Abstract
Cysteine catabolism in mammals is dependent upon cysteine dioxygenase (CDO), an enzyme that adds molecular oxygen to the sulfur of cysteine, converting the thiol to a sulfinic acid known as cysteinesulfinic acid (3-sulfinoalanine). CDO is one of the most highly regulated metabolic enzymes responding to diet that is known. It undergoes up to 45-fold changes in concentration and up to 10-fold changes in catalytic efficiency. This provides a remarkable responsiveness of the cell to changes in sulfur amino acid availability: the ability to decrease CDO activity and conserve cysteine when cysteine is scarce and to rapidly increase CDO activity and catabolize cysteine to prevent cytotoxicity when cysteine supply is abundant. CDO in both liver and adipose tissues responds to changes in dietary intakes of protein and/or sulfur amino acids over a range that encompasses the requirement level, suggesting that cysteine homeostasis is very important to the living organism.
Keywords: Cysteine, Cysteine dioxygenase, Amino acid derived cofactor, Hypotaurine, Taurine, Sulfate
Introduction
Cysteine catabolism in mammals is dependent upon cysteine dioxygenase (CDO), an enzyme that adds molecular oxygen to the sulfur of cysteine, converting the thiol to a sulfinic acid known as cysteinesulfinic acid (3-sulfinoalanine), as shown in Figure 1. CDO is one of the most highly regulated metabolic enzymes responding to diet that is known. In response to a change in dietary protein or sulfur amino acid intake, CDO concentrations change by up to 45-fold (Bella et al. 1999a, 1999b; Lee et al. 2004) and catalytic efficiency of the enzyme changes by up to 10-fold (Dominy et al. 2008), resulting in an overall dynamic range of change in CDO activity of up to 450-fold. Hepatic CDO activity is very low in animals fed a low protein diet (e.g., 100 g casein per kg diet) but increases dramatically when the protein level in the diet is increased to 200 g/kg (near the requirement) and even more when it is increased to 400g/kg (excess of the requirement), with the new steady state levels of CDO activity being reached within hours of the diet change (Bella et al. 1999a, 1999b; Stipanuk et al. 2002; Lee et al. 2004). This robust regulation of CDO activity over a physiological range of protein and/or sulfur amino acid intakes suggests that cysteine homeostasis is very important to the living organism.
Figure 1.
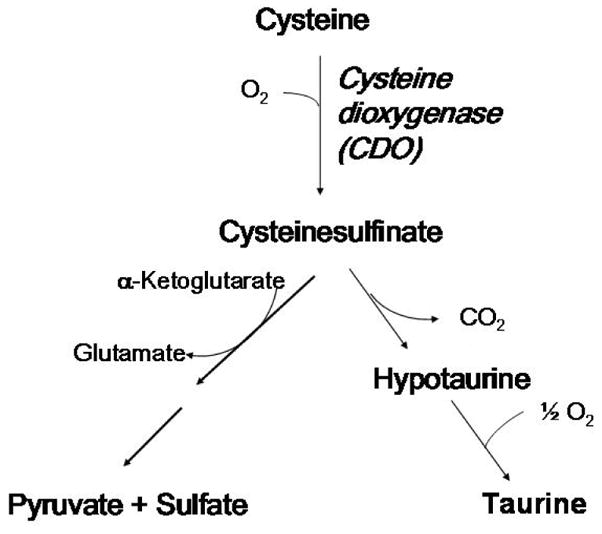
The metabolism of cysteine by cysteine dioxygenase-catalyzed conversion to cysteinesulfinate followed by further metabolism to either pyruvate + sulfate or to taurine + CO2.
Elevated levels of cysteine have been shown to be both cytotoxic and neurotoxic, and CDO plays a critical and highly regulated role in degradation of excess cysteine obtained from the diet or synthesized from methionine sulfur and serine by the transsulfuration pathway. The product of the reaction catalyzed by CDO, cysteinesulfinic acid, is either decarboxylated to hypotaurine, which is further oxidized to taurine by a poorly understood mechanism, or transaminated to the putative intermediate 3-sulfinylpyruvate that spontaneously decomposes to pyruvate and sulfite, with sulfite being further oxidized to sulfate by sulfite oxidase. Thus, CDO not only removes excess cysteine but is necessary for hypotaurine/taurine and sulfite/sulfate production from cysteine. It is likely that both roles of CDO have physiological significance and that the major role of CDO may vary with cell type.
Cells that have the capacity for taurine synthesis, from either cysteine or cysteamine, synthesize taurine via the intermediate formation of hypotaurine (Stipanuk 1986, 2004a). Because hypotaurine is readily oxidized to taurine, the accumulation of hypotaurine in tissues almost always represents de novo synthesis, and hypotaurine accumulation in tissues or cells has been observed to be highly correlated with flux through CDO (Ueki and Stipanuk, unreported; Dominy et al. 2006b; Stipanuk et al. 2006). The search for a specific enzymatic system capable of oxidizing the sulfinic acid group of hypotaurine (or its carboxylated analog, cysteinesulfinic acid, that is formed from cysteine) has been unsuccessful (Stipanuk 2004a), but both hypotaurine and cysteinesulfinic acid can be oxidized to sulfonic acids by non-specific oxidizing agents (e.g., hydroxyl radicals, singlet oxygen, peroxynitrite) in vitro and presumably in vivo (Fontana et al. 2005; Green et al. 1991). Whether hypotaurine plays a unique physiological role apart from its conversion to taurine has not been extensively explored.
