Abstract
Study Design
This study evaluated whether the aggregation behavior of a thermally responsive elastin-like polypeptide (ELP) prolongs protein residence time at the dorsal root ganglion (DRG). This work involves development of a sustained-release drug delivery vehicle to provide high and sustained levels of biologic therapeutics to the dorsal root ganglion while minimizing systemic exposure.
Objective
To study the potential of the ELP biopolymer to sustain release and lower systemic exposure of bioactive peptides following perineural administration.
Summary of Background Data
Anticytokine treatment for lumbar radiculopathy may offer clinical improvement, but exposes patients to systemic toxicities of immunosuppression. ELPs are environmentally responsive polypeptides that undergo a phase transition on heating to form an insoluble aggregate. Drug conjugates with ELP exhibit both temperature-sensitivity and in vitro bioactivity. Monomer resolubilization yields solution-phase molecules, and this reversible aggregation behavior may create a perineural drug depot to sustain drug delivery to an inflamed nerve.
Methods
This experiment involved 48 rats in which radiolabeled ELPs (aggregating or soluble) were injected overlying the L5 dorsal root ganglion. Animals were killed at 6 different time points, and radioactivity associated with the injected segment, serum, and other tissues was evaluated.
Results
The aggregating ELP demonstrated a 7-fold longer perineural half-life compared with the soluble ELP. This supports the hypothesis that the aggregating ELP forms a depot from which slow resolubilization and clearance provides sustained, local protein release. Furthermore, serum radioactivity reached a lower peak for the aggregating group, demonstrating slower absorption of the aggregating protein into the systemic circulation.
Conclusion
These results suggest that ELP aggregation confer the benefit of perineural compartment longevity for bioactive therapeutics delivered fused with this carrier. This may sustain release of potent immunomodulator therapeutics to treat local neuroinflammation. Desirable features include delivery of high local doses and protection against systemic exposure and associated toxicity.
Keywords: perineural, radiculopathy, controlled release, drug delivery, elastin-like polypeptide, biodistribution, dorsal root ganglion
The pathophysiology of radiculopathy associated with nucleus pulposus (NP) herniation from the intervertebral disc may involve both mechanical deformation and biochemical irritation.1 Whereas mechanical compression of the nerve root causes histopathological changes and pain behavior in animal models,2,3 the trigger and sequence of biochemical events accompanying noncompressive disc-herniation remain unclear.1 Contact of nucleus pulposus tissue with the dorsal root ganglion (DRG) elicits inflammatory histopathology, slowed nerve conduction velocities, and pain behavior4–6 that may be mediated by tumor necrosis factor alpha (TNFα).7 Higher biomarker expression of astrocyte and microglia activation3 and histopathological evidence of macrophage infiltration8 further implicate the nonspecific inflammatory basis of this disease. Systemic immunomodulatory therapeutics targeting TNFα attenuate these effects in animal models9–11 and may provide clinical relief of sciatica in humans.12–14 However, conventional systemic delivery of anti-TNFα medications expose patients to toxicity associated with relative immunosuppression.15 While systemic inflammatory diseases such as rheumatoid arthritis, Crohn disease, and psoriasis demand parenteral treatment, absent serum and cerebrospinal fluid biomarkers of inflammation suggest that disc-herniation radiculopathy is primarily a local inflammatory process.16 These considerations motivate development of perineural drug delivery vehicles that maximize dose and sustain release of therapeutic agents at the DRG.
Local drug depots can sustain drug release at a target site. Epidural liposomes containing alfentanil,17 morphine,18,19 and bupivacaine,20,21 prolong analgesic effects with few systemic toxicities. These systems are highly effective for entrapment of small molecules, sustaining release for several hours after epidural administration. Biodegradable copolymers of glycolic acid and lactones increase the duration of epidural lidocaine block from 61 to 170 minutes in rabbits while decreasing serum exposure.22 Difficulty arises when applying these systems to delivery of protein biomacromolecules because of the need for organic solvents and exposure to drying and shear stresses during the microencapsulation process that make protein instability and maintenance of native structure significant concerns. Furthermore, these polymers suffer from low drug-to-carrier ratio, and the entrapped protein may be simultaneously degraded during the release process. Drugs with low aqueous solubility will phase-separate and undergo spontaneous depot release, such as butamben suspensions that prolong antinociceptive effect in a rat model.23 Thermally responsive polymers may be delivered in aqueous solution below physiologic temperature and spontaneously undergo a phase change on heating to form a gel-like depot. Poly-(N-isopropylacrylamide) and polyoxyethylene-polyoxypropylene block copolymers effectively entrap drugs through this mechanism and provide for sustained release.24 The elastin-like polypeptides (ELPs) are thermally responsive polypentapeptide biopolymers of sequence Val-Pro-Gly-Xaa-Gly where the Xaa residue can be any amino acid other than proline.25,26 The phase transition temperature can be specifically genetically designed by modifying 2 orthogonal variables: Xaa amino acid residue identity, and ELP molecular weight.27 As a recombinant biopolymer, ELPs have advantages of high-yield production in Escherichia coli,28 demonstrated biocompatibility and nonimmunogenicity,29 and facile genetic engineering to produce recombinant proteins with bioactive peptide fusion partners.30,31 This carries the potential advantage of simultaneous expression of the protein drug and polypeptide carrier to yield chimeric proteins with bidomain functionality. Of specific interest for this study, the ELP phase transition leads a soluble polypeptide below a characteristic transition temperature (Tt) to form insoluble, micron-sized, supramolecular complexes on being raised above Tt·32 This aggregation behavior prolongs the half-life of ELPs after intra-articular administration.33
The objective of this study is to evaluate biodistribution of a depot-forming ELP following perineural delivery in a rat model. Two ELPs of similar molecular weight were selected—one transitioning below physiologic temperature to ensure depot formation, and a second with a transition temperature above 75°C to guarantee solubility. Biodistribution of tritium-labeled ELPs was quantified over 2 weeks, with the specific interests of quantifying prolongation of protein residence at the injected segment, attenuation of serum exposure, and bioaccumulation in remote tissues.
