Abstract
Objective
To evaluate diagnostic properties of International Classification of Diseases, Version 9 (ICD-9) diagnosis codes and infection criteria to identify bacterial infections among rheumatoid arthritis (RA) patients.
Study Design and Setting
We performed a cross- sectional study of RA patients with and without ICD-9 codes for bacterial infections. Sixteen bacterial infection criteria were developed. Diagnostic properties of comprehensive and restrictive sets of ICD-9 codes and the infection criteria were tested against an adjudicated review of medical records.
Results
Records on 162 RA patients with and 50 without purported bacterial infections were reviewed. Positive (PPV) and negative predictive values (NPVs) of ICD-9 codes ranged from 54% – 85% and 84% – 100%, respectively. PPVs of the medical records-based criteria were: 84% and 89% for “definite” and “definite or empirically treated” infections, respectively. PPV of infection criteria increased by 50% as disease prevalence increased using ICD-9 codes to enhance infection likelihood.
Conclusion
ICD-9 codes alone may misclassify bacterial infections in hospitalized RA patients. Misclassification varies with the specificity of the codes used and strength of evidence required to confirm infections. Combining ICD-9 codes with infection criteria identified infections with greatest accuracy. Novel infection criteria may limit the requirement to review medical records.
Keywords: Bacterial Infections, Diagnostic coding, Administrative claims data, Rheumatoid Arthritis, Bacterial infection classification criteria, Validation study
Bacterial infections in rheumatoid arthritis (RA) are common [1, 2] and are of growing interest based on an increasing number of serious infections reported in patients receiving biologic therapies [3–6]. A comprehensive understanding of the associations between infection with RA and the use of specific therapeutic agents has been limited by the absence of objective criteria to correctly identify infection in studies of large populations. Misclassifying infections may mask the risks related to use of particular arthritis medications [6] or could introduce bias if outcome assessment is subjective and reviewers are not blinded to medication exposure. A validated set of diagnostic criteria for a broad range of infections has been lacking in the medical literature and exists mainly for isolated infections such as the Duke criteria for endocarditis [7]. Additionally, although the accuracy of administrative claims data has been studied for various conditions [8–10], their ability to accurately identify bacterial infections in a hospitalized rheumatoid arthritis population is largely unknown.
To address these methodological gaps, we sought to evaluate the accuracy of the International Classification of Diseases, Version 9, Clinical Modification (ICD-9) codes commonly used to identify infection outcomes in epidemiologic research, by evaluating a population of RA patients who was hospitalized. Additionally, we constructed medical records- based infection criteria for bacterial infections that could be used to validate the presence of infection when applied to abstracted medical information. Both the claims-based algorithms and the medical records- based infection criteria were validated against a standard of physician panel review of medical records for hospitalized RA patients.
METHODS
Study population and infection case identification
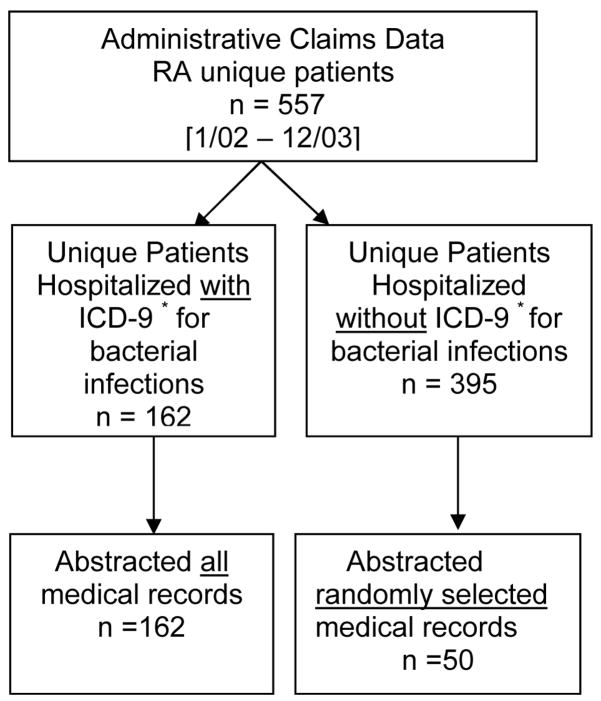
After local institutional review board approval, we used the administrative claims data from the University of Alabama at Birmingham (UAB) health system to identify adults (age ≥ 18 years at the time of hospitalization) with one or more diagnostic codes for RA (ICD-9, 714.X in any position on the hospital discharge claim), who were hospitalized at our institution between January 2002 and December 2003. We compiled two sets of ICD-9 codes for infections (Appendix 1) based on expert consensus, 1) a “comprehensive” set that included a wide range of codes, with the goal of maximizing sensitivity; 2) a “restricted” set that included presumably more specific ICD-9 codes, as used by Schneeweiss and colleagues to validate infections in a Veterans Affairs (VA) hospital [11]. Both these lists were grouped by anatomical site. Because hospitals may code the principal diagnosis as the one that leads to the highest reimbursement rather than the etiologic event that prompted the hospitalization, we allowed these infection codes to be in any position on the billing claim. We abstracted medical records of the first hospitalization during the study period that had an ICD-9 code for any bacterial infection (Figure 1). To ascertain the sensitivity of the administrative data to identify bacterial infections, medical records of 50 RA patients without a discharge diagnosis of infection in any position on the claims were randomly selected and similarly studied. The primary discharge diagnoses on the claims for these patients ranged from codes for arthritis related hospitalizations to codes for other systemic conditions such as multiple sclerosis, myocardial infarctions, and surgical treatments. None of the primary discharge diagnoses codes suggested admissions for treatment of putative infections.
Figure 1.
Selection of rheumatoid arthritis (RA) patient population for assessing diagnostic properties of ICD-9* codes and medical records-based infection criteria for serious bacterial infections.
* ICD-9, International Classification of Diseases, Version 9, Clinical Modification.
Medical record abstraction and diagnostic criteria for bacterial infections
We developed medical records-based infection criteria that encompassed 16 groups of bacterial infections commonly treated in clinical practice. As part of this process, we conducted a comprehensive literature review and integrated clinical, laboratory, microbiological, and radiological criteria used to diagnose infections (Appendix 2). These medical records-based infection criteria were further refined in collaboration with infectious disease specialists (N.A., M.S., A.R.), a gastroenterologist (C.E.), and a radiologist (R.L.). We excluded those microbes from bacterial cultures that were likely “contaminants” and whenever possible, we required objective criteria (e.g. culture data) to fulfill the infection criteria in order to maximize specificity. A medical record abstraction form was developed to collect relevant details of all bacterial infections that comprised these infection criteria. It included (among numerous data elements) results from up to six bacterial cultures and also the name and duration of use of all antibiotic treatments. Our medical record abstraction form though very similar to that used by Schneeweiss and colleagues [11], additionally included intra-abdominal bacterial infections requiring hospitalization that occurred in the presence of cholecystitis, diverticulitis or gastroenteritis. However, we did not capture non-bacterial atypical or opportunistic infections, as was done by Schneeweiss and colleagues [11]. The medical record abstraction form was extensively pilot tested using medical records from RA patients hospitalized in our university facility.