Evidence of abnormal or deficient CDO activity has been reported in individuals with a variety of autoimmune and neurodegenerative diseases, including rheumatoid arthritis, Parkinson’s disease, Alzheimer’s disease, and motor neuron diseases. Investigators conducting clinical studies on these patient populations have chosen to look primarily at impairments in the conversion of cysteine to sulfate (but not to taurine), and have reported depressed levels of sulfate in plasma, elevated fasting plasma cysteine concentrations, elevated plasma cysteine:sulfate ratios, and lower sulfate-conjugate:glucuronide-conjugate ratios for acetaminophen detoxification products, all of which are consistent with impaired cysteine oxidation (Bradley et al. 1994; Davies et al. 1995; Heafield et al. 1990; Emery et al. 1992). The etiology of these diseases could be linked to functional impairment of cysteine dioxygenase, leading to elevated levels of cysteine and possibly elevated production of H2S via alternative cysteinesulfinate-independent pathways of cysteine metabolism as well as to deficiencies of hypotaurine, taurine, and sulfate that are normally products of cysteinesulfinate-dependent metabolism of cysteine..
CDO concentration in liver and adipose tissue is regulated by cysteine availability via regulation of CDO degradation
Dietary regulation of hepatic CDO
CDO is mainly regulated at the level of protein turnover rather than at the level of mRNA in liver of rats in vivo (Bella et al. 1999a, 1999b; Stipanuk et al. 2002; Stipanuk and Dominy 2006). CDO is very low in liver of rats fed a low protein diet (e.g., 100 g protein/kg diet) but accumulates in liver of rats fed diets with adequate or excess protein or supplemental sulfur-containing amino acids despite little or no change in hepatic CDO mRNA levels. Changes in hepatic CDO concentration occur within hours of a diet switch, reaching a new steady-state by 24 h (Lee et al., 2004), and hepatic CDO concentration in vivo appears to respond specifically to changes in cysteine availability (Cresenzi et al. 2003). In addition, primary hepatocytes and rat CDO heterologously expressed in human HepG2/C3A cells (which do not express detectable endogenous CDO) respond to a deficiency (or excess) of cysteine in the culture medium with a decrease (or increase) in CDO protein level but little or no change in CDO mRNA level (Kwon and Stipanuk 2001; Stipanuk et al. 2002; Dominy et al. 2006a). The cysteine analog cysteamine (i.e., “decarboxylated cysteine”, or 2-aminoethanethiol), but not other cysteine metabolites or thiol reagents, was also effective in increasing CDO concentration in these cells (Dominy et al. 2006a). Inhibitors of the 26S proteasome (e.g., proteasome inhibitor 1 and lactacystin) blocked CDO degradation in cysteine-deficient cells but had little or no effect on CDO concentration in hepatocytes cultured with excess cysteine. High-molecular-mass CDO-ubiquitin conjugates that reacted with both anti-ubiquitin and anti-CDO were observed in cells cultured in cysteine-deficient medium, whether or not proteasome inhibitor was present, but these CDO-ubiquitin conjugates were not observed or were much lower in cells cultured in cysteine-supplemented medium with or without proteasome inhibitor (Stipanuk et al. 2002; Dominy et al. 2006). We further evaluated the contribution of the ubiquitin-26S proteasome pathway to the diet-induced changes in CDO half-life that we have observed in rats (Dominy et al. 2006). In the rat, as in cultured cells, a cysteine-supplemented diet (100 g casein + 8.1 g L-cysteine/kg) or a high protein diet (400 g casein/kg) led to markedly higher hepatic CDO concentrations than were observed in rats fed a low protein diet (100 g casein/kg). Additionally, inhibition of the proteasome in vivo with proteasome inhibitor 1 dramatically stabilized CDO in the liver under dietary conditions (i.e., low protein) that normally favor its degradation, and ubiquitinated CDO intermediates accumulated in the liver of these rats. Metabolic analyses showed that proteasome inhibitor 1 had a significant effect on sulfoxidation flux (accumulation of hypotaurine, and to a much lesser extent taurine) secondary to the stabilization of CDO. Because proteasome inhibitor 1 had no significant effect on the intracellular cysteine pool, the increased sulfoxidation flux can be attributed to increased enzyme (CDO) activity, demonstrating the physiological relevance of an increase in CDO activity, apart from increased cysteine concentration, in determining cysteine flux to taurine. Rats switched to a diet with excess cysteamine (2-aminoethanethiol, 7.2g/kg, equimolar to a cysteine supplement of 8.1g/kg or the sulfur amino acid equivalent in 300g casein/kg) had hepatic cysteamine levels that at 6 h after the onset of the meal were 19-times those of rats fed the low protein diet, or 3-times those of rats fed the high protein diet, but these rats did not exhibit any stabilization of CDO, clearly demonstrating that physiological concentrations of cysteamine would be insufficient to regulate CDO turnover in vivo. Thus, regulation of hepatic CDO levels in response to dietary cyst(e)ine involves a block in the (poly)ubiquitination of CDO that in turn diminishes its rate of proteasomal degradation, allowing it to accumulate in the cell. This regulation of CDO is remarkably robust, involving rapid changes in CDO concentration when rats adapted to a low protein/low sulfur amino acid diet are given a high protein/high sulfur amino acid meal (Dominy et al., 2006a, 2006b;Lee et al. 2004).
