Abstract
Pigeons are used frequently as subjects in behavioral pharmacology research. An advantage of the pigeon is an exceedingly vascular breast muscle, which is easily accessible for injections. The purpose of these studies was to provide a profile of the pharmacokinetics of (+)-methamphetamine (METH) and (+)-amphetamine (AMP), a pharmacologically active metabolite, in pigeons (n = 6) after intramuscular (IM) and intravenous (IV) dosing (0.8 mg/kg). LC-MS/MS analysis was used to determine serum concentrations of METH and AMP. A modified crossover design was used to determine the bioavailability, time to maximum concentration, total clearance, the volume of distribution, the maximal concentration, the area under the concentration-time curve (AUC), and terminal elimination half-life for METH. The route of administration did not significantly affect these pharmacokinetic parameters. The time to maximum concentration for METH and AMP following IM administration was 0.3 h. Maximum AMP serum concentrations were achieved in 2 h, irrespective of the route of administration, and these concentrations remained essentially constant for an additional 6 h. The metabolism of METH to AMP was not affected by the route of administration, and the molar ratio AMP to METH AUC values were the same (IV=0.57; IM=0.41). These results show that METH pharmacokinetics after IM administration in the pigeon are similar to IV administration. Thus IM is a reasonable route of administration for METH behavioral assays in the pigeon if sufficient time for absorption is given following the dose, and the behavioral endpoint is not dependent on the rapid input of METH following an IV dose.
Keywords: Pigeon, Methamphetamine, Amphetamine, Pharmacokinetics, Bioavailability, LC-MS/MS
1. Introduction
(+)-Methamphetamine (METH) abuse is one of the most serious health problems in United States and Europe because of its abuse liability and potential neurotoxic effects (Sekine et al. 2001; Sekine et al. 2006; Volkow et al. 2001). While METH abuse in the general population appears to have stabilized, or decreased slightly, abuse in certain populations is increasing (Halkitis et al. 2005; Shoptaw et al. 2006). Abuse potential of drugs, as well as treatments for drug abuse, are often tested in animal behavioral pharmacology models. Drug discrimination is one of several behavioral assays used to test these drugs in animals. In a simple drug discrimination study, an animal is trained to make a different response depending on whether a drug is present or not (i.e., to discriminate whether a drug is present or absent). Once stimulus control of responding is established, a different drug can be substituted for the training drug. Drugs that produce the same externally observed stimulus control are thought to also produce internal stimuli that can be detected by the subject, (Overton et al. 1986; Overton et al. 1999; Schuster & Johanson 1988). In this manner, drugs can be classified into pharmacological classes.
It has long been recognized the study procedural factors can affect the drug stimulus. In fact, procedural changes can affect the results of many behavioral assays (e.g., locomotor activity, self-administration). Järbe and Kroon-Järbe have shown that the speed with which rats established discriminative responding to AMP and cocaine was dependent on the whether the incentive was food or electrical shock (Jarbe & Kroon-Jarbe 1983). Green-Jordan et al., have shown that the discriminative stimulus affects of cocaine were influenced by the pretreatment time of various μ opioid agonists (Green-Jordan et al. 2001). Several investigators have shown that the route of administration can affect the outcome of the behavioral endpoint. For example, the dose required for METH or AMP stimulus control in rats was 5-fold lower when these drugs were administered IV or subcutaneous (SC) compared with intraperitoneal (IP) administration (Ando 1975; Ando et al. 1994). Gentry et al., found that the locomotor effects and stereotypical behavior of METH was dependant on the route of administration (i.e., IP or SC). These authors attributed the changes in behavior to the observed changes in METH pharmacokinetics (Gentry et al. 2004). In general, route-dependent behavioral outcomes have been attributed to differences in absorption and a higher degree of first-pass metabolism with some routes of administration. First-pass metabolism occurs when a drug with metabolic potential passes through sites of elimination like the gastrointestinal tract or the liver prior to reaching systemic circulation. First-pass metabolism is avoided following IV and IM administration. The absorption of small molecules, like METH, from muscle is dissolution and perfusion rate-limited. Therefore, the absorption of METH into the breast muscle of a pigeon, with many capillaries, is expected to be extensive.