Medical record review to confirm infections
All medical records with any ICD-9 code for bacterial infection(s) were reviewed by a team of three trained reviewers consisting of two physicians (N.M.P., G.G.T.) and one physician assistant (K.C.). Each medical record was independently abstracted by two of the three reviewers. Reviewers assessed the medical records for all categories of bacterial infection, not limited to just those pre-identified by the initial ICD-9 code(s). Based on their clinical judgment, the reviewers’ findings for each type of infection were assigned to three categories, “definite”, “empirically treated” or “no” bacterial infection(s). Anticipating some clinical uncertainty and an occasional paucity of information in medical records, “empirically treated” was listed by the reviewers if after reading the medical record, clear evidence for an infection was lacking, but the treating health care providers appeared to be managing a putative infection. Discordances in the reviewers’ assessment were adjudicated by a consensus of two physicians (K.G.S or J.R.C). These two physicians assigned the case status only among the range of diagnostic categories in discordance.
Statistical analysis
The reviewers’ assessment of certainty of the bacterial infections (“definite”, “empirically treated” or “no”) was used as the standard against which the ICD-9 codes and medical records-based infection criteria were compared to determine sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Inter-rater reliability of the standard was assessed using Cohen’s Kappa [12] as the measure of agreement for the certainty of the bacterial infections between the reviewers’ independent assessment of the medical records. Kappa values lower than 0.40 represent a poor agreement beyond chance, values between 0.40 and 0.75 as fair to good agreement beyond chance and Kappa values higher than 0.75 represent excellent agreement beyond chance. Since there may be circumstances where the high specificity of an infection is desired, one analysis considered only “definite” infections according to our gold standard definition as the outcome point. However, because the high specificity of this approach reduces sensitivity, we also grouped “definite” together with “empirically treated” infections in a separate analysis. Using these two separate standards, we calculated sensitivity, specificity, positive and negative predictive values of the infection criteria and the comprehensive and the restricted sets of ICD-9 codes for at least 1 infection of any type occurring during that hospitalization and organ specific infections (e.g. lung infections such as pneumonia). The predictive values vary with the prevalence of the disease as per the Bayes’ Theorem. For a known prevalence of the disease in a study population, the predictive values can be calculated using the sensitivity and specificity for the test [13]. The findings from the 50 patients without a discharge diagnosis of infection were extrapolated to the additional hospitalized RA patients without ICD-9 evidence of bacterial infections to determine the prevalence of infection in the cohort of 557 patients. The sensitivity and specificity of ICD-9 codes were used to calculate the predictive values of these codes for a range of assumed prevalence of these infections [13].
RESULTS
Of the total 557 RA patients observed in the study period, we abstracted 100% of the relevant records of 162 RA patients hospitalized for the first time with a purported bacterial infection (based on ICD-9 codes) and 50 RA admitted patients without claims for infection (Figure 1). The mean ± standard deviation (SD) age of the patients was 63 ± 14 years and 73% were women. Using claims data, a median of 1.0 bacterial infection was identified per patient per hospitalization; the range of the number of infections per hospitalization was 1 to 5 among 13 possible discharge codes. Only one of the 50 (2%) medical records without claims for infection that were reviewed identified a bacterial infection.
Assessment of infections, inter-rater reliability, and infection prevalence
Prior to adjudication, the inter-rater reliability of the reviewers’ assessment for the presence of any bacterial infection(s) was 0.85 (95% CI 0.74–0.97). Of persons hospitalized with a suspected infection, 41% (n = 87) had “definite” infection using both the restricted and comprehensive codes. In contrast, 64% (n = 135) and 62% (n = 132) had a “definite or empirically treated” infection with the comprehensive and the restrictive ICD-9 codes, respectively. Among all 557 RA patients hospitalized for any condition, the prevalence of “definite” and “definite or empirically treated” infection was 16% and 25%, respectively.
Diagnostic performance characteristics of the ICD-9 codes
The sensitivity of comprehensive ICD-9 codes was higher than that of the restricted set for both “definite”, (100% vs. 59%) and “definite or empirically treated” infections (99% vs. 48%) respectively. The specificities of the comprehensive set of codes as expected were lower than those of the restricted set of codes (Table 1).
Table 1.
Diagnostic properties of comprehensive and restricted set of International Classification of Diseases, Version 9, clinical modification (ICD-9) codes* and medical records-based infection criteria for bacterial infection compared to the gold standard of reviewers’ assessment as “definite” and “definite or empirically treated” infections.
| Comprehensive ICD-9 codes | Restricted ICD-9 codes | Medical Records-based Infection Criteria |
||||
|---|---|---|---|---|---|---|
| Gold Standard of Reviewers’ Assessment |
Sensitivity (%) (95%CI) |
Specificity (%) (95%CI) |
Sensitivity (%) (95%CI) |
Specificity (%) (95%CI) |
Sensitivity (%) (95%CI) |
Specificity (%) (95%CI) |
| Definite Infection (n= 87) | 100 (96–100) | 40 (31–49) | 59 (48–69) | 81 (73–87) | 48 (38–60) | 94 (90–100) |
| Definite or Empirically treated (n= 135) | 99 (96–100) | 64 (52–74) | 48 (40–57) | 86 (77–93) | 41 (32–50) | 91 (82–96) |
Comprehensive and restricted ICD-9 codes are hospital discharge diagnoses codes in any position of the discharge claims and are listed in Appendix 1.
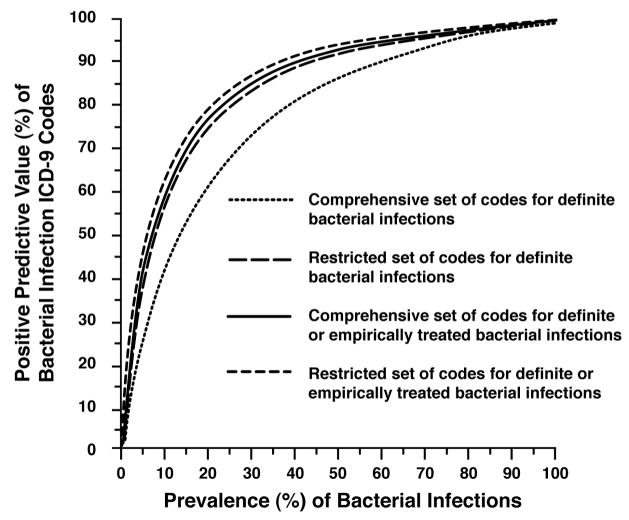
The PPV and NPV of the two sets of ICD-9 codes ranged from 54%–85% and 84% –100% respectively. The effect of varying the prevalence of infections on the PPVs of the comprehensive and the restricted sets of ICD-9 codes are shown in Figure 2. Across infectious disease prevalence rates, the PPVs of the more restricted set of ICD-9 codes to identify either “definite”, or “definite or empirically treated” infections compared to the PPVs of the comprehensive set of ICD-9 codes to identify definite or empirically treated infections were very similar and were consistently greater than 80% at a disease prevalence of 25% (“definite or empiric” infections). Thus these restricted codes demonstrated greater accuracy over using the comprehensive codes in identifying infections. The positive predictive value of the more comprehensive codes to identify only “definite” infections did not exceed 80% unless the expected disease prevalence was at least 40%.