Methods
Synthesis of ELPs
Two different ELPs were expressed in E. coli and purified using inverse transition cycling (ITC).34 ELP-(V)120 (MW = 49.9 kDa) exclusively contained Val as the guest residue and was selected because of a subphysiological Tt of 29°C. The soluble ELP-(VA8G7)128 (MW = 49.0 kDa) contained Val, AL, and Gly as guest residues with a frequency of 1:8:7, and served as a control because of similar molecular weight but a Tt of 78°C ensuring in vivo solubility.
Radiolabeling of ELPs by Reductive Methylation
Purified ELPs were reconstituted in PBS at 36 µmol/L and reductively methylated using a method described by Jentoft and Dearborn35 with3[H]-sodium cyanoborohydride (American Radiochemical Corp., St. Louis, MO) as the radioactivity source. The reaction mixture was dialyzed 5 times against 3 L of deionized water for 8 hours each, concentrated using an Amicon Ultra-15 centrifugal filter of 5 kDa MWCO (Millipore, Billerica, MA), and then subjected to 2 further rounds of ITC. Proteins were sterile-filtered through a 0.22 µmol/L filter and tritium incorporation was evaluated using absorbance spectro-photometry to quantify ELP concentration and liquid scintillation counting to quantify associated radioactivity (5 mL of Bio-Safe II, RPI Corp., Mt. Prospect, IL) using a Packard Tricarb B1900-TR scintillation counter (Packard Instruments, CT). Analogous nonradioactive alkylation permitted evaluation of the transitioning behavior of the modified proteins
In Vitro Stability of ELPs
Proteolytic degradation by active enzymes under physiologic or pathologic conditions could lead to loss of the tritium radiolabel from the ELP molecule. Consequently, an experiment was designed to evaluate the in vitro stability of both3[H]-ELP-(V)120 and3[H]-ELP-(VA8G7)128 by incubating quadruplicate replicates of3[H]-ELP (4 nmol in 1 mL) at 37°C in rat serum (Invitrogen, Carlsbad, CA) or PBS. One hundred microliters aliquots were withdrawn at 1, 2, 4, 7, 10, and 14 days. A 10 kDa MWCO microcentrifugation filter (Millipore, Billerica, MA) was used to separate radioactivity associated with smaller degradation products from larger parent ELP molecules. Total radioactivity in both the filtrate and retentate was measured by liquid scintillation counting (LSC) in 5 mL of Bio-Safe II, and the fraction of radioactivity in the filtrate was computed. Two-factor analysis of variance (ANOVA) and post hoc Dunn’s tests evaluated ELP sequence and incubation buffer dependency of filtrate fraction of radioactivity. An average degradation rate was computed for each ELP in serum using linear least-squares regression, and derived parameters were compared using a Student t test.
In Vivo Perineural Administration of Radiolabeled ELPs
Forty-eight Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used for biodistribution studies of3[H]-ELP following perineural administration. Rats in the experimental group received the aggregating3[H]-ELP-(V)120 and those in the control group received soluble3[H]-ELP-(VA8G7)128. While drug administration to the disc-nerve interface is accomplished by transforaminal injection under fluoroscopic guidance,36 the small size of these structures in the rodent model precluded our use of a guided injection procedure.37 The DRG was exposed using an open procedure involving paraspinal muscle dissection followed by drilling a 1 mm hole in the transverse process using a Midas A3 drill bit.38–40 Each ELP sequence had 4 animals for each of 6 sacrifice time points. Animals were anesthetized with intraperitoneal pentobarbital (40 mg/kg), opened with a 15 mm midline dorsal lumbar incision, and a 10 mm paramedian incision through the dorsal lumbar fascia. Twenty-five microliters of 25 µmol/L3[H]-ELP solution was injected through the defect after which the hole was sealed with bone wax. Dorsal lumbar fascia was closed with 3-0 silk sutures, and skin was closed with staples. Animals in the experimental group receiving3[H]-ELP-(V)120 were killed at 0, 6 hours, and 1, 4, 7, and 14 days. Animals in the control group receiving3[H]-ELP-(VA8G7)128 were killed at 0, 1, 4, 12, 24, and 48 hours. For sacrifice, animals were administered 60 mg/kg intraperitoneal pentobarbital after which a blood sample was obtained via retro-orbital bleed. After this, the following tissues were harvested in order: brain, C2 motion segment, left gastrocnemius muscle, liver, kidney, adjacent segment (L4), and injected segment (L5).
