysosomal storage disorder that results in an accumulation of globotriaosylceramide in vascular tissue secondary to a deficiency in α-galactosidase A. The glycolipid-associated vasculopathy results in strokes and cardiac disease, but the basis for these complications is poorly understood. Recent studies in the α-galactosidase A–knockout mouse suggested that a decrease in nitric oxide (NO) bioavailability may play a role in the abnormal thrombosis, atherogenesis, and vasorelaxation that are characteristic of these mice. To understand better the association between impaired NO bioavailability and glycolipid accumulation, we studied α-galactosidase A–knockout mice or primary cultures of their aortic endothelial cells. Treatment of knockout mice with a potent inhibitor of glucosylceramide synthase reversed accumulation of globotriaosylceramide but failed to normalize the defect in vasorelaxation. Basal and insulin-stimulated endothelial NO synthase (eNOS) activities in endothelial cells derived from knockout mice were lower than those observed from wild-type mice; normalization of glycolipid only partially reversed this reduction in eNOS activity. The loss of eNOS activity associated with a decrease in high molecular weight caveolin oligomers in endothelial cells and isolated caveolae, suggesting a role for glycolipids in caveolin assembly. Finally, concentrations of ortho-tyrosine and nitrotyrosine in knockout endothelial cells were markedly elevated compared with wild-type endothelial cells. These findings are consistent with a loss of NO bioavailability, associated with eNOS uncoupling, in the α-galactosidase A–knockout mouse.
Anderson-Fabry disease is a rare lysosomal storage disorder that arises secondary to an X-linked inherited deficiency in the enzyme α-galactosidase A (Gla).1 As a consequence of this enzyme deficiency, levels of globotriaosylceramide (Gb3) accumulate in the vascular tissues and kidneys of affected patients. A major manifestation of this disease is a large and small vessel vasculopathy with premature death resulting from strokes and cardiac disease. In a recently reported analysis of the Fabry disease registry, 21% of men with Gla deficiency reported significant cardiovascular events.2 Of note is the finding that approximately 40% of male patients with Fabry disease experience cerebrovascular complications.3
The existence of a primary defect in endothelial function in patients with Fabry disease manifest by abnormalities in regional blood flow is supported by several clinical studies. Patients with Fabry disease are also reported to have abnormalities in regional cerebral blood flow at rest.4 These abnormalities are usually associated with hyperperfusion. In addition, a decrement in coronary artery reserve flow and impaired postischemic forearm perfusion have been reported.5
The Gla knockout mouse has provided a useful model for studying vascular disease. Although these mice do not display a spontaneous vascular phenotype, recent reports have detailed three inducible models of vasculopathy. These models include oxidant-stimulated thrombosis in response to activation of rose Bengal,6 accelerated atherogenesis in mice bred on apolipoprotein E null background,7 and impaired vascular reactivity in aortic rings in response to α adrenergic agonists and vasorelaxation in response to acetylcholine.8,9
Recently, our group has been studying the basis for the vasoreactive defect in the Gla knockout mouse.9 The defect in vascular responsiveness was observed to reside in the endothelium on the basis of three experimental observations. First, both the vasoconstrictive and vasorelaxation abnormalities were abolished when the endothelium was removed. Second, no difference between wild-type and Gla knockout mice was observed when relaxation was induced with sodium nitroprusside. Third, when aortic rings were exposed to low concentrations of ionomycin, no difference in calcium-induced vasorelaxation was observed between wild-type and null mice. These data suggest that the vasoreactive defects in the Gla knockout mice reside not only in the endothelium but also at the level of plasma membrane.
Although the thrombotic, atherogenic, and vasoreactive abnormalities seen in the Gla knockout mice may result from independent and disparate mechanisms, a common mechanism could account for the observed phenotypes, viz. the loss of endothelial nitric oxide synthase (eNOS) activity. The loss of normal NOS signaling has been associated with each of these phenotypes, and several mechanisms for the loss or impairment of normal eNOS activity have been identified. In this report, we studied the vasoreactivity of aortas in knockout mice in which Gb3 levels were reduced by a potent inhibitor of glucosylceramide synthase. We observed no statistically significant correction of the vasorelaxation in response to acetylcholine after normalization of Gb3 levels. We subsequently studied the expression, activity, and functional coupling of eNOS in the aortas and aortic endothelium of wild-type and Gla knockout mice to ascertain whether these might account for the partial response and the induced vascular phenotypes.
Results
Gb3 Reduction by Systemic Administration of Glucosylceramide Synthase Inhibitor Fails to Reverse the Defect in Relaxation to Acetylcholine
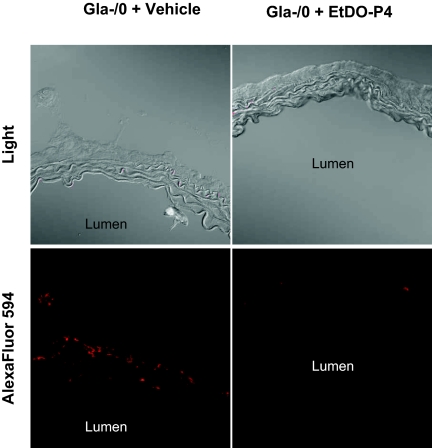
To explore the association between elevated Gb3 and the vasorelaxation defect of the Gla−/0 null mice, we used the potent glucosylceramide synthase inhibitor D-threo-ethylenedioxyphenyl-2-palmitoylamino-3-pyrrilidino-propanol (EtDO-P4). This small molecule inhibitor of glucosylceramide-based glycosphingolipid synthesis was designed and demonstrated by our group to reduce Gb3 potently in Gla−/0 mouse liver, kidney, and heart.10,11 Seventeen to 22-wk-old Gla−/0 mice were treated with 10 mg/kg per d inhibitor in sulfobutyl ether β-cyclodextrin (Captisol) carrier or with the carrier alone by daily intraperitoneal injections for 28 d.12 Twenty-four hours after the last injection, the aortas were isolated for Gb3 detection by immunofluorescence and contractility studies. When Gb3 was measured by Shiga toxin B subunit binding, we observed a near-complete reduction in the aortic Gb3 content (Figure 1). This reduction was evident throughout the aortic wall and at the luminal edge, suggesting that the inhibitor blocked glucosylceramide-based glycolipid synthesis throughout all levels of the artery.
Figure 1.
Reduction of aortic ring Gb3 content in Gla−/0 mice treated with EtDO-P4. Gla−/0 mice were treated with EtDO-P4 (10 mg/kg per d intraperitoneally) in Captisol or with Captisol alone for 28 d. Twenty-four hours after the final injection, the mice were killed and the aortic rings were isolated, embedded, and frozen in O.C.T. compound. Gb3 was detected by biotinylated STX binding and Alexa Fluor 546 conjugated to streptavidin before immunofluorescence microscopy.