Figure 2.
Positive predictive value (%) of ICD-9* codes according to the prevalence of bacterial Infections, the categories of reviewers’ assessment for infection†, and the groupings‡ of diagnostic codes (based on extrapolation to full cohort of 557 patients with rheumatoid arthritis).
* ICD-9, International Classification of Diseases, Version 9, Clinical Modification
† Category of reviewers’ assessment of medical records for identifying “definite” and “definite or empirically treated” bacterial infections.
‡ Comprehensive and restricted sets of ICD-9 codes of potential bacterial infections.
Diagnostic performance characteristics of the medical records- based infection criteria
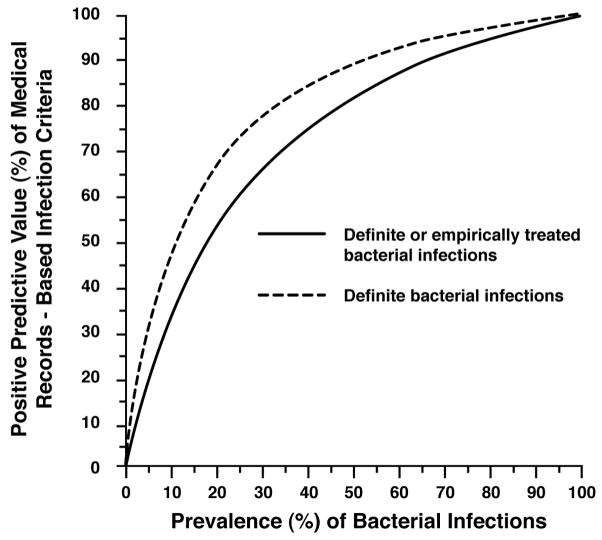
The medical records-based infection criteria were intentionally constructed to favor specificity over sensitivity. The specificity of both “definite” and “definite or empirically treated” infections was very high at 94% and 91% respectively; the sensitivity was similarly low (48% and 41% respectively) (Table 1). The PPV of the infection criteria was 84% for “definite” infections and 89% for “definite or empirically treated” infections. The PPVs of the infection criteria across a range of infection prevalence rates is shown in Figure 3. Figure 3 also shows that bacterial infection detection can be improved by using the ICD-9 codes simultaneously with the medical records-based infection criteria. For example, at a 16% infectious disease prevalence, the PPV of the infection criteria to identify definite bacterial infections was just 60%. By first screening with ICD-9 codes to increase the prevalence of definite bacterial infections to 54%, the PPV of the infection criteria then increased to 90%. Similarly, for identifying “definite or empirically treated” infections, the PPV of these criteria was 60% at a 25% disease prevalence. Using the ICD-9 codes to prescreen potential cases, the disease prevalence increased to 83%, and the PPV was then very high (96%).
Figure 3.
Positive Predictive Value (%) of medical records-based infection criteria according to the prevalence of bacterial Infections and the categories of reviewers’ assessment for infection*.
*Category of reviewers’ assessment of medical records for identifying “definite” and “definite or empirically treated” bacterial infections.
For the most common site-specific infections, the specificities for urinary tract infection, bacteremia, and device-associated medical record infection criteria were each 100% and the specificities of the other sites of infection were only slightly lower (Table 2). The other site-specific infections that we examined occurred less frequently and were therefore not reported separately. These included postoperative infections (n = 11), upper respiratory tract infections (n = 9), osteomyelitis (n = 8), meningitis (n = 7), gastroenteritis (n = 5), septic arthritis and intraabdominal abscesses (n = 3), diverticulitis (n = 2), endocarditis and cholecystitis (n = 1).
Table 2.
Diagnostic properties of medical records-based infection criteria for site-specific bacterial infections* compared to a gold standard of reviewers’ assessment as “definite or empirically treated” infections.
| Treated Infection Type | Sensitivity (%) (95%CI) | Specificity (%) (95%CI) | PPV (%) (95%CI) | NPV (%) (95%CI) |
|---|---|---|---|---|
| Urinary Tract (n = 68) | 5 (1–14) | 100 (63–100) | 100 (29–100) | 12 (5–23) |
| Lower Respiratory Tract (n = 44) | 52 (34–69) | 82 (48–98) | 89 (67–99) | 36 (18–57) |
| Cellulitis (n = 39) | 52 (33–71) | 70 (35–93) | 83 (59–96) | 33 (15–57) |
| Bacteremia (n = 28) | 89 (67–99) | 100 (66–100) | 100 (80–100) | 82 (48–98) |
| Septicemia (n = 26) | 65 (38–86) | 67 (30–93) | 79 (49–95) | 50 (21–79) |
| Device-Associated (n =21) | 24 (7–50) | 100 (40–100) | 100 (40–100) | 24 (7–50) |
Each patient may have ≥ 1 bacterial infections.
PPV= positive predictive value, NPV = negative predictive value.
Discussion
Our study evaluated the diagnostic properties of ICD-9 codes to identify putative bacterial infections in hospitalized rheumatoid arthritis patients. We found that using ICD- 9 codes alone to identify bacterial infections in hospitalized rheumatoid arthritis patients may misclassify 15% to 46% of the infections, depending on the set of codes and the strength of the evidence desired to identify infections. We also developed diagnostic criteria for bacterial infections based on medical records abstraction, and showed that they had very high positive predictive values. A two-stage process where potential cases are identified using claims data and these medical records classified based on the infection criteria, result in a PPV of 96% and eliminate the need for physicians to review each medical record.
Complementary to our work, Schneeweiss and colleagues developed infection criteria and identified suspected infections among general medical patients at a Veterans Administration (VA) hospital using the restricted list of ICD-9 codes that we adopted for this study [11]. In their study, the PPV of the claims data to identify selected bacterial infections combined was similar (90%) to our findings (85%). However, compared to the comprehensive set of codes, this high PPV and its corresponding specificity come at the expense of lower sensitivity (48%). Factors that might account for the modest differences in our studies include inherent dissimilarity in the coding practices between a VA and a university hospital, and differences in the characteristics of general medical patients versus RA patients. In addition, since both these studies were focused on assessing validity of the infection codes neither studies separately assessed possible nosocomial infections. Distinguishing nosocomial from community-acquired infections would be important when assessing susceptibility to infections with use of particular therapeutic interventions for managing RA. Schneeweiss and colleagues selected their study population using only the primary discharge diagnoses codes identified in VA data, excluding nosocomial infections [11]. In contrast, as we had to overcome the possibility of financial incentives in coding for higher reimbursements, we included bacterial infection codes at any position of the discharge claims.