Tissue Solubilization and Liquid Scintillation Counting
The isolated tissues and serum samples were weighed and incubated in an alkaline tissue solubilizer (GE Healthcare, Piscataway, NJ) at 55°C until they were completely solubilized. The solutions were decolorized (3% hydrogen peroxide, 55°C for 1 hour) and the radioactivity was quantified by LSC. Radioactivity in the injected segment at the earliest time point was interpreted as an average injected dose (ID) for experimental and control groups. Each tissue or fluid sample was normalized by the corresponding injected dose. Radioactivity associated with the L5 (injected) and L4 (adjacent) regions were expressed as fractions of ID associated with the segment. Data for organs (brain, liver, and kidney) and the C2 motion segment were expressed as fraction of ID per gram of tissue. The fraction of injected3[H]-ELP in the serum compartment was estimated by scaling up the measured radioactivity in a serum sample to the total blood volume predicted by animal body mass.41
Biodistribution Analysis
The clearance half-life (t1/2) of the protein depot was evaluated by fitting the time-dependent radioactivity at the injected segment to first-order kinetics by a monoexponential decay model using nonlinear least squares regression. The derived rate constant of absorption (ka) is related to the half-life by the equality t1/2 = ln2/ka. The t1/2 values for the aggregating ELP-(V)120 and the soluble ELP-(VA8G7)128 were compared by a Student t test. One-factor ANOVA with post hoc Dunn’s tests were used to detect an effect of time on the presence of3[H]-ELP in the noninjected tissues (muscle, liver, kidney, C2 segment, L4 segment) and serum. The maximum serum fraction observed for each ELP was also evaluated.
Results
Tritium-Radiolabeling of ELPs by Reductive Methylation
The specific radioactivity of the ELP-(V)120 and ELP-(VA8G7)128 proteins after reductive methylation were 2628 and 876 mCi/mmol, respectively. The inverse phase transition behavior of both ELPs was only slightly modified following nonradioactive methylation, with observe transitions occurring at 26°C for ELP-(V)120 and at 82°C for ELP-(VA8G7)128. This observation suggests that the important physical properties of ELPs were not modified following radiolabeling, thus permitting their use to contrast biodistribution of aggregating and soluble ELP formulations in this study.
In Vitro Stability of ELPs
There was evidence of degradation of3[H]-ELP to fragments of molecular weight less than 10 kDa for both soluble and aggregating ELP formulations in rat serum (Figure 1, ANOVA, P < 0.05). There was no corresponding degradation effects noted for incubation of either3[H]-ELP in PBS. Post hoc analysis reveals that filtrate fraction of radioactivity rises above background levels for the soluble ELP from 24 hours onwards (Dunn’s, α =0.05), whereas the aggregating ELP reaches such difference at 7 days and beyond (Dunn’s, α = 0.05). The soluble ELP was more extensively degraded than the aggregating ELP at every sampling time, likely because of greater accessibility to serum proteases than aggregating ELPs that physically separate from aqueous solution into an insoluble coacervate phase. The degradation rate for ELP-(VA8G7)128 averaged 0.56% ± 0.05%/day, significantly faster (Student t test, P = 0.002) than for than for ELP-(V)120 averaging 0.33% ± 0.05%/day.
Figure 1.
Incubation of both soluble and aggregating ELP sequences in PBS or rat serum at 37°C. Significant effects (ANOVA) were detected for both tested factors: ELP sequence (P < 0.01) and incubation conditions (P < 0.01). Serum degradation exceeded baseline from day 1 onwards for the soluble ELP (*, Dunn’s tests, α = 0.05) and from day 7 onwards (*, Dunn’s tests, α = 0.05). Greater fraction of degradation products were measured for the soluble ELP at nearly all time points (#, Dunn’s tests, α = 0.05).
In Vivo Biodistribution of Radiolabeled ELPs After Perineural Administration of Radiolabeled ELPs
All 48 rats survived to the desired time point, with no deaths, infections, permanent neurologic deficits, or wound dehiscence. Drilling through the transverse process was consistently performed without gross damage to the underlying exiting nerve root.
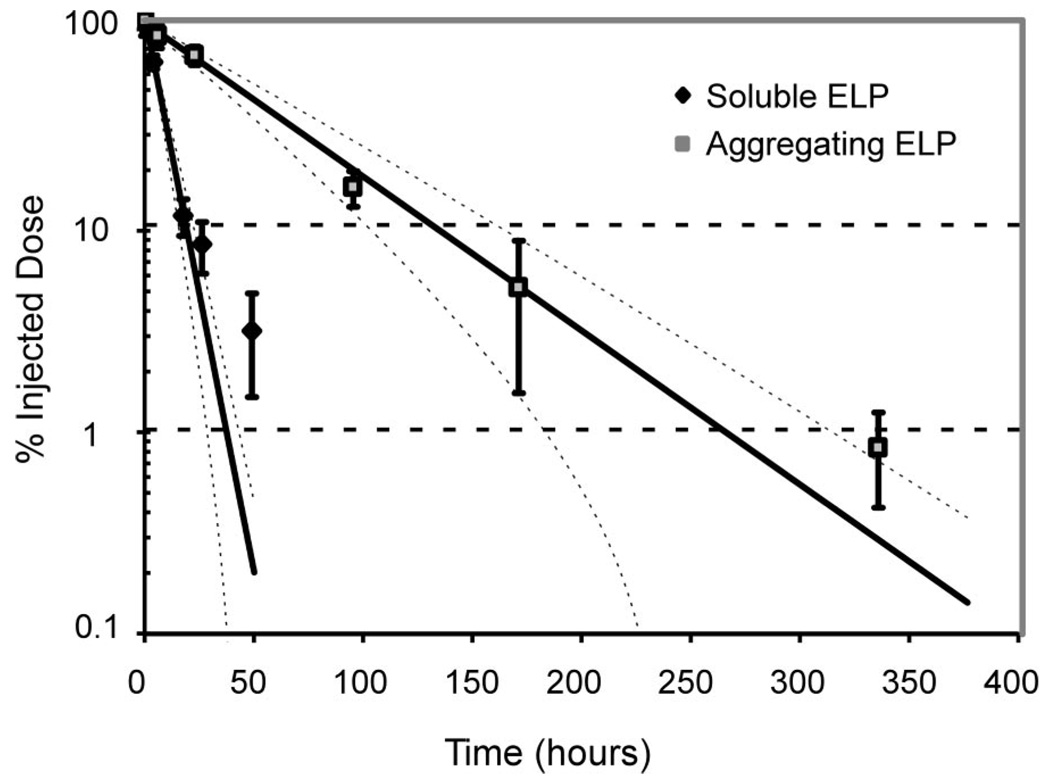
Fractions of soluble and aggregating3[H]-ELP per ID at the L5 injected level decreased monotonically and was well described by first-order exponential kinetics (Figure 2). The computed half-life of release for the soluble ELP was 5.5 ± 0.5 hours whereas a significantly longer 39 ± 5 hours characterized the aggregating ELP as summarized in Table 1 (Student t test, P < 0.01).
