Abstract
Dysfunction of the serotonin 1A receptor (5-HT1A) may play a role in the genesis of major depressive disorder (MDD). Here we review the pharmacological, post-mortem, positron-emission tomography (PET), and genetic evidence in support of this statement. We also touch briefly on two MDD-associated phenotypes, cognitive impairment and somatic pain. The results of pharmacological challenge studies with 5-HT1A receptor agonists are indicative of blunted endocrine responses in depressed patients. Lithium, valproate, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and other treatment, such as electroconvulsive shock therapy (ECT), all increase post-synaptic 5-HT1A receptor signaling through either direct or indirect effects. Reduced somatodendritic and postsynaptic 5-HT1A receptor numbers or affinity have been reported in some post-mortem studies of suicide victims, a result consistent with well-replicated PET analyses demonstrating reduced 5-HT1A receptor binding potential in diverse regions such as the dorsal raphe, medial prefrontal cortex (mPFC), amygdala and hippocampus. 5-HT1A receptor knockout (KO) mice display increased anxiety-related behavior, which, unlike in their wild-type counterparts, cannot be rescued with AD treatment. In humans, the G allele of a single nucleotide polymorphism (SNP) in the 5-HT1A receptor gene (HTR1A; rs6295), which abrogates a transcription factor binding site for Deaf-1 and Hes5, has been reported to be over-represented in MDD cases. Conversely, the C allele has been associated with better response to AD drugs. We raise the possibility that 5-HT1A receptor dysfunction represents one potential mechanism underpinning MDD and other stress-related disorders.
Introduction
The decreased levels of serotonin metabolites in cerebrospinal fluid (CSF), coupled with the mood-lowering effects of tryptophan depletion and the efficacy of serotonin-modulating antidepressants, have lent support to the notion that a dysfunctional serotonergic system is a vulnerability factor for major depressive disorder (MDD; or “unipolar depression”) and other forms of affective illness (Jans et al 2006).
At least 14 different serotonin receptors have been identified (Hoyer et al 2002). These receptors can be divided into distinct families - denoted, 1, 2, 3, 4, 5, 6 and 7, with subtypes in each family denoted by letters such as a, b, and c. A number of these receptors may play a role in the genesis of psychiatric illness. For example, polymorphisms of the HTR2A gene [Entrez ID 3356], which codes for the 5-HT2A receptor, have been associated with major depressive illness (MDD) (Christiansen et al 2007) and the efficacy of antidepressant drug (AD) treatment (McMahon et al 2006). In addition, variants of the HTR3A gene [Entrez ID 3359], which codes for the A subunit of the 5-HT3 receptor, have been associated with bipolar disorder and schizophrenia (Niesler et al 2008). This study will, however, focus on one of the best characterized of these receptors, the serotonin 1A receptor (5-HT1A), which is a key mediator of serotonergic signaling in the central nervous system..
Central serotonergic neurons are located in the raphe nuclei in the brain stem (Jans et al 2006). The 5-HT1A receptor is a G-protein-coupled receptor widely distributed in regions that receive serotonergic input from the raphe nuclei: the frontal cortex, septum, amygdala, hippocampus, and hypothalamus (Lesch and Gutknecht 2004) (Sharp et al 2007). In these cortico-limbic regions 5-HT1A is distributed post-synaptically (Celada et al 2004) (Sharp et al 2007). The 5-HT1A receptor also serves as the predominant (somatodendritic) autoreceptor of the raphe nuclei, reducing the firing rate of these neurons, the amount of serotonin released per action potential, and the synthesis of the neurotransmitter; and thus by implication, the serotonergic activity of its projection areas (Wang and Aghajanian 1977) (Verge et al 1985) (Sprouse and Aghajanian 1986) (Blier and de Montigny 1987) (Hutson et al 1989) (Meller et al 1990) (Hjorth and Sharp 1991) (Kreiss and Lucki 1994). Activation of the somatodendritic (i.e. presynaptic) 5-HT1A receptors may also indirectly reduce serotonergic transmission through the inhibition of tyrosine hydroxylase synthesis [reviewed in (Drevets et al 2007)], as well as an excitatory, glutamatergic pathway that originates in the medial prefrontal cortex (PFC) and projects to the raphe nuclei (Sharp et al 2007).
The 5-HT1A receptor not only contributes not only to the dynamic modulation of serotonergic activity impacting diverse functions such as cognition and emotion, but is thought to play a crucial role in the neuronal migration, neurite outgrowth and synapse formation inherent to the neurodevelopmental process (Whitaker-Azmitia et al 1996). The 5-HT1A receptor is therefore a natural candidate for impacting an array of phenotypes, among them affective disorders.
There are four strands of evidence implicating the 5-HT1A receptor in affective illness, especially MDD: pharmacological challenge studies; post-mortem studies; neuroreceptor imaging, and genetic analyses. We will discuss each of these in turn.
Pharmacological Challenge Studies
The serotonin system has a facilitatory influence on cortisol, adrenocorticotrophic hormone (ACTH) and prolactin release. It also plays a role in the regulation of body temperature, and therefore a number of studies have evaluated 5-HT1A receptor function in depression through the administration of 5-HT1A agonists which elicit these endocrine and hypothermic responses. The data are difficult to interpret because known 5-HT1A agonists and antagonists bind to both somatodendritic and post-synaptic 5-HT1A receptor types (see page 10 for a more detailed discussion).
(Young et al 1992) has argued that 5-HT1A agonist temperature changes are a better model of somatodendritic than postsynaptic 5-HT1A function because hypothermic responses have been shown to be precipitated by the injection of the 5-HT1A agonist 5-OH-DPAT into the dorsal raphe. (Cowen et al 1994) reported that buspirone (which behaves as a full agonist at the somatodendritic 5-HT1A autoreceptor, but a partial agonist at post-synaptic receptors) reduced hypothermic responses, but not growth hormone or adrenocorticotrophic hormone (ACTH) levels in their depressed sample, a result the authors attribute to a desensitization of the 5-HT1A autoreceptor, but not the post-synaptic receptor. On the other hand, (Blier et al 2002) reject the notion that buspirone-induced hypothermia is purely a measure of 5-HT1A autoreceptor function, and ascribe the effects of the drug to enhanced post-synaptic receptor activation.
There is greater consensus that the endocrine and ACTH release that follows 5-HT1A receptor agonist administration is indicative of postsynaptic 5-HT1A receptor signaling (Van de Kar et al 1985) (Pan and Gilbert 1992) (Cowen 2000) (Blier et al 2002). Ipsapirone, a partial agonist at both somatodendritic and post-synaptic 5-HT1A receptors, was found to produce attenuated hormonal or temperature responses in depressed patients, possibly indicative of decreased sensitivity of the post-synaptic 5-HT1A receptor (Lesch et al 1990) (Meltzer and Maes 1995) (Shapira et al 2000) (Riedel et al 2002). Depressed patients with a history of suicidal behavior were shown to exhibit a blunted hormonal and temperature response to flesinoxan, a full pre- and post-synaptic 5-HT1A receptor agonist (Pitchot et al 2005). Additionally, injection of flesinoxan into the amygdala and hippocampus has been shown to ameliorate prototypical stress-related behavior in rats, suggesting that the anxiolytic effects of the drug are mediated by the post-synaptic 5-HT1A receptor (Li et al 2006). This hypothesis is supported by the finding that rats exposed to prenatal stress show decreased 8-OH-DPAT binding in the hippocampus compared with non-stressed controls (Griffin et al 2005).
However, some studies measuring hormonal responses to buspirone (Navines et al 2007)or ipsapirone (Price et al 1997) (Shiah et al 1998) have questioned whether the post-synaptic 5-HT1A receptor is truly desensitized in depression. Other studies have hypothesized that the somatodendritic 5-HT1A receptor mediates the serotonergic response to challenge studies. The submissive and defensive behavior characteristic of socially-defeated Syrian hamsters was shown to be attenuated by injection of the agonist flesinoxon into the dorsal raphe before the animal is subjected to a social defeat, while injection of the antagonist WAY 100635 into the dorsal raphe enhanced defeat-related conditioning (Cooper et al 2008). Similarly, earlier studies had reported that injection of the 5-HT1A receptor agonist 8-OH-DPAT into the dorsal raphe (but also the ventral hippocampus) had alleviated the anxiety of rats in a maze task (File and Gonzalez 1996) and reduced the efficacy of fear conditioning using inescapable shock (Maier et al 1995) (Jolas et al 1995).
In addition to the lack of specificity of 5-HT1A receptor agonists or antagonists for somatodendritic versus post-synaptic 5-HT1A receptors - a phenomenon influenced by the dose of the ligand administered as well as the intrinsic properties of the compound (Ogren et al 2008) - subtype of depression may be a confounding variable (Navines et al 2007). Melancholic patients are thought to suffer from a greater dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis than their non-melancholic counterparts (Barden 2004). (Robinson et al 1990) found that MDD patients with melancholia responded better to treatment with buspirone than patients with other subtypes of depression. A similar finding has been obtained in a trial of ipsapirone (Lapierre et al 1998). Further, a functional genetic variant of the HTR1A gene, which codes for the 5-HT1A receptor, was recently reported to be associated with antidepressant treatment response in melancholic MDD but not non-melancholic MDD patients (Baune et al 2008). In another study, however, plasma and cortisol response to buspirone reportedly failed to differentiate melancholic individuals from other MDD patients (Meltzer and Maes 1994), while gepirone, a somatodendritic and post-synaptic 5-HT1A receptor partial agonist, was reportedly ineffective in alleviating depressive symptoms in a melancholic MDD sample (Amsterdam 1992).
The potential impact of HPA axis dysregulation on 5-HT1A receptor function is illustrated by animal (Young et al 1992) (Young et al 1994a) (Laaris et al 1999) (Lanfumey et al 1999) (McAllister-Williams et al 2001) (Fairchild et al 2003) (Leitch et al 2003) (Hensler et al 2007) and human (Young et al 1994b) (McAllister-Williams et al 2007) studies which have demonstrated that the physiological effects of 5-HT1A receptor agonists are attenuated after chronic treatment with glucocorticoids. These data are interpreted by the authors of the above studies to be suggestive of down-regulation or desensitization of the somatodendritic 5-HT1A receptor.
Other studies have shown that postsynaptic 5-HT1A receptors are also impacted by stress or corticosteroids. 5-HT1A receptors in the rodent hippocampus (Chalmers et al 1994) (Lopez et al 1998) (Karten et al 1999) (van Riel et al 2003) (van Riel et al 2004) frontal cortex (Watanabe et al 1993), and basolateral anterior, basolateral ventral and basomedial amygdaloid nuclei as well as the hypothalamus (Vicentic et al 2006) are desensitized or down-regulated after chronic stress or corticosterone administration. In addition, tree shrews, which show a “primate-like” distribution of glucocorticoid receptors, exposed to psychosocial stress show a decrease in 5-HT1A receptor numbers in the posterior cingulate cortex, parietal cortex, hippocampus, and PFC (Flugge 1995) (Flugge et al 1998). These stress effects are prevented by adrenalectomy (Chalmers et al 1994) and suggest that the hypothetical dysregulation of both somatodendritic and post-synaptic 5-HT1A receptor function is intimately linked to stress.
Exposure to a stressor increases secretion of cortisol partly through the action of the post-synaptic 5-HT1A receptors of raphe -originating serotonergic fibres which terminate in the corticotrophin-releasing hormone (CRH)-producing neurons of the hypothalamus (Raap and Van de Kar 1999) (Lesch and Gutknecht 2004) (Schule 2007). Cortisol binds to two different receptor types found in multiple brain regions such as the hippocampus, amygdala, mPFC, and hypothalamus. High-affinity and occupancy mineralocorticoid receptors (MR) tonically inhibit serotonergic neurotransmission, while lower-affinity and occupancy glucocorticoid receptors (GR) are activated only by rising cortisol levels (De Kloet et al 1998). Cortisol-induced activation of GR receptors results in the formation of GR-MR dimers which are transported to the nucleus where they bind to glucocorticoid response elements (GREs) on the 5-HT1A gene promoter, inhibiting gene expression (Flugge 1995) (Lopez et al 1998) (Ou et al 2001).
The possibility that glucocorticoids might attenuate somatodendritic as well as post-synaptic 5-HT1A receptor function is interesting as GR have traditionally not been viewed as playing an important role in the modulation of raphe activity. The distribution of the GR receptor in the brain is not entirely clear. In two studies, the GR receptor gene was found to be highly expressed in the PFC and limbic region of rats but less so in the raphe (Reul and de Kloet 1985) (Sah et al 2005). On the other hand, (Harfstrand et al 1986) reported significant levels of GR immunoreactivity in the dorsal raphe, a result echoed by (Morimoto et al 1996) who found high densities of the GR in the dorsal raphe as well as cerebral cortex, limbic structures and cerebellum in the rat. Further, moderate to high immunoreactivity of the GR in the raphe of neonatal rats was detected by (Cintra et al 1991).