Given their higher sensitivity, a comprehensive set of administrative codes are better able to maximally identify putative bacterial infections. This is necessary when initially researching large administrative databases to identify any potential infections. A restricted set of codes, however, given their better specificity will decrease the number of falsely identified cases of infections. The resultant risk estimates vary with differing sensitivity and specificity. It is important to note though that even if sensitivity is low, high specificity will result in un-biased relative effect measures [14, 15]. In our past experience, we used a “more sensitive” method of applying the medical and pharmacy administrative claims codes from a health-care insurers’ database as a first step for identifying as many cases of presumed serious bacterial infections [6]. Once the plausible bacterial infection cases were identified, these cases where further evaluated by applying a “more specific” medical record criteria for bacterial infections. As the specificity of the infection criteria increased, fewer cases met the criteria for an infection (i.e. sensitivity decreased and the adjusted hazard ratio for bacterial infection associated with TNFα antagonist increased).
To our knowledge, only few other studies have assessed the diagnostic accuracy of administrative data alone to identify patients with suspected bacterial infections. One study compared 5 different claims- based algorithms to a standard of clinically diagnosed pneumonia. The prevalence of pneumonia in that study was 2.5%, and the PPVs of the claims algorithms ranged from 73% to 81% compared to ours of 89% [16]. In another study using data from the General Practice Research Database, 62% of pneumococcal pneumonia administrative codes were confirmed using medical record review [17]. In both these studies the patients were healthier general medical populations in contrast to our RA patients that are commonly on immunosuppressant medications. Despite these differences in patient populations, the results of our study are similar, suggesting that ICD-9 codes are capable of identifying pneumonia accurately.
Our newly developed medical records-based infection criteria will be a particularly valuable resource when uniform classification criteria are needed. Examples of settings in which this may be particularly useful include multi-site trials where infectious adverse events are classified subjectively by individual site investigators or open label studies where investigators are not blinded to drug exposure status. They also may be useful in retrospective epidemiologic studies where abstractors can collect data and classify infections according to these validated medical records-based infection criteria without requiring case-by-case determinations from a physician.
Some bacterial infections have objective, pathognomonic laboratory findings and are therefore relatively simple to classify. For example, the diagnosis of bacteremia required presence of a positive blood culture, and thus the specificity of its infection criteria was 100%. Classification of some other infections however, was more challenging. For example, cellulitis, a predominantly clinical diagnosis, was defined mainly based on history and physical examination findings. These were sometimes inadequately documented in the medical records, and supporting microbiologic evidence for cellulitis is typically scant and not useful. Thus, the specificity of the cellulitis criteria was only 70%. In comparison, diagnosis of lower respiratory tract infections (mainly pneumonia), relied both on objective (laboratory & culture) and more subjective (i.e. clinical) evidence, and the infection criteria demonstrated an intermediate specificity of 82%.
As is true for any diagnostic test, higher specificity comes at the expense of lower sensitivity. This was made especially clear by the criteria for urinary tract infection (UTI) which had 100% specificity but a sensitivity of only 5%. The likely explanation for this very low sensitivity was the requirement for at least 10,000 colonies of an organism pathologic to the genitourinary tract, or for lesser colony counts, antibiotic administration of at least 7 days. Many of the suspected UTIs we found were treated on the basis of an abnormal urinalysis and no positive culture results were ever documented (even after hospital discharge).
Varying approaches have been used to diagnose bacterial infections in observational studies. Infections have been defined by using a combination of patient self-report, hospital records, physician reports, mortality records, antibiotic prescriptions, or by the opinion of treating physicians [5, 6, 11, 18–20]. A few of these studies developed operational criteria to identify their outcomes [2, 11, 19, 20]. For example, Doran and colleagues required the presence of specific symptoms and laboratory values to define any infectious adverse events in RA patients in a retrospective longitudinal cohort study [2]. Leveille and colleagues [19] compared antibiotic prescription fills in automated pharmacy records with medical record review as the standard for infections. Although the face validity of these approaches is reasonable, these approaches were not rigorously evaluated and validated, as we did in this study, nor have they been widely adopted by subsequent investigators.
The major strengths of our study are its sample size and the full access we had to all the information in the medical records, including culture results that became positive even after patient discharge. All medical records were independently abstracted by two healthcare providers who had complete access to all the desired medical records and had high agreement on the classification of infections. Additionally, we studied RA patients who may experience unique patterns of infections compared to general medical patients.
Despite its strengths, our results must be interpreted in the context of our study design. Since this project was undertaken in one university health system, the results may not be generalizable to some other health care settings where medical record documentation or ICD-9 coding practices differ. We were unable to assess the medical records of all patients without an ICD-9 code for infection, hence verification bias might exist [21, 22], which might increase the apparent sensitivity and decrease the apparent specificity of the ICD-9 codes. Based though on an expectation that claims data may have high specificity [23], and the observation of only one infection (2%) from those medical records without an ICD-9 code for infection that were reviewed, this bias is likely lessened. Additionally, the medical record infection criteria were intentionally designed to favor specificity at the expense of sensitivity; thus, they under-ascertained infections that did not have objective diagnostic details in the medical records. For some particular sites of bacterial infections (e.g. meningitis), the low prevalence of these infections restricted drawing conclusions about the diagnostic accuracy of these groups of infections. Finally, our administrative data examined only single ICD-9 codes; more complex claims-based algorithms may be able to achieve higher specificities and PPVs.
In summary, the use of ICD-9 diagnosis codes alone may mis-classify bacterial infections in hospitalized RA populations, although the level of misclassification varies depending on the codes used. To improve accuracy in identifying infections by the administrative claims codes alone, a more restricted set of codes with higher specificity will be more efficient. Our novel, validated, medical records-based infection criteria for a broad range of serious bacterial infections, may limit the need for physicians’ manual review of medical records. These criteria may have usefulness both in clinical trials and observational study populations, either as the primary outcome or as part of a sensitivity analysis.
Acknowledgments
We thank Drs. Nenad Avramovski and Ari Robicsek M.D. for guiding in constructing the medical records-based infection criteria, Dr. Robert Lopez M.D. for guiding in interpreting the radiological findings, Dr. Charles Elson M.D. for his expertise in gastroenterology, Ms. Karen Connor, physician assistant who helped in abstracting medical records, Mr. Bart Prevallet MBA, for creating the data abstraction instrument, and Mr. Jorge Nunez for helping with the data entry.