Dietary regulation of CDO in adipose tissues
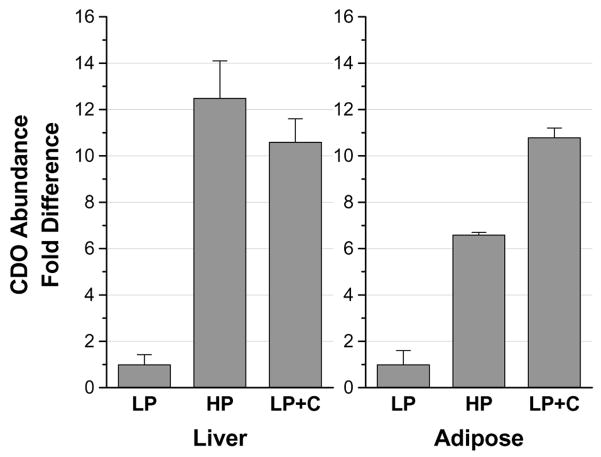
In screening tissues for expression of CDO, we found that adipose tissue contained relatively high levels of CDO, generally lower than those in liver but equal to or greater than those in kidney and lung (Ueki and Stipanuk, unreported). High levels of CDO mRNA in adipose tissue of rats had previously been reported by Ide et al. (2002). We further screened different adipose depots for CDO expression and found high levels of CDO protein in various white adipose depots and in brown fat in the rat (Ueki and Stipanuk, unreported). We further screened the epididymal fat of male rats fed diets with varying cyst(e)ine or protein levels to determine if CDO in adipose tissue is regulated in response to dietary intake of sulfur amino acids. As shown in Figure 2, we found markedly higher amounts of CDO in adipose tissue from rats fed high protein and high cystine diets. In further work with 3T3-L1 cells, preadipocytes were found to have very low levels of CDO expression (mRNA and protein) whereas differentiated adipocytes showed high levels of CDO expression that corresponded with the appearance of fatty acid binding protein 4, which served as a marker for mature adipocytes. As shown in Figure 3, mature adipocytes also displayed cysteine- and proteasome inhibitor (lactacystin)-mediated increases in CDO concentration. Much like our observations in cultured hepatocytes, cysteamine was also effective in stabilizing CDO whereas 2-mercaptoethanol was not. However, methionine had no effect, presumably due to the lack of an active transsulfuration pathway in adipocytes as addition of S-adenosylhomocysteine or cystathionine also did not substitute for cysteine in preventing CDO degradation. Consistent with previous results for a strong association of hypotaurine and CDO levels in other tissues, hypotaurine + taurine accumulated markedly both intracellularly (mainly hypotaurine) and in the culture medium of mature (but not of undifferentiated) adipocytes when medium was supplemented with cysteine. These observations on adipose tissue, along with our observations of significant expression of cysteinesulfinic acid decarboxylase in adipose tissue and adipocytes, indicate that adipose tissue, like liver, may be an important site in the body for regulation of cysteine levels and for hypotaurine/taurine synthesis.
Figure 2.
Fold changes in CDO abundance in liver and epidydimal fat of rats adapted for 7 days to either a low protein (LP, 100 g casein per kg diet), high protein (HP, 400 g casein per kg diet), or low protein plus L-cystine (LP+C, 100 g casein + 8.1 g L-cystine per kg diet) diet. CDO abundance was determined by Western blotting. Bands were quantified by densitometry. Values are means ± standard deviations for 3 (adipose) or 6 (liver) rats. Based on data of Dominy et al. (2006b) and Ueki and Stipanuk, unreported.
Figure 3.
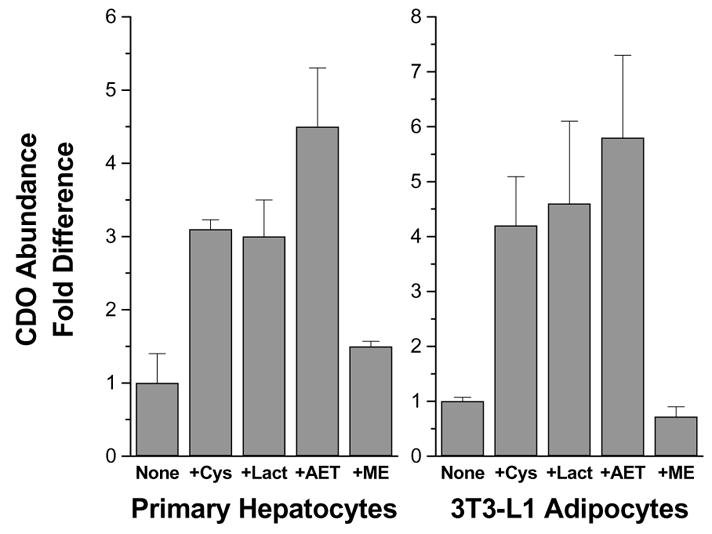
CDO abundance in primary hepatocytes or 3T3-L1 differentiated adipocytes cultured in medium with 0.1 mM L-methionine and no cysteine (None) or with the addition of 1 mM L-cysteine (+Cys), 5 μM lactacystin (+Lact), 1 mM cysteamine (2-aminoethanethiol) (+AET), or 1 mM 2-mercaptoethanol (+ME) for 12 h (hepatocytes) or 4 h (adipocytes). CDO abundance was determined by western blotting and densitometry. Values are means ± standard deviations for 3 separate experiments. Based on data of Dominy et al. (2006b) and Ueki and Stipanuk, unreported.