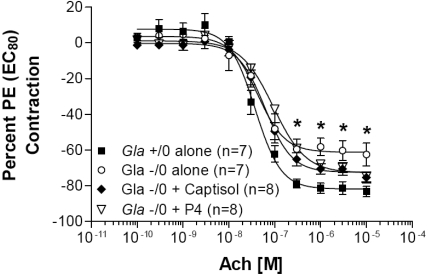
Previously, we had demonstrated that when aortic rings were precontracted with phenylephrine to 80% of their maximal contraction, Gla−/0 aortic rings failed to relax to the same degree as those from Gla+/0 mice. When the vehicle- and inhibitor-treated mouse aortic rings were similarly studied, no significant improvement in the relaxation response was observed (Figure 2).
Figure 2.
Acetylcholine (Ach)-mediated endothelium-dependent relaxation in endothelium-intact mouse aortic rings. Rings from wild-type (Gla+/0) or knockout (Gla−/0) mice were precontracted with an EC80 concentration of phenylephrine. Gla−/0 mice were untreated or were treated for 28 d with Captisol carrier or with 10 mg/kg per d EtDO-P4 (P4) in Captisol carrier before isolation of the aortic rings. Data are expressed as a percentage of the contraction elicited by PE EC80. *P < 0.05 versus Gla+/0 by one-way ANOVA followed by Bonferroni post hoc test.
Age-Dependent Decrease of High Molecular Mass Oligomerization of Caveolin-1 in Gla−/0, not Gla+/0, Mouse Aortas
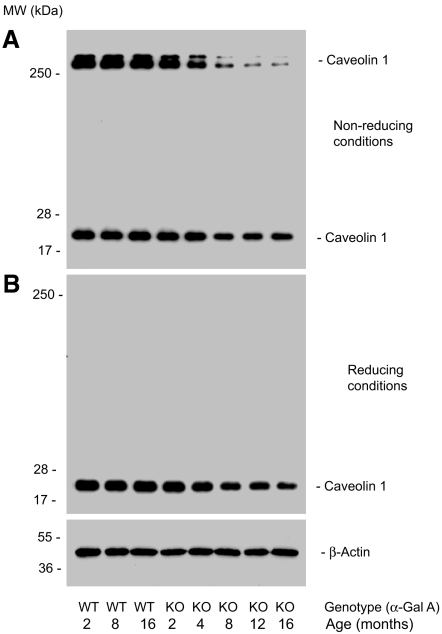
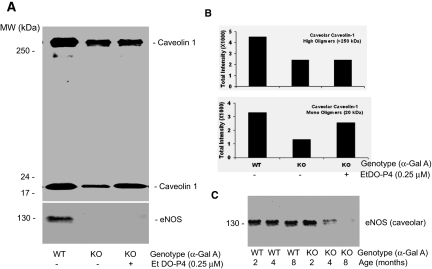
To understand better the molecular basis of the endothelial dysfunction in Gla−/0 mice, we compared the age-dependent expression of caveolin-1 and eNOS in wild-type and Gla−/0 mouse aortas. Freshly isolated mouse aortas were lysed in 1% Triton X-100 lysate buffer, and the clarified aortic lysates were subjected to a gradient SDS-PAGE separation. Caveolin-1 was detected with a polyclonal antibody directly against the N-terminus of mouse caveolin-1 (MSGGKYVDSEGHLYTVP-C). Under nonreducing (0% β-mercaptoethanol) and without heating, two high molecular mass homo-oligomers of caveolin-1 (>250 kD) were readily detected in Gla+/0 and younger Gla−/0 mouse aortas (Figure 3A, lanes 1 through 4); however, the detection of high molecular mass oligomers of caveolin-1 was significantly decreased in the aged aortas from 4- to 16-mo-old Gla−/0 mice (Figure 3A, lanes 5 through 8). The expression of 20-kD monomeric caveolin-1 was also decreased as a function of age. The high molecular mass oligomers were not detected under reducing conditions (Figure 3B).
Figure 3.
Age-associated decrease of high and low molecular weight caveolin-1 in aged Gla−/0 mouse aortas as measured by immunoblotting. (A) When caveolin-1 was separated under nonreducing and nonheating conditions, two high molecular weight bands of >250 kD and one low molecular weight band of 20 kD were detected in control aortas isolated from C57Bl/6 mice at ages of 2 to 16 mo (lanes 1 through 3). Aortic caveolin-1 from Gla−/0 mice exhibited a reduction in levels that was more pronounced as a function of age. The reduction in the high molecular weight caveolin-1 was more pronounced than in the monomeric 20-kD form (lanes 4 through 8). The age-associated reduction in the 20- kD caveolin-1 in Gla−/0 aortas was observed under both nonreducing (A, lanes 6 through 8) and reducing (B, lanes 6 through 8) conditions. Aortic β-actin (bottom) served as loading controls. The data shown are representative of immunoblots that were repeated four times from four different sets of mouse aortas.
Reduced Immunoreactivity of eNOS in Aged Gla−/0 but not Gla+/0 Mouse Aortas
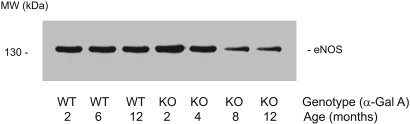
We next analyzed the age-dependent immunoreactivities of eNOS in Gla+/0 and Gla−/0 mouse aortas. The aortic proteins were solubilized in 1% Triton X-100 lysate buffer mixed with loading buffer containing 1% β-mercaptoethanol; after heating at 80°C for 5 min, the samples were separated in SDS-PAGE with a 6 to 12% gradient. The immunoblot analysis revealed that in aged Gla−/0 mice (8 and 12 mo old), the expression of eNOS was significantly decreased compared with age-matched wild-type mouse aortas (Figure 4).
Figure 4.
Reduced eNOS in the aged Gla−/0 mouse aortas. The immunoblotting of eNOS in C57Bl/6 and Gla−/0 mouse aortas was performed by probing with a monoclonal anti-eNOS antibody followed by reduction with a 1% β-mercaptoethanol and heating at 80°C for 5 min. With increasing age, the immunoreactive bands (133 kD) of eNOS in the Gla−/0 mouse aortas were significantly reduced (lanes 6 and 7) compared with C57Bl/6 Gla+/0 mouse aortas (lanes 2 and 3). The blot is representative of three independent experiments that yielded equivalent results.