Figure 2.
Clearance of ELP from the perineural delivery to the right L5 DRG is well described by a first-order exponential model for both soluble (upper, r2 = 0.97) and aggregating (lower, r2 = 0.94) sequences. Data are expressed as mean ± SD (n = 4) and presented with first-order exponential decay fits and 95% confidence intervals. Half-lives are 5.5 ± 0.5 hours for the soluble ELP and 39 ± 5 hours for the aggregating ELP (Student t test, P < 0.01).
Table 1.
Physical Properties of Aggregating and Soluble ELP and Their Pharmacokinetic Parameters After Perineural Injection
| ELP-(VA8G7)128 | ELP-(V)120 | P | |
|---|---|---|---|
| MW (Da) | 48990 | 49867 | |
| Tt (°C) | 26 | 82 | |
| ka (h−1) | 0.125 ± 0.012 | 0.017 ± 0.002 | <0.01* |
| t1/2 (h) | 5.5 ± 0.5 | 39 ± 5 | <0.01* |
| t10% (h) | 18.3 ± 1.7 | 126 ± 14 | <0.01* |
| Cmax,serum (%ID) | 23 ± 5 | 1.6 ± 0.3 |
Significantly different by Student’s t test at 0.05 level of significance.
MW indicates molecular weight; Tt, transition temperature; ka, absorption rate constant; t1/2, half-life; t10%, time to 10% dose; Cmax,serum, maximum observed serum fraction of radioactivity.
The fractions of3[H]-ELP per ID in the serum compartment are shown in Figure 3, with both formulations peaking at 24 hours and steadily thereafter decaying to below detectable values. The maximum fraction of soluble3[H]-ELP-(VA8G7)128 was 23 ± 5% ID, while that for the aggregating3[H]-ELP-(V)120 was only 1.6 ± 0.3% ID.
Figure 3.
Serum fraction of soluble and aggregating ELP, normalized to the injected dose. Data are expressed as mean ± SD (n = 4) with the maximum serum fraction of both ELPs observed at 24 hours. Maximum magnitudes were 23 ± 5% ID for the soluble ELP and 1.6 = 0.3% ID for the aggregating ELP.
Distribution of the3[H]-ELP to peripheral tissues are shown in Figure 4, and immediate postinjection analysis is summarized in Table 2. Time-dependent variation in radioactivity was observed in the kidney for both soluble (ANOVA, significant effect of time, P < 0.01) and aggregating (ANOVA, significant effect of time, P < 0.05) proteins. Maximum values for the soluble ELP were reached at 24 hours with a value of 1.2 ± 0.4%ID/g (or 1.0 ± 0.1%ID/kidney), whereas maximum values for the aggregating ELP were reached at 96 hours with a value of 1.8 ± 0.5%ID/g (or 1.9 ± 0.8%ID/kidney). All levels of3[H]-ELP dropped below1%for the soluble ELP by 48 hours and for the aggregating ELP by 168 hours. Neither aggregating nor soluble ELPs accumulated in the brain, muscle, liver, and C2 motion segment above baseline detection; and none of these tissues demonstrated time-dependent radioactivity (ANOVA, no significant effects of time, α = 0.05). Table 3 summarizes the residual tissue radioactivity after 14 days among the animals injected with aggregating ELP.
Figure 4.
Profile of soluble (upper) and aggregating (lower) ELP distribution to peripheral tissues, normalized to the injected dose. Data are expressed as mean ± SD (n = 4) Accumulation of radioactivity was noted in the kidney for soluble ELP (significantly higher at 24 hours than all other times, Dunn’s tests, α = 0.05) and for aggregating ELP (significantly higher at 96 hours than 0, 6, and 336 hours, Dunn’s tests, α = 0.05). The soluble ELP did not accumulate in the brain, liver, or muscle (ANOVA, P > 0.05); but did peak at the C2 motion segment at 24 hours (significantly higher than at 0 and 48 hours, Dunn’s test, α = 0.05). The aggregating ELP did not accumulate in the brain, liver, C2 motion segment, or muscle (ANOVA, P > 0.05).
Table 2.