Thus, CDO levels in both liver and adipose tissue of intact rats are regulated by ubiquitination and proteasomal degradation of CDO when cysteine availability is low (Dominy et al. 2006a, 2006b; Stipanuk et al. 2006, 2004a; Ueki and Stipanuk, unreported). Ubiquitination of CDO, and hence its degradation, is inhibited by elevated cysteine levels. In both tissues, metabolites or precursors of cysteine or other thiol analogs, with the exception of cysteamine in vitro, are ineffective in blocking CDO ubiquitination/degradation, demonstrating the specificity of this regulation for CDO’s substrate molecule (Dominy et al. 2006a, 2006b; Stipanuk et al. 2006, 2004a; Ueki and Stipanuk, unreported).
The CDO activity state is regulated by cysteine concentration via a substrate-turnover-dependent formation of a CDO cofactor that enhances CDO’s catalytic efficiency
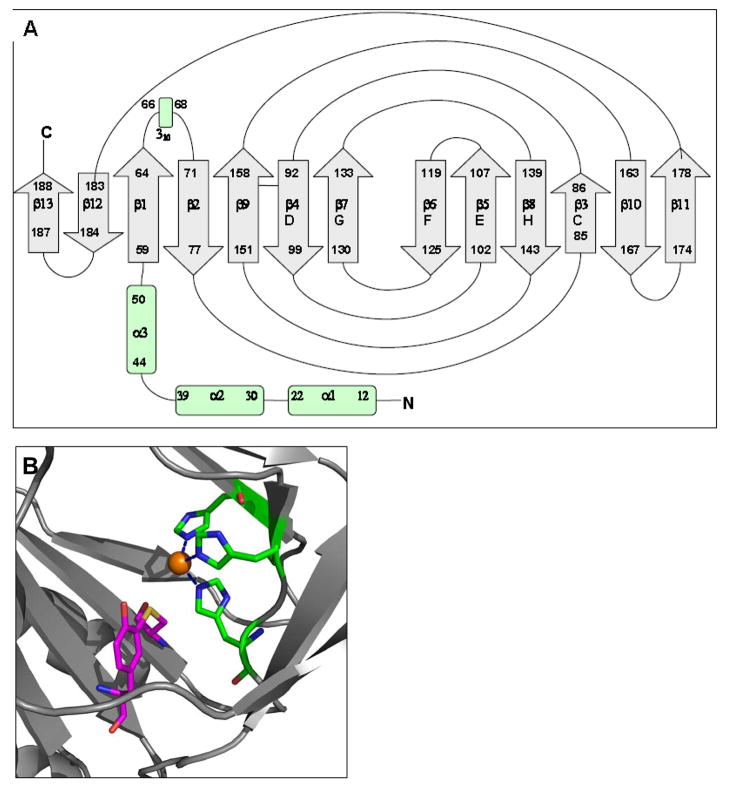
Two major CDO bands are routinely observed when CDO from animal tissues or recombinant CDO expressed in bacterial systems is analyzed by electrophoretic methods (Stipanuk et al. 2004b). Although these two CDO species appeared to have different degrees of catalytic activity, the physical difference in these two apparent isoforms was elusive (Simmons et al. 2006a; Stipanuk et al. 2004b). Efforts to detect known posttranslational modifications, proteolytic cleavage, or oxidative modifications only yielded negative results. Furthermore, mass spectrometry measurements indicated that the two species could not be resolved by mass spectrometry instrumentation with a mass accuracy of ± 4 Da after deconvolution for the 23-kDa protein, making the addition of a functional group or proteolytic cleavage unlikely possibilities (Stipanuk et al. 2004b; Dominy et al. 2008). The discovery of a rare intramolecular crosslink between active site residues Tyr157 and Cys93 in the x-ray crystal structure of CDO, as shown in Figure 4, offered a new potential explanation that was consistent with our observation of negligible mass difference; the predicted mass difference between crosslinked an dnon-crosslinked CDO isoforms would be only 2 Da. To test whether the two populations of CDO were due to the presence or absence of a thioether bond, we disrupted thioether bond formation by mutation of either Cys93 or Tyr157. This produced a protein species that migrated as a single (upper, 23 kDa) band on SDS-PAGE. We then set up further experiments to determine whether the second (lower band, “22.5” kDa) isoform of CDO was, in fact, the thioether-crosslinked or “mature” enzyme (Dominy et al. 2008). Isoelectric focusing revealed that the 22.5 kDa band had a very slightly higher pI (pH 5.85 versus pH 5.80 for the 23 kDa band), which was consistent with Cys-Tyr crosslink formation (Dominy et al. 2008). 2-D gel electrophoresis to separate isoforms followed by mass spectrometric studies of trypsin- and chymotrypsin-digests of the two isoforms showed the presence of the predicted unlinked peptides containing Cys93 and Tyr157 in the upper but not lower band (Dominy et al., 2008). All of these findings were consistent with the resolution of CDO into a no n-thioether-containing immature isoform that migrates at the predicted molecular mass of ~23 kDa and a Cys-Tyr thioether-containing mature isoform that migrates with an apparent mass of ~22.5 kDa. The electrophoretic migration patterns for these two forms of CDO are similar to those previously reported for galactose oxidase, which also forms an intramolecular Cys-Tyr crosslink (Whittaker and Whittaker 2003).