Supported by the Engalitcheff Arthritis Outcomes Initiative, Maryland Chapter, Arthritis Foundation grant HS10389 from the Agency for Healthcare Research and Quality, from grant award 5K24AR052361-03,1-K23-AR053351-01-A1,PhRMA Foundation, Research Starter Grant in Health Outcomes
Appendix 1
International Classification of Diseases, Version 9, Clinical Modification codes for Serious Bacterial Infections
| Discharge Diagnosis | Sensitive set | Specific set |
|---|---|---|
| Meningitis | 003.21 | 003.21 |
| 036.0 | 036.0 | |
| 049.0 | 049.0 | |
| 091.81 | 091.81 | |
| 094.2 | 094.2 | |
| 098.82 | 098.82 | |
| 320.X X | 320.XX | |
| Encephalitis | 036.1 | 036.1 |
| 323.X | 323.X | |
| 094.81 | 094.81 | |
| 130 | 054.3 | |
| 062 | ||
| 063 | ||
| 064 | ||
| 066.4 | ||
| Cellulitis | 040.0 | 040.0 |
| 569.61 | 569.61 | |
| 681.XX | 681.XX | |
| 682.X | 682.X | |
| 785.4 | 785.4 | |
| 728.86 | 728.86 | |
| 035 | 035 | |
| 608.4 | ||
| 681.XX | ||
| 614.3 | ||
| 528.3 | ||
| 566 | ||
| 597.0 | ||
| Endocarditis | 036.42 | 36.42 |
| 093.2X | 093.2X | |
| 98.84 | 98.84 | |
| 391.1 | 391.1 | |
| 397.9 | 397.9 | |
| 421.X | 421.X | |
| 421.9 | 421.9 | |
| 422.92 | 422.92 | |
| Pneumonia | 003.22 | 003.22 |
| 481.0 | 481.0 | |
| 482.XX | 482.XX | |
| 483.X | 483.X | |
| 485.X | 485.X | |
| 486.X | 486.X | |
| 513.0 | 513.0 | |
| 480.X | ||
| Pyelonephritis/Urinary Tract Infection | 590.XX | 590.X |
| 599.0 | ||
| Septic Arthritis | 003.23 | 003.23 |
| 056.71 | 056.71 | |
| 711.9X | 711.9X | |
| 711.0X | 711.0X | |
| 098.5X | 098.5X | |
| Osteomyelitis | 003.24 | 003.24 |
| 730.2X | 730.2X | |
| 526.4 | 526.4 | |
| 730.0X | 730.0X | |
| 730.1X | 730.1X | |
| 376.03 | 376.03 | |
| Bacteremia/Septicemia | 038.XX | 038.X |
| 041.XX | 790.7 | |
| 790.7 | ||
| Upper Respiratory Tract Infection | 34 | |
| 381.5X | ||
| 382. X | ||
| 383.0X | ||
| 383.1 | ||
| 383.9 | ||
| 461.X | ||
| 462.X | ||
| 463 | ||
| 465.X | ||
| 466 | ||
| 464.0X | ||
| 472.X | ||
| 473.X | ||
| 475 | ||
| 510 | ||
| 510.9 | ||
| Abdominal Abscess | 95.2 | |
| 540.1 | ||
| 569.5 | ||
| 567.X | ||
| 572 | ||
| 590.2 | ||
| 601.2 | ||
| 614.4 | ||
| 998.59 | ||
| Brain Abscess | 324.X | |
| Cholecystitis | 574.X | |
| 575 | ||
| 575 | ||
| 576.1 | ||
| 575.1X | ||
| 575.1 | ||
| 575.1 | ||
| 575.12 | ||
| 575.11 | ||
| Prostate Infections | 98.32 | |
| 98.12 | ||
| 131.03 | ||
| 601.X | ||
| Gastroenteritis | 001 | |
| 002 | ||
| 003 | ||
| 003.0 | ||
| 004 | ||
| 005 | ||
| 008 | ||
| 008.1 | ||
| 008.2 | ||
| 008.4 | ||
| 008.5 | ||
| 009.X | ||
| Infectious Conjunctivitis | 372.0X | |
| 77.9 | ||
| 32.81 | ||
| Device Associated Infections | 996.6X | |
| Local Infections of skin and subcutaneous Tissue | 686.1 | |
| 686.8 | ||
| 686.9 | ||
| Gangrene | 785.4 | |
| Retropharyngeal Abscess | 478.21 | |
| 478.24 | ||
| 478.22 | ||
| Breast Abscess | 611.0 | |
| Splenic Abscess | 289.5 | |
| 289.59 | ||
| Pyogenic Granuloma | 686.1 | |
| Post Traumatic Wound Infection | 958.3 | |
| Postoperative Wound Infection | 998.5 | |
| Infective Myositis | 40.81 | |
| Necrotizing Fascitis | 728.86 |
Appendix 2. Medical Records-Based Infection Criteria for Serious Bacterial Infections
-
Infection Criteria for Septicemia (1–3)
-
If at least one of the following is present:
Patient meets criteria for another infection using criteria in the abstraction tool
-
Patient meets criteria for bacteremia
AND
-
At least one of:
History of fever or documented fever >38.0 °C/100.4 °F
Hypothermia (Temperature <36.0 °C/96.8 °F)
Tachycardia (Heart rate >90/min)
Tachypnea (Respiratory rate >20/min)
Diminished level of consciousness, confusion or delirium
-
Blood glucose >120mg/dl
AND
-
At least one of:
Hypotension (Systolic BP< 90mmHg) or decrease of SBP > 40mmHg from the baseline or mean arterial blood pressure > 40 mmHg from baseline( i.e. patient’s otherwise “normal” blood pressure)
-
Administration of inotropic/vasopressor therapy (dopamine, vasopressin, norepinephrine, epinephrine, amrinone)
AND
-
At least one of:
WBC >12,000cells/mm3
WBC< 4000cells/mm3
-
Normal WBC with >10% Bands or immature forms
OR
At least one of:
Mechanical ventilation
Oliguria (low urine output < 30 ml/hour)
Increase in serum creatinine increase >0.5mg/dl within 48 hours of a previous report
Thrombocytopenia <100,000 platelets/microL
-
-
Infection Criteria for Bacteremia
-
Any one of:
At least one positive peripheral blood culture for any Gram negative organism
At least one positive peripheral blood culture for any Gram positive organisms except for coagulase negative Staphylococcus, Bacillus species., Corynebacterium species., Propionibacterium species., Micrococcus
Two or more positive consecutive peripheral blood cultures for coagulase negative Staphylococcus, Bacillus species., Corynebacterium species., Propionibacterium species., Micrococcus AND Patient treated for at least seven days with antibiotics for putative bacteremia
-
-
Infection Criteria for Devise associated infections (4–9)
Any one of
Erythema/Redness in area surrounding location of the device
Edema/Swelling in area surrounding location of the device
-
Presence of pus/purulent discharge in area surrounding location of the device
AND
Patient meets criteria for bacteremia or septicemia
-
Infection Criteria for Post-operative/Peri-operative infection
If surgery or procedure is performed at least thirty days prior to infection
AND
If patient meets criteria for any other infection using any other listed pre-defined criteria in this appendix, OR Patient meets criteria for bacteremia.
-
Infection Criteria for Intraabdominal Abscess/intra-abdominal infection/intra-peritoneal infection/peritonitis/Liver abscess (10–13)
If positive aspirate cultures* for the polymicrobial+ infection, e.g. enteric gram negative rods, enterococci, bacteroids fragilis, streptococcus milleri, staphylococcus aureus, klebsiella pneumoniae
OR
Any one of:
Fever >101.0°F
Chills
Weight loss
Weakness/malaise
Abdominal pain/tenderness
Elevated alkaline phosphatase ( > upper limit of laboratory normal)
-
Leukocytocis/White Blood Cells ((WBC) > 12,000/cubic mm
AND
If radiographic findings on the computed tomography or ultrasonography of the abdomen are consistent with liver abscess.