The behavioral pharmacology of METH and other amphetamines has been studied extensively in a number of species, including the rat, pigeon, mouse, and monkey (Itzhak & Ali 2002; McMillan & Wessinger 1989; Suzuki et al. 2004; Woolverton et al. 1989). Discernment of the psychostimulant properties of METH is complicated by the fact that amphetamine (AMP) is a metabolite of METH with similar psychomotor stimulant properties. Sasaki et al. have shown that pigeons attained stimulus control with METH and subsequently generalized on the drug appropriate key with AMP (Sasaki et al. 1995), suggesting that METH and AMP share the same discriminative stimulus. Sasaki et al. further suggested that because METH and AMP shared discriminative stimulus cues, these compounds shared a similar mechanism of action. Similarly, Milesi-Hallé et al. have recently shown that AMP and METH were equally potent in inducing locomotor activity in rats (Milesi-Halle et al. 2007). It is unknown how much METH is metabolized to AMP following IM administration of METH to pigeons. If a sufficient amount of METH were metabolized to AMP to cause an AMP response, it would be difficult to discern the individual or combined effects of METH and AMP behavioral endpoints in these animals.
Pigeons are used for drug discrimination studies due in part to the animal’s ability to distinguish color and space (McClure et al. 2005; McMillan 1990; Stubbs 1968). IM administration of drugs is the preferred route in pigeons because it is convenient and the breast muscle of the pigeon is well vascularized. Most experimental protocols allow a certain amount of time to elapse after administration of the drug, usually 10-min before the behavioral session begins. This 10-min pre-session interval is incorporated into the experiment to allow absorption of the drug, but often without prior knowledge of true absorption characteristics of the drug. Despite the potential impact of administration route on the pharmacokinetics (i.e., bioavailability, time to maximum concentration, maximum concentration, and pharmacodynamics), most behavioral assays are conducted using an extravascular route of drug administration (i.e., IM or IP) (Gentry et al. 2004; Kitaichi et al. 2003; Samaha et al. 2005). Extravascular routes of administration can bias the desired effects of the drug if the actions of the drug are due to rapid penetration into the brain or route specific differences in metabolism (Mactutus et al. 2000). There is a growing body of evidence that rapid penetration into the brain is particularly important to the abuse liability of stimulants in humans (Samaha & Robinson 2005; Volkow et al. 2000). Therefore, it is important to understand the relationship of the route of drug administration to pharmacokinetics and behavioral pharmacology.
The purpose of these studies was to characterize METH pharmacokinetics after IM and IV dosing to determine if these routes of administration affected the pharmacokinetic parameters and bioavailability of METH in pigeons. Because AMP is a potent metabolite of METH, AMP serum concentrations were determined as well. The molar ratio of AMP to METH for each route was assessed using the area under the concentration time curve for each compound. The implications of these results, as they relate to the behavioral experimental approach is also discussed.