Co-localization of eNOS and Caveolin-1 in Mouse Aortic Extracts
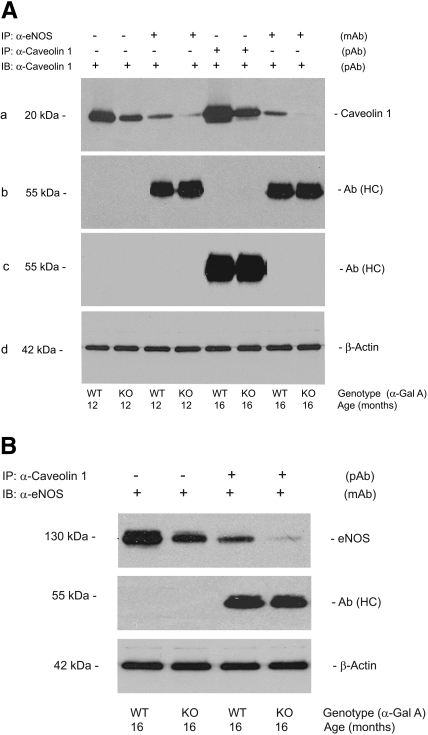
To ascertain whether there was a correlation between the decreased expression of eNOS and caveolin-1, we performed immunoprecipitation studies on the aortas of Gla+/0 and Gla−/0 mice. Caveolin-1 expression was compared in the aortas from 12- and 16-mo-old wild-type and knockout mice (Figure 5). The decrease in caveolin-1 expression was observed after immunoprecipitation of eNOS (Figure 5A), and, conversely, the decrease in eNOS expression was observed after the immunoprecipitation of caveolin-1 (Figure 5B). These data suggest that eNOS and caveolin-1 co-localize in the wild-type and knockout aortas and that the decreased expression of eNOS may result, in part, from the lower content of caveolin-1.
Figure 5.
eNOS/caveolin-1 immunocomplex formation in the aortas of Gla+/0 and Gla−/0 mice. (A, a) Caveolin-1 co-immunoprecipitation with eNOS was compared between the aortas from Gla+/0 (lanes 3 and 7) and Gla−/0 mice (lanes 4 and 8). Caveolin-1 immunoblotting without immunoprecipitation (lanes 1 and 2) served as a positive control. Recovered caveolin-1 (lanes 5 and 6) after immunoprecipitation served as a second positive control. (b) The heavy chain of eNOS antibody [Ab (HC)] was detected with a goat anti-mouse Ig antibody (lanes 3 and 4, 7 and 8). (c) Ab (HC) was detected with a goat anti-rabbit Ig antibody (lanes 5 and 6). (B, top) eNOS co-immunoprecipitation with caveolin-1 was compared between the aortas from Gla+/0 (lane 3) and Gla−/0 mice (lane 4). eNOS immunoblotting without immunoprecipitation (lanes 1 and 2) served as a positive control. (Middle) The Ab (HC) was detected with a goat anti-rabbit Ig antibody (lanes 3 and 4). Aortic β-actin in A (d) and B (bottom) served as an internal loading control. The blots are representative of three independent experiments that yielded equivalent results.
High Levels of Gb3 Accumulate in the Cultured Mouse Aortic Endothelial Cells Isolated from Aged Gla−/0, not Gla+/0, Mice
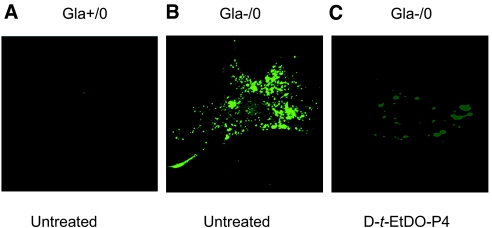
Our previous studies on the Gla−/0 mouse aortas suggested that the primary vascular reactivity defect resides in the endothelium. To explore further the basis for this defect and to determine whether changes in caveolin and eNOS expression could be localized to the endothelium, we isolated and grew primary cultures of mouse aortic endothelial cells (MAECs) as described in the Concise Methods section. Each batch of Gla+/0 or Gla−/0 MAECs was isolated and pooled from a total of 12 wild-type or null mice at 8 mo of age. At the third passage, the cells were grown on gelatin-coated glass coverslips and maintained in 10% FBS-RPMI 1640 medium for 3 d. Gla−/0 but not Gla+/0 MAECs expressed high levels of Gb3 as detected by verotoxin B subunit binding (Figure 6). The treatment of the MAECs with 200 nM EtDO-P4 for 48 h resulted in an almost complete elimination of the verotoxin binding. This finding is consistent with previous reports in which the Gb3 mass was significantly reduced by inhibitor treatment.11,13
Figure 6.
STX binding and Gb3 accumulation in cultured Gal−/0 MAECs. MAECs grown on glass coverslips precoated with gelatin were overlain with purified STX-1 and subsequently with anti–STX-1 (13C4) mAb and stained with Alexa Fluor 546–conjugated secondary antibody. (A) Cultured Gla+/0 MAECs isolated from C57Bl/6 control mice at age of 8 mo. (B) Cultured Gla−/0 MAECs isolated from 8-mo-old mice. (C) Cultured Gla−/0 MAECs isolated from 8-mo-old mice treated for 48 h with 0.2 μM EtDO-P4.
Decreased Expression and Activity of eNOS in Cultured Primary MAECs Isolated from Aged Gla−/0
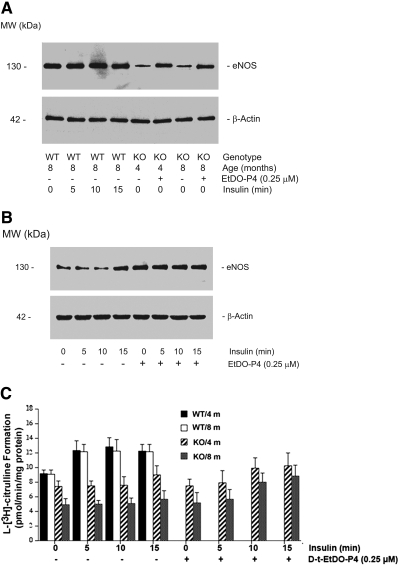
The expression and activity of eNOS was measured in MAECs from Gla+/0 and Gla−/0 mice of 4 or 8 mo of age. Cultured primary MAECs at passage 3 were grown to 80% confluence in 100-mm culture dishes for immunoblot analysis and in 60-mm dishes for measurements of eNOS activity. The concentration of serum in medium was gradually decreased from 20 to 0%, and endothelial growth factors were omitted for 12 h before eNOS was measured. With heating (80°C for 5 min) and nonreducing conditions, eNOS in cultured MAECs was not detected (data not shown). Under reducing conditions (1% β-mercaptoethanol) and in the absence of heating, the eNOS expression in cultured MAECs was detected with a polyclonal antibody against the C-terminal amino acids 1178 through 1194 of mouse eNOS. Stable and consistent expression of eNOS was detected in cultured MAECs obtained from 2- to 8-mo-old wild-type mice (data not shown); however, the eNOS levels were significantly lower in cultured Gla−/0 MAECs from 4- and 8-mo-old Gla null mice (Figure 7A, lanes 5 and 7). The treatment of cultured Gla−/0 MAECs with EtDO-P4 restored partially the eNOS levels in the Gla-deficient cells (Figure 7A, lanes 6 and 8).
Figure 7.