Figure 4.
Overall Structure of CDO. (A) Topology diagram of CDO. (B) Model of the CDO active site. The CDO iron (orange sphere) is coordinated by the Nε2 atoms of three histidine ligands (His86, His88, and His140) and a water molecule (not shown) in an unusual tetrahedral geometry. The covalent bond between the γS of Cys93 (yellow) and the (ortho-position) Cε2 of Tyr157 is also shown. The phenolic hydroxyl of Tyr157 appears to be involved in catalysis, serving as a H-bond donor or proton donor. Based on data of Simmons et al. (2006b).
Confirmation of the correspondence of the presence of the intramolecular Cys-Tyr crosslink with a shift in CDO’s electrophoretic migration provided an easy means to “assay” thioether crosslink formation. This, in turn, allowed us to evaluate (i) the exact requirements for intramolecular thioether synthesis, (ii) to demonstrate that thioether crosslink formation increased catalytic efficiency, establishing the Cys-Tyr moiety as an amino acid-derived cofactor, and (iii) to evaluate the contribution of thioether cofactor to the catalytic activity and stability of the enzyme. Some catalytic activity in the immature CDO was necessary for thioether crosslink formation; inactive mutant forms of CDO (e.g., His86Ala, leading to loss of the His that serves as a metal ligand in the active site) did not form any crosslink, and mutant forms of CDO with low activity (e.g., Arg60Ala, which has a markedly decreased affinity for cysteine, probably due to the loss of the hydrogen bonding partner for the carboxylate of the substrate) formed the crosslink more slowly. Mutations of nonessential residues (e.g., Ser153Ala; Cys164Ala) had little effect. Like other amino acid cofactor-containing enzymes, formation of CDO’s Cys-Tyr crosslink required a transition metal cofactor (Fe2+) and O2, but unlike other amino acid cofactor-containing enzymes, biogenesis of the CDO crosslink did not occur immediately upon exposure to metal and O2,, was not essential for basal catalytic activity, and was strictly dependent upon the presence of its specific substrate cysteine. Substrate and substrate turnover were essential for CDO cofactor formation both in assays of purified CDO and of CDO in intact cells. Cofactor formation was much slower than the rates reported for other amino acid cofactor-containing enzymes and, in fact, took hundreds of catalytic turnover cycles to occur. Nevertheless, the CDO Cys-Tyr moiety seems to serve a cofactor function in CDO. Although CDO possessed appreciable catalytic activity in the absence of the Cys-Tyr cofactor, cofactor formation increased CDO catalytic efficiency by ~10-fold (e.g., 3200 M−1s−1 for wild-type CDO versus 220 M−1s−1 for Cys93Ser mutant CDO) and additionally increased its catalytic life time (~3-fold as many catalytic cycles, as measured in vitro). A transition of hepatic isoforms of CDO from mainly nonthioether form in liver of rats fed a low protein or low cyst(e)ine diet to mainly thioether form in liver of rats fed a diet containing a moderate to high level of cysteine or protein indicated that the presence of the two isoforms is physiologically relevant, and several studies from our laboratory have demonstrated the relationship of cysteine concentration to mature CDO isoform formation in vivo (Stipanuk et al. 2004b; Dominy et al. 2006a, 2006b; Ueki and Stipanuk, unreported).
We also tested the possibility that Cys-Tyr thioether cofactor formation might alter the susceptibility of CDO to (poly)ubiquitination and proteasomal degradation, by expressing wild-type and Cys93Ser mutant CDO in HepG2/C3A cells using a Tet-off system and cysteine-supplemented medium. Following expression, both the wild-type CDO (converted to the mature form as a consequence of being cultured in cysteine-supplemented medium) and the Cys93Ser mutant CDO (which could not by converted to the mature form due to the loss of the cysteine residue involved in thioether crosslink formation) appeared to be degraded at a similar rate when cells were placed in cysteine-deficient medium. The maintenance of a high rate of turnover of the mature CDO is consistent with the cell’s retention of its ability to rapidly degrade CDO when cysteine supply abruptly decreases. Nevertheless, coupling the >10-fold increase in CDO concentration that can be achieved by regulation of CDO’s ubiquitination/degradation with the >10-fold increase in CDO’s catalytic efficiency that can be achieved by Cys-Tyr cofactor formation plus the increased catalytic life time of the mature enzyme provides the cell with a capacity for a many-fold increase in CDO activity in response to a high intake of protein or sulfur amino acids. Additionally, changes in CDO activity state can be observed within minutes, and changes in CDO concentration can be observed within a few hours of changes in cysteine availability, both in cells in culture and in liver of rats switched from a diet containing a low, inadequate level of sulfur-containing amino acids to one containing a high, excess level.
