Abstract
The human skin contacts molecules from house dust mites that are ubiquitous in many environments. These mite-derived molecules may penetrate the skin epidermis and dermis and contact microvascular endothelial cells and influence their function. The purpose of this study was to determine the response of normal human dermal microvascular endothelial cells to extracts of the dust mites, Dermatophagoides farinae, D. pteronyssinus, and Euroglyphus maynei with and without endotoxin (lipopolysaccharide). Endothelial cells were stimulated with mite extracts and the expression of surface molecules and the secretion of cytokines were measured in the absence and presence of polymyxin B to bind endotoxin. All three mite extracts stimulated endothelial cells to express intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin and to secrete interleukin (IL)-6, IL-8, monocyte chemoattractant protein (MCP-1), and granulocyte/macrophage colony stimulating factor (GM-CSF). Euroglyphus maynei-induced expression of all the cell surface molecules was not inhibited when the endotoxin activity in the mite extract was inhibited. In contrast, endothelial cells challenged with D. farinae or D. pteronyssinus extract depleted of endotoxin activity expressed only constitutive levels of ICAM-1, VCAM-1, and E-selectin. D. farinae and E. maynei extracts depleted of endotoxin activity still induced secretion of IL-8 and MCP-1 but at reduced levels. Only constitutive amounts of IL-6, G-CSF, and GM-CSF were secreted in response to any of the endotoxin-depleted mite extracts. Extracts of D. farinae, D. pteronyssinus, and E. maynei contain both endotoxins and other molecules that can stimulate expression of cell adhesion molecules and chemokine receptors and the secretion of cytokines by normal human microvascular endothelial cells.
Keywords: cytokine, chemokine, mite, endotoxin, lipopolysaccharide
House dust mites are the source of molecules that can influence innate and immune responses in humans. Some of these molecules are allergenic and can sensitize and induce allergic reactions that may manifest as atopic dermatitis, rhinitis (nasal and ocular), or asthma in genetically predisposed individuals. Approximately 20 of these allergens have been characterized (Thomas et al. 2007). It is likely that there are other bioreactive molecules from house dust mites that are not allergenic. These bioreactive allergenic and nonallergenic molecules in extracts of house dust mites can potentially modulate the function of many cell types they may contact in the skin, nasal and ocular mucus membranes, and lung epithelium. Previous research has shown that molecules in whole mite extracts or selected allergens (e.g., Der 1 allergen) induce the release of proinflammatory cytokines and the expression of adhesion molecules on many different cell types. Numerous studies have shown that molecules in house dust mite extracts can effect the function of human fibroblasts, keratinocytes, skin microvascular postcapillary endothelial cells, dendritic cells, macrophages, mast cells, basophils, eosinophils, and lung epithelial cells (Machado et al. 1996, Shimizu et al. 1998, Chen et al. 2003, Boasen et al. 2005, Wong et al. 2006, Sohn et al. 2007, Arlian et al. 2008, Lee et al. 2008). Thus, molecules from dust mites may contribute to or amplify immune and inflammatory pathological reactions or the course of allergic reactions.
The mechanisms used by mite molecules to modulate the function of cells in the skin, nasal and ocular mucus membranes, lung epithelium, and others are largely unknown. Some of these molecules are serine and cysteine protease enzymes. Previous studies have shown that Der p 1 (cysteine protease), Der p 3 (trypsin), Der p 6 (chymotrypsin), and Der p 9 (collagenolytic serine protease) activate lung epithelial cells and this is mediated through cleavage of protease-activated receptors (PARs) (Sun et al. 2001, Reed and Kita 2004, Adam et al. 2006, Kauffman et al. 2006, Liu 2007, Thomas et al. 2007). However, Kauffman et al. (2006) reported that house dust mite extracts induce responses in human alveolar type II epithelial-like A549 cells that are mediated by both protease-dependent and protease-independent signaling pathways. Der p 1 can activate airway epithelial cells through a PAR-2-independent pathway (Adam et al. 2006).