Tissue Distribution of Soluble and Aggregating ELP Immediately After Injection
| Tissue | Scale | ELP-(VA8G7)128 (% ID) |
ELP-(V)120 (% ID) |
|---|---|---|---|
| Injected segment | Per organ | 100 ± 8 | 100 ± 16 |
| Adjacent segment | Per organ | 9 ± 4 | 8 ± 5 |
| C2 segment | Per gram | 0.16 ± 0.15 | 0.08 ± 0.04 |
| Brain | Per gram | 0.6 ± 1.0 | 0.20 ± 0.14 |
| Kidney | Per gram | 0.08 ± 0.04 | 0.14 ± 0.18 |
| Liver | Per gram | 0.10 ± 0.07 | 0.10 ± 0.15 |
| Muscle | Per gram | 0.05 ± 0.07 | 0.12 ± 0.07 |
| Serum | Per organ | 1.6 ± 1.1 | 0.5 ± 0.3 |
No significant differences between ELP distribution between soluble and aggregating ELPs immediately after injection as assessed by Student’s t test at 0.05 level of significance.
Table 3.
Tissue Distribution of Aggregating ELP at 2 Weeks
| Tissue | Scale | ELP-(V)120 (% ID) |
|---|---|---|
| Injected segment | Per organ | 0.8 ± 0.4 |
| Adjacent segment | Per organ | 0.10 ± 0.06 |
| C2 segment | Per gram | 0.11 ± 0.08 |
| Brain | Per gram | 0.049 ± 0.005 |
| Kidney | Per gram | 0.43 ± 0.09 |
| Liver | Per gram | 0.06 ± 0.04 |
| Muscle | Per gram | 0.08 ± 0.03 |
| Serum | Per organ | 0.44 ± 0.12 |
Discussion
Local irritation of the DRG can generate symptomatic changes of painful radiculopathy, detected as histologic changes in the DRG and dorsal horn,42,43 electrophysiological changes in nerve conduction,44 and behavioral1 changes in the affected subject. Animal models have demonstrated therapeutic effect on systemic pretreatment with anticytokine agents.9,45,46 Local therapeutic delivery to this region is attractive to treat the local pathology and minimize systemic toxicities. In this study, a thermally responsive polypeptide, ELP, was evaluated for its ability to prolong the residence time of a potential drug carrier delivered locally to the DRG. ELP was hypothesized to increase polypeptide residence time by forming insoluble aggregates at temperatures above body temperature that reversibly separate from an aqueous phase, thus slowing their clearance. As a consequence, a significant 7-fold prolongation in perineural residence time was observed compared with an ELP that remains soluble at body temperature. Vascular and lymphatic absorption of ELP is expected to only clear solution-phase particles, which includes only a fraction of the aggregating ELP-(V)120. This process of clearing the soluble fraction overlying an ELP aggregate thermodynamically drives disaggregation forming the basis of this drug delivery system. This contrasts with the soluble ELP-(V5A2G3)128 molecules that do not aggregate in vivo and are hence all accessible for clearance. The formation of multimeric particles following delivery to the perineural space will also increase the apparent molecular weight of ELP with concomitant slowing of diffusion, as assessed by dynamic light scattering.24 Prolongation in protein residence time suggests that aggregation occurs in the perineural space and is responsible for delayed clearance from around the DRG.
This delayed absorption of the aggregating ELP also lowers serum fraction of radiolabel (maximum 1.6 ± 0.3% ID) compared with the soluble ELP (maximum 23 ± 5% ID). Reduction of serum drug concentration is important when for local drug depots to reduce systemic toxicity. This is particularly important when delivering bioactive peptides to the perineural space, such as anti-TNF agents or other immunomodulating proteins. These drugs elicit untoward immunosuppression on significant systemic exposure, and the ability of the aggregating ELP to reduce serum compartment peak exposure is an important feature of this delivery system. Liu et al47 have shown intravascular ELP elimination half-life to be 8.37 hours. The clearance mechanism is unclear, but the rise and fall of kidney-associated radioactivity in synchrony with serum in our experiment suggests a glomerular filtration route. Although additional studies are necessary to definitively implicate renal or hepatic clearance, we have demonstrated feasibility of delivering high local drug concentrations while attenuating systemic exposure. We further observe the remote C2 level did not accumulate radiolabel above background for either ELP. This supports local peptide delivery because even high circulating protein doses very inefficiently reaches desired neural structures.
Drug clearance after perineural injection is predominantly by vascular and lymphatic absorption, with the latter more prominent for large soluble polypeptides. While previous work suggests ELP aggregation to prolong residence time after injection into the anatomically confined knee joint,33 the perineural region is less anatomically defined and access is more challenging because of complex bony anatomy. The lumbar DRG lies distal to the confluence of dura and peripheral nerve sheath, medial to the transverse process in the rat37–39 and either foraminal or intraspinal in human radiographic48 and cadaveric49 studies. The foraminal zone, an osteofibrous canal, is bounded superiorly and inferiorly by pedicles of adjacent vertebrae. The intervertebral disc and posterior margins of adjacent vertebrae form the anterior boundary. The posterior limit comprises the pars interarticularis, the superior facet joint, and the ligamentum flavum.50 Target neural structures are directly visualized by surgical procedures such as lumbar laminectomy, or less invasively accessed by fluoroscopy-guided injection specifically delivering therapeutics to the disc-nerve interface.