2. Methods
2.1. Animals
Six male White Carneau pigeons (Palmetto Pigeon Plant, Sumter, SC) served as subjects in these experiments. All birds were housed individually, and allowed free access to food and water. The birds had been used in drug discrimination studies prior to the present experiments. (McMillan et al. 2001; McMillan et al. 2002). The vivarium was temperature and humidity controlled, with a light cycle from 06.00 to 18.00 hours and a dark cycle of 18.00 to 06.00 hours. Pharmacokinetic studies were initiated at 09.00 hours. All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences and were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
2.2. Pharmacokinetic Experiments
(+)-Methamphetamine hydrochloride and (+)-amphetamine sulfate, 4-hydroxyamphetamine hydrochloride and 4-hydroxymethamphetamine hydrochloride were obtained from the National Institute on Drug Abuse (Rockville, MD). Pharmacokinetic parameters for METH were determined in 6 pigeons following administration of 0.8 mg/kg METH (expressed as the free base) by the IV and IM routes of administration in sterile saline (1 ml/kg body weight). The IV dose was administered as a bolus in the right brachial vein using a 26 G needle and 1 ml tuberculin syringe. Blood samples were collected from the left brachial vein following IV administration through an indwelling catheter (20 G × 1 in) (Introcan Safety Braun Medical Co., Bethlehem, PA). The IM dose was administered 1 week later as a bolus in the breast muscle using a 1 ml tuberculin syringe (26 G). A catheter was used to take blood samples from the right brachial vein following IM administration of METH. The IM dose was always administered on the second week because this route did not require a viable vein for dosing. The 20 G needle on the catheter caused a hematoma, which required 4 weeks to heal before the vein was viable again. Therefore, the IV dose always preceded the IM dose. Blood samples (100 – 200 μl) were collected prior to METH injection and at 1, 2, 5, 10, 20, 60, 120, 240, 360, and 420 min after the injection. No more than 2.5 ml of blood was obtained from each bird during the 7 hr pharmacokinetic study. The catheter was flushed with 200 μl of saline after each blood sample was taken. The catheter was kept patent by occasionally flushing with 100 μl of saline. The blood was allowed to clot (30 – 45 min) at room temperature and the serum was collected after centrifugation (10621 × g for 10 min). The serum samples were stored at −30 °C and analyzed within 24 hr by liquid chromatography with tandem mass spectrometric detection (LC-MS/MS).
2.3 LC-MS/MS of METH, AMP, and 4-hydroxylated metabolites in serum
METH and AMP serum concentrations were determined using LC-MS/MS methodology published previously (Hendrickson et al. 2006). A Waters Alliance 2690 HPLC system coupled to a Micromass Quattro LC triple quadrupole mass spectrometer (Waters Corp, Beverly MA) was used for LC-MS/MS analysis. Pigeon serum samples (50 μl) were prepared for LC-MS/MS analysis by first diluting each sample with 50 μl of normal rat serum (Pel Freez Biologicals, Rogers, AR). Internal standard (10 μl), containing amphetamine-d11 (Sigma/Isotech, St. Louis, MO) and methamphetamine-d5 (Sigma/Isotech), was added to each sample. METH, AMP, OH-METH, and OH-AMP were separated and detected using a Waters 2695 Alliance System (Waters Corp., Milford, MA) and a Micromass Quattro LC triple quadrupole mass spectrometer equipped with an electrospray interface (Waters Corp). Analytical separation was achieved on 100 × 2.1 mm, 3μm Hypersil BDS C8 column with a guard column (10 × 2.1 mm, 3 μm) (Thermo Electron Corp, Bellefonte, PA). The mobile phase consisted of solvent A (5 mM ammonium acetate (pH 3.7) with 5% (v/v) acetonitrile) and solvent B (5 mM ammonium acetate (pH 3.7) with 95% (v/v) acetonitrile). The flow rate was 0.3 ml/min. The linear gradient was as follows: 0 – 2 min, 0% B; 2 –4 min: 0 – 65% B; 4 – 8 min: 65% B; 8 – 10 min: 65% - 0% B; 10 – 14 min 0% B. The MS/MS experiments were performed by collision-induced dissociation with argon as the target gas (2 × 10−3 torr). METH, AMP, OH-METH, and OH-AMP were quantitated using the following precursor → product m/z values: 150 → 91, 136 → 91, 166 → 107, and 152 → 107, respectively. Quantitation was achieved using the internal standard approach. Calibration standards (0.3 ng/ml – 1000 ng/ml) were prepared in normal rat serum and treated as described above for authentic pigeon samples. A separate set of quality control samples (3, 10, and 800 ng/ml) were also prepared in rat serum and analyzed as described above. The predicted values at the lower limit of quantitation (1 ng/ml) were within ± 25% of the nominal concentration. The precision at the lower limit of quantitation was ± 12%.