Other in vitro studies have shown that house dust mite extracts or specific allergens affect bronchial epithelial cells (Herbert et al. 1995) and mast cells (Stewart et al. 1994). Der p 1 can also disrupt the IgE network by cleavage of CD23 on activated B cells (Hewitt et al. 1995). Likewise, Der p 1 can cleave CD25, the human T-cell interleukin-2 (IL-2) receptor, and it may also affect the function of B cells (Schulz et al. 1998, Shakib et al. 1998). Molecules in extracts of Dermatophagoides farinae (Hughes) or D. pteronyssinus (Trouessart) can modulate the secretion of growth-related oncoprotein-α(GRO-α) by normal human epidermal keratinocytes and IL-6, IL-8, monocyte chemoattractant protein (MCP-1), and macrophage colony stimulating factor (M-CSF) by normal human dermal fibroblasts (Arlian et al. 2008). Dust mite proteolytic allergens induce cultured airway epithelial cells to release IL-6 and IL-8 and produce prostaglandin E2, granulocyte/macrophage colony stimulating factor (GM-CSF), and eotaxin (CCL11) (King et al. 1998, Tomee et al. 1998, Knight et al. 2000, Sun et al. 2001, Mascia et al. 2002). Human eosinophils are activated and degranulate in response to the mite cysteine protease allergens (Miike and Kita 2003). Molecules in an extract of D. pteronyssinus induce human monocytic THP-1 cells (monocyte-like) to increase expression of MCP-1, IL-6, and IL-8 (Lee et al. 2008). House dust mite extracts activate bone marrow–derived dendritic cells to produce IL-6 and IL-12 and to express CD40, CD80, CD86, and MHC-II molecules (Boasen et al. 2005). Der p 1 induced secretion of IL-1β, IL-6, IL-10, tumor necrosis factor-α (TNFα), and GM-CSF from human blood eosinophils and surface expression of CD18 and intercellular adhesion molecule-1 (ICAM-1) (Wong et al. 2006). Basophils from mite-sensitive asthmatics secreted IL-4 and IL-13 when stimulated with dust mite antigen (Shimizu et al. 1998). Alveolar macrophages stimulated with whole body extract of D. farinae released IL-6, TNFα, and NO (Chen et al. 2003). Airway-derived epithelial cells gave a dose-dependent increased secretion of IL-6 and IL-8 when stimulated with house dust mite extract, Der p 1, and Der p 5 (Kauffman et al. 2006). Thus, there is much evidence that molecules from house dust mites can modulate the function of cells they contact.
Extracts of storage and house dust mites contain molecules that modulate the secretion of proinflammatory cytokines and chemokines by cultured normal human dermal fibroblasts and epidermal keratinocytes (Arlian et al. 2008). Likewise, molecules in extracts of the related scabies mite influence the secretion of cytokines and chemokines and expression of adhesion molecules and chemokine receptors by cultured normal human skin microvascular endothelial cells (Elder et al. 2006). Postcapillary venule endothelial cells of the skin play a key role in regulating the migration of inflammatory and immune cells from the blood stream into the dermis and thus anything that influences their function also influences the course of pathogenesis in the skin (Steinhoff et al. 2006). The effect that soluble molecules in extracts of the common house dust mites have on dermal microvascular endothelial cells is unknown. The purpose of this study was to determine the response of normal human microvascular endothelial cells of the skin to molecules in extracts of the house dust mites D. farinae, D. pteronyssinus, and Euroglyphus maynei (Cooreman).
Materials and Methods
House Dust Mite Extracts
Mite material was harvested from pure colonies that were maintained on a high-protein diet at room temperature (75°F) and 75% RH. Extracts were prepared from mites that migrated to the lids of thriving cultures (live mites of all life stages) as previously described (Arlian et al. 2008). Briefly, aqueous whole body extracts of the house dust mites D. farinae (DF), D. pteronyssinus (DP), and E. maynei (EM) were prepared by extraction into endotoxin-free distilled water for 48 h at 4°C. After homogenization in a TenBroeck homogenizer (Fisher, Pittsburgh, PA), extracts were centrifuged to remove insoluble material, and the supernatants were collected and sterile-filtered (0.22 μm) into sterile vials for storage at 4°C. The total protein concentration of each extract was determined using the Bradford protein assay with bovine serum albumin as standard (Bradford 1976).
Levels of Endotoxin Present in Dust Mite Extracts
House dust mites have previously been reported to contain endosymbiont bacteria and endotoxins (Trivedi et al. 2003, Valerio et al. 2005). Therefore, we measured endotoxin levels in the mite extracts using a chromogenic endpoint LAL (limulus amebocyte lysate) assay kit (QCL-1000; Lonza Walkersville, Walkersville, MD) according to the manufacturer’s directions. We also prepared a set of mite extracts that were preincubated with polymyxin B at 30 μg/ml (Cardoso et al. 2007) for 18 h at 4°C to bind and inactivate any endotoxin present in them.
Reagents
Biotinylated monoclonal antibodies directed against human ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and recombinant human TNFα were purchased from eBioscience (San Diego, CA). The biotinylated monoclonal antibody directed against human E-selectin, unlabeled antibodies to CXCR-1 and CXCR-2, and all ELISA kits for cytokine detection (Duo-Set kits) were purchased from R&D Systems (Minneapolis, MN). Lipopolysaccharide (LPS), polymyxin B sulfate, aprotinin, and E-64 were purchased from Sigma-Aldrich (St. Louis, MO). Horseradish peroxidase-conjugated streptavidin (HRP-SA) and goat anti-mouse Ig were purchased from Fisher (Pittsburgh, PA).
Endothelial Cells
Cryopreserved adult dermal microvascular endothelial cells (HMVEC-D) and all media and growth factors were purchased from Lonza. Cells were cultured in Clonetics endothelial cell basal medium-2 supplemented with EGM-2-MV growth factors (EGM-2) at 37°C in 5–7% CO2. Cells were plated and grown to confluency in Costar 96-well culture plates (Corning, Corning, NY) for subsequent cell ELISA and cytokine secretion studies. All studies were performed on cells between passages 7 and 10.