Decreased eNOS expression and activity in cultured MAECs prepared from Gla−/0 mice. (A) eNOS was compared by Western blots of gels from wild-type and Gla−/0 MAECs. β-Actin levels were used as a control. Lanes 1 through 4, no change in eNOS levels in Gla+/0 MAECs in response to insulin stimulation; lanes 5 and 7, reduced expression of eNOS in cultured Gla−/0 MAECs prepared from 4- and 8-mo-old mice; lanes 6 and 8, partial restoration of the immunoreactivity of eNOS in EtDO-P4–treated Gla−/0 MAECs isolated from 4- and 8-mo-old Fabry mice. (B) Immunoreactivity of eNOS in Gla−/0 MAECs from 4-mo-old Fabry mice stimulated with insulin for 0, 5, 10, and 15 min in the absence (lanes 1 through 4) and presence (lanes 5 through 8) of 0.25 μM EtDO-P4. Cellular β-actin served as a loading control. The blots are representative of three independent experiments with similar results. (C) eNOS activity in cultured wild-type and Gla−/0 MAECs isolated from 4- and 8-mo-old mice. Data are means ± SE (n = 6).
We next examined the eNOS expression in response to insulin in Gla−/0 MAECs from 4-mo-old mice. No significant difference was observed in the eNOS levels between unstimulated Gla−/0 MAECs and MAECs exposed to 100 nM insulin for 5 or 10 min (Figure 7B, lanes 1 to 3); however, insulin stimulation increased eNOS at 15 min (Figure 7B, lane 4). EtDO-P4 pretreatment (0.25 μM for 2 d) significantly increased eNOS levels in cultured Gla−/0 MAECs (Figure 7B, lane 5) compared with untreated Gla−/0 MAECs (Figure 7B, lane 1); however, stimulation with insulin did not further increase the levels of eNOS in EtDO-P4–treated Gla−/0 MAECs (Figure 7B, lanes 6 through 8).
Although there was no significant difference in eNOS levels as measured by immunoblotting in the control and insulin-stimulated Gla+/0 MAECs, the eNOS activity increased significantly in response to insulin within 5 min (Figure 7C). By contrast, eNOS activity was significantly reduced in MAECs from 4- and 8-mo-old Gla−/0 mice (23 and 53% reduced, respectively, compared with wild-type; Figure 7C). The treatment of Gla−/0 MAECs with EtDO-P4 for 2 d failed to restore significantly the basal measured eNOS activity even though the detectable protein levels of eNOS were partially restored by reduction of Gb3.
Insulin stimulation increased eNOS activity by 21% in 4-mo-old and 16% in 8-mo-old Gla−/0 MAECs with a time course that was delayed compared with the wild-type MAECs. Although insulin stimulation did not increase the levels of eNOS in EtDO-P4–treated Gla−/0 MAECs, treatment with EtDO-P4 enhanced the response of eNOS enzyme in Gla−/0 mice to insulin; however, the absolute levels of eNOS activity in EtDO-P4–treated and insulin-stimulated Gla−/0 MAECs were still significantly below the eNOS activity in corresponding Gla+/0 MAECs.
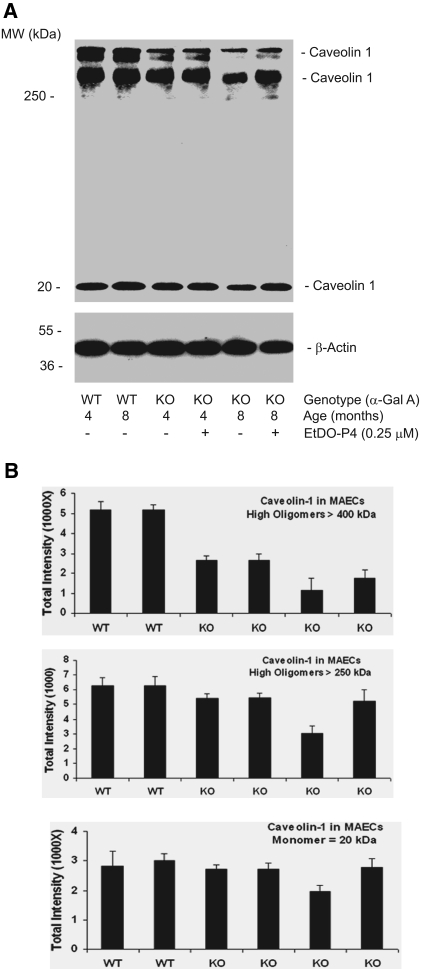
Decreased Expression of Caveolin-1 in Aged Gla−/0 MAECs
We performed immunoblots on MAECs from 4- and 8-mo-old Gla−/0 and Gla+/0 mice to determine whether there was a loss of monomeric and oligomeric caveolin-1 reflecting the findings observed in intact aortas. High molecular weight oligomers of caveolin-1 were markedly decreased in the Gla−/0 MAECs compared with those from wild-type mice (Figure 8A). Repeated measurements of caveolin-1 expression demonstrated an age-dependent decrease in both monomeric and oligomeric forms of caveolin-1. Gb3 depletion with 250 nM EtDO-P4 only partially increased caveolin recovery in the MAECs from 8-mo-old knockout mice without any significant effect in the cells from the 4-mo-old mice (Figure 8B).
Figure 8.
Decreased formation of high molecular mass oligomers of caveolin-1 in cultured Gla−/0 MAECs. (A) Representative immunoblots of high and low molecular weight caveolin-1 in wild-type and Gla−/0 MAECs cultured from 4- and 8-mo-old mice. Gla−/0 cells were also treated for 48 h with 0.25 μM EtDO-P4. (B) Densitometric data from the immunoblots (n = 3). Data are means ± SE.
Caveolar Localization of Caveolin-1 and eNOS in Cultured MAECs
Previously, we examined the compartmentalization of glycosphingolipids and caveolin-1 in caveolar fractions of cultured Gla−/0 and Gla+/0 MAECs. Caveolin-1 was observed to decrease in cultured Gla−/0 MAECs as a function of glycosphingolipid content and age. The recovery of eNOS in caveolae was also evaluated. No difference in eNOS levels was observed in caveolar fractions in MAECs isolated from 2-mo-old Gla−/0 and Gla+/0 mice; however, an age-dependent decrease in caveolar eNOS was observed in cells from 4- and 8-mo-old knockout mice compared with their wild-type counterparts (Figure 9A). Monomeric and oligomeric caveolin-1 was assayed from 8-mo-old mouse MAECs. A decrement in both high and low molecular weight caveolin-1 was observed in the Gla−/0 MAEC caveolar fractions in association with the loss of detectable eNOS. The depletion of Gb3 with 250 nM EtDO-P4 partially normalized the levels of the monomeric caveolin-1 but did not significantly increase the recovery of oligomeric caveolin-1 or eNOS (Figure 9, B and C).
Figure 9.