Notably, lithium and valproate may exert part of their therapeutic effect by attenuating GR signaling through the up-regulation of BCL2-associated athanogene (BAG1: Entrez Gene ID, 573) expression. BAG1 inhibits the chaperone protein, HSP70, which facilitates the translocation of the GR-MR complex to the nucleus after cortisol binding (Zhou et al 2005). In line with this hypothesis, we recently showed that lithium and valproate increase baseline levels of 5-HT1A receptor binding in the parieto-occipital cortex, anterior cingulate, and anterior temporopolar cortex of bipolar depressives (Carlson et al 2007).
Most antidepressant drugs (AD), in contrast, appear to exert their therapeutic effects via a more direct mechanism. Several weeks of treatment with selective serotonin reuptake inhibitors (SSRIs) and monoamine oxidase inhibitors (MAOIs) leads to desensitization of the raphe 5-HT1A autoreceptors (but not cortical or limbic post-synaptic receptors), thereby resulting in a stable upturn in post-synaptic serotonergic transmission (Blier et al 1987) (Chaput et al 1991) (Invernizzi et al 1994) (Stahl 1994) (Kreiss and Lucki 1995) (Lerer et al 1999) (Pineyro and Blier 1999) (Riad et al 2004) (Giovacchini et al 2005) (Rausch et al 2006). Moreover, the desensitizing effect of fluoxetine on somatodendritic receptors is reportedly blocked by concurrent administration of the 5-HT1A receptor antagonist WAY100635, this despite the fact that WAY100635 had no effect on 5-HT1A somatodendritic receptor sensitivity in the absence of fluoxetine (Hervas et al 2001) (Castro et al 2008).
Nevertheless, a recent study has questioned the homogeneity of therapeutic mechanism within the SSRI class of drugs. Although both fluoxetine and sertraline reduce the binding of 5-HT1A agonists (presumably indicative of receptor desensitization) in the raphe, chronic treatment with fluoxetine (Hensler 2002) (Pejchal et al 2002) (Shen et al 2002) (Elena Castro et al 2003) but not sertraline (Rossi et al 2008) limits the capacity of somatodendritic autoreceptors to activate G proteins.
Tricyclic antidepressants (TCAs) have been hypothesized to act via a distinct mechanism to that of SSRIs, enhancing tonic serotonergic transmission by facilitating the activation of G proteins by the postsynaptic 5-HT1A receptor, thereby increasing the sensitivity of the post-synaptic receptor rather than desensitizing the 5-HT1A somatodendritic autoreceptor (Gravel and de Montigny 1987) (Chaput et al 1991) (Blier and Bouchard 1994) (Rossi et al 2006).
The increased sensitivity of post-synaptic 5-HT1A receptors has also been shown to occur in limbic structures such as the hippocampus following repeated administration of electroconvulsive shock (ECS) (Hayakawa et al 1994) (Ishihara et al 1999) or the selective norepinephrine reuptake inhibitor reboxetine (Szabo and Blier 2001).
Long-term treatment with 5-HT1A receptor agonists such as buspirone have been reported to exert modest antidepressant and anxiolytic effects in both animal and human studies, possibly through a combination of their desensitizing effects on 5-HT1A autoreceptors and their activation of normally sensitive post-synaptic receptors (Lucki 1991) (Detke et al 1995) (Kreiss and Lucki 1997) (Sussman 1998) (Blier and Ward 2003). Further, the 5-HT1A autoreceptor antagonist pindolol has been reported to accelerate SSRI-driven AD response by blocking the initial negative feedback mediated by increased raphe 5-HT1A autoreceptor binding (Artigas et al 1994) (Zanardi et al 1997) (Zanardi et al 1998) (Hjorth et al 2000) (Zanardi et al 2001) (Berney et al 2008). Nevertheless, a number of clinical trials have questioned the clinical utility of both buspirone and pindolol (Berman et al 1997) (Berman et al 1999) (Perry et al 2004) which appear to show more promise as augmentation agents than first-line treatments (Trivedi et al 2006) (Shelton 2007) (Thase et al 2007) (Geretsegger et al 2008).
Nonetheless, the importance of the 5-HT1A receptor agonist treatment data for testing the the hypothesis that enhanced post-synaptic 5-HT1A receptor function produces antidepressant effects in depression is limited by two factors. Firstly, the available 5-HT1A agonists are only partial agonists, because full 5-HT1A receptor agonists produce intolerable gastrointestinal side effects in humans. Consequently, such agents have mixed agonist/antagonist effects, and do not exert the full agonist effects of serotonin at post-synaptic 5-HT1A receptors. Thus, under conditions of increased serotonin release, the occupancy of post-synaptic 5-HT1A receptors by a partial agonist may actually reduce 5-HT1A receptor transmission by blocking the direct effects of serotonin while inducing only a partial agonist effect instead.
Secondly, the trans-synaptic nature of the 5-HT1A receptor system complicates the interpretation of clinical responses to 5-HT1A receptor agonists. Such agents stimulate both somatodendritic autoreceptors and post-synaptic 5-HT1A receptors, effects that would exert opposing influences on post-synaptic 5-HT1A receptor transmission. Stimulation of somatodendritic autoreceptors reduces serotonin release and synthesis, resulting in reduced 5-HT transmission. Although desensitization of the somatodendritic autoreceptor in response to 5-HT1A receptor partial agonists (e.g., buspirone) has been demonstrated in rodents, whether this effect fully offsets the effect of stimulating somatodendritic autoreceptors on serotonin release remains unclear. Moreover, the reduction in serotonin transmission is only partly compensated by direct stimulation of post-synaptic 5-HT1A receptors by partial agonists, for the reasons listed above.
A minority of ADs have been hypothesized to impact the 5-HT1A receptor signaling indirectly. For example, nefazodone acts as a selective antagonist at post-synaptic 5-HT2A receptors, blocking these receptors, and thus theoretically shifting the balance of receptor activation in favor of 5-HT1A (Artigas et al 2002). There is a possibility that the selectivenoradrenaline (NA) reuptake inhibitor reboxetine may also modulate 5-HT1A receptor signaling via its effect on noradrenergic neurons of the locus coeruleus which communicate with their serotonergic counterparts in the raphe (Harkin et al 1999) (Szabo and Blier 2001) (Troelsen et al 2005). Further evidence for the interaction between these two systems comes from the study of (Detke et al 1995) who found that the anti-depressant effect of the NA reuptake inhibitor desipramine was reduced by concurrent administration of 5-HT1A receptor antagonists.
In summary, despite potential differences in the specific physiological effects exerted by AD, increased limbic serotonin turnover and post-synaptic 5-HT1A receptor function/transmission appear to be one possible pathway of AD action (Detke et al 1995) (Haddjeri et al 1998). These theoretical changes in 5-HT1A receptor-mediated signaling may lead to a restoration of top-down control over hyperactive limbic nuclei and with it physiological homeostasis (Gartside et al 1995) (Albert et al 1996) (Artigas et al 1996) (Pineyro and Blier 1999) (Albert and Lemonde 2004) (Celada et al 2004).
While changes in neurotransmission have traditionally been invoked as the mechanism of action underlying AD response, psychopharmacology appears to be in the early stages of a paradigm shift driven by evidence of depression or stress-associated neuronal atrophy. New data indicate that psychiatric medication, in particular mood stabilizers (Moore et al 2000) (Manji et al 2000), but also various AD drugs (McEwen and Olie 2005) (Duman and Monteggia 2006), may ameliorate this neuronal atrophy by encouraging cell proliferation (Malberg and Duman 2003), neurogenesis (Malberg et al 2000), and the up-regulation of neurotrophins such as brain-derived neurotrophic factor (BDNF) (Duman and Monteggia 2006).
Interestingly, extant evidence suggests that chronic activation of post-synaptic 5-HT1A receptors may stimulate neurite outgrowth in the hippocampus (Huang and Herbert 2005), although the effect may be dependent on HPA axis function (Huang and Herbert 2006). In earlier studies, (Santarelli et al 2003) had shown that administration of a 5-HT1A receptor agonist increased cell proliferation in the dentate gyrus of wild-type but not 5-HT1A knockout mice. Similarly, (Fricker et al 2005) reported that hippocampal cell survival was enhanced by 5-HT1A receptor agonists; an effect which was blocked by the receptor antagonist WAY-100635. This result supports the study of (Radley and Jacobs 2002) who found that 5-HT1A receptor antagonists were associated with a 30% decline in cell proliferation in the dentate gyrus.
Stimulation of 5-HT1A receptors may also protect against glutamate-induced apoptosis. (Yuen et al 2005) have shown that 5-HT1A receptor activation disturbs microtubule stability, thereby inhibiting the microtubule-associated transport of the N-methyl-D-aspartate (NMDA) receptor to the dendritic surface, and by implication, reducing glutamate signaling. These data may explain the results of previous studies which showed that administration of 5-HT1A receptor agonists rescues neurons from glutamate or ischemia-induced cell death (Nakata et al 1997) (Harkany et al 2001) (Gandolfi et al 2002) (Madhavan et al 2003). (Salazar-Colocho et al 2008) recently postulated that these neuroprotective effects result from reduced 5-HT1A receptor-mediated phosphorylation of the N-methyl-D-aspartate (NMDA) receptor NR1 subunit, as well as increased expression of brain-derived neurotrophic factor (BDNF). The latter finding is certainly consistent with a report of attenuated hippocampal 5-HT1A receptor signaling in BDNF knockout (KO) mice exposed to stress (Hensler et al 2007), as well as a burgeoning literature detailing AD-associated changes in BDNF gene regulation [reviewed in (Martinowich and Lu 2008)].
Post-Mortem Analyses
An early post-mortem study reported reduced 5-HT1A receptor numbers and lowered receptor affinity in the hippocampi and amygdalae of depressed suicide victims, respectively [N=19; 6 medicated with antidepressants (AD) (Cheetham et al 1990)]. Other post-mortem studies have also produced evidence for reduced 5-HT1A receptor gene expression, binding and/or numbers in the ventrolateral prefrontal cortex and the temporal polar cortex [all subjects received AD, tranquilizers or ECT prior to death (Bowen et al 1989)]; caudal aspects of the dorsal raphe nucleus [no subjects medicated at the time of death in the Boldrini et al. study (Arango et al 2001) (Boldrini et al 2008)]; dorsolateral prefrontal cortex (DLPFC) and hippocampus [“subset” of sample on medication at time of death (Lopez-Figueroa et al 2004)], Brodmann area (BA) 10 of the PFC in females but not males [none of whom were medicated with AD based on toxicology (Szewczyk et al 2008)], hippocampus [subjects not “chronically” taking medication at time of death (Lopez et al 1998)], and orbital frontal cortex (OFC) [subjects did not use AD for at least 2 months prior to death (Anisman et al 2008)]. In a similar vein, a small group (n=4) of severely depressed patients, surgically treated by excision of DLPFC tissue in vivo, displayed a 29% reduction in receptor binding in this region of the brain (Francis et al 1993). Most recently, (Albert et al 2008) found evidence for increased methylation of the promoter region of the 5-HT1A receptor gene in the PFC region of MDD subjects who had committed suicide. The increased methylation was interpreted by the authors as being indicative of decreased PFC 5-HT1A receptor expression in MDD.
On the other hand, some post-mortem analyses of depressed suicides have reported increased 5-HT1A ligand binding in the ventrolateral prefrontal cortex [(Arango et al 1995); although this elevation in 5-HT1A receptor binding was later shown to be accounted for by the depressed cases with co-morbid alcoholism in this sample; V. Arango, personal communication]. Increased 5-HT1A receptor binding has also been \noted in BA 8 and 9 of the PFC [(Matsubara et al 1991); although this sample was not psychiatrically characterized], the rostral aspects of the raphe (Boldrini et al 2008), including the ventrolateral and dorsal nuclei [majority of sample medicated before death (Stockmeier et al 1998)], frontal polar cortex (males only) and paraventricular nucleus of the hypothalamus (Anisman et al 2008), and the outermost cortical layers {BA 9, 10, 40 and 46) of a sample of bipolar I subjects [half treated with lithium before death (Gray et al 2006)]. A recent paper presented in abstract form has reported an increase in neurons immunoreactive for the 5-HT1A receptor in BA 10 (rostral orbital frontal cortex) of depressed suicides (Liu et al 2008).
Negative reports have also surfaced in the literature: frontal cortex (BA 9, 10, and 11) [at least some of the sample was medicated before death (Arranz et al 1994)]; amygdala, hippocampus, frontal and occipital cortex [in both AD-treated and AD-free samples (Lowther et al 1997)]; right prefrontal cortex (BA 10) and hippocampus [3 out of 13 subjects taking AD at time of death (Stockmeier et al 1997)]; and DRN, hippocampus and amygdala (Anisman et al 2008).