2.4. Pharmacokinetic data analysis
METH and AMP serum concentration-time data were analyzed by model-independent pharmacokinetic analysis methods using PK Solutions 2.0 (Summit Research Services, Montrose, CO). Cmax was the maximum observed concentration and tmax was the time point at Cmax. The METH elimination half-life (t1/2λz) was determined from the slope of the linear terminal portion of the log concentration-time curve. The area under the METH concentration time curve (AUC) from the time of dosing to the last measure time point (tn) was determined by the linear trapezoidal rule. The remaining area to time infinity was determined from last measured concentration (Cn) and the terminal elimination rate constant (λz). Thus, the equation for AUC∞ was
The apparent volume of distribution (Vd) was determined from the AUC and λz according to the equation,
where D equaled the dose. Total clearance (CL) was determined from the dose and AUC according to the equation,
The bioavailability (F) of METH was determined using the equation F = (AUCIM/AUCIV). All values are presented as the mean ± S.D. The area under the AMP concentration time curve was determined using the linear trapezoidal rule on the observed data only. This same method was used to determine the area the METH concentration time curve when calculating the AMP-METH ratio. Curves were fit to the data using a two or three component exponential and took the general form,
2.5. Statistics
The pharmacokinetic values (i.e., AUC, Cmax, Vd, CL, and tmax) for METH and AMP were compared using a two-tailed matched pairs Wilcoxon test. A Wilcoxon rank sum was used to compare the median bioavailability (F) to 1.0. The level of significance was set at the 95% confidence interval (P < 0.05). Statistical analysis was performed using Prism v4.0c (Graphpad Software, Inc., San Diego, CA).
3. Results
3.1. Bioavailability of METH
Serum concentration-time curves for METH and AMP are shown for each of the six birds in Figure 1. Blood samples were not collected for a sufficient amount of time to determine the elimination half-life for AMP. Serum concentrations of the hydroxylated metabolites, OH-METH and OH-AMP, were below the lower limit of quantitation (< 1 ng/ml). The most striking feature of the data shown in Figure 1 is that METH concentrations did not reach a maximum until 20 ± 19 min after IM administration of METH, but the characteristics of METH elimination were not effected by the route of administration. But in the first 10-min following drug administration, the METH serum concentration was always higher following IV administration in 4 of the 6 birds tested. On average (n = 6), this concentration was 3-fold higher following an IV dose of METH. Pharmacokinetic parameters (AUC, Cmax, tmax, Vd, and CL, ) for METH following IV and IM administration in each of the pigeons are shown in Table 1. AUCIV and AUCIM were not significantly different from each other, indicating that the IM bioavailability for METH approached 100%. In fact, no significant differences were observed for any of the pharmacokinetic parameters.