Caveolin-1 and eNOS levels in caveolar fractions from cultured wild-type and Gla−/0 MAECs. (A) Immunoblots of caveolin-1 and eNOS in caveolar fractions of wild-type and Gla−/0 MAECs. Reduced caveolin-1 levels are observed in the caveolar fraction of Gla−/0 MAECs compared with Gla+/0 MAECs from 8-mo-old mice. Partial restoration of monomeric caveolar caveolin-1 is observed after treatment with EtDO-P4 (top, lane 3). (B) Densitometric measurements of high and low molecular weight caveolin-1 from multiple experiments. Data are means (n = 3). (C) Age-associated decrease of caveolar eNOS in cultured Gla+/0 and Gla−/0 MAECs.
eNOS Uncoupling in Gla−/0 MAECs
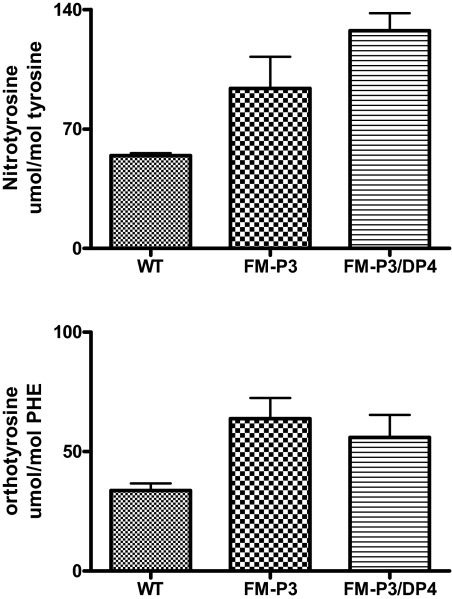
We sought to determine whether the decrease in eNOS activity was accompanied by an uncoupling of eNOS. Uncoupling of the enzyme results in the formation of the highly reactive oxidant peroxynitrite, which forms when NO reacts with superoxide. Previous studies demonstrated that ortho-tyrosine and nitrotyrosine are markers of peroxynitrite-mediated oxidation. To determine whether proteins from endothelial cells contain oxidized amino acid products of peroxynitrite oxidation, we isolated endothelial cells from Gla+/0 and Gla−/0 aortas. After delipidating the cells and hydrolysis with acid, we isolated amino acids. The content of the two oxidized amino acids—ortho-tyrosine and 3-nitrotyrosine—we analyzed “molecular signatures” characteristic of peroxynitrite-mediated oxidation by isotope dilution liquid chromatography tandem mass spectrometry (Figure 10). The samples from the endothelial cells of Gla−/0 mice contained 90% more ortho-tyrosine than those from the wild-type animals (Gla−/0, 63.8 ± 8.6 μmol/mol phenylalanine [the precursor of ortho-tyrosine]; Gla+/0, 33.7 ± 3.0 μmol/mol phenylalanine; n = 3 per group). Similarly, levels of nitrotyrosine were 72% greater in the Gla−/0 endothelial cells (Gla−/0, 93.70 ± 18.60 μmol/mol tyrosine [the precursor of nitrotyrosine]; Gla+/0, 54.37 ± 1.40 μmol/mol tyrosine; n = 3 per group). The marked elevation of both of these oxidized amino acids is consistent with peroxynitrite oxidation of proteins in the endothelial cells. The increases in ortho-tyrosine and nitrotyrosine were not corrected when the Gb3 levels in the cells were normalized with treatment with EtDO-P4. These observations suggest that the eNOS is uncoupled in this in vitro model.
Figure 10.
Oxidized amino acid levels in cultured MAECs from 8-mo-old Gla+/0 and Gla−/0 mice. Nitro- and ortho-tyrosine levels were measured by mass spectrometry as described in the Concise Methods section. Both nitrotyrosine and ortho-tyrosine concentrations were significantly elevated in the knockout cells compared with the wild-type controls (n = 3; P < 0.05). Cultured cells from Gla−/0 mice were also treated from 48 h with 0.25 μM EtDO-P4 to deplete cellular Gb3 levels.
Discussion
Previous in vivo studies with the Gla null mouse demonstrated three primary phenotypes when compared with strain-specific wild-type mice. These include enhanced thrombosis secondary to oxidant-induced endothelial injury, accelerated atherogenesis when mice are bred on an apolipoprotein E null background, and impaired vasorelaxation to acetylcholine in precontracted aortic rings. Although these phenotypes may result from disparate and complex abnormalities, a potential common mechanism linking these abnormalities is the decreased bioavailability of NO. There are four principal causes of diminished NO bioactivity.14 These causes include decreased expression or activity of eNOS, uncoupling of eNOS, enhanced breakdown or scavenging of NO, and impaired transmission of NO-mediated signaling processes. In these studies, we considered the possibility that decreased NO bioavailability might partially explain these vascular phenotypes by first measuring caveolin-1 and eNOS expression in the aortas of the Gla null mice and their isogenic littermates.
To probe this possibility, Gla null mice were treated with a potent inhibitor of glucosylceramide synthase. EtDO-P4 potently reversed the Gb3 accumulation in aortic rings (and cultured endothelial cells) but did not reverse the vascular reactivity abnormality observed in the Gla knockout mouse aortic rings. The failure to correct the vasorelaxation defect is contrary to a previous report using N-butyldeoxynojirimycin. In that study, the inhibitor had minimal effects in lowering aortic Gb3 content but partially improved the vasodilatory response to acetylcholine.8
Our recently reported studies comparing aortic ring reactivity Gla null with wild-type mice demonstrated that the vascular defect resided within the endothelium of the aortas; therefore, to probe further a possible difference in NO bioavailability, we studied primary cultures of aortic endothelial cells from 4- and 8-mo-old mice. A decrease in basal and insulin-stimulated eNOS activity was observed in the Gla null cells compared with the wild-type MAECs. This decrement in NO formation was paralleled by similar decreases in caveolin-1 and eNOS expression. When Gb3 levels were lowered by exposure of the MAECs to EtDO-P4, a potent inhibitor of glucosylceramide synthase, we observed a partial but incomplete reversal of the defect in insulin stimulated NO formation, consistent with the in vivo observations; however, when the caveolar fractions were isolated from these cells and probed for both caveolin-1 and eNOS, we observed no change in the recovery of either protein.