-
Infection Criteria for Cellulitis
-
If both:
Acute skin erythema (redness) or tenderness for < 14 days
-
Treatment with Antibiotics for ≥ 7 days
AND
-
Any one of:
History of fever or documented fever >38.0 °C/100.4 °F
Leukocytosis/White blood cells (WBC) > 12,000/cubic mm
Positive blood cultures* for typical pathogens (Streptococci, Staph Aureus, Clostridia…)
-
-
Infection Criteria for Cholecystitis/Cholangitis (14–17)
-
If visible pus draining from the papilla
OR
-
Any one of:
History of fever or documented fever (temperature > 38.0 °C/100.4 °F)
Right upper quadrant pain
Jaundice
Leukocytosis/white blood cells (WBC) > 10,000/cubic mm
Elevated alkaline phosphatase (> upper limit of laboratory normal)
Elevated amylase (> upper limit of laboratory normal)
-
Ultrasonographic Murphy’s sign (right upper quadrant tenderness)
AND
-
Any one of:
Ultrasonography (US) of the abdomen consistent with cholecystitis
Endoscopic retrograde cholangiopancreatography (ERCP) consistent with cholangitis
Magnetic resonance cholangiopancreatography (MRCP) consistent with cholangitis
-
Hydroxyl iminodiacetic acid (HIDA) scan consistent with cholecystitis
OR
-
Any one of:
Positive gram stains from cholecystectomy specimens AND Patient treated for at least 7 days with antibiotics
-
Positive Cultures for the infectious process from cholecystectomy specimens
OR
If histopathology findings consistent with “cholecystitis”
-
-
Infection Criteria for Diverticulitis (18–20)
-
Any one of:
Current lower abdominal pain
Nausea/vomiting/emesis
Palpable tender mass in abdomen
-
Leukocytosis/white blood cells > 12,000/cubic mm
AND
-
If history of the previous episodes of lower abdominal pain/History of previous diagnosis of diverticulitis
OR
-
Any one of:
CT scan showing “consistent with diverticulitis”
CT scan showing increased soft tissue density within pericolic fat
CT scan showing colonic diverticula’s
-
CT scan showing bowel wall thickening
OR
-
If history of the previous episodes of lower abdominal pain/History of previous diagnosis of diverticulitis
AND
-
Any one of:
e) CT scan showing “consistent with diverticulitis”
f) CT scan showing increased soft tissue density within pericolic fat
g) CT scan showing colonic diverticula’s
h) CT scan showing bowel wall thickening
-
-
Infection Criteria for Meningitis (21, 22)
If any one of:
CSF cultures growing bacterial pathogens without recent history* of any surgery involving the brain or spinal cord or head injury. Coag. Neg. Staph, Diphteroids, Propionibacteria, and Bacillus spp. are excluded.
any positive CSF culture in the setting of recent brain or spinal cord surgery (e.g. neurosurgery, ENT surgery) or history of head trauma
-
CSF Serology+ positive for Syphilis
OR
At least any two of:
History of: Headaches, nausea, emesis, photophobia, neck stiffness, altered mental status
Presence of clinical signs: petechial rash, rigid neck, fever >38.0 °C/100.4 °F
-
Patients who were treated for meningitis with at least 7 days of IV Abx
AND
Any one of:
CSF WBC >5cells/mm3
CSF Glucose < 40 mg/dl
CSF/Glucose ratio of ≤ 0.4
CSF Protein > 45 mg/dl
-
Infection Criteria for Osteomyelitis (23–28)
If any one of:
Culture positive* bone biopsy
Positive gram stain of the bone biopsy
-
Histopathology findings consistent with osteomyelitis
OR
At least any two of:
History of fever or documented fever >38.0 ° C/100.4 ° F
Bone pain or/and tenderness
Positive probing of the wound to the bone
Draining sinus over the affected area
Elevated ESR >100mm/hr
-
Diabetic foot ulceration larger then 2cm in diameter
AND
Any one of:
X- ray with specific findings c/w osteomyelitis
CT findings consistent with osteomyelitis
MRI findings consistent with osteomyelitis
Bone scan consistent with osteomyelitis
-
Infection Criteria for Septic Arthritis/Septic Bursitis (29):
If any one of:
Acutely swollen joint
-
Acute pain and/tenderness in the affected joint
AND
If Leukocytosis/white blood cells (WBC)> 100,000cells/mm3 and polymorphonuclear cells >75%
OR
If any one of*:
Positive Gram Stain of the synovial fluid.
Positive culture of the synovial fluid
-
Infection Criteria for Renal Abscess/Pyelonephritis
-
At least any two of:
History of fever or documented fever >38.0 °C/104.0 °F
Dysuric complaints
Flank pain/costovertebral angle tenderness
Leukocytosis/white blood cells (WBC) > 12,000/cubic mm
-
Abnormal urine (cloudy, frank pus or blood in urine, foul smell)
AND
-
Any one of:
Computed Tomography (CT), Magnetic Resonance Imaging (MRI) or Ultrasonography (US) findings consistent with renal inflammation
Computed Tomography (CT), Magnetic Resonance Imaging (MRI) or Ultrasonography (US) findings consistent with renal abscess
-
Computed Tomography (CT), Magnetic Resonance Imaging (MRI) or Ultrasonography (US) findings consistent with hydronephrosis
OR
-
Any one of:
Blood cultures and urine cultures positive for the same organism
Blood cultures positive for the GNR, Enterococci or S. Saphrophyticus
Urine culture positive for the >105 GNR, Enterococci or S. Saphrophyticus
Urine culture positive for < 105 any organism AND Patient treated for ≥ 7 days with antibiotics
-
-
Infection Criteria for Pneumonia
Radiology- If radiological or historical evidence during current hospitalization consistent with pneumonia
-
Organism- Any one of*:
-
Sputum/Expectoration/bronchial secretions/endotracheal tube secretions/bronchoalveolar lavage (BAL) culture* report (source labeled as sputum or expectoration or bronchial secretions or endotracheal tube secretions or bronchioalveolar lavage/BAL) are Positive EXCEPT Staphylococcus epidermidis and Diphtheroids
Positive Sputum culture (source labeled as sputum or expectoration or bronchial secretions or endotracheal tube secretions or bronchioalveolar lavage/BAL) for S. Pneumoniae,
Positive Sputum culture (source labeled as sputum or expectoration or bronchial secretions or endotracheal tube secretions or bronchioalveolar lavage/BAL) for H. Influenzae,
Positive Sputum culture (source labeled as sputum or expectoration or bronchial secretions or endotracheal tube secretions or bronchioalveolar lavage/BAL) for M. Catarrhalis,
Positive Sputum culture (source labeled as sputum or expectoration or bronchial secretions or endotracheal tube secretions or bronchioalveolar lavage/BAL) for B. Pertussis
Positive Sputum culture (source labeled as sputum or expectoration or bronchial secretions or endotracheal tube secretions or bronchioalveolar lavage/BAL) for Legionella spp.
-
Positive Serum IgM antibodies for Mycoplasma Pneumoniae
Positive Serum IgM antibodies for Chlamydia Pneumoniae
Positive Serum IgM antibodies for Legionella Pneumophila
Positive Urine Antigen test for Legionella
Positive PCR for Mycoplasma Pneumoniae or Legionella Pneumophil
-
-
Symptoms- Any one of:
New or worsened cough
New sputum production
Shortness of breath
Pleurisy
-
Examination- Any one of:
History of fever or documented fever >38.0 °C/100.4 °F
Findings on auscultation of crackles or rales or egophony, or whispered pectoriloquy
Findings of dullness to percussion
-
White Blood Count- Any one of:
White blood cell count (WBC) > 11.0 × 109/L
White blood cell count (WBC) < 3.0 × 109/L
-
Infection Criteria for Lung Abscess/Empyema
-
Any one of:
Pleural fluid culture Positive for gram negative organisms EXCEPT S. Epidermidis, Bacillus, Propionibacterium, corynbacterium, Micrococcus
-
Pleural fluid culture Positive for gram positive organisms EXCEPT S. Epidermidis, Bacillus, Propionibacterium, corynbacterium, Micrococcus
OR
-
Any one of:
Concurrent pneumonia
History of fever or documented fever >38.0 °C/104.0 °F
Chills
-
Night sweat
AND
-
Any one of:
CT scan report findings consistent with lung abscess
-
CT scan report findings consistent with presence of pus in the pleural cavity
OR
At least any three of:
Purulent fluid or Pus
pH of pleural fluid/empyema <7.29
Pleural fluid white blood cell count > 1000 cells/cubic mm
Pleural fluid/empyema glucose <40 mg/dl
Pleural fluid/empyema LDH >1000 IU/l
-
-
Infection Criteria for Acute Bacterial Sinusitis (30–32)
If sinus culture of 105 or more of organisms/ml of likely respiratory pathogen (e.g. S. Pneumonia, H. Influenzae, S. Aureus, Brahmela Catharallis,..)
OR
If at least two of:
Purulent nasopharyngeal discharge
Maxillary toothache (dental pain)
Poor response to decongestants
3 of: anosmia or hyposmia, ear fullness, cough, fatigue
History of fever or documented fever >38.0 °C/100.4 °F
AND If abnormal sinus imaging showing sinus opacification or air-fluid levels on sinus X-rays or CT scan.
-
Infection Criteria for Gastroenteritis (33–35):
-
If any one of:
Abdominal pain
Dehydration
Diarrhea
Nausea
Vomiting/Emesis
Treated with ≥ 7 days of antibiotics
-
AND If any one of:
≥ 1 stool culture positive for bacteria
≥ 1 blood culture positive for bacteria
≥ 1 stool gram stain positive for leukocytes AND RBCs
-
-
Infection Criteria for Endocarditis (36)
-
Major Criteria
Positive 2 separate Blood Culture for typical organisms: Viridans Streptococci, S. Bovis, HACEK group or Community-acquired S. Aureus or Enterococci w/o other obvious focus or Persistently positive blood culture for any microorganism from the blood cultures drawn 12 hours apart
All of 3, or 3 of 4 or more positive blood cultures with first and last set being drawn at least 1 hour apart.
-
Endovascular involvement
ECHO findings positive for IE such as vegetation on the valve, vegetation in the path of the regurgitant blood flow jets, on iatrogenic device, paravalvular abscess,
New dehiscence of the prosthetic valve
New valve regurgitation murmur (changes in the old murmur are not sufficient)
-
Minor Criteria
Predisposing heart condition or IVDU
Fever > 38°C/100.4°F)
Vascular phenomena: arterial embolism, septic pulmonary infarcts, mycotic aneurysm, secondary intracranial hemorrhage, Janeway lesions
Immunologic phenomena: GN, Osler’s nodes, Roth spots
ECHO findings c/w IE but not meeting major criteria
Microbiologic criteria not meeting major criteria or serologic evidence of infection with organism c/w IE (Brucella, Bartonella, Coxiella Burnetti…)
Infection Criteria for Infective Endocarditis:
At least any 2 of Major criteria OR at least 1 of Major criteria AND at least 3 of Minor criteria
-
- 1.Systemic inflammatory response syndrome (SIRS) definition and diagnosis. UpToDate website [Google Scholar]
- 2.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 4.Bryant JK, Strand CL. Reliability of blood cultures collected from intravascular catheter versus venipuncture. Am J Clin Pathol. 1987;88(1):113–6. doi: 10.1093/ajcp/88.1.113. [DOI] [PubMed] [Google Scholar]
- 5.DesJardin JA, Falagas ME, Ruthazer R, et al. Clinical utility of blood cultures drawn from indwelling central venous catheters in hospitalized patients with cancer. Ann Intern Med. 1999;131(9):641–7. doi: 10.7326/0003-4819-131-9-199911020-00002. [DOI] [PubMed] [Google Scholar]
- 6.Everts RJ, Vinson EN, Adholla PO, Reller LB. Contamination of catheter-drawn blood cultures. J Clin Microbiol. 2001;39(9):3393–4. doi: 10.1128/JCM.39.9.3393-3394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki DG. The prevention and management of device-related infection in infusion therapy. J Med. 1980;11(4):239–53. [PubMed] [Google Scholar]
- 8.Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296(23):1305–9. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 9.O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR10):1–29. [PubMed] [Google Scholar]
- 10.Gastrointestinal Infections: Diagnosis and Management. New York: Marcel Dekker; 1997. p. 397. [Google Scholar]
- 11.Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223(5):600–7. doi: 10.1097/00000658-199605000-00016. discussion 607–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin RH, Swartz MN, Malt R. Hepatic abscess: changes in clinical, bacteriologic and therapeutic aspects. Am J Med. 1974;57(4):601–10. doi: 10.1016/0002-9343(74)90012-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26(6):1434–8. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 14.Chan YL, Chan AC, Lam WW, et al. Choledocholithiasis: comparison of MR cholangiography and endoscopic retrograde cholangiography. Radiology. 1996;200(1):85–9. doi: 10.1148/radiology.200.1.8657949. [DOI] [PubMed] [Google Scholar]
- 15.Lee MG, Lee HJ, Kim MH, et al. Extrahepatic biliary diseases: 3D MR cholangiopancreatography compared with endoscopic retrograde cholangiopancreatography. Radiology. 1997;202(3):663–9. doi: 10.1148/radiology.202.3.9051013. [DOI] [PubMed] [Google Scholar]
- 16.Saik RP, Greenburg AG, Farris JM, Peskin GW. Spectrum of cholangitis. Am J Surg. 1975;130(2):143–50. doi: 10.1016/0002-9610(75)90362-1. [DOI] [PubMed] [Google Scholar]
- 17.Sinanan MN. Acute cholangitis. Infect Dis Clin North Am. 1992;6(3):571–99. [PubMed] [Google Scholar]
- 18.Fischer MG, Farkas AM. Diverticulitis of the cecum and ascending colon. Dis Colon Rectum. 1984;27(7):454–8. doi: 10.1007/BF02555537. [DOI] [PubMed] [Google Scholar]
- 19.Konvolinka CW. Acute diverticulitis under age forty. Am J Surg. 1994;167(6):562–5. doi: 10.1016/0002-9610(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 20.Rao PM, Rhea JT, Novelline RA, et al. Helical CT with only colonic contrast material for diagnosing diverticulitis: prospective evaluation of 150 patients. AJR Am J Roentgenol. 1998;170(6):1445–9. doi: 10.2214/ajr.170.6.9609151. [DOI] [PubMed] [Google Scholar]
- 21.Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? Jama. 1999;282(2):175–81. doi: 10.1001/jama.282.2.175. [DOI] [PubMed] [Google Scholar]
- 22.Leib SL, Boscacci R, Gratzl O, Zimmerli W. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis. 1999;29(1):69–74. doi: 10.1086/520184. [DOI] [PubMed] [Google Scholar]
- 23.Gold RH, Hawkins RA, Katz RD. Bacterial osteomyelitis: findings on plain radiography, CT, MR, and scintigraphy. AJR Am J Roentgenol. 1991;157(2):365–70. doi: 10.2214/ajr.157.2.1853823. [DOI] [PubMed] [Google Scholar]
- 24.Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. Jama. 1995;273(9):721–3. [PubMed] [Google Scholar]
- 25.Mackowiak PA, Jones SR, Smith JW. Diagnostic value of sinus-tract cultures in chronic osteomyelitis. Jama. 1978;239(26):2772–5. doi: 10.1001/jama.239.26.2772. [DOI] [PubMed] [Google Scholar]
- 26.Perry CR, Pearson RL, Miller GA. Accuracy of cultures of material from swabbing of the superficial aspect of the wound and needle biopsy in the preoperative assessment of osteomyelitis. J Bone Joint Surg Am. 1991;73(5):745–9. [PubMed] [Google Scholar]
- 27.Perry M. Erythrocyte sedimentation rate and C reactive protein in the assessment of suspected bone infection--are they reliable indices? J R Coll Surg Edinb. 1996;41(2):116–8. [PubMed] [Google Scholar]
- 28.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282(4):198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 29.Shmerling RH, Delbanco TL, Tosteson AN, Trentham DE. Synovial fluid tests. What should be ordered? Jama. 1990;264(8):1009–14. [PubMed] [Google Scholar]
- 30.Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. Bmj. 1995;311(6999):233–6. doi: 10.1136/bmj.311.6999.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Low DE, Desrosiers M, McSherry J, et al. A practical guide for the diagnosis and treatment of acute sinusitis. Cmaj. 1997;156 (Suppl 6):S1–14. [PubMed] [Google Scholar]
- 32.Snow V, Lascher S, Mottur-Pilson C. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001;134(7):595–9. doi: 10.7326/0003-4819-134-7-200104030-00015. [DOI] [PubMed] [Google Scholar]
- 33.Barbut F, Leluan P, Antoniotti G, Collignon A, Sedallian A, Petit JC. Value of routine stool cultures in hospitalized patients with diarrhea. Eur J Clin Microbiol Infect Dis. 1995;14(4):346–9. doi: 10.1007/BF02116530. [DOI] [PubMed] [Google Scholar]
- 34.Slutsker L, Ries AA, Greene KD, Wells JG, Hutwagner L, Griffin PM. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126(7):505–13. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 35.Talan D, Moran GJ, Newdow M, et al. Etiology of bloody diarrhea among patients presenting to United States emergency departments: prevalence of Escherichia coli O157:H7 and other enteropathogens. Clin Infect Dis. 2001;32(4):573–80. doi: 10.1086/318718. [DOI] [PubMed] [Google Scholar]
- 36.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–9. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
Footnotes
this is not an exhaustive list, any organism irrespective of colony count
Polymicrobial infection = any number of organisms
Any number of organisms irrespective of the colony count
Recent history = 4 to 6 weeks prior to meningitis
“positive” CSF serology for syphilic meningitis = CSF VDRL or RPR or FTA any one positive
= presence of any organism irrespective of colony counts
presence of any organism irrespective of colony counts
IGNORE any gram stain report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14(1):135–7. doi: 10.1002/art.1780140119. [DOI] [PubMed] [Google Scholar]
- 2.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CD. Infectious complications of treatment with biologic agents. Curr Opin Rheumatol. 2004;16(4):393–8. doi: 10.1097/01.bor.0000127594.92432.7c. [DOI] [PubMed] [Google Scholar]
- 4.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. New Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 5.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, Gromnica-Ihle E, Antoni C, Herzer P, Kekow J, Schneider M, Zink A. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52(11):3403–12. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 6.Curtis JR, Patkar N, Xie A, Martin C, Allison JJ, Saag M, Shatin D, Saag KG. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56(4):1125–33. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 7.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–9. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 8.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 9.Geraci JM, Ashton CM, Kuykendall DH, Johnson ML, Wu L. International Classification of Diseases, 9th Revision, Clinical Modification codes in discharge abstracts are poor measures of complication occurrence in medical inpatients. Med Care. 1997;35(6):589–602. doi: 10.1097/00005650-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60(4):397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J. A coefficient of agreement for nominal scale. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 13.Woolson RF. Statistical Methods for the Analysis of Biomedical Data. New York: John Wiley & Sons; 1987. [Google Scholar]
- 14.Kelsey JL, Thompson WD, Evans AS. Methods in Observational Epidemiology. New York: Oxford University Press; 1986. [Google Scholar]
- 15.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–28. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 17.Metlay JP, Kinman JL. Failure to validate pneumococcal pneumonia diagnoses in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2003;12:S163–4. [Google Scholar]
- 18.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54(2):628–34. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 19.Leveille SG, Gray S, Black DJ, LaCroix AZ, Ferrucci L, Volpato S, Guralnik JM. A new method for identifying antibiotic-treated infections using automated pharmacy records. J Clin Epidemiol. 2000;53(10):1069–75. doi: 10.1016/s0895-4356(00)00235-3. [DOI] [PubMed] [Google Scholar]
- 20.Schneeweiss S, Setoguchi S, Weinblatt ME, Katz J, Avorn J, Sax PE, Levin R, Solomon DH. Anti-tumor necrosis factor alpha therapy and the risk of serious baterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(6):1754–64. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Greenes RA. Assessment of diagnostic tests when disease verification is subject to selection bias. Biometrics. 1983;39(1):207–15. [PubMed] [Google Scholar]
- 22.Punglia RS, D’Amico AV, Catalona WJ, Roehl KA, Kuntz KM. Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med. 2003;349(4):335–42. doi: 10.1056/NEJMoa021659. [DOI] [PubMed] [Google Scholar]
- 23.Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care. 1994;32(1):81–90. doi: 10.1097/00005650-199401000-00006. [DOI] [PubMed] [Google Scholar]