Figure 1.
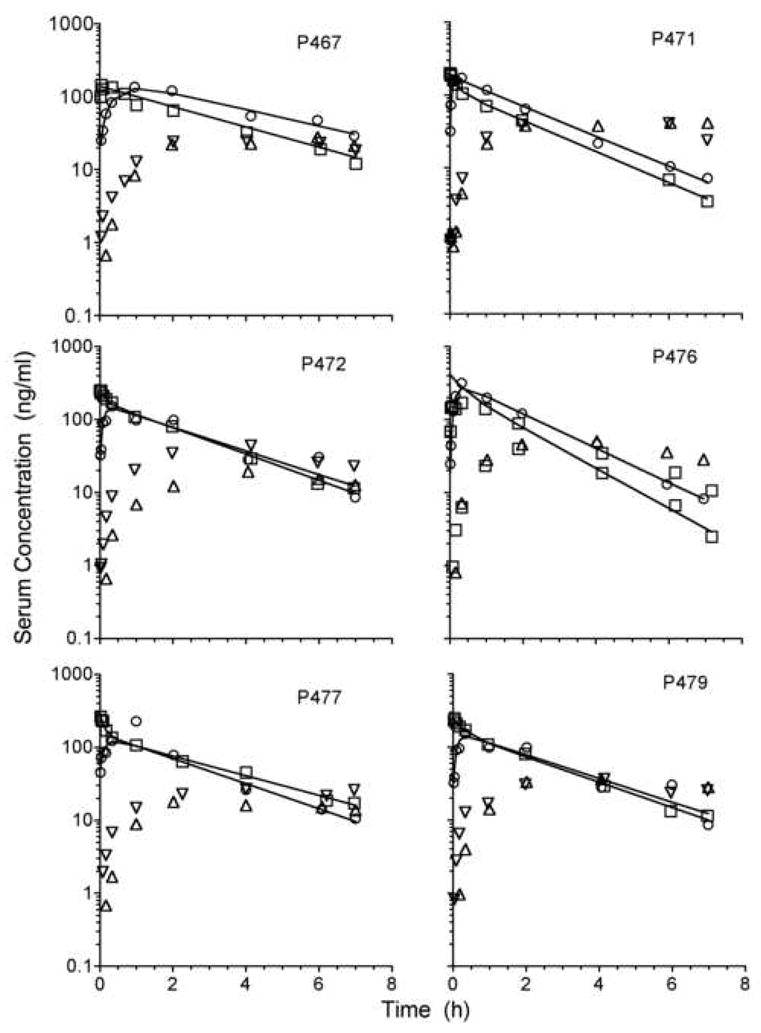
Serum concentration-time profiles for (+)-methamphetamine (METH) and (+)-amphetamine (AMP) following either intravenous (▽, AMP and □, METH) or intramuscular (△, AMP and ○, METH) administration of 0.8 mg/kg METH to pigeons.
Table 1.
Effects of route of administration on pharmacokinetic parametersa for METH and its metabolite, AMP, following a single 0.8 mg/kg METH dose.
| IV | IM | |||
|---|---|---|---|---|
| Parameter | METH | AMP | METH | AMP |
| AUC∞ (h*ng/mL) | 412 ± 72b | 508 ± 112 | ||
| Vd (ml) | 4930 ± 1268 | 4209 ± 980 | ||
| CL (ml/h) | 2006 ± 438 | 1633 ± 339 | ||
| t1/2λz (h) | 1.73 ± 0.46 | 1.81 ± 0.48 | ||
| tmax (h) | N.A. | 2.0 ± 0.8 | 0.3 ± 0.3 | 2.0 ± 0.9 |
| Cmax (ng/mL) | N.A. | 35 ± 9 | 162 ± 69 | 28 ± 11 |
| AUC(obs) (h*nmol/mL) | 2.8 ± 0.4 | 1.4 ± 0.3 | 3.0 ± 0.6 | 1.2 ± 0.5 |
| Molar AMP:METH Ratio | 0.56 ± 0.21 | 0.41 ± 0.19 | ||
Model-independent pharmacokinetic analysis of METH and AMP concentration-time data collected after IM and IV METH administration. The AUC∞ was determined using the trapezoidal rule on the observed data (AUCobs) plus the area of the extrapolated data (Cn/λz).
Values are the mean ± the standard deviation (n=6).
Complete AMP serum concentration-time curves were not collected in this study, and, therefore, the total amount of AMP formed in the pigeons from the parent compound METH could not be determined with a high degree of certainty. An AMP elimination half-life could not be determined from the data so these AUC0→t values were calculated from the observed data only. In order to assess whether METH metabolism to AMP was affected by the route of administration, tmax and AUC values were determined. The area under the concentration (AUC)-time curve for AMP was slightly higher following IV administration versus IM administration, but not significantly so. The observed mole equivalent AUC ratio of AMP:METH was 0.57 and 0.41 for the IV and IM routes, respectively. The concentration of AMP at tmax was not affected by the route either, thus supporting the suggestion that metabolism of METH to AMP was unaffected by the route of administration (Table 1). The AUC value for AMP was significantly underestimated since complete serum concentration curves were not obtained for AMP. This means that the actual AUC ratio may be higher than reported here and AMP may contribute more extensively to the pharmacodynamic response at later times after the METH dose.