eNOS synthesizes NO in endothelial cells, and its uncoupling has been described in various conditions, including diabetes, hypertension, and hypercholesterolemia. Under those conditions, eNOS transfers electrons to molecular oxygen, generating superoxide. Several mechanisms have been proposed for this uncoupling. One important mechanism involves oxidation of its co-factor, BH4.15 An alternative mechanism for uncoupling eNOS involves overproduction of angiotensin II, which can induce dihydrofolate reductase deficiency. Because dihydrofolate reductase maintains BH4 in its reduced form, its deficiency uncouples eNOS.16
When both NO and superoxide are generated by eNOS, it can result in the formation of the highly reactive oxidant peroxynitrite.17 Our data demonstrating marked elevations of protein-bound ortho-tyrosine and nitrotyrosine in MAECs isolated from Gla null mice is consistent with peroxynitrite-induced protein damage.18–20 In addition to eNOS uncoupling, other cellular sources of excess superoxide production include mitochondria and NADPH oxidase. Peroxynitrite can be generated in the presence of NO under these conditions as well.21 The effects of Gla deficiency on these pathways remain to be elucidated. Peroxynitrite once generated can also oxidize BH4 and perpetuate eNOS uncoupling22; however, the mechanism by which elevated Gb3 results in aberrant eNOS function is not known. Posttranscriptional events are important for eNOS function. These events include the phosphorylation of the enzyme by one of several kinases,23 the routing of the enzyme to the caveolus,24 proper dissociation of eNOS from caveolin-1,25 and the availability of co-factors and substrate. Among the phosphorylation events are the phosphorylation of serine, threonine, and tyrosine residues, primarily located in the reductase domain of eNOS.26 The localization of eNOS to caveolae is also an important regulatory mechanism. The association of eNOS with these specialized lipid rafts occurs through palmitoylation and myristoylation sites and seems to depend on the lipid composition of the caveolae.25–27 Cholesterol and sphingolipids are critical components of lipid rafts and at least the former lipid is believed to be critical for caveolus formation.28 Approximately 100 to 200 caveolin molecules are present in each caveolus, most in an oligomeric form.29 In general, the anchorage of eNOS to caveolin results in the inactivation of the enzyme, and the dissociation results in activation. The consequences of eNOS-caveolin dissociation for signal transduction have been previously reviewed.30
The importance of caveolar lipid is based primarily on studies restricted to evaluating the role of cholesterol in caveolae. Cholesterol depletion with cyclodextrin or HDL or cholesterol displacement with oxidized LDL lowers eNOS activity by redistribution of eNOS from caveolae28; however, the role of other caveolar lipid components in eNOS redistribution, most notable sphingomyelin and glycosphingolipids, does not seem to have been studied.
In this study, we observed a decrease in high molecular weight caveolin-1 oligomers observed in the Gla null mouse endothelium. This finding suggests that Gb3 may regulate the assembly or maintenance of caveolar structure. This regulation may be direct, conceivably as a result of a direct interaction between the glycosphingolipid and caveolin-1, or indirect secondary to the displacement of caveolar lipids that are normally present. The displacement of cholesterol by Gb3 in Gla null endothelial cells has been reported.13
Caveolins are synthesized on the rough endoplasmic reticulum and transit through the Golgi complex before being trafficked to the plasma membrane. At some point during this synthetic route, caveolins change from a monomeric form to an oligomeric form. Studies using a caveolin-1–GFP fusion construct are consistent with the formation of the caveolae in the Golgi complex.31 Cholesterol seems to be important for this process. The addition of cholesterol seems to decrease the transit time through the Golgi complex. Cholesterol binds directly to caveolin-1 as demonstrated by photoactivatable cross-linking studies, plasma membrane cholesterol enrichment in cells expressing caveolin-1, and numerous structural studies defining cholesterol-binding motifs. Whether glycosphingolipids such as Gb3 also bind caveolin-1 directly and are important for caveolin assembly, trafficking, and structure is unknown but clearly worthy of further investigation.
In summary, direct measurements of eNOS activity and caveolin-1 oligomerization in aortic endothelial cells from Gla null mice demonstrate a loss of activity consistent with decreased NO bioavailability. The loss of NO, through either decreased production by eNOS or scavenging through the formation of superoxide and conversion to peroxynitrite, may account for aberrant vascular phenotypes in the Gla null mouse model that include atherogenesis, thrombosis, and impaired relaxation.
Concise Methods
Reagents
Polyclonal anti-eNOS and anti–caveolin-1 antibodies were obtained from Abcam (Cambridge, MA), and a mAb directly against eNOS was from BD Transduction Laboratories (Lexington, KY). Shiga toxin-1 (STX) and anti-STX mAb (13C4) were obtained from Toxin Technology (Sarasota, FL). Monoclonal anti–β-actin antibody P2714 (a protease inhibitor cocktail with a broad specificity for the inhibition of serine, cysteine, and metalloproteases) and a 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), a water-soluble and more stable form of PMSF, were purchased from Sigma-Aldrich (St. Louis, MO). The glucosylceramide synthase inhibitor EtDO-P4 was designed and synthesized in our laboratory as described previously.10 [3H]l-arginine was from Amersham (Arlington Heights, IL). Unless otherwise specified, the cell culture reagents and media were from Invitrogen (Carlsbad, CA).
Animals and Genotyping
Gla-deficient mice, originally developed by Ohshima et al.,32 and wild-type C57BL/6 mice were bred, housed, and maintained in the animal facility of the University of Michigan as described previously.33 The mouse genotypes were routinely confirmed using a simple, one-step PCR procedure. Genomic DNA was prepared from mouse tails by using DirectPCR Lysis Reagent (Viagen Biotech, Los Angeles, CA) following the instructions provided by the supplier. Crude DNA released in DirectPCR reagent was directly subjected to PCR amplification without further purification because this reagent inhibits the certain compounds that interfere with PCR reactions in animal tissues. The sequences of designed primers and PCR conditions for the Gla gene were as follows: forward primer 5′-ACTGGGTATCCTGGCTCTATCC-3′, reverse primer 5′-GATCTACGCCCCAGTCAGCAAATG-3′, and primer for PGKneo sequence 5′-TCCATCTGCACGAGACTAGT-3′. The PCR reaction mixture (25 μl) contained 0.2 μM of each primer, 1× PCR buffer (Applied Biosystems, Foster City, CA), 1.5 mM MgCl2, 0.2 mM dNTPs, 1.0 U Taq DNA polymerase, and 0.5 μl of genomic DNA (0.2 to 0.3 μg). Thirty cycles were performed for the PCR amplification. The cycling temperatures and timings for Gla gene were 94°C for 30 s, 60°C for 30 s, and 68°C for 60 s. The generated PCR products—a 550-bp fragment for wild-type and an 885-bp fragment for knockout Gla, respectively—were separated on a 1.5% agarose gel. Animal studies were performed under the review of the University of Michigan committee on the use and care of animals and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
MAEC Cultures
Primary MAECs were isolated, cultured, and maintained as described previously.33 Cultured MAECs were either studied at passages 3 to 4 or frozen at density of 1.0 to 1.5 × 106 cells/ml in frozen medium consisting of 50% FBS, 10% DMSO, and 40% RPMI-1640. After thawing at 37°C for 2 to 3 min, frozen MAECs were recovered in RPMI-1640 medium supplemented with 15% FBS only for 3 to 4 h to allow cells to adhere to the culture dishes or preincubated in RPMI-1640 medium containing 15% FBS, 2 mM l-glutamine and 1× nonessential amino acid for overnight, if necessary. Recovery medium and unattached cells were then removed by replacement with RPMI-1640 medium containing 15% FBS, 2 mM l-glutamine, 1× nonessential amino acid, 0.05 mg/ml endothelial cell growth supplement (ECGS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.05 mg/ml heparin. Under those conditions, the recovery rate of frozen MAECs was usually >90%.