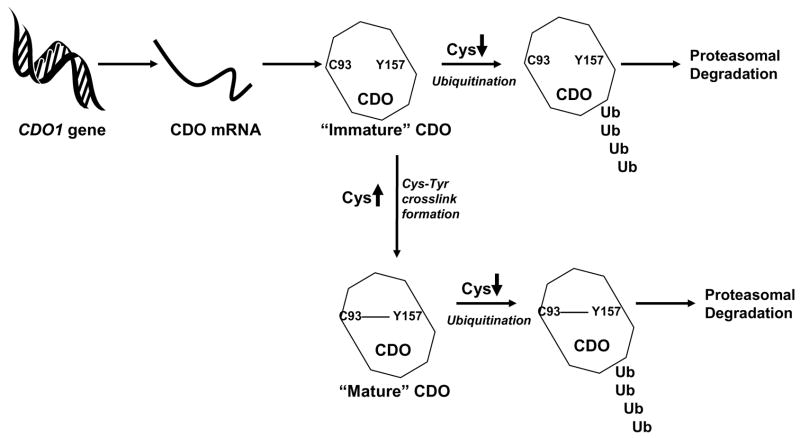
Overall, the results indicate that cysteine’s ability to modulate internal Cys-Tyr cofactor formation represents a very unusual form of feed-forward activation of enzyme activity. This is a unique finding as substrate-regulation of protein cofactor formation has never before been reported, to our knowledge, as a means of regulating a protein’s normal function. This, along with the regulation of CDO concentration by cysteine-mediated inhibition of CDO ubiquitination and proteasomal degradation, provides a remarkable responsiveness of the cell to changes in sulfur amino acid availability – the ability to decrease CDO activity and conserve cysteine when cysteine is scarce and to rapidly increase CDO activity and catabolize cysteine to prevent cytotoxicity when cysteine supply is abundant. Clearly, CDO is remarkable among metabolic enzymes in the degree to which its activity can be regulated in response to its substrate, suggesting that tight regulation of cysteine levels is a critical function of the body. The regulation of CDO concentration and activity state by cysteine is summarized in Figure 5.
Figure 5.
Diagram showing the regulation of CDO concentration and activity via effects of cysteine (substrate) on CDO degradation via the ubiquitin-proteasome system and on thioether cofactor formation.
CDO controls intracellular cysteine levels
Although we have postulated that CDO is a major regulator of intracellular cysteine levels in mammals, direct experimental evidence has been lacking to support the hypothesis that CDO is capable of altering steady-state intracellular cysteine levels. To directly test this hypothesis, we expressed either the wild-type or a catalytically inactivated mutant (His86Ala) CDO in HepG2/C3A cells (which do not express endogenous CDO protein) and cultured them in different concentrations of extracellular cysteine (Dominy et al. 2007). Wild-type CDO, but not inactive His86Ala CDO, was capable of reducing intracellular cysteine levels in cells incubated in physiologically relevant concentrations of cysteine. Because cellular glutathione levels are highly dependent upon cellular cysteine levels, we also measured glutathione concentrations in these cells. Expression of wild-type CDO decreased the glutathione pool and potentiated the toxicity of CdCl2 (Dominy et al. 2007) In addition, in studies with 3T3-L1 cells, supplementation of the culture medium with cysteine led to a marked elevation of the intracellular cysteine level in preadipocytes that express little or no CDO, whereas differentiating and differentiated adipocytes that do express CDO were able to maintain intracellular cysteine levels that were not significantly greater (P>0.05) than those of cells cultured in basal medium (Ueki and Stipanuk, unreported). These results demonstrate that CDO is capable of altering intracellular steady-state cysteine levels as well as glutathione levels in cells and support the belief that CDO is an important regulator of cellular and whole body cysteine levels. This observation underscores the significant roles of liver and adipose tissue CDO in the regulation of body cysteine levels, as well as in the production of critical cysteine metabolites (taurine and sulfate).
Tissue-specific localization and function of CDO
CDO appears to be expressed in a very tissue- and cell-specific manner. Although there have been no extensive studies of CDO in tissues other than liver and adipose tissue, CDO may play specialized functions in certain tissues or cell types. Previous immunohistochemical studies of CDO localization were done using CDO antibodies that apparently were not specific for CDO as they detected a 68-kDa protein that was assumed to be a complex containing CDO (Parsons et al. 2001a, 2001b; Millard et al. 2003). CDO has now been shown to clearly migrate on SDS-PAGE as the predicted 23-kDa monomer, albeit as a doublet (Stipanuk et al. 2004b, Simmons et al. 2006a, 2006b; Dominy et al. 2006b). Nevertheless, in these earlier studies, in-situ hybridization to detect CDO mRNA demonstrated the localization of CDO mRNA to particular cell types in various tissues (Parsons et al. 2001a, 2001b; Shimada et al. 1998; Millard et al. 2003).