The effects of medication confound most published studies, and thus treatment differences are unlikely to be the only explanation for the discrepancy in results across studies. Another possibility, at least in the case of the dorsal raphe, is sub-nucleus specific changes. The increase in 5-HT1A receptor binding seen in the suicide samples of (Stockmeier et al 1998) and (Boldrini et al 2008) may be specific to rostral, ventrolateral and dorsal sub-nuclei: the latter group reported lower 5-HT1A receptor binding in the caudal sub-nucleus, making up the pontine portion of the raphe (Boldrini et al 2008). Sex differences, substance abuse, medication use, the psychiatric status of the suicide cases at the time of death, post-mortem interval, and variations in techniques used to measure receptor binding may also be important (Stockmeier et al 1997) (Stockmeier et al 1998) (Boldrini et al 2008). The collection from which the post-mortem samples were obtained may be another source of noise. (Deep-Soboslay et al 2008) point out that in the case of bipolar disorder, the sample characteristics of the two largest collections, the Harvard Brain Tissue Resource Center, and the Stanley Foundation brain collection, differ with respect to age at death and illness onset, number of suicides, tissue pH and post-mortem interval.
Peripheral attention has also been paid to potential abnormalities in 5-HT1A signal transduction. 5-HT1A receptor agonist-induced activation of adenylyl cyclase (AC) as well as phosphatidylinositol 3-kinase and its downstream effector, Akt, was found to be blunted in depressed suicide victims (Hsiung et al 2003). Since the activity of AC has been reported to be inhibited by 5-HT1A receptor binding (Hoyer et al 1994) (Albert et al 1996) (Barnes and Sharp 1999) (Lisinicchia and Watts 2003), the expression of AC and its downstream effector, cyclic-AMP dependent protein kinase A (PKA), may prove to be an indirect marker of 5-HT1A receptor function.
Reduced levels of AC in the temporal (Reiach et al 1999) and occipital (Cowburn et al 1994) cortices, as well as PKA in the PFC and nucleus accumbens (but not the hippocampus), have been recorded in depressed suicide samples (Dwivedi et al 2002) (Dwivedi et al 2004) (Pandey et al 2005). Other studies have, however, returned non-significant results (Dowlatshahi et al 1999) (Gonzalez-Maeso et al 2002). Nevertheless, 5-HT1A receptor-induced inhibition of AC may be a regionally specific phenomenon (Marazziti et al 2002) (Hensler 2003), and, furthermore, stimulation of other receptor types may impact AC activation, rendering the relevance of these data to 5-HT1A function, debatable.
The evidence for reduced 5-HT1A receptor affinity or numbers in the hippocampus and diverse regions of the frontal cortex at post-mortem is congruent with the proposed mechanism of action of TCAs and ECS: facilitating the activation of G proteins at the post-synaptic 5-HT1A receptor. Similarly, the studies of (Stockmeier et al 1998) and (Boldrini et al 2008) that found decreased 5-HT1A receptor numbers in the rostral aspects of the raphe are consistent with the hypothesized mechanism of action of SSRIs such as fluoxetine and sertraline which either desensitize the somatodendritic autoreceptor, or limit the somatodendritic receptor’s capacity to activate G proteins. On the other hand, reports of increased 5-HT1A receptor binding or numbers in regions of the PFC at post-mortem (Arango et al 1995) (Anisman et al 2008) are not consistent with the hypothesis that depression is associated with reduced post-synaptic 5-HT1A receptor signaling. Perhaps a more resilient subset of patients with MDD is able to compensate for the reduction in post-synaptic 5-HT1A receptor signaling. Nevertheless, the fact that the post-mortem samples are largely composed of suicide victims, many of whom suffered from chronic alcoholism, weakens this argument.
Positron Emission Tomography (PET) Studies of Receptor Binding Potential
In an [11C]WAY-100635 5-HT1A receptor antagonist positron emission tomography (PET) study, (Drevets et al 1999) (Drevets et al 2000) showed that unipolar and bipolar depressives with a familial form of illness exhibited reduced binding potential (BP) in the medial temporal cortex and hippocampus (25–33%), as well as the midbrain raphe (42%), compared with healthy controls. This result has been independently replicated: (Sargent et al 2000) reported a wide-spread reduction (frontal, temporal and limbic cortices) in 5-HT1A receptor binding in both medicated and unmedicated individuals with major depressive disorder (MDD), and a follow-up study indicated that this effect holds true for remitted patients (Bhagwagar et al 2004). A similar conclusion was reached by (Hirvonen et al 2008) who reported 9–25% reductions in receptor binding across large regions of the brain (but not the raphe) in drug-naïve individuals with MDD.
A sample of elderly, depressed patients provided further evidence for this effect, showing reduced BP in the dorsal raphe nucleus (Meltzer et al 2004). Moreover, in a mixed sample of women with post-partum MDD and bipolar disorder (BD), (Moses-Kolko et al 2007) reported post-synaptic 5-HT1A receptor binding reductions in the order of 20% in the subgenual and pregenual anterior cingulate, lateral orbital, and mesiotemporal cortices. Mostly recently, (Drevets et al 2007) replicated their previous results (Drevets et al 1999), detecting a mean 5-HT1A receptor BP reduction of 26% in the medial temporal cortex and 43% in the raphe nucleus in non-medicated recurrent depressives.
This pattern of depression-associated 5-HT1A receptor dysregulation is equally salient in non-human primates. PET imaging (using the antagonist [18F]MPPF) of subordinate cynomolgous monkeys, who showed behavioral signs of depression after exposure to social defeat, was indicative of reduced 5-HT1A receptor binding in diverse areas of the brain, including the raphe nuclei, amygdala, hippocampus, and anterior cingulate cortex (Shively et al 2006). Moreover, parentally-deprived monkeys display reduced hippocampal WAY100635 binding, and mRNA expression relative to their normally-reared siblings (Law et al 2008). Nonetheless, 5-HT1A receptor binding was actually increased in the dentate gyrus and CA3 field in males, raising the possibility of gender-stress interactions (Law et al 2008). Reports of reduced 5-HT1A receptor BP are congruent with a non-PET study using the agonist ipsapirone, which reported a long-term desensitization of 5-HT1A autoreceptors in mice exposed to chronic mild stress (Lanfumey et al 1999).
The phenomenon of reduced 5-HT1A BP has been extended to panic disorder [anterior and posterior cingulate cortex and raphe nuclei, irrespective of past history and treatment] (Neumeister et al 2004), [raphe, OFC, temporal cortex and amygdala] (Nash et al 2008), social anxiety disorder [amygdala, anterior cingulate, insula, raphe] (Lanzenberger et al 2007), chronic fatigue syndrome [hippocampus] (Cleare et al 2005), and the depressive symptoms of temporal lobe epilepsy [anterior and posterior cingulate cortex; anterior insula, raphe, right hippocampus and amygdala] (Hasler et al 2007), but not post-traumatic stress disorder (Bonne et al 2005), schizophrenia (Frankle et al 2006), or anorexia nervosa (Bailer et al 2005).
On the other hand, (Parsey et al 2006) recorded higher 5-HT1A receptor BP across all measured brain regions using [11C]WAY-100635 PET in AD naïve, but not AD-exposed patients with MDD. (Parsey et al 2008) recently extended their original finding in an expanded sample. The AD naïve subjects showed a greater—approximately 25%higher—5-HT1A receptor BP in 12 different brain regions, including the raphe, the mPFC, hippocampus, and amygdala. Nevertheless, these data conflict with the recent report by (Hirvonen et al 2008) indicating that AD naïve subjects with MDD have decreased 5-HT1A receptor BP relative to controls (by 9 to 25%) in these and other brain regions.
A sample of patients with panic disorder and social phobia displayed decreased 5-HT1A BP (measured with [11C]WAY-100635) in the subgenual cortex, hippocampus and posterior cingulate cortex after 12 weeks of treatment with escitalopram (Spindelegger et al 2008). Nevertheless, the relevance of these data to MDD is questionable because subjects with a history of co-morbid depression were specifically excluded from the study. Further, the methods applied by (Spindelegger et al 2008) did not allow for assessment of the reference tissue (cerebellum) distribution volume (DV). Further, 5-HT1A receptor specific binding of [11C]WAY100635 is relatively insensitive to changes in intrasynaptic serotonin concentrations (Hume et al 2001). Thus the (Spindelegger et al 2008) finding may not reflect the elevated serotonin transmission expected with SSRI treatment.
In sum, the data suggest that a dysregulation of 5-HT1A receptor-mediated serotonergic activity may constitute one potential common disease pathway for psychiatric disorders associated with chronic hypothalamic-pituitary-adrenal (HPA) axis dysfunction. Since not all patients with MDD display HPA axis abnormalities, illness subtype may contribute to variability in study outcome. For example, the Drevets group participants suffered from familial, primary, recurrent mood disorders and as a group showed abnormally elevated stress plasma cortisol concentrations (Drevets et al 1999), while other studies have included patients with depression secondary to other medical conditions [reviewed in (Drevets et al 2007)]. Genetic factors may also predispose to dysfunction of the 5-HT1A receptor signaling system, and by implication, MDD [see (Drago et al 2007) for a recent review].
The molecular correlates of 5-HT1A receptor BP changes are unclear. Most of the PET studies discussed above have been completed using the potent antagonist, [11C]WAY-100635. Antagonists bind with equal affinity to receptors in both high and low affinity states while agonists bind preferentially to receptors in the high affinity state. Thus the decreased BP seen in many 5-HT1A receptor [11C]WAY-100635 PET analyses of MDD could theoretically be indicative of a reduction in receptor density rather than desensitization of the 5-HT1A receptor.
The PET data, which like the post-mortem data, are largely indicative of decreased 5-HT1A receptor BP in the frontal, temporal and limbic cortices (although see (Parsey et al 2006)), support the hypothesis of a MDD-associated reduction in postsynaptic 5-HT1A receptor signaling. The evidence for reduced 5-HT1A receptor BP in the dorsal raphe (Drevets et al 1999) (Meltzer et al 2004) (Shively et al 2006) (Drevets et al 2007) is not necessarily at odds with the received wisdom that SSRI medications such as fluoxetine normalize postsynaptic 5-HT1A receptor signaling by desensitizing the somatodendritic 5-HT1A receptor. Post-mortem studies have shown that 5-HT1A receptor binding is increased only in the rostral aspects of the dorsal raphe but decreased in the caudal raphe nuclei (Boldrini et al 2008). PET does not possess the necessary resolution to differentiate subnuclei within the raphe and thus the decrease in raphe BP seen in most PET studies is indicative of 5-HT1A receptor function across the entire raphe.
Genetic Studies
Serotonin transporter (SERT) KO mice show behavioral effects akin to depression in humans (Holmes et al 2003) (Lira et al 2003) (Wellman et al 2007). Congruent with the PET imaging data, these SERT KO mice have also been reported to display a reduced density of 5-HT1A receptors in the hypothalamus, amygdala and dorsal raphe nucleus (Li et al 2004). Similarly, an earlier study reported a desensitization of somatodendritic 5-HT1A receptors in the dorsal raphe nucleus, but not post-synaptic receptors of the hippocampus, in SERT KO mice (Mannoury la Cour et al 2001). Further, the depressive behavior and sleep abnormalities of SERT KO mice have been demonstrated to be “durably” normalized by the postnatal administration of a 5-HT1A receptor antagonist which prevented excessive stimulation of the 5-HT1A receptor early in life (Alexandre et al 2006), thus presumably preventing a compensatory desensitization of post-synaptic receptors.
5-HT1A receptor KO mice display reduced exploratory behavior and enhanced reactivity to fear cues; a potential anxious phenotype (Heisler et al 1998) (Parks et al 1998) (Ramboz et al 1998) (Gross et al 2000) (Olivier et al 2001) (Toth 2003) (Klemenhagen et al 2006). In line with these data, (Mayorga et al 2001) showed that fluoxetine and paroxetine fail to ameliorate immobility behavior in 5-HT1A receptor KO mice exposed to a stressor, suggesting that 5-HT1A receptor activation is a necessary feature of the AD response.
A single-nucleotide promoter polymorphism (SNP) found within a 26bp palindromic promoter sequence of the intronless 5-HT1A receptor gene (HTR1A:–1019C/G; rs6295), (Wu and Comings 1999) has been reported to regulate gene expression (Lemonde et al 2003). The G allele putatively disrupts a transcription factor-binding site for two transcription factors: deformed epidermal autoregulatory factor-1 (Deaf-1) [Entrez ID 10522] (sometimes known as NUDR), and hairy and enhancer of split 5 (Drosophila) (Hes5) [Entrez ID 388585]. (Czesak et al 2006) hypothesize that Deaf-1 recruits DNA-binding protein complexes in a cell-specific manner, leading to differential exposure of critical functional domains. More specifically, Deaf-1 shows differential activity at somatodendritic and postsynaptic 5-HT1A receptors. In the raphe, Deaf-1 is inhibitory but acts as an enhancer of transcription in non-serotonergic neurons, that is, neurons expressing postsynaptic 5-HT1A receptors (Le Francois et al 2008). Thus, in the raphe, the G allele up-regulates autoreceptor expression, decreasing the firing rate of these cells, and reducing serotonergic neurotransmission in projection areas (Lemonde et al 2003). Consistent with this hypothesis, the G/G genotype has been associated with an increase in raphe 5-HT1A autoreceptor expression (David et al 2005) (Parsey et al 2006). Moreover, the G allele putatively results in lowered expression of the postsynaptic 5-HT1A receptor because of the abrogation of enhancer function (Czesak et al 2006) (Le Francois et al 2008). This hypothesis is congruent with the post-mortem study of (Szewczyk et al 2008) who reported both decreased 5-HT1A receptor and Deaf-1 protein numbers in the PFC of depressed female suicide victims. The G allele also prevents the binding of Hes5 which represses transcription at both somatodendritic and postsynaptic 5-HT1A receptor expressing neurons (Czesak et al 2006) (Le Francois et al 2008).