4. Discussion
METH has been routinely administered to rats (SC, IP and IV) at doses of 3.0 mg/kg METH (Gentry et al. 2004) and to pigeons (IM) at doses up to 4.8 mg/kg METH (Li & McMillan 1998) with no observable toxicity. However Byrnes-Blake et al. have reported that doses of 5.6 mg/kg (IV) caused self-mutilation in rats and a dose of 10 mg/kg (IV) was lethal (Byrnes-Blake et al. 2003). Attempts to administer 3.6 and 6.7 mg/kg (IM or IV) in the present METH pharmacokinetic study was also lethal to pigeons. The primary difference between the pharmacokinetic studies described here, and the drug discrimination studies conducted at higher METH doses, was that the birds were not handled to the same degree in the previous drug discrimination studies. The added handling, which was necessary for these pharmacokinetic studies, may have caused additional stress to the birds, and led to a more potent toxic METH response.
The principal findings of this study were that METH intramuscular bioavailability approaches 100%, but as expected there was delay in the absorption of METH following IM administration. This delay in absorption may impact the design of some METH behavioral studies. Although a systematic study of the effects of pre-session interval time on METH responding has not been conducted, it has generally been assumed that METH concentrations reach a point which produces a stable pharmacodynamic response in 10 to 20 min following IM or intraperitoneal administration of METH. The current pharmacokinetic studies suggest that a 10 min pre-session time may not be appropriate, if affects from the high concentrations achieved following IV administration of METH are what are needed for the behavioral endpoint. This high concentration might be achieved with an increase in the IM dose, but the pharmacokinetics could not be determined at higher doses without significant toxicity to the pigeons. Therefore, behavioral affects caused by route-dependent pharmacokinetics could not be determined at these higher doses.
The data presented herein have shown that the serum pharmacokinetics METH were the same regardless of whether METH was administered IV or IM, and suggest that the behavioral effects of METH should be the same regardless of the route of administration. Indeed, the discriminative stimulus properties of stimulant drugs have been studied in pigeons and non-human primates following various routes of administration. For example, de la Garza et al. investigated the effects of three routes of administration on the discriminative stimulus properties of AMP in Rhesus monkeys (de la Garza et al. 1984). A 10-min pre-session was used following IV and IM administration, while a 60-min pre-session was used when AMP was administered intragastrically. These investigators found no difference in ED50 values if AMP was administered IV or IM, but found that AMP was a less potent stimulus if given intragastrically. While METH is a different than AMP, the serum pharmacokinetic study presented herein, supports the notion that METH behavioral affects should be the same regardless of whether the METH is administered IV or IM, if 20-min is allotted for absorption of the drug, since there were no differences in clearance, elimination half-life, or volume of distribution between the two routes.
Most studies that have compared the stimulus properties of METH and AMP have found these compounds have the same stimuli with equal potency. For example, AMP and METH were equipotent as discriminative stimuli in the pigeon (Li & McMillan 2001) and there was cross generalization between these drugs (Sasaki et al. 1995) if the pre-session time was relatively long (i.e., 10 to 20 min). Because METH and AMP have been shown to be equal in potency with most behavioral assays, it may be difficult to discern whether AMP or METH is responsible for the drug appropriate responding when METH is administered. But most drug affects are complete within two hours or less and the studies presented herein indicate that AMP and METH serum concentrations are not equal until 4 hours after METH administration. Therefore, it is unlikely that AMP impacts the behavioral affects due to METH in the pigeon at the dose used in this study. This conclusion is supported by data recently reported by Gentry et al. (2004), where the effects of SC and IP METH administration on the pharmacokinetics and behavior were studied in rats. These investigators reported that the efficiency of the metabolism of