While herniated disc material triggers proinflammatory cascades and contributes to radiculopathy pain and disability,51–54 it is unclear whether addressing cytokine expression at the disc-nerve interface is sufficient or whether subepineurial effects have greater importance. Symptomatic resolution after disc resorption implicates the former,55–57 and local perineural therapeutics address the interaction between nucleus pulposus and the DRG epineurium. An ongoing clinical trial is evaluating epidural etanercept to treat painful radiculopathy. This biodistribution study shows that an ELP depot sustains release and attenuates extraspinal spread. Applications include gel phase entrapment of small molecules, conjugation of these molecules to ELP to further slow clearance, or expression of immunomodulator proteins fused with ELP. This delivery system is limited by the fixed volume in which spinal agents must be delivered beyond which extraspinal spread occurs. Maximum doses are limited by drug solubility and the effects of high doses on surrounding tissue.37 This biodistribution study is limited in its ability to address the animal’s in vivo response to ELP exposure because of the small mass of radiolabeled peptides used and the short follow-up. An evaluation of nonspecific complement response and inflammatory cell activation will require both in vitro testing of ELP material safety and in vivo testing with exposure to higher doses. However, it is noteworthy that a prior study of recombinant ELP application as a cardiovascular device coating demonstrated reduced platelet microparticle release with fewer thrombotic emboli.58 Furthermore, additional studies of host generation of antibodies against bioactive peptide fusions with ELP must be evaluated once a desired therapeutic and the anticipated doses are selected.
Covalent attachment of active drugs to ELP carriers is readily performed,30,59,60 and in vitro bioactivity of ELP-based therapeutics has been thoroughly reviewed.61 Chemical conjugation of ELP with doxorubicin yields a thermally sensitive compound that retains in vitro cytotoxicity against squamous cell carcinoma (FaDu) cells equivalent to free doxorubicin.59 Fusion protein activity has been demonstrated for oligopeptide cell penetrating peptides and oncogene inhibitors recombinantly expressed with an ELP partner. Penetratin-ELP readily translocates across cell membranes, while ELP-H1 decreases c-myc transcriptional activation and inhibits in vitro proliferation of breast carcinoma (MCF-7) cells. Ternary fusion penetratin-ELP-H1 exhibits tridomain functionality.30 Finally, ELP has been expressed fused with the anti-inflammatory protein, interleukin-1 receptor antagonist (IL1Ra), with effective antagonism of IL1 pro-inflammatory effects on thymocytes, lymphocytes, and fibrochondrocytes.62 The retention of ELP-IL1Ra bidomain functionality are especially relevant as they suggest the possibility of local delivery of complex biologic therapeutics for radiculopathy.
This study demonstrated feasibility of a locally administered drug delivery system based on thermally induced ELP phase separation. Perineural injection of an aqueous protein solution triggers formation of an insoluble aggregate from which individual molecules disaggregate over time. This behavior substantially increases ELP half-life in the perineural compartment, while also reducing ELP fraction in the serum compartment with little extraspinal redistribution. This biocompatible and nonimmunogenic29,63 biopolymer has the additional benefit of facile expression fused with bioactive polypeptides. While this work demonstrates ELP aggregation to sustain protein release with slowed elimination from the perineural compartment, future work will evaluate therapeutic efficacy of this drug delivery system in an animal model of lumbar radiculopathy secondary to disc herniation.
Key Points
This study demonstrates the feasibility of sustaining delivery of peptide-based therapeutics to the dorsal root ganglion.
This recombinant biopolymer is biocompatible and nonimmunogenic, and undergoes supramolecular aggregation to form an insoluble coacervate phase on reaching body temperature.
We show that this aggregation behavior prolongs local residence time of the ELP after perineural delivery, and slows its absorption into systemic circulation as reflected in its lower peak serum concentration as compared with a soluble, control protein.
Acknowledgments
The authors thank Ms. Charlene Flahiff and Mr. Steve Johnson for assistance with animal studies.
Federal and Institutional funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. Supported with funds from the NIH (R01EB002263, R21AR052745 and R01GM061232) and a Pratt-Gardner Predoctoral Research Fellowship.