Aortic and MAEC Lysates
Aortas of Gla knockout and wild-type mice at ages 2 to 20 mo were isolated as described previously.13 Each aorta, gently dissected from fat and connective tissue in RPMI-1640 medium, was minced in 0.4 ml of ice-cold lysis buffer (25 mM Tris-HCl [pH 7.4], 137 mM NaCl, 2 mM EDTA, 2 mM Na3VO4, 20 mM NaF, 1% Triton X-100, 10% glycerol, 5% P2714, and 1 mM AEBSF) for 10 min at 4°C. Aortic samples were sonicated using a probe sonicator for 1 s five times at 4°C. Tissue debris was removed by centrifugation at 12,000 × g for 10 min at 4°C. MAEC lysates were prepared from cultured wild-type and Fabry MAECs (passage 3) either treated or untreated with EtDO-P4 (0.25 μM) for 2 d. After removal of serum and growth factors, MAECs were stimulated with insulin (100 nM) for the indicated durations. The cells in 100-mm culture dishes were then lysed in 0.5 ml of 1% Triton X-100 lysate buffer as described already for 10 min at 4°C and collected by scraping. After a brief sonication (3 × 1 s), the cell debris was precipitated by centrifugation at 12, 000 × g for 10 min. The clarified aortic and MAEC lysates were subjected to immunoblot analysis after the determination of protein concentration by BCA assay using BSA as a standard.
Immunoprecipitation and Immunoblotting
For immunoprecipitation, equal amounts of aortic lysate proteins (100 to 150 μg) were first incubated with a polyclonal anti–caveolin-1 antibody (1 μg/ml) or with a monoclonal anti-eNOS antibody (2 μg/ml) overnight at 4°C with gentle rotation and subsequently incubated with protein A agarose beads (50 μl of a 50% suspension) for another 2 h at 4°C. The protein A agarose beads were then centrifuged at 7000 × g for 20 s and washed three times with a washing buffer consisting of 25 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 1 mM Na3VO4. The recovered immunocomplexes were dissolved in SDS sample buffer containing 0.5% β-mercaptoethanol and separated in SDS-PAGE with a 7 to 13% gradient. Immunoblots were performed as described previously.34 Equal amounts of either aortic or MAEC lysates were directly resolved by a 7 to 13% gradient SDS-PAGE. After membrane transfer, the nylon membranes were blocked in 5% skim milk in TBS buffer (20 mM Tris-HCl [pH 7.6] and 150 mM NaCl) for 1 h at room temperature and probed with selected antibodies. The immunoreactive bands were detected with the ECL-plus system (PerkinElmer Life Sciences, Waltham, MA) and quantified by densitometry using NIH Image 1.62 software (Bethesda, MD).
eNOS Activity Measurements
Enzyme activities of eNOS in cultured MAECs were determined as the formation of [3H]l-citrulline from [3H]l-arginine by adopting a protocol with high recovery rate of [3H]l-citrulline developed and described by Xiao et al.35 Briefly, MAECs (passage 3) untreated or treated with EtDO-P4 (2 d) were cultured in 60-mm dishes to 70 to 75% confluence. ECGS was first withdrawn from culture medium for 12 h. The concentration of FBS in the culture medium was then gradually reduced from 10 to 0%. Subsequently, cells were preincubated in 3 ml of l-arginine–deficient, serum-free, and ECGS-omitted RPMI-1640 for 2 h at 37°C. At a final 15 min period, cells were stimulated either with insulin (100 nM) or with buffer. The preincubation was stopped by removing the medium followed by washing once with 2 ml of Krebs-HEPES buffer consisting of 25.0 mM HEPES (pH 7.40, 37°C), 140.0 mM NaCl, 5.4 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, 1.0 mM Na2HPO4, 25.0 mM NaHCO3, and 5.5 mM glucose. Cells were then incubated in Krebs-HEPES buffer (2 ml), and the reaction was initiated by addition of 3.3 μCi of [3H]l-arginine (specific activity 59 Ci/mmol) diluted with 10 μM unlabeled l-arginine. After a 30-min incubation at 37°C, the reaction was terminated by removing the reaction buffer and rapidly rinsing the cells with 2 ml of ice-cold PBS containing 5 mM unlabeled l-arginine and 4 mM EDTA three times. Washed cells were solubilized but not denatured in 1.0 ml of 1% Triton X-100. Two aliquots (50 μl) were withdrawn for quantification of the total protein content and total cellular tritium incorporation. Two portions of cell lysate (0.3 ml) were taken, and each was mixed with 0.7 ml of 1:1 (vol/vol) H2O/AG 50WX8-400 cation exchange resin (Na+ form [pH 5.5]) to remove unconverted [3H]l-arginine. After a 5-min mixing, the samples were centrifuged at 2000 × g for 2 min. The neutrally charged [3H]l-citrulline in the supernatants was collected, measured for radioactivity in a liquid scintillation counter, normalized to protein concentration, and expressed as pmol/min per mg protein. The samples were incubated in the presence of the competitive eNOS inhibitor, NG-nitro-l-arginine methyl ester (L-NAME), to account for nonspecific activity. The L-NAME–inhibitable (2 mM) activity was determined as specific eNOS activity.
Caveolae Isolation
Caveolar fractions were isolated from cultured MAECs either untreated or treated with EtDO-P4 at indicated times and concentrations by using a nondetergent method taking advantage of the unique buoyant density of caveolar membrane as described previously.36
Analysis of Oxidized Amino Acids
Cells were washed with ice-cold antioxidant buffer (100 μM diethylene tetramino pentaacetic acid [metal chelator], 50 μM butylated hydroxyl toluene [lipid-soluble antioxidant], 1% [vol/vol] ethanol, and 10 mM 3-amino-1,2,4-triazole [peroxidase inhibitor] in 50 mM sodium phosphate buffer [pH 7.4]). Cellular proteins were precipitated with ice-cold TCA (10% vol/vol), collected by centrifugation, washed with 10% TCA, and delipidated twice with water/methanol/water-washed diethyl ether (1:3:7; vol/vol/vol). Isotopically labeled internal standards were added, and samples were hydrolyzed with 4 N methane sulfonic acid. All samples were manually injected using an on column injector and a Hewlett Packard 6890 gas chromatograph equipped with a 15-m DB-5 capillary column (0.25 mm id, 0.33-μ film thickness; J&W Scientific, Fulsom, CA) interfaced with a Hewlett Packard (Palo Alto, CA) 5973 mass detector. The t-butyl dimethylsilyl derivatives of amino acids were quantified by selected ion monitoring, using isotope dilution negative-ion chemical ionization gas chromatography mass spectrometry as described previously.18,37 Results are normalized to protein content of tyrosine, the precursor of 3-nitrotyrosine, or phenylalanine, the precursor for ortho-tyrosine.