METH to AMP in rats was independent of the route of administration when the dose was relatively small (i.e., 0.3 or 1 mg/kg). A comparison of the factors controlling AMP elimination in the rat and pigeon shows that there are differences in AMP elimination between these two animals. Serum AMP concentration time-curves in the pigeon (Figure 1) clearly show that the rate-limiting step in the overall AMP elimination was elimination-limited, and not formation-limited. In other words, AMP was formed at a rate that was faster than it was being eliminated (Figure 1). The characteristics of AMP elimination following a single METH dose in the rat have also been reported by several investigators (Byrnes-Blake et al. 2003; Cho et al. 2001; Milesi-Halle et al. 2005; Riviere et al. 2000). Milesi-Halle et al. have reported a much lower AMP to METH ratio in the male and female rat following a 1 mg/kg IV dose (i.e., 0.29 and 0.22) when compared to the pigeon (i.e., 0.47 with an IV dose) (Milesi-Halle et al. 2005). The Cmax for AMP was higher in the pigeon (35 ng/ml) relative to the rat (15 ng/ml) suggesting that AMP might contribute to the behavioral affects observed in the pigeon to a greater extent than possible in the rat. But any contribution from AMP to the behavioral affects are still unlikely in the first two hours, following METH administration, since AMP did not reach a maximum concentration until two hours. AMP elimination was rate-limited by the formation of AMP, but the rate of AMP elimination was significantly faster in the rat when compared to the pigeon. Despite the faster rate of AMP accumulation in the pigeon, it is unlikely that AMP does in fact affect METH responding since the AMP concentrations are low during the time behavior is typically monitored.
In summary, these experiments suggest that the serum pharmacokinetics of METH and AMP after METH IV or IM administration are not different. Because METH distribution into the brain was not evaluated in this study, there may still exist route-related differences in the overall distribution. Because IV administration of METH produces rapid and extensive distribution of METH into the brain, when compared with other routes of administration it is likely that IV dosing of METH will produce a unique behavioral response (Riviere et al. 2000).
Acknowledgments
This work was supported by NIDA grants P01DA14361, R01DA2251, and a Mentored Basic Scientist Award (K25DA14601).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando K. The discriminative control of operant behavior by intravenous administration of drugs in rats. Psychopharmacologia. 1975;45:47–50. [Google Scholar]
- Ando K, Miyata H, Yanagita T. Effects of methamphetamine, dopamine and noradrenaline administered into the nucleus accumbens of rats discriminating subcutaneous methamphetamine. Jpn J Pharmacol. 1994;64:35–40. doi: 10.1254/jjp.64.35. [DOI] [PubMed] [Google Scholar]
- Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, et al. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol. 2003;461:119–28. doi: 10.1016/s0014-2999(03)01313-x. [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39:161–6. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE, Schuster CR. The discriminative stimulus properties of cocaine and d-amphetamine: a comparison of three routes of administration. NIDA Res Monogr. 1984;49:150–5. [PubMed] [Google Scholar]
- Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol Biochem Behav. 2004;79:751–60. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Green-Jordan K, Warren L, Kantak KM. Temporal factors affecting cocaine-opioid interactions: a cocaine drug discrimination study in rats. Psychopharmacology (Berl) 2001;156:427–34. doi: 10.1007/s002130100732. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Shrem MT, Martin FW. Sexual behavior patterns of methamphetamine-using gay and bisexual men. Subst Use Misuse. 2005;40:703–19. doi: 10.1081/ja-200055393. [DOI] [PubMed] [Google Scholar]
- Hendrickson H, Laurenzana E, Owens SM. Quantitative determination of total methamphetamine and active metabolites in rat tissue by liquid chromatography with tandem mass spectrometric detection. AAPS J. 2006;8:E709–17. doi: 10.1208/aapsj080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Behavioral consequences of methamphetamine-induced neurotoxicity in mice: relevance to the psychopathology of methamphetamine addiction. Ann N Y Acad Sci. 2002;965:127–35. doi: 10.1111/j.1749-6632.2002.tb04156.x. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Kroon-Jarbe ER. Discriminability and procedure: effects of cocaine and amphetamine. Psychol Rep. 1983;52:611–6. doi: 10.2466/pr0.1983.52.2.611. [DOI] [PubMed] [Google Scholar]