Consistent with the rs6295 G allele-associated decrease in postsynaptic 5-HT1A receptor expression, (Lemonde et al 2003) reported a two-fold increase in the frequency of the rs6295 G/G genotype in patients with MDD. The association was accentuated in an independent sample of suicide attempters, with a four-fold increase in the frequency of the G/G genotype. The latter finding, in particular, has attracted some interest in more recent studies. (Parsey et al 2006) partially replicated these data, finding a trend towards over-representation of the G allele in their MDD group, while (Kraus et al 2007) noted that Hepatitis C patients homozygous for the G allele showed an increased incidence and severity of interferon-induced depression. Further, an elderly sample of G allele carriers with hip fractures were reportedly more likely to become depressed than C/C homozygotes (Lenze et al 2008).
A recent paper reported an over-representation of the G allele and G/G genotype in an MDD sample (effect size of 1.10–1.34), and a subsequent linkage analysis conditional on possession of at least one G allele generated a significant signal on chromosome 10, suggesting a possible epistatic interaction with a gene in this region (Neff et al 2008). (Zhang et al 2008) reported that individuals homozygous for the L/L 5-HTTLPR polymorphisms of the SLC6A4 gene who were also homozygous for the rs6295 G allele, and were exposed to negative life events, had an increased odds ratio for MDD. In support of the genetic association data, the rs6295 G/G genotype was found to be associated with greater methylation of the HTR1A promoter (indicative of decreased gene expression) in a post-mortem sample of suicide attempters (Albert et al 2008). Nevertheless, other studies do not indicate that the G allele is over-represented in MDD or suicide attempters (Videtic et al 2006) (Wasserman et al 2006) (Serretti et al 2007).
fMRI studies have begun to shed light on the broader functional correlates of 5-HT1A receptor activity. (Dannlowski et al 2006) report that patients with MDD who carry the G allele of the –1019C/G SNP show hyperactivity of the amygdala in response to emotional stimuli compared to their C/C genotype counterparts. In a similar vein, the G allele was recently reported to be negatively associated with amygdala volume in depressed subjects with borderline personality disorder, but not healthy controls (Zetzsche 2007).
These data are consistent with a report detailing an inverse relationship between 5-HT1Areceptor BP in the raphe, and amygdala reactivity: autoreceptor BP accounted for 30–44% of the variance in amygdala reactivity (Fisher et al 2006). In contrast, post-synaptic receptor BP in the amygdala accounted for only 8% of the variation in amygdala activity (Fisher et al 2006).
The G allele of -1019C/G SNP has also been associated with a variety of other psychiatric phenotypes, including substance abuse (Huang et al 2004), panic disorder (Rothe et al 2004) (Domschke et al 2006), the depression-associated personality trait neuroticism (Strobel et al 2003) and greater autonomic arousal during reward and punishment, a trait putatively correlated with anxiety (Schmitz et al 2008). The polymorphism has also been shown to impact response to pharmacological treatments—although see (Peters et al 2004) and (Levin et al 2007) for negative reports.
Depressed patients homozygous for the C allele have been reported to respond better to fluoxetine than their counterparts (Hong et al 2006) (Yu et al 2006). Furthermore, the antidepressants nefazodone and flibanserin (Lemonde et al 2004), fluvoxamine (Hong et al 2006) (Serretti et al 2004), and citalopram (Arias et al 2005) may be more efficacious in carriers of the C allele. (Arias et al 2005) proposed an epistatic effect: S/S and G/G homozygotes for the serotonin transporter promoter (5-HTTLPR) and rs6295 HTR1A polymorphisms, respectively, were less likely than their counterparts to achieve clinical remission. In another analysis of gene-gene interactions, the rs6295 GG genotype was reported to predispose to treatment resistant depression in individuals who also carried the met allele of the val66met BDNF SNP (Anttila et al 2007). The effect may hold equally true for anti-psychotic medication. The C allele has been reported to be associated with a greater improvement in depressive and negative symptoms in a group of first-episode psychosis patients (Reynolds et al 2006).
The treatment-genotype associations reported above should, however, be interpreted with caution. (Kato et al 2008) detected a putative error in the allele-calling of the rs6295 SNP in the studies of (Arias et al 2005), (Hong et al 2006) and (Yu et al 2006). According to (Kato et al 2008), the G allele and not the C allele, improved response to antidepressant medication in these studies, supporting their finding of greater fluvoxamine, paroxetine, and milnaciplan efficacy in carriers of a 3-SNP hapotype that included the rs6295 G allele. Similarly, C allele carriers with melancholic depression reportedly demonstrated an attenuated response to a variety of AD medication in the sample of (Baune et al 2008).
On the other hand, two studies comparing the frequencies of the –1019C/G alleles in MDD and healthy cases have returned negative results (Huang et al 2004) (Zill P 2001), while another study reported an over-representation of the putatively protective C/C genotype in women with premenstrual dysphoric disorder (Dhingra et al 2007). Most recently, negative results were also obtained in a large sample of 589 MDD cases and 539 controls (Hettema et al 2007).
The fact that the rs6295 G allele both decreases postsynaptic 5-HT1A receptor expression, and increases the risk of developing MDD, is consistent with pharmacological, postmortem and PET data suggesting impaired serotonergic signaling at the postsynaptic 5-HT1A receptor. Nonetheless, one important caveat in the interpretation of the genetic association studies is that genes other than HTR1A might exert a signficant effect on 5-HT1A receptor binding. A quantification of 5-HT1A receptor binding in suicide cases failed to produce a significant association with the –1019C/G polymorphism (Huang et al 2004). Further, (Lesch and Gutknecht 2004) reported that the –1019C/G variant did not predict neuroendocrine responses to a 5-HT1A receptor agonist challenge in healthy individuals. In fact, (David et al 2005) found that the 5-HTTLPR polymorphism of the serotonin transporter gene, but not the –1019C/G HTR1A variant, impacts 5-HT1A receptor binding. Most recently, (Mickey et al 2008) found that a putatively functional variable number tandem repeat (VNTR) polymorphism in the monoamine oxidase A gene (MAOA; Entrez ID 4128) predicted 42–74% of in vivo 5-HT1A receptor availability in the raphe, mPFC, temporal cortex, hippocampus, amygdala and anterior cingulate cortex. Nevertheless, the effect of MAOA genotype on 5-HT1A receptor BP was restricted to women, and the frequency of the MAOA VNTR alleles did not differ significantly between healthy controls and patients with MDD. In fact, the MAOA gene has been not been convincingly associated with MDD in previously published genetic association studies. We raise the possibility that the size of the phenotypic effect exerted by a polymorphism cannot always be equated with the importance of this effect for the development of psychiatric illness.
MDD-Associated Phenotypes
The 5-HT1A receptor has been implicated in cognition and the regulation of pain perception. Since cognitive dysfunction and somatic symptoms are associated with mood disorders (Savitz et al 2005) (Vaccarino et al 2008), understanding the impact of the 5-HT1A receptor on these two phenotypes may have relevance for MDD.
Long-term potentiation (LTP) is believed to be the neurobiological mechanism underlying learning and memory. At least one type of LTP is dependent on glutamatergic neurotransmission at the N-methyl-D-aspartate (NMDA) receptor (Lisman 2003) (Pare 2004). 5-HT1A receptor mRNA has been detected in the excitatory pyramidal cells of the hippocampus (Pompeiano et al 1992), possibly explaining why 5-HT1A receptor agonists like 8-OH-DPAT have been reported to inhibit hippocampal pyramidal cell firing and suppress LTP (Sakai and Tanaka 1993) (Edagawa et al 1998). One would therefore expect 5-HT1A receptor antagonists to enhance LTP. LTP has, however, been found to be attenuated by administration of the 5-HT1A receptor antagonist WAY-100635 into the dentate gyrus of rats (Sanberg et al 2006). One explanation for this finding is that the majority of postsynaptic 5-HT1A receptors are found on GABAergic interneurons (Freund et al 1990) (Halasy et al 1992). Blocking these GABAergic interneurons with 5-HT1A receptor antagonists may enhance excitatory neurotransmission and LTP. Conversely, suppression of interneuron GABAergic neurotransmission with 5-HT1A receptor agonists may enhance LTP. The situation is rendered even more complicated, however, by the fact that agonists like 8-OH-DPAT could theoretically block LTP, not by acting directly on postsynaptic 5-HT1A receptors at pyramidal neurons or GABAergic interneurons, but by activating somatodendritic 5-HT1A receptors and thus reducing serotonergic signaling (Sanberg et al 2006).
The relationship between LTP and the cognitive impairment seen in MDD is unknown. The 5-HT1A receptor does, however, appear to play some role in less specific, psychometrically-defined cognitive performance [reviewed in (Borg 2008)]. The 5-HT1A receptor agonist tandospirone administered together with typical neuroleptics has been reported to improve verbal memory and executive function in people with schizophrenia (Sumiyoshi et al 2001) (Sumiyoshi et al 2007). In contrast, verbal recall (but not working) memory was reported to be impaired by tandospirone in a sample of healthy volunteers (Yasuno et al 2003); raising the possibility of differential effects of 5-HT1A receptor agonists on cognition in healthy and psychiatrically ill populations (Riedel et al 2002).
In sum, extant data suggest that serotonergic signaling at the 5-HT1A receptor may moderate the process of LTP through a complex series of mutually antagonistic pathways. Interpreting the impact of the 5-HT1A receptor on LTP and neuropsychological task performance using pharmacological challenge studies faces the same drawback as discussed previously: known 5-HT1A receptor agonists and antagonists bind to both somatodendritic and postsynaptic receptors. Another approach to addressing the role of the 5-HT1A receptor in cognition is to make use of molecular imaging techniques such as PET.
(Borg 2008) identified 4 published PET studies that examined the relationship between 5-HT1A receptor BP and cognition. One study using the antagonist, [11C]WAY100635 found a negative association between 5-HT1A receptor BP and verbal and visual memory performance in healthy volunteers (Yasuno et al 2003), while another study using the same radioligand returned negative results using a broad range of cognitive tests in a sample of healthy individuals (Borg et al 2006). Two studies were performed with the [18F]MPPF ligand in Alzheimer’s disease (AD). A positive correlation between 5-HT1A receptor BP in the raphe and hippocampus was detected by (Kepe et al 2006) but negative results were noted by (Truchot et al 2007).
Epidemiological studies demonstrate that up to 8 out of 10 patients with MDD suffer from chronic pain or somatic symptoms (Hotopf et al 1998) (Lepine and Briley 2004) (Gureje et al 2008). Conversely, people suffering from chronic, painful physical conditions have a significantly increased risk of developing MDD (Moldin et al 1993) (Ohayon and Schatzberg 2003).
Extant evidence implicates the 5-HT1A receptor in the modulation of pain. Lesions to the raphe were shown more than 30 years ago to block morphine-derived analgesia (Yaksh et al 1977). Preclinical studies have demonstrated the analgesic effects of 5-HT1A receptor agonists such as buspirone, gepirone and OH-DPAT (Giordano and Rogers 1992) (Robles et al 1996) (Galeotti et al 1997) (Bardin et al 2001), while the selective 5-HT1A receptor agonist F-13640 reportedly exerts potent, long-term analgesic effects [reviewed in (Colpaert 2006)]. ADs such as SSRIs and dual reuptake inhibitors such as venlafaxine, have been used with some success in the treatment of fibromyalgia, neuropathies, somatoform pain disorder, and rheumatoid arthritis (Jann and Slade 2007).
The raphe nuclei have been postulated to integrate nociceptive and affective stimuli via ascending and descending spinal projections (Wang and Nakai 1994). Descending projections from the raphe and the locus ceruleus act to suppress (especially minor) pain signals from the body (Millan 2002). (Berrocoso and Mico 2008) postulate that the analgesic effects of AD such as venlafaxine can be partially attributed to a desensitization of somatodendritic 5-HT1A receptors leading to an increase in serotonergic signaling at descending spinal projections. Thus, just as dysfunction of the raphe nuclei may lead to abnormal serotonin signaling at postsynaptic 5-HT1A receptors in the PFC and limbic regions, so too dysfunction of the raphe may disrupt descending serotonergic signaling at the dorsal horn of the spinal cord, and hence lead to more permissive pain input from the periphery (Stahl and Briley 2004).