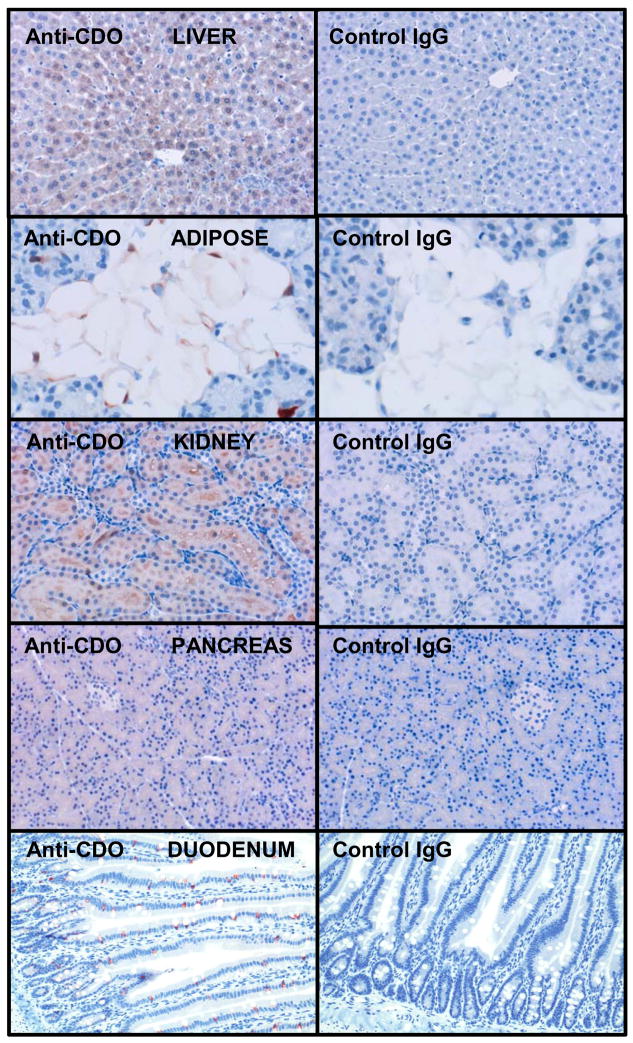
We have looked further at CDO localization in rat tissues using our affinity-purified polyclonal CDO antibody that recognizes the ~23 kDa CDO. Some examples of our immunohistochemistry are shown in Figure 6. CDO was found in the cytoplasm of hepatocytes and adipocytes, both of which respond to dietary protein or sulfur amino acid intake by increased cellular CDO concentrations. CDO also is expressed in many secretory and/or epithelial cell types. In the kidney, CDO was found in the epithelial cells of the renal tubules. In the pancreas, CDO was found in exocrine cells but not in islet endocrine cells. In the intestinal epithelium, CDO was found specifically in the mucus-secreting goblet cells. For mammary gland, we have reported intense staining for CDO in ductal cells of pregnant rats but not in other mammary epithelial cells or in ductal cells of nonpregnant rats (Ueki and Stipanuk, 2007). Further exploration of the physiological functions of CDO in these or other specialized cells within tissues will likely provide additional insights into biological functions of CDO. Further studies using conditional CDO knockout mice are underway.
Figure 6.
CDO immunohistochemistry showing localization of CDO. Rat tissues were fixed in 4% (w/v) paraformaldehyde, embedded in paraffin and sectioned into 4 μm sections. After deparaffinization, sections were immersed in 0.5% (v:v) hydrogen peroxide in methanol to block endogenous peroxidases and steamed in 0.01 mol/L citrate buffer, pH 6.0 for 15 min for antigen retrieval. Sections were blocked with 10% (v/v) nonimmune goat serum (Zymed, Invitrogen) in 2x casein (Vector Laboratories) prior to incubation, for 2 h at 37°C, with a 1:90 dilution of an affinity-purified rabbit- anti-rat CDO antibody or an equivalent concentration of nonimmune rabbit IgG (IgG control) diluted in 1x casein in PBS. Application of biotinylated secondary antibody followed by streptavidin peroxidase and AEC chromagen substrate (Zymed) was performed for color development, and sections were counterstained with Gill’s #2 hematoxylin.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases through Public Health Service Grant # DK056649 (to M. H. S.).
Abbreviations
- CDO
Cysteine dioxygenase
- SDS-PAGE
Sodium dodecyl sulfate- polyacrylamide gel electrophoresis
References
- Bella DL, Hahn C, Stipanuk MH. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol. 1999a;277:E144–E153. doi: 10.1152/ajpendo.1999.277.1.E144. [DOI] [PubMed] [Google Scholar]
- Bella DL, Hirschberger LL, Hosokawa Y, Stipanuk MH. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol. 1999b;276:E326–E335. doi: 10.1152/ajpendo.1999.276.2.E326. [DOI] [PubMed] [Google Scholar]
- Bradley H, Gough A, Sokhi RS, Hassell A, Waring R, Emery P. Sulfate metabolism is abnormal in patients with rheumatoid arthritis. Confirmation by in vivo biochemical findings. J Rheumatol. 1994;21:1192–1196. [PubMed] [Google Scholar]
- Cresenzi CL, Lee JI, Stipanuk MH. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr. 2003;133:2697–2702. doi: 10.1093/jn/133.9.2697. [DOI] [PubMed] [Google Scholar]
- Davies MH, Ngong JM, Pean A, Vickers CR, Waring RH, Elias E. Sulphoxidation and sulphation capacity in patients with primary biliary cirrhosis. J Hepatol. 1995;22:551–560. doi: 10.1016/0168-8278(95)80450-1. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr, Hirschberger LL, Coloso RM, Stipanuk MH. In vivo regulation of cysteine dioxygenase via the ubiquitin-26S proteasome system. Adv Exp Med Biol. 2006a;583:37–47. doi: 10.1007/978-0-387-33504-9_4. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Stipanuk MH. New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr Rev. 2004;62:348–353. doi: 10.1111/j.1753-4887.2004.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr, Hirschberger LL, Coloso RM, Stipanuk MH. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26S proteasome system in the living rat. Biochem J. 2006b;394(Pt 1):267–273. doi: 10.1042/BJ20051510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy JE, Jr, Hwang J, Stipanuk MH. Overexpression of cysteine dioxygenase reduces intracellular cysteine and glutathione pools in HepG2/C3A cells. Am J Physiol Endocrinol Metab. 2007;293:E62–E69. doi: 10.1152/ajpendo.00053.2007. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr, Hwang J, Guo S, Hirschberger LL, Zhang S, Stipanuk MH. Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. J Biol Chem. 2008;283:12188–12201. doi: 10.1074/jbc.M800044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, Salmon M, Bradley H, Wordsworth P, Tunn E, Bacon PA, Waring R. Genetically determined factors as predictors of radiological change in patients with early symmetrical arthritis. Bmj. 1992;305(6866):1387–1389. doi: 10.1136/bmj.305.6866.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana M, Amendola D, Orsini E, Boffi A, Pecci L. Oxidation of hypotaurine and cysteine sulphinic acid by peroxynitrite. Biochem J. 2005;389:233–240. doi: 10.1042/BJ20041696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TR, Fellman JH, Eicher AL, Pratt KL. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim Biophys Acta. 1991;1073:91–97. doi: 10.1016/0304-4165(91)90187-l. [DOI] [PubMed] [Google Scholar]