Fluorescence Microscopy
Wild-type and Gla null MAECs at passage 3 were grown on glass coverslips precoated with 1% gelatin to 80% confluence. After washing twice with cold PBS, the cells were fixed with 1 ml of 4% cold fresh-prepared paraformaldehyde for 5 min at 4°C. The fixative was then removed by washing with ice-cold PBS, and cells were incubated with diluted STX (1:50) for 1 h at room temperature. The cells were subsequently blotted with anti-STX (13C4) mAb at 1:50 dilution for 1 h at room temperature and stained with goat anti-mouse antibody conjugated with Alexa Fluor 546 (1:50) for 1 h at room temperature. The mounted coverslips were viewed under fluorescence microscopy and photographed as described previously.33
Statistical Analysis
The data were collected from three or four individual experiments, analyzed by the t test and expressed as mean ± SEM. The differences between control and treated samples were considered statistically significant at P < 0.05.
Disclosures
J.A.S. is an inventor of D-threo-ethylenedioxyphenyl-P4. The University of Michigan holds the patent to this compound and has licensed this inhibitor to Genzyme Corporation.
Acknowledgments
This work was support by National Institutes of Health grant RO1DK055823-09.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Shayman JA, Killen PD:Fabry disease. In: Molecular and Genetic Basis of Renal Disease, edited by Mount DB, Pollak MR.Philadelphia, Saunders Elsevier, 2008, pp 195–199 [Google Scholar]
- 2.Eng CM, Fletcher J, Wilcox WR, Waldek S, Scott CR, Sillence DO, Breunig F, Charrow J, Germain DP, Nicholls K, Banikazemi M:Fabry disease: Baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis 30: 184–192, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kolodny EH, Pastores GM:Anderson-Fabry disease: Extrarenal, neurologic manifestations. J Am Soc Nephrol 13[ Suppl 2]: S150–S153, 2002 [PubMed] [Google Scholar]
- 4.Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease-Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R:Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: Reversal by enzyme replacement therapy. Circulation 104: 1506–1512, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R, Tome MT, McKenna WJ, Lee P, Camici PG:Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 92: 357–360, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eitzman DT, Bodary PF, Shen Y, Khairallah CG, Wild SR, Abe A, Shaffer-Hartman J, Shayman JA:Fabry disease in mice is associated with age-dependent susceptibility to vascular thrombosis. J Am Soc Nephrol 14: 298–302, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bodary PF, Shen Y, Vargas FB, Bi X, Ostenso KA, Gu S, Shayman JA, Eitzman DT:Alpha-galactosidase A deficiency accelerates atherosclerosis in mice with apolipoprotein E deficiency. Circulation 111: 629–632, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Heare T, Alp NJ, Priestman DA, Kulkarni AB, Qasba P, Butters TD, Dwek RA, Clarke K, Channon KM, Platt FM:Severe endothelial dysfunction in the aorta of a mouse model of Fabry disease: Partial prevention by N-butyldeoxynojirimycin treatment. J Inherit Metab Dis 30: 79–87, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Park JL, Whitesall SE, D'Alecy LG, Shu L, Shayman JA:Vascular dysfunction in the alpha-galactosidase A-knockout mouse is an endothelial cell-, plasma membrane-based defect. Clin Exp Pharmacol Physiol 35: 1156–1163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee L, Abe A, Shayman JA:Improved inhibitors of glucosylceramide synthase. J Biol Chem 274: 14662–14669, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Abe A, Gregory S, Lee L, Killen PD, Brady RO, Kulkarni A, Shayman JA:Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest 105: 1563–1571, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe A, Gregory S, Lee L, Shayman JA:Use of sulfobutyl ether beta-cyclodextrin as a vehicle for D-threo-1-phenyl-2-decanoylamino-3-morpholinopropanol-related glucosylceramide synthase inhibitors. Anal Biochem 287: 344–347, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Shu L, Shayman JA:Caveolin-associated accumulation of globotriaosylceramide in the vascular endothelium of alpha-galactosidase A null mice. J Biol Chem 282: 20960–20967, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Braam B, Verhaar MC:Understanding eNOS for pharmacological modulation of endothelial function: A translational view. Curr Pharm Des 13: 1727–1740, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG:Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA, Jr:Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci U S A 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckman JS, Chen J, Ischiropoulos H, Crow JP:Oxidative chemistry of peroxynitrite. Methods Enzymol 233: 229–240, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW:A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest 107: 853–860, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennathur S, Heinecke JW:Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal 9: 955–969, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Pennathur S, Jackson-Lewis V, Przedborski S, Heinecke JW:Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o'-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson's disease. J Biol Chem 274: 34621–34628, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Vivekanadan-Giri A, Wang JH, Byun J, Pennathur S:Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Rev Endocr Metab Disord 9: 275–287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennathur S, Heinecke JW:Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep 7: 257–264, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Michel T, Li GK, Busconi L:Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 90: 6252–6256, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das K, Lewis RY, Scherer PE, Lisanti MP:The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers: Implications for the assembly of caveolae membranes in vivo. J Biol Chem 274: 18721–18728, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Garcia-Cardena G, Sessa WC:Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: Implications for caveolae localization. Biochemistry 35: 13277–13281, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG:Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res 79: 984–991, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Robinson LJ, Michel T:Mutagenesis of palmitoylation sites in endothelial nitric oxide synthase identifies a novel motif for dual acylation and subcellular targeting. Proc Natl Acad Sci U S A 92: 11776–11780, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaul PW:Regulation of endothelial nitric oxide synthase: Location, location, location. Annu Rev Physiol 64: 749–774, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Parton RG, Hanzal-Bayer M, Hancock JF:Biogenesis of caveolae: A structural model for caveolin-induced domain formation. J Cell Sci 119: 787–796, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Goligorsky MS, Li H, Brodsky S, Chen J:Relationships between caveolae and eNOS: Everything in proximity and the proximity of everything. Am J Physiol Renal Physiol 283: F1–F10, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Schlegel A, Lisanti MP:A molecular dissection of caveolin-1 membrane attachment and oligomerization: Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem 275: 21605–21617, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, Sugimoto Y, Pastan I, Gottesman MM, Brady RO, Kulkarni AB:Alpha-galactosidase A deficient mice: A model of Fabry disease. Proc Natl Acad Sci U S A 94: 2540–2544, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu L, Murphy HS, Cooling L, Shayman JA:An in vitro model of Fabry disease. J Am Soc Nephrol 16: 2636–2645, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Shu L, Shayman JA:Src kinase mediates the regulation of phospholipase C-gamma activity by glycosphingolipids. J Biol Chem 278: 31419–31425, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Xiao S, Gillespie DG, Baylis C, Jackson EK, Dubey RK:Effects of estradiol and its metabolites on glomerular endothelial nitric oxide synthesis and mesangial cell growth. Hypertension 37: 645–650, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Shu L, Lee L, Chang Y, Holzman LB, Edwards CA, Shelden E, Shayman JA:Caveolar structure and protein sorting are maintained in NIH 3T3 cells independent of glycosphingolipid depletion. Arch Biochem Biophys 373: 83–90, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW:Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: A potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem 280: 22706–22714, 2005 [DOI] [PubMed] [Google Scholar]