In a recent [11C]WAY100635 PET study of 11 healthy males, (Martikainen et al 2007)reported that the intensity of cold pressor pain was inversely correlated with 5-HT1A receptor BP in the raphe, amygdala, insula, posterior cingulate cortex, and PFC. The authors interpret their data to suggest that individuals with a greater availability of 5-HT1A receptors in these regions have a greater ability to suppress pain.
Cause or Effect?
It remains unclear whether the depression-related changes in 5-HT1A receptor binding discussed above are developmentally or genetically-driven, or whether they are simply an adaptation to increased or decreased serotonergic neurotransmission. At least in the case of the dorsal raphe, receptor density is modified by pharmacological interventions. Acute administration of fluoxetine to rats may cause approximately one third of 5-HT1A receptors in the raphe to become internalized (Riad et al 2001). Similarly, a single dose of the 5-HT1A receptor agonist 8-OH-DPAT has been reported to induce a 34% increase in the internalization of the autoreceptor in the raphe (Zimmer et al 2004), while (Cahir et al 2007) reported a 14% decrease in 5-HT1A receptor binding in the raphe (but no cortical or hippocampal changes) after tryptophan depletion.
Nevertheless, there is also evidence to support the role of genetic or developmental influences. The G allele of the –1019C/G polymorphism has been fairly consistently associated with psychopathology. It could be hypothesized that the variant in question impacts neurodevelopment: the 5-HT1A gene is highly expressed early in development (del Olmo and Pazos 2001), and some reports suggest that, at least in the rat, the 5-HT1A receptor is found in regions of the fetal brain such as the cerebellum from which it is absent in mature animals (Daval et al 1987) (Miquel et al 1994). In a 5-HT1A KO paradigm that allowed for temporal manipulation of gene activation, (Gross et al 2002) found that eliminating gene expression during development, but not adulthood, led to anxiety-related behaviors. When the timing of gene activity was reversed, however, no salient abnormalities were recorded.
Conclusion
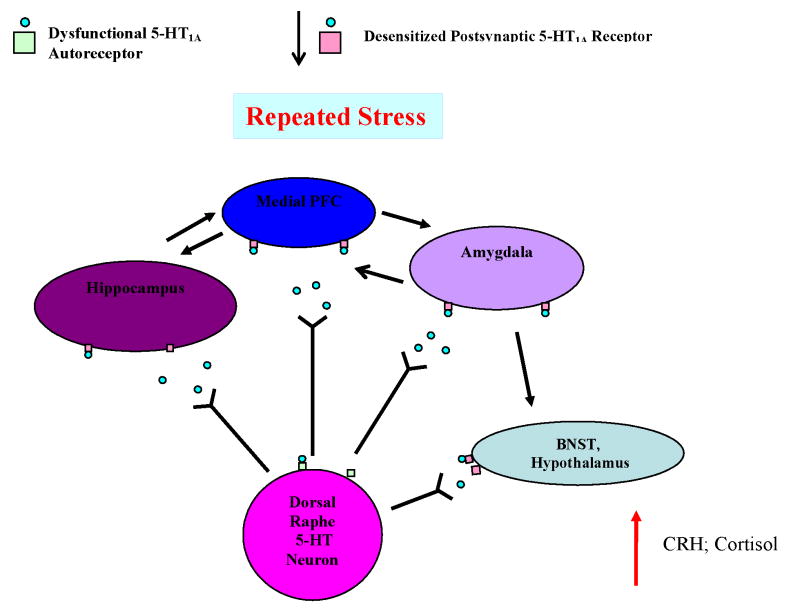
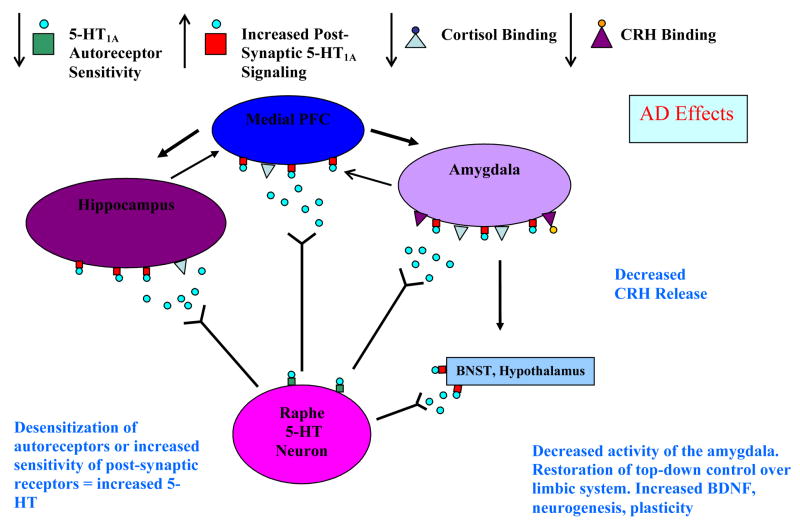
The preclinical literature suggests that acute stress or administration of corticosteroids down-regulates or desensitizes both somatodendritic and postsynaptic 5-HT1A receptors (Figure 1). This phenomenon may explain why MDD and other psychiatric conditions that have been associated with elevated secretion of cortisol show blunted endocrine or hormonal responses to 5-HT1A receptor agonists, reduced somatodendritic and postsynaptic 5-HT1A receptor numbers or binding at post-mortem, and reduced 5-HT1A receptor binding in the raphe, hippocampus and PFC in PET studies. Antidepressant medications are believed to enhance serotonergic signaling at the postsynaptic receptor either by desensitizing the somatodendritic autoreceptor or by facilitating the activation of G proteins at the postsynaptic 5-HT1A receptor (Figure 2). Desensitization of the somatodendritic 5-HT1A receptor may increase serotonergic signaling in descending spinal projections and ameliorate somatic pain but more data are needed to confirm this hypothesis. The G allele of the rs6295 HTR1A polymorphism has been postulated to up-regulate autoreceptor expression in the raphe but decrease expression of the postsynaptic 5-HT1A receptor; perhaps explaining why the G variant is over-represented in some MDD samples. Nevertheless, contradictory pharmacological, post-mortem, PET and genetic studies can be found in the literature, illustrating the complexity of the 5-HT1A receptor signaling system.
Figure 1. Somatodendritic Autoreceptors and Postsynaptic 5-HT1A Receptors Down-Regulate or Desensitize in Response to Stress.
Excess glucocorticoid receptor stimulation inhibits 5-HT1A receptor mRNA expression and leads to a down-regulation of both somatodendritic and postsynaptic 5-HT1A receptors. Increased stress-induced serotonin release may also contribute to the desensitization of post-synaptic 5-HT1A receptor binding. Decreased 5-HT1A receptor signaling may in turn.attenuate inhibitory control over the limbic system leading to many of the somatic and psychological symptoms associated with depression.
Figure 2. Simplified Model of 5-HT1A Receptor-Mediated Therapeutic Effects of AD.
AD are hypothesised to increase serotonergic signaling at the postsynaptic 5-HT1A receptor either by desensitizing the somatodendritic 5-HT1A receptor in the raphe, or by facilitating the activation of G proteins by the postsynaptic 5-HT1A receptor. Normalized signaling at the postsynaptic 5-HT1A receptor reduces cortisol and CRH release, restores endocrine function, and improves mood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert P, Lu J, Lefrancois B, Burns AM, Stockmeier CA, Austin MC, et al. Increased DNA methylation of the 5-HT1A receptor promoter in suicide brain. International Journal of Neuropsychopharmacology. 2008;11:105–106. [Google Scholar]
- Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, et al. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam JD. Gepirone, a selective serotonin (5HT1A) partial agonist in the treatment of major depression. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:271–280. doi: 10.1016/0278-5846(92)90079-t. [DOI] [PubMed] [Google Scholar]
- Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, et al. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- Anttila S, Huuhka K, Huuhka M, Rontu R, Hurme M, Leinonen E, Lehtimaki T. Interaction between 5-HT1A and BDNF genotypes increases the risk of treatment-resistant depression. J Neural Transm. 2007;114:1065–1068. doi: 10.1007/s00702-007-0705-9. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arias B, Catalan R, Gasto C, Gutierrez B, Fananas L. Evidence for a combined genetic effect of the 5-HT(1A) receptor and serotonin transporter genes in the clinical outcome of major depressive patients treated with citalopram. J Psychopharmacol. 2005;19:166–172. doi: 10.1177/0269881105049037. [DOI] [PubMed] [Google Scholar]
- Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol Psychiatry. 1994;35:457–463. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Artigas F, Nutt DJ, Shelton R. Mechanism of action of antidepressants. Psychopharmacol Bull. 2002;36(Suppl 2):123–132. [PubMed] [Google Scholar]
- Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, et al. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [carbonyl11C]WAY-100635. Arch Gen Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- Bardin L, Tarayre JP, Koek W, Colpaert FC. In the formalin model of tonic nociceptive pain, 8-OH-DPAT produces 5-HT1A receptor-mediated, behaviorally specific analgesia. Eur J Pharmacol. 2001;421:109–114. doi: 10.1016/s0014-2999(01)01029-9. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Baune BT, Hohoff C, Roehrs T, Deckert J, Arolt V, Domschke K. Serotonin receptor 1A -1019C/G variant: Impact on antidepressant pharmacoresponse in melancholic depression? Neurosci Lett. 2008;436:111–115. doi: 10.1016/j.neulet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Berman RM, Anand A, Cappiello A, Miller HL, Hu XS, Oren DA, Charney DS. The use of pindolol with fluoxetine in the treatment of major depression: final results from a double-blind, placebo-controlled trial. Biol Psychiatry. 1999;45:1170–1177. doi: 10.1016/s0006-3223(98)00383-7. [DOI] [PubMed] [Google Scholar]
- Berman RM, Darnell AM, Miller HL, Anand A, Charney DS. Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: a double-blind, placebo-controlled trial. Am J Psychiatry. 1997;154:37–43. doi: 10.1176/ajp.154.1.37. [DOI] [PubMed] [Google Scholar]
- Berney A, Nishikawa M, Benkelfat C, Debonnel G, Gobbi G, Diksic M. An index of 5-HT synthesis changes during early antidepressant treatment: alpha-[(11)C]methyl-l-tryptophan PET study. Neurochem Int. 2008;52:701–708. doi: 10.1016/j.neuint.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA. Role of serotonin 5-HT1A receptors in the antidepressant-like effect and the antinociceptive effect of venlafaxine in mice. Int J Neuropsychopharmacol. 2008:1–11. doi: 10.1017/S1461145708008766. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Blier P, Bouchard C. Modulation of 5-HT release in the guinea-pig brain following long-term administration of antidepressant drugs. Br J Pharmacol. 1994;113:485–495. doi: 10.1111/j.1476-5381.1994.tb17015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Chaput Y. Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol. 1987;7:24S–35S. [PubMed] [Google Scholar]
- Blier P, Seletti B, Gilbert F, Young SN, Benkelfat C. Serotonin 1A receptor activation and hypothermia in humans: lack of evidence for a presynaptic mediation. Neuropsychopharmacology. 2002;27:301–308. doi: 10.1016/S0893-133X(02)00318-4. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O, Bain E, Neumeister A, Nugent AC, Vythilingam M, Carson RE, et al. No change in serotonin type 1A receptor binding in patients with posttraumatic stress disorder. Am J Psychiatry. 2005;162:383–385. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- Borg J. Molecular imaging of the 5-HT(1A) receptor in relation to human cognition. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Borg J, Andree B, Lundberg J, Halldin C, Farde L. Search for correlations between serotonin 5-HT1A receptor expression and cognitive functions--a strategy in translational psychopharmacology. Psychopharmacology (Berl) 2006;185:389–394. doi: 10.1007/s00213-006-0329-z. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Najlerahim A, Procter AW, Francis PT, Murphy E. Circumscribed changes of the cerebral cortex in neuropsychiatric disorders of later life. Proc Natl Acad Sci U S A. 1989;86:9504–9508. doi: 10.1073/pnas.86.23.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir M, Ardis T, Reynolds GP, Cooper SJ. Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT 2A receptor binding in the rat. Psychopharmacology (Berl) 2007;190:497–506. doi: 10.1007/s00213-006-0635-5. [DOI] [PubMed] [Google Scholar]
- Carlson P, Bain E, Tinsley R, Nugent A, Luckenbaugh D, Carson R, et al. Serotonin-1A receptor binding in bipolar depression before and after mood stabilizer treatment. Bipolar Disorders. 2007;9:26. [Google Scholar]
- Castro E, Diaz A, Rodriguez-Gaztelumendi A, Del Olmo E, Pazos A. WAY100635 prevents the changes induced by fluoxetine upon the 5-HT(1A) receptor functionality. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.08.038. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lopez JF, Vazquez DM, Akil H, Watson SJ. Regulation of hippocampal 5-HT1A receptor gene expression by dexamethasone. Neuropsychopharmacology. 1994;10:215–222. doi: 10.1038/npp.1994.24. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology. 1991;5:219–229. [PubMed] [Google Scholar]
- Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT1 binding sites in depressed suicides. Psychopharmacology (Berl) 1990;102:544–548. doi: 10.1007/BF02247138. [DOI] [PubMed] [Google Scholar]
- Christiansen L, Tan Q, Iachina M, Bathum L, Kruse TA, McGue M, Christensen K. Candidate gene polymorphisms in the serotonergic pathway: influence on depression symptomatology in an elderly population. Biol Psychiatry. 2007;61:223–230. doi: 10.1016/j.biopsych.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Cintra A, Solfrini V, Agnati LF, Gustafsson JA, Fuxe K. Strongly glucocorticoid receptor immunoreactive neurons in the neonatal rat brain. Neuroreport. 1991;2:85–88. doi: 10.1097/00001756-199102000-00005. [DOI] [PubMed] [Google Scholar]
- Cleare AJ, Messa C, Rabiner EA, Grasby PM. Brain 5-HT1A receptor binding in chronic fatigue syndrome measured using positron emission tomography and [11C]WAY-100635. Biol Psychiatry. 2005;57:239–246. doi: 10.1016/j.biopsych.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. 5-HT(1A) receptor activation: new molecular and neuroadaptive mechanisms of pain relief. Curr Opin Investig Drugs. 2006;7:40–47. [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowburn RF, Marcusson JO, Eriksson A, Wiehager B, O’Neill C. Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res. 1994;633:297–304. doi: 10.1016/0006-8993(94)91552-0. [DOI] [PubMed] [Google Scholar]
- Cowen PJ. Psychopharmacology of 5-HT(1A) receptors(1) Nucl Med Biol. 2000;27:437–439. doi: 10.1016/s0969-8051(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Power AC, Ware CJ, Anderson IM. 5-HT1A receptor sensitivity in major depression. A neuroendocrine study with buspirone. Br J Psychiatry. 1994;164:372–379. doi: 10.1192/bjp.164.3.372. [DOI] [PubMed] [Google Scholar]
- Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J Neurosci. 2006;26:1864–1871. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, et al. Serotonergic genes modulate amygdala activity in major depression. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- Daval G, Verge D, Becerril A, Gozlan H, Spampinato U, Hamon M. Transient expression of 5-HT1A receptor binding sites in some areas of the rat CNS during postnatal development. Int J Dev Neurosci. 1987;5:171–180. doi: 10.1016/0736-5748(87)90027-x. [DOI] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, Iglesias B, Hyde TM, Bigelow LB, Imamovic V, Herman MM, Kleinman JE. Evaluation of tissue collection for postmortem studies of bipolar disorder. Bipolar Disord. 2008;10:822–828. doi: 10.1111/j.1399-5618.2008.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Olmo E, Pazos A. Aminergic receptors during the development of the human brain: the contribution of in vitro imaging techniques. J Chem Neuroanat. 2001;22:101–114. doi: 10.1016/s0891-0618(01)00097-7. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Wieland S, Lucki I. Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT1A receptor antagonists. Psychopharmacology (Berl) 1995;119:47–54. doi: 10.1007/BF02246053. [DOI] [PubMed] [Google Scholar]
- Dhingra V, Magnay JL, O’Brien PM, Chapman G, Fryer AA, Ismail KM. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110:788–792. doi: 10.1097/01.AOG.0000284448.73490.ac. [DOI] [PubMed] [Google Scholar]
- Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, Bauer J, et al. Association of the functional -1019C/G 5-HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3 T fMRI in panic disorder. Int J Neuropsychopharmacol. 2006;9:349–355. doi: 10.1017/S1461145705005869. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen GM, Wang JF, Reiach JS, Young LT. G Protein-coupled cyclic AMP signaling in postmortem brain of subjects with mood disorders: effects of diagnosis, suicide, and treatment at the time of death. J Neurochem. 1999;73:1121–1126. doi: 10.1046/j.1471-4159.1999.0731121.x. [DOI] [PubMed] [Google Scholar]
- Drago A, Ronchi DD, Serretti A. 5-HT1A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Int J Neuropsychopharmacol. 2007:1–21. doi: 10.1017/S1461145707008218. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN. [(3)H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am J Psychiatry. 2002;159:66–73. doi: 10.1176/appi.ajp.159.1.66. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Shukla PK, Lyons J, Faludi G, Palkovits M, et al. Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry. 2004;55:234–243. doi: 10.1016/j.biopsych.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K. 5-HT1A receptor-mediated inhibition of long-term potentiation in rat visual cortex. Eur J Pharmacol. 1998;349:221–224. doi: 10.1016/s0014-2999(98)00286-6. [DOI] [PubMed] [Google Scholar]
- Elena Castro M, Diaz A, del Olmo E, Pazos A. Chronic fluoxetine induces opposite changes in G protein coupling at pre and postsynaptic 5-HT1A receptors in rat brain. Neuropharmacology. 2003;44:93–101. doi: 10.1016/s0028-3908(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Leitch MM, Ingram CD. Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology. 2003;45:925–934. doi: 10.1016/s0028-3908(03)00269-7. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE. Anxiolytic effects in the plus-maze of 5-HT1A-receptor ligands in dorsal raphe and ventral hippocampus. Pharmacol Biochem Behav. 1996;54:123–128. doi: 10.1016/0091-3057(95)02108-6. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Flugge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugge G, Kramer M, Rensing S, Fuchs E. 5HT1A-receptors and behaviour under chronic stress: selective counteraction by testosterone. Eur J Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- Francis PT, Pangalos MN, Stephens PH, Bartlett JR, Bridges PK, Malizia AL, et al. Antemortem measurements of neurotransmission: possible implications for pharmacotherapy of Alzheimer’s disease and depression. J Neurol Neurosurg Psychiatry. 1993;56:80–84. doi: 10.1136/jnnp.56.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, Kegeles LS, Slifstein M, Martin JH, Huang Y, et al. Serotonin 1A receptor availability in patients with schizophrenia and schizo-affective disorder: a positron emission tomography imaging study with [11C]WAY 100635. Psychopharmacology (Berl) 2006;189:155–164. doi: 10.1007/s00213-006-0543-8. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI, Acsady L, Gorcs T, Toth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc Natl Acad Sci U S A. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res. 2005;138:228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Bartolini A. 5-HT1A agonists induce central cholinergic antinociception. Pharmacol Biochem Behav. 1997;57:835–841. doi: 10.1016/s0091-3057(96)00401-7. [DOI] [PubMed] [Google Scholar]
- Gandolfi O, Gaggi R, Voltattorni M, Dall’Olio R. The activation of serotonin receptors prevents glutamate-induced neurotoxicity and NMDA-stimulated cGMP accumulation in primary cortical cell cultures. Pharmacol Res. 2002;46:409–414. doi: 10.1016/s1043661802002050. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geretsegger C, Bitterlich W, Stelzig R, Stuppaeck C, Bondy B, Aichhorn W. Paroxetine with pindolol augmentation: A double-blind, randomized, placebo-controlled study in depressed in-patients. Eur Neuropsychopharmacol. 2008;18:141–146. doi: 10.1016/j.euroneuro.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Giordano J, Rogers L. Putative mechanisms of buspirone-induced antinociception in the rat. Pain. 1992;50:365–372. doi: 10.1016/0304-3959(92)90042-A. [DOI] [PubMed] [Google Scholar]
- Giovacchini G, Lang L, Ma Y, Herscovitch P, Eckelman WC, Carson RE. Differential effects of paroxetine on raphe and cortical 5-HT1A binding: a PET study in monkeys. Neuroimage. 2005;28:238–248. doi: 10.1016/j.neuroimage.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ, Garcia-Sevilla JA, Guimon J. Neurotransmitter receptor-mediated activation of G-proteins in brains of suicide victims with mood disorders: selective supersensitivity of alpha(2A)-adrenoceptors. Mol Psychiatry. 2002;7:755–767. doi: 10.1038/sj.mp.4001067. [DOI] [PubMed] [Google Scholar]
- Gravel P, de Montigny C. Noradrenergic denervation prevents sensitization of rat forebrain neurons to serotonin by tricyclic antidepressant treatment. Synapse. 1987;1:233–239. doi: 10.1002/syn.890010303. [DOI] [PubMed] [Google Scholar]
- Gray L, Scarr E, Dean B. Serotonin 1a receptor and associated G-protein activation in schizophrenia and bipolar disorder. Psychiatry Res. 2006;143:111–120. doi: 10.1016/j.psychres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Skinner HD, Birkle DL. Prenatal stress influences 8-OH-DPAT modulated startle responding and [3H]-8-OH-DPAT binding in rats. Pharmacol Biochem Behav. 2005;81:601–607. doi: 10.1016/j.pbb.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, et al. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasy K, Miettinen R, Szabat E, Freund TF. GABAergic Interneurons are the Major Postsynaptic Targets of Median Raphe Afferents in the Rat Dentate Gyrus. Eur J Neurosci. 1992;4:144–153. doi: 10.1111/j.1460-9568.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Mulder J, Horvath KM, Keijser J, van der Meeberg EK, Nyakas C, Luiten PG. Oral post-lesion administration of 5-HT(1A) receptor agonist repinotan hydrochloride (BAY x 3702) attenuates NMDA-induced delayed neuronal death in rat magnocellular nucleus basalis. Neuroscience. 2001;108:629–642. doi: 10.1016/s0306-4522(01)00444-4. [DOI] [PubMed] [Google Scholar]
- Harkin A, Kelly JP, McNamara M, Connor TJ, Dredge K, Redmond A, Leonard BE. Activity and onset of action of reboxetine and effect of combination with sertraline in an animal model of depression. Eur J Pharmacol. 1999;364:123–132. doi: 10.1016/s0014-2999(98)00838-3. [DOI] [PubMed] [Google Scholar]
- Hasler G, Bonwetsch R, Giovacchini G, Toczek MT, Bagic A, Luckenbaugh DA, et al. 5-HT(1A) Receptor Binding in Temporal Lobe Epilepsy Patients With and Without Major Depression. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa H, Shimizu M, Nishida A, Motohashi N, Yamawaki S. Increase in serotonin 1A receptors in the dentate gyrus as revealed by autoradiographic analysis following repeated electroconvulsive shock but not imipramine treatment. Neuropsychobiology. 1994;30:53–56. doi: 10.1159/000119413. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci. 2003;72:1665–1682. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Advani T, Monteggia LM. Regulation of Serotonin-1A Receptor Function in Inducible Brain-Derived Neurotrophic Factor Knockout Mice After Administration of Corticosterone. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Hervas I, Vilaro MT, Romero L, Scorza MC, Mengod G, Artigas F. Desensitization of 5-HT(1A) autoreceptors by a low chronic fluoxetine dose effect of the concurrent administration of WAY-100635. Neuropsychopharmacology. 2001;24:11–20. doi: 10.1016/S0893-133X(00)00175-5. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, van den Oord EJ, Neale MC, Kendler KS, Chen X. Association study between the serotonin 1A receptor (HTR1A) gene and neuroticism, major depression, and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol. 2000;14:177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 1991;48:1779–1786. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hong CJ, Chen TJ, Yu YW, Tsai SJ. Response to fluoxetine and serotonin 1A receptor (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2006;6:27–33. doi: 10.1038/sj.tpj.6500340. [DOI] [PubMed] [Google Scholar]
- Hotopf M, Mayou R, Wadsworth M, Wessely S. Temporal relationships between physical symptoms and psychiatric disorder. Results from a national birth cohort. Br J Psychiatry. 1998;173:255–261. doi: 10.1192/bjp.173.3.255. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hsiung SC, Adlersberg M, Arango V, Mann JJ, Tamir H, Liu KP. Attenuated 5-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. J Neurochem. 2003;87:182–194. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. The role of 5-HT1A receptors in the proliferation and survival of progenitor cells in the dentate gyrus of the adult hippocampus and their regulation by corticoids. Neuroscience. 2005;135:803–813. doi: 10.1016/j.neuroscience.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G, et al. Human 5-HT1A receptor C(-1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol. 2004;7:441–451. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V, Grasby P. Effect of 5-HT on binding of [(11)C] WAY 100635 to 5-HT(IA) receptors in rat brain, assessed using in vivo microdialysis nd PET after fenfluramine. Synapse. 2001;41:150–159. doi: 10.1002/syn.1069. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Sarna GS, O’Connell MT, Curzon G. Hippocampal 5-HT synthesis and release in vivo is decreased by infusion of 8-OHDPAT into the nucleus raphe dorsalis. Neurosci Lett. 1989;100:276–280. doi: 10.1016/0304-3940(89)90698-8. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Bramante M, Samanin R. Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical serotonin output: role of presynaptic 5-HT1A receptors. Eur J Pharmacol. 1994;260:243–246. doi: 10.1016/0014-2999(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Amano T, Hayakawa H, Yamawaki S, Sasa M. Enhancement of serotonin(1A) receptor function following repeated electroconvulsive shock in young rat hippocampal neurons in vitro. Int J Neuropsychopharmacol. 1999;2:101–104. doi: 10.1017/S1461145799001467. [DOI] [PubMed] [Google Scholar]
- Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007;27:1571–1587. doi: 10.1592/phco.27.11.1571. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2006 doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Jolas T, Schreiber R, Laporte AM, Chastanet M, De Vry J, Glaser T, et al. Are postsynaptic 5-HT1A receptors involved in the anxiolytic effects of 5-HT1A receptor agonists and in their inhibitory effects on the firing of serotonergic neurons in the rat? J Pharmacol Exp Ther. 1995;272:920–929. [PubMed] [Google Scholar]
- Karten YJ, Nair SM, van Essen L, Sibug R, Joels M. Long-term exposure to high corticosterone levels attenuates serotonin responses in rat hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 1999;96:13456–13461. doi: 10.1073/pnas.96.23.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Fukuda T, Wakeno M, Okugawa G, Takekita Y, Watanabe S, et al. Effect of 5-HT1A gene polymorphisms on antidepressant response in major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30783. [DOI] [PubMed] [Google Scholar]