- Heafield MT, Fearn S, Steventon GB, Waring RH, Williams AC, Sturman SG. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson’s and Alzheimer’s disease. Neurosci Lett. 1990;110:216–220. doi: 10.1016/0304-3940(90)90814-p. [DOI] [PubMed] [Google Scholar]
- Hirschberger LL, Daval S, Stover PJ, Stipanuk MH. Murine cysteine dioxygenase gene: structural organization, tissue-specific expression and promoter identification. Gene. 2001;277:153–161. doi: 10.1016/s0378-1119(01)00691-6. [DOI] [PubMed] [Google Scholar]
- Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S. mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism. 2002;51:1191–1197. doi: 10.1053/meta.2002.34036. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Stipanuk MH. Cysteine regulates expression of cysteine dioxygenase and gamma-glutamylcysteine synthetase in cultured rat hepatocytes. Am J Physiol Endocrinol Metab. 2001;280(5):E804–E815. doi: 10.1152/ajpendo.2001.280.5.E804. [DOI] [PubMed] [Google Scholar]
- Lee JI, Londono M, Hirschberger LL, Stipanuk MH. Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. J Nutr Biochem. 2004;15:112–122. doi: 10.1016/j.jnutbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Millard J, Parsons RB, Waring RH, Williams AC, Ramsden DB. Expression of cysteine dioxygenase (EC 1.13.11.20) and sulfite oxidase in the human lung: a potential role for sulfate production in the protection from airborne xenobiotica. Mol Pathol. 2003;56:270–274. doi: 10.1136/mp.56.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RB, Sampson D, Huggins CC, Waring RH, Williams AC, Ramsden DB. Renal localisation of rat cysteine dioxygenase. Nephron. 2001a;88:340–346. doi: 10.1159/000046018. [DOI] [PubMed] [Google Scholar]
- Parsons RB, Waring RH, Williams AC, Ramsden DB. Cysteine dioxygenase: regional localisation of protein and mRNA in rat brain. J Neurosci Res. 2001b;65:78–84. doi: 10.1002/jnr.1130. [DOI] [PubMed] [Google Scholar]
- Shimada M, Koide T, Kuroda E, Tsuboyama N, Hosokawa Y, Watanabe M. Expression and localization of cysteine dioxygenase mRNA in the liver, lung, and kidney of the rat. Amino Acids. 1998;15:143–150. doi: 10.1007/BF01345287. [DOI] [PubMed] [Google Scholar]
- Simmons CR, Hirschberger LL, Machi MS, Stipanuk MH. Expression, purification, and kinetic characterization of recombinant rat cysteine dioxygenase, a non-heme metalloenzyme necessary for regulation of cellular cysteine levels. Protein Expr Purif. 2006a;47:74–81. doi: 10.1016/j.pep.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Simmons CR, Liu Q, Huang Q, Hao Q, Begley TP, Karplus PA, Stipanuk MH. Crystal structure of mammalian cysteine dioxygenase. A novel mononuclear iron center for cysteine thiol oxidation. J Biol Chem. 2006b;281:18723–18733. doi: 10.1074/jbc.M601555200. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Metabolism of sulfur-containing amino acids. Annu Rev Nutr. 1986;6:179–209. doi: 10.1146/annurev.nu.06.070186.001143. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004a;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem Res. 2004b;29:105–110. doi: 10.1023/b:nere.0000010438.40376.c9. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Dominy JE., Jr Surprising insights that aren’t so surprising in the modeling of sulfur amino acid metabolism. Amino Acids. 2006;30:251–256. doi: 10.1007/s00726-005-0288-4. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr. 2002;132:3369–3378. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am J Physiol Endocrinol Metab. 2004a;286:E439–E448. doi: 10.1152/ajpendo.00336.2003. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Londono M, Hirschberger LL, Hickey C, Thiel DJ, Wang L. Evidence for expression of a single distinct form of mammalian cysteine dioxygenase. Amino Acids. 2004b;26:99–106. doi: 10.1007/s00726-003-0001-4. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Dominy JE, Jr, Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr. 2006;136:1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- Ueki I, Stipanuk MH. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr. 2007;137:1887–1894. doi: 10.1093/jn/137.8.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker MM, Whittaker JW. Cu(I)-dependent biogenesis of the galactose oxidase redox cofactor. J Biol Chem. 2003;278:22090–22101. doi: 10.1074/jbc.M300112200. [DOI] [PubMed] [Google Scholar]