- Kitaichi K, Morishita Y, Doi Y, Ueyama J, Matsushima M, Zhao YL, et al. Increased plasma concentration and brain penetration of methamphetamine in behaviorally sensitized rats. Eur J Pharmacol. 2003;464:39–48. doi: 10.1016/s0014-2999(03)01321-9. [DOI] [PubMed] [Google Scholar]
- Li M, McMillan DE. The effects of drug discrimination history on drug discrimination and on punished and unpunished responding. Pharmacol Biochem Behav. 1998;61:93–105. doi: 10.1016/s0091-3057(98)00077-x. [DOI] [PubMed] [Google Scholar]
- Li M, McMillan DE. Four-choice drug discrimination in pigeons. Behav Pharmacol. 2001;12:621–8. doi: 10.1097/00008877-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Booze RM, Dowell RT. The influence of route of administration on the acute cardiovascular effects of cocaine in conscious unrestrained pregnant rats. Neurotoxicol Teratol. 2000;22:357–68. doi: 10.1016/s0892-0362(99)00084-7. [DOI] [PubMed] [Google Scholar]
- McClure EA, Saulsgiver KA, Wynne CD. Effects of D-amphetamine on temporal discrimination in pigeons. Behav Pharmacol. 2005;16:193–208. doi: 10.1097/01.fbp.0000171773.69292.bd. [DOI] [PubMed] [Google Scholar]
- McMillan DE. The pigeon as a model for comparative behavioral pharmacology and toxicology. Neurotoxicol Teratol. 1990;12:523–9. doi: 10.1016/0892-0362(90)90017-7. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M. Discrimination of pentobarbital doses and drug mixtures under fixed-ratio and fixed-interval reinforcement schedules. Behav Pharmacol. 2001;12:195–208. doi: 10.1097/00008877-200105000-00005. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M, Owens SM. Pharmacokinetic antagonism of (+)-methamphetamine discrimination by a low-affinity monoclonal anti-methamphetamine antibody. Behav Pharmacol. 2002;13:465–73. doi: 10.1097/00008877-200209000-00019. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Wessinger WD. Interaction of the discriminative stimulus effects of phencyclidine with those of (+)-N-allylnormetazocine, pentobarbital and d-amphetamine. Pharmacol Biochem Behav. 1989;32:711–5. doi: 10.1016/0091-3057(89)90022-1. [DOI] [PubMed] [Google Scholar]
- Milesi-Halle A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex-and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005 doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–9. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton DA, Leonard WR, Merkle DA. Methods for measuring the strength of discriminable drug effects. Neurosci Biobehav Rev. 1986;10:251–63. doi: 10.1016/0149-7634(86)90012-6. [DOI] [PubMed] [Google Scholar]
- Overton DA, Rosecrans JA, Barry H., 3rd Creation and first 20 years of the society for the stimulus properties of drugs (SSPD) Pharmacol Biochem Behav. 1999;64:347–52. doi: 10.1016/s0091-3057(99)00074-x. [DOI] [PubMed] [Google Scholar]
- Riviere GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292:1042–7. [PubMed] [Google Scholar]
- Samaha A-N, Yau W-YW, Yang P, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biological Psychiatry. 2005;57:351–60. doi: 10.1016/j.biopsych.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–7. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Sasaki JE, Tatham TA, Barrett JE. The discriminative stimulus effects of methamphetamine in pigeons. Psychopharmacology (Berl) 1995;120:303–10. doi: 10.1007/BF02311178. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser. 1988;4:161–75. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–14. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Klausner JD, Reback CJ, Tierney S, Stansell J, Hare CB, et al. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health. 2006;6:214. doi: 10.1186/1471-2458-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs A. The discrimination of stimulus duration by pigeons. J Exp Anal Behav. 1968;11:223–38. doi: 10.1901/jeab.1968.11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Fukuoka Y, Mori T, Miyatake M, Narita M. Behavioral sensitization to the discriminative stimulus effects of methamphetamine in rats. Eur J Pharmacol. 2004;498:157–61. doi: 10.1016/j.ejphar.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–15. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–8. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]