- Kepe V, Barrio JR, Huang SC, Ercoli L, Siddarth P, Shoghi-Jadid K, et al. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Al-Taie O, Schafer A, Pfersdorff M, Lesch KP, Scheurlen M. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Differential regulation of serotonin (5-HT) release in the striatum and hippocampus by 5-HT1A autoreceptors of the dorsal and median raphe nuclei. J Pharmacol Exp Ther. 1994;269:1268–1279. [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharmacol Exp Ther. 1995;274:866–876. [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Chronic administration of the 5-HT1A receptor agonist 8-OH-DPAT differentially desensitizes 5-HT1A autoreceptors of the dorsal and median raphe nuclei. Synapse. 1997;25:107–116. doi: 10.1002/(SICI)1098-2396(199702)25:2<107::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Laaris N, Le Poul E, Laporte AM, Hamon M, Lanfumey L. Differential effects of stress on presynaptic and postsynaptic 5-hydroxytryptamine-1A receptors in the rat brain: an in vitro electrophysiological study. Neuroscience. 1999;91:947–958. doi: 10.1016/s0306-4522(98)00674-5. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Pardon MC, Laaris N, Joubert C, Hanoun N, Hamon M, Cohen-Salmon C. 5-HT1A autoreceptor desensitization by chronic ultramild stress in mice. Neuroreport. 1999;10:3369–3374. doi: 10.1097/00001756-199911080-00021. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Lapierre YD, Silverstone P, Reesal RT, Saxena B, Turner P, Bakish D, et al. A Canadian multicenter study of three fixed doses of controlled-release ipsapirone in outpatients with moderate to severe major depression. J Clin Psychopharmacol. 1998;18:268–273. doi: 10.1097/00004714-199808000-00002. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, et al. Early Parental Deprivation in the Marmoset Monkey Produces Long-Term Changes in Hippocampal Expression of Genes Involved in Synaptic Plasticity and Implicated in Mood Disorder. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Leitch MM, Ingram CD, Young AH, McQuade R, Gartside SE. Flattening the corticosterone rhythm attenuates 5-HT1A autoreceptor function in the rat: relevance for depression. Neuropsychopharmacology. 2003;28:119–125. doi: 10.1038/sj.npp.1300016. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol. 2004;7:501–506. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Shardell M, Ferrell RE, Orwig D, Yu-Yahiro J, Hawkes W, et al. Association of serotonin-1A and 2A receptor promoter polymorphisms with depressive symptoms and functional recovery in elderly persons after hip fracture. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19(Suppl 1):S3–7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT1A receptor function in normal subjects on clinical doses of fluoxetine: blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology. 1999;20:628–639. doi: 10.1016/S0893-133X(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Focus on The 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int J Neuropsychopharmacol. 2004;7:381–385. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mayer S, Disselkamp-Tietze J, Hoh A, Wiesmann M, Osterheider M, Schulte HM. 5-HT1A receptor responsivity in unipolar depression. Evaluation of ipsapirone-induced ACTH and cortisol secretion in patients and controls. Biol Psychiatry. 1990;28:620–628. doi: 10.1016/0006-3223(90)90400-v. [DOI] [PubMed] [Google Scholar]
- Levin GM, Bowles TM, Ehret MJ, Langaee T, Tan JY, Johnson JA, Millard WJ. Assessment of human serotonin 1A receptor polymorphisms and SSRI responsiveness. Mol Diagn Ther. 2007;11:155–160. doi: 10.1007/BF03256237. [DOI] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 2004;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Inoue T, Abekawa T, Weng S, Nakagawa S, Izumi T, Koyama T. 5-HT1A receptor agonist affects fear conditioning through stimulations of the postsynaptic 5-HT1A receptors in the hippocampus and amygdala. Eur J Pharmacol. 2006;532:74–80. doi: 10.1016/j.ejphar.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Lisinicchia JG, Watts VJ. Sensitization of adenylate cyclase by short-term activation of 5-HT1A receptors. Cell Signal. 2003;15:1111–1117. doi: 10.1016/s0898-6568(03)00115-3. [DOI] [PubMed] [Google Scholar]
- Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bakalian MJ, Tamir H, Dwork AJ, Hamon M, Arango V. Society for Neuroscience Abstracts. Washington, DC: Society for Neuroscience; 2008. Neuronal density and volume in Brodmann area 10 of depressed suicides: A comparison study of glia, neurons and 5-HT1A receptor immunolabeled neurons; p. 843.816. [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lowther S, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls. J Affect Disord. 1997;42:199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- Lucki I. Behavioral studies of serotonin receptor agonists as antidepressant drugs. J Clin Psychiatry. 1991;52(Suppl):24–31. [PubMed] [Google Scholar]
- Madhavan L, Freed WJ, Anantharam V, Kanthasamy AG. 5-hydroxytryptamine 1A receptor activation protects against N-methyl-D-aspartate-induced apoptotic cell death in striatal and mesencephalic cultures. J Pharmacol Exp Ther. 2003;304:913–923. doi: 10.1124/jpet.102.044370. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000;48:740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. J Neurosci. 2001;21:2178–2185. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Palego L, Giromella A, Mazzoni MR, Borsini F, Mayer N, et al. Region-dependent effects of flibanserin and buspirone on adenylyl cyclase activity in the human brain. Int J Neuropsychopharmacol. 2002;5:131–140. doi: 10.1017/S1461145702002869. [DOI] [PubMed] [Google Scholar]
- Martikainen IK, Hirvonen J, Kajander J, Hagelberg N, Mansikka H, Nagren K, et al. Correlation of human cold pressor pain responses with 5-HT(1A) receptor binding in the brain. Brain Res. 2007;1172:21–31. doi: 10.1016/j.brainres.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. J Neural Transm Gen Sect. 1991;85:181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–1107. [PubMed] [Google Scholar]
- McAllister-Williams RH, Anderson AJ, Young AH. Corticosterone selectively attenuates 8-OH-DPAT-mediated hypothermia in mice. Int J Neuropsychopharmacol. 2001;4:1–8. doi: 10.1017/S1461145701002218. [DOI] [PubMed] [Google Scholar]
- McAllister-Williams RH, Massey AE, Fairchild G. Repeated cortisol administration attenuates the EEG response to buspirone in healthy volunteers: evidence for desensitization of the 5-HT1A autoreceptor. J Psychopharmacol. 2007;21:826–832. doi: 10.1177/0269881107078292. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Olie JP. Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine. Mol Psychiatry. 2005;10:525–537. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller E, Goldstein M, Bohmaker K. Receptor reserve for 5-hydroxytryptamine1A-mediated inhibition of serotonin synthesis: possible relationship to anxiolytic properties of 5-hydroxytryptamine1A agonists. Mol Pharmacol. 1990;37:231–237. [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Maes M. Effects of buspirone on plasma prolactin and cortisol levels in major depressed and normal subjects. Biol Psychiatry. 1994;35:316–323. doi: 10.1016/0006-3223(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Maes M. Effects of ipsapirone on plasma cortisol and body temperature in major depression. Biol Psychiatry. 1995;38:450–457. doi: 10.1016/0006-3223(94)00370-i. [DOI] [PubMed] [Google Scholar]
- Mickey BJ, Ducci F, Hodgkinson CA, Langenecker SA, Goldman D, Zubieta JK. Monoamine oxidase A genotype predicts human serotonin 1A receptor availability in vivo. J Neurosci. 2008;28:11354–11359. doi: 10.1523/JNEUROSCI.2391-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Kia HK, Boni C, Doucet E, Daval G, Matthiessen L, et al. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Brain Res Dev Brain Res. 1994;80:149–157. doi: 10.1016/0165-3806(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Moldin SO, Scheftner WA, Rice JP, Nelson E, Knesevich MA, Akiskal H. Association between major depressive disorder and physical illness. Psychol Med. 1993;23:755–761. doi: 10.1017/s0033291700025526. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH, et al. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata N, Suda H, Izumi J, Tanaka Y, Ikeda Y, Kato H, et al. Role of hippocampal serotonergic neurons in ischemic neuronal death. Behav Brain Res. 1997;83:217–220. doi: 10.1016/s0166-4328(97)86073-1. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Navines R, Gomez-Gil E, Martin-Santos R, de Osaba MJ, Escolar G, Gasto C. Hormonal response to buspirone is not impaired in major depression. Hum Psychopharmacol. 2007;22:389–395. doi: 10.1002/hup.862. [DOI] [PubMed] [Google Scholar]
- Neff CD, Abkevich V, Packer JC, Chen Y, Potter J, Riley R, et al. Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.8. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B, Kapeller J, Hammer C, Rappold G. Serotonin type 3 receptor genes: HTR3A, B, C, D, E. Pharmacogenomics. 2008;9:501–504. doi: 10.2217/14622416.9.5.501. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekstrom JC, Svenningsson P, et al. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- Olivier B, Pattij T, Wood SJ, Oosting R, Sarnyai Z, Toth M. The 5-HT(1A) receptor knockout mouse and anxiety. Behav Pharmacol. 2001;12:439–450. doi: 10.1097/00008877-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem. 2001;276:14299–14307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- Pan L, Gilbert F. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinology. 1992;56:797–802. doi: 10.1159/000126332. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Mondal AC, Shukla PK, Conley RR. Brain region specific alterations in the protein and mRNA levels of protein kinase A subunits in the post-mortem brain of teenage suicide victims. Neuropsychopharmacology. 2005;30:1548–1556. doi: 10.1038/sj.npp.1300765. [DOI] [PubMed] [Google Scholar]
- Pare D. Presynaptic induction and expression of NMDA-dependent LTP. Trends Neurosci. 2004;27:440–441. doi: 10.1016/j.tins.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Tin A, Sullivan G, Oquendo MA, Mann JJ. Serotonin 1A binding potential in major depression: A second patient cohort. Society for Neuroscience Abstracts. 2008:414.418. [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Pejchal T, Foley MA, Kosofsky BE, Waeber C. Chronic fluoxetine treatment selectively uncouples raphe 5-HT(1A) receptors as measured by [(35)S]-GTP gamma S autoradiography. Br J Pharmacol. 2002;135:1115–1122. doi: 10.1038/sj.bjp.0704555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EB, Berman RM, Sanacora G, Anand A, Lynch-Colonese K, Charney DS. Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitors: a double-blind, randomized, controlled trial. J Clin Psychiatry. 2004;65:238–243. doi: 10.4088/jcp.v65n0215. [DOI] [PubMed] [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- Pitchot W, Hansenne M, Pinto E, Reggers J, Fuchs S, Ansseau M. 5-Hydroxytryptamine 1A receptors, major depression, and suicidal behavior. Biol Psychiatry. 2005;58:854–858. doi: 10.1016/j.biopsych.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Cappiello A, Malison RT, McDougle CJ, Pelton GH, Schollnhammer G, Heninger GR. Effects of antiglucocorticoid treatment on 5-HT1A function in depressed patients and healthy subjects. Neuropsychopharmacology. 1997;17:246–257. doi: 10.1016/S0893-133X(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Raap DK, Van de Kar LD. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 1999;65:1217–1235. doi: 10.1016/s0024-3205(99)00169-1. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch JL, Johnson ME, Kasik KE, Stahl SM. Temperature regulation in depression: functional 5HT1A receptor adaptation differentiates antidepressant response. Neuropsychopharmacology. 2006;31:2274–2280. doi: 10.1038/sj.npp.1301088. [DOI] [PubMed] [Google Scholar]
- Reiach JS, Li PP, Warsh JJ, Kish SJ, Young LT. Reduced adenylyl cyclase immunolabeling and activity in postmortem temporal cortex of depressed suicide victims. J Affect Disord. 1999;56:141–151. doi: 10.1016/s0165-0327(99)00048-8. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Arranz B, Templeman LA, Fertuzinhos S, San L. Effect of 5-HT1A receptor gene polymorphism on negative and depressive symptom response to antipsychotic treatment of drug-naive psychotic patients. Am J Psychiatry. 2006;163:1826–1829. doi: 10.1176/ajp.2006.163.10.1826. [DOI] [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J Neurosci. 2001;21:8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci. 2004;24:5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, Griez E, Honig A, Menheere PP, van Praag HM. Dissociable hormonal, cognitive and mood responses to neuroendocrine challenge: evidence for receptor-specific serotonergic dysregulation in depressed mood. Neuropsychopharmacology. 2002;26:358–367. doi: 10.1016/S0893-133X(01)00361-X. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Rickels K, Feighner J, Fabre LF, Jr, Gammans RE, Shrotriya RC, et al. Clinical effects of the 5-HT1A partial agonists in depression: a composite analysis of buspirone in the treatment of depression. J Clin Psychopharmacol. 1990;10:67S–76S. doi: 10.1097/00004714-199006001-00013. [DOI] [PubMed] [Google Scholar]
- Robles LI, Barrios M, Del Pozo E, Dordal A, Baeyens JM. Effects of K+ channel blockers and openers on antinociception induced by agonists of 5-HT1A receptors. Eur J Pharmacol. 1996;295:181–188. doi: 10.1016/0014-2999(95)00643-5. [DOI] [PubMed] [Google Scholar]
- Rossi DV, Burke TF, McCasland M, Hensler JG. Serotonin-1A receptor function in the dorsal raphe nucleus following chronic administration of the selective serotonin reuptake inhibitor sertraline. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2007.05201.x. [DOI] [PubMed] [Google Scholar]
- Rossi DV, Valdez M, Gould GG, Hensler JG. Chronic administration of venlafaxine fails to attenuate 5-HT1A receptor function at the level of receptor-G protein interaction. Int J Neuropsychopharmacol. 2006;9:393–406. doi: 10.1017/S1461145705005754. [DOI] [PubMed] [Google Scholar]
- Rothe C, Gutknecht L, Freitag C, Tauber R, Mossner R, Franke P, et al. Association of a functional 1019C>G 5-HT1A receptor gene polymorphism with panic disorder with agoraphobia. Int J Neuropsychopharmacol. 2004;7:189–192. doi: 10.1017/S1461145703004061. [DOI] [PubMed] [Google Scholar]
- Sah R, Pritchard LM, Richtand NM, Ahlbrand R, Eaton K, Sallee FR, Herman JP. Expression of the glucocorticoid-induced receptor mRNA in rat brain. Neuroscience. 2005;133:281–292. doi: 10.1016/j.neuroscience.2005.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Tanaka C. Inhibitory modulation of long-term potentiation via the 5-HT1A receptor in slices of the rat hippocampal dentate gyrus. Brain Res. 1993;613:326–330. doi: 10.1016/0006-8993(93)90921-9. [DOI] [PubMed] [Google Scholar]
- Salazar-Colocho P, Del Rio J, Frechilla D. Neuroprotective effects of serotonin 5-HT(1A) receptor activation against ischemic cell damage in gerbil hippocampus: Involvement of NMDA receptor NR1 subunit and BDNF. Brain Res. 2008 doi: 10.1016/j.brainres.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Sanberg CD, Jones FL, Do VH, Dieguez D, Jr, Derrick BE. 5-HT1A receptor antagonists block perforant path-dentate LTP induced in novel, but not familiar, environments. Learn Mem. 2006;13:52–62. doi: 10.1101/lm.126306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disord. 2005;7:216–235. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Kirsch P, Reuter M, Alexander N, Kozyra E, Kuepper Y, et al. The 5-HT1A C(-1019)G polymorphism, personality and electrodermal reactivity in a reward/punishment paradigm. Int J Neuropsychopharmacol. 2008:1–10. doi: 10.1017/S1461145708009401. [DOI] [PubMed] [Google Scholar]
- Schule C. Neuroendocrinological mechanisms of actions of antidepressant drugs. J Neuroendocrinol. 2007;19:213–226. doi: 10.1111/j.1365-2826.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- Serretti A, Artioli P, Lorenzi C, Pirovano A, Tubazio V, Zanardi R. The C(-1019)G polymorphism of the 5-HT1A gene promoter and antidepressant response in mood disorders: preliminary findings. Int J Neuropsychopharmacol. 2004;7:453–460. doi: 10.1017/S1461145704004687. [DOI] [PubMed] [Google Scholar]
- Serretti A, Mandelli L, Giegling I, Schneider B, Hartmann AM, Schnabel A, et al. HTR2C and HTR1A gene variants in German and Italian suicide attempters and completers. Am J Med Genet B Neuropsychiatr Genet. 2007;144:291–299. doi: 10.1002/ajmg.b.30432. [DOI] [PubMed] [Google Scholar]
- Shapira B, Newman ME, Gelfin Y, Lerer B. Blunted temperature and cortisol responses to ipsapirone in major depression: lack of enhancement by electroconvulsive therapy. Psychoneuroendocrinology. 2000;25:421–438. doi: 10.1016/s0306-4530(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Shelton RC. Augmentation strategies to increase antidepressant efficacy. J Clin Psychiatry. 2007;68(Suppl 10):18–22. [PubMed] [Google Scholar]
- Shen C, Li H, Meller E. Repeated treatment with antidepressants differentially alters 5-HT1A agonist-stimulated [35S]GTP gamma S binding in rat brain regions. Neuropharmacology. 2002;42:1031–1038. doi: 10.1016/s0028-3908(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Shiah IS, Yatham LN, Lam RW, Tam EM, Zis AP. Cortisol, hypothermic, and behavioral responses to ipsapirone in patients with bipolar depression and normal controls. Neuropsychobiology. 1998;38:6–12. doi: 10.1159/000026510. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Spindelegger C, Lanzenberger R, Wadsak W, Mien LK, Stein P, Mitterhauser M, et al. Influence of escitalopram treatment on 5-HT(1A) receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.35. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. (−)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- Stahl S. 5HT1A receptors and pharmacotherapy. Is serotonin receptor down-regulation linked to the mechanism of action of antidepressant drugs? Psychopharmacol Bull. 1994;30:39–43. [PubMed] [Google Scholar]
- Stahl S, Briley M. Understanding pain in depression. Hum Psychopharmacol. 2004;19(Suppl 1):S9–S13. doi: 10.1002/hup.619. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, et al. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm. 2003;110:1445–1453. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, et al. The effect of tandospirone, a serotonin(1A) agonist, on memory function in schizophrenia. Biol Psychiatry. 2001;49:861–868. doi: 10.1016/s0006-3223(00)01025-8. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Park S, Jayathilake K, Roy A, Ertugrul A, Meltzer HY. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95:158–168. doi: 10.1016/j.schres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Sussman N. Anxiolytic antidepressant augmentation. J Clin Psychiatry. 1998;59(Suppl 5):42–48. discussion 49–50. [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effects of the selective norepinephrine reuptake inhibitor reboxetine on norepinephrine and serotonin transmission in the rat hippocampus. Neuropsychopharmacology. 2001;25:845–857. doi: 10.1016/S0893-133X(01)00284-6. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2008:1–14. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739–752. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- Toth M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur J Pharmacol. 2003;463:177–184. doi: 10.1016/s0014-2999(03)01280-9. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Troelsen KB, Nielsen EO, Mirza NR. Chronic treatment with duloxetine is necessary for an anxiolytic-like response in the mouse zero maze: the role of the serotonin transporter. Psychopharmacology (Berl) 2005;181:741–750. doi: 10.1007/s00213-005-0032-5. [DOI] [PubMed] [Google Scholar]
- Truchot L, Costes SN, Zimmer L, Laurent B, Le Bars D, Thomas-Anterion C, et al. Up-regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology. 2007;69:1012–1017. doi: 10.1212/01.wnl.0000271377.52421.4a. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Sills TL, Evans KR, Kalali AH. Prevalence and association of somatic symptoms in patients with Major Depressive Disorder. J Affect Disord. 2008;110:270–276. doi: 10.1016/j.jad.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Karteszi M, Bethea CL, Ganong WF. Serotonergic stimulation of prolactin and corticosterone secretion is mediated by different pathways from the mediobasal hypothalamus. Neuroendocrinology. 1985;41:380–384. doi: 10.1159/000124205. [DOI] [PubMed] [Google Scholar]
- van Riel E, Meijer OC, Steenbergen PJ, Joels M. Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience. 2003;120:649–658. doi: 10.1016/s0306-4522(03)00355-5. [DOI] [PubMed] [Google Scholar]
- van Riel E, van Gemert NG, Meijer OC, Joels M. Effect of early life stress on serotonin responses in the hippocampus of young adult rats. Synapse. 2004;53:11–19. doi: 10.1002/syn.20033. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol. 1985;113:463–464. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, et al. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Videtic A, Pungercic G, Pajnic IZ, Zupanc T, Balazic J, Tomori M, Komel R. Association study of seven polymorphisms in four serotonin receptor genes on suicide victims. Am J Med Genet B Neuropsychiatr Genet. 2006;141:669–672. doi: 10.1002/ajmg.b.30390. [DOI] [PubMed] [Google Scholar]
- Wang QP, Nakai Y. The dorsal raphe: an important nucleus in pain modulation. Brain Res Bull. 1994;34:575–585. doi: 10.1016/0361-9230(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Inhibiton of neurons in the amygdala by dorsal raphe stimulation: mediation through a direct serotonergic pathway. Brain Res. 1977;120:85–102. doi: 10.1016/0006-8993(77)90499-1. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Geijer T, Sokolowski M, Rozanov V, Wasserman J. The serotonin 1A receptor C(-1019)G polymorphism in relation to suicide attempt. Behav Brain Funct. 2006;2:14. doi: 10.1186/1744-9081-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Sakai RR, McEwen BS, Mendelson S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Comings DE. A common C-1018G polymorphism in the human 5-HT1A receptor gene. Psychiatr Genet. 1999;9:105–106. doi: 10.1097/00041444-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Plant RL, Rudy TA. Studies on the antagonism by raphe lesions of the antinociceptive action of systemic morphine. Eur J Pharmacol. 1977;41:399–408. doi: 10.1016/0014-2999(77)90260-6. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Nakayama T, Ichimiya T, Okubo Y, Takano A, et al. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am J Psychiatry. 2003;160:334–340. doi: 10.1176/appi.ajp.160.2.334. [DOI] [PubMed] [Google Scholar]
- Young AH, Goodwin GM, Dick H, Fink G. Effects of glucocorticoids on 5-HT1A presynaptic function in the mouse. Psychopharmacology (Berl) 1994a;114:360–364. doi: 10.1007/BF02244859. [DOI] [PubMed] [Google Scholar]
- Young AH, MacDonald LM, St John H, Dick H, Goodwin GM. The effects of corticosterone on 5-HT receptor function in rodents. Neuropharmacology. 1992;31:433–438. doi: 10.1016/0028-3908(92)90080-9. [DOI] [PubMed] [Google Scholar]
- Young AH, Sharpley AL, Campling GM, Hockney RA, Cowen PJ. Effects of hydrocortisone on brain 5-HT function and sleep. J Affect Disord. 1994b;32:139–146. doi: 10.1016/0165-0327(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Liou YJ, Hong CJ, Chen TJ. Association study of two serotonin 1A receptor gene polymorphisms and fluoxetine treatment response in Chinese major depressive disorders. Eur Neuropsychopharmacol. 2006;16:498–503. doi: 10.1016/j.euroneuro.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardi R, Artigas F, Franchini L, Sforzini L, Gasperini M, Smeraldi E, Perez J. How long should pindolol be associated with paroxetine to improve the antidepressant response? J Clin Psychopharmacol. 1997;17:446–450. doi: 10.1097/00004714-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Franchini L, Gasperini M, Lucca A, Smeraldi E, Perez J. Faster onset of action of fluvoxamine in combination with pindolol in the treatment of delusional depression: a controlled study. J Clin Psychopharmacol. 1998;18:441–446. doi: 10.1097/00004714-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Serretti A, Rossini D, Franchini L, Cusin C, Lattuada E, et al. Factors affecting fluvoxamine antidepressant activity: influence of pindolol and 5-HTTLPR in delusional and nondelusional depression. Biol Psychiatry. 2001;50:323–330. doi: 10.1016/s0006-3223(01)01118-0. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Preuss UW, Bondy B, Frodl T, Zill P, Schmitt G, Koutsouleris N, Rujescu D, Born C, Reiser M, Möller H-J, Meisenzahl EM. 5-HT1A receptor gene C–1019 G polymorphism and amygdala volume in borderline personality disorder. Genes Brain Behavior. 2007 doi: 10.1111/j.1601-183X.2007.00353.x. In Press. [DOI] [PubMed] [Google Scholar]
- Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y, et al. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill PBT, Zwanzger P, Schule C, Minov C, Behrens S, Rupprecht R, Bondy B. Association study of a common polymorphism in the human 5HT1A receptor gene in major depression. Pharmacopsychiatry. 2001;34:238. [Google Scholar]
- Zimmer L, Riad M, Rbah L, Belkacem-Kahlouli A, Le Bars D, Renaud B, Descarries L. Toward brain imaging of serotonin 5-HT1A autoreceptor internalization. Neuroimage. 2004;22:1421–1426. doi: 10.1016/j.neuroimage.2004.03.020. [DOI] [PubMed] [Google Scholar]