References
- 1.Olmarker K, Storkson R, Berge OG. Pathogenesis of sciatic pain: a study of spontaneous behavior in rats exposed to experimental disc herniation. Spine. 2002;27:1312–1317. doi: 10.1097/00007632-200206150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Winkelstein BA, Weinstein JN, DeLeo JA. The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine. 2002;27:27–33. doi: 10.1097/00007632-200201010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Hashizume H, DeLeo JA, Colburn RW, et al. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Aoki Y, Rydevik B, Kikuchi S, et al. Local application of disc-related cytokines on spinal nerve roots. Spine. 2002;27:1614–1617. doi: 10.1097/00007632-200208010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi T, Kikuchi S, Shubayev V, et al. Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Murata Y, Onda A, Rydevik B, et al. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor-alpha to the dorsal root ganglion in rats. Spine. 2006;31:530–535. doi: 10.1097/01.brs.0000201260.10082.23. [DOI] [PubMed] [Google Scholar]
- 7.Weiler C, Nerlich AG, Bachmeier BE, et al. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30:44–53. doi: 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- 8.Woertgen C, Rothoerl RD, Brawanski A. Influence of macrophage infiltration of herniated lumbar disc tissue on outcome after lumbar disc surgery. Spine. 2000;25:871–875. doi: 10.1097/00007632-200004010-00017. [DOI] [PubMed] [Google Scholar]
- 9.Murata Y, Onda A, Rydevik B, et al. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced histologic changes in the dorsal root ganglion. Spine. 2004;29:2477–2484. doi: 10.1097/01.brs.0000144406.17512.ea. [DOI] [PubMed] [Google Scholar]
- 10.Onda A, Murata Y, Rydevik B, et al. Immunoreactivity of brain-derived neurotrophic factor in rat dorsal root ganglion and spinal cord dorsal horn following exposure to herniated nucleus pulposus. Neurosci Lett. 2003;352:49–52. doi: 10.1016/j.neulet.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Onda A, Yabuki S, Kikuchi S. Effects of neutralizing antibodies to tumor necrosis factor-alpha on nucleus pulposus-induced abnormal nociresponses in rat dorsal horn neurons. Spine. 2003;28:967–972. doi: 10.1097/01.BRS.0000061984.08703.0C. [DOI] [PubMed] [Google Scholar]
- 12.Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63:1120–1123. doi: 10.1136/ard.2003.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korhonen T, Karppinen J, Malmivaara A, et al. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine. 2004;29:2115–2119. doi: 10.1097/01.brs.0000141179.58778.6c. [DOI] [PubMed] [Google Scholar]
- 14.Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med Wkly. 2003;133:170–177. doi: 10.4414/smw.2003.10163. [DOI] [PubMed] [Google Scholar]
- 15.Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat. 2004;15:280–294. doi: 10.1080/09546630410017275. [DOI] [PubMed] [Google Scholar]
- 16.Brisby H, Olmarker K, Larsson K, et al. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernards CM, Luger TJ, Malmberg AB, et al. Liposome encapsulation prolongs alfentanil spinal analgesia and alters systemic redistribution in the rat. Anesthesiology. 1992;77:529–535. doi: 10.1097/00000542-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Kim T, Murdande S, Gruber A, et al. Sustained-release morphine for epidural analgesia in rats. Anesthesiology. 1996;85:331–338. doi: 10.1097/00000542-199608000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho B, Riley E, Cohen SE, et al. Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg. 2005;100:1150–1158. doi: 10.1213/01.ANE.0000149544.58230.FF. [DOI] [PubMed] [Google Scholar]
- 20.Lafont ND, Legros FJ, Boogaerts JG. Use of liposome-associated bupivacaine in a cancer pain syndrome. Anaesthesia. 1996;51:578–579. doi: 10.1111/j.1365-2044.1996.tb12569.x. [DOI] [PubMed] [Google Scholar]
- 21.Boogaerts JG, Lafont ND, Declercq AG, et al. Epidural administration of liposome-associated bupivacaine for the management of postsurgical pain: a first study. J Clin Anesth. 1994;6:315–320. doi: 10.1016/0952-8180(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 22.Sato S, Baba Y, Tajima K, et al. Prolongation of epidural anesthesia in the rabbit with the use of a biodegradable copolymer paste containing lidocaine. Anesth Analg. 1995;80:97–101. doi: 10.1097/00000539-199501000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Shulman M, Joseph NJ, Haller CA. Effect of epidural and subarachnoid injections of a 10% butamben suspension. Reg Anesth. 1990;15:142–146. [PubMed] [Google Scholar]
- 24.Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 25.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by protein-based polymers. J Phys Chem. 1997;101:11007–11028. [Google Scholar]
- 26.Urry DW. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog Biophys Mol Biol. 1992;57:23–57. doi: 10.1016/0079-6107(92)90003-o. [DOI] [PubMed] [Google Scholar]
- 27.Meyer DE, Chilkoti A. Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides. Biomacromolecules. 2004;5:846–851. doi: 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- 28.Chow DC, Dreher MR, Trabbic-Carlson K, et al. Ultra-high expression of a thermally responsive recombinant fusion protein in E. coli. Biotechnol Prog. 2006;22:638–646. doi: 10.1021/bp0503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urry DW, Parker TM, Reid MC, et al. Biocompatibility of the bioelastic materials, Poly(GVGVP) and its gamma-irradiation cross-linked matrix: summary of generic biological test results. J Bioact Compat Polym (USA) 1991;6:263–282. [Google Scholar]
- 30.Bidwell GL, III, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4:1076–1085. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 31.Massodi I, Bidwell GL, III, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J Control Release. 2005;108:396–408. doi: 10.1016/j.jconrel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Deliv Rev. 2002;54:1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 33.Betre H, Liu W, Zalutsky MR, et al. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release. 2006;115:175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 34.McPherson DT, Xu J, Urry DW. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr Purif. 1996;7:51–57. doi: 10.1006/prep.1996.0008. [DOI] [PubMed] [Google Scholar]