Cell Stimulations and Assays
Cell stimulations were performed as previously described (Elder et al. 2006). Briefly, at the beginning of each experimental period, one half of the culture medium was removed from the wells and was replaced with medium containing the test stimuli prepared in EGM-2 to provide the desired final concentrations in the test wells with mite extract doses based on the protein concentrations. When present, TNFα was added at a final concentration of 4 ng/ml alone or along with the mite extracts at varying doses. At the end of the stimulation periods (6, 12, or 24 h), culture supernatants were removed and frozen at −80°C for subsequent cytokine ELISA. The expression of cellular adhesion molecules and receptors was determined using a cellular ELISA procedure modified from Yokote et al. (1993) and described previously (Elder et al. 2006). Briefly, cells were washed and wells were blocked for 30 min at 37°C in 5–7% CO2. The desired concentration of primary antibody in 50 μl was added to each well. All incubations were carried out for 30 min at 37°C in 5–7% CO2 with washing between each step. Binding of biotinylated antibodies was detected with 50 μl of a 1:2,000 dilution of HRP-SA, whereas binding of nonbiotinylated antibodies was detected with 50 μl of a 1:1,000 dilution of HRP-labeled goat anti-mouse antibody. After the final wash, 50 μl of TMB ELISA substrate (Sigma-Aldrich) was added to each well, and the plate was incubated for up to 20 min before color development was stopped with the addition of 50 μl of 1 M sulfuric acid. Absorbance was measured at 450 nm using a BioTek EL800X microplate reader.
For experiments to determine the effects of endogenous endotoxin in mite extracts, extracts (100 μg/ml) or control endotoxin (LPS at 250 EU/ml final) was preincubated with polymyxin B at 30 μg/ml (Cardoso et al. 2007) for 18 h at 4°C to bind and inactivate any endotoxin present in the extracts before 6-h cell exposure. To determine whether proteases in the mite extracts were mediating their effects through proteolytic cleavage of PARs, we added aprotinin (serine protease inhibitor) or E-64 (cysteine protease inhibitor) to the mite extracts at 50 μg/ml (Lee et al. 2008) and used these mixtures to stimulate the cells.
Data Analysis
All dose and time course experiments were performed twice with triplicate wells for each individual test. In one experiment, varying doses of the three different mite extracts were tested simultaneously at each of the three times (6, 12, or 24 h). In the other experiment, both the doses and times were varied, and tests were performed separately for each mite extract. The data presented here are the average for triplicate wells of cells from the experiment in which both the doses and times were varied.
Results
Effects Induced by Whole Mite Extracts on Expression of Cell Adhesion Molecules and Cytokine Receptors
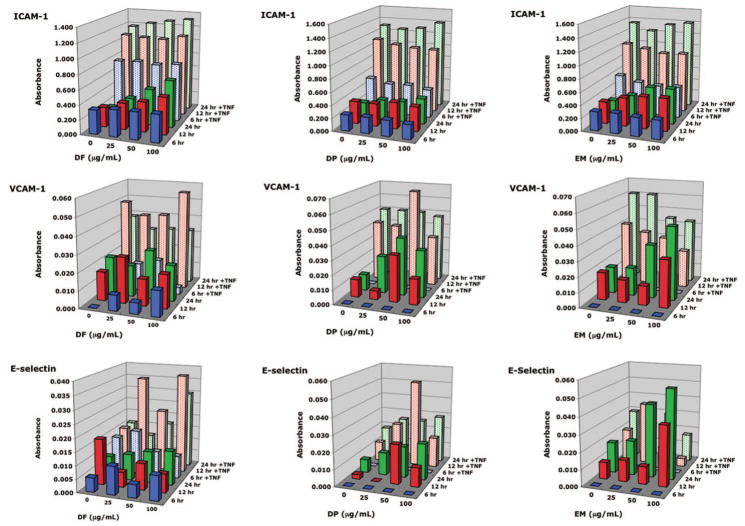
Dermatophagoides farinae, D. pteronyssinus, and E. maynei extracts stimulated endothelial cells to increase expression of ICAM-1 and VCAM-1 above constitutive levels in both a dose- and time-dependent manner (Fig. 1). All three mite extracts also stimulated the expression of E-selectin. Likewise, TNFα induced increased expression of ICAM-1, VCAM-1, and E-selectin. Generally, endothelial cells co-stimulated with both TNFα and any of the house dust mite extracts expressed greater amounts of ICAM-1 and VCAM-1 compared with either any of the house dust mite extracts alone or TNFα alone. D. farinae extract stimulated greater expression of ICAM-1 than either D. pteronyssinus or E. maynei extracts.
Fig. 1.
Dose-dependent expression of adhesion molecules by human microvascular endothelial cells stimulated for 6, 12, or 24 h with varying doses of extracts of the house dust mites D. farinae (DF), D. pteronyssinus (DP), or E. maynei (EM) in the absence or presence of TNFα at 4 ng/ml. Data are presented as means of three replicate wells of cells. (Online figure in color.)
The chemokine receptors CXCR-1 and CXCR-2 were constitutively expressed by endothelial cells (Fig. 2). D. farinae and E. maynei extracts induced expression of CXCR-1 and CXCR-2 above constitutive levels. TNFα did not induce increased expression of CXCR-1. Co-stimulation with TNFα and D. pteronyssinus, but not D. farinae or E. maynei, induced expression of CXCR-2 above the levels expressed in the presence of either TNFα alone or D. pteronyssinus alone.
Fig. 2.
Expression of chemokine receptors by human microvascular endothelial cells stimulated for 6, 12, or 24 h with varying doses of extracts of the house dust mites D. farinae (DF), D. pteronyssinus (DP), or E. maynei (EM) in the absence or presence of TNFα at 4 ng/ml. Data are presented as means of three replicate wells of cells. (Online figure in color.)
Secretion of Cytokines Induced by Stimulation With Mite Extracts
IL-8 was constitutively secreted by endothelial cells, whereas very little IL-6, MCP-1, or GM-CSF was constitutively produced (Fig. 3). D. farinae, D. pteronyssinus, and E. maynei extracts induced dose- and time-dependent secretion of IL-6, IL-8, MCP-1, and GM-CSF. TNFα enhanced secretion of IL-6, IL-8, MCP-1, and GM-CSF. Co-stimulation with the house dust mite extracts and TNFα considerably increased the TNFα-induced IL-6 production but did not effect the level of secretion of GM-CSF induced by TNFα. At comparable doses, D. farinae stimulated greater secretion of IL-6, IL-8, and MCP-1 than did D. pteronyssinus. Interestingly, E. maynei extract stimulated greater secretion of IL-6 and MCP-1 than did D. pteronyssinus extract, but D. pteronyssinus stimulated greater secretion of IL-8 than E. maynei. D. farinae, D. pteronyssinus, and E. maynei did not stimulate secretion of IL-1α, IL-1β, macrophage inflammatory protein-1α (MIP-1α), or eotaxin (CCL-11) (data not shown).
Fig. 3.
Dose-dependent secretion of cytokines by human microvascular endothelial cells stimulated for 6, 12, or 24 h with varying doses of extracts of the house dust mites D. farinae (DF), D. pteronyssinus (DP), or E. maynei (EM) in the absence or presence of TNFα at 4 ng/ml. Data are presented as means of three replicate wells of cells. (Online figure in color.)
Vascular endothelial growth factor (VEGF) secretion was highest after 6-h incubations and thereafter declined over time (Fig. 4). Neither the addition of house dust mite extracts or of TNFα reversed this effect.
Fig. 4.
VEGF secretion by human microvascular endothelial cells stimulated for 6, 12, or 24 h with varying doses of extracts of the house dust mites D. farinae (DF), D. pteronyssinus (DP), or E. maynei (EM) in the absence or presence of TNFα at 4 ng/ml. Data are presented as means of three replicate wells of cells. (Online figure in color.)
Effects of Mite Extracts Depleted of Endotoxin Activity on Cell Adhesion Molecule Expression and Cytokine Secretion
We measured the endotoxin levels in our extracts and determined that the D. farinae and E. maynei extracts contained >45,000 EU/ml, whereas the D. pteronyssinus extract had 29,400 EU/ml. Control endotoxin (LPS) induced expression of ICAM-1, VCAM-1, and E-selectin by endothelial cells, and this LPS-induced expression was inhibited by polymyxin B returning to the levels expressed constitutively (Fig. 5). Endothelial cells stimulated with E. maynei extract alone expressed ICAM-1, VCAM-1, and E-selectin, and the E. maynei-induced expression was not inhibited by including polymyxin B in the extract to bind and inhibit endotoxin activity. In contrast, endothelial cells stimulated with D. farinae or D. pteronyssinus extract containing polymyxin B to inhibit any activity caused by endogenous endotoxin showed expression of ICAM-1, VCAM-1, and E-selectin that was reduced to constitutive levels. This indicates that for the Dermatophagoides mites, but not for E. maynei, the stimulatory effects on adhesion molecule expression were caused by the presence of endotoxin in the extracts.
Fig. 5.
Effect of endotoxin inactivation by polymyxin B on mite-induced adhesion molecule expression and cytokine secretion by human microvascular endothelial cells. Cells were stimulated for 6 h with D. farinae (DF), D. pteronyssinus (DP), or E. maynei (EM) extract (100 μg/ml) or endotoxin control (LPS at 250 EU/ml) in the absence or presence of polymyxin B (30 μg/ml) to bind and inactivate endotoxin. Data are presented as mean ± SEM of three replicate wells of cells.
In the presence of polymyxin B, extracts of D. farinae, D. pteronyssinus, and E. maynei stimulated little or no secretion of IL-6, G-CSF, and GM-CSF (data not shown). However, polymyxin B–treated (endotoxin-depleted) extracts of D. farinae and E. maynei still stimulated secretion of IL-8 and MCP-1 above constitutive levels (Fig. 5). Control LPS by itself stimulated secretion of large amounts of IL-6, IL-8, G-CSF, GM-CSF, and MCP-1, but treatment of these solutions with polymyxin B inhibited the LPS-induced secretion of cytokines to constitutive or near-constitutive levels.
Role of PARs
Endothelial cells stimulated with the mite extracts in the presence of either aprotinin (serine protease inhibitor) or E-64 (cysteine protease inhibitor) did not exhibit decreased expression of adhesion molecules or cytokine secretion, indicating that proteolytic cleavage of PARs was not responsible for the induction of these effects (data not shown).
Discussion
The house dust mites D. farinae, D. pteronyssinus, and to a lesser extent E. maynei are important sources of potent allergens in homes throughout the world. The human skin regularly contacts house dust mites and molecules from house dust mites that are ubiquitous in human dwellings in fabrics of soft furniture (couches and recliners), beds (mattress, pillows and bedding), carpets, clothing, automobile seats, and in public places (seats in theaters, buses, trains and airplanes). Dust mites and their allergens have been recovered from clothing and human skin (Naspitz et al. 1997, Riley et al. 1998, Siebers et al. 1998, Neal et al. 2002, Yasueda et al. 2003). Disturbance of the epidermis and properties of some of the mite molecules would allow mite molecules to penetrate the epidermis and dermis and contact resident (e.g., keratinocytes, fibroblasts, and endothelial cells of the microvasculature) and migratory (e.g., macrophages, mast cells, Langerhans cells, lymphocytes and other leukocytes) cells in the skin. House dust mite extracts contain many enzymes that may disrupt the skin barrier function and facilitate the penetration of molecules from mites through the skin (Cork et al. 2006). Physical abrasions, soaps and detergents, skin care products, solvents, cleaning products, and volatile organic compounds can also disrupt the skin barrier and facilitate penetration of mite molecules (Cork et al. 2006, Huss-Marp et al. 2006). Positive patch tests are direct evidence that dust mite molecules can penetrate the epidermis and dermis (Deleuran et al. 1998, Yasueda et al. 2003, Darsow et al. 2004, Fuiano et al. 2008).
Previous studies found that extracts of storage and house dust mites, as well as the related scabies mites, influence the release of some proinflammatory cytokines and chemokines from epidermal keratinocytes and dermal fibroblasts and monocytic THP-1 cells (Arlian et al. 2003, 2008; Lee et al. 2008). It has been shown that Der p 1 induced expression of ICAM-1 and VCAM-1 on human umbilical vein endothelial cells and that Der p 2 moderately upregulated ICAM-1 but not ICAM-2 (Mastrandrea et al. 2003). Likewise, molecules in an extract of the related mite Sarcoptes scabiei (De Geer) can influence the expression of VCAM-1, E-selectin, CXCR-1, and CXCR-2 on the surface of dermal endothelial cells and the secretion of IL-6 and IL-8 by these same cells (Elder et al. 2006).
In this study, we investigated the effect of D. farinae, D. pteronyssinus, and E. maynei on human microvascular endothelial cells because these cells regulate the extravasation of inflammatory and immune cells and permeability of fluids (leakage and edema) into the dermis. The important finding is that molecules from D. farinae, D. pteronyssinus, and E. maynei extracts directly induced dose- and time-dependent expression of the adhesion molecules ICAM-1, VCAM-1, and E-selectin by normal human endothelial cells. Likewise, D. farinae, D. pteronyssinus, and E. maynei extracts induced secretion of IL-6, IL-8, MCP-1, and GM-CSF. IL-6 is a general promoter of inflammation and activates other cell types in the skin. IL-8 is chemotaxic for neutrophils. MCP-1 (CCL2) functions include attracting monocytes and eosinophils (Dunzen-dorfer et al. 2001) and provoking the aggregation of mast cells (Conti et al. 1995). GM-CSF locally may activate leukocytes at sites of inflammation. Therefore, these mites are the source of molecules that promote inflammation by stimulation of skin endothelial cells.
Human dermal microvascular endothelial cells express toll-like receptors (TLRs) (Faure et al. 2000, Henneke and Golenbock 2002, Fitzner et al. 2008). These cells constitutively express TLR4 that is activated by LPS and LPS may stimulate increased expression of TLR2 that is not normally constitutively expressed by these endothelial cells (Henneke and Golenbock 2002). Previous studies have shown that extracts of D. farinae and D. pteronyssinus mites contain endotoxins, D. farinae much more so than D. pteronyssinus (Michel et al. 1996, Trivedi et al. 2003, Valerio et al. 2005) and house dust mite extracts elicit TLR-dependent dendritic cell responses (Boasen et al. 2005). It was possible that endotoxins in the mite extracts activated TLRs (particularly TLR4) and this induced the upregulated expression of cellular adhesion molecules and secreted cytokines that was observed. Therefore, we preincubated our D. farinae, D. pteronyssinus, and E. maynei extracts with polymyxin B to bind and inactivate the LPS in these extracts (Cardoso et al. 2007, Fitzner et al. 2008). However, using LPS-depleted extracts, our current studies showed that the effect induced by the mite extracts was not entirely induced by LPS in the extracts. In this study, LPS alone induced the secretion of IL-6, IL-8, G-CSF, GM-CSF, and MCP-1, and polymyxin B inhibited this LPS-induced effect. Extracts of D. farinae, D. pteronyssinus, and E. maynei also induced secretion of IL-6, IL-8, G-CSF, GM-CSF, and MCP-1. Cells stimulated with D. farinae, D. pteronyssinus, or E. maynei in the presence of polymyxin B to inactivate endotoxin continued to secrete only constitutive levels of IL-6, G-CSF, and GM-CSF. However, D. farinae and E. maynei extracts treated with polymyxin B still secreted elevated levels of IL-8 and MCP-1, whereas only E. maynei--stimulated cells expressed ICAM-1, VCAM-1, and E-selectin at elevated levels. Taken together, these results indicate that these house dust mites induced effects on endothelial cells that were not entirely induced by endotoxin acting through TLR4 and therefore there are molecules in mite extracts that must induce their effects by other receptors and signaling pathways.
It is interesting that extracts of D. farinae, D. pteronyssinus, and E. maynei did not stimulate endothelial cells to the same degree despite the fact the extracts were formulated identically and that equal protein doses were tested. Overall, D. farinae extract had greater stimulating ability than extracts of D. pteronyssinus or E. maynei, but in some cases, E. maynei had greater stimulating ability than D. pteronyssinus. Although the mechanism for this is unknown, it suggests that extracts from the different species may have different molecules in them or that they have different concentration/amounts of similar molecules that can modulate cytokine and adhesion molecule expression. Our D. farinae and E. maynei extracts contained higher levels of endotoxin than D. pteronyssinus and this may be a contributing factor.
The reaction by endothelial cells to house dust mites is likely caused by bioreactive molecules present in these extracts that are both allergenic and nonallergenic. In some cases, the bioreactive molecules may be enzymes. Any of these molecules may influence endothelial cell function as well as many other cells in the skin and the blood that are involved in inflammation and immune reactions. Previous studies using lung epithelial cells indicate that allergenic enzymes of dust mites can exert an effect by activation of PARs (Reed and Kita 2004). Likewise, endothelial cells may express PARs that are cleaved and activated by enzymes in an extract of house dust mites. Although house dust mites are the source of some protease allergens, we have not previously detected serine protease activity in our dust mite extracts (Morgan and Arlian 2006). When we attempted to inhibit the proteases in the extracts used here, the expression of cellular adhesion molecules and secretion of cytokines was not reduced compared with stimulation with mite extracts alone. These results suggested that D. farinae, D. pteronyssinus, and E. maynei modulate endothelial cells by serine and cysteine protease-independent mechanisms and not by proteolytic cleavage of PARs.
The processes by which dermal endothelial cells regulate cell extravasation is mediated by cytokines and chemokines from other cells in the vicinity (e.g., keratinocytes, fibroblasts, and macrophages). Inflammation in the skin involves the participation of many different cell types. We previously showed that dust and storage mites induce keratinocytes and fibroblasts to release multiple proinflammatory cytokines, some of which are known to stimulate endothelial cells (Arlian et al. 2008). D. farinae, D. pteronyssinus, and E. maynei can induce secretion of growth-related oncoprotein-α (GROα) from normal human epidermal keratinocytes and IL-8 from normal dermal fibroblasts. D. farinae can also induce IL-6, MCP-1, and M-CSF secretion from fibroblasts, whereas D. pteronyssinus induces M-CSF secretion (Arlian et al. 2008). Thus, in vivo, there is simultaneous co-stimulation of many cell types. How the multiple mite-induced effects from fibroblasts, keratinocytes, and endothelial cells collectively modulate and orchestrate the response to these mites in vivo in the skin is not clear. In vivo, the effects of mites on several other cell types along with the direct effects of mites on endothelial cells could together orchestrate endothelial cell function. In the culture system used here, we could not determine the effects contributed by other cell types that are also stimulated by house dust mite extract.
In this study, we showed that D. farinae, D. pteronyssinus, and E. maynei extracts contain molecules that are potent stimulators of skin endothelial cells. Some of these effects are likely caused by endotoxin present in the extracts and mediated through TLR4, whereas the mechanisms responsible for the other responses are not yet clear. It does not seem that the effects of these mite extracts are Significantly mediated through PARs.
The expression of cellular adhesion molecules and release of cytokines and chemokines induced by D. farinae, D. pteronyssinus, and E. maynei have significant implications in understanding the immunopathology of allergic reactions associated with atopic dermatitis and local inflammation in the skin. This study and our previous work (Arlian et al. 2008) clearly show that house dust mites can provide exogenous inflammatory stimuli in the skin to fibroblasts, keratinocytes, and endothelial cells, and these cells can produce a milieu of proinflammatory cytokines or express cellular adhesion molecules in response to mite molecules. Although these were in vitro studies, our results suggest that these mites can induce a similar response in vivo and contribute to inflammation in the skin should molecules penetrate the epidermis and dermis.
Acknowledgments
This research was partially supported by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Disease (AI-017252) to L.G.A. We thank D. L. Vyszenski-Moher for culturing the mites.
References Cited
- Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Neal JS. Modulation of cytokine expression in human keratinocytes and fibroblasts by extracts of scabies mites. Am J Trop Med Hyg. 2003;69:652–656. [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Peterson KT. House dust and storage mite extracts influence skin keratinocyte and fibroblast function. Int Arch Allergy Immunol. 2008;145:33–42. doi: 10.1159/000107464. [DOI] [PubMed] [Google Scholar]
- Boasen J, Chisholm D, Lebet L, Akira S, Horner AA. House dust extracts elicit Toll-like receptor-dependent dendritic cell responses. J Allergy Clin Immunol. 2005;116:185–191. doi: 10.1016/j.jaci.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cardoso LS, Araujo MI, Goes AM, Pacifico LG, Oliveira RR, Oliveira SC. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb Cell Fact. 2007;6:1. doi: 10.1186/1475-2859-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Lee CT, Liu YC, Wang JY, Lei HY, Yu CK. House dust mite Dermatophagoides farinae augments proinflammatory mediator productions and accessory function of alveolar macrophages: implications for allergic sensitization and inflammation. J Immunol. 2003;170:528–536. doi: 10.4049/jimmunol.170.1.528. [DOI] [PubMed] [Google Scholar]
- Conti P, Boucher W, Letourneau R, Feliciani C, Reale M, Barbacane RC, Vlagopoulos P, Bruneau G, Thibault J, Theoharides TC. Monocyte chemotactic protein-1 provokes mast cell aggregation and [3H]5HT release. Immunology. 1995;86:434–440. [PMC free article] [PubMed] [Google Scholar]
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Darsow U, Laifaoui J, Kerschenlohr K, Wollenberg A, Przybilla B, Wuthrich B, Borelli S, Jr, Giusti F, Seidenari S, Drzimalla K, et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy. 2004;59:1318–1325. doi: 10.1111/j.1398-9995.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Deleuran M, Ellingsen AR, Paludan K, Schou C, Thestrup-Pedersen K. Purified Der p1 and p2 patch tests in patients with atopic dermatitis: evidence for both allergenicity and proteolytic irritancy. Acta Derm Venereol. 1998;78:241–243. doi: 10.1080/000155598441783. [DOI] [PubMed] [Google Scholar]
- Dunzendorfer S, Kaneider NC, Kaser A, Woell E, Frade JM, Mellado M, Martinez-Alonso C, Wiedermann CJ. Functional expression of chemokine receptor 2 by normal human eosinophils. J Allergy Clin Immunol. 2001;108:581–587. doi: 10.1067/mai.2001.118518. [DOI] [PubMed] [Google Scholar]
- Elder BL, Arlian LG, Morgan MS. Sarcoptes scabiei (Acari: Sarcoptidae) mite extract modulates expression of cytokines and adhesion molecules by human dermal microvascular endothelial cells. J Med Entomol. 2006;43:910–915. doi: 10.1603/0022-2585(2006)43[910:ssasme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- Fitzner N, Clauberg S, Essmann F, Liebmann J, Kolb-Bachofen V. Human skin endothelial cells can express all 10 TLR genes and respond to respective ligands. Clin Vaccine Immunol. 2008;15:138–146. doi: 10.1128/CVI.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuiano N, Incorvaia C, Prodam F, Procaccini DA, Bona G. Relationship between the atopy patch test and clinical expression of the disease in children with atopic eczema/dermatitis syndrome and respiratory symptoms. Ann Allergy Asthma Immunol. 2008;101:174–178. doi: 10.1016/S1081-1206(10)60206-2. [DOI] [PubMed] [Google Scholar]
- Henneke P, Golenbock DT. Innate immune recognition of lipopolysaccharide by endothelial cells. Crit Care Med. 2002;30:S207–S213. doi: 10.1097/00003246-200205001-00006. [DOI] [PubMed] [Google Scholar]
- Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, Robinson C. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995;12:369–378. doi: 10.1165/ajrcmb.12.4.7695916. [DOI] [PubMed] [Google Scholar]
- Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182:1537–1544. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss-Marp J, Eberlein-Konig B, Breuer K, Mair S, Ansel A, Darsow U, Kramer U, Mayer E, Ring J, Behrendt H. Influence of short-term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clin Exp Allergy. 2006;36:338–345. doi: 10.1111/j.1365-2222.2006.02448.x. [DOI] [PubMed] [Google Scholar]
- Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 2006;4:5. doi: 10.1186/1476-7961-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161:3645–3651. [PubMed] [Google Scholar]
- Knight DA, Asokananthan N, Watkins DN, Misso NL, Thompson PJ, Stewart GA. Oncostatin M synergises with house dust mite proteases to induce the production of PGE(2) from cultured lung epithelial cells. Br J Pharmacol. 2000;131:465–472. doi: 10.1038/sj.bjp.0703612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim IS, Ryu JS, Yun CY. House dust mite, Dermatophagoides pteronyssinus, increases expression of MCP-1, IL-6, and IL-8 in human monocytic THP-1 cells. Cytokine. 2008;42:365–371. doi: 10.1016/j.cyto.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Machado DC, Horton D, Harrop R, Peachell PT, Helm BA. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur J Immunol. 1996;26:2972–2980. doi: 10.1002/eji.1830261224. [DOI] [PubMed] [Google Scholar]
- Mascia F, Mariani V, Giannetti A, Girolomoni G, Pastore S. House dust mite allergen exerts no direct proinflammatory effects on human keratinocytes. J Allergy Clin Immunol. 2002;109:532–538. doi: 10.1067/mai.2002.121830. [DOI] [PubMed] [Google Scholar]
- Mastrandrea F, Nicotra MR, De Vita L, Coradduzza G, Minardi A, Scarcia G, Manelli M, Cadario G, Parmiani S, Natali PG. Mite antigens enhance ICAM-1 and induce VCAM-1 expression on human umbilical vein endothelium. Allergol Immunopathol (Madr) 2003;31:259–264. doi: 10.1016/s0301-0546(03)79193-9. [DOI] [PubMed] [Google Scholar]
- Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;154:1641–1646. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- Miike S, Kita H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol. 2003;111:704–713. doi: 10.1067/mai.2003.1332. [DOI] [PubMed] [Google Scholar]
- Morgan MS, Arlian LG. Enzymatic activity in extracts of allergy-causing astigmatid mites. J Med Entomol. 2006;43:1200–1207. doi: 10.1603/0022-2585(2006)43[1200:eaieoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Naspitz CK, Diniz C, Candida Rizzo M, Fernandez-Caldas E, Sole D. Human scalps as a reservoir of domestic mites. Lancet. 1997;349:404. doi: 10.1016/s0140-6736(97)80026-8. [DOI] [PubMed] [Google Scholar]
- Neal JS, Arlian LG, Morgan MS. Relationship among house-dust mites, Der 1, Fel d 1, and Can f 1 on clothing and automobile seats with respect to densities in houses. Ann Allergy Asthma Immunol. 2002;88:410–415. doi: 10.1016/S1081-1206(10)62373-3. [DOI] [PubMed] [Google Scholar]
- Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Riley G, Siebers R, Rains N, Crane J, Fitzharris P. House-dust mite antigen on skin and sheets. Lancet. 1998;351:649–650. doi: 10.1016/S0140-6736(05)78433-6. [DOI] [PubMed] [Google Scholar]
- Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med. 1998;187:271–275. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–316. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Shichijo M, Hiramatsu K, Takeuchi M, Nagai H, Takagi K. Mite antigen-induced IL-4 and IL-13 production by basophils derived from atopic asthma patients. Clin Exp Allergy. 1998;28:497–503. doi: 10.1046/j.1365-2222.1998.00267.x. [DOI] [PubMed] [Google Scholar]
- Siebers RW, Rains N, Fitzharris P, Crane J. House dust mite allergen (Der p 1) in human hair. J Allergy Clin Immunol. 1998;101:421–422. doi: 10.1016/S0091-6749(98)70257-X. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Lee KE, Kim KW, Kim ES, Park JY, Kim KE. Calcium-calmodulin mediates house dust mite-induced ERK activation and IL-8 production in human respiratory epithelial cells. Respiration. 2007;74:447–453. doi: 10.1159/000099264. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126:1705–1718. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- Stewart GA, Boyd SM, Bird CH, Krska KD, Kollinger MR, Thompson PJ. Immunobiology of the serine protease allergens from house dust mites. Am J Ind Med. 1994;25:105–107. doi: 10.1002/ajim.4700250128. [DOI] [PubMed] [Google Scholar]
- Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Heinrich TK, Smith WA, Hales BJ. Pyroglyphid house dust mite allergens. Protein Pept Lett. 2007;14:943–953. doi: 10.2174/092986607782541169. [DOI] [PubMed] [Google Scholar]
- Tomee JF, van Weissenbruch R, deMonchy JG, Kauffman HF. Interactions between inhalant allergen extracts and airway epithelial cells: effect on cytokine production and cell detachment. J Allergy Clin Immunol. 1998;102:75–85. doi: 10.1016/s0091-6749(98)70057-0. [DOI] [PubMed] [Google Scholar]
- Trivedi B, Valerio C, Slater JE. Endotoxin content of standardized allergen vaccines. J Allergy Clin Immunol. 2003;111:777–783. doi: 10.1067/mai.2003.1338. [DOI] [PubMed] [Google Scholar]
- Valerio CR, Murray P, Arlian LG, Slater JE. Bacterial 16S ribosomal DNA in house dust mite cultures. J Allergy Clin Immunol. 2005;116:1296–1300. doi: 10.1016/j.jaci.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Wong CK, Li ML, Wang CB, Ip WK, Tian YP, Lam CW. House dust mite allergen Der p 1 elevates the release of inflammatory cytokines and expression of adhesion molecules in co-culture of human eosinophils and bronchial epithelial cells. Int Immunol. 2006;18:1327–1335. doi: 10.1093/intimm/dxl065. [DOI] [PubMed] [Google Scholar]
- Yasueda H, Saito A, Nishioka K, Kutsuwada K, Akiyama K. Measurement of Dermatophagoides mite allergens on bedding and human skin surfaces. Clin Exp Allergy. 2003;33:1654–1658. doi: 10.1111/j.1365-2222.2003.01820.x. [DOI] [PubMed] [Google Scholar]
- Yokote K, Morisaki N, Zenibayashi M, Ueda S, Kanzaki T, Saito Y, Yoshida S. The phospholipase-A2 reaction leads to increased monocyte adhesion of endothelial cells via the expression of adhesion molecules. Eur J Biochem. 1993;217:723–729. doi: 10.1111/j.1432-1033.1993.tb18298.x. [DOI] [PubMed] [Google Scholar]