- 35.Jentoft N, Dearborn DG. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979;254:4359–4365. [PubMed] [Google Scholar]
- 36.Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79:1362–1366. doi: 10.1016/s0003-9993(98)90228-3. [DOI] [PubMed] [Google Scholar]
- 37.Yaksh T. Spinal Drug Delivered. Amsterdam: Elsevier Science; 1999. [Google Scholar]
- 38.Dobretsov M, Hastings SL, Stimers JR, et al. Mechanical hyperalgesia in rats with chronic perfusion of lumbar dorsal root ganglion with hyperglycemic solution. J Neurosci Methods. 2001;110:9–15. doi: 10.1016/s0165-0270(01)00410-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JM, Homma Y, Ackerman WE, et al. Topical application of acidic bupivacaine to the lumbar ganglion induces mechanical hyperalgesia in the rat. Anesth Analg. 2001;93:466–471. doi: 10.1097/00000539-200108000-00045. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JM, Li H, Brull SJ. Perfusion of the mechanically compressed lumbar ganglion with lidocaine reduces mechanical hyperalgesia and allodynia in the rat. J Neurophysiol. 2000;84:798–805. doi: 10.1152/jn.2000.84.2.798. [DOI] [PubMed] [Google Scholar]
- 41.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 42.Murata Y, Rydevik B, Takahashi K, et al. Macrophage appearance in the epineurium and endoneurium of dorsal root ganglion exposed to nucleus pulposus. J Peripher Nerv Syst. 2004;9:158–164. doi: 10.1111/j.1085-9489.2004.09305.x. [DOI] [PubMed] [Google Scholar]
- 43.Olmarker K, Nordborg C, Larsson K, et al. Ultra-structural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine. 1996;21:411–414. doi: 10.1097/00007632-199602150-00002. [DOI] [PubMed] [Google Scholar]
- 44.Iwabuchi M, Rydevik B, Kikuchi S, et al. Effects of anulus fibrosus and experimentally degenerated nucleus pulposus on nerve root conduction velocity: relevance of previous experimental investigations using normal nucleus pulposus. Spine. 2001;26:1651–1655. doi: 10.1097/00007632-200108010-00003. [DOI] [PubMed] [Google Scholar]
- 45.Murata Y, Olmarker K, Takahashi I, et al. Effects of selective tumor necrosis factor-alpha inhibition to pain-behavioral changes caused by nucleus pulposus-induced damage to the spinal nerve in rats. Neurosci Lett. 2005;382:148–152. doi: 10.1016/j.neulet.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26:863–869. doi: 10.1097/00007632-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, Dreher MR, Chow DC, et al. Tracking the in vivo fate of recombinant polypeptides by isotopic labeling. J Control Release. 2006;114:184–192. doi: 10.1016/j.jconrel.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Shen J, Wang HY, Chen JY, et al. Morphologic analysis of normal human lumbar dorsal root ganglion by 3D MR imaging. AJNR Am J Neuroradiol. 2006;27:2098–2103. [PMC free article] [PubMed] [Google Scholar]
- 49.Viswanathan R, Swamy NK, Tobler WD, et al. Extraforaminal lumbar disc herniations: microsurgical anatomy and surgical approach. J Neurosurg. 2002;96:206–211. doi: 10.3171/spi.2002.96.2.0206. [DOI] [PubMed] [Google Scholar]
- 50.Cinotti G, De Santis P, Nofroni I, et al. Stenosis of lumbar intervertebral foramen: anatomic study on predisposing factors. Spine. 2002;27:223–229. doi: 10.1097/00007632-200202010-00002. [DOI] [PubMed] [Google Scholar]
- 51.Mulleman D, Mammou S, Griffoul I, et al. Pathophysiology of disk-related low back pain and sciatica. II. Evidence supporting treatment with TNF-alpha antagonists. Joint Bone Spine. 2006;73:270–277. doi: 10.1016/j.jbspin.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Mulleman D, Mammou S, Griffoul I, et al. Pathophysiology of disk-related sciatica. I.-Evidence supporting a chemical component. Joint Bone Spine. 2006;73:151–158. doi: 10.1016/j.jbspin.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Rhee JM, Schaufele M, Abdu WA. Radiculopathy and the herniated lumbar disc. Controversies regarding pathophysiology and management. J Bone Joint Surg Am. 2006;88:2070–2080. doi: 10.2106/00004623-200609000-00023. [DOI] [PubMed] [Google Scholar]
- 54.Slipman CW, Isaac Z, Lenrow DA, et al. Clinical evidence of chemical radiculopathy. Pain Physician. 2002;5:260–265. [PubMed] [Google Scholar]
- 55.Komori H, Shinomiya K, Nakai O, et al. The natural history of herniated nucleus pulposus with radiculopathy. Spine. 1996;21:225–229. doi: 10.1097/00007632-199601150-00013. [DOI] [PubMed] [Google Scholar]
- 56.Mochida K, Komori H, Okawa A, et al. Regression of cervical disc herniation observed on magnetic resonance images. Spine. 1998;23:990–995. doi: 10.1097/00007632-199805010-00005. [DOI] [PubMed] [Google Scholar]
- 57.Slavin KV, Raja A, Thornton J, et al. Spontaneous regression of a large lumbar disc herniation: report of an illustrative case. Surg Neurol. 2001;56:333–336. doi: 10.1016/s0090-3019(01)00607-3. [DOI] [PubMed] [Google Scholar]
- 58.Woodhouse KA, Klement P, Chen V, et al. Investigation of recombinant human elastin polypeptides as non-thrombogenic coatings. Biomaterials. 2004;25:4543–4553. doi: 10.1016/j.biomaterials.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 59.Dreher MR, Raucher D, Balu N, et al. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 60.Trabbic-Carlson K, Liu L, Kim B, et al. Expression and purification of recombinant proteins from Escherichia coli: comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 2004;13:3274–3284. doi: 10.1110/ps.04931604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simnick AJ, Lim DW, Chow DC, et al. Biomedical and biotechnological applications of elastin-like polypeptides. Part C: polymer reviews. J Macromol Sci. 2007;47:121–154. [Google Scholar]
- 62.Shamji MF, Betre H, Kraus VB, et al. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist. Arthritis Rheum. 2007;56:3